Abstract
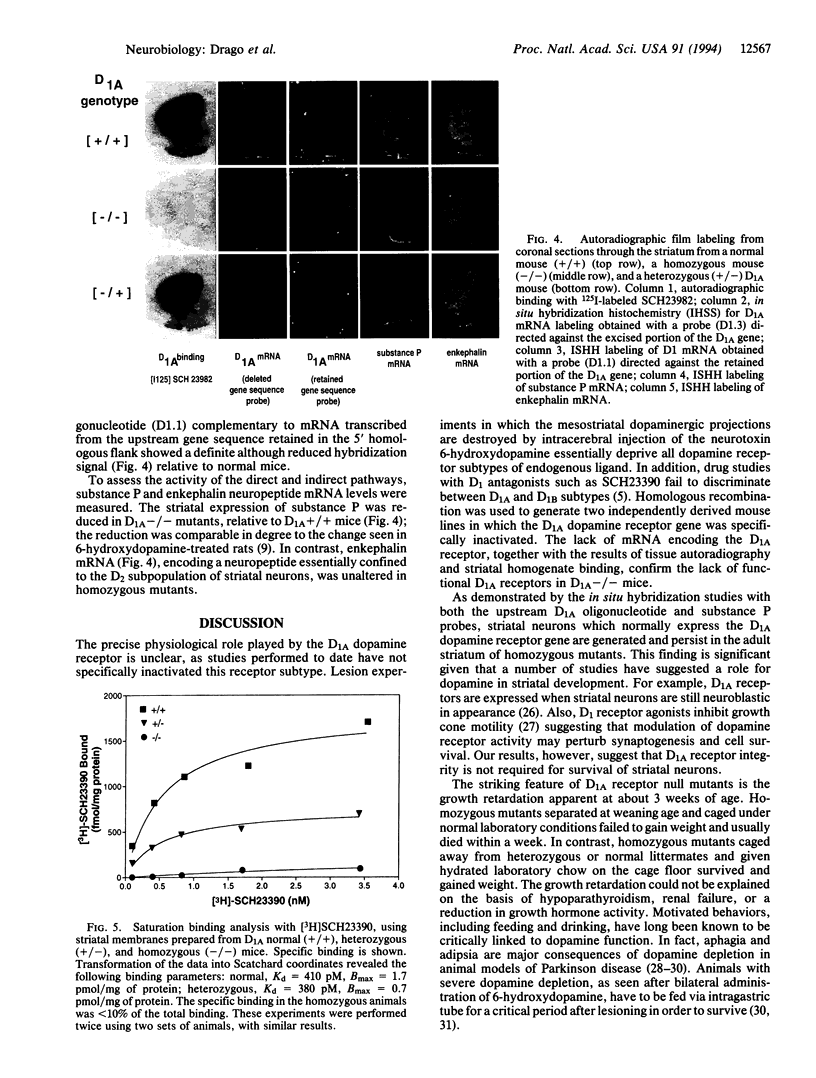
Of the five known dopamine receptors, D1A and D2 represent the major subtypes expressed in the striatum of the adult brain. Within the striatum, these two subtypes are differentially distributed in the two main neuronal populations that provide direct and indirect pathways between the striatum and the output nuclei of the basal ganglia. Movement disorders, including Parkinson disease and various dystonias, are thought to result from imbalanced activity in these pathways. Dopamine regulates movement through its differential effects on D1A receptors expressed by direct output neurons and D2 receptors expressed by indirect output neurons. To further examine the interaction of D1A and D2 neuronal pathways in the striatum, we used homologous recombination to generate mutant mice lacking functional D1A receptors (D1A-/-). D1A-/- mutants are growth retarded and die shortly after weaning age unless their diet is supplemented with hydrated food. With such treatment the mice gain weight and survive to adulthood. Neurologically, D1A-/- mice exhibit normal coordination and locomotion, although they display a significant decrease in rearing behavior. Examination of the striatum revealed changes associated with the altered phenotype of these mutants. D1A receptor binding was absent in striatal sections from D1A-/- mice. Striatal neurons normally expressing functional D1A receptors are formed and persist in adult homozygous mutants. Moreover, substance P mRNA, which is colocalized specifically in striatal neurons with D1A receptors, is expressed at a reduced level. In contrast, levels of enkephalin mRNA, which is expressed in striatal neurons with D2 receptors, are unaffected. These findings show that D1A-/- mice exhibit selective functional alterations in the striatal neurons giving rise to the direct striatal output pathway.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albin R. L., Young A. B., Penney J. B. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989 Oct;12(10):366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Barton A. C., Kang H. C., Rinaudo M. S., Monsma F. J., Jr, Stewart-Fram R. M., Macinko J. A., Jr, Haugland R. P., Ariano M. A., Sibley D. R. Multiple fluorescent ligands for dopamine receptors. I. Pharmacological characterization and receptor selectivity. Brain Res. 1991 May 3;547(2):199–207. doi: 10.1016/0006-8993(91)90963-v. [DOI] [PubMed] [Google Scholar]
- Breese G. R., Duncan G. E., Napier T. C., Bondy S. C., Iorio L. C., Mueller R. A. 6-hydroxydopamine treatments enhance behavioral responses to intracerebral microinjection of D1- and D2-dopamine agonists into nucleus accumbens and striatum without changing dopamine antagonist binding. J Pharmacol Exp Ther. 1987 Jan;240(1):167–176. [PMC free article] [PubMed] [Google Scholar]
- Chandler C. J., Wohab W., Starr B. S., Starr M. S. Motor depression: a new role for D1 receptors? Neuroscience. 1990;38(2):437–445. doi: 10.1016/0306-4522(90)90040-b. [DOI] [PubMed] [Google Scholar]
- Chin E., Zhou J., Dai J., Baxter R. C., Bondy C. A. Cellular localization and regulation of gene expression for components of the insulin-like growth factor ternary binding protein complex. Endocrinology. 1994 Jun;134(6):2498–2504. doi: 10.1210/endo.134.6.7515002. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Dragunow M., Faull R. The use of c-fos as a metabolic marker in neuronal pathway tracing. J Neurosci Methods. 1989 Sep;29(3):261–265. doi: 10.1016/0165-0270(89)90150-7. [DOI] [PubMed] [Google Scholar]
- Dreher J. K., Jackson D. M. Role of D1 and D2 dopamine receptors in mediating locomotor activity elicited from the nucleus accumbens of rats. Brain Res. 1989 May 22;487(2):267–277. doi: 10.1016/0006-8993(89)90831-7. [DOI] [PubMed] [Google Scholar]
- Fibiger H. C., Zis A. P., McGeer E. G. Feeding and drinking deficits after 6-hydroxydopamine administration in the rat: similarities to the lateral hypothalamic syndrome. Brain Res. 1973 May 30;55(1):135–148. doi: 10.1016/0006-8993(73)90493-9. [DOI] [PubMed] [Google Scholar]
- Gerfen C. R., Engber T. M., Mahan L. C., Susel Z., Chase T. N., Monsma F. J., Jr, Sibley D. R. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990 Dec 7;250(4986):1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- Gerfen C. R. The neostriatal mosaic: multiple levels of compartmental organization. Trends Neurosci. 1992 Apr;15(4):133–139. doi: 10.1016/0166-2236(92)90355-c. [DOI] [PubMed] [Google Scholar]
- Gerfen C. R., Young W. S., 3rd Distribution of striatonigral and striatopallidal peptidergic neurons in both patch and matrix compartments: an in situ hybridization histochemistry and fluorescent retrograde tracing study. Brain Res. 1988 Sep 13;460(1):161–167. doi: 10.1016/0006-8993(88)91217-6. [DOI] [PubMed] [Google Scholar]
- Graybiel A. M., Moratalla R., Robertson H. A. Amphetamine and cocaine induce drug-specific activation of the c-fos gene in striosome-matrix compartments and limbic subdivisions of the striatum. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6912–6916. doi: 10.1073/pnas.87.17.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guennoun R., Bloch B. Ontogeny of D1 and DARPP-32 gene expression in the rat striatum: an in situ hybridization study. Brain Res Mol Brain Res. 1992 Jan;12(1-3):131–139. doi: 10.1016/0169-328x(92)90076-n. [DOI] [PubMed] [Google Scholar]
- Heikkila R. E., Orlansky H., Cohen G. Studies on the distinction between uptake inhibition and release of (3H)dopamine in rat brain tissue slices. Biochem Pharmacol. 1975 Apr 15;24(8):847–852. doi: 10.1016/0006-2952(75)90152-5. [DOI] [PubMed] [Google Scholar]
- Hoffman D. C., Beninger R. J. The D1 dopamine receptor antagonist, SCH 23390 reduces locomotor activity and rearing in rats. Pharmacol Biochem Behav. 1985 Feb;22(2):341–342. doi: 10.1016/0091-3057(85)90401-0. [DOI] [PubMed] [Google Scholar]
- Kebabian J. W., Calne D. B. Multiple receptors for dopamine. Nature. 1979 Jan 11;277(5692):93–96. doi: 10.1038/277093a0. [DOI] [PubMed] [Google Scholar]
- Kozlowski M. R., Marshall J. F. Plasticity of [14C]2-deoxy-D-glucose incorporation into neostriatum and related structures in response to dopamine neuron damage and apomorphine replacement. Brain Res. 1980 Sep 15;197(1):167–183. doi: 10.1016/0006-8993(80)90442-4. [DOI] [PubMed] [Google Scholar]
- Lankford K. L., DeMello F. G., Klein W. L. D1-type dopamine receptors inhibit growth cone motility in cultured retina neurons: evidence that neurotransmitters act as morphogenic growth regulators in the developing central nervous system. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4567–4571. doi: 10.1073/pnas.85.12.4567-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levey A. I., Hersch S. M., Rye D. B., Sunahara R. K., Niznik H. B., Kitt C. A., Price D. L., Maggio R., Brann M. R., Ciliax B. J. Localization of D1 and D2 dopamine receptors in brain with subtype-specific antibodies. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):8861–8865. doi: 10.1073/pnas.90.19.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li E., Bestor T. H., Jaenisch R. Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell. 1992 Jun 12;69(6):915–926. doi: 10.1016/0092-8674(92)90611-f. [DOI] [PubMed] [Google Scholar]
- Love P. E., Tremblay M. L., Westphal H. Targeting of the T-cell receptor zeta-chain gene in embryonic stem cells: strategies for generating multiple mutations in a single gene. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9929–9933. doi: 10.1073/pnas.89.20.9929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J. F., Teitelbaum P. A comparison of the eating in response to hypothermic and glucoprivic challenges after nigral 6-hydroxydopamine and lateral hypothalamic electrolytic lesions in rats. Brain Res. 1973 May 30;55(1):229–233. doi: 10.1016/0006-8993(73)90507-6. [DOI] [PubMed] [Google Scholar]
- Meyer M. E., Cottrell G. A., Van Hartesveldt C. Dopamine D1 antagonists potentiate the durations of bar and cling catalepsy and the dorsal immobility response in rats. Pharmacol Biochem Behav. 1992 Mar;41(3):507–510. doi: 10.1016/0091-3057(92)90365-m. [DOI] [PubMed] [Google Scholar]
- Meyer M. E., Cottrell G. A., Van Hartesveldt C., Potter T. J. Effects of dopamine D1 antagonists SCH23390 and SK&F83566 on locomotor activities in rats. Pharmacol Biochem Behav. 1993 Feb;44(2):429–432. doi: 10.1016/0091-3057(93)90486-d. [DOI] [PubMed] [Google Scholar]
- Meyer M. E., Van Hartesveldt C., Potter T. J. Locomotor activity following intra-accumbens microinjections of dopamine D1 agonist SK&F 38393 in rats. Synapse. 1993 Apr;13(4):310–314. doi: 10.1002/syn.890130403. [DOI] [PubMed] [Google Scholar]
- Niznik H. B. Dopamine receptors: molecular structure and function. Mol Cell Endocrinol. 1987 Nov;54(1):1–22. doi: 10.1016/0303-7207(87)90134-1. [DOI] [PubMed] [Google Scholar]
- Robertson G. S., Vincent S. R., Fibiger H. C. D1 and D2 dopamine receptors differentially regulate c-fos expression in striatonigral and striatopallidal neurons. Neuroscience. 1992 Jul;49(2):285–296. doi: 10.1016/0306-4522(92)90096-k. [DOI] [PubMed] [Google Scholar]
- Robertson G. S., Vincent S. R., Fibiger H. C. Striatonigral projection neurons contain D1 dopamine receptor-activated c-fos. Brain Res. 1990 Jul 23;523(2):288–290. doi: 10.1016/0006-8993(90)91498-6. [DOI] [PubMed] [Google Scholar]
- Scibilia R. J., Lachowicz J. E., Kilts C. D. Topographic nonoverlapping distribution of D1 and D2 dopamine receptors in the amygdaloid nuclear complex of the rat brain. Synapse. 1992 Jun;11(2):146–154. doi: 10.1002/syn.890110208. [DOI] [PubMed] [Google Scholar]
- Seeman P., Ulpian C., Bergeron C., Riederer P., Jellinger K., Gabriel E., Reynolds G. P., Tourtellotte W. W. Bimodal distribution of dopamine receptor densities in brains of schizophrenics. Science. 1984 Aug 17;225(4663):728–731. doi: 10.1126/science.6147018. [DOI] [PubMed] [Google Scholar]
- Sibley D. R., Monsma F. J., Jr Molecular biology of dopamine receptors. Trends Pharmacol Sci. 1992 Feb;13(2):61–69. doi: 10.1016/0165-6147(92)90025-2. [DOI] [PubMed] [Google Scholar]
- Steiner H., Gerfen C. R. Cocaine-induced c-fos messenger RNA is inversely related to dynorphin expression in striatum. J Neurosci. 1993 Dec;13(12):5066–5081. doi: 10.1523/JNEUROSCI.13-12-05066.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas K. R., Capecchi M. R. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell. 1987 Nov 6;51(3):503–512. doi: 10.1016/0092-8674(87)90646-5. [DOI] [PubMed] [Google Scholar]
- Tybulewicz V. L., Crawford C. E., Jackson P. K., Bronson R. T., Mulligan R. C. Neonatal lethality and lymphopenia in mice with a homozygous disruption of the c-abl proto-oncogene. Cell. 1991 Jun 28;65(7):1153–1163. doi: 10.1016/0092-8674(91)90011-m. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U. Adipsia and aphagia after 6-hydroxydopamine induced degeneration of the nigro-striatal dopamine system. Acta Physiol Scand Suppl. 1971;367:95–122. doi: 10.1111/j.1365-201x.1971.tb11001.x. [DOI] [PubMed] [Google Scholar]
- Wooten G. F., Collins R. C. Effects of dopaminergic stimulation on functional brain metabolism in rats with unilateral substantia nigra lesions. Brain Res. 1983 Mar 21;263(2):267–275. doi: 10.1016/0006-8993(83)90319-0. [DOI] [PubMed] [Google Scholar]
- Yamaguchi I., Jose P. A., Mouradian M. M., Canessa L. M., Monsma F. J., Jr, Sibley D. R., Takeyasu K., Felder R. A. Expression of dopamine D1A receptor gene in proximal tubule of rat kidneys. Am J Physiol. 1993 Feb;264(2 Pt 2):F280–F285. doi: 10.1152/ajprenal.1993.264.2.F280. [DOI] [PubMed] [Google Scholar]
- Young W. S., 3rd, Bonner T. I., Brann M. R. Mesencephalic dopamine neurons regulate the expression of neuropeptide mRNAs in the rat forebrain. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9827–9831. doi: 10.1073/pnas.83.24.9827. [DOI] [PMC free article] [PubMed] [Google Scholar]