Abstract
Giardia lamblia is a leading protozoan cause of diarrheal disease worldwide. It colonizes the lumen and epithelial surface of the small intestine, but does not invade the mucosa. Acute infection causes only minimal mucosal inflammation. Effective immune defenses exist, yet their identity and mechanisms remain incompletely understood. Interleukin (IL)-17A has emerged as an important cytokine involved in inflammation and antimicrobial defense against bacterial pathogens at mucosal surfaces. In this study, we demonstrate that IL-17A has a crucial function in host defense against Giardia infection. Using murine infection models with Giardia muris and G. lamblia, we observed marked and selective induction of intestinal IL-17A with peak expression after two weeks. Th17 cells in the lamina propria and innate immune cells in the epithelial compartment of the small intestine were responsible for the IL-17A response. Experiments in gene-targeted mice revealed that the cytokine, and its cognate receptor IL-17RA, were required for eradication of the parasite. The actions of the cytokine were mediated by hematopoietic cells, and were required for the transport of IgA into the intestinal lumen, since IL-17A deficiency led to marked reduction of fecal IgA levels, as well as for increased intestinal expression of several other potential effectors, including β-defensin 1 and resistin-like molecule β. In contrast, intestinal hypermotility, another major antigiardial defense mechanism, was not impacted by IL-17A loss. Taken together, these findings demonstrate that IL-17A and IL-17 receptor signaling are essential for intestinal defense against the important lumen-dwelling intestinal parasite Giardia.
Keywords: Protozoan parasites, mucosal immunity, small intestine, cytokines
Graphical abstract
INTRODUCTION
Giardiasis, caused by the parasitic protozoon, Giardia lamblia, is one of the most common waterborne diarrheal diseases in the world, with more than 280 million people annually infected (Baldursson and Karanis, 2011; Feng and Xiao, 2011; Upcroft and Upcroft, 2001). The parasite colonizes predominantly the upper small intestine, where it attaches to the surface of the epithelium and undergoes asexual replication, but does not penetrate the epithelial barrier. Symptomatic disease is characterized by watery diarrhea, abdominal cramps, bloating, malabsorption, and weight loss. Infection is generally self-limiting, but can become persistent and severe in high-risk groups, especially young children and pregnant women, and re-infections occur frequently in endemic areas (Lengerich, et al., 1994).
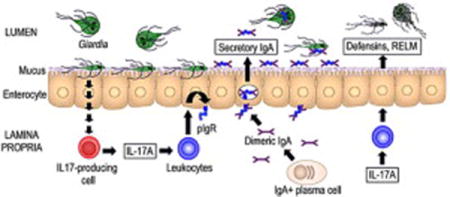
The normally self-limiting course of Giardia infection in humans, and studies in murine infection models, demonstrate that effective immune defenses exist against the parasite, yet they remain relatively poorly understood (Eckmann, 2003; Solaymani-Mohammadi and Singer, 2010). The parasite is located in the intestinal lumen and at the epithelial surface, suggesting that effective host defenses must be active at those “off-shore” sites. One important defensive mechanism that protects the epithelial surface is secretory IgA, which is produced by plasma cells in the lamina propria and transported to the lumen via the polymeric immunoglobulin receptor (pIgR) expressed on epithelial cells. Several murine studies have demonstrated essential functions of B cells (Stager and Muller, 1997), secretory IgA (Langford, et al., 2002), and pIgR (Davids, et al., 2006) in controlling parasite burden and eliminating infection. In contrast, in other mouse models, B cells were not required for clearance (Singer and Nash, 2000), suggesting that IgA can be dispensable in antigiardial defense and that antibody-independent effectors such as α-defensins may be important (Tako, et al., 2013). In humans, giardiasis is associated with hypogammaglobulinemia due to common variable immunodeficiency or X-linked agammaglobulinemia (Agarwal and Mayer, 2013; Stark, et al., 2009), whereas the association between selective IgA deficiency and infection is mostly anecdotal and has not been systematically investigated (Eren, et al., 2007; Fisher, et al., 1975). Independent of secretory IgA, increased intestinal motility has been shown to contribute to clearance of Giardia (Andersen, et al., 2006; Li, et al., 2006), presumably by reducing the chances of the parasite to attach to the epithelium and resist the luminal bulk flow.
Beyond direct effector mechanisms, several immune cells and regulators are known to be involved in antigiardial immune defense. Mast cells and CD4+ T cells, but not CD8+ T cells, are required for clearing Giardia infection (Heyworth, et al., 1987; Li, et al., 2004). CD4+ T cells may act in part by controlling antigiardial IgA responses (Heyworth, 1989), while their functions are not related to classical Th1 or Th2 subsets, since their signature cytokines, IFN-γ or IL-4, play no role in immune defense (Singer and Nash, 2000). In contrast, IL-6 is important in Giardia clearance (Bienz, et al., 2003; Zhou, et al., 2003). The cytokine appears to act by promoting dendritic cell functions during infection (Kamda, et al., 2012), although it has many other activities, including activation of neutrophils and monocytes, enhancement of follicular helper T cell responses, and stimulation of B cell proliferation and antibody production (Eto, et al., 2011; Mihara, et al., 2012). IL-6 is also a key inducer of Th17 cell responses and IL-17 production by innate and adaptive immune cells (Bettelli, et al., 2006; Geddes, et al., 2011; Mangan, et al., 2006; Passos, et al., 2010; Taylor, et al., 2014).
The IL-17 family of cytokines has six members (A–F), of which IL-17A is the founding and best studied one. IL-17A was initially identified as a pro-inflammatory cytokine that is produced by Th17 cells and stimulates neutrophils recruitment and production of inflammatory factors (Kolls and Khader, 2010; Onishi and Gaffen, 2010). In addition, IL-17A induces secretion of antimicrobial peptides (Onishi and Gaffen, 2010) and regulates intestinal pIgR expression and IgA production (Cao, et al., 2012; Hirota, et al., 2013). The cytokine can have both protective and pathogenic effects in different infection and inflammation models and organ systems (Onishi and Gaffen, 2010). In the intestinal tract, IL-17A is required for protective immunity against Helicobacter pylori (Algood, et al., 2009), Citrobacter rodentium (Ishigame, et al., 2009), Salmonella enterica serovar Typhimurium (Mayuzumi, et al., 2010; Raffatellu, et al., 2008), and enteroaggregative Escherichia coli (Philipson, et al., 2013), indicating that IL-17A is an important regulator of mucosal immune defenses associated with mucosal inflammation. Although Giardia infection is typically devoid of acute inflammatory events (Oberhuber, et al., 1997), a recent study found that IL-17A contributes to clearance of the murine pathogen Giardia muris (Dreesen, et al., 2014). The cellular source of IL-17A and the involvement of specific host defenses remain unknown. The present studies were conducted to elucidate the cellular requirements of IL-17A expression and signaling, and to determine how IL-17A signaling can impact intestinal defenses against different Giardia species.
MATERIALS AND METHODS
Mice and infection protocols
C57BL/6 (wild-type), Rag2−/− and Cd4−/− mice (The Jackson Laboratory), Il17a−/− (Nakae, et al., 2002) and Il17ra−/− (Ye, et al., 2001) were housed under specific pathogen-free conditions. At 8–12 weeks of age, mice were infected by oral gavage with either G. muris cysts (104 cysts/mouse) or G. lamblia GS/M trophozoites (106 trophozoites/mouse) as previously described (Davids, et al., 2006). Parasite loads were assessed at different times after infection by enumerating trophozoites in the small intestine (Langford, et al., 2002). Animals were housed according to institutional guidelines, as detailed in the current Guide for the Care and Use of Laboratory Animals. All animal studies were reviewed and approved by the University of California, San Diego Institutional Animal Care and Use Committee.
Bone marrow chimeras
Eight- to ten-week-old wild-type, Il17a−/−, and I117ra−/− recipient mice received a single dose of 9 Gy total body irradiation from a 137Cesium source. Eight hours later, mice were intravenously injected with 4–6 × 106 bone marrow cells from sex-matched wild-type, Il17a−/−, and Il17ra−/− donor mice, and then rested for 8 weeks before use.
Analysis of mRNA levels
Whole tissue was collected from the mid-third region of the small intestine, snap-frozen, and stored at −80°C until use. Total RNA was isolated from the tissue using TRIzol reagent (Life Technologies). RNA samples were treated with Turbo DNA-free (Ambion), assessed for quantity and purity using a NanoDrop spectrophotometer (NanoDrop Technologies), and reverse-transcribed into cDNA using the High Capacity cDNA Reverse Transcription kit (Applied Biosystems). Microarray analysis was performed to assess changes in expression of IL-17 family member genes after infection. Total RNA was processed and hybridized to CodeLink Mouse Whole Genome Bioarrays (carrying 34,790 unique probes; GE Healthcare) as described before (Spehlmann, et al., 2009). Hybridized slides were stained with streptavidin-Alexa 647 conjugate (Invitrogen/Molecular Probes) and scanned at 10 μm resolution with an Axon 4000B Scanner (Molecular Devices). Background correction was performed using Codelink software. Assays were performed on RNA collected from uninfected mice (Week 0) and at weeks 1, 2, and 3 post-infection. For each array, RNA was pooled from six female C57BL/6 mice. Microarray studies were also performed to identify genes differentially expressed between C57BL/6 mice and Il17ra−/− mice. For these studies, pooled total RNAs (six female mice/group) were analyzed using the Whole Mouse Gene Expression Microarray (4 × 44,000; Agilent Technologies), following the manufacturer’s protocols. Intensities were normalized and the means of array replicates were calculated. The microarray data were deposited with the Gene Expression Omnibus (GEO) database of the National Center for Biotechnology Information under accession number GSE68868.
Selected microarray data were confirmed by quantitative PCR analysis (qPCR) using Mesa Green 2 × SYBR mix (Eurogentc) in a real-time PCR machine. Primers and expected PCR product sizes were as follows: IL-17A, 5′- ACT ACC TCA ACC GTT CCA CG-3′ (sense), 5′-TTC CCT CCG CAT TGA CAC AG-3′ (antisense), 120 bp; IL-17E, 5′- CAG CAA AGA GCA AGA ACC CC-3′ (sense), 5′- ACC CGA TTC AAG TCC CTG TC-3′ (antisense), 180 bp; IL-17F, 5′- TGA AGT GCA CCC GTG AAA CA-3′ (sense), 5′- CTG CTT TGG GGT TCT TCC GA-3′ (antisense), 102 bp; GAPDH, 5′-TGT GAT GGG TGT GAA CCA CGA CAA-3′ (sense), 5′-AGT GAT GGC ATG GAC TGT GGT CAT-3′ (antisense), 209 bp. Relative changes in target mRNA levels were calculated by the 2ΔΔCt method, with GAPDH mRNA as the reference standard (CT values for GAPDH varied by <1 between different samples and experiments).
Isolation of epithelial and lamina propria cells
The mid-third region of the small intestine was opened, rinsed in PBS, and cut into 5 mm pieces, which were incubated in HBSS (without Ca and Mg) with 5 mM EDTA, 5% FCS, 10 mM HEPES (pH 7.3) twice for 20 min each at 37°C with gentle shaking. Detached cells within the epithelial compartment were passed through a nylon mesh strainer and collected by centrifugation. Cells were kept on ice prior to staining (see below). The remaining tissue pieces were washed in PBS, diced, and incubated three times at 37°C for 20 min each in RPMI 1640 medium containing 1 mg/ml collagenase D (Roche Applied Science) and 100 μg/ml DNase I (Worthington Biochemical). After each incubation, tissue suspensions were vortexed and passed through a nylon strainer. Cells from all digestions were combined and collected by centrifugation.
Flow cytometry and cell sorting
Isolated lamina propria cells were stimulated for 6 h with 50 ng/ml phorbol myristate acetate and 750 ng/ml ionomycin in complete RPMI 1640 medium at 37°C. GolgiStop (BD Biosciences) was added for the last 2 h of incubation. Stimulated cells were washed and stained with anti-CD4 PeCy5, fixed and permeabilized, and stained with PE-labeled anti-IL-17A. All antibodies were purchased from eBioscience. Cells were analyzed on a FACSCalibur flow cytometer (BD Biosciences) followed by data analysis with FlowJo software (TreeStar Inc.). For cell sorting, isolated cells were washed in PBS, FcR-blocked with anti-CD16/CD32 Mab (eBioscience) prior to incubation with anti-CD45 FITC and anti-EpCAM PE in PBS with 1% BSA for 30 min at 4°C. After washing in PBS, cells were resuspended in RPMI 1640 supplemented with 5% FBS and 1% penicillin/streptomycin, and sorted using a MoFLO XDP cell sorter (Beckman Coulter).
ELISA
For quantitation of tissue IL-17A levels, the mid-third region of the small intestine was divided into quadrants, and 0.5 cm long pieces were excised, snap-frozen, and stored at −80°C until use. The tissue was weighed and homogenized in PBS containing 1% Triton X-100 and Complete Protease Inhibitor (Roche). Following centrifugation, supernatants were collected and assayed by ELISA (Mouse IL-17A Duoset, R&D Systems). Results were normalized to wet weight of tissue.
To measure fecal antibodies, stool pellets were collected, homogenized in PBS containing 0.04 mg/ml soybean trypsin inhibitor, 20 mM EDTA, and 2 mM PMSF, and centrifuged to remove bacteria and debris (Cong, et al., 1998). Two-fold dilutions in PBS were added to 96-well plates coated with 1 μg/ml anti-IgA (Kirkegaard & Perry Labs), and incubated for 2 h at room temperature. Biotinylated anti-IgA (KPL; 0.25 μg/ml) was added for 1 h, followed by incubation with HRP-conjugated streptavidin. TMB substrate was used to visualize specific antibody binding. Optical density (OD) was determined at 450 nm, and results were normalized to the total protein concentrations determined by BCA assay (BioRad). We found in preliminary studies that protein levels are stable between individual animals (8.7 ± 2.1 μg protein/mg feces; n=5) and are therefore suitable for standardization.
To determine antigiardial IgA titers in serum, microtiter plates were coated with whole trophozoites by overnight drying and glutaraldehyde fixation as described (Davids, et al., 2006). After quenching with 0.15 M glycine and blocking with 5% dried nonfat milk and 1% goat serum in PBS, plates were incubated with 1:10 diluted sera (in PBS with 1% BSA) for 1 h at room temperature, washed with PBS, and further incubated with HRP-labeled goat anti-mouse IgA (Southern Biotech; 1:1,000 dilution in 1% goat serum in PBS) for 1 h at room temperature. Bound HRP was detected with H2O2 and TMB in 0.1 M sodium acetate buffer (pH 6.0), followed by addition of 1.2 M sulfuric acid and OD reading at 450 nm.
Assessment of intestinal motility
Intestinal motility was assessed by the transit of carmine dye through the intestine (Andersen, et al., 2006). Mice were fasted overnight and given a single oral dose of 0.2 ml of carmine solution (6% carmine dye and 5% gum arabic in PBS). Twenty minutes later, the small intestine was removed, and the position of the carmine dye front and the length of the entire small intestine were recorded. The distance traveled by the dye front was expressed as a percentage of the entire length of the small intestine.
Statistical analysis
Trophozoite numbers were log10 transformed, and mean and SEM were calculated. Samples without detectable trophozoites were assigned a log value of 3, which is equivalent to half of the detection limit for the assay (which was ~2 × 103 trophozoites/small intestine). Mann-Whitney rank sum test was used to assess differences in trophozoite counts between groups of mice. Student’s t test was used for all other comparisons. Differences with a p-value of <0.05 were considered significant.
RESULTS
G. muris infection induces intestinal IL-17A expression
To begin to define the role of IL-17 in the mucosal response to Giardia infection, we inoculated adult C57BL/6 mice with G. muris cysts by the natural oral route. Maximal trophozoite numbers in this model are observed by one week in the small intestine, which is followed by gradual clearance, with a >100 fold reduction in the infectious load by three weeks after infection (Langford, et al., 2002). Total RNA was extracted from the mucosa of the middle third of the small intestine (where the majority of Giardia trophozoites are localized) at different times after infection, and assayed for expression of IL-17 family members by microarray analysis. Levels of IL-17A mRNA increased after one week and peaked after two weeks (Fig. 1A). IL-17F, which is structurally and functionally related to IL-17A, was also induced two weeks after infection, but the increase was more modest compared to IL-17A (Fig. 1A). Other IL-17 cytokine family members, IL-17B, IL-17C, and IL-17D, were only minimally or not at all altered after infection (Fig. 1A).
Figure 1. Increased IL-17A expression after G. muris infection.
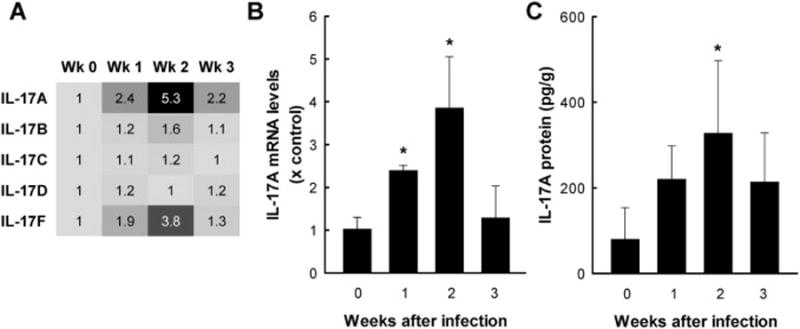
C57BL/6 mice were orally infected with 104 G. muris cysts, and the small intestine was removed at the indicated times. Uninfected mice were used as controls (Week 0). (A) Heat map of IL-17 family member mRNA expression determined by microarray analysis. Numbers represent the fold increases relative to uninfected controls (Wk 0). Data represent RNA pooled from 6 mice/group. (B) mRNA expression was analyzed by qPCR for IL-17A. Levels are presented as mean + SE of three separate experiments, which each experiment performed on total RNA pooled from 3–5 mice/time point. (C) Levels of IL-17A protein were assessed by ELISA in tissue extracts of the small intestine. Data represent mean + SE of 5–6 mice/time point. Data are representative of results from two independent experiments. *p<0.05 relative to uninfected controls.
Because IL-17A was the most strongly induced IL-17 family member after infection, we focused on this cytokine in subsequent studies. Increased intestinal expression was confirmed by qPCR, with maximal induction again seen after two weeks (Fig. 1B). Moreover, IL-17A protein levels were significantly elevated in tissue extracts after two weeks (Fig. 1C). Together, these results demonstrate that G. muris infection induces intestinal expression of IL-17A, and to a lesser extent IL-17F, with peak levels at two weeks, a time that corresponds to the maximal immune response to infection and the greatest reduction in parasite load.
Cellular sources of IL-17A after G. muris infection
IL-17A is the signature cytokine of Th17 cells. To determine whether they were responsible for the IL-17A response after infection, we prepared single-cell suspensions from the lamina propria of the small intestine and analyzed the CD4+ T cell population for IL-17A production by flow cytometry. Infection induced a moderate (2–3 fold) increase in IL-17A+ CD4+ T cells after two weeks (Fig. 2A), suggesting that Th17 cells in the lamina propria account for some but perhaps not all of the observed increase in IL-17A expression after infection.
Figure 2. Cellular sources of intestinal IL-17A in response to G. muris infection.
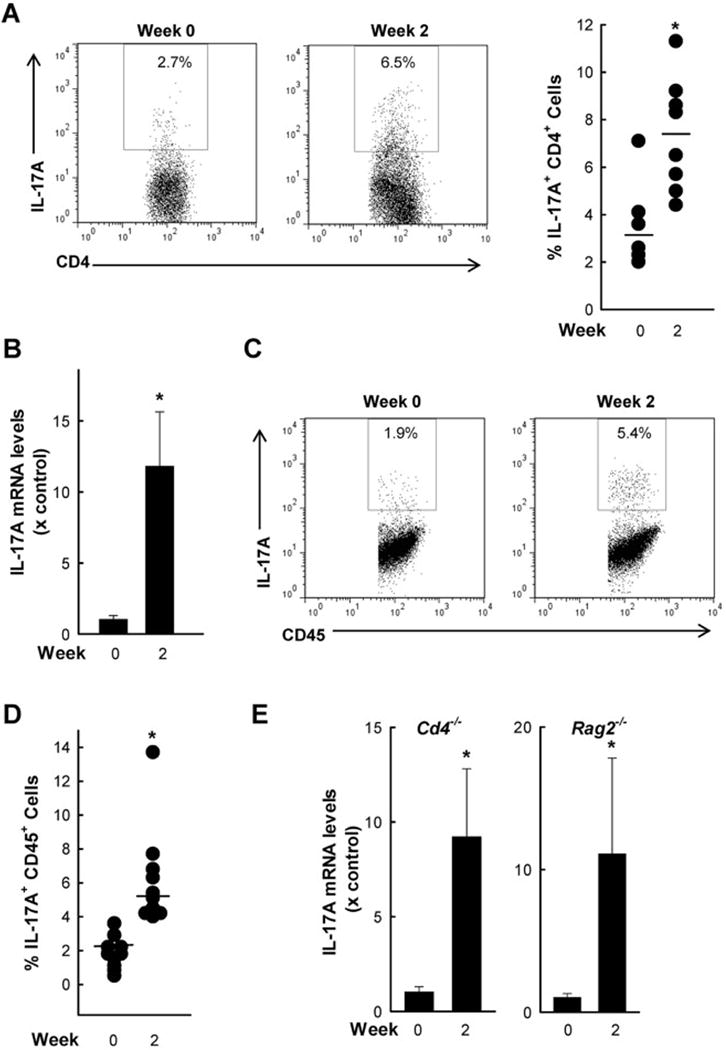
C57 mice were orally infected with G. muris cysts and examined after two weeks. Uninfected mice were used as a control. (A) CD4+ T cells were isolated from the lamina propria of the small intestine and analyzed by flow cytometry for CD4 and IL-17A expression. Representative FACS dot plots (left) and percentages of IL-17A producing CD4+ cells of individual mice (right). Horizontal bars indicate means. (B–D) Cells were isolated from the epithelial compartment of the small intestine of groups of 3–4 mice, and assessed for IL-17A mRNA expression by qPCR (B; bar graphs represent mean + SE for three independent experiments) and by flow cytometry for co-staining of IL-17A and CD45 (representative FACS dot plot in C, and data on cell percentages from 8–10 individual mice in D; Horizontal bars indicate means). (E) Quantitative PCR analysis of IL-17A mRNA in the small intestine of CD4-deficient (Cd4−/−) and Rag 2-deficient (Rag2−/−) mice infected with G muris. Bar graphs show fold induction (mean + SE; n=3–4) compared to uninfected controls. Data from A–D are representative of the results from three independent experiments; and data from E is representative of the results from two independent experiments; *p <0.05 compared to uninfected controls.
To determine whether other cells might also be involved in the IL-17A response, we isolated cells from the epithelial compartment and determined cytokine expression by qPCR. A marked increase in IL-17A was observed in the cells by two weeks after infection (Fig. 2B). Furthermore, flow cytometric analysis of these cells demonstrated IL-17A induction among the CD45+ cells, suggesting that IL-17A was increased in leukocytes within the epithelial compartment (Fig. 2C,D). Because Th17 cells are not known to be present in the epithelial compartment, we speculated that other leukocytes, such as innate immune cells (Cua and Tato, 2010), may be involved in the IL-17A response to infection. To address this possibility, we infected CD4 deficient (Cd4−/−) mice, which lack Th17 and other Th subsets, and cannot clear Giardia infection (Solaymani-Mohammadi and Singer, 2010), and mice deficient in recombination-activating gene 2 (Rag2−/−), which lack all T and B cells but have intact innate immune cells (Shinkai, et al., 1992). Marked induction of intestinal IL-17A expression was observed after infection in both groups of mutant mice (Fig. 2E). Collectively, these data suggest that non-Th17 leukocytes, probably innate immune cells residing within the small intestinal epithelium, contribute to the intestinal IL-17A response after G. muris infection.
IL-17A deficiency impairs G. muris clearance
We next sought to determine the physiological function of IL-17A in host defense against G. muris. Mice deficient for IL-17A (Il17a−/−) were infected with G. muris cysts, and trophozoite numbers were determined in the small intestine at different times after infection. Parasite levels were not significantly different from wild-type controls after one week, but Il17a−/− mice had a marked clearance defect thereafter, with 100- to 1,000-fold higher trophozoite numbers at week 7 (Fig. 3A). Furthermore, IL-17A production by hematopoietic cells was important for mediating its functions in host protection against Giardia, because bone marrow chimeric mice lacking IL-17A in hematopoietic cells displayed a significant defect in clearing G. muris after 7 weeks, whereas chimeric mice lacking IL-17A in non-hematopoietic cells had only a modest clearance defect that did not reach significance after 7 weeks (Fig. 3B). These data provide functional support for our earlier observation that Th17 cells as well as non-Th17, innate immune cells are responsible for the IL-17A response to infection.
Figure 3. G. muris clearance is impaired in the absence of IL-17A.
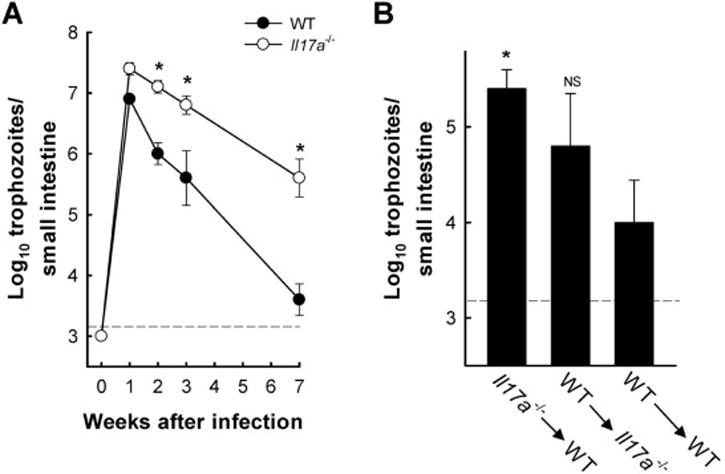
(A) IL-17A-deficient (open circles) and wild-type (closed circles) mice were infected with G muris, and trophozoite numbers in the small intestine were determined at the indicated times. Data are mean ± SE (n ≥6 mice/time point) from three independent experiments; *p<0.05 compared to wild-type controls. (B) Bone marrow chimeric mice were generated by isolating and transferring bone marrow cells from Il17a−/− mice into C57BL/6 (WT) mice (Il17a−/−→WT), from WT to Il17a−/− mice (WT→Il17a−/−), or as controls from WT to WT mice (WT→WT). Following an 8 week reconstitution period, mice were infected orally with G muris cysts. Trophozoite numbers were determined 7 weeks after infection. Data are mean + SE (n≥6 mice/time point) from two independent experiments; *p<0.05 relative to WT→WT controls. The black dashed lines depict the detection limit of the assays.
Role of IL-17A in host defense against G. lamblia
To expand the relevance of our findings, we tested another Giardia species, G. lamblia, which is a natural human pathogen. After oral infection of wild-type mice with G. lamblia GS/M trophozoites, small intestinal IL-17A mRNA levels were significantly increased 5 days after infection and remained elevated after two weeks (Fig. 4A; note that G. lamblia is known to have an accelerated time course of infection compared to G. muris). No significant changes were observed in IL-17E or IL-17F mRNA expression (with relative peak levels of 1.5 and 1.4, respectively, occurring after 5 days compared to uninfected controls). Oral infection of Il17a−/− and wild-type mice with G. lamblia GS/M revealed similar parasite numbers early (day 5) after infection, but Il17a−/− mice were markedly impaired in their ability to clear G. lamblia after two weeks (Fig. 4B). Together, these results indicate that IL-17A is critical in immune defense against two different Giardia species, including the human pathogen G. lamblia.
Figure 4. IL-17A deficiency impairs eradication of G. lamblia.
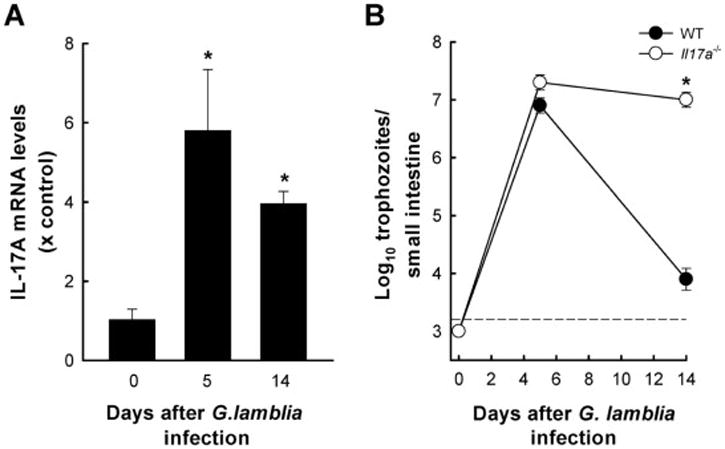
(A) Wild-type C57BL/6 mice were orally infected with 106 G lamblia GS/M trophozoites. Total RNA was extracted from the small intestine and analyzed for IL-17A mRNA expression by qPCR. Data are mean + SE (n=3–4 mice/time point) from two independent experiments, *p<0.05 relative to uninfected controls (Day 0). (B) Mice deficient in IL-17A (Il17a−/−, open circles) and wild-type (WT, closed circles) mice were infected with G lamblia, and trophozoite numbers were determined at the indicated times. Data are mean ± SE (n=3–4 mice/time point); *p<0.05 relative to wild-type controls. The black dashed line depicts the detection limit of the assay.
Importance of IL-17RA in host defense against G. muris
IL-17A has multiple effects on different cell types, including epithelial cells, endothelial cells, lymphocytes and granulocytes (Onishi and Gaffen, 2010), raising the question which of these may be important for mediating its functions in antigiardial defense. To begin to address this question, we utilized mice lacking a critical subunit, IL-17RA, of the cognate IL-17 receptor (Song and Qian, 2013). IL-17RA-deficient (Il17ra−/−) mice were strongly compromised in their ability to control and clear infection, as demonstrated by >9,000-fold higher G. muris numbers in the small intestine after 7 weeks compared to wild-type controls (Fig. 5A). Next, we generated bone marrow chimeric mice deficient in IL-17RA expression on either hematopoietic or non-hematopoietic cells. IL-17RA deficient mice reconstituted with wild-type hematopoietic cells exhibited no impairment in G. muris elimination, whereas wild-type mice reconstituted with IL-17RA-deficient hematopoietic cells showed a significant clearance defect after 5 weeks (Fig. 5B). These results indicate that IL-17RA signaling in hematopoietic cells is required for mediating immune protection against G. muris.
Figure 5. IL-17RA signaling in hematopoietic cells is essential for G. muris eradication.
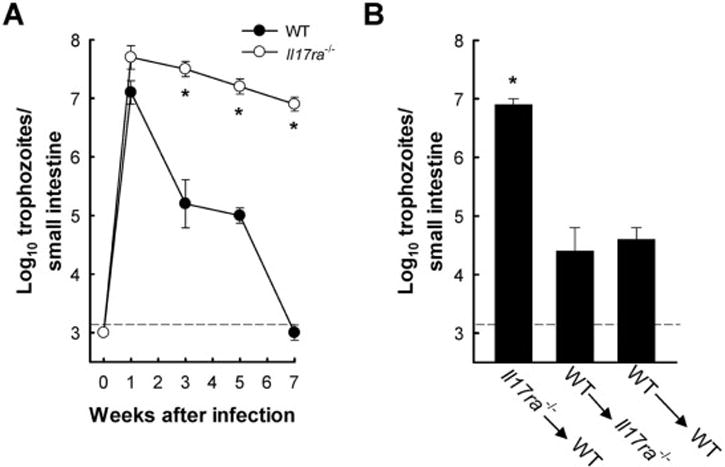
(A) Mice deficient in IL-17RA (IL17ra−/−, open circles) and wild-type (WT, closed circles) mice were infected with 104 G muris cysts, and trophozoite numbers in the small intestine were determined at the indicated times. Data are mean ± SE (n=3–4 mice/time point) from three independent experiments; *p<0.05 relative to uninfected controls (Day 0). (B) Bone marrow chimeric mice were generated by isolating and transferring bone marrow cells from IL17ra−/− mice into C57BL/6 (WT) mice (IL17ra−/−→WT), from WT to IL17ra−/− mice (WT→ IL17ra−/−), or as controls from WT to WT mice (WT→WT). Following an 8 week reconstitution period, mice were infected orally with G muris cysts, and trophozoite numbers were determined after 5 weeks. Data are mean + SE (n≥6 mice/time point) from three independent experiments; *p<0.05 relative to WT→WT chimeras. The black dashed lines depict the detection limit of the assays.
IL-17A deficiency compromises IgA transport into the lumen but not intestinal motility
Having established that IL-17A and IL-17RA signaling are required for Giardia clearance, we sought to elucidate the underlying mechanisms. To date, only a small number of effectors have been identified in antigiardial immune defense, including intestinal hypermotility (Andersen, et al., 2006; Li, et al., 2006), secretory IgA (Davids, et al., 2006; Langford, et al., 2002), α-defensins (Aley, et al., 1994; Tako, et al., 2013), and nitric oxide (NO) (Eckmann, et al., 2000; Tako, et al., 2013), although their relative importance under different conditions remains to be clarified (Langford, et al., 2002; Singer and Nash, 2000; Solaymani-Mohammadi and Singer, 2010). To address the potential role of intestinal hypermotility in IL-17 dependent defense, we assayed small intestinal motility in Il17a−/− and wild-type mice after G. muris infection. Both strains of mice displayed enhanced small intestinal transit two weeks after infection, but no significant difference was observed between the groups (Fig. 6A). Thus, intestinal hypermotility was unlikely to be responsible for impaired parasite clearance in Il17a−/− mice.
Figure 6. Mechanisms of IL-17A signaling-dependent antigiardial defense.
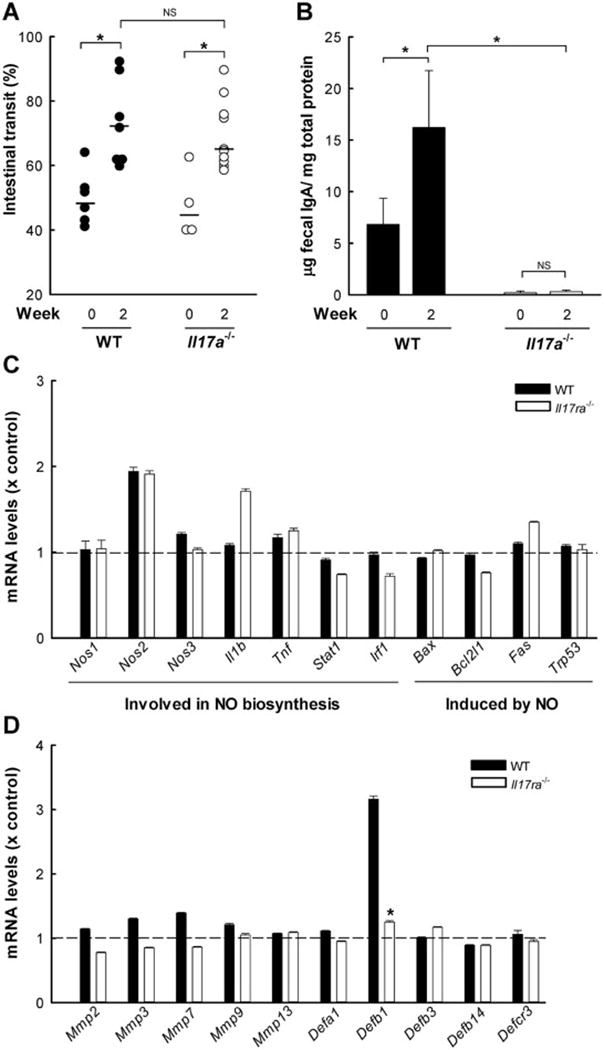
IL-17A-deficient (Il17a−/−, open circles and bars in A and B), 17RA-deficient mice (IL17ra−/−, open bars in C and D), and wild-type mice (WT, closed circles and bars in A–D) were infected with 104 G. muris cysts or left uninfected as controls (Week 0), and analyzed two weeks after infection. (A) Small intestinal motility was evaluated by determining the distance traveled by a carmine dye-containing test meal relative to the length of the entire small intestine over a 20-minute period. Each point represents an individual animal from three independent experiments. Horizontal bars indicate medians; *p<0.05 relative to indicated control; NS, not significant compared to wild-type controls. (B) IgA levels were determined in stool homogenates by ELISA and normalized to total protein. Results are mean + SE (n=5–6 mice/time point) from two independent experiments; *p<0.05 relative to uninfected controls. (C, D) Microarray analysis of small intestinal expression of the indicated genes after G. muris infection. Bars show relative expression compared to the respective uninfected control mice. Error bars represent SD for genes with array replicates (n=3–12 replicates/gene); *p<0.05 relative to wild-type controls.
We then examined secretory IgA as another potential effector mechanism. Fecal extracts were analyzed for IgA levels by ELISA. Il17a−/− mice had lower levels of fecal IgA prior to and after G. muris infection in comparison to wild-type controls (Fig. 6B). In contrast, levels of anti-giardial IgA in the serum were higher in Il17a−/− mice compared to controls (median OD450 = 0.50 vs. 0.15, respectively; 1:10 dilution, n=3–4 mice/group). These data indicate that the loss of fecal IgA in Il17a−/− mice was not related to diminished induction of antigen-specific IgA, but probably caused by impaired IgA delivery into the intestinal lumen due to reduced pIgR expression in the absence of IL-17A (Cao, et al., 2012; Davids, et al., 2006).
To determine whether other known mechanisms may be involved in IL-17A-dependent antigiardial defense, we examined the expression of α-defensins and NO synthase (NOS) 2 (Tako, et al., 2013). Microarray analysis showed that the absence of IL-17RA signaling had no differential impact on the expression of genes directly involved in NO synthesis, or genes whose products regulate NO production or are affected by it (Fig. 6C). Minor differences were observed in the expression of matrix metalloproteinase (MMP) 7 (also known as matrilysin), which is required for activating epithelial α-defensins (Wilson, et al., 1999), and several other members of the MMP family of endopeptidases potentially involved in host defense (Fig. 6D). Thus, differential MMP7-dependent activation of α-defensins may make a minor contribution to the IL-17A functions in this system.
Further analysis of the microarray data revealed that wild-type mice had an increase in β-defensin 1 expression that was absent in Il17ra−/− mice after infection (Fig. 6D). In addition, several other genes were preferentially induced in wild-type but not Il17ra−/− mice (Table 1). Interestingly, the strongest induction was observed for the genes Retnlb, Saa1, and Saa2, whose respective products, resistin-like molecule β, serum amyloid A1, and serum amyloid A2, contribute to antimicrobial defense (Eckhardt, et al., 2010; Herbert, et al., 2009). Conversely, a number of genes were preferentially induced in Giardia-infected Il17ra−/− mice, which shows that the absence of IL-17R signaling can lead to compensatory gene expression (Table 1). Furthermore, several genes were similarly induced in both groups of mice, underlining the specificity of the observed expression differences (Table 1). Taken together, our findings suggest that IL-17A signaling is not required for intestinal hypermotility or NOS expression after infection, but is critical for secretion of protective IgA into the intestinal lumen and expression of several other candidate effectors.
Table 1.
Differentially expressed genes in WT and Il17ra−/− mice two weeks after G. muris infection
| Fold Induction
|
|||||
|---|---|---|---|---|---|
| Gene Symbol | GenBank ID | WT | Il17ra−/− | Gene Product | Functions |
| Preferentially Induced in WT | |||||
| Retnlb | NM_023881 | 77.9 | 5.9 | Resistin-like molecule β | Hormone activity, intestinal innate immunity |
| Saa2 | NM_011314 | 26.2 | 5.8 | Serum amyloid A2 | Chemoattractant activity, G-protein coupled receptor binding |
| Saa1 | NM_009117 | 24.8 | 5.0 | Serum amyloid A1 | Chemoattractant activity, G-protein coupled receptor binding |
| U90926 | U90926 | 21.5 | 1.2 | Putative TNF-resistance related protein | Not known |
| Pla2g4c | NM_001004762 | 17.4 | 1.0 | Phospholipase A2, group IVC | Glycerophospholipid, catabolic process |
| Preferentially Induced in IL17ra−/− | |||||
| Ms4a1 | NM_007641 | 1.1 | 20.5 | Membrane spanning 4-domain, subfamily A, member 1/CD40 | Development and differentiation of B cells into plasma cells |
| Ighg | BC092269 | 4.1 | 13.2 | Immunoglobulin heavy chain (gamma polypeptide) | Antigen binding |
| Blr1 | NM_007551 | 1.1 | 11.2 | Burkitt lymphoma receptor 1/C-X-C chemokine receptor type 5 (CXCR5) | G-protein coupled receptor, has role in B cell migration |
| Oxt | NM_011025 | 1.7 | 10.4 | Oxytocin | Neuromodulator, intestinal motility |
| Tnfrsf13c | NM_028075 | 1.3 | 9.2 | Tumor necrosis factor receptor superfamily, member 13c/B-cell activating factor receptor (BAFF-R) | B cell regulator |
| Similarly Induced in WT and IL17ra−/− | |||||
| L20961 | L20961 | 8.2 | 5.8 | Ig rearranged H-chain | Antigen binding |
| S100a9 | NM_009114 | 4.2 | 3.0 | S100 calcium binding protein A9 (calgranulin B) | Complexes with S100A8 to regulate myeloid cell function |
| Egln3 | NM_028133 | 3.7 | 2.6 | Egl-9 family hypoxia-inducible factor 3 | Cellular oxygen sensor |
| Not induced in WT or IL17ra−/− | |||||
| Ccl1 | NM_011329 | 1.1 | 1.2 | Chemokine (C-C motif) ligand 1 | Chemotactic for monocytes, NK cells, immature B cells, and dendritic cells |
| Ccl3 | NM_011337 | 0.9 | 0.9 | Chemokine (C-C motif) ligand 3/macrophage inflammatory protein 1α (MP-1α) | Recruitment and activation of polymorphonuclear leukocytes |
| Ccl5 | NM_013653 | 0.8 | 0.9 | Chemokine (C-C motif) ligand 5/RANTES (regulated on activation, normal T cell expressed and secreted) | Chemotactic for T cells, eosinophils, and basophils |
DISCUSSION
IL-17A is a multi-functional cytokine involved in regulating mucosal homeostasis, inflammation, and immunity (Kolls and Khader, 2010; Onishi and Gaffen, 2010). Although the cytokine has been shown to be critical for host protection against various pulmonary and urogenital pathogens, efforts to determine the importance of IL-17A in defense against enteric pathogens have yielded varying results (Cypowyj, et al., 2012). Our studies, extending the findings in a recent report (Dreesen, et al., 2014), demonstrate that IL-17A plays a critical role in mucosal defense against both Giardia species, G. muris and G. lamblia. Protective functions of IL-17A have also been reported for several enteric bacterial pathogens, including C. rodentium and S. Typhimurium (Ishigame, et al., 2009; Mayuzumi, et al., 2010; Raffatellu, et al., 2008), although these pathogens differ substantially from Giardia in their interactions with the host. Both bacterial species are invasive and cause inflammatory responses in the intestinal tract (Mittrucker and Kaufmann, 2000; Mundy, et al., 2005). In contrast, Giardia is non-invasive and acute infection causes only minimal inflammation (Eckmann, 2003; Oberhuber, et al., 1997). Despite these differences, our data, together with prior findings (Dreesen, et al., 2014; Ishigame, et al., 2009; Mayuzumi, et al., 2010; Raffatellu, et al., 2008), demonstrate that IL-17A is a critical mediator of intestinal defense against both bacterial and protozoan pathogens.
The timing and magnitude of the IL-17A response to Giardia were compatible with the kinetics of a prototypic adaptive Th17 immune response, and Th17 cell numbers were accordingly increased after infection. However, using Rag-2-deficient mice that lack T and B cells, we discovered that innate immune cells residing within the epithelial compartment are additional producers of IL-17A in response to infection. Consistent with this observation, several subsets of Rag 2-independent cells, including innate lymphoid cells, neutrophils, NK cells, and mast cells, have been shown to express IL-17A in the mucosa under different conditions (Cua and Tato, 2010). The localization of these cells within the epithelium may enable them to interact with Giardia attached to the apical epithelial surface, or with factors released locally by the parasite, and respond by activating IL-17A expression. This idea is supported by our observation that the IL-17A response was localized to the site of infection and not observed in mesenteric lymph nodes (data not shown). Expression of the cytokine in most innate immune cells, except mast cells, is dependent on IL-6 and the nuclear receptor RORγt (Cua and Tato, 2010). Consistent with this, we found in preliminary studies that IL-6-deficient mice and mice lacking functional RORγt failed to induce IL-17A expression in response to Giardia infection, indicating that the canonical pathway is involved in IL-17A induction in this situation. This observation might also provide a connection between the known role of IL-6 in antigiardial defense (Bienz, et al., 2003; Zhou, et al., 2003) and our findings on IL-17A involvement.
Deficiency in IL-17A signaling had little impact on parasite burden in the first week after Giardia infection, suggesting that innate defenses, such as antimicrobial peptides produced by Paneth cells or neutrophils, which may control initial infection (Aley, et al., 1994), are not IL-17A dependent. Instead, the cytokine had a critical function 2–3 weeks after infection, suggesting that it mediates protection through controlling adaptive immune responses. Indeed, IL-17RA expression on bone marrow-derived hematopoietic cells was required for parasite clearance. Although the underlying effector mechanisms are not known, IL-17A was not important for controlling intestinal NOS expression, which can contribute to antigiardial defense (Tako, et al., 2013), or for mediating intestinal hypermotility, a key effector of antigiardial immune defense (Andersen, et al., 2006; Li, et al., 2006). This is in contrast to the findings with another lumen-dwelling parasite, the nematode Trichinella spiralis, where IL-17A promotes hypermotility during infection (Fu, et al., 2009), underlining that the actions of IL-17A can be mediated by different immune effectors.
Another important, albeit controversial, effector mechanism of antigiardial defense is secretory IgA (Davids, et al., 2006; Langford, et al., 2002; Singer and Nash, 2000). Our data suggest that IL-17A is required for optimal mucosal IgA secretion, because fecal IgA was markedly diminished in IL-17A-deficient mice, while antigen-specific IgA was increased in the serum. This observation is consistent with the observation that the cytokine regulates expression of the IgA transport receptor, pIgR, required for IgA secretion into the intestinal lumen (Cao, et al., 2012; Hirota, et al., 2013), although the regulation is predictably indirect since we found that the cognate IL-17 receptor is required on hematopoietic but not non-hematopoietic cells, such as pIgR-expressing epithelial cells. Beyond IgA, we found that IL-17A signaling may play a minor role in controlling MMP7 dependent α-defensin actions, which have been shown to contribute to controlling Giardia infection (Tako, et al., 2013), but is critical for expression of several other potential effectors, including β-defensin 1 and resistin-like molecule β, which can contribute to antimicrobial defenses in the intestine (Eckhardt, et al., 2010; Herbert, et al., 2009), although this remains to be established for Giardia.
The observed antigiardial function of IL-17A, which is apparently active in the absence of mucosal inflammation (Oberhuber, et al., 1997), broadens the understanding of the complexity of IL-17-mediated immunological protection against enteric infections. Importantly, we demonstrated that a lumen-dwelling pathogen can activate immune cells in the mucosa and within the epithelium to produce IL-17A, which helps to orchestrate protective intestinal immune defenses. The insights gained from these studies provide new mechanistic ideas about the pathophysiology and immunology of giardiasis, and are expected to be important for designing future mucosal vaccination strategies against this common worldwide cause of diarrheal disease.
Highlights.
The protozoan parasite Giardia dwells in the intestinal lumen and causes diarrhea
Mucosal defenses exist against the parasite but remain poorly understood
We found that intestinal IL-17A is induced by infection and needed for eradication
IL-17A signaling promotes transport of protective IgA into the intestinal lumen
It also induces other possible effectors, β-defensin 1 and resistin-like molecule β
Acknowledgments
We thank Kim Nguyen and the staff of the VA San Diego Flow Cytometer Core Laboratory for technical assistance, and Lucia Hall and the staff of the UCSD Animal Care Program for animal husbandry.
This work was supported by NIH grants DK035108, AI075527, and AI112594. SMD was supported by the Institute for Translational Sciences at the University of Texas Medical Branch, supported in part by an NIH Clinical and Translational Science Award (UL1TR000071, KL2TR000072). CFM was supported by a fellowship from the German Research Foundation (MA 4980/1-1).
Abbreviations
- pIgR
polymeric immunoglobulin receptor
- NO
nitric oxide
- Rag 2
recombination-activating gene 2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agarwal S, Mayer L. Diagnosis and treatment of gastrointestinal disorders in patients with primary immunodeficiency. Clin Gastroenterol Hepatol. 2013;11:1050–1063. doi: 10.1016/j.cgh.2013.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aley SB, Zimmerman M, Hetsko M, Selsted ME, Gillin FD. Killing of Giardia lamblia by cryptdins and cationic neutrophil peptides. Infect Immun. 1994;62:5397–5403. doi: 10.1128/iai.62.12.5397-5403.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aley SB, Zimmerman M, Hetsko M, Selsted ME, Gillin FD. Killing of Giardia lamblia by cryptdins and cationic neutrophil peptides. Infect Immun. 1994;62:5397–5403. doi: 10.1128/iai.62.12.5397-5403.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Algood HM, Allen SS, Washington MK, Peek RM, Jr, Miller GG, Cover TL. Regulation of gastric B cell recruitment is dependent on IL-17 receptor A signaling in a model of chronic bacterial infection. J Immunol. 2009;183:5837–5846. doi: 10.4049/jimmunol.0901206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen YS, Gillin FD, Eckmann L. Adaptive immunity-dependent intestinal hypermotility contributes to host defense against Giardia spp. Infect Immun. 2006;74:2473–2476. doi: 10.1128/IAI.74.4.2473-2476.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldursson S, Karanis P. Waterborne transmission of protozoan parasites: review of worldwide outbreaks – an update 2004–2010. Water Res. 2011;45:6603–6614. doi: 10.1016/j.watres.2011.10.013. [DOI] [PubMed] [Google Scholar]
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- Bienz M, Dai WJ, Welle M, Gottstein B, Muller N. Interleukin-6-deficient mice are highly susceptible to Giardia lamblia infection but exhibit normal intestinal immunoglobulin A responses against the parasite. Infect Immun. 2003;71:1569–1573. doi: 10.1128/IAI.71.3.1569-1573.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao AT, Yao S, Gong B, Elson CO, Cong Y. Th17 cells upregulate polymeric Ig receptor and intestinal IgA and contribute to intestinal homeostasis. J Immunol. 2012;189:4666–4673. doi: 10.4049/jimmunol.1200955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong Y, Brandwein SL, McCabe RP, Lazenby A, Birkenmeier EH, Sundberg JP, Elson CO. CD4+ T cells reactive to enteric bacterial antigens in spontaneously colitic C3H/HeJBir mice: increased T helper cell type 1 response and ability to transfer disease. J Exp Med. 1998;187:855–864. doi: 10.1084/jem.187.6.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol. 2010;10:479–489. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- Cypowyj S, Picard C, Marodi L, Casanova JL, Puel A. Immunity to infection in IL-17-deficient mice and humans. Eur J Immunol. 2012;42:2246–2254. doi: 10.1002/eji.201242605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davids BJ, Palm JE, Housley MP, Smith JR, Andersen YS, Martin MG, Hendrickson BA, Johansen FE, Svard SG, Gillin FD, Eckmann L. Polymeric immunoglobulin receptor in intestinal immune defense against the lumen-dwelling protozoan parasite Giardia. J Immunol. 2006;177:6281–6290. doi: 10.4049/jimmunol.177.9.6281. [DOI] [PubMed] [Google Scholar]
- Dreesen L, De Bosscher K, Grit G, Staels B, Lubberts E, Bauge E, Geldhof P. Giardia muris infection in mice is associated with a protective IL-17A response and induction of the Peroxisome Proliferator-Activated Receptor Alpha. Infect Immun. 2014 doi: 10.1128/IAI.01536-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhardt ER, Witta J, Zhong J, Arsenescu R, Arsenescu V, Wang Y, Ghoshal S, de Beer MC, de Beer FC, de Villiers WJ. Intestinal epithelial serum amyloid A modulates bacterial growth in vitro and pro-inflammatory responses in mouse experimental colitis. BMC Gastroenterol. 2010;10:133. doi: 10.1186/1471-230X-10-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckmann L. Mucosal defences against Giardia. Parasite Immunol. 2003;25:259–270. doi: 10.1046/j.1365-3024.2003.00634.x. [DOI] [PubMed] [Google Scholar]
- Eckmann L, Laurent F, Langford TD, Hetsko ML, Smith JR, Kagnoff MF, Gillin FD. Nitric oxide production by human intestinal epithelial cells and competition for arginine as potential determinants of host defense against the lumen-dwelling pathogen Giardia lamblia. J Immunol. 2000;164:1478–1487. doi: 10.4049/jimmunol.164.3.1478. [DOI] [PubMed] [Google Scholar]
- Eren M, Saltik-Temizel IN, Yuce A, Caglar M, Kocak N. Duodenal appearance of giardiasis in a child with selective immunoglobulin A deficiency. Pediatr Int. 2007;49:409–411. doi: 10.1111/j.1442-200X.2007.02357.x. [DOI] [PubMed] [Google Scholar]
- Eto D, Lao C, DiToro D, Barnett B, Escobar TC, Kageyama R, Yusuf I, Crotty S. IL-21 and IL-6 are critical for different aspects of B cell immunity and redundantly induce optimal follicular helper CD4 T cell (Tfh) differentiation. PLoS One. 2011;6:e17739. doi: 10.1371/journal.pone.0017739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Xiao L. Zoonotic potential and molecular epidemiology of Giardia species and giardiasis. Clin Microbiol Rev. 2011;24:110–140. doi: 10.1128/CMR.00033-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher CH, Oh KS, Bayless TM, Siegelman SS. Current perspectives on giardiasis. Am J Roentgenol Radium Ther Nucl Med. 1975;125:207–217. doi: 10.2214/ajr.125.1.207. [DOI] [PubMed] [Google Scholar]
- Fu Y, Wang W, Tong J, Pan Q, Long Y, Qian W, Hou X. Th17 cells influence intestinal muscle contraction during Trichinella spiralis infection. J Huazhong Univ Sci Technolog Med Sci. 2009;29:481–485. doi: 10.1007/s11596-009-0418-4. [DOI] [PubMed] [Google Scholar]
- Geddes K, Rubino SJ, Magalhaes JG, Streutker C, Le Bourhis L, Cho JH, Robertson SJ, Kim CJ, Kaul R, Philpott DJ, Girardin SE. Identification of an innate T helper type 17 response to intestinal bacterial pathogens. Nat Med. 2011;17:837–844. doi: 10.1038/nm.2391. [DOI] [PubMed] [Google Scholar]
- Herbert DR, Yang JQ, Hogan SP, Groschwitz K, Khodoun M, Munitz A, Orekov T, Perkins C, Wang Q, Brombacher F, Urban JF, Jr, Rothenberg ME, Finkelman FD. Intestinal epithelial cell secretion of RELM-beta protects against gastrointestinal worm infection. J Exp Med. 2009;206:2947–2957. doi: 10.1084/jem.20091268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyworth MF. Intestinal IgA responses to Giardia muris in mice depleted of helper T lymphocytes and in immunocompetent mice. J Parasitol. 1989;75:246–251. [PubMed] [Google Scholar]
- Heyworth MF, Carlson JR, Ermak TH. Clearance of Giardia muris infection requires helper/inducer T lymphocytes. J Exp Med. 1987;165:1743–1748. doi: 10.1084/jem.165.6.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota K, Turner JE, Villa M, Duarte JH, Demengeot J, Steinmetz OM, Stockinger B. Plasticity of Th17 cells in Peyer’s patches is responsible for the induction of T cell-dependent IgA responses. Nat Immunol. 2013;14:372–379. doi: 10.1038/ni.2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishigame H, Kakuta S, Nagai T, Kadoki M, Nambu A, Komiyama Y, Fujikado N, Tanahashi Y, Akitsu A, Kotaki H, Sudo K, Nakae S, Sasakawa C, Iwakura Y. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity. 2009;30:108–119. doi: 10.1016/j.immuni.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Kamda JD, Nash TE, Singer SM. Giardia duodenalis: dendritic cell defects in IL-6 deficient mice contribute to susceptibility to intestinal infection. Exp Parasitol. 2012;130:288–291. doi: 10.1016/j.exppara.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolls JK, Khader SA. The role of Th17 cytokines in primary mucosal immunity. Cytokine Growth Factor Rev. 2010;21:443–448. doi: 10.1016/j.cytogfr.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford TD, Housley MP, Boes M, Chen J, Kagnoff MF, Gillin FD, Eckmann L. Central importance of immunoglobulin A in host defense against Giardia spp. Infect Immun. 2002;70:11–18. doi: 10.1128/IAI.70.1.11-18.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengerich EJ, Addiss DG, Juranek DD. Severe giardiasis in the United States. Clin Infect Dis. 1994;18:760–763. doi: 10.1093/clinids/18.5.760. [DOI] [PubMed] [Google Scholar]
- Li E, Zhou P, Petrin Z, Singer SM. Mast cell-dependent control of Giardia lamblia infections in mice. Infect Immun. 2004;72:6642–6649. doi: 10.1128/IAI.72.11.6642-6649.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li E, Zhou P, Singer SM. Neuronal nitric oxide synthase is necessary for elimination of Giardia lamblia infections in mice. J Immunol. 2006;176:516–521. doi: 10.4049/jimmunol.176.1.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- Mayuzumi H, Inagaki-Ohara K, Uyttenhove C, Okamoto Y, Matsuzaki G. Interleukin-17A is required to suppress invasion of Salmonella enterica serovar Typhimurium to enteric mucosa. Immunology. 2010;131:377–385. doi: 10.1111/j.1365-2567.2010.03310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihara M, Hashizume M, Yoshida H, Suzuki M, Shiina M. IL-6/IL-6 receptor system and its role in physiological and pathological conditions. Clin Sci (Lond) 2012;122:143–159. doi: 10.1042/CS20110340. [DOI] [PubMed] [Google Scholar]
- Mittrucker HW, Kaufmann SH. Immune response to infection with Salmonella typhimurium in mice. J Leukoc Biol. 2000;67:457–463. doi: 10.1002/jlb.67.4.457. [DOI] [PubMed] [Google Scholar]
- Mundy R, MacDonald TT, Dougan G, Frankel G, Wiles S. Citrobacter rodentium of mice and man. Cell Microbiol. 2005;7:1697–1706. doi: 10.1111/j.1462-5822.2005.00625.x. [DOI] [PubMed] [Google Scholar]
- Nakae S, Komiyama Y, Nambu A, Sudo K, Iwase M, Homma I, Sekikawa K, Asano M, Iwakura Y. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 2002;17:375–387. doi: 10.1016/s1074-7613(02)00391-6. [DOI] [PubMed] [Google Scholar]
- Oberhuber G, Kastner N, Stolte M. Giardiasis: a histologic analysis of 567 cases. Scand J Gastroenterol. 1997;32:48–51. doi: 10.3109/00365529709025062. [DOI] [PubMed] [Google Scholar]
- Onishi RM, Gaffen SL. Interleukin-17 and its target genes: mechanisms of interleukin-17 function in disease. Immunology. 2010;129:311–321. doi: 10.1111/j.1365-2567.2009.03240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passos ST, Silver JS, O’Hara AC, Sehy D, Stumhofer JS, Hunter CA. IL-6 promotes NK cell production of IL-17 during toxoplasmosis. J Immunol. 2010;184:1776–1783. doi: 10.4049/jimmunol.0901843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipson CW, Bassaganya-Riera J, Viladomiu M, Pedragosa M, Guerrant RL, Roche JK, Hontecillas R. The role of peroxisome proliferator-activated receptor gamma in immune responses to enteroaggregative Escherichia coli infection. PLoS One. 2013;8:e57812. doi: 10.1371/journal.pone.0057812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffatellu M, Santos RL, Verhoeven DE, George MD, Wilson RP, Winter SE, Godinez I, Sankaran S, Paixao TA, Gordon MA, Kolls JK, Dandekar S, Baumler AJ. Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat Med. 2008;14:421–428. doi: 10.1038/nm1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinkai Y, Rathbun G, Lam KP, Oltz EM, Stewart V, Mendelsohn M, Charron J, Datta M, Young F, Stall AM, et al. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- Singer SM, Nash TE. T-cell-dependent control of acute Giardia lamblia infections in mice. Infect Immun. 2000;68:170–175. doi: 10.1128/iai.68.1.170-175.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solaymani-Mohammadi S, Singer SM. Giardia duodenalis: the double-edged sword of immune responses in giardiasis. Exp Parasitol. 2010;126:292–297. doi: 10.1016/j.exppara.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Qian Y. The activation and regulation of IL-17 receptor mediated signaling. Cytokine. 2013;62:175–182. doi: 10.1016/j.cyto.2013.03.014. [DOI] [PubMed] [Google Scholar]
- Spehlmann ME, Dann SM, Hruz P, Hanson E, McCole DF, Eckmann L. CXCR2-dependent mucosal neutrophil influx protects against colitis-associated diarrhea caused by an attaching/effacing lesion-forming bacterial pathogen. J Immunol. 2009;183:3332–3343. doi: 10.4049/jimmunol.0900600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stager S, Muller N. Giardia lamblia infections in B-cell-deficient transgenic mice. Infect Immun. 1997;65:3944–3946. doi: 10.1128/iai.65.9.3944-3946.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark D, Barratt JL, van Hal S, Marriott D, Harkness J, Ellis JT. Clinical significance of enteric protozoa in the immunosuppressed human population. Clin Microbiol Rev. 2009;22:634–650. doi: 10.1128/CMR.00017-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tako EA, Hassimi MF, Li E, Singer SM. Transcriptomic analysis of the host response to Giardia duodenalis infection reveals redundant mechanisms for parasite control. MBio. 2013;4:e00660–00613. doi: 10.1128/mBio.00660-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor PR, Roy S, Leal SM, Jr, Sun Y, Howell SJ, Cobb BA, Li X, Pearlman E. Activation of neutrophils by autocrine IL-17A-IL-17RC interactions during fungal infection is regulated by IL-6, IL-23, RORgammat and dectin-2. Nat Immunol. 2014;15:143–151. doi: 10.1038/ni.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upcroft P, Upcroft JA. Drug targets and mechanisms of resistance in the anaerobic protozoa. Clin Microbiol Rev. 2001;14:150–164. doi: 10.1128/CMR.14.1.150-164.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CL, Ouellette AJ, Satchell DP, Ayabe T, Lopez-Boado YS, Stratman JL, Hultgren SJ, Matrisian LM, Parks WC. Regulation of intestinal alpha-defensin activation by the metalloproteinase matrilysin in innate host defense. Science. 1999;286:113–117. doi: 10.1126/science.286.5437.113. [DOI] [PubMed] [Google Scholar]
- Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, Oliver P, Huang W, Zhang P, Zhang J, Shellito JE, Bagby GJ, Nelson S, Charrier K, Peschon JJ, Kolls JK. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P, Li E, Zhu N, Robertson J, Nash T, Singer SM. Role of interleukin-6 in the control of acute and chronic Giardia lamblia infections in mice. Infect Immun. 2003;71:1566–1568. doi: 10.1128/IAI.71.3.1566-1568.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]


