Abstract
Background
Obesity may be associated with worse clinical outcomes, including chronic kidney disease. It is unclear if this association is modified by age.
Methods
In a national cohort of over 3·3 million (n=3,376,187) US veterans with estimated glomerular filtration rate (eGFR) >60ml/min/1·73m2, we examined the association of body mass index (BMI) in patients of different age (<40, 40–<50, 50–<60, 60–<70, 70–<80, and ≥80 years old) with loss of kidney function and with all-cause mortality in logistic regression models and proportional hazards models adjusted for race, gender, comorbidities, medications, and baseline eGFR.
Findings
A U-shaped association between BMI and loss of kidney function was somewhat consistent and more prominent with advancing age, except in the patients ≤40 years old, in whom BMI did not appear to predict renal function impairment. The lowest risk for loss of kidney function was observed in patients with BMI 25– <30 kg/m2. BMI also displayed a U-shaped association with mortality, which was similar in all age groups.
Interpretation
BMI ≥30 kg/m2 is associated with rapid loss of kidney function in patients with eGFR ≥60 ml/min/1·73m2, and BMI ≥35 kg/m2 is also associated with high mortality. The former association is accentuated in older patients. A BMI of 25– <30 kg/m2 is associated with optimal clinical outcomes.
Keywords: Body Mass Index, Chronic Kidney Disease, Age
INTRODUCTION
Obesity is associated with increased risk of incident chronic kidney disease (CKD),1,2 end stage renal disease (ESRD),3,4 and mortality,5,6 according to some but not all studies. In past decades, many observational studies examined the associations with BMI in individuals of different age7 and with various clinical conditions.8 Paradoxical associations were observed in persons with pre-existing chronic illnesses.9–11 The optimal BMI for survival has also varied from study to study.5,12 Besides obesity, very low BMI levels have been consistently associated with high all-cause mortality.6,13 Some, but not all clinical trials reported improved kidney function after intentional weight loss in obese individuals.14,15
Obesity is a chronic condition which could persist for decades in most affected individuals. Older age is associated with a higher prevalence of comorbid conditions and a high short-term mortality, and it is therefore possible that age may modify the association of BMI with outcomes such as kidney disease. The heterogeneity of the study populations in most previous studies, in which subjects were different not only by age but also by their comorbidities, makes it difficult to determine the independent effect of age on the risk imparted by obesity. In order to determine whether the risk of adverse clinical outcomes in relation to obesity would differ by age, we examined the association of BMI with progressive loss of kidney function and with all-cause mortality in a large national cohort of US veterans with estimated glomerular filtration rate (eGFR) of ≥60 ml/min/1·73m2 grouped by age. We hypothesized that the association of BMI with clinical outcomes will be attenuated in older patients, especially in individuals with a high chronic comorbidity burden.
METHODS
Study Population
Data was extracted from a historic cohort study (Racial and Cardiovascular Risk Anomalies in CKD (RCAV) study), as previously described.16,17 Briefly, the cohort consisted of 3,582,478 US veterans, selected from among all veterans who received clinical care in any of the VA healthcare facilities, and who had an eGFR >60 ml/min/1·73m2 recorded during October 1, 2004–September 30, 2006, calculated by using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.18 After excluding patients with no available weight and height measurement, those starting renal replacement therapy before the cohort entry, and those with extreme BMI values, our final study population included 3,376,187 individuals. Cohort entry was defined as the first date of eGFR ≥60 ml/min/1·73m2. Information about baseline demographic characteristics, vital signs, comorbid conditions (defined based on ICD9 codes recorded during October 1, 2004–September 30, 2006) and medication usage was extracted from various national VA research databases, as previously described.19–21 Information about race was cross referenced with data obtained from Medicare through the VA-Medicare data merge project.22 Baseline blood pressure was defined as the mean of all measurements performed in the first 90 days after cohort entry. Medication usage was examined both at cohort entry and throughout the follow-up period.
We calculated the BMI of every subject as the weight in kilograms divided by the height in meters squared. BMI levels were stable over time, with baseline BMI (29.1±5.6 kg/m2) and the mean of all intra-individual BMI measurements throughout the entire follow-up period (29.1±5.6 kg/m2) being similar; the average change in BMI over time was −0.0518 kg/m2/year (95% confidence interval: −0.0524, −0.0512). Therefore, in order to minimize the effects of random variations and erroneous entries, in primary analyses we used the mean of all available BMI measurements collected for each patient between cohort entry and the end of follow-up, and excluded extreme values over 55 or lower than 15 kg/m2 (a total of 7,800 patients were excluded due to extreme BMI values). We used baseline BMI as predictor in sensitivity analyses. BMI was divided into 6 a-priori defined categories: <20, 20–<25, 25–<30, 30–<35, 35–<40, and ≥40 kg/m2. We used this categorization instead of the standard classification23 in order to allow for the examination of more granular associations with extremely elevated BMI values, which is justified by the steady elevation of mean BMI levels observed in recent years.24 Age at baseline was stratified into six groups with ten-year increments starting from <40 years old to >80 years old. In order to examine the independent effects of both age and BMI on the studied outcomes, we constructed joint categories based on all possible one-to-one combinations of BMI categories and age groups, using patients with BMI <20 kg/m2 and age<40 years old as referent in all analyses. In sensitivity analyses we examined the association of BMI with the studied end points in separate subgroups of age, using BMI 20–<25 kg/m2 as referent within each subgroup.
Outcomes
Our co-primary outcomes were rapid decline of kidney function and all-cause mortality. Rapid decline in kidney function was defined as the presence of an average decrease (slope) in eGFR of more than 5 ml/min/1·73m2/year during the follow-up period.25 Slopes were calculated from minimum 3 available serum creatinine measurements by using least square regression. All-cause mortality was ascertained from the VA Vital Status Files22 which record dates of death or dates of last encounter based on all available sources in the VA system. Using the US National Death index as referent gold standard, the sensitivity and specificity of the VA Vital Status Files were shown to be 98·3% and 99·8% respectively.22
Statistical Analysis
Descriptive analyses were performed by using means ± standard deviation (SD), medians (interquartile range, IQR) and proportions as appropriate. The association of mutually exclusive categories of BMI-age combinations with the presence of rapid loss of kidney function and with mortality was examined in logistic regression models, and in Cox proportional hazards models, respectively. Patients were followed in survival analyses from the date of the baseline eGFR until the first occurrence of death and were censored at the date of last healthcare service, or on July 31, 2013.
The effect of potential confounders was analyzed by constructing multivariable adjusted models. Our main multivariable model included gender, race, baseline eGFR, median income, marital status, comorbidities (coronary heart disease, cerebrovascular disease, congestive heart failure (CHF), peripheral artery disease, rheumatologic disease, malignancy, depression, liver disease, chronic lung disease, HIV, and the Deyo-modified Charlson comorbidity index (CCI)),26 and baseline medication use (angiotensin-converting-enzyme inhibitors/angiotensin receptor blockers (ACEI/ARB), statins, and the use of any antihypertensive medications). Models were also adjusted for urine albumin-creatinine ratio (UACR) in subgroup analyses of patients with available measurements for this variable. Diabetes mellitus (DM) and blood pressure were not included in these models because these are likely effect mediators of obesity, rather than confounders. In order to examine whether DM and/or blood pressure have an effect on the association of BMI with progressive loss of kidney function, we constructed an additional model adjusting for these two variables in addition to all the other ones. In order to clarify the effect of pre-existing chronic illness on the association of BMI with progressive loss of kidney function, a subgroup analysis was conducted in patients lacking comorbid conditions (defined as CCI=0),in patients with and without hypertension. The effect of albuminuria on the association of BMI with both outcomes was examined in the subset of patients (29% of the total cohort) with available UACR, by adjusting for UACR level in addition to the other covariates.
Data points were missing for race (8·7%), marital status (3·6%), income (6·1%), and comorbidities (0·2%). 87·5% (for slopes of eGFR) and 86·6% (for mortality) of the patients included in crude models had complete data for multivariable analysis. Missing values were not imputed in primary analyses, and were substituted by using multiple imputation procedures in sensitivity analyses. Missing values were replaced by multiple imputations with a multivariate normal regression method using data augmentation with an iterative Markov chain Monte Carlo procedure.27,28 Five imputed datasets were generated, primary analyses were performed on each imputed dataset and Rubin’s combination rules were used to form one set of results.29
Statistical analyses were performed using Stata MP Version 12 (Stata Corporation, College Station, TX).
Role of the funding source: The sponsors had no role in the design and conduct of the study, in the collection, analysis, and interpretation of the data, and in the preparation, review, or approval of the manuscript. JLL, MZM, MKM, KKZ and CPK had access to raw data. The corresponding author (CPK) had full access to all of the data and the final responsibility to submit for publication.
RESULTS
The mean age of the cohort was 60·0±14·0 years, 72% were white and the mean baseline eGFR was 83·8±15·6 ml/min/1·73m2. The mean of the intra-individual mean BMI was 29·1±5·6 kg/m2, and BMI was measured a median of 18 (IQR: 10–30) times/patient. Baseline characteristics of patients categorized by their BMI and age are described in Tables 1a and 1b. Younger patients were more likely to be female and to be unmarried. Systolic blood pressure was incrementally higher with increasing BMI and age, but diastolic blood pressure increased with higher BMI but decreased with age. Older patients with higher BMI had higher medication use, higher prevalence of CHF, cardiovascular disease, DM, and hypertension. Depression was more common in younger and more obese individuals, and lung disease, malignancies, and cerebrovascular disease were more prevalent in slimmer and older patients.
Table 1.
| a: Baseline characteristics in patients grouped according to mutually exclusive categories of baseline age and body mass index | ||||||
|---|---|---|---|---|---|---|
| Number of patients | BMI (kg/m2) | |||||
| <20 kg/m2 | 20–<25 kg/m2 | 25–<30 kg/m2 | 30–<35 kg/m2 | 35–<40 kg/m2 | ≥40 kg/m2 | |
| <40 | 5,284 | 57,632 | 107,898 | 77,030 | 32,467 | 14,096 |
| 40–<50 | 8,520 | 71,687 | 148,400 | 117,231 | 52,143 | 27,404 |
| 50–<60 | 27,678 | 173,941 | 364,094 | 272,786 | 118,113 | 61,920 |
| 60–<70 | 20,528 | 132,535 | 299,402 | 198,035 | 73,946 | 31,620 |
| 70–<80 | 22,798 | 159,263 | 276,900 | 127,394 | 33,770 | 9,661 |
| ≥80 | 15,844 | 95,764 | 104,018 | 30,084 | 5,299 | 1,002 |
| Gender (%Male) | ||||||
| <40 | 56.04 | 69.61 | 81.50 | 82.13 | 79.31 | 79.21 |
| 40–<50 | 81.12 | 83.18 | 87.15 | 86.49 | 83.56 | 82.50 |
| 50–<60 | 94.14 | 94.10 | 95.38 | 94.79 | 93.29 | 91.87 |
| 60–<70 | 96.82 | 97.18 | 98.04 | 97.75 | 96.90 | 95.53 |
| 70–<80 | 97.66 | 98.76 | 99.16 | 99.05 | 98.44 | 97.45 |
| ≥80 | 94.07 | 97.22 | 97.94 | 97.39 | 96.38 | 92.81 |
| Race (%black) | ||||||
| <40 | 22.90 | 22.22 | 22.42 | 25.64 | 28.99 | 31.47 |
| 40–<50 | 35.05 | 32.21 | 31.74 | 32.52 | 31.67 | 30.29 |
| 50–<60 | 28.95 | 23.75 | 19.25 | 18.01 | 16.61 | 15.67 |
| 60–<70 | 20.73 | 14.21 | 10.94 | 10.71 | 10.69 | 11.31 |
| 70–<80 | 19.24 | 11.15 | 8.38 | 8.76 | 9.97 | 11.24 |
| ≥80 | 16.54 | 8.90 | 6.63 | 7.41 | 8.96 | 13.74 |
| Marital status (%married) | ||||||
| <40 | 27.01 | 30.40 | 38.56 | 44.51 | 46.17 | 44.78 |
| 40–<50 | 23.94 | 29.61 | 39.43 | 44.58 | 45.53 | 43.65 |
| 50–<60 | 28.29 | 37.61 | 49.81 | 55.13 | 55.90 | 54.26 |
| 60–<70 | 37.37 | 51.19 | 61.93 | 64.33 | 63.10 | 60.02 |
| 70–<80 | 48.46 | 62.88 | 69.17 | 68.58 | 65.77 | 62.31 |
| ≥80 | 50.17 | 59.81 | 62.33 | 59.65 | 56.60 | 51.90 |
| Median income($) | ||||||
| <40 | 17,000 | 17,967 | 19,218 | 19,172 | 18,849 | 18,837 |
| 40–<50 | 12,641 | 14,539 | 17,387 | 18,514 | 18,969 | 18,383 |
| 50–<60 | 13,719 | 17,495 | 22,514 | 23,895 | 23,862 | 22,774 |
| 60–<70 | 15,571 | 23,059 | 28,034 | 28,108 | 27,193 | 25,446 |
| 70–<80 | 15,787 | 24,287 | 28,404 | 28,008 | 25,974 | 24,159 |
| ≥80 | 16,649 | 22,535 | 24,936 | 23,307 | 21,994 | 20,967 |
| eGFR (EPI) (ml/min/1.73m2) | ||||||
| <40 | 108.0±18.0 | 103.6±17.0 | 99.8±16.4 | 98.4±16.5 | 98.8±16.6 | 99.8±16.7 |
| 40–<50 | 101.9±17.0 | 96.8±15.7 | 93.1±15.4 | 91.7±15.4 | 92.0±15.5 | 93.2±15.8 |
| 50–<60 | 95.6±15.4 | 90.1±14.6 | 86.1±13.9 | 85.0±13.7 | 85.1±13.9 | 85.9±14.2 |
| 60–<70 | 87.4±14.0 | 81.9±12.7 | 79.3±11.9 | 78.9±11.9 | 79.2±12.0 | 79.9±12.4 |
| 70–<80 | 79.5±11.8 | 75.8±10.2 | 74.5±9.7 | 74.3±9.8 | 74.4±10.0 | 74.9±10.4 |
| ≥80 | 73.9±9.9 | 71.5±8.9 | 70.6±8.5 | 70.6±8.6 | 70.5±8.6 | 71.4±9.3 |
| SBP (mmHg) | ||||||
| <40 | 117±15 | 122±15 | 127±15 | 131±15 | 133±16 | 137±17 |
| 40–<50 | 125±20 | 127±18 | 130±17 | 134±17 | 136±18 | 139±19 |
| 50–<60 | 130±22 | 132±20 | 134±19 | 137±19 | 139±19 | 140±19 |
| 60–<70 | 133±22 | 135±20 | 136±19 | 138±19 | 140±19 | 141±19 |
| 70–<80 | 134±22 | 137±20 | 138±19 | 139±19 | 140±19 | 140±19 |
| ≥80 | 136±22 | 138±21 | 139±20 | 140±20 | 140±20 | 140±21 |
| DBP (mmHg) | ||||||
| <40 | 71±11 | 73±11 | 76±11 | 78±11 | 80±11 | 81±12 |
| 40–<50 | 77±13 | 78±12 | 80±12 | 82±12 | 82±12 | 82±12 |
| 50–<60 | 78±13 | 79±12 | 80±11 | 81±11 | 81±12 | 80±12 |
| 60–<70 | 76±13 | 76±12 | 77±11 | 78±11 | 78±11 | 77±12 |
| 70–<80 | 72±12 | 72±11 | 73±11 | 74±11 | 74±11 | 74±11 |
| ≥80 | 70±12 | 71±11 | 72±11 | 72±11 | 72±11 | 73±12 |
| Statin (%) | ||||||
| <40 | 5.19 | 9.85 | 19.23 | 28.54 | 34.68 | 40.98 |
| 40–<50 | 22.04 | 31.98 | 44.80 | 53.21 | 57.97 | 61.60 |
| 50–<60 | 30.54 | 46.65 | 59.92 | 66.62 | 69.77 | 70.51 |
| 60–<70 | 37.29 | 55.12 | 64.01 | 67.66 | 69.24 | 68.64 |
| 70–<80 | 38.74 | 55.43 | 61.73 | 63.77 | 64.85 | 63.03 |
| ≥80 | 31.79 | 46.22 | 53.10 | 54.98 | 56.39 | 48.70 |
| ACEI/ARB (%) | ||||||
| <40 | 4.45 | 6.20 | 11.88 | 21.46 | 31.28 | 41.95 |
| 40–<50 | 21.40 | 24.29 | 33.67 | 45.77 | 56.57 | 66.59 |
| 50–<60 | 34.30 | 40.74 | 51.98 | 64.06 | 73.21 | 79.91 |
| 60–<70 | 39.83 | 48.85 | 58.94 | 69.21 | 76.69 | 81.42 |
| 70–<80 | 43.81 | 54.23 | 61.38 | 69.28 | 75.74 | 80.19 |
| ≥80 | 42.47 | 52.23 | 58.43 | 64.95 | 70.41 | 73.15 |
| Anti-hypertensive medication (%) | ||||||
| <40 | 21.54 | 25.13 | 34.12 | 46.29 | 57.69 | 69.13 |
| 40–<50 | 52.23 | 54.15 | 62.07 | 71.98 | 80.13 | 87.38 |
| 50–<60 | 70.97 | 73.44 | 79.97 | 86.92 | 91.39 | 94.14 |
| 60–<70 | 76.55 | 80.06 | 85.01 | 89.94 | 93.09 | 94.52 |
| 70–<80 | 79.81 | 84.55 | 87.95 | 91.32 | 93.62 | 94.72 |
| ≥80 | 78.04 | 84.06 | 87.58 | 90.16 | 91.77 | 90.42 |
| b: Baseline characteristics in patients grouped according to mutually exclusive categories of baseline age and body mass index | ||||||
|---|---|---|---|---|---|---|
| BMI (kg/m2) | ||||||
| <20 kg/m2 | 20–<25 kg/m2 | 25–<30 kg/m2 | 30–<35 kg/m2 | 35–<40 kg/m2 | ≥40 kg/m2 | |
| DM | ||||||
| <40 | 2.07 | 2.10 | 2.89 | 5.26 | 8.51 | 13.04 |
| 40–<50 | 5.90 | 6.88 | 10.38 | 16.60 | 23.93 | 33.00 |
| 50–<60 | 8.49 | 12.39 | 20.20 | 30.96 | 42.07 | 52.42 |
| 60–<70 | 9.55 | 16.97 | 25.34 | 36.77 | 48.26 | 57.61 |
| 70–<80 | 12.08 | 21.24 | 28.16 | 38.35 | 48.82 | 55.96 |
| ≥80 | 12.76 | 19.71 | 25.80 | 34.59 | 43.89 | 45.16 |
| Hypertension | ||||||
| <40 | 4.64 | 6.52 | 13.14 | 22.20 | 31.51 | 42.62 |
| 40–<50 | 23.89 | 26.08 | 35.36 | 47.52 | 57.49 | 67.34 |
| 50–<60 | 40.36 | 45.20 | 55.94 | 67.34 | 75.88 | 81.28 |
| 60–<70 | 51.25 | 58.16 | 67.88 | 77.17 | 83.35 | 86.57 |
| 70–<80 | 58.92 | 67.74 | 74.74 | 81.49 | 85.94 | 88.16 |
| ≥80 | 61.87 | 69.66 | 75.66 | 80.61 | 83.81 | 83.57 |
| CHF | ||||||
| <40 | 0.21 | 0.17 | 0.17 | 0.25 | 0.46 | 1.05 |
| 40–<50 | 1.50 | 1.00 | 0.96 | 1.28 | 2.08 | 4.27 |
| 50–<60 | 3.49 | 2.73 | 2.47 | 3.33 | 5.23 | 9.41 |
| 60–<70 | 5.77 | 4.25 | 3.74 | 5.14 | 8.02 | 13.04 |
| 70–<80 | 8.29 | 6.36 | 5.60 | 7.75 | 12.07 | 17.51 |
| ≥80 | 11.47 | 9.43 | 9.02 | 12.86 | 16.82 | 24.60 |
| Cardiovascular disease | ||||||
| <40 | 0.17 | 0.31 | 0.48 | 0.63 | 0.88 | 1.34 |
| 40–<50 | 2.47 | 2.73 | 3.36 | 4.26 | 5.13 | 5.78 |
| 50–<60 | 7.00 | 7.86 | 9.54 | 11.67 | 13.47 | 14.02 |
| 60–<70 | 10.82 | 12.81 | 14.51 | 16.68 | 18.06 | 18.28 |
| 70–<80 | 13.78 | 16.29 | 17.32 | 18.65 | 19.00 | 18.18 |
| ≥80 | 14.79 | 17.34 | 18.12 | 19.24 | 18.64 | 15.12 |
| Cerebrovascular disease | ||||||
| <40 | 0.70 | 0.56 | 0.47 | 0.52 | 0.61 | 0.60 |
| 40–<50 | 2.87 | 2.19 | 1.89 | 1.90 | 1.99 | 2.07 |
| 50–<60 | 6.43 | 5.37 | 4.63 | 4.55 | 4.57 | 4.14 |
| 60–<70 | 10.65 | 8.78 | 7.16 | 6.67 | 6.37 | 5.66 |
| 70–<80 | 13.67 | 11.40 | 9.57 | 8.88 | 8.32 | 7.10 |
| ≥80 | 13.85 | 13.14 | 12.02 | 11.43 | 10.05 | 9.38 |
| PAD | ||||||
| <40 | 0.65 | 0.55 | 0.42 | 0.39 | 0.41 | 0.73 |
| 40–<50 | 2.88 | 1.89 | 1.49 | 1.43 | 1.64 | 2.09 |
| 50–<60 | 7.50 | 5.55 | 4.30 | 4.21 | 4.53 | 5.47 |
| 60–<70 | 12.32 | 8.54 | 6.43 | 6.21 | 6.74 | 7.34 |
| 70–<80 | 13.51 | 9.49 | 7.86 | 7.98 | 8.81 | 9.52 |
| ≥80 | 11.67 | 9.79 | 9.17 | 9.80 | 10.33 | 9.68 |
| Malignancy | ||||||
| <40 | 2.11 | 1.46 | 1.30 | 1.26 | 1.19 | 1.33 |
| 40–<50 | 7.85 | 3.88 | 2.68 | 2.55 | 2.44 | 2.39 |
| 50–<60 | 15.92 | 9.02 | 6.69 | 5.97 | 5.58 | 5.13 |
| 60–<70 | 23.10 | 15.57 | 12.15 | 11.11 | 10.40 | 9.15 |
| 70–<80 | 25.25 | 20.25 | 17.91 | 16.85 | 16.22 | 14.55 |
| ≥80 | 23.61 | 21.44 | 20.73 | 20.11 | 19.19 | 18.65 |
| Depression | ||||||
| <40 | 13.49 | 13.77 | 13.48 | 14.45 | 15.93 | 17.04 |
| 40–<50 | 14.81 | 16.89 | 16.76 | 16.72 | 17.73 | 18.47 |
| 50–<60 | 11.14 | 12.97 | 12.86 | 13.20 | 13.85 | 14.05 |
| 60–<70 | 6.58 | 6.16 | 5.65 | 5.94 | 6.69 | 7.14 |
| 70–<80 | 3.90 | 3.30 | 2.97 | 2.93 | 3.37 | 3.92 |
| ≥80 | 3.12 | 2.76 | 2.67 | 2.97 | 3.03 | 4.13 |
| Liver disease | ||||||
| <40 | 0.23 | 0.25 | 0.20 | 0.22 | 0.22 | 0.28 |
| 40–<50 | 2.51 | 2.02 | 1.54 | 1.27 | 1.12 | 1.06 |
| 50–<60 | 3.60 | 3.11 | 2.12 | 1.77 | 1.53 | 1.30 |
| 60–<70 | 2.19 | 1.47 | 0.94 | 0.86 | 0.85 | 0.70 |
| 70–<80 | 0.90 | 0.70 | 0.53 | 0.51 | 0.62 | 0.52 |
| ≥80 | 0.39 | 0.32 | 0.31 | 0.31 | 0.47 | 0.30 |
| Rheumatologic disease | ||||||
| <40 | 0.67 | 0.56 | 0.53 | 0.49 | 0.65 | 0.53 |
| 40–<50 | 1.30 | 1.14 | 1.03 | 0.93 | 1.02 | 1.08 |
| 50–<60 | 1.89 | 1.45 | 1.31 | 1.27 | 1.27 | 1.21 |
| 60–<70 | 2.09 | 1.93 | 1.53 | 1.40 | 1.23 | 1.27 |
| 70–<80 | 2.54 | 2.38 | 1.79 | 1.53 | 1.28 | 1.45 |
| ≥80 | 2.35 | 2.15 | 1.82 | 1.55 | 1.78 | 2.12 |
| Lung disease | ||||||
| <40 | 7.35 | 7.22 | 8.07 | 9.81 | 11.30 | 13.94 |
| 40–<50 | 21.47 | 14.44 | 12.09 | 13.03 | 15.40 | 19.04 |
| 50–<60 | 37.22 | 21.78 | 15.81 | 15.92 | 18.49 | 22.65 |
| 60–<70 | 50.39 | 26.75 | 17.99 | 17.93 | 20.88 | 25.17 |
| 70–<80 | 50.36 | 26.08 | 18.54 | 19.38 | 22.98 | 26.62 |
| ≥80 | 39.47 | 22.73 | 18.63 | 21.29 | 25.05 | 29.44 |
| HIV | ||||||
| <40 | 2.45 | 1.72 | 1.05 | 0.51 | 0.40 | 0.29 |
| 40–<50 | 6.45 | 3.90 | 1.92 | 0.88 | 0.55 | 0.37 |
| 50–<60 | 3.07 | 1.94 | 0.74 | 0.30 | 0.18 | 0.15 |
| 60–<70 | 1.23 | 0.64 | 0.20 | 0.09 | 0.06 | 0.05 |
| 70–<80 | 0.33 | 0.13 | 0.05 | 0.02 | 0.01 | 0 |
| ≥80 | 0.06 | 0.02 | 0.01 | 0.01 | 0.02 | 0 |
Loss of Kidney Function
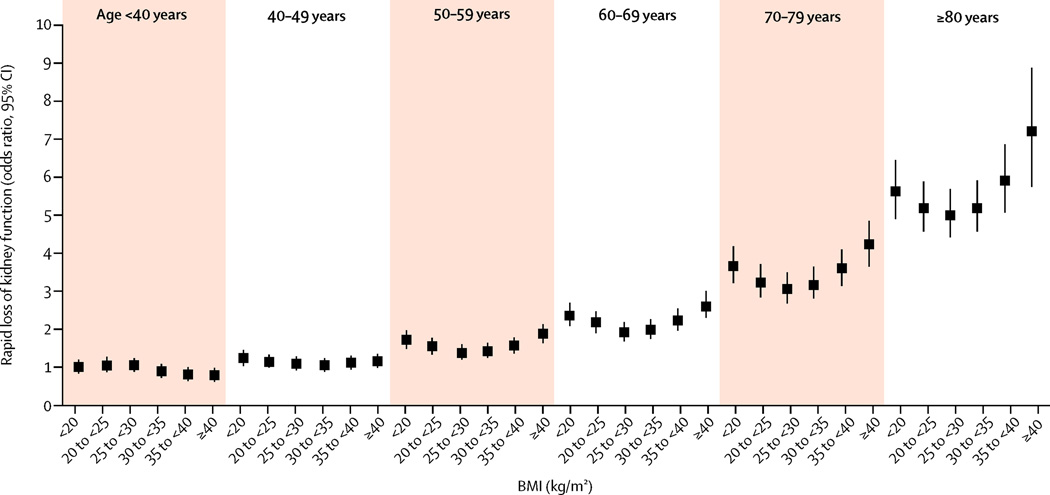
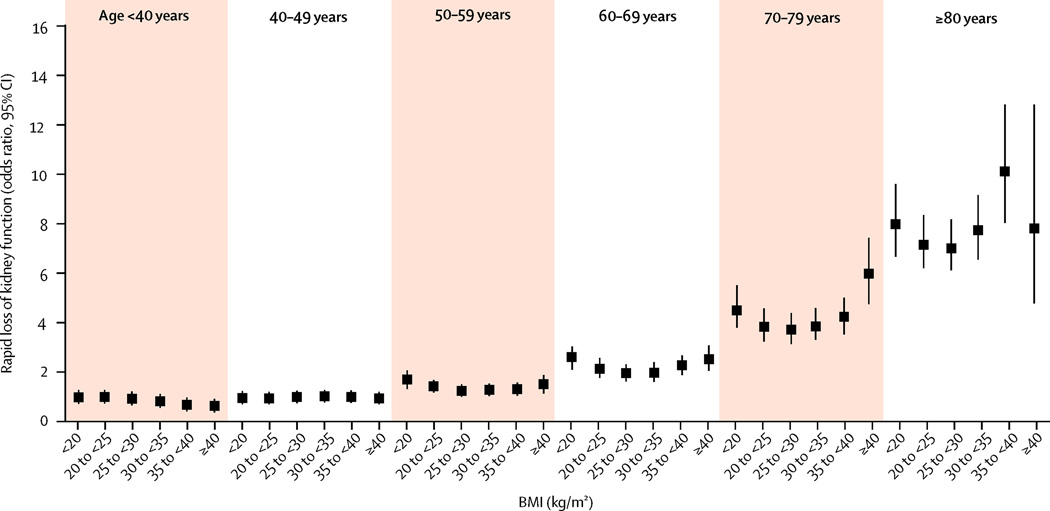
274,764 (8·8%) veterans in this cohort had steeper slopes (≤−5 ml/min/1·73m2). Figure 1 and supplemental Table 1 illustrates the multivariable-adjusted odds ratios (95%CI) of progressive loss of kidney function associated with the various combined age-BMI groups. Older patients had a higher risk of progressive loss of kidney function, independent of BMI levels. The association between BMI and faster decline of kidney function was U-shaped in patients older than 40 years old, with a marked accentuation of the risk associated with higher BMI as age increased. In those younger than 40 years old there was no association between BMI and progressive loss of kidney function. The lowest risk of kidney function deterioration was seen in individuals with a BMI of 25–30 kg/m2. The observed associations were consistent in various sensitivity analyses; such as after multiple imputations to account for missing covariates (supplemental Figure 1), when adjusting for DM and blood pressure in addition to other covariates (supplemental Figure 2), in subgroup analyses of individuals without chronic medical conditions (Figure 3) or history of hypertension (supplemental Figure 3), in patients with available UACR adjusted for UACR levels (supplemental Figure 4), when using baseline BMI as predictor (supplemental Figure 5), and when examining the associations in separate subgroups of age (supplemental Figure 6). Adjustment for DM and baseline blood pressure did not change the nature of the observed associations (supplemental Figure 2).
Figure 1.
Multivariable adjusted odds ratios (95% confidence intervals) of steeper slopes of estimated GFR vs. time (defined as slopes <−5 ml/min/1.73m2/year), associated with various BMI-age joint categories in logistic regression models. Model adjusted for gender, race, baseline eGFR, marital and income status, comorbidities, and medications except for diabetes mellitus and baseline blood pressure. Patients with BMI <20 kg/m2 and age <40yrs served as referent.
Figure 3.
Multivariable adjusted odds ratios (95% confidence intervals) of steeper slopes of estimated GFR vs. time (defined as slopes <−5 ml/min/1.73m2/year), associated with various BMI-age joint categories in logistic regression models in subjects with Charlson comorbdity index of 0. Model adjusted for gender, race, baseline eGFR, marital and income status, comorbidities, and medications except for diabetes mellitus and baseline blood pressure. Patients with BMI <20 kg/m2 and age <40yrs served as referent.
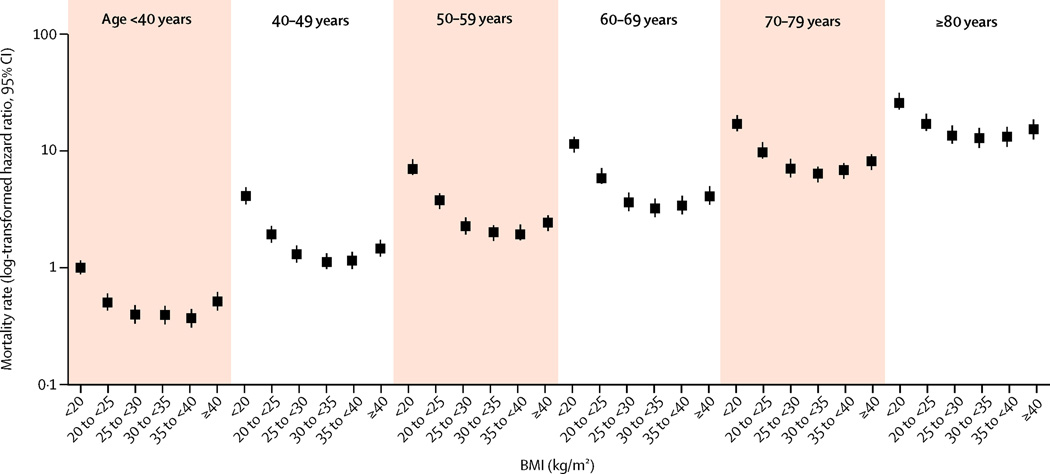
Mortality
A total of 672,341 patients died (mortality rate: 28·7/1000 patient years (PY), 95% CI: 28·6–28·7) over a median follow-up time of 6·8±1·6 years. Both BMI <20 kg/m2 and BMI >35 kg/m2 were associated with higher mortality (Figure 2 and supplemental Table 2), and these associations were similar for patients in all age groups. The results were qualitatively unchanged in various sensitivity analyses, except for an attenuation of the association between elevated BMI and mortality in older age groups after adjustment for UACR (supplemental Figures 7–10).
Figure 2.
Multivariable adjusted log-transformed hazard ratios (95% confidence intervals) of all-cause mortality associated with various BMI-age joint categories in Cox models. Model adjusted for gender, race, baseline eGFR, marital and income status, comorbidities, and medications except for diabetes mellitus and baseline blood pressure. Patients with BMI <20 kg/m2 and age <40yrs served as referent.
DISCUSSION
In this large national cohort of veterans with eGFR ≥60 ml/min/1·73m2, we describe an incrementally accentuated U-shaped association of BMI with progressive loss of kidney function in patients older than 40 years old. Patients younger than 40 years old showed no detrimental association between BMI and loss of kidney function over this relatively short follow-up period of approximately 7 years. A similar marked U-shaped association was present between BMI and all-cause mortality, with an especially high mortality seen in patients with BMI <20 kg/m2 in all age groups, and with an attenuated association with higher BMI in older patients after adjustment for UACR. Optimal outcomes were associated with overweight-to-mild obesity status.
Our study supports earlier findings of associations between obesity and higher prevalence of metabolic syndrome30 and CKD.31 Other measures of obesity such as the Waist-Hip-Ratio (WHR) have also been associated with renal impairment independent of BMI.32 However, few studies have examined the effect of age on the association of obesity with renal outcomes. In a cross sectional analysis from Turkey, ageing strengthened the association between BMI and CKD,33 but to the best of our knowledge there are no studies examining the association of BMI with future loss of kidney function in patients of different ages.
Our findings pertaining to mortality should be interpreted in the context of general population cohorts showing a higher risk of mortality and cardiovascular outcomes associated with obesity,34 and studies done in patients with severe chronic comorbidities indicating paradoxically lower mortality associated with obesity.9,35–37 The effect of age on the BMI-mortality relationship has been examined in previous studies, indicating that obesity was associated with a higher mortality rate in healthy non-smoking young persons than in sicker smoking old individuals.30 In our study, only 19% of the subjects did not have major chronic illnesses and only 20% were younger than 50 years old, so the entire cohort was a relatively sicker and older. Furthermore, even patients without measured comorbidities might have suffered from unmeasured illnesses, since our cohort was recruited from patients who received medical care in the VA system. It is therefore possible that the high mortality associated with the lowest BMI levels is due to measured and unmeasured disease states associated with malnutrition in all age categories, as previously described in other observational studies.5,38 Another possible explanation for the high mortality seen in patients with low BMI is the presence of the metabolically obese normal weight (MONW)39 status in some of them. Relatively weaker associations between BMI and mortality in older patients have also been described in other observational studies.7,40 These latter observations could be explained by short term survival benefits of high muscle mass or even body fat in individuals with elevated BMI.41
The accentuation of the association between high BMI and faster decline in kidney function in older individuals could have several explanations. There is strong evidence that obesity exerts negative effects through various mechanisms, either directly (increased renal sinus fat,42,43 focal or segmental glomerulosclerosis,44 glomerulomegaly,45 and glomerular hypertension or increased glomerular permeability caused by hyperfiltration related glomerular filtration barrier (GFB) injury46), or indirectly (obesity related hypertension47 or diabetes48). The glomerular hyperfiltration characteristic of higher BMI typically starts at a young age,49 and in stages of pre-hypertension or pre-diabetes.50 However, significant physiological and structural changes in nephrons resulting from obesity may only occur after a longer exposure,48,51,52 followed by irreversible pathological changes after even longer time.53 It is thus possible that progressive loss of kidney function becomes most obvious in individuals who were exposed to the effects of obesity for the longest time, which could explain the accentuation of this association with older age in our study. Another potential explanation for our findings is that the effects of aging and obesity on kidney function may conspire and accentuate each other. Aging itself causes increased glomerular permeability, decreased individual glomerular volume, glomerular sclerosis, and decreased nephron numbers.54 These effects could superimpose on the changes induced by obesity and result in a more marked effect on kidney function in older individuals. The observation that older age was independently associated with higher risk of progressive loss of kidney function in our cohort also supports this hypothesis.
Based on these competing hypotheses, it is unclear whether weight-reduction strategies aimed at renoprotection should start in younger vs. older age. This, and the optimal target BMI for interventions, will need further evaluation by clinical trials. Considering the effects of obesity on both kidney function and mortality (with the latter being most accentuated in younger patients), it is plausible to hypothesize that weight reduction in very obese individuals should be implemented at an early age, and maintained for a prolonged duration of time (potentially for several decades) in order to reap optimal benefits. Inadequate duration of weight-loss intervention may be one of the explanations why previous studies of intentional weight management14,15,55 did not universally improve mortality or comorbidity conditions. Conversely, it is also possible that obese older individuals could experience renoprotection from weight-loss interventions, by alleviating the burden of obesity on the ageing kidneys. However, any weight-reducing intervention (regardless of patients’ age) would have to be implemented cautiously, due to the uncertainty of the ideal therapeutic target, and the possibility of higher mortality associated with low BMI levels.
Our study has limitations. Most individuals in our cohort were male US veterans and suffered from a high prevalence of comorbid conditions, which limits the generalizability of our findings to women, the general population, or to individuals from other geographic regions of the world. We adjusted for multiple potential confounders, but we cannot rule out the effect of unmeasured confounders. We defined CKD using the CKD-EPI equation as it is more accurate than other estimating equations (such as the Modification of Diet in renal Disease (MDRD) equation) in patients with normal and mildly decreased GFR. The CKD-EPI equation was, however, meant to be used with serum creatinine measured by the IDMS-traceable method, which was not ubiquitous at the time when our cohort was defined (2005–2006), and hence it is unclear how accurate the estimation of GFR in our cohort was. BMI is not an ideal marker of obesity. A study from Taiwan used body fat as predictor of outcomes instead of BMI, since their mean BMIs were closer to the normal range.56 Indeed, some studies also assessed the WHR or visceral fat and found these to be better associated with kidney function than BMI.32,57–60 We used estimated GFR to assess kidney function, which could lead to inaccuracies at extreme BMI levels. However, our main renal outcome was intra-individual change in eGFR, which should not have been affected by such inaccuracies assuming stable BMI over time. BMI’s association with mortality could affect competing end points such as the development of CKD, but our use of eGFR slopes with sufficient number of measurements over time eliminates such competing risk.
In conclusion, the association between BMI and progressive loss of kidney function in this cohort of patients with normal baseline eGFR is U-shaped. Age had a strong effect on this association, which became more accentuated in older individuals. BMI also had a U-shaped association with mortality, but the association of higher BMI with mortality was attenuated (but not negated) in older individuals. It will be important to study in randomized controlled trials the optimal age when weight-loss interventions should start, and the optimal duration for which such interventions should be maintained in order to achieve the best clinical outcomes.
Supplementary Material
ACKNOWLEDGMENTS
This study is supported by grant R01DK096920 to CPK and KKZ and utilized resources provided by the Memphis VAMC and the Long Beach VA Healthcare System. Support for VA/CMS data is provided by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Health Services Research and Development, VA Information Resource Center (Project Numbers SDR 02-237 and 98-004).
KKZ reports personal fees from Abbott, Abbvie, Fresenius, Genentech, Genzyme/Sanofi, Hospira, Keryx, Amgen, Shire and Vifor, non-financial support from DaVita, and grants from NIH, outside the submitted work. CPK reports personal fees from Sanofi Aventis, NPS, ZS Pharma, Relypsa and Amgen, royalties from UpToDate for a review article on metabolic acidosis, and grants from the US National Institute of Health, Abbvie, Amgen, Shire, OPKO, outside the submitted work. CPK and KKZ are employees of the US Department of Veterans affairs. Opinions expressed in this paper are those of the authors’ and do not necessarily represent the opinion of the Department of Veterans Affairs.
Funding: This study is supported by grant R01DK096920 to CPK and KKZ and utilized resources provided by the Memphis VAMC and the Long Beach VA Healthcare System. Support for VA/CMS data is provided by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Health Services Research and Development, VA Information Resource Center (Project Numbers SDR 02-237 and 98-004).
Footnotes
CONFLICT OF INTEREST STATEMENT
The other authors report no relevant conflicts of interest.
AUTHOR CONTRIBUTIONS
Study concept and design: JLL, MZM, KKZ and CPK.
Literature search: JLL and AN.
Acquisition of data: CPK, JLL, MZM.
Analysis and interpretation of data: JLL, MZM, AN, MKM, KKZ and CPK.
Drafting of the manuscript and approval of the final version: JLL, MKM and CPK.
Critical revision of the manuscript for important intellectual content and approval of the final version: MZM, AN and KKZ.
REFERENCES
- 1.Foster MC, Hwang SJ, Larson MG, et al. Overweight, obesity, and the development of stage 3 CKD: the Framingham Heart Study. Am J Kidney Dis. 2008;52(1):39–48. doi: 10.1053/j.ajkd.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fox CS, Larson MG, Leip EP, Culleton B, Wilson PW, Levy D. Predictors of new-onset kidney disease in a community-based population. JAMA. 2004;291(7):844–850. doi: 10.1001/jama.291.7.844. [DOI] [PubMed] [Google Scholar]
- 3.Vivante A, Golan E, Tzur D, et al. Body Mass Index in 1.2 Million Adolescents and Risk for End-Stage Renal Disease. Arch Intern Med. 2012:1–7. doi: 10.1001/2013.jamainternmed.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iseki K, Ikemiya Y, Kinjo K, Inoue T, Iseki C, Takishita S. Body mass index and the risk of development of end-stage renal disease in a screened cohort. Kidney Int. 2004;65(5):1870–1876. doi: 10.1111/j.1523-1755.2004.00582.x. [DOI] [PubMed] [Google Scholar]
- 5.de Gonzalez AB, Hartge P, Cerhan JR, et al. Body-Mass Index and Mortality among 1.46 Million White Adults. N Engl J Med. 2010;363(23):2211–2219. doi: 10.1056/NEJMoa1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Visscher TLS, Seidell JC, Menotti A, et al. Underweight and overweight in relation to mortality among men aged 40–59 and 50–69 years - The seven countries study. Am J of Epidemiol. 2000;151(7):660–666. doi: 10.1093/oxfordjournals.aje.a010260. [DOI] [PubMed] [Google Scholar]
- 7.Stevens J, Cai JW, Pamuk ER, Williamson DF, Thun MJ, Wood JL. The effect of age on the association between body-mass index and mortality. N Engl J Med. 1998;338(1):1–7. doi: 10.1056/NEJM199801013380101. [DOI] [PubMed] [Google Scholar]
- 8.Patel AV, Hildebrand JS, Gapstur SM. Body mass index and all-cause mortality in a large prospective cohort of white and black U.S. Adults. PLoS One. 2014;9(10):e109153. doi: 10.1371/journal.pone.0109153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark AL, Fonarow GC, Horwich TB. Waist circumference, body mass index, and survival in systolic heart failure: the obesity paradox revisited. J Card Fail. 2011;17(5):374–380. doi: 10.1016/j.cardfail.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 10.Escalante A, Haas RW, del Rincon I. Paradoxical effect of body mass index on survival in rheumatoid arthritis: role of comorbidity and systemic inflammation. Arch Intern Med. 2005;165(14):1624–1629. doi: 10.1001/archinte.165.14.1624. [DOI] [PubMed] [Google Scholar]
- 11.Leavey SF, McCullough K, Hecking E, Goodkin D, Port FK, Young EW. Body mass index and mortality in 'healthier' as compared with 'sicker' haemodialysis patients: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS) Nephrol Dial Transplant. 2001;16(12):2386–2394. doi: 10.1093/ndt/16.12.2386. [DOI] [PubMed] [Google Scholar]
- 12.Janssen I, Mark AE. Elevated body mass index and mortality risk in the elderly. Obes Rev. 2007;8(1):41–59. doi: 10.1111/j.1467-789X.2006.00248.x. [DOI] [PubMed] [Google Scholar]
- 13.Taylor DH, Jr, Ostbye T. The effect of middle- and old-age body mass index on short-term mortality in older people. J Am Geriatr Soc. 2001;49(10):1319–1326. doi: 10.1046/j.1532-5415.2001.49259.x. [DOI] [PubMed] [Google Scholar]
- 14.Bolignano D, Zoccali C. Effects of weight loss on renal function in obese CKD patients: a systematic review. Nephrol Dial Transplant. 2013;28(Suppl 4):iv82–iv98. doi: 10.1093/ndt/gft302. [DOI] [PubMed] [Google Scholar]
- 15.Navaneethan SD, Yehnert H, Moustarah F, Schreiber MJ, Schauer PR, Beddhu S. Weight loss interventions in chronic kidney disease: a systematic review and meta-analysis. Clin J Am Soc Nephrol. 2009;4(10):1565–1574. doi: 10.2215/CJN.02250409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molnar MZ, Alhourani HM, Wall BM, et al. Association of hepatitis C virus infection with incidence and progression of chronic kidney disease in a large cohort of US veterans. Hepatology. 2014 doi: 10.1002/hep.27664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gosmanova EO, Lu JL, Streja E, Cushman WC, Kalantar-Zadeh K, Kovesdy CP. Association of medical treatment nonadherence with all-cause mortality in newly treated hypertensive US veterans. Hypertension. 2014;64(5):951–957. doi: 10.1161/HYPERTENSIONAHA.114.03805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Molnar MZ, Kalantar-Zadeh K, Lott EH, et al. Angiotensin-converting enzyme inhibitor, angiotensin receptor blocker use, and mortality in patients with chronic kidney disease. J Am Coll Cardiol. 2014;63(7):650–658. doi: 10.1016/j.jacc.2013.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kovesdy CP, Bleyer AJ, Molnar MZ, et al. Blood pressure and mortality in U.S. veterans with chronic kidney disease: a cohort study. Ann Intern Med. 2013;159(4):233–242. doi: 10.7326/0003-4819-159-4-201308200-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kovesdy CP, Lu JL, Molnar MZ, et al. Observational modeling of strict vs conventional blood pressure control in patients with chronic kidney disease. JAMA Intern Med. 2014;174(9):1442–1449. doi: 10.1001/jamainternmed.2014.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arnold N, Sohn M, Maynard C, Hynes DM. Edward Hines jr. VA hospital, Hines, IL: VA Information Resource Center; 2006. VIReC technical report 2: VA-NDI mortality data merge project. [Google Scholar]
- 23.Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults--The Evidence Report. National Institutes of Health. Obes Res. 1998;6(Suppl 2):51S–209S. [PubMed] [Google Scholar]
- 24.Kuczmarski RJ, Flegal KM. Criteria for definition of overweight in transition: background and recommendations for the United States. Am J Clin Nutr. 2000;72(5):1074–1081. doi: 10.1093/ajcn/72.5.1074. [DOI] [PubMed] [Google Scholar]
- 25.KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013 doi: 10.1038/ki.2013.243. [DOI] [PubMed] [Google Scholar]
- 26.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 27.Li K. Imputation using Markov chains. J Stat Comput Simul. 1988;30(1):57–79. [Google Scholar]
- 28.Tanner M, Wong WH. The calculation of posterior distributions by data augmentation. J Am Stat Assoc. 1987;82:528–550. [Google Scholar]
- 29.Rubin D. Multiple imputation for nonresponse in surveys. New York: JWiley & Sons. 1987 [Google Scholar]
- 30.Canning KL, Brown RE, Jamnik VK, Kuk JL. Relationship between obesity and obesity-related morbidities weakens with aging. J Gerontol A Biol Sci Med Sci. 2014;69(1):87–92. doi: 10.1093/gerona/glt026. [DOI] [PubMed] [Google Scholar]
- 31.Lu JL, Kalantar-Zadeh K, Ma JZ, Quarles LD, Kovesdy CP. Association of body mass index with outcomes in patients with CKD. J Am Soc Nephrol. 2014;25(9):2088–2096. doi: 10.1681/ASN.2013070754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwakernaak AJ, Zelle DM, Bakker SJ, Navis G. Central body fat distribution associates with unfavorable renal hemodynamics independent of body mass index. J Am Soc Nephrol. 2013;24(6):987–994. doi: 10.1681/ASN.2012050460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suleymanlar G, Utas C, Arinsoy T, et al. A population-based survey of Chronic REnal Disease In Turkey--the CREDIT study. Nephrol Dial Transplant. 2011;26(6):1862–1871. doi: 10.1093/ndt/gfq656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW., Jr Body-mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med. 1999;341(15):1097–1105. doi: 10.1056/NEJM199910073411501. [DOI] [PubMed] [Google Scholar]
- 35.Tseng CH. Obesity paradox: differential effects on cancer and noncancer mortality in patients with type 2 diabetes mellitus. Atherosclerosis. 2013;226(1):186–192. doi: 10.1016/j.atherosclerosis.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 36.Wilson DO, Rogers RM, Wright EC, Anthonisen NR. Body weight in chronic obstructive pulmonary disease. The National Institutes of Health Intermittent Positive-Pressure Breathing Trial. Am Rev Respir Dis. 1989;139(6):1435–1438. doi: 10.1164/ajrccm/139.6.1435. [DOI] [PubMed] [Google Scholar]
- 37.Kwan BC, Murtaugh MA, Beddhu S. Associations of body size with metabolic syndrome and mortality in moderate chronic kidney disease. Clin J Am Soc Nephrol. 2007;2(5):992–998. doi: 10.2215/CJN.04221206. [DOI] [PubMed] [Google Scholar]
- 38.Nagai M, Kuriyama S, Kakizaki M, et al. Effect of Age on the Association between Body Mass Index and All-Cause Mortality: The Ohsaki Cohort Study. J Epidemiol. 2010;20(5):398–407. doi: 10.2188/jea.JE20090204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Conus F, Rabasa-Lhoret R, Peronnet F. Characteristics of metabolically obese normal-weight (MONW) subjects. Appl Physiol Nutr Metab. 2007;32(1):4–12. doi: 10.1139/h06-092. [DOI] [PubMed] [Google Scholar]
- 40.de Mutsert R, Snijder MB, van der Sman-de Beer F, et al. Association between body mass index and mortality is similar in the hemodialysis population and the general population at high age and equal duration of follow-up. J Am Soc Nephrol. 2007;18(3):967–974. doi: 10.1681/ASN.2006091050. [DOI] [PubMed] [Google Scholar]
- 41.Kalantar-Zadeh K, Kuwae N, Wu DY, et al. Associations of body fat and its changes over time with quality of life and prospective mortality in hemodialysis patients. Am J Clin Nutr. 2006;83(2):202–210. doi: 10.1093/ajcn/83.2.202. [DOI] [PubMed] [Google Scholar]
- 42.Henegar JR, Bigler SA, Henegar LK, Tyagi SC, Hall JE. Functional and structural changes in the kidney in the early stages of obesity. J Am Soc Nephrol. 2001;12(6):1211–1217. doi: 10.1681/ASN.V1261211. [DOI] [PubMed] [Google Scholar]
- 43.Foster MC, Hwang SJ, Porter SA, Massaro JM, Hoffmann U, Fox CS. Fatty kidney, hypertension, and chronic kidney disease: the Framingham Heart Study. Hypertension. 2011;58(5):784–790. doi: 10.1161/HYPERTENSIONAHA.111.175315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kambham N, Markowitz GS, Valeri AM, Lin J, D'Agati VD. Obesity-related glomerulopathy: an emerging epidemic. Kidney Int. 2001;59(4):1498–1509. doi: 10.1046/j.1523-1755.2001.0590041498.x. [DOI] [PubMed] [Google Scholar]
- 45.Tsuboi N, Utsunomiya Y, Kanzaki G, et al. Low glomerular density with glomerulomegaly in obesity-related glomerulopathy. Clin J Am Soc Nephrol. 2012;7(5):735–741. doi: 10.2215/CJN.07270711. [DOI] [PubMed] [Google Scholar]
- 46.Knight SF, Quigley JE, Yuan J, Roy SS, Elmarakby A, Imig JD. Endothelial dysfunction and the development of renal injury in spontaneously hypertensive rats fed a high-fat diet. Hypertension. 2008;51(2):352–359. doi: 10.1161/HYPERTENSIONAHA.107.099499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ribstein J, du Cailar G, Mimran A. Combined renal effects of overweight and hypertension. Hypertension. 1995;26(4):610–615. doi: 10.1161/01.hyp.26.4.610. [DOI] [PubMed] [Google Scholar]
- 48.Zhang C, Meng Y, Liu Q, et al. Injury to the endothelial surface layer induces glomerular hyperfiltration rats with early-stage diabetes. J Diabetes Res. 2014;2014:953740. doi: 10.1155/2014/953740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tomaszewski M, Charchar FJ, Maric C, et al. Glomerular hyperfiltration: a new marker of metabolic risk. Kidney Int. 2007;71(8):816–821. doi: 10.1038/sj.ki.5002160. [DOI] [PubMed] [Google Scholar]
- 50.Okada R, Yasuda Y, Tsushita K, Wakai K, Hamajima N, Matsuo S. Glomerular hyperfiltration in prediabetes and prehypertension. Nephrol Dial Transplant. 2012;27(5):1821–1825. doi: 10.1093/ndt/gfr651. [DOI] [PubMed] [Google Scholar]
- 51.Ruggenenti P, Porrini EL, Gaspari F, et al. Glomerular hyperfiltration and renal disease progression in type 2 diabetes. Diabetes Care. 2012;35(10):2061–2068. doi: 10.2337/dc11-2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Okada R, Yasuda Y, Tsushita K, Wakai K, Hamajima N, Matsuo S. The number of metabolic syndrome components is a good risk indicator for both early- and late-stage kidney damage. Nutr Metab Cardiovasc Dis. 2014;24(3):277–285. doi: 10.1016/j.numecd.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 53.Chen A, Sheu LF, Ho YS, et al. Experimental focal segmental glomerulosclerosis in mice. Nephron. 1998;78(4):440–452. doi: 10.1159/000044974. [DOI] [PubMed] [Google Scholar]
- 54.McNamara BJ, Diouf B, Hughson MD, Hoy WE, Bertram JF. Associations between age, body size and nephron number with individual glomerular volumes in urban West African males. Nephrol Dial Transplant. 2009;24(5):1500–1506. doi: 10.1093/ndt/gfn636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shea MK, Nicklas BJ, Houston DK, et al. The effect of intentional weight loss on all-cause mortality in older adults: results of a randomized controlled weight-loss trial. Am J Clin Nutr. 2011;94(3):839–846. doi: 10.3945/ajcn.110.006379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tsai YW, Ho CI, Chen JY, et al. Impact of body composition on estimated glomerular filtration rate in relatively healthy adults in Taiwan. Eur J Clin Nutr. 2014 doi: 10.1038/ejcn.2014.66. [DOI] [PubMed] [Google Scholar]
- 57.Evans PD, McIntyre NJ, Fluck RJ, McIntyre CW, Taal MW. Anthropomorphic Measurements That Include Central Fat Distribution Are More Closely Related with Key Risk Factors than BMI in CKD Stage 3. Plos One. 2012;7(4) doi: 10.1371/journal.pone.0034699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pinto-Sietsma SJ, Navis G, Janssen WM, et al. A central body fat distribution is related to renal function impairment, even in lean subjects. Am J Kidney Dis. 2003;41(4):733–741. doi: 10.1016/s0272-6386(03)00020-9. [DOI] [PubMed] [Google Scholar]
- 59.Elobeid MA, Desmond RA, Thomas O, Keith SW, Allison DB. Waist circumference values are increasing beyond those expected from BMI increases. Obesity. 2007;15(10):2380–2383. doi: 10.1038/oby.2007.282. [DOI] [PubMed] [Google Scholar]
- 60.Elsayed EF, Sarnak MJ, Tighiouart H, et al. Waist-to-hip ratio, body mass index, and subsequent kidney disease and death. Am J Kidney Dis. 2008;52(1):29–38. doi: 10.1053/j.ajkd.2008.02.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.