Abstract
Emerging evidence links the receptor for advanced glycation endproducts (RAGE) to the pathogenesis of tissue damage in chronic metabolic and inflammatory diseases. In human subjects, multiple reports suggest that in the plasma/serum, circulating levels of distinct forms of soluble RAGEs may be biomarkers of the presence or absence, and the extent of chronic disease. These considerations prompt us to consider in this review, what are soluble RAGEs; how are they formed; what might be their natural functions; and may they serve as biomarkers of inflammatory and metabolic disease activity? In this brief review, we seek to address what is known and suggest new areas for scientific investigation to uncover the biology of soluble RAGEs.
Graphical abstract
The families of RAGE ligands
The pathobiology of the receptor for advanced glycation endproducts (RAGE) is tightly coupled to chronic disease and in particular, to long-standing inflammation. RAGE, a multiligand receptor, interacts with and transduces the biological signals of distinct molecules that are generated and/or accumulated in chronic cellular stress. RAGE was first described as a receptor for the advanced glycation endproducts (AGEs), the products of nonenzymatic glycation and oxidation of proteins and lipids. Although originally coupled mechanistically largely to hyperglycemia and diabetes, it is established that AGEs accumulate in oxidative and lipid stresses as well [1–4]. Studies in human subjects and animal models suggest the accelerated formation and accumulation of AGEs, especially carboxy methyl lysine (CML) AGE, in obese adipose tissue, even in the absence of diabetes [5, 6]. One of the principal AGE precursors, methylglyoxal (MG), is regulated by the enzyme glyoxalase1 (Glo1). Glo1 detoxifies MG, thereby suppressing ongoing formation of AGE. In diabetic kidney, mice devoid of Ager (gene encoding RAGE) display significantly higher levels of Glo1 mRNA and activity, in parallel with significantly lower levels of MG compared to wild-type (WT) Ager-expressing mouse kidney, despite the same degrees of hyperglycemia [7]. Hence, a possible “feed-forward loop’ of AGE production and accumulation leading to upregulation of RAGE, thereby sustaining AGE production through downregulation of Glo1 may contribute to the pathogenesis of chronic diseases, such as diabetes. Interestingly, it has been shown that in brain of human Alzheimer’s Disease (AD) subjects, GLO1 activity becomes progressively lower as the disease progresses [8]. In AD, another of the RAGE ligand families, amyloid-β peptide (Ap) and β-sheet fibrils have been strongly linked to neuronal damage and microglial activation in this disorder [9]. It does remain to be tested, however, whether the reduced levels of GLO1 in AD brain are RAGE-dependent, or not and whether they contribute to overall pathology and cognitive dysfunction.
Multiple members of the S100/calgranulin family and high mobility group box 1 (HMGB1) are ligands of RAGE; their formation and release upon cell death and cellular activation implicate their subsequent interaction with cell surface RAGE as means to perpetuate cellular damage and the failure of inflammatory resolution [4]. Much evidence points to lack of direct roles for RAGE in innate/acute immune responses, but, rather, to chronic and tissue-damaging inflammation. Experiments in mice devoid of Ager or Myd88 subjected to massive liver resection (a mechanism to deliver substantial quantities of lipopolysaccharide (LPS) to the liver remnant) revealed that whereas mice devoid of Myd88 succumbed rapidly to massive liver resection, those mice devoid of Ager displayed increased survival and higher levels of activated NF-kB in the remnant, thereby supporting potent anti-apoptotic defense [10]. In mice devoid of both Myd88 and Ager, survival upon massive liver resection was similar to that in mice devoid of Myd88 alone. Further, in mice subjected to cecal ligation and puncture (sepsis) or high doses of pathogens, deletion of Ager was nearly uniformly linked to improved survival without deleterious effects on pathogen load [11].
Recent work has suggested that lipid moieties may interact with RAGE. Phosphatidylserine (PS), an amino acid-derived phospholipid on the outer leaflet of the cell membrane is linked to clearance of apoptotic cells. It was recently shown that RAGE is a receptor for PS and that it contributes to the clearance of apoptotic cells, as mice devoid of Ager revealed impaired phagocytosis of apoptotic thymocytes and impaired clearance of apoptotic neutrophils [12]. Direct binding of RAGE to PS was established using surface plasmon resonance and distinct biological techniques. In addition, RAGE is a receptor for lysophosphatidic acid (LPA); although LPA is typically linked to binding through specific G-protein coupled receptors (GPCRs), it also binds RAGE and in both vascular and tumor cells, appears to mediate fundamental properties linked to cellular migration and signaling [13].
RAGE is expressed in multiple cell types including vascular cells, immune cells such as monocytes/macrophages, dendritic cells and lymphocytes, as well as target cell types in diabetes including cardiomyocytes, neurons, podocytes of the kidney and epidermal cells. In homeostasis, the highest levels of RAGE expression are noted in the lung, where it is expressed especially in pneumocytes and in alveolar macrophages [1–4]. Hence, RAGE’s ability to bind to and transduce the signals of its ligand families implicates the receptor in metabolism, cancer and chronic inflammation, both by exacerbation of inflammatory mechanisms and at least in some settings, through impaired resolution of tissue-damaging inflammation (Figure 1).
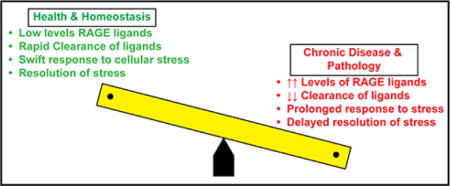
Figure 1. Balancing Cellular Stress & Resolution: Roles for RAGE.
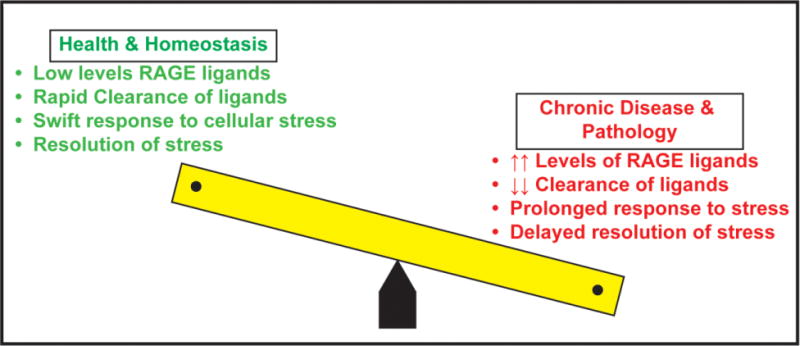
In this “see-saw” depiction of health and homeostasis versus chronic disease and pathology, data support that in health and homeostasis, low levels of RAGE ligands, likely both due to low level of production and/or effective mechanisms of clearance, are present in the circulation and in the tissues. In response to acute stress, rapid homeostatic responses to cellular stress and rapid resolution occur. However, on the other side of the balance, in chronic disease and pathological settings, such as obesity, diabetes, Alzheimer’s disease, and chronic inflammation, as examples, higher levels of RAGE ligands pervade the circulation and tissue, accounted for by accelerated production and/or reduced clearance. In such settings, compounded by oxidative and inflammatory stresses, we predict that the RAGE ligands contribute to prolonged responses to stress and, ultimately, to delayed resolution – all processes that mediate tissue damage.
Soluble Forms of RAGE: tracking receptor activity in health and disease
RAGE is a transmembrane receptor, composed of an extracellular domain (1 variable (V)-type domain followed by 2 constant (C)-type domains); while most of the ligands bind at the V-type domain, evidence does suggest that the V-C1 motif forms a key structural unit for ligand binding. V-C1 is followed by a second C-type domain, referred to as C2. The extracellular domain of the receptor is followed by a single transmembrane spanning domain and lastly, by a highly charged, 43 amino acid cytoplasmic domain (ctRAGE) which is essential for RAGE signaling (Figure 2) [1–4].
Figure 2. The Multiple Ligands, Domains and Forms of RAGE.
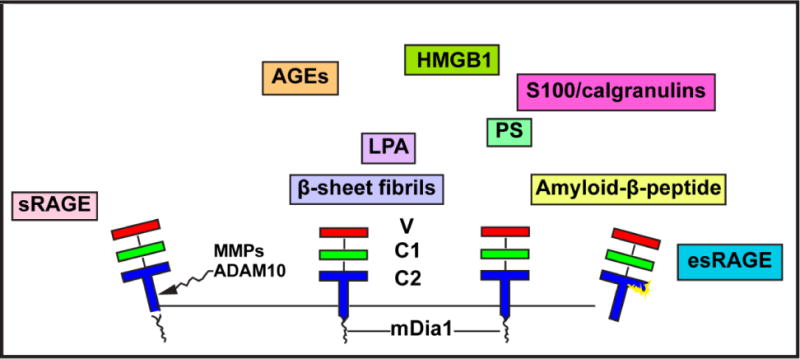
RAGE is an immunoglobulin superfamily receptor that binds multiple families of ligands such as the AGEs, HMGB1, S100/calgranulins, LPA, PS, amyloid-beta peptide and beta-sheet fibrils. The ligands bind at the extracellular domain, largely the V-type domain. The extracellular domain is followed by a single transmembrane spanning domain and a short cytoplasmic domain, which binds to the formin mDia1 to enact cellular signaling. Two forms of soluble RAGE have been identified, soluble or sRAGE is formed by cell surface shedding actions of ADAM10 and MMPs. The second form is an alternatively spliced form called endogenous secretory (esRAGE), also known as RAGEv1. A unique 16 amino acid span in the C2 domain distinguishes esRAGE from sRAGE. Abbreviations: AGEs, advanced glycation endproducts; HMGB1, high mobility group box 1; LPA, lysophosphatidic acid; PS, phosphatidylserine; MMP, matrix metalloproteinase; and ADAM10, A-Distintegrin and Metalloprotease 10.
In addition to the full-length RAGE, at least two distinct forms of “soluble RAGE” have been detected in the circulation and in body fluids such as that from the bronchoalveolar compartment and the joint space (Figure 2) [14–16]. The first form of soluble RAGE is derived from cell surface cleavage mechanisms, such as that induced by matrix metalloproteinases (MMPs) and A-Distintegrin and Metalloprotease (ADAM)-10. This form of sRAGE, by virtue of its derivation from the full length form of RAGE, is composed of the V-C1-C2 domains [17, 18]. The second form of soluble RAGE is derived from pre-mRNA alternative splicing. Known as endogenous secretory (es) RAGE or RAGEv1, this form of RAGE contains a unique span of 16 amino acids in the C-terminal region of the molecule [19]. Generally, of the total sRAGE species, esRAGE comprises about 20%. Many published studies have examined levels of sRAGE and/or esRAGE in multiple distinct populations in health and in disorders in which RAGE ligands are known to accumulate. What is often not reported in these studies, however, is the parallel levels of RAGE ligands. Presumably, as has been shown in animals, at least pharmacological levels of sRAGE (administered to the animals most often parenterally) exerted protective effects in a wide range of settings in which RAGE ligands accumulate, such as cardiovascular disease, diabetes complications, inflammatory joint diseases, infection and tumors. The working hypothesis is that soluble RAGE may exert its benefit by sequestering RAGE ligands and blocking their interaction not only with RAGE, but, perhaps, with other cell surface receptors. For example, HMGB1 and certain S100s have been shown to bind to both RAGE and to toll like receptors [20, 21]. Soluble RAGE treatment would thus be expected to limit the ability of these ligands to bind to these receptors, thereby attenuating inflammation and cellular stress.
Many studies have examined the relationship between sRAGE and esRAGE levels and the status of diseases, from diseases of the cardiovasculature; endocrine system (such as types 1 and 2 diabetes, with and without complications); neurodegenerative disorders (such as Alzheimer’s disease and amyotrophic lateral sclerosis); autoimmune/inflammatory disorders; and chronic obstructive pulmonary diseases, as examples [14–16].
In this edition of Vascular Pharmacology, Santilli and colleagues cross-sectionally compared 60 patients with and 50 patients without non-alcoholic fatty liver disease (NAFLD) [22]. Within these groups were subjects with familial combined hyperlipidemia alone or with metabolic syndrome. The authors reported that among these subjects, those with NAFLD displayed lower plasma esRAGE, IL10, and adiponectin, in parallel with higher levels of CD40 ligand, endogenous thrombin potential, and oxidized LDL. The authors concluded that lower levels of esRAGE were associated with higher degrees of atherothrombotic abnormalities. In that work, however, total levels of sRAGE were not examined, nor were levels of any of the ligand families linked to metabolic and hyperlipidemic stress tested. Thus, Santilli and co-workers show that low levels of esRAGE track with higher degrees of inflammation, oxidative stress and prothrombotic potential and with the disease, NAFLD.
These and other published studies on the relationship of levels of soluble RAGEs do raise fundamental questions, however. Chief among them is, why are there conflicting reports on the directionality of soluble RAGE levels (i.e., “higher” or “lower”) with respect to protected or vulnerable phenotypes? In the section to follow, we address this question.
Soluble RAGEs: high or low – confounding factors?
A number of key considerations may help us to sort through the quandary. In the section to follow, we review some of these mitigating factors (summarized in Figure 3). First, it may be important to consider the interplay between gender and the RAGE axis. In a recent report, Pertynska-Marczewska and Merhi reviewed current published evidence on the effects of hormonal therapies in women on the AGE/RAGE and soluble RAGEs axis [23]. Whereas administration of estradiol valerate for six months to post-menopausal women resulted in reduced levels of vaginal AGEs versus placebo treatment, incubation of human endothelial cells with 17β-estradiol (10 nM) resulted in induction of RAGE expression [24, 23, 25]. Hence, irrespective of the effect of these therapies on vaginal AGEs (presumably mostly reflective of epithelial cell physiology), the upregulation of RAGE expression in cultured human endothelial cells might signal a higher pro-inflammatory potential.
Figure 3. Multiple Factors May Modulate Levels of sRAGE and esRAGE.
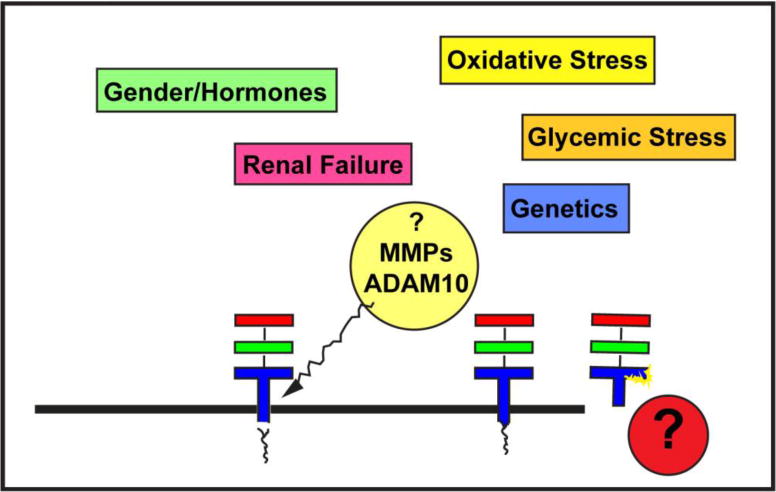
As described earlier, sRAGE is largely formed by membrane shedding of the extracellular RAGE by ADAM10 and MMPs. In contrast, esRAGE is an alternatively spliced form of the extracellular receptor, which contains a 16 amino acid novel segment in the C2 domain. Multiple factors are likely to regulate sRAGE and esRAGE production and clearance, including but not limited to gender and the effects of hormones; oxidative stress; glycemic stress; genetic predilection; and renal disease. Likely, additional yet-to-be-discovered factors play contributory roles in the regulation of soluble RAGEs.
In a distinct study, postmenopausal women with rheumatoid arthritis were treated with estradiol (2 mg) plus noretisterone acetate (form of progestin) versus placebo over 2 years. Increased levels of serum estradiol were associated with lower levels of sRAGE at the end of the study, which appeared to reflect less bone resorption and turnover markers [26]. These conclusions were based on the finding that higher levels of baseline sRAGE were associated with greater degrees of bone resorption at the baseline. Hence, these studies suggest that the gender factor, menopausal or pre-menopausal state, and treatment with hormones in post-menopausal women should be considered in the context of potential factors that might influence levels of sRAGE and RAGE ligands. Most published studies do not discern the specific effects of disease or interventions on male versus female subjects; perhaps this might be considered to enhance overall data interpretation.
Multiple investigators have shown that chronic kidney disease and significant renal impairment may increase the levels of both sRAGE and esRAGE [27–30]. The mechanisms underlying these findings are not clarified. For example, do the elevations in sRAGE and esRAGE reflect changes in clearance mechanisms? More importantly, do even modest degrees of renal insufficiency that are not infrequently observed in diabetic subjects result in gradual elevations in these forms of soluble RAGE? Alternatively, but not mutually exclusively, is it plausible that increased accumulation of RAGE ligands, particularly AGEs, in increasing renal insufficiency may tip the balance of soluble RAGEs production, cell surface cleavage, and alternative splicing mechanisms?
Of all the homeostasis/disease areas in which soluble RAGE levels have been tested to date, it appears that the most “discrepancies” between “high” versus “low” levels as they associate with protection versus vulnerability to disease and disease complications appears to be in the area of diabetes. In general, most of the published work suggests that levels of sRAGE are higher in diabetic versus non-diabetic subjects; data on esRAGE levels are mixed, but in general, esRAGE levels appear to be lower in subjects with types 1 or 2 diabetes versus controls [31]. However, examination of sRAGE and esRAGE levels in type 2 diabetic subjects enrolled in the CARDS trial revealed that there was a positive correlation between levels of both sRAGE and esRAGE in these subjects with incident cardiovascular disease [32]. Of note, the positive correlation with renal insufficiency was noted. If one examines the fate of sRAGE in subjects without diabetes, but with cardiovascular disease, post-percutaneous coronary intervention restenosis (PCI), essential hypertension, Alzheimer’s disease, amyotropic lateral sclerosis, abdominal aortic aneurysm disease, juvenile arthritis, systemic lupus erythematosus, pancreatic cancer, and chronic obstructive pulmonary disease, as examples, the general trend is that lower plasma/serum levels of sRAGE are associated with disease state versus the otherwise healthy controls [33–42]. In fact, supportive of “low” levels of sRAGE and the association to disease state or burden, it was shown in the cerebrospinal fluid of multiple sclerosis patients, low levels of sRAGE were observed compared to control subjects [43]. Hence, the “opposite” is generally true in diabetes. What might be the reasons? We would speculate that factors such as even modest degrees of renal insufficiency may contribute to regulation of sRAGE and esRAGE levels; other confounders might include gender representation in the studies. On a more fundamental level, it is possible that glucose and/or its downstream consequences, such as oxidative stress, might selectively alter splicing mechanisms, thereby directly affecting esRAGE levels. Additionally, diabetes specific factors might contribute to more dynamic and significant changes in levels and activity of MMPs. In addition, the cell type(s) most likely to be releasing/generating sRAGEs might differ to some degree in diabetic vs. non-diabetic states. For example, it is possible that in general, endothelial cells are the major producers of soluble RAGEs but that in diabetes, peripheral blood monocytes and lymphocytes may also contribute substantially to soluble RAGE production. In this context, in subjects with sepsis, a dramatic form of disease manifestation with heightened inflammatory linked to hemodynamic abnormalities, sRAGE levels in plasma were found to be significantly higher than in control subjects [44]. Hence, we might speculate, is diabetes somewhere in the middle?
Finally, consistent with this concept, it has been proposed that in clinically stable disease, soluble RAGE levels may be abruptly altered by superimposed acute events. Piarulli and colleagues proposed a model in which sRAGE and esRAGE levels might steadily decline during the progression from early stages of atherosclerosis to advanced stages of atherosclerosis (presumably due, at least in part, to gradual rises in RAGE ligand levels (such as AGEs) [45]. However, upon a superimposed acute event, these authors proposed that inflammation and oxidative stress might stimulate an abrupt rise in sRAGE levels, but no such rise in esRAGE levels was predicted. This model predicts potentially protective roles for esRAGE in blocking the adverse effects of glycation, inflammation and oxidative stress in the vasculature. These concepts reinforce the need to delineate the cellular mechanisms regulating ecotodomain cleavage of sRAGE in health, in diabetes and in disease, and the cellular mechanisms regulating alternative splicing and release of esRAGE in these same states. As suggested above, however, it is essential to consider that these mechanisms may differ in vascular cells versus immune cells such as monocytes and lymphocytes.
Taken together, these considerations strongly suggest that understanding the specific mechanisms that drive generation of sRAGE and esRAGE is a critical step in determining whether or not harnessing the potential power of soluble RAGEs as therapeutic targets may be feasible or wise. Cellular studies have begun to address this key question.
Modulation of soluble RAGEs: studies in cultured cells
Multiple cell types have been used in attempts to identify stimuli that modulate sRAGE release and/or production. There is evidence that expression of soluble RAGE forms in cultured cells may alter cellular signaling. Expression of esRAGE (RAGEv1) in human hepatocellular carcinoma Hepg2 cells resulted in reduced expression of NF-kB and Tumor Necrosis Factor-α, even without exogenous treatments [46]. In distinct experiments, cells were treated with stimuli to mimic glycemic or inflammatory stresses. In some cases, cells were also treated with potential therapeutic agents, in an attempt to test if levels of soluble RAGEs might be mutable.
In human K562 cells, treatment with high glucose or AGE resulted in reduced levels of sRAGE, which was restored by pre-treatment with resveratrol [47]. In mouse alveolar epithelial cells or HEK cells, treatment with the statin lovastatin stimulated RAGE shedding, which was completely prevented by an inhibitor of ADAM10 [48]. In human monocyte like THP-1 cells, treatment with insulin increased esRAGE and sRAGE levels in the culture medium, which was prevented by GM6001, a metalloproteinase inhibitor [49]. In microvascular endothelial cells, treatment with angiotensin-II (angII) upregulated RAGE and increased the expression of soluble RAGE n the culture medium. Both of these effects of angII were prevented by treatment with the angII type 1 receptor antagonist, telmisartan [50].
Taken together, these considerations indicate that soluble RAGE production in cultured cells is mutable by pathogenic stimuli and by therapeutic treatments. The next logical step was testing these concepts in vivo. Indeed, considerable evidence is accruing that medications and interventions may modulate soluble RAGE levels. In the section to follow, we review the available evidence on this point from multiple reported studies.
Modulation of soluble RAGEs: studies in vivo
In a study of type 2 diabetic subjects, the effects of treatment with pioglitazone or a sulfonylurea (glimepiride) on mononuclear cell RAGE expression and circulating levels of sRAGE and esRAGE were studied. Pioglitazone resulted in reduced RAGE expression in mononuclear cells and in higher levels of circulating sRAGE and esRAGE compared to glimepiride treatment [51]. In other studies, treatment of subjects with impaired glucose tolerance or type 2 diabetes with pioglitazone resulted in increased levels of sRAGE and high density lipoprotein and improved insulin sensitivity [52]. In type 2 diabetic subjects, the levels of sRAGE and esRAGE were examined after six months of either rosiglitazone or sulfonylurea treatment. Although both treatments resulted in reduced levels of glycosylated hemoglobin, only rosiglitazone treatment resulted in increased levels of total sRAGE and esRAGE [53]. These results strongly suggest that changes in sRAGE levels are not simply induced by changes in glycemic control; hence, more fundamental aspects of the overall mechanisms of actions of the drugs in question appears to be critical in mediating changes in sRAGE/esRAGE biology.
The effects of distinct forms of insulin were also tested in 49 type 1 diabetic subjects and 10 type 2 diabetic subjects. Multiple injections per day of glulisine improved glycemic control in basal insulin glargine-injected subjects in parallel with reduced levels of AGEs and sRAGE [54]. In critically ill hyperglycemic subjects (both diabetic and non-diabetic), intensive insulin treatment resulted in significant decreases in sRAGE levels as well, suggesting fundamental effects of insulin on reducing sRAGE levels [55].
In type 2 diabetic subjects, administration of the dipeptidyl peptidase-4 (DPP4) inhibitor alogliptin resulted in reduced fasting glucose and reduced levels of sRAGE in parallel with reduced urinary albumin to creatinine ratio [56].
In pre-type 1 diabetic subjects in the FinnDiane study, declining levels of sRAGE were noted prior to the development of frank diabetes; this was prevented by treatment with alagebrium, an AGE cross link breaker [57]. Hence, by lowering levels of AGEs, the continued decline in sRAGE levels was prevented [57].
The effects of calcium channel blocker therapy in non-diabetic subjects with stage I or II chronic kidney disease was tested with the agent azelnidipine; treatment resulted in decreased levels of circulating AGE and sRAGE, in parallel with reduced proteinuria [58]. In non-diabetic subjects with essential hypertension, renin angiotensin blockade with telmisartan resulted in reduced levels of sRAGE [50]. In type 1 diabetic subjects with nephropathy, treatment with angiotensin converting enzyme inhibition (perindopril), resulted in increased levels of sRAGE [59].
In a mixed population of diabetic and non-diabetic subjects with acute coronary syndrome, treatment with pitavastatin or atorvastatin resulted in reduced levels of circulating AGEs (independent of diabetic state); no change in levels of sRAGE were noted [60]. These findings were analogous to results in the CARDS trial of subjects with type 2 diabetes. In these subjects, higher levels of sRAGE and esRAGE were associated with greater incident cardiovascular disease. Treatment with atorvastatin, however, exerted no effect on levels of sRAGE [32]. A third study reported different results; six months treatment with atorvastatin in Chinese type 2 diabetic subjects resulted in increased levels of sRAGE and esRAGE [61]. In a fourth study, treatment of non-diabetic hypercholesterolemic subjects with atorvastatin but not pravastatin, resulted in increased levels of sRAGE [62].
Other studies addressed the effects of green tea components such as epigallocatechin-3-gallate (EGCG) in type 2 diabetic subjects. EGCG stimulated increases in sRAGE in the circulation through enhanced ADAM10 ectodomain shedding of extracellular RAGE [63].
In healthy subjects, treatment with endotoxin induces inflammation, in parallel with increased levels of sRAGE. This was prevented by treatment with adenosine [64]. In subjects with rheumatoid arthritis, treatment with methotrexate results in significantly higher levels of sRAGE in synovial joint fluid compared with subjects treated with non-disease modifying agents [65].
In subjects without diabetes, but with advanced renal failure on hemodialysis with secondary hyperparathyroidism, administration of calcitrol was found to increase serum sRAGE levels, in parallel with decreased levels of IL-6 [66].
Interventions have also been tested for their ability to modulate levels of sRAGE. Subjects with morbid obesity displayed lower levels of sRAGE compared to normal control lean subjects. However, after bariatric surgery, levels of sRAGE increased significantly and were associated with improved measures of metabolic function [67]. In a distinct study, Parikh and colleagues reported that in type 2 diabetic obese subjects, the higher the level of baseline sRAGE, the greater the metabolic response to surgery [68].
The findings summarized in Table 1 reveal a mixed picture on the effects of interventions on levels of soluble RAGEs. As is evident from the table, a number of points may be deduced: (1) It is unusual that both sRAGE and esRAGE levels are examined in the same study; (2) Most studies do not capture levels of RAGE ligands; (3) Most studies only capture data in type 2 but not type 1 diabetic subjects; (4) Most studies may not be powered to examine the effects of interventions independently in male vs. female subjects; and (5) The renal status of most of the subjects enrolled in these studies, although it may be known, is rarely if ever factored into the reporting of the levels of sRAGE or esRAGE. More importantly, if interventions yield improvements in renal function, such changes were not considered in the reporting of the changes in soluble RAGE levels. Taken together, as depicted in Figure 3, multiple confounding factors might impact on regulation of sRAGE and esRAGE production and clearance. Much more research is required to delineate the specific underlying mechanisms.
Table 1.
Summary of the effects of intervention on levels of soluble RAGEs (note that studies reflect human subjects)
| Intervention | Ligand | sRAGE | esRAGE | Reference |
|---|---|---|---|---|
| Pioglitazone (vs. glimepiride) T2D |
NT | ↑ | ↑ | [51] |
| Pioglitazone IGT or T2D |
NT | ↑ | NT | [52] |
| Pioglitazone T2D |
NT | ↑ | ↑ | [53] |
| Multiple injections Glulisine vs. Basal Glargine T2D & T1D |
AGEs↓ | ↓ | NT | [54] |
| Intensive Insulin Critically ill subjects Hyperglycemic (Diabetic & Non-diabetic) |
↑HMGB1 | ↓D* only | NT | [55] |
| DPP4 Inhibition Alogliptin T2D |
NT | ↓ | NT | [56] |
| Alagebrium Pre-diabetic T1D Vulnerable |
↓AGE | ↓ | NT | [57] |
| Ca2+ channel blockade Azelnidipine Non-diabetic + CKD |
↓AGE | ↓ | NT | [58] |
| Telmisartan Non-diabetic Hypertensive |
NT | ↓ | NT | [50] |
| Perindopril T1D |
↓ | ↑ | NT | [59] |
| Statins Pitavastatin & Atorvastatin (Non-diabetic & Diabetic with Acute coronary Syndrome) |
↓ | No Δ | NT | [60] |
| T2D Atorvastatin |
NT | No Δ | NT | [32] |
| T2D Atorvastatin |
NT | ↑(trend) | ↑ | [61] |
| Atorvastatin Vs. Pravastatin (Non-diabetic with Hypercholesterolemia) | NT | ↑ | NT | [62] |
| EGCG T2D |
↓S100A12 | ↑ | NT | [63] |
| Adenosine Healthy subject + LPS |
NT | ↑ | NT | [64] |
| Methotrexate (Rheumatoid arthritis) | NT | ↑ (synovial fluid) | NT | [65] |
| Calcitrol Renal Failure/Non-diabetic |
NT | ↑ | NT | [66] |
| Bariatric Surgery Morbidly Obese |
NT | ↑ | NT | [67] |
indicates only in D or Diabetic Subjects NT=not tested
Summary and Future Directions
In summary, multiple questions remain regarding soluble RAGEs (Figure 4). First and foremost, what is their purpose, and, do they even have a purpose? No doubt soluble RAGEs may bind RAGE ligands; in pharmacological studies, administration of sRAGE to animals resulted in attenuation of inflammation and metabolic dysfunction. Do sRAGE and esRAGE, as detected in human subject plasma/serum, have the same function, that is do they sequester RAGE ligands and prevent their biological impact? Does activation of sheddase function serve to curtail and tightly regulate RAGE signaling? It would seem both possibilities are plausible (Figure 4A). Second, is shedding or increased expression of esRAGE a mechanism to divert cellular machinery from producing more cell surface RAGE, that is, molecular forms capable of signaling (Figure 4B)? Finally, perhaps the endogenous function of soluble RAGEs, albeit evolutionarily conserved, is, on a practical level, relatively limited and these molecular forms, are, in practice, largely biomarkers of the state of metabolic or inflammatory stresses and the response to therapeutic interventions. In this context, irrespective of the caveats discussed above, what is interesting to speculate is that depending on the mechanism of action of the interventions shown in Table 1, is it possible that a main driver of the outcome on soluble RAGE levels is whether the agent may directly affect ADAM10 or MMP activities at the cellular surface, or whether they may affect alternative splicing? Additionally or alternatively, is one of the main drivers of soluble RAGE levels, the degree of ligand burden? At this stage of investigation, review of the data to date do not allow us to make firm connections on potential relationships between the effects of interventions on levels of ligands of RAGE, with the changes in soluble RAGE levels. Finally, although the effects of treatment interventions in diabetic subjects reveal a truly mixed picture on “increases” or “decreases” in soluble RAGEs, in general, in non-diabetic subjects, otherwise beneficial interventions appear to raise levels of soluble RAGEs. It is reasonable to surmise that particularly in the diabetic subjects, the effects of renal dysfunction on measures of soluble RAGEs must not be inconsequential.
Figure 4. Why Soluble RAGEs?
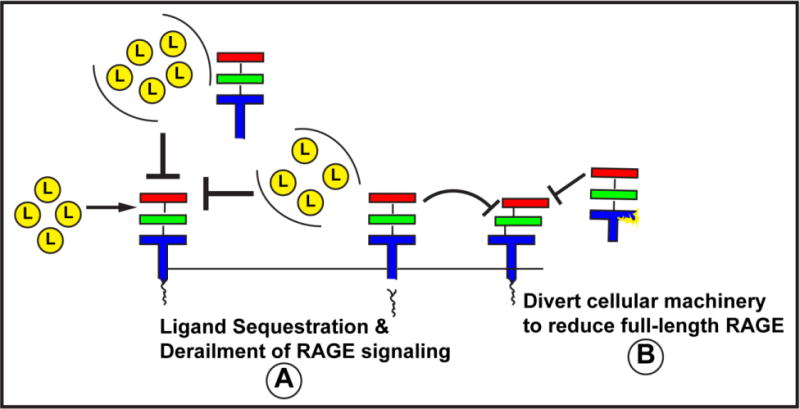
Evidence suggests that sRAGE is produced by sheddases cleaving cell surface RAGE and that esRAGE is an alternatively spliced RAGE form. Our understanding of the mechanisms of regulation of production and clearance of these forms is relatively limited but, no doubt, holds the key to whether it is possible to harness the power of sRAGEs to sequester RAGE ligands, reduce the overall potential of ligand stimulated RAGE signaling (by disengaging the ligand binding forms of RAGE from the intracellular effectors) and/or divert cellular machinery away from generation of more full-length RAGE. At the same time or alternatively, measures of soluble RAGEs may be mirrors of the activation state of the receptor and, as such, may be amenable to tracking the status of inflammatory and metabolic diseases. More research is needed to uncover the purpose of soluble RAGEs.
We conclude that in future directions, perhaps by reporting both sRAGE and esRAGE levels, along with a ligand surrogate (that is, AGEs or S100s or HMGB1, as examples) and measures of renal function, a more complete picture of the predictive value of measures of sRAGE and esRAGE in each disease setting and in the context of therapeutic interventions may be uncovered. Such strategies, coupled with fundamental basic science experimentation to uncover the mechanisms of production and functions of soluble RAGEs, may help us to discover the potential benefit of measuring these molecules as bona fide biomarkers, or not, in metabolic and inflammatory disorders and the responses to therapeutic interventions.
Acknowledgments
The author is grateful to Ms. Latoya Woods for her assistance in the preparation of this manuscript. Research in the Schmidt laboratory is funded by grants from the United States Public Health Service, the JDRF and the American Diabetes Association.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Daffu G, del Pozo CH, O’Shea KM, Ananthakrishnan R, Ramasamy R, Schmidt AM. Radical roles for RAGE in the pathogenesis of oxidative stress in cardiovascular diseases and beyond. Int J Mol Sci. 2013;14:19891–910. doi: 10.3390/ijms141019891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Litwinoff EM, Hurtado Del, Pozo C, Ramasamy R, Schmidt AM. Emerging targets for therapeutic development in diabetes and its complications: The RAGE signaling pathway. Clin Pharmacol Ther. 2015 doi: 10.1002/cpt.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manigrasso MB, Juranek J, Ramasamy R, Schmidt AM. Unlocking the biology of RAGE in diabetic microvascular complications. Trends Endocrinol Metab. 2014;25:15–22. doi: 10.1016/j.tem.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramasamy R, Yan SF, Schmidt AM. The diverse ligand repertoire of the receptor for advanced glycation endproducts and pathways to the complications of diabetes. Vascul Pharmacol. 2012;57:160–7. doi: 10.1016/j.vph.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaens KH, Goossens GH, Niessen PM, van Greevenbroek MM, van der Kallen CJ, Niessen HW, et al. Nepsilon-(carboxymethyl)lysine-receptor for advanced glycation end product axis is a key modulator of obesity-induced dysregulation of adipokine expression and insulin resistance. Arterioscler Thromb Vasc Biol. 2014;34:1199–208. doi: 10.1161/ATVBAHA.113.302281. [DOI] [PubMed] [Google Scholar]
- 6.Song F, Hurtado del Pozo C, Rosario R, Zou YS, Ananthakrishnan R, Xu X, et al. RAGE regulates the metabolic and inflammatory response to high-fat feeding in mice. Diabetes. 2014;63:1948–65. doi: 10.2337/db13-1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reiniger N, Lau K, McCalla D, Eby B, Cheng B, Lu Y, et al. Deletion of the receptor for advanced glycation end products reduces glomerulosclerosis and preserves renal function in the diabetic OVE26 mouse. Diabetes. 2010;59:2043–54. doi: 10.2337/db09-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.More SS, Vartak AP, Vince R. Restoration of glyoxalase enzyme activity precludes cognitive dysfunction in a mouse model of Alzheimer’s disease. ACS Chem Neurosci. 2013;4:330–8. doi: 10.1021/cn3001679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yan SD, Chen X, Fu J, Chen M, Zhu H, Roher A, et al. RAGE and amyloid-beta peptide neurotoxicity in Alzheimer’s disease. Nature. 1996;382:685–91. doi: 10.1038/382685a0. [DOI] [PubMed] [Google Scholar]
- 10.Zeng S, Zhang QY, Huang J, Vedantham S, Rosario R, Ananthakrishnan R, et al. Opposing roles of RAGE and Myd88 signaling in extensive liver resection. FASEB J. 2012;26:882–93. doi: 10.1096/fj.11-192997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liliensiek B, Weigand MA, Bierhaus A, Nicklas W, Kasper M, Hofer S, et al. Receptor for advanced glycation end products (RAGE) regulates sepsis but not the adaptive immune response. J Clin Invest. 2004;113:1641–50. doi: 10.1172/JCI18704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He M, Kubo H, Morimoto K, Fujino N, Suzuki T, Takahasi T, et al. Receptor for advanced glycation end products binds to phosphatidylserine and assists in the clearance of apoptotic cells. EMBO Rep. 2011;12:358–64. doi: 10.1038/embor.2011.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rai V, Toure F, Chitayat S, Pei R, Song F, Li Q, et al. Lysophosphatidic acid targets vascular and oncogenic pathways via RAGE signaling. J Exp Med. 2012;209:2339–50. doi: 10.1084/jem.20120873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maillard-Lefebvre H, Boulanger E, Daroux M, Gaxatte C, Hudson BI, Lambert M. Soluble receptor for advanced glycation end products: a new biomarker in diagnosis and prognosis of chronic inflammatory diseases. Rheumatology (Oxford) 2009;48:1190–6. doi: 10.1093/rheumatology/kep199. [DOI] [PubMed] [Google Scholar]
- 15.Vazzana N, Santilli F, Cuccurullo C, Davi G. Soluble forms of RAGE in internal medicine. Intern Emerg Med. 2009;4:389–401. doi: 10.1007/s11739-009-0300-1. [DOI] [PubMed] [Google Scholar]
- 16.Yamagishi S, Matsui T, Nakamura K. Kinetics, role and therapeutic implications of endogenous soluble form of receptor for advanced glycation end products (sRAGE) in diabetes. Curr Drug Targets. 2007;8:1138–43. doi: 10.2174/138945007782151298. [DOI] [PubMed] [Google Scholar]
- 17.Raucci A, Cugusi S, Antonelli A, Barabino SM, Monti L, Bierhaus A, et al. A soluble form of the receptor for advanced glycation endproducts (RAGE) is produced by proteolytic cleavage of the membrane-bound form by the sheddase a disintegrin and metalloprotease 10 (ADAM10) FASEB J. 2008;22:3716–27. doi: 10.1096/fj.08-109033. [DOI] [PubMed] [Google Scholar]
- 18.Zhang L, Bukulin M, Kojro E, Roth A, Metz VV, Fahrenholz F, et al. Receptor for advanced glycation end products is subjected to protein ectodomain shedding by metalloproteinases. J Biol Chem. 2008;283:35507–16. doi: 10.1074/jbc.M806948200. [DOI] [PubMed] [Google Scholar]
- 19.Yonekura H, Yamamoto Y, Sakurai S, Petrova RG, Abedin MJ, Li H, et al. Novel splice variants of the receptor for advanced glycation end-products expressed in human vascular endothelial cells and pericytes, and their putative roles in diabetes-induced vascular injury. Biochem J. 2003;370:1097–109. doi: 10.1042/BJ20021371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hiratsuka S, Watanabe A, Sakurai Y, Akashi-Takamura S, Ishibashi S, Miyake K, et al. The S100A8-serum amyloid A3-TLR4 paracrine cascade establishes a pre-metastatic phase. Nat Cell Biol. 2008;10:1349–55. doi: 10.1038/ncb1794. [DOI] [PubMed] [Google Scholar]
- 21.Park JS, Gamboni-Robertson F, He Q, Svetkauskaite D, Kim JY, Strassheim D, et al. High mobility group box 1 protein interacts with multiple Toll-like receptors. Am J Physiol Cell Physiol. 2006;290:C917–24. doi: 10.1152/ajpcell.00401.2005. [DOI] [PubMed] [Google Scholar]
- 22.Santilli F, Blardi P, Scapellato C, Bocchia M, Guazzi G, Terzuoli L, et al. Decreased plasma endogenous soluble RAGE, and enhanced adipokine secretion, oxidative stress and platelet/coagulative activation identify non-alcoholic fatty liver disease among patients with Familial Combined Hyperlipidemia and/or Metabolic syndrome. Vascul Pharmacol. 2015 doi: 10.1016/j.vph.2015.04.004. In press. [DOI] [PubMed] [Google Scholar]
- 23.Pertynska-Marczewska M, Merhi Z. Relationship of Advanced Glycation End Products With Cardiovascular Disease in Menopausal Women. Reprod Sci. 2014 doi: 10.1177/1933719114549845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mukhopadhyay S, Mukherjee TK. Bridging advanced glycation end product, receptor for advanced glycation end product and nitric oxide with hormonal replacement/estrogen therapy in healthy versus diabetic postmenopausal women: a perspective. Biochim Biophys Acta. 2005;1745:145–55. doi: 10.1016/j.bbamcr.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka N, Yonekura H, Yamagishi S, Fujimori H, Yamamoto Y, Yamamoto H. The receptor for advanced glycation end products is induced by the glycation products themselves and tumor necrosis factor-alpha through nuclear factor-kappa B, and by 17beta-estradiol through Sp-1 in human vascular endothelial cells. J Biol Chem. 2000;275:25781–90. doi: 10.1074/jbc.M001235200. [DOI] [PubMed] [Google Scholar]
- 26.Pullerits R, d’Elia HF, Tarkowski A, Carlsten H. The decrease of soluble RAGE levels in rheumatoid arthritis patients following hormone replacement therapy is associated with increased bone mineral density and diminished bone/cartilage turnover: a randomized controlled trial. Rheumatology (Oxford) 2009;48:785–90. doi: 10.1093/rheumatology/kep079. [DOI] [PubMed] [Google Scholar]
- 27.Humpert PM, Papadopoulos G, Schaefer K, Djuric Z, Konrade I, Morcos M, et al. sRAGE and esRAGE are not associated with peripheral or autonomic neuropathy in type 2 diabetes. Horm Metab Res. 2007;39:899–902. doi: 10.1055/s-2007-993155. [DOI] [PubMed] [Google Scholar]
- 28.Koyama H, Shoji T, Fukumoto S, Shinohara K, Shoji T, Emoto M, et al. Low circulating endogenous secretory receptor for AGEs predicts cardiovascular mortality in patients with end-stage renal disease. Arterioscler Thromb Vasc Biol. 2007;27:147–53. doi: 10.1161/01.ATV.0000251502.88818.4b. [DOI] [PubMed] [Google Scholar]
- 29.Koyama H, Yamamoto H, Nishizawa Y. RAGE and soluble RAGE: potential therapeutic targets for cardiovascular diseases. Mol Med. 2007;13:625–35. doi: 10.2119/2007-00087.Koyama. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Semba RD, Ferrucci L, Fink JC, Sun K, Beck J, Dalal M, et al. Advanced glycation end products and their circulating receptors and level of kidney function in older community-dwelling women. Am J Kidney Dis. 2009;53:51–8. doi: 10.1053/j.ajkd.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prasad K. Low levels of serum soluble receptors for advanced glycation end products, biomarkers for disease state: myth or reality. Int J Angiol. 2014;23:11–6. doi: 10.1055/s-0033-1363423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Colhoun HM, Betteridge DJ, Durrington P, Hitman G, Neil A, Livingstone S, et al. Total soluble and endogenous secretory receptor for advanced glycation end products as predictive biomarkers of coronary heart disease risk in patients with type 2 diabetes: an analysis from the CARDS trial. Diabetes. 2011;60:2379–85. doi: 10.2337/db11-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bobek D, Grcevic D, Kovacic N, Lukic IK, Jelusic M. The presence of high mobility group box-1 and soluble receptor for advanced glycation end-products in juvenile idiopathic arthritis and juvenile systemic lupus erythematosus. Pediatr Rheumatol Online J. 2014;12:50. doi: 10.1186/1546-0096-12-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Emanuele E, D’Angelo A, Tomaino C, Binetti G, Ghidoni R, Politi P, et al. Circulating levels of soluble receptor for advanced glycation end products in Alzheimer disease and vascular dementia. Arch Neurol. 2005;62:1734–6. doi: 10.1001/archneur.62.11.1734. [DOI] [PubMed] [Google Scholar]
- 35.Falcone C, Emanuele E, D’Angelo A, Buzzi MP, Belvito C, Cuccia M, et al. Plasma levels of soluble receptor for advanced glycation end products and coronary artery disease in nondiabetic men. Arterioscler Thromb Vasc Biol. 2005;25:1032–7. doi: 10.1161/01.ATV.0000160342.20342.00. [DOI] [PubMed] [Google Scholar]
- 36.Geroldi D, Falcone C, Emanuele E, D’Angelo A, Calcagnino M, Buzzi MP, et al. Decreased plasma levels of soluble receptor for advanced glycation end-products in patients with essential hypertension. J Hypertens. 2005;23:1725–9. doi: 10.1097/01.hjh.0000177535.45785.64. [DOI] [PubMed] [Google Scholar]
- 37.Ilzecka J. Serum-soluble receptor for advanced glycation end product levels in patients with amyotrophic lateral sclerosis. Acta Neurol Scand. 2009;120:119–22. doi: 10.1111/j.1600-0404.2008.01133.x. [DOI] [PubMed] [Google Scholar]
- 38.Jiao L, Weinstein SJ, Albanes D, Taylor PR, Graubard BI, Virtamo J, et al. Evidence that serum levels of the soluble receptor for advanced glycation end products are inversely associated with pancreatic cancer risk: a prospective study. Cancer Res. 2011;71:3582–9. doi: 10.1158/0008-5472.CAN-10-2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma CY, Ma JL, Jiao YL, Li JF, Wang LC, Yang QR, et al. The plasma level of soluble receptor for advanced glycation end products is decreased in patients with systemic lupus erythematosus. Scand J Immunol. 2012;75:614–22. doi: 10.1111/j.1365-3083.2012.02691.x. [DOI] [PubMed] [Google Scholar]
- 40.McNair ED, Wells CR, Mabood Qureshi A, Basran R, Pearce C, Orvold J, et al. Soluble receptors for advanced glycation end products (sRAGE) as a predictor of restenosis following percutaneous coronary intervention. Clin Cardiol. 2010;33:678–85. doi: 10.1002/clc.20815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miniati M, Monti S, Basta G, Cocci F, Fornai E, Bottai M. Soluble receptor for advanced glycation end products in COPD: relationship with emphysema and chronic cor pulmonale: a case-control study. Respir Res. 2011;12:37. doi: 10.1186/1465-9921-12-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yao Y, Zhuang J, Li Y, Jing B, Li H, Li J, et al. Association of polymorphisms of the receptor for advanced glycation end products gene and susceptibility to sporadic abdominal aortic aneurysm. Biomed Res Int. 2015;2015:394126. doi: 10.1155/2015/394126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Glasnovic A, Cvija H, Stojic M, Tudoric-Deno I, Ivcevic S, Romic D, et al. Decreased level of sRAGE in the cerebrospinal fluid of multiple sclerosis patients at clinical onset. Neuroimmunomodulation. 2014;21:226–33. doi: 10.1159/000357002. [DOI] [PubMed] [Google Scholar]
- 44.Narvaez-Rivera RM, Rendon A, Salinas-Carmona MC, Rosas-Taraco AG. Soluble RAGE as a severity marker in community acquired pneumonia associated sepsis. BMC Infect Dis. 2012;12:15. doi: 10.1186/1471-2334-12-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Piarulli F, Sartore G, Lapolla A. Glyco-oxidation and cardiovascular complications in type 2 diabetes: a clinical update. Acta Diabetol. 2013;50:101–10. doi: 10.1007/s00592-012-0412-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lertwittayapon T, Tencomnao T, Santiyanont R. Inhibitory effect of alternatively spliced RAGEv1 on the expression of NF-kB and TNF-alpha in hepatocellular carcinoma cells. Genet Mol Res. 2012;11:1712–20. doi: 10.4238/2012.June.29.3. [DOI] [PubMed] [Google Scholar]
- 47.Shemirani F, Yazdanparast R. The interplay between hyperglycemia-induced oxidative stress markers and the level of soluble receptor for advanced glycation end products (sRAGE) in K562 cells. Mol Cell Endocrinol. 2014;393:179–86. doi: 10.1016/j.mce.2014.05.019. [DOI] [PubMed] [Google Scholar]
- 48.Quade-Lyssy P, Kanarek AM, Baiersdorfer M, Postina R, Kojro E. Statins stimulate the production of a soluble form of the receptor for advanced glycation end products. J Lipid Res. 2013;54:3052–61. doi: 10.1194/jlr.M038968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lam JK, Wang Y, Shiu SW, Wong Y, Betteridge DJ, Tan KC. Effect of insulin on the soluble receptor for advanced glycation end products (RAGE) Diabet Med. 2013;30:702–9. doi: 10.1111/dme.12166. [DOI] [PubMed] [Google Scholar]
- 50.Nakamura K, Yamagishi S, Nakamura Y, Takenaka K, Matsui T, Jinnouchi Y, et al. Telmisartan inhibits expression of a receptor for advanced glycation end products (RAGE) in angiotensin-II-exposed endothelial cells and decreases serum levels of soluble RAGE in patients with essential hypertension. Microvasc Res. 2005;70:137–41. doi: 10.1016/j.mvr.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 51.Koyama H, Tanaka S, Monden M, Shoji T, Morioka T, Fukumoto S, et al. Comparison of effects of pioglitazone and glimepiride on plasma soluble RAGE and RAGE expression in peripheral mononuclear cells in type 2 diabetes: randomized controlled trial (PioRAGE) Atherosclerosis. 2014;234:329–34. doi: 10.1016/j.atherosclerosis.2014.03.025. [DOI] [PubMed] [Google Scholar]
- 52.Tahara N, Yamagishi S, Mizoguchi M, Tahara A, Imaizumi T. Pioglitazone decreases asymmetric dimethylarginine levels in patients with impaired glucose tolerance or type 2 diabetes. Rejuvenation Res. 2013;16:344–51. doi: 10.1089/rej.2013.1434. [DOI] [PubMed] [Google Scholar]
- 53.Tan KC, Chow WS, Tso AW, Xu A, Tse HF, Hoo RL, et al. Thiazolidinedione increases serum soluble receptor for advanced glycation end-products in type 2 diabetes. Diabetologia. 2007;50:1819–25. doi: 10.1007/s00125-007-0759-0. [DOI] [PubMed] [Google Scholar]
- 54.Yanagisawa K, Ashihara J, Obara S, Wada N, Takeuchi M, Nishino Y, et al. Switching to multiple daily injection therapy with glulisine improves glycaemic control, vascular damage and treatment satisfaction in basal insulin glargine-injected diabetic patients. Diabetes Metab Res Rev. 2014;30:693–700. doi: 10.1002/dmrr.2537. [DOI] [PubMed] [Google Scholar]
- 55.Arabi YM, Dehbi M, Rishu AH, Baturcam E, Kahoul SH, Brits RJ, et al. sRAGE in diabetic and non-diabetic critically ill patients: effects of intensive insulin therapy. Crit Care. 2011;15:R203. doi: 10.1186/cc10420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sakata K, Hayakawa M, Yano Y, Tamaki N, Yokota N, Eto T, et al. Efficacy of alogliptin, a dipeptidyl peptidase-4 inhibitor, on glucose parameters, the activity of the advanced glycation end product (AGE) – receptor for AGE (RAGE) axis and albuminuria in Japanese type 2 diabetes. Diabetes Metab Res Rev. 2013;29:624–30. doi: 10.1002/dmrr.2437. [DOI] [PubMed] [Google Scholar]
- 57.Forbes JM, Soderlund J, Yap FY, Knip M, Andrikopoulos S, Ilonen J, et al. Receptor for advanced glycation end-products (RAGE) provides a link between genetic susceptibility and environmental factors in type 1 diabetes. Diabetologia. 2011;54:1032–42. doi: 10.1007/s00125-011-2058-z. [DOI] [PubMed] [Google Scholar]
- 58.Nakamura T, Sato E, Fujiwara N, Kawagoe Y, Koide H, Ueda Y, et al. Calcium channel blocker inhibition of AGE and RAGE axis limits renal injury in nondiabetic patients with stage I or II chronic kidney disease. Clin Cardiol. 2011;34:372–7. doi: 10.1002/clc.20885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Forbes JM, Thorpe SR, Thallas-Bonke V, Pete J, Thomas MC, Deemer ER, et al. Modulation of soluble receptor for advanced glycation end products by angiotensin-converting enzyme-1 inhibition in diabetic nephropathy. J Am Soc Nephrol. 2005;16:2363–72. doi: 10.1681/ASN.2005010062. [DOI] [PubMed] [Google Scholar]
- 60.Fukushima Y, Daida H, Morimoto T, Kasai T, Miyauchi K, Yamagishi S, et al. Relationship between advanced glycation end products and plaque progression in patients with acute coronary syndrome: the JAPAN-ACS sub-study. Cardiovasc Diabetol. 2013;12:5. doi: 10.1186/1475-2840-12-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tam HL, Shiu SW, Wong Y, Chow WS, Betteridge DJ, Tan KC. Effects of atorvastatin on serum soluble receptors for advanced glycation end-products in type 2 diabetes. Atherosclerosis. 2010;209:173–7. doi: 10.1016/j.atherosclerosis.2009.08.031. [DOI] [PubMed] [Google Scholar]
- 62.Santilli F, Bucciarelli L, Noto D, Cefalu AB, Davi V, Ferrante E, et al. Decreased plasma soluble RAGE in patients with hypercholesterolemia: effects of statins. Free Radic Biol Med. 2007;43:1255–62. doi: 10.1016/j.freeradbiomed.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 63.Huang SM, Chang YH, Chao YC, Lin JA, Wu CH, Lai CY, et al. EGCG-rich green tea extract stimulates sRAGE secretion to inhibit S100A12-RAGE axis through ADAM10-mediated ectodomain shedding of extracellular RAGE in type 2 diabetes. Mol Nutr Food Res. 2013;57:2264–8. doi: 10.1002/mnfr.201300275. [DOI] [PubMed] [Google Scholar]
- 64.Soop A, Sunden-Cullberg J, Albert J, Hallstrom L, Treutiger CJ, Sollevi A. Adenosine infusion attenuates soluble RAGE in endotoxin-induced inflammation in human volunteers. Acta Physiol (Oxf) 2009;197:47–53. doi: 10.1111/j.1748-1716.2009.01985.x. [DOI] [PubMed] [Google Scholar]
- 65.Pullerits R, Bokarewa M, Dahlberg L, Tarkowski A. Decreased levels of soluble receptor for advanced glycation end products in patients with rheumatoid arthritis indicating deficient inflammatory control. Arthritis Res Ther. 2005;7:R817–24. doi: 10.1186/ar1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sung JY, Chung W, Kim AJ, Kim HS, Ro H, Chang JH, et al. Calcitriol treatment increases serum levels of the soluble receptor of advanced glycation end products in hemodialysis patients with secondary hyperparathyroidism. Tohoku J Exp Med. 2013;230:59–66. doi: 10.1620/tjem.230.59. [DOI] [PubMed] [Google Scholar]
- 67.Brix JM, Hollerl F, Kopp HP, Schernthaner GH, Schernthaner G. The soluble form of the receptor of advanced glycation endproducts increases after bariatric surgery in morbid obesity. Int J Obes (Lond) 2012;36:1412–7. doi: 10.1038/ijo.2012.107. [DOI] [PubMed] [Google Scholar]
- 68.Parikh M, Chung M, Sheth S, McMacken M, Zahra T, Saunders JK, et al. Randomized pilot trial of bariatric surgery versus intensive medical weight management on diabetes remission in type 2 diabetic patients who do NOT meet NIH criteria for surgery and the role of soluble RAGE as a novel biomarker of success. Ann Surg. 2014;260:617–22. doi: 10.1097/SLA.0000000000000919. discussion 22–4. [DOI] [PMC free article] [PubMed] [Google Scholar]


