Abstract
Thrombosis and restenosis are the most prevalent late complications of coronary artery stenting. Current standards of clinical care focus on prevention of smooth muscle proliferation by the use of drug-eluting stents able to release anti-proliferative drugs. Unfortunately, these drugs also block endothelial cell proliferation and, in this manner, prevent recovery of endothelial cell coverage. Continued lack of endothelial repair leaves the root cause of thrombosis and restenosis unchanged, creating a vicious cycle where drug-mediated prevention of restenosis simultaneously implies promotion of thrombosis.
In this issue of Vascular Pharmacology, Hussner and colleagues provide in vitro evidence and a mechanistic basis for the use of atorvastatin in stents as a way to bypass this roadblock. Here we review the pathological mechanisms and therapeutic approaches to restore flow in occluded arteries. We argue that rational design of drug eluting stents should focus on specific inhibition of smooth muscle proliferation with concurrent stimulation of endothelial regeneration. We comment on our poor understanding of the cellular and molecular regulation of endothelial cell proliferation in the context of a functional artery, and on the pitfalls of extrapolating from the well-studied process of neovascularization by sprouting vessel formation.
Keywords: endothelial regeneration, restenosis, stenosis, thrombosis, vascular injury
Graphical abstract
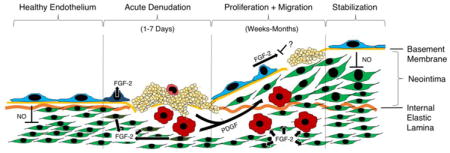
1. Introduction
Evolution has endowed vertebrate blood vessels with a fragile inner lining that interfaces with circulating blood [1]. This endothelial lining is naturally protected from mechanical trauma because of its location. However, endovascular balloon angioplasty and stent deployment mechanically damage the lining. These procedures create areas of denudation injury where stent struts, basement membrane, and/or vascular smooth muscle cells are exposed to the blood [2]. Unlike the intact endothelium, these surfaces do not offer an anti-thrombotic surface and instead promote platelet binding, activation of the clotting cascade, and thrombosis [3]. In addition to thrombosis, loss of endothelial signaling and subsequent cytokine release by platelets and macrophages stimulates the migration and proliferation of smooth muscle cells, resulting in the formation of a neointimal layer that further occludes the lumen and promotes stenosis of the involved vessel [4].
Balloon angioplasty, bare metal stents, and drug eluting stents have been sequentially developed in an effort to prevent vessel restenosis (Fig 1). Drug eluting stents first approved in the early 2000s effectively do so: they achieve local, non-specific inhibition of cell proliferation and halt neointima formation. However, they exacerbate the pro-thrombotic phenotype [5]. Research efforts in this area have resulted in incremental improvements. These include changes in stent structure and materials to reduce the inflammatory response, as well as the development of drug derivatives with modified pharmacokinetics [6,7]. However, the root cause of both thrombosis and restenosis remains unaddressed: poor regeneration of the endothelial lining.
Figure 1.
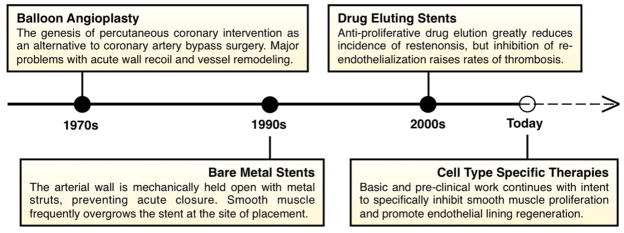
The evolution of angioplasty and stenting. Listed are the major developments and clinical procedures applied to address arterial occlusion.
2. Consequences of arterial denudation: Thrombosis and restenosis
Approximately one million coronary artery stents are placed in the United States each year, dwarfing the incidence of all other sources of denudation injury [8]. Typically a balloon-tipped catheter wrapped with a collapsed stent is inserted through the skin into a peripheral artery, advanced retrograde to flow to the root of the aorta, and then to the site of occlusion. While the coronary arteries are the major site of stent deployment, other sites are also frequent. Vessels that are common targets for stent placement include the proximal carotid artery and carotid bifurcation, the renal branches, and lower extremity arterial bifurcations [8].
Current guidelines recommend aggressive balloon inflation to restore the artery to its full diameter (angioplasty) and ensure stents are completely expanded to be flush with the arterial wall [9]. Balloon angioplasty alone is known to cause loss of the endothelial lining and damage to the arterial media [10]; intuitively, stenting results in comparable or more severe damage [11–14]. It is likely that additional undocumented endothelial denudation injuries are created with high incidence during the process of stent placement. In fact, studies in animal models have indicated that surgical clamping alone is sufficient to cause loss of the endothelial layer [15,16]. Catheter contact with the vessel wall is also sufficient to cause denudation, an underappreciated source of endothelial injury [17]. In sum, physician-induced injury of the coronary arteries during stent deployment is an extremely common cause of endothelial lining loss. This loss creates clinical complications without adequate solutions.
Currently, one of the most important late complications of denudation injury is thrombosis (Figure 2). The causal link between denudation and thrombosis is well established: thrombosis occurs upon platelet activation due to exposure to non-endothelial surfaces and initiation of the coagulation cascade [18]. Following stenting and accompanying denudation injury, patients are at great risk for a thrombotic event leading to myocardial infarct or death [3,19]. Currently, patients receive dual antiplatelet therapy with aspirin and a platelet P2Y12 receptor designed to prevent thrombus formation [20]. Beyond the significant morbidity and mortality risks associated with thrombosis (stroke, infarct), the therapy to prevent thrombosis has additional shortcomings. Illustrating these, a recent phase III trial found no upper bound on the duration of benefit from dual antiplatelet therapy when patients were followed to 30 months, even when considering only the subgroup who had state of the art “second generation” stents placed [20]. Another study found that thrombosis risk from implantation of sirolimus eluting stents extends to at least five years [21]. The findings indicate that patients face a careful, prolonged balancing act between bleeding and thrombosis risks, as well as an indefinite requirement for medication.
Figure 2.
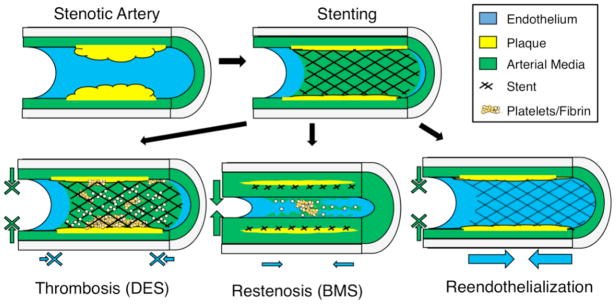
Biological responses following stent deployment. Bare metal stents (BMS) elicit a reendothelialization response, but also induce smooth muscle cell proliferation leading to restenosis (bottom left). Drug eluting stents (DES) releasing sirolimus and derivatives inhibit smooth muscle cell proliferation, but also prevent reendothelialization. Lack of endothelial coverage results in thrombosis (bottom middle). Regeneration of the endothelial lining prevents thrombosis and inhibits smooth muscle proliferation (bottom right).
In addition to thrombosis, denudation injury frequently causes restenosis. Evidence for a causal link between denudation and neointima formation in humans is limited. However, animal models clearly show correlation between the two [22,23]. The advent of anti-proliferative drug eluting stents has reduced the incidence of restenosis to an estimated 2–20% of stented patients from an estimated 30–40% when using bare metal stents, depending on selection criteria [4]. In absolute terms, restenosis remains prevalent due to the large population receiving interventions [4] – and it often necessitates a costly and dangerous second vascular intervention [24].
In summary, stent placement is a common procedure which causes denudation injury of the arterial wall. Thrombosis and restenosis are natural consequences of endothelial denudation which continue to severely impact human health. Next, we discuss the evidence that regeneration of the endothelial lining effectively prevents thrombosis and restenosis.
3. Regeneration of the endothelial lining prevents thrombosis and restenosis
Effective repair of the endothelial lining restores an antithrombotic inner vessel surface and effectively inhibits thrombosis. At autopsy [11–14] and in animal models [25], increased coverage of stent struts by endothelium correlates with reduced rates of thrombotic events. In the case where drug-eluting stents are used, and regeneration is inhibited, increased rates of thrombosis are observed [11,26–29]. A causal relationship has been found in animal models. Experimental inhibition of endothelial repair after denudation results in dramatically elevated rates of thrombosis [30].
Evidence for the effect of endothelial lining repair on restenosis is more limited, albeit still consistent. Gentle removal of the endothelial layer was found to be sufficient to promote neointima formation [22]. Furthermore, denudation injury models with endothelial lining regeneration correlate closely with reduced or absent neointima formation [31–33], and there is evidence that it may even cause regression [32].
The findings above prompted experiments to test whether endothelial re-seeding at the site of injury would be beneficial and promote repair of the intima. Unfortunately, the process is not simple. Attempts to regenerate endothelial lining by seeding cells was found functionally ineffective and still permissive of neointima formation [34]. Though not demonstrated, it is likely that the newly seeded endothelium is “dysfunctional” in the sense of being unable to release nitric oxide and other anti-proliferative vasodilators [35]. Notably, such dysfunction also appears to occur in humans at sites of stenting [36]. Therefore, regeneration of a functional endothelial lining (as defined by flow-mediated dilation) is likely to prevent restenosis, while a dysfunctional endothelial lining remains permissive to neointima formation. Thus, rapid and complete stent reendothelialization by proliferation of the endothelium from the wounded margins, but not necessarily through seeding would be expected to prevent restenosis.
4. Drug eluting stents: Trading restenosis for thrombosis
The use of drug eluting stents creates a therapeutic trade-off: restenosis is prevented by inhibition of smooth muscle proliferation, but thrombosis is promoted by inhibition of endothelial proliferation [5]. This is true even for state of the art “second generation” DES which elute everolimus [37] or zotarolimus [38]. Sirolimus and its derivatives act through disruption of the mTOR metabolic sensor complex to inhibit downstream Akt signaling, ultimately resulting in impaired cell migration and cell cycle arrest in G1 [39]. Evidence for the effect of DES implantation on endothelial lining regeneration is strong, in both humans and animals. Autopsy evaluations suggests that lack of reendothelialization is a common trait among thrombotic events occurring out to five years post stent placement, and even beyond [11,13,14,21,29]. Clinical follow-up of stented patients under prolonged dual antiplatelet therapy suggests that endothelial lining regeneration was not completed years after placement [20]. Even the slowest eluting stents come to release an undetectable level of drug after six months [40]. Suggesting that thrombosis is the outcome of a permanent defect in endothelial proliferation. In sum, while effective, drug eluting stents designed to block cell proliferation result in uncovered thrombogenic surfaces, leading to elevated and prolonged risk for clotting events.
5. Cellular and molecular mechanisms of endothelial lining regeneration
At the cellular level, the biological response to stent-induced denudation injury proceeds through defined stages [19,41] (Figure 3). Acutely, platelets adhere to the denuded vessel wall [42]. Provided the medial layer is injured [43], over the following days neutrophils and monocytes infiltrate the arterial media [44–48]. Subsequently, and depending on the presence or absence of pharmacological inhibition, the intact endothelial border adjacent to the injury undergoes coordinated migration as a “front” of cells to begin covering the denuded area [32,33]. For unknown reasons, this migratory front stops at a certain point, and the injury stabilizes with an area that remains uncovered by endothelium (a “vascular ulcer”) [22,43] resulting in thrombosis, release of PDGF and neointima formation.
Figure 3.
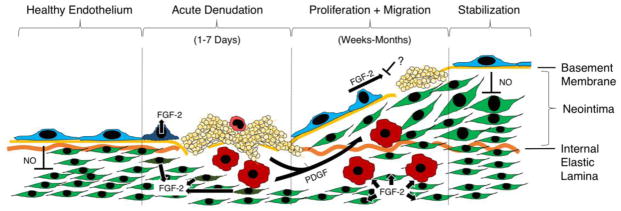
Major molecular signals driving endothelial lining regeneration and neointima formation following denudation injury. Leftmost: Healthy endothelial lining suppresses smooth muscle proliferation through release of nitric oxide (NO). Middle left: Following denudation injury, damaged endothelial and smooth muscle cells release stored basic fibroblast growth factor (FGF-2). Platelets adhere to the site of denudation and degranulate to release stored platelet derived growth factor (PDGF). Monocytes adhere and enter the vessel wall, where they also secrete PDGF. Middle right: Following acute injury, PDGF stimulation results in smooth muscle cell migration from the arterial media toward the lumen, forming a neointima. FGF-2 stimulation results in the proliferation of both endothelial cells and smooth muscle cells. Endothelial proliferation is frequently incomplete. Rightmost: Depending on size and degree of medial damage, a functional endothelial lining may eventually regenerate and resume inhibition of smooth muscle proliferation via NO release.
The origin of the endothelial cells that form the regenerated lining remains controversial. It has been attributed to circulating progenitor cells (themselves controversial [49]), tissue resident stem or progenitor cells [50], and simple proliferation of pre-existing differentiated endothelial cells [33].
It is important to note that extent and characteristics of the injury are dominant factors in determining the healing response. If the denudation injury is small and involves the tunica media, or if larger and created without damaging the media. Usually, lack of damage to the media results in complete regeneration of the endothelial lining with minimal neointima formation [43]. Larger injuries involving the media, as typical of humans, often never achieve complete endothelial coverage [21]. Moreover, human denudation injuries heal more slowly than in animal models [42]. In animal models, a stent-sized denudation injury heals in approximately one month [42]. In humans, autopsy reveals a healing time of at least three months [51]. The reasons for slower healing kinetics in human are not clear, but variation in patient characteristics such as age and extent of atherosclerotic plaque likely have a significant effect on the ability of the endothelial layer to regenerate.
At the molecular level, the signals and pathways underlying endothelial lining regeneration remain murky – though at least some growth factors and other molecules with important roles in endothelial lining regeneration have been identified (Figure 3).
Basic fibroblast growth factor (FGF2) is an important mitogen for both endothelial cells and smooth muscle cells following denudation injury [52]. In endothelial cells, FGF2 signaling occurs at the leading edge of lining regeneration [43]. In cases where regeneration is incomplete, FGF2 is absent from the border of existing lining [43]. Stimulation of incompletely regenerated endothelial lining with FGF2 causes further mitotic activity and extends lining coverage [53]. While in vivo data are lacking, in vitro a gradient of FGF2 is sufficient to promote endothelial cell migration [54]. In smooth muscle, FGF2 signaling similarly results in increased mitotic activity, leading to neointima formation [55]. Stimulation with FGF2 results in increased neointima thickness [55]. Antibody-based inhibition results in reduced neointimal thickness due to a decrease in vascular smooth muscle proliferation [56]. Further, FGF2 has a role in at least vascular smooth muscle migration [57]. Antibody-based inhibition of FGF-2 signaling results in blockade of smooth muscle proliferation [56]. Importantly, FGF-2 knockout mice still form hyperplastic neointima following denudation injury [58], proving that other, unknown factors are sufficient to cause this pathology.
Platelet derived growth factor is a smooth muscle specific mitogen and migratory factor occurring in A and B isoforms. It is predominately expressed as the PDGF-AB heterodimer by human platelets, and PDGF-BB in most other cell types and species [59]. Sources of PDGF include platelets [60], macrophages [61], and endothelial cells [62]. PDGF dimers are in turn detected primarily by PDGF receptors on smooth muscle cells [63]. These receptors are upregulated in response to denudation injury [63]. The migration of vascular smooth muscle cells from media to intima is primarily driven by platelet derived growth factor B dimers (PDGF-BB) [64–66]; antibody inhibition reduces neointima thickness by preventing migration, but has no effect on proliferative indices [65].
VEGF-A
Despite its role as master regulator and potent endothelial cell mitogen during sprouting vessel formation [67], evidence for efficacy of VEGF-A in stimulating endothelial lining repair is mixed [25,68–78]. One way to reconcile existing data on VEGF-A effects in regenerating endothelium is to consider the factor as effective only in combination with other growth factors. There is good evidence that this is the case. FGF-2 causes upregulation of the transcription factor ATF-4, which in turn controls transcription of VEGF-A. Thus, regulation of VEGF-A expression occurs downstream of FGF-2 signaling in the arterial endothelial lining – a marked contrast to angiogenesis, in which VEGF-A is the initiator endothelial sprouting and proliferation [67]. VEGF-A and FGF-2 have a synergistic effect on cellular proliferation in both endothelial and smooth muscle cells [74,75]. The same is true of VEGF-A and PDGF-B. In vivo, the two have a synergistic effect on cellular proliferation [70]. In vitro, PDGF treatment of smooth muscle cells results in VEGF-A expression, placing VEGF-A downstream of PDGF-B similar to its relation to FGF-2, and again in contrast to sprouting angiogenesis [79,80].
Nitric oxide (NO)
A potent vasodilator produced by endothelial cells with extremely short range paracrine action, nitric oxide effectively inhibits smooth muscle proliferation in animal models of denudation injury [81]. One mechanism of this inhibition has been determined in an in vitro co-culture system, namely that endothelial cell derived NO inhibits the function of ornithine decarboxylase in smooth muscle cells [23], preventing polyamine synthesis and cellular proliferation.
Other molecules
Additional molecular signaling pathways have been implicated in regeneration of the endothelial lining, but we have chosen not to emphasize them in this review due to limited space and relatively weaker evidence speaking to biological relevance. This is not to say that they are unimportant. These factors include, but are not limited to, the TGF-β family, estradiol, angiotensin, intermedin, the Notch receptor family and its ligands, fibronectin and other extracellular matrix molecules, and VE-Cadherin/Wnt signaling.
Inhibition of endothelial repair
The reason(s) as to why endothelial lining repair is often incomplete is unknown. It is not, however, due to a limited replicative potential of endothelial cells bordering the denudation injury: inhibition does not occur in the absence of injury to the medial wall, even for large areas of denudation [43]. A fascinating study performed by Reidy et al. suggests that endothelial cells’ proliferation decision is not determined by an inhibitory factor release from smooth muscle cells [82], and opens the possibility of recapitulating and studying the defect in vitro using an arterial endothelial culture monolayer under flow conditions.
6. State of basic scientific research in endothelial repair
Information on how a functional blood vessel promotes repair and regeneration of its endothelial lining is presently scant. Most of the experimental data available were obtained in the 1980s and 1990s, before the widespread application of powerful transcriptome analysis tools and transgenic animal models. A clear molecular and mechanistic definition of the endothelial lining regeneration process is overdue and key to both the development of therapeutics with differential inhibition of vascular smooth muscle as compared to endothelial cells, and to agents which directly stimulate endothelial cell proliferation and lining repair. Unbiased approaches to identifying the molecular signaling pathways regulating endothelial regeneration are now possible, and are likely to uncover additional important regulators. Context, however, is critical. In many cases, knowledge from angiogenesis models is anticipated to directly apply to a fully functional, pulsatile artery with flowing blood. The differences between the invading front of a sprout and the inner lining of an injured aorta are broad and meaningful. New animal models that can provide accurate molecular information are essential to make concrete advances in basic science to support this unmet clinical need. Meanwhile, a few efforts with pre-clinical and clinical models appear promising but information on basic biological mechanisms is urgently needed.
7. Therapeutic promotion of endothelial lining regeneration
A number of attempts have been made to specifically inhibit vascular smooth muscle proliferation and promote endothelial lining repair. Below we discuss the genetic and pharmacological approaches undertaken in the last ten years.
Genetic
Tools for delivering recombinant DNA in vivo are still at the early stages of pre-clinical development, but offer unprecedented power to regulate cell specificity and target particular molecular pathways. A recent study provided proof-of-concept for the elegant technique of including an endothelial-specific microRNA target sequence on the transcript of a cell cycle blockade protein [30]. Using this approach, only non-endothelial cells exhibited cell cycle arrest; while endothelial cells showed quick degradation of the transcript. Cleverly, the authors used expression of the same cell cycle blockade protein upregulated by sirolimus and its derivatives to achieve inhibition of cell cycle progression. In concept, a similar specificity for endothelial cell proliferation driven by FGF-2 could be achieved by viral transduction of a downstream proliferation driver under the control of an endothelial specific promoter. Unfortunately genetic approaches, while powerful, represent major changes in methodology and involve significant obstacles, both technical and regulatory, to bring to the clinic.
Statins
In this issue Hussner et al., report that specific statins may have differential uptake by smooth muscle cells and endothelial cells, promoting differential inhibition of proliferation. This represents an attractive strategy, as existing drug eluting stents designs might be adapted for statin elution, or statins might even be given orally following stent deployment. Cerivastatin eluting stents have been tested in animal models with moderate success [83,84]. Hussner et al. report that OAT2B1 transporter mediated uptake in vascular smooth muscle is specific to atorvastatin, meriting further investigation for increased efficacy and vascular smooth muscle specificity relative to cerivastatin in vivo.
CD34 coated stents
Stents coated with an anti-CD34 antibody have been developed in an attempt to capture circulating “endothelial progenitor cells” (EPCs) at the site of stent placement [85–87]. However, in vivo animal model evidence does not support the function of so-called EPCs as precursors to endothelial cells [49,88]. Despite their adoption of an endothelial phenotype in culture, these cells appear to be lineage-restricted monocytes which secrete multiple paracrine growth factors affecting endothelial cells [89]. Whether these cells have favorable paracrine effect on endothelial cells at the site of denudation injury remains to be determined. Nonetheless, pre-clinical data from animal models is promising: restenosis rates appear to be similar to first generation paclitaxel eluting stents, and reendothelialization appears to be augmented [87].
8. Conclusions
Loss of the endothelial lining is at the root of thrombosis and restenosis following stent deployment. Regeneration of the endothelial lining after denudation is possible in humans and animal models, and effectively prevents both complications. However, current stent designs and a great deal of ongoing research neglect regeneration of the endothelial lining as a therapeutic goal. Doing so limits progress to only incremental improvements in patient outcomes. Therapeutically promoting regeneration of the endothelial lining may be possible. Further understanding of the cellular and molecular mechanisms driving regeneration of the endothelium in adult arteries is likely to uncover novel means of therapeutically stimulate endothelial growth.
Acknowledgments
This work was supported by funds from MSTP Institutional Training Grant: 5T32GM008042 and 5T32HL069766.
Abbreviations
- BMS
bare metal stents
- DES
drug eluting stents
- FGF-2
basic fibroblast growth factor
- PDGF
platelet derived growth factor
- NO
nitric oxide
- VEGF
vascular endothelial growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Monahan-Earley R, Dvorak aM, Aird WC. Evolutionary origins of the blood vascular system and endothelium. J Thromb Haemost. 2013;11:46–66. doi: 10.1111/jth.12253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rogers C, Parikh S, Seifert P, Edelman ER. Endogenous Cell Seeding: Remnant Endothelium After Stenting Enhances Vascular Repair. Circulation. 1996;94:2909–14. doi: 10.1161/01.CIR.94.11.2909. [DOI] [PubMed] [Google Scholar]
- 3.Otsuka F, Finn AV, Yazdani SK, Nakano M, Kolodgie FD, Virmani R. The importance of the endothelium in atherothrombosis and coronary stenting. Nat Rev Cardiol. 2012;9:439–53. doi: 10.1038/nrcardio.2012.64. [DOI] [PubMed] [Google Scholar]
- 4.Farooq V, Gogas BD, Serruys PW. Restenosis: Delineating the numerous causes of drug-eluting stent restenosis. Circ Cardiovasc Interv. 2011;4:195–205. doi: 10.1161/CIRCINTERVENTIONS.110.959882. [DOI] [PubMed] [Google Scholar]
- 5.Bavry AA, Bhatt DL. Appropriate use of drug-eluting stents: balancing the reduction in restenosis with the concern of late thrombosis. Lancet. 2008;371:2134–43. doi: 10.1016/S0140-6736(08)60922-8. [DOI] [PubMed] [Google Scholar]
- 6.Stefanini GG, Taniwaki M, Windecker S. Coronary stents: novel developments. Heart. 2014;100:1051–61. doi: 10.1136/heartjnl-2012-303522. [DOI] [PubMed] [Google Scholar]
- 7.Escárcega RO, Baker NC, Lipinski MJ, Magalhaes MA, Minha S, Omar A-F, et al. Current application and bioavailability of drug-eluting stents. Expert Opin Drug Deliv. 2014;11:689–709. doi: 10.1517/17425247.2014.888054. [DOI] [PubMed] [Google Scholar]
- 8.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. 2014;129 doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl Ja, Cercek B, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention. J Am Coll Cardiol. 2011;58 doi: 10.1016/j.jacc.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 10.Austin GE, Ratliff NB, Hollman J, Tabei S, Phillips DF. Intimal proliferation of smooth muscle cells as an explanation for recurrent coronary artery stenosis after percutaneous transluminal coronary angioplasty. J Am Coll Cardiol. 1985;6:369–75. doi: 10.1016/S0735-1097(85)80174-1. [DOI] [PubMed] [Google Scholar]
- 11.Finn AV, Joner M, Nakazawa G, Kolodgie F, Newell J, John MC, et al. Pathological correlates of late drug-eluting stent thrombosis: strut coverage as a marker of endothelialization. Circulation. 2007;115:2435–41. doi: 10.1161/CIRCULATIONAHA.107.693739. [DOI] [PubMed] [Google Scholar]
- 12.Finn AV, Nakazawa G, Joner M, Kolodgie FD, Mont EK, Gold HK, et al. Vascular responses to drug eluting stents: importance of delayed healing. Arterioscler Thromb Vasc Biol. 2007;27:1500–10. doi: 10.1161/ATVBAHA.107.144220. [DOI] [PubMed] [Google Scholar]
- 13.Nakazawa G, Finn AV, Joner M, Ladich E, Kutys R, Mont EK, et al. Delayed arterial healing and increased late stent thrombosis at culprit sites after drug-eluting stent placement for acute myocardial infarction patients: an autopsy study. Circulation. 2008;118:1138–45. doi: 10.1161/CIRCULATIONAHA.107.762047. [DOI] [PubMed] [Google Scholar]
- 14.Farb A, Burke AP, Kolodgie FD, Virmani R. Pathological mechanisms of fatal late coronary stent thrombosis in humans. Circulation. 2003;108:1701–6. doi: 10.1161/01.CIR.0000091115.05480.B0. [DOI] [PubMed] [Google Scholar]
- 15.Jelev L, Romansky R, Guirov K, Minkov M. Morphological changes in the rat aorta endothelium at the clamping sites. Scr Sci Medica. 2013;45:57–9. doi: 10.14748/ssm.v45i0.840. [DOI] [Google Scholar]
- 16.Gucu A, Cavusoglu I, Bozkurt O, Eris C, Toktas F, Goncu T, et al. Effects of temporary vascular occluder poloxamer 407 gel on the endothelium. J Cardiothorac Surg. 2013;8:16. doi: 10.1186/1749-8090-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forauer AR, Theoharis C. Histologic Changes in the Human Vein Wall Adjacent to Indwelling Central Venous Catheters. J Vasc Interv Radiol. 2003;14:1163–8. doi: 10.1097/01.RVI.0000086531.86489.4C. [DOI] [PubMed] [Google Scholar]
- 18.Cutlip DE, Nakazawa G, Krucoff MW, Vorpahl M, Mehran R, Finn AV, et al. Autopsy validation study of the academic research consortium stent thrombosis definition. JACC Cardiovasc Interv. 2011;4:554–9. doi: 10.1016/j.jcin.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 19.Chaabane C, Otsuka F, Virmani R, Bochaton-Piallat ML. Biological responses in stented arteries. Cardiovasc Res. 2013;99:353–63. doi: 10.1093/cvr/cvt115. [DOI] [PubMed] [Google Scholar]
- 20.Mauri L, Kereiakes DJ, Yeh RW, Driscoll-Shempp P, Cutlip DE, Steg PG, et al. Twelve or 30 Months of Dual Antiplatelet Therapy after Drug-Eluting Stents. N Engl J Med. 2014;371:2155–66. doi: 10.1056/NEJMoa1409312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kimura T, Morimoto T, Nakagawa Y, Kawai K, Miyazaki S, Muramatsu T, et al. Very Late Stent Thrombosis and Late Target Lesion Revascularization after Sirolimus-Eluting Stent Implantation: Five-Year Outcome of the j-Cypher Registry. Circulation. 2012;125:584–91. doi: 10.1161/CIRCULATIONAHA.111.046599. [DOI] [PubMed] [Google Scholar]
- 22.Fingerle J, Au YP, Clowes AW, Reidy MA. Intimal lesion formation in rat carotid arteries after endothelial denudation in absence of medial injury. Arteriosclerosis. 10:1082–7. doi: 10.1161/01.atv.10.6.1082. [DOI] [PubMed] [Google Scholar]
- 23.Ignarro LJ, Buga GM, Wei LH, Bauer PM, Wu G, del Soldato P. Role of the arginine-nitric oxide pathway in the regulation of vascular smooth muscle cell proliferation. Proc Natl Acad Sci U S A. 2001;98:4202–8. doi: 10.1073/pnas.071054698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piccolo R, Galasso G, Piscione F, Esposito G, Trimarco B, Dangas GD, et al. Meta-Analysis of Randomized Trials Comparing the Effectiveness of Different Strategies for the Treatment of Drug-Eluting Stent Restenosis. Am J Cardiol. 2014;114:1339–46. doi: 10.1016/j.amjcard.2014.07.069. [DOI] [PubMed] [Google Scholar]
- 25.Joner M, Nakazawa G, Finn AV, Quee SC, Coleman L, Acampado E, et al. Endothelial cell recovery between comparator polymer-based drug-eluting stents. J Am Coll Cardiol. 2008;52:333–42. doi: 10.1016/j.jacc.2008.04.030. [DOI] [PubMed] [Google Scholar]
- 26.Vorpahl MK, Yazdani S, Nakano M, Ladich ED, Kolodgie FV, Finn A, et al. Pathobiology of Stent Thrombosis after Drug-Eluting Stent Implantation. Curr Pharm Des. 2010;16:4064–71. doi: 10.2174/138161210794454879. [DOI] [PubMed] [Google Scholar]
- 27.Yahagi K, Joner M, Virmani R. Insights into very late stent thrombosis from the wisdom of pathology. J Invasive Cardiol. 2014;26:417–9. [PubMed] [Google Scholar]
- 28.Nakano M, Otsuka F, Yahagi K, Sakakura K, Kutys R, Ladich ER, et al. Human autopsy study of drug-eluting stents restenosis: histomorphological predictors and neointimal characteristics. Eur Heart J. 2013;34:3304–13. doi: 10.1093/eurheartj/eht241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaliyadan A, Siu H, Fischman DL, Ruggiero NJ, Jasti B, Walinsky P, et al. “Very” very late stent thrombosis: acute myocardial infarction from drug-eluting stent thrombosis more than 5 years after implantation. J Invasive Cardiol. 2014;26:413–6. [PubMed] [Google Scholar]
- 30.Santulli G, Wronska A, Uryu K, Diacovo TG, Gao M, Marx SO, et al. A selective microRNA-based strategy inhibits restenosis while preserving endothelial function. J Clin Invest. 2014;124 doi: 10.1172/JCI76069DS1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fishman JA, Ryan GB, Karnovsky MJ. Endothelial regeneration in the rat carotid artery and the significance of endothelial denudation in the pathogenesis of myointimal thickening. Lab Invest. 1975;32:339–51. [PubMed] [Google Scholar]
- 32.Stemerman MB, Spaet TH, Pitlick F, Cintron J, Lejnieks I, Tiell ML. Intimal healing. The pattern of reendothelialization and intimal thickening. Am J Pathol. 1977;87:125–42. [PMC free article] [PubMed] [Google Scholar]
- 33.Haudenschild CC, Schwartz SM. Endothelial regeneration. II.Restitution of endothelial continuity. Lab Invest. 1979;41:407–18. [PubMed] [Google Scholar]
- 34.Conte MS, Choudhry RP, Shirakowa M, Fallon JT, Birinyi LK. Endothelial cell seeding fails to attenuate intimal thickening in balloon-injured rabbit arteries. J Vasc Surg. 1995;21:413–21. doi: 10.1016/S0741-5214(95)70283-0. [DOI] [PubMed] [Google Scholar]
- 35.Lerman A. Restenosis: another “dysfunction” of the endothelium. Circulation. 2005;111:8–10. doi: 10.1161/01.CIR.0000152694.27996.3E. [DOI] [PubMed] [Google Scholar]
- 36.Patti G, Pasceri V, Melfi R, Goffredo C, Chello M, D’Ambrosio A, et al. Impaired flow-mediated dilation and risk of restenosis in patients undergoing coronary stent implantation. Circulation. 2005;111:70–5. doi: 10.1161/01.CIR.0000151308.06673.D2. [DOI] [PubMed] [Google Scholar]
- 37.Schuler W, Sedrani R, Cottens S, Häberlin B, Schulz M, Schuurman HJ, et al. SDZ RAD, a new rapamycin derivative: pharmacological properties in vitro and in vivo. Transplantation. 1997;64:36–42. doi: 10.1097/00007890-199707150-00008. [DOI] [PubMed] [Google Scholar]
- 38.Chen Y-W, Smith ML, Sheets M, Ballaron S, Trevillyan JM, Burke SE, et al. Zotarolimus, a novel sirolimus analogue with potent anti-proliferative activity on coronary smooth muscle cells and reduced potential for systemic immunosuppression. J Cardiovasc Pharmacol. 2007;49:228–35. doi: 10.1097/FJC.0b013e3180325b0a. [DOI] [PubMed] [Google Scholar]
- 39.Guertin DA, Sabatini DM. The Pharmacology of mTOR Inhibition. Sci Signal. 2009;2:pe24–pe24. doi: 10.1126/scisignal.267pe24. [DOI] [PubMed] [Google Scholar]
- 40.Yang C, Burt HM. Drug-eluting stents: factors governing local pharmacokinetics. Adv Drug Deliv Rev. 2006;58:402–11. doi: 10.1016/j.addr.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 41.Welt FGP. Inflammation and Restenosis in the Stent Era. Arterioscler Thromb Vasc Biol. 2002;22:1769–76. doi: 10.1161/01.ATV.0000037100.44766.5B. [DOI] [PubMed] [Google Scholar]
- 42.Virmani R. Drug eluting stents: are human and animal studies comparable? Heart. 2003;89:133–8. doi: 10.1136/heart.89.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lindner V, Reidy MA, Fingerle J. Regrowth of arterial endothelium. Denudation with minimal trauma leads to complete endothelial cell regrowth. Lab Invest. 1989;61:556–63. [PubMed] [Google Scholar]
- 44.Carter AJ, Laird JR, Farb A, Kufs W, Wortham DC, Virmani R. Morphologic characteristics of lesion formation and time course of smooth muscle cell proliferation in a porcine proliferative restenosis model. J Am Coll Cardiol. 1994;24:1398–405. doi: 10.1016/0735-1097(94)90126-0. [DOI] [PubMed] [Google Scholar]
- 45.Taylor AJ, Gorman PD, Kenwood B, Hudak C, Tashko G, Virmani R. A comparison of four stent designs on arterial injury, cellular proliferation, neointima formation, and arterial dimensions in an experimental porcine model. Catheter Cardiovasc Interv. 2001;53:420–5. doi: 10.1002/ccd.1194. [DOI] [PubMed] [Google Scholar]
- 46.Rogers C, Welt FGP, Karnovsky MJ, Edelman ER. Monocyte Recruitment and Neointimal Hyperplasia in Rabbits: Coupled Inhibitory Effects of Heparin. Arterioscler Thromb Vasc Biol. 1996;16:1312–8. doi: 10.1161/01.ATV.16.10.1312. [DOI] [PubMed] [Google Scholar]
- 47.Farb A. Morphological Predictors of Restenosis After Coronary Stenting in Humans. Circulation. 2002;105:2974–80. doi: 10.1161/01.CIR.0000019071.72887.BD. [DOI] [PubMed] [Google Scholar]
- 48.Moreno PR, Bernardi VH, Lopez-Cuellar J, Newell JB, McMellon C, Gold HK, et al. Macrophage Infiltration Predicts Restenosis After Coronary Intervention in Patients With Unstable Angina. Circulation. 1996;94:3098–102. doi: 10.1161/01.CIR.94.12.3098. [DOI] [PubMed] [Google Scholar]
- 49.Purhonen S, Palm J, Rossi D, Kaskenpää N, Rajantie I, Ylä-Herttuala S, et al. Bone marrow-derived circulating endothelial precursors do not contribute to vascular endothelium and are not needed for tumor growth. Proc Natl Acad Sci U S A. 2008;105:6620–5. doi: 10.1073/pnas.0710516105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Basile DP, Yoder MC. Circulating and tissue resident endothelial progenitor cells. J Cell Physiol. 2014;229:10–6. doi: 10.1002/jcp.24423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grewe PH, Deneke T, Machraoui A, Barmeyer J, Müller KM. Acute and chronic tissue response to coronary stent implantation: pathologic findings in human specimen. J Am Coll Cardiol. 2000;35:157–63. doi: 10.1016/s0735-1097(99)00486-6. [DOI] [PubMed] [Google Scholar]
- 52.Lindner V, Reidy MA. Expression of basic fibroblast growth factor and its receptor by smooth muscle cells and endothelium in injured rat arteries. An en face study. Circ Res. 1993;73:589–95. doi: 10.1161/01.res.73.3.589. [DOI] [PubMed] [Google Scholar]
- 53.Lindner V, Majack RA, Reidy MA. Basic fibroblast growth factor stimulates endothelial regrowth and proliferation in denuded arteries. J Clin Invest. 1990;85:2004–8. doi: 10.1172/JCI114665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barkefors I, Le Jan S, Jakobsson L, Hejll E, Carlson G, Johansson H, et al. Endothelial cell migration in stable gradients of vascular endothelial growth factor A and fibroblast growth factor 2: effects on chemotaxis and chemokinesis. J Biol Chem. 2008;283:13905–12. doi: 10.1074/jbc.M704917200. [DOI] [PubMed] [Google Scholar]
- 55.Lindner V, Lappi Da, Baird a, Majack Ra, Reidy Ma. Role of basic fibroblast growth factor in vascular lesion formation. Circ Res. 1991;68:106–13. doi: 10.1161/01.RES.68.1.106. [DOI] [PubMed] [Google Scholar]
- 56.Lindner V, Reidy MA. Proliferation of smooth muscle cells after vascular injury is inhibited by an antibody against basic fibroblast growth factor. Proc Natl Acad Sci U S A. 1991;88:3739–43. doi: 10.1073/pnas.88.9.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jackson CL, Reidy MA. Basic fibroblast growth factor: its role in the control of smooth muscle cell migration. Am J Pathol. 1993;143:1024–31. [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou M, Sutliff RL, Paul RJ, Lorenz JN, Hoying JB, Haudenschild CC, et al. Fibroblast growth factor 2 control of vascular tone. Nat Med. 1998;4:201–7. doi: 10.1038/nm0298-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hughes AD, Clunn GF, Refson J, Demoliou-Mason C. Platelet-derived growth factor (PDGF): Actions and mechanisms in vascular smooth muscle. Gen Pharmacol Vasc Syst. 1996;27:1079–89. doi: 10.1016/S0306-3623(96)00060-2. [DOI] [PubMed] [Google Scholar]
- 60.Ross R, Glomset J, Kariya B, Harker L. A platelet-dependent serum factor that stimulates the proliferation of arterial smooth muscle cells in vitro. Proc Natl Acad Sci U S A. 1974;71:1207–10. doi: 10.1073/pnas.71.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shimokado K, Raines EW, Madtes DK, Barrett TB, Benditt EP, Ross R. A significant part of macrophage-derived growth factor consists of at least two forms of PDGF. Cell. 1985;43:277–86. doi: 10.1016/0092-8674(85)90033-9. [DOI] [PubMed] [Google Scholar]
- 62.Collins T, Ginsburg D, Boss JM, Orkin SH, Pober JS. Cultured human endothelial cells express platelet-derived growth factor B chain: cDNA cloning and structural analysis. Nature. 316:748–50. doi: 10.1038/316748a0. [DOI] [PubMed] [Google Scholar]
- 63.Sjölund M, Rahm M, Claesson-Welsh L, Sejersen T, Heldin CH, Thyberg J. Expression of PDGF alpha- and beta-receptors in rat arterial smooth muscle cells is phenotype and growth state dependent. Growth Factors. 1990;3:191–203. doi: 10.3109/08977199009043904. [DOI] [PubMed] [Google Scholar]
- 64.Friedman RJ, Stemerman MB, Wenz B, Moore S, Gauldie J, Gent M, et al. The effect of thrombocytopenia on experimental arteriosclerotic lesion formation in rabbits. Smooth muscle cell proliferation and re-endothelialization. J Clin Invest. 1977;60:1191–201. doi: 10.1172/JCI108872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ferns Ga, Raines EW, Sprugel KH, Motani aS, Reidy Ma, Ross R. Inhibition of neointimal smooth muscle accumulation after angioplasty by an antibody to PDGF. Science. 1991;253:1129–32. doi: 10.1126/science.1653454. [DOI] [PubMed] [Google Scholar]
- 66.Jawien A, Bowen-Pope DF, Lindner V, Schwartz SM, Clowes AW. Platelet-derived growth factor promotes smooth muscle migration and intimal thickening in a rat model of balloon angioplasty. J Clin Invest. 1992;89:507–11. doi: 10.1172/JCI115613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146:873–87. doi: 10.1016/j.cell.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 68.Lindner V, Reidy MA. Expression of VEGF receptors in arteries after endothelial injury and lack of increased endothelial regrowth in response to VEGF. Arterioscler Thromb Vasc Biol. 1996;16:1399–405. doi: 10.1161/01.atv.16.11.1399. [DOI] [PubMed] [Google Scholar]
- 69.Swanson N, Hogrefe K, Javed Q, Malik N, Gershlick AH. Vascular endothelial growth factor (VEGF)-eluting stents: in vivo effects on thrombosis, endothelialization and intimal hyperplasia. J Invasive Cardiol. 2003;15:688–92. [PubMed] [Google Scholar]
- 70.Shibata M, Suzuki H, Nakatani M, Koba S, Geshi E, Katagiri T, et al. The involvement of vascular endothelial growth factor and flt-1 in the process of neointimal proliferation in pig coronary arteries following stent implantation. Histochem Cell Biol. 2001;116:471–81. doi: 10.1007/s00418-001-0336-4. [DOI] [PubMed] [Google Scholar]
- 71.Van Belle E, Tio FO, Couffinhal T, Maillard L, Passeri J, Isner JM. Stent Endothelialization: Time Course, Impact of Local Catheter Delivery, Feasibility of Recombinant Protein Administration, and Response to Cytokine Expedition. Circulation. 1997;95:438–48. doi: 10.1161/01.CIR.95.2.438. [DOI] [PubMed] [Google Scholar]
- 72.Asahara T, Chen D, Tsurumi Y, Kearney M, Rossow S, Passeri J, et al. Accelerated restitution of endothelial integrity and endothelium-dependent function after phVEGF165 gene transfer. Circulation. 1996;94:3291–302. doi: 10.1161/01.cir.94.12.3291. [DOI] [PubMed] [Google Scholar]
- 73.Asahara T, Bauters C, Pastore C, Kearney M, Rossow S, Bunting S, et al. Local Delivery of Vascular Endothelial Growth Factor Accelerates Reendothelialization and Attenuates Intimal Hyperplasia in Balloon-Injured Rat Carotid Artery. Circulation. 1995;91:2793–801. doi: 10.1161/01.CIR.91.11.2793. [DOI] [PubMed] [Google Scholar]
- 74.Couper LL, Bryant SR, Eldrup-Jørgensen J, Bredenberg CE, Lindner V. Vascular endothelial growth factor increases the mitogenic response to fibroblast growth factor-2 in vascular smooth muscle cells in vivo via expression of fms-like tyrosine kinase-1. Circ Res. 1997;81:932–9. doi: 10.1161/01.res.81.6.932. [DOI] [PubMed] [Google Scholar]
- 75.Asahara T, Bauters C, Zheng LP, Takeshita S, Bunting S, Ferrara N, et al. Synergistic Effect of Vascular Endothelial Growth Factor and Basic Fibroblast Growth Factor on Angiogenesis In Vivo. Circulation. 1995;92:365–71. doi: 10.1161/01.CIR.92.9.365. [DOI] [PubMed] [Google Scholar]
- 76.Lazarous DF, Shou M, Scheinowitz M, Hodge E, Thirumurti V, Kitsiou AN, et al. Comparative Effects of Basic Fibroblast Growth Factor and Vascular Endothelial Growth Factor on Coronary Collateral Development and the Arterial Response to Injury. Circulation. 1996;94:1074–82. doi: 10.1161/01.CIR.94.5.1074. [DOI] [PubMed] [Google Scholar]
- 77.Van Belle E, Maillard L, Tio FO, Isner JM. Accelerated endothelialization by local delivery of recombinant human vascular endothelial growth factor reduces in-stent intimal formation. Biochem Biophys Res Commun. 1997;235:311–6. doi: 10.1006/bbrc.1997.6772. [DOI] [PubMed] [Google Scholar]
- 78.Malabanan KP, Kanellakis P, Bobik A, Khachigian LM. Activation transcription factor-4 induced by fibroblast growth factor-2 regulates vascular endothelial growth factor-A transcription in vascular smooth muscle cells and mediates intimal thickening in rat arteries following balloon injury. Circ Res. 2008;103:378–87. doi: 10.1161/CIRCRESAHA.107.168682. [DOI] [PubMed] [Google Scholar]
- 79.Brogi E, Wu T, Namiki A, Isner JM. Indirect angiogenic cytokines upregulate VEGF and bFGF gene expression in vascular smooth muscle cells, whereas hypoxia upregulates VEGF expression only. Circulation. 1994;90:649–52. doi: 10.1161/01.cir.90.2.649. [DOI] [PubMed] [Google Scholar]
- 80.Stavri GT, Hong Y, Zachary IC, Breier G, Baskerville PA, Ylä-Herttuala S, et al. Hypoxia and platelet-derived growth factor-BB synergistically upregulate the expression of vascular endothelial growth factor in vascular smooth muscle cells. FEBS Lett. 1995;358:311–5. doi: 10.1016/0014-5793(94)01458-d. [DOI] [PubMed] [Google Scholar]
- 81.Janssens S, Flaherty D, Nong Z, Varenne O, van Pelt N, Haustermans C, et al. Human Endothelial Nitric Oxide Synthase Gene Transfer Inhibits Vascular Smooth Muscle Cell Proliferation and Neointima Formation After Balloon Injury in Rats. Circulation. 1998;97:1274–81. doi: 10.1161/01.CIR.97.13.1274. [DOI] [PubMed] [Google Scholar]
- 82.Reidy MA. Endothelial regeneration. VIII. Interaction of smooth muscle cells with endothelial regrowth. Lab Invest. 1988;59:36–43. [PubMed] [Google Scholar]
- 83.Jaschke B, Michaelis C, Milz S, Vogeser M, Mund T, Hengst L, et al. Local statin therapy differentially interferes with smooth muscle and endothelial cell proliferation and reduces neointima on a drug-eluting stent platform. Cardiovasc Res. 2005;68:483–92. doi: 10.1016/j.cardiores.2005.06.029. [DOI] [PubMed] [Google Scholar]
- 84.Miyauchi K, Kasai T, Yokayama T, Aihara K, Kurata T, Kajimoto K, et al. Effectiveness of statin-eluting stent on early inflammatory response and neointimal thickness in a porcine coronary model. Circ J. 2008;72:832–8. doi: 10.1253/circj.72.832. [DOI] [PubMed] [Google Scholar]
- 85.Aoki J, Serruys PW, Van Beusekom H, Ong ATL, McFadden EP, Sianos G, et al. Endothelial progenitor cell capture by stents coated with antibody against CD34: The HEALING-FIM (Healthy Endothelial Accelerated Lining Inhibits Neointimal Growth-First in Man) registry. J Am Coll Cardiol. 2005;45:1574–9. doi: 10.1016/j.jacc.2005.01.048. [DOI] [PubMed] [Google Scholar]
- 86.Nakazawa G, Granada JF, Alviar CL, Tellez A, Kaluza GL, Guilhermier MY, et al. Anti-CD34 Antibodies Immobilized on the Surface of Sirolimus-Eluting Stents Enhance Stent Endothelialization. JACC Cardiovasc Interv. 2010;3:68–75. doi: 10.1016/j.jcin.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 87.Zarpak R, Sanchez OD, Joner M, Guy LG, Leclerc G, Virmani R. A novel “pro-healing” approach: the COMBO™ dual therapy stent from a pathological view. Minerva Cardioangiol. 2015;63:31–43. [PubMed] [Google Scholar]
- 88.Padfield GJ, Short A, Mills NL, Samuel K, Turner M, Newby DE, et al. The constituents and mechanisms of generation of “endothelial cell--colony forming units”. Cardiovasc Res. 2013;100:288–96. doi: 10.1093/cvr/cvt182. [DOI] [PubMed] [Google Scholar]
- 89.Yoder MC. Human endothelial progenitor cells. Cold Spring Harb Perspect Med. 2012;2:a006692. doi: 10.1101/cshperspect.a006692. [DOI] [PMC free article] [PubMed] [Google Scholar]


