Abstract
The spatial distribution and strength of information processing 'hubs' are essential features of the brain’s network topology, and may thus be particularly susceptible to neuropsychiatric disease. Despite growing evidence that drug addiction alters functioning and connectivity of discrete brain regions, little is known about whether chronic drug use is associated with abnormalities in this network-level organization, and if such abnormalities could be targeted for intervention. We used functional connectivity density (FCD) mapping to evaluate how chronic and acute stimulants affect brain hubs (i.e., regions with many short-range or long-range functional connections). Nineteen individuals with cocaine use disorders (CUD) and 15 healthy controls completed resting-state fMRI scans following a randomly assigned dose of methylphenidate (MPH; 20 mg) or placebo. Short-range and long-range FCD maps were computed for each participant and medication condition. CUD participants had increased short-range and long-range FCD in the ventromedial prefrontal cortex, posterior cingulate/precuneus, and putamen/amygdala, which in areas of the default mode network correlated with years of use. Across participants, MPH decreased short-range FCD in the thalamus/putamen, and decreased long-range FCD in the supplementary motor area and postcentral gyrus. Increased density of short-range and long-range functional connections to default mode hubs in CUD suggests an overrepresentation of these resource-expensive hubs. While the effects of MPH on FCD were only partly overlapping with those of CUD, MPH-induced reduction in the density of short-range connections to the putamen/thalamus, a network of core relevance to habit formation and addiction, suggests that some FCD abnormalities could be targeted for treatment.
Keywords: cocaine, addiction, dopamine, methylphenidate, brain hubs, resting-state connectivity, functional magnetic resonance imaging
1. Introduction
The human brain is organized into specialized topological components, termed “hubs,” which facilitate efficiency of information flow [for a theoretical perspective on this topic, see (Sporns et al., 2007)]. These hubs are brain regions or groups of brain regions that are ideally positioned as throughways to sensory cortical and subcortical brain regions, and are therefore central to basic and more complex emotional, regulatory, and higher-order cognitive processes. As such integral components of the brain’s network architecture, brain hubs are responsible for the consumption of a large portion of the brain’s overall energy demands, even at rest (Liang et al., 2013; Tomasi et al., 2013). The higher energy demands of brain hubs, such as those part of the default mode network, may render them particularly susceptible to the effects of neuropsychiatric disease [e.g., Alzheimer’s disease (Buckner et al., 2009) and schizophrenia (Crossley et al., 2014; Rubinov and Bullmore, 2013)] and normal aging (Tomasi and Volkow, 2012). Currently, there is limited knowledge about the ability to directly modulate these hubs pharmacologically or via other means. Such knowledge could contribute to a better understanding of hub topology and strength, susceptibility to disease, and potentially inform systems-level biomarkers of interventions aimed to revert or delay pathology by improving the efficiency of hub function.
We recently proposed a data-driven method to identify hubs in the human brain based on functional connectivity density (FCD) mapping performed on functional magnetic resonance imaging (fMRI) data collected at rest. Resting-state connectivity captures the synchronicity of low-frequency, spontaneous fluctuations in blood-oxygen-level-dependent (BOLD) fMRI signals that reflect fluctuations in neuronal activity (Shmuel and Leopold, 2008) between brain regions in the absence of external stimulation (Fox and Raichle, 2007). That is, this measure captures functional coherence between regions that may not necessarily map onto direct anatomical connections. More classical connectivity approaches typically define a certain number of a priori seed regions and examine which other regions’ activity co-varies, over time, with that of the seed region. FCD expands upon these seed-based approaches by agnostically examining connectivity between every voxel in the brain with every other voxel. More specifically, FCD can be used to estimate the number of global (analogous to degree centrality in graph theory) (Tomasi and Volkow, 2011) and local (Tomasi and Volkow, 2010) functional connections to a given region that exceed a specified correlation strength. The former captures the total number of functional connections of every voxel with every other voxel but does not distinguish between short-range and long-range connections. The latter captures the number of short-range connections but does not account for long-range connections. Thus, subtracting each region’s local FCD from global FCD provides an estimate of the number of remote (long-range) connections. Both local and global FCD have good test-retest reliability [with lower variability observed across sessions for local FCD (~12%) than global FCD (~20%)] (Tomasi and Volkow, 2010; Tomasi and Volkow, 2011), making these metrics well suited to investigate changes in hub topology.
In the present study, we were interested in examining the effects of stimulants on brain functional connectivity hubs. Previous seed-based studies have reported resting-state connectivity perturbations in individuals addicted to nicotine (Cole et al., 2010a; Hong et al., 2009), opioids (Ma et al., 2010; Ma et al., 2011; Upadhyay et al., 2010), and cocaine (Gu et al., 2010; Kelly et al., 2011; Konova et al., 2013). In the current study, beyond testing the effects of chronic stimulant exposure [i.e., as defined by a diagnosis of cocaine use disorder (CUD)] on FCD, we explored the effects of acute stimulants using a randomized design where we administered oral methylphenidate (MPH) or placebo prior to resting-state fMRI in individuals with CUD and healthy individuals without a history of drug addiction. Like cocaine, MPH blocks dopamine and norepinephrine transporters, thereby increasing extracellular concentration of these neuromodulatory neurotransmitters (Kuczenski and Segal, 1997). However, unlike cocaine, the rate of clearance of orally administered MPH from the brain is substantially slower (90 min half-life versus 20 min for cocaine), contributing to its lower abuse potential (Volkow et al., 2004). Based on our previous study in CUD (Konova et al., 2013), where using a seed-based approach we found that MPH reduced abnormally strong connectivity in a key basal ganglia circuit relevant to the severity of addiction while strengthening several, in some cases abnormally weak, cortico-limbic and cortico-cortical connections relevant to behavioral control, we hypothesized differential (opposing) effects on FCD of diagnosis and MPH in at least partly overlapping regions.
2. Methods
2.1. Participants
Participants were 19 (17 males and 2 females) non-treatment seeking CUD and 15 (all male) healthy controls with no history of drug or psychiatric illness, as described in detail elsewhere (Konova et al., 2013). All participants were right-handed native English speakers, and provided their written consent to participate in the study in accordance with the Stony Brook University Institutional Review Board. The diagnostic groups did not differ in race, gender, socioeconomic status, or non-verbal IQ (all P>0.16); however controls were on average younger than CUD [controls: 39.0 ± 7.4 (mean ± standard deviation); CUD: 46.2 ± 7.5, T32=2.78, P=0.009]. Therefore, age was included as a covariate in all analyses involving the two groups as described further below.
Participants were in good health and not taking any medications. Their psychiatric history was ascertained by a comprehensive clinical interview, consisting of the: Structured Clinical Interview for DSM-IV Axis I Disorders [research version (First et al., 1996; Ventura et al., 1998)] and Addiction Severity Index (McLellan et al., 1992). Exclusion criteria were: (A) history of head trauma or loss of consciousness (>30 min) or neurological disease; (B) abnormal vital signs at time of screening; (C) history of major medical conditions; (D) history of major psychiatric disorder (other than substance abuse or dependence in CUD); (E) pregnancy as tested with a urine test in all females; (F) contraindications to the MRI environment; (G) history of glaucoma; and (H) except for cocaine, positive urine screens for psychoactive drugs or their metabolites. An evaluation by medical and/or trained research staff confirmed participants were not currently intoxicated.
The CUD participants were currently using cocaine and identified cocaine as their primary drug of choice, meeting criteria for current cocaine dependence (n=18) or abuse (n=1). Average scores on the Severity of Dependence scale (Gossop et al., 1992) were 7.3 ± 2.4. Participants reported an average of 15.8 ± 7.5 years of cocaine use and 2.7 ± 2.1 days/week of cocaine use in the previous month. Nine participants tested positive for cocaine on MPH day, 8 tested positive for cocaine on placebo day, and 7 tested positive for cocaine on both study days (McNemar within-subjects χ2 test, P=1.0); however, there was a small but significant difference between the study days in self-reported days since last use of any drugs (MPH: 5.11 ± 6.15; placebo: 7.21 ± 9.13, T18=2.12, P=0.048). We therefore tested if days since last use modified the effects of MPH on FCD in the CUD group. Controls tested negative for all drugs on both study days. Current comorbidities for the CUD group included heroin dependence (n=1), marijuana abuse (n=2), alcohol abuse (n=1), and nicotine dependence (n=12). Controls did not regularly use any drugs or alcohol. One control was a past smoker; the remaining were non-smokers. Because of this large disparity, we were unable to control for differences in smoking between the groups (Miller and Chapman, 2001). As nicotine use rates in our sample of CUD are comparable to those reported in the literature [e.g., (Grant et al., 2004; Jia et al., 2011; Kalman et al., 2005; Weinberger and Sofuoglu, 2009)], this may be an inherent feature of this population and future studies are needed that recruit more cigarette smoking controls to better address this important confound.
2.2. Study Sessions
We re-analyzed the data from a previously published seed-based functional connectivity study in cocaine users administered MPH or placebo (Konova et al., 2013), here further extending the data set to include healthy controls. Briefly, at each of the two study sessions (conducted 8.1 ± 3.0 days apart), participants were randomized to receive a 20 mg dose of oral MPH or placebo (lactose). The study was initially performed such that only participants were blinded to the administered challenge (n=24). Once it became clear that risks were minimal, we transitioned to double-blind administration (n=10), where study personnel were also blinded to the medication condition.
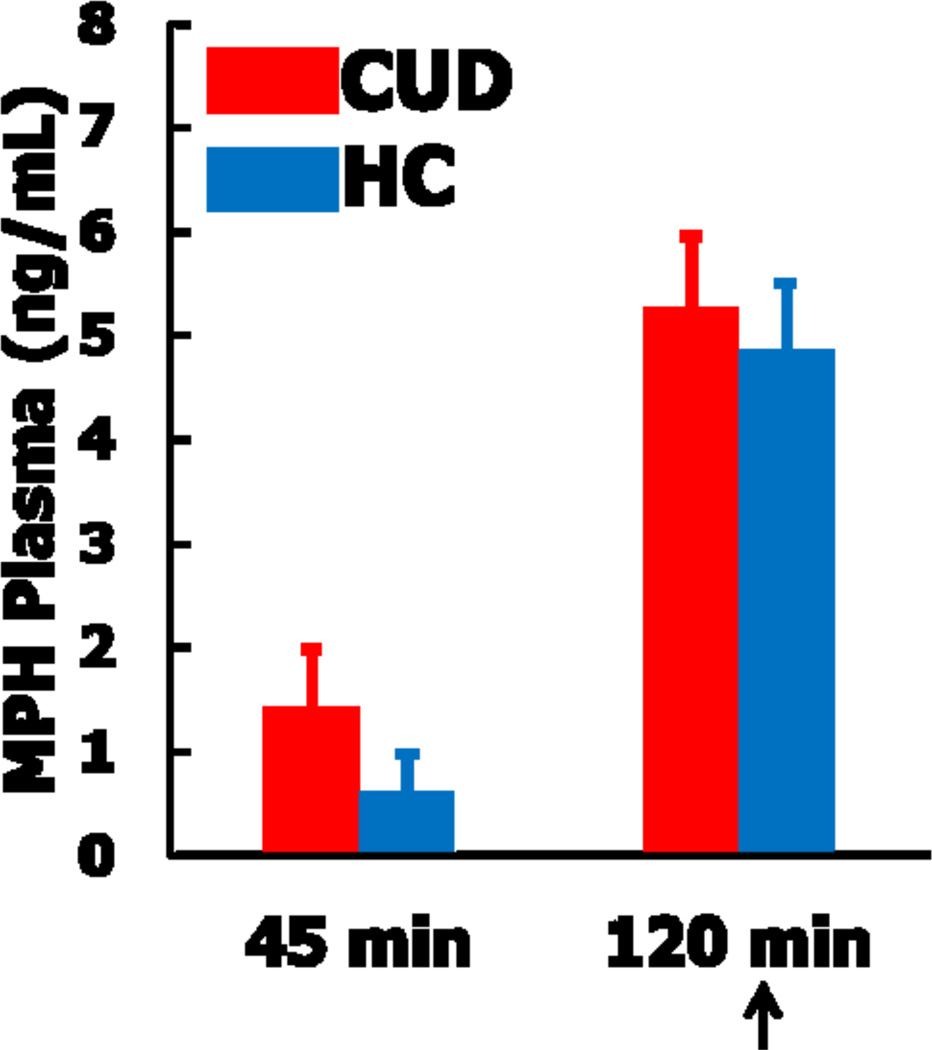
The MPH and placebo sessions consisted of identical study procedures. Resting fMRI scans were acquired approximately 120 minutes after medication administration, within the window of peak MPH effects (Volkow et al., 1998). To confirm peak levels, plasma concentrations of MPH were collected via venous blood draws at 0 min, 45 min, and 120 min post administration using capillary GC-MS (see Figure 1).
Figure 1.
Methylphenidate plasma (MPH) levels at 45 min and 120 min post drug administration in individuals with cocaine use disorders (CUD) and healthy controls (HC). Values at time 0 (not shown) were null for all participants. Arrow indicates time of the resting-state scan, during peak MPH levels.
2.3. Image Acquisition
Functional imaging was performed with a 4T Varian/Siemens MRI scanner using a coronal T2*-weighted single shot gradient-echo EPI sequence (TE/TR=20/1600 ms, 3.125×3.125 mm2 in-plane resolution, 4 mm slice thickness, 1 mm gap, 33 coronal slices, 20 cm FOV, 64×64 matrix size, 90°-flip angle, 200 kHz bandwidth with ramp sampling). Padding was used to minimize motion, and earplugs and headphones were used to minimize the influence of scanner noise on brain activation (Tomasi et al., 2005). Participants were instructed to keep their eyes open, lie as still as possible, and remain awake during the resting scans. Each resting scan was approximately 8 min in duration (see further below).
2.4. Image Processing and Construction of the Functional Connectivity Density Maps
Image processing and analyses were performed in SPM8 (Wellcome Trust Centre for Neuroimaging, London UK). The data were first realigned, slice time corrected, and coregistered and spatially normalized to a standard EPI template in the Montreal Neurological Institute (MNI) frame, resulting in a final voxel size of 3×3×3 mm. Other preprocessing steps were carried out in IDL (ITT Visual Information Solutions, Boulder, CO) and included motion correction using the six time-varying realignment parameters (3 translations and 3 rotations), global signal normalization, and band-pass filtering (0.01–0.10 Hz). All images were visually inspected for image quality/spikes. Additional motion was corrected with “scrubbing” (Power et al., 2012), where individual time points contaminated by motion were also removed. For this purpose, we computed the root mean square variance across voxels (DVARS) of the differences in % BOLD intensity, Ii, between adjacent time points,, where the brackets denote the average intensity across voxels. We also computed frame-wise displacements, FDi = |Δdix| + |Δdiy| + |Δdiz| + r|Δαi| + r|Δβi| + r|Δγi|, for every time point, i, from head translations (dix, diy, diz) and rotations (αi, βi, γi), the six realignment parameters obtained from SPM8. A radius r = 50 mm, approximately the mean distance from the center of the MNI space to the cortex, was used to convert angle rotations to displacements. Time points with FDi > 0.5 mm and DVARSi > 0.5% were considered potentially contaminated by motion artifacts [as recommended by (Power et al., 2012)] and excluded from the time series, resulting in a variable number of time points for analysis (276–311, or 7–8 min time series) for each participant. The number of remaining time points did not differ by diagnosis or medication condition (P>0.16).
The FCD analyses were performed as previously described (Tomasi and Volkow, 2010; Tomasi and Volkow, 2011) [freely available software download forthcoming]. Voxels with signal-to-noise ratio (SNR) as a function of time < 50 were first eliminated to minimize unwanted effects from susceptibility-related signal-loss artifacts. The remaining preprocessed data then underwent global (Tomasi and Volkow, 2011) FCD mapping, where we computed the total number of functional connections to every voxel in the brain, and local FCD mapping (Tomasi and Volkow, 2010) where we computed the total number of neighbor (short-range) functional connections to every voxel.
The Pearson correlation was used to assess the strength of the functional connectivity of each voxel with each other voxel, and a correlation threshold of 0.6 was used to compute the binary undirected connectivity coefficient, aij. The global FCD, also called "degree," at every voxel was calculated from the N × (N − 1)/2 binary matrices (N = 57,713 voxels) as ki = ∑aij, using a C-algorithm and parallel computing (Tomasi and Volkow, 2011). The local FCD at x0 was computed as the number of elements in the local functional connectivity cluster, (ki = ∑aij), using a “growing” algorithm written in IDL (Tomasi and Volkow, 2010). That is, a voxel xj was added to the list of voxels functionally connected with xi only if it was adjacent to a voxel that was linked to xi by a continuous path of functionally connected voxels. This calculation was repeated in an iterative manner for all voxels that were adjacent to voxels that belonged to the list of voxel functionally connected to xi until no new voxels could be added to the list. The calculation was then initiated for a different xi. Thus, subtracting each region’s local FCD from global FCD gave us an estimate of the number of remote (long-range) connections. The short-range (local) and long-range FCD maps were then smoothed with an 8-mm full-width at half-maximum Gaussian kernel prior to group-level analyses in SPM8.
2.5. Statistical Analyses
Two 2 (diagnosis: CUD, healthy controls) × 2 (medication: MPH, placebo) repeated measures analyses of covariance in SPM8, with age as covariate, were used to analyze differences in short-range and long-range FCD, respectively, as a function of addiction and MPH administration. A voxel-wise threshold of P<0.005 was applied, combined with a minimum cluster-extent of 26 contiguous voxels (702 mm3), to yield a corrected cluster-level false positive rate of P<0.05 as determined by Monte Carlo simulations (http://www2.bc.edu/~slotnics/scripts.htm).
The average signal in significant clusters was extracted with the EasyROI toolbox (http://www.sbirc.ed.ac.uk/cyril/cp_download.html) and used for visual representation of the data and correlation analyses with years of cocaine use in CUD. For these latter analyses, we correlated the average number of connections in clusters exhibiting higher FCD in CUD relative to controls and years of cocaine use, controlling for chronological age and current drug use severity with partial correlations.
3. Results
3.1. Spatial Distribution of Functional Hubs
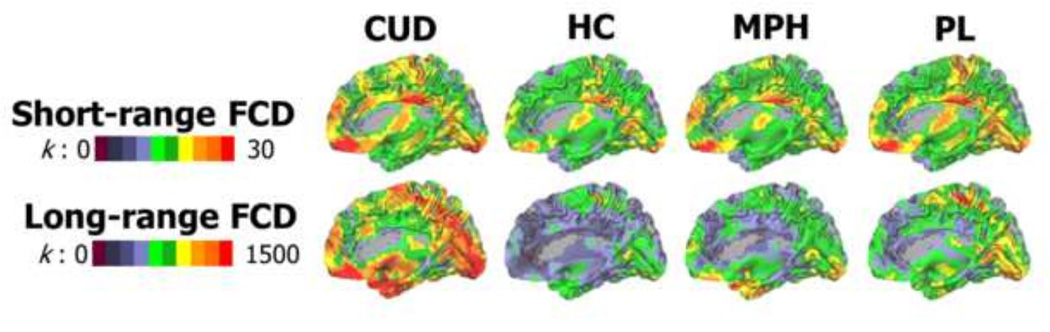
Across the diagnostic groups and medication conditions, the posterior cingulate cortex (PCC)/precuneus, ventromedial prefrontal cortex (VMPFC), cerebellum, sensorimotor cortex, visual cortex, thalamus, and putamen showed the highest FCD (Figure 2), supporting these regions’ previously established roles as brain hubs and consistent with our prior studies in a large sample of healthy individuals (Tomasi and Volkow, 2010; Tomasi and Volkow, 2011).
Figure 2.
Surface rendering showing the distribution of short-range and long-range FCD hubs in the human brain as a function of chronic cocaine use [cocaine use disorders (CUD) and healthy controls (HC)] and acute methylphenidate [MPH and placebo (PL)]. Color maps reflect the average number (k) of functional connections to neighbor (short-range) or remote (long-range) voxels. The images were created using the Computerized Anatomical Reconstruction and Editing Toolkit (CARET) 5.65 (http://brainvis.wustl.edu/wiki/index.php/Caret:About).
3.2. Diagnosis Differences in Short-Range and Long-Range FCD
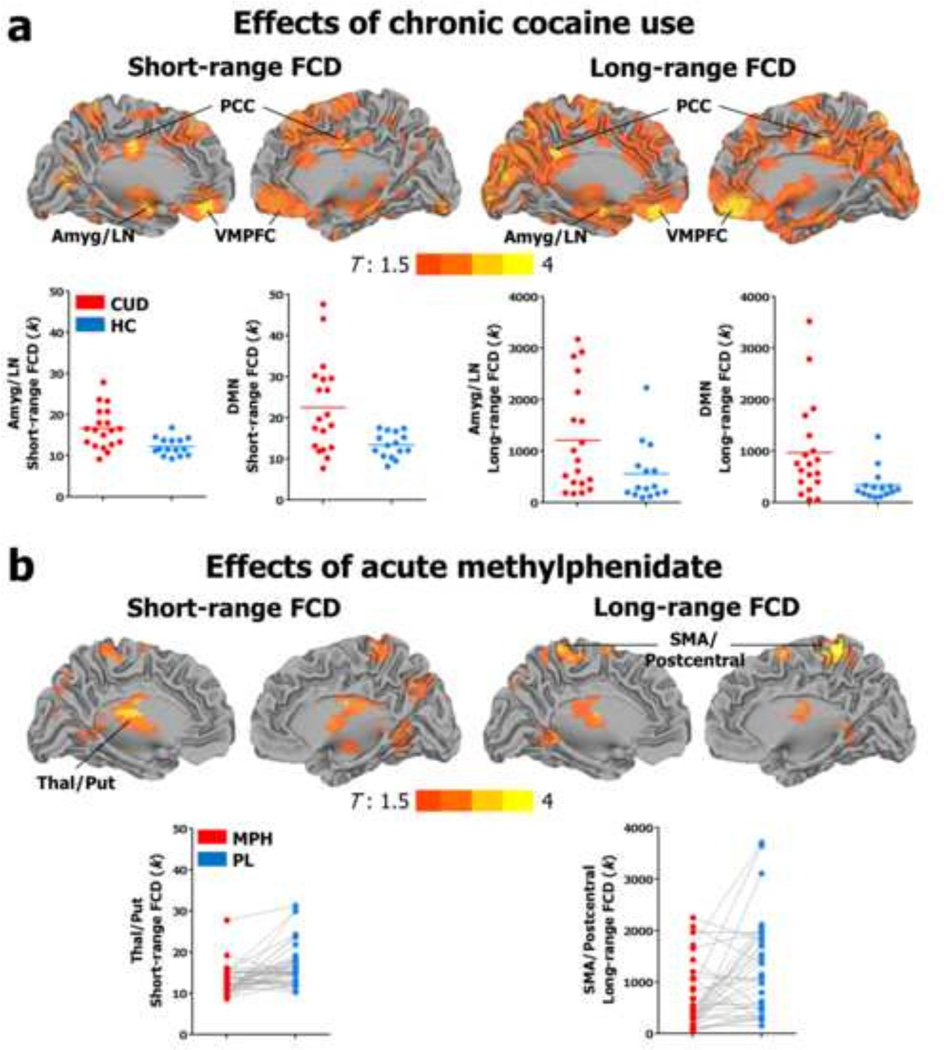
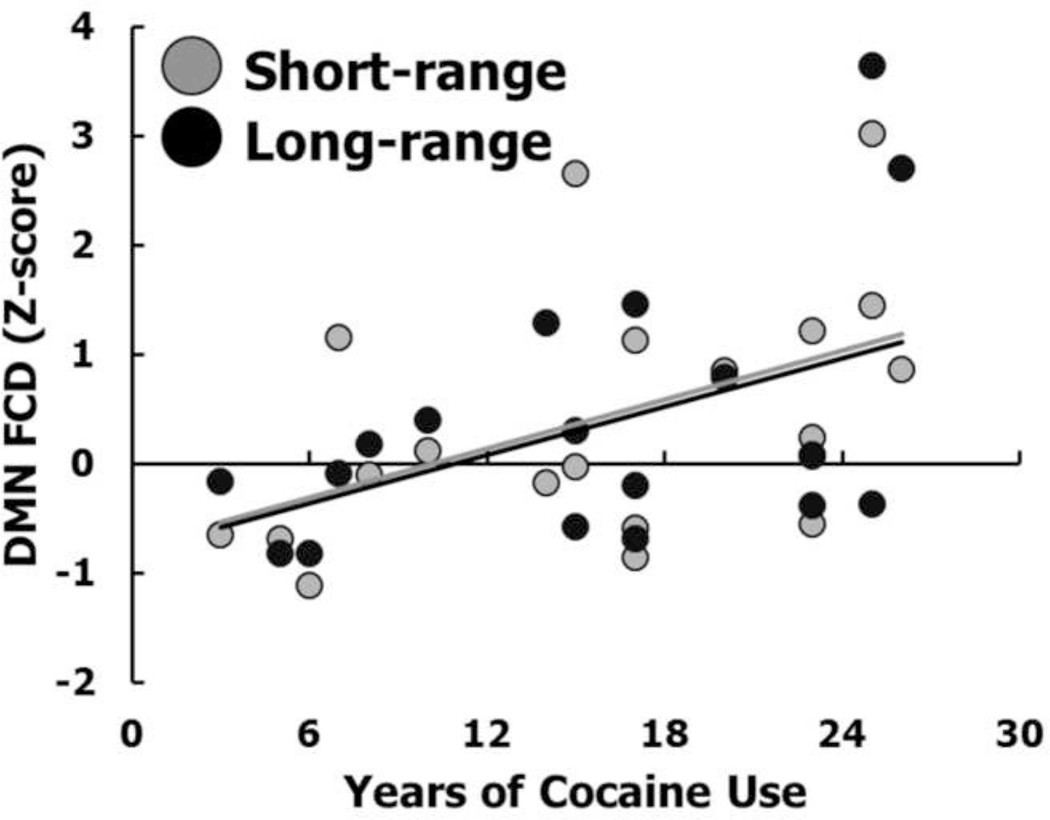
Chronic cocaine use was associated with an abnormal count of short-range and long-range connections to default mode network (VMPFC, PCC) and subcortical/basal ganglia hubs (thalamus and putamen/amygdala), suggesting an overrepresentation of these energy-expensive topological components in addiction (Figure 3a). Consistent with the idea that at least some of these changes are due to drug use rather than preexisting factors, every one year increase in cocaine use was associated with a corresponding increase in the number of connections to default mode hubs at a rate of 0.7 for short-range FCD and 57 for long-range FCD (Figure 4).
Figure 3.
Surface rendering showing significant differences in short-range and long-range functional connectivity density (FCD) (a) in individuals with cocaine use disorders (CUD) as compared with healthy controls (HC) and (b) across participants, following a single 20 mg dose of oral methylphenidate (MPH) as compared with placebo (PL). Color maps reflect T-values ranging from 1.5 to 4. Results are significant at P<0.05 cluster corrected for multiple comparisons. The images were created using the Computerized Anatomical Reconstruction and Editing Toolkit (CARET) 5.65 (http://brainvis.wustl.edu/wiki/index.php/Caret:About). Abbreviations: DMN, default mode network; LN, lenticular nucleus; SMA, supplementary motor area; PCC, posterior cingulate; VMPFC, ventromedial prefrontal cortex.
Figure 4.
Positive relationship between years of cocaine use and average functional connectivity density (FCD) in regions of the default mode network (DMN). For both the short- and long-range FCD, R>0.46, P<0.05. When age and drug use severity (encompassing frequency of use, perceived control over use, level of craving, and withdrawal symptoms) are included as covariates in partial correlation analyses, the results for short-range FCD remain significant (P=0.024) while the results for long-range FCD are somewhat attenuated (P=0.08).
3.3. Effects of Methylphenidate on Short-Range and Long-Range FCD
Opposing effects were observed for the acute dose of MPH, which reduced the number of short-range and long-range connections to hubs relevant to automatic action plans and the formation of habits in the basal ganglia (bilateral thalamus/putamen) and sensorimotor cortex (supplementary motor area and postcentral gyrus) across participants (Figure 3b), suggesting that hub strength could be reduced by the acute administration of this drug.
No hubs showed increased FCD in controls relative to CUD or following MPH relative to placebo. Diagnosis × medication interactions were also non-significant, suggesting that MPH did not differentially affect FCD in CUD. See Tables 1 & 2 for a complete summary of results of the whole-brain analyses on short-range and long-range FCD, respectively. Because time since last use of any drugs differed between the MPH and placebo days, we also tested whether this difference was likely to modify any of the observed effects of MPH on FCD in the CUD group. There were not significant interactions between time since last use and medication condition in any of our regions of interest (i.e., those showing main effects of MPH in Figure 3; P>0.31). FCD in these same regions and those showing main effects of diagnosis also did not differ by cocaine urine status (positive/negative) at either study day (P>0.07). These control analyses suggest that the effects of recent cocaine (or any drug) use are not likely to have confounded those of diagnosis or MPH.
Table 1.
Main Effects of Diagnostic Group and Medication Condition on Short-Range Functional Connectivity Density (FCD)a
| BA | Side | Cluster Size, mm3 |
Peak Z | x | y | z | |
|---|---|---|---|---|---|---|---|
| Diagnosis Main Effect | |||||||
| Inf. Frontal Oper./Inf. Frontal Tri. | 48 | L | 169 | +3.9 | −54 | 12 | 30 |
| −57 | 21 | 15 | |||||
| −57 | 27 | 3 | |||||
| Precuneus/Calcarine Sulcus | 23,17 | R | 106 | +3.7 | 21 | −60 | 27 |
| 12 | −72 | 15 | |||||
| Amygdala | 24 | R | 48 | +3.4 | 24 | −6 | −12 |
| Mid. Temporal G. | 21 | 57 | +3.4 | 54 | 6 | −21 | |
| Sup. Parietal Cortex | 5 | R | 48 | +3.4 | 21 | −57 | 69 |
| Cerebellum | 18 | R | 45 | +3.1 | 12 | −84 | −18 |
| Mid. Cingulate G. | R | 65 | +3.1 | 9 | −12 | 33 | |
| −12 | −12 | 36 | |||||
| Mid. Frontal G. | 9,44 | R | 138 | +3.1 | 27 | 30 | 48 |
| 36 | 21 | 33 | |||||
| 39 | 30 | 45 | |||||
| Mid. Occipital/Angular G. | 37,39,40 | L | 42 | +3.0 | −42 | −63 | 18 |
| −39 | −60 | 27 | |||||
| −33 | −57 | 36 | |||||
| Inf. Orb. Frontal | 47 | L | 84 | +2.9 | −30 | 27 | −21 |
| Rolandic Oper./Postcentral | 6,43 | R | 50 | +2.9 | 60 | 6 | 12 |
| 63 | 0 | 24 | |||||
| Inf. Occipital/Lingual | 19,18 | L | 48 | +2.8 | −45 | −75 | −15 |
| −15 | −87 | −9 | |||||
| −27 | −87 | −15 | |||||
| Sup. Frontal G./ Sup. Med. Frontal G. | 9,32 | L | 45 | +2.7 | −18 | 33 | 48 |
| −9 | 30 | 42 | |||||
| Med. Orb. Frontal/ Ant. Cingulate G. | 11,10 | R | 65 | +2.7 | 6 | 42 | −6 |
| −6 | 45 | −3 | |||||
| Medication Main Effect | |||||||
| Angular G. | 40 | L | 30 | −3.2 | −39 | −48 | 33 |
| Putamen/Thalamus | L | 66 | −3.1 | −24 | −6 | 12 | |
| −18 | −21 | 18 | |||||
| Thalamus | R | 122 | −3.0 | −3 | −12 | 21 | |
| 18 | −9 | 12 | |||||
| Mid. Occipital/ Mid. Temporal G. | 39,37,40 | R | 106 | −2.8 | 39 | −63 | 27 |
| 48 | −57 | 48 | |||||
| 33 | −48 | 33 |
Note. Statistical Threshold: cluster-level P<0.05 corrected using a combined voxel-level P<0.005 uncorrected height threshold and k=26 contiguous voxels;
+Z score indicates increased short-range FCD with chronic cocaine use (cocaine users>controls) or acute methylphenidate (methylphenidate>placebo);
−Z score indicates decreased short-range FCD with chronic cocaine use (cocaine users<controls) or acute methylphenidate (methylphenidate<placebo);
Analysis of covariance (covariate: age);
Coordinates follow Montreal Neurological Institute (MNI) convention.
Table 2.
Main Effects of Diagnostic Group and Medication Condition on Long-Range Functional Connectivity Density (FCD)a
| BA | Side | Cluster Size, mm3 |
Peak Z | x | y | z | |
|---|---|---|---|---|---|---|---|
| Diagnosis Main Effect | |||||||
| Sup. Parietal G./ Precuneus | 7 | L | 175 | +3.6 | −33 | −69 | 54 |
| −15 | −72 | 60 | |||||
| Sup. Orb. G./ Med. Orb.l G. | 11 | R | 397 | +3.1 | −18 | 45 | −21 |
| 0 | 48 | −12 | |||||
| Mid. Orb. G./ Inf. Orb. G./ Mid. Frontal G. | 47,10 | R | 321 | +3.0 | −39 | 48 | 0 |
| −36 | 42 | −15 | |||||
| −36 | 60 | 9 | |||||
| Precentral G. | 6 | L | 228 | +3.0 | −36 | −12 | 45 |
| −27 | −18 | 57 | |||||
| −18 | −6 | 63 | |||||
| Cuneus | 18/19 | R | 235 | +3.0 | 6 | −81 | 24 |
| 18 | −87 | 42 | |||||
| 27 | 84 | 9 | |||||
| Sup. Temporal G. | 21 | R | 47 | +2.9 | 51 | 0 | −12 |
| Mid. Cingulate G./ Post. Cingulate G./ Thalamus | 23,26 | R | 60 | +2.9 | −12 | −27 | 33 |
| −9 | −39 | 21 | |||||
| −12 | −15 | 33 | |||||
| Calcarine Sulcus | 17 | R | 61 | +2.9 | 15 | −105 | −3 |
| 0 | −93 | 6 | |||||
| Post. Cingulate G. | 23 | L | 34 | +2.8 | 12 | −39 | 30 |
| Amygdala/ Putamen | L | 49 | +2.7 | 21 | 3 | −18 | |
| 30 | 6 | −9 | |||||
| Sup. Occipital G./ Angular G. | 7 | R | 54 | +2.7 | 30 | −69 | 45 |
| 39 | −72 | 42 | |||||
| Mid. Occipital/ Sup. Occipital | 18,19 | L | 77 | +2.6 | −33 | −84 | 18 |
| −36 | −87 | 9 | |||||
| −15 | −90 | 21 | |||||
| Sup. Frontal G. | 6 | L | 38 | +2.6 | 15 | −12 | 69 |
| 18 | 3 | 72 | |||||
| Parahippocampal G. | 30 | R | 27 | +2.6 | −24 | −18 | −27 |
| −30 | −18 | −39 | |||||
| Medication Main Effect | |||||||
| Postcentral G./Supp. Motor Area | 5,6 | L | 188 | −3.4 | −6 | −42 | 75 |
| 0 | −12 | 78 | |||||
| 0 | 9 | 72 |
Note. Statistical Threshold: cluster-level P<0.05 corrected using a combined voxel-level P<0.005 uncorrected height threshold and k=26 contiguous voxels;
+Z score indicates increased long-range FCD with chronic cocaine use (cocaine users>controls) or acute methylphenidate (methylphenidate>placebo);
−Z score indicates decreased long-range FCD with chronic cocaine use (cocaine users<controls) or acute methylphenidate (methylphenidate<placebo);
Analysis of covariance (covariate: age);
Coordinates follow Montreal Neurological Institute (MNI) convention.
4. Discussion
We used an agnostic, data-driven approach to investigate the effects of stimulants on the density of functional connections to brain hubs in humans. This approach improves upon commonly used seed-based approaches by enabling network-level conclusions to be drawn, obviating susceptibility to biases related to variations in seed positioning, and eliminating the requirement of a priori hypotheses about particular brain regions (Buckner et al., 2008; Cole et al., 2010b). In the current study, across all participants and medication conditions, we observed higher FCD in regions comprising the default mode network (PCC/precuneus, VMPFC) (Andrews-Hanna et al., 2010; Buckner et al., 2008), in regions central to information processing and cortico-striatal-thalamo-cortical communication (thalamus) (Jakab et al., 2012), and in regions central to motor coordination and action plans (cerebellum, sensorimotor cortex) (Hardwick et al., 2013; Pisotta and Molinari, 2014), all purported to serve as critical brain hubs.
The main goals of the current study, however, were to test whether such functional brain hubs are abnormal in disease (cocaine addiction) and whether they can be modified by an acute stimulant challenge (single-dose of MPH). For the former (addiction), we found that a diagnosis of CUD was associated with widespread increases in both short-range and long-range FCD, particularly in regions of the default mode network (VMPFC and PCC) and subcortical/basal ganglia hubs (thalamus, putamen, and amygdala). Consistent with these findings, functional connectivity alterations were observed in the default mode network including the VMPFC (e.g., to/from a salience network) in nicotine dependence as a function of abstinence length (Ding and Lee, 2013; Lerman et al., 2014). Interestingly, a recent seed-based approach showed that specific alteration of connections between the amygdala and VMPFC prospectively predicted 30-day relapse in CUD (McHugh et al., 2014). Our findings showing a higher than normal number of connections to these default mode- and subcortical/basal ganglia hubs in CUD, as directly associated with the length of cocaine exposure in the case of the default mode, suggests that cost-value tradeoffs between efficiency and metabolic cost may be altered in these individuals. While it remains to be determined whether these abnormalities are due to the effects of cocaine or pre-existing factors, the presence of such alterations is consistent with the diverse range of impairments in cognitive and emotional processes observed in addiction (Goldstein and Volkow, 2011), and with the idea that high cost network components are likely most vulnerable to disease (Buckner et al., 2009; Crossley et al., 2014; Rubinov and Bullmore, 2013) and aging (Tomasi and Volkow, 2012) (and also most likely to result in the greatest deficits).
For the latter goal of this study (brain hub modulation by acute MPH), across all participants MPH generally, albeit more selectively, reduced the number of short-range and long-range connections, with significant reductions emerging subcortically in the basal ganglia (putamen but also the thalamus for short-range FCD) and sensorimotor cortex (supplementary motor area and postcentral gyrus for long-range FCD). Effects in these regions have also been observed with complementary methodologies. For example, administration of a different psychostimulant (acute cocaine infusion) reduced resting-state connectivity of the visual and primary motor cortices in a small sample of CUD (Li et al., 2000). In our prior study (Konova et al., 2013), MPH reduced abnormally strong connectivity as assessed by seed-based methods between the ventral striatum and putamen, a pathway implicated in the formation of habits and drug addiction (Belin and Everitt, 2008). It is possible that these MPH-induced changes in hub strength (locally in the striatum and more distributed in the sensory cortices) could facilitate willful behavior or make cortical processing underlying behavior more efficient, or less difficult to override. This interpretation is consistent with the idea that MPH enhances attention and goal-directed action (Moeller et al., 2014; Pauls et al., 2012) and reduces brain-energy requirements needed for these kinds of functions (Swanson et al., 2011; Volkow et al., 2008). While this hypothesis remains to be directly tested, in support of this idea a previous study (Giessing et al., 2013) found that acute nicotine modified resting state network topology by increasing global efficiency and decreasing clustering (number of local connections) in the basal ganglia and thalamus among other regions, as indicative of a more integrative network configuration, whereas continuous performance on a cognitively demanding task had the opposite effects. Both manipulations (acute nicotine and time-on-task) were further differentially associated with attentional performance in the expected direction (enhanced by nicotine and impaired by cognitive fatigue).
In summary, we examined the effects of stimulants on functional brain hubs at rest by comparing hub strength between individuals with CUD and healthy controls (to indirectly isolate chronic stimulant effects) and following a single dose of MPH (to directly isolate acute stimulant effects). Our data-driven methodology has the potential to produce a more complete (and potentially less biased) picture of the brain’s topology than can be provided by traditional seed-based approaches. Here, the opposing effects of chronic and acute stimulants on brain functional connectivity hubs – that is, many hubs were increased in CUD, while some of the same hubs were reduced with acute MPH administration – speak to the use of hub resting-state connectivity as a tool to monitor disease course and treatment in drug addiction, with possible applications to other neuropsychiatric diseases as well. While we cannot at present speak to whether MPH-induced modulation of connectivity is a viable treatment target in CUD, the observed changes in hub strength with short-term MPH, possibly due to transient changes in synaptic density due to dopaminergic modulation, highlight the malleable nature of the brain’s topological organization and suggest an exciting window of opportunity for intervention.
Highlights.
Hubs are energy-expensive nodes in the brain with dense functional connections.
We tested the effects of cocaine addiction and acute methylphenidate on brain hubs.
Addiction linked to higher density of connections to default mode and striatal hubs.
Methylphenidate decreased density of connections to striatal and sensorimotor hubs.
Hub topology is malleable: it can be altered by addiction and dopamine treatment.
Acknowledgments
Funding/Support
This research was conducted with grant support from the National Institute on Drug Abuse (1R01DA023579 to R.Z.G. and 1F32DA030017-01 to S.J.M) and the National Institute of Mental Health (T32MH019524 training award in Systems and Integrative Neuroscience to A.B.K.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure
No conflicts declared.
References
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain's default network. Neuron. 2010;65:550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Everitt BJ. Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron. 2008;57:432–441. doi: 10.1016/j.neuron.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, Andrews-Hanna JR, Sperling RA, Johnson KA. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer's disease. J Neurosci. 2009;29:1860–1873. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole DM, Beckmann CF, Long CJ, Matthews PM, Durcan MJ, Beaver JD. Nicotine replacement in abstinent smokers improves cognitive withdrawal symptoms with modulation of resting brain network dynamics. Neuroimage. 2010a;52:590–599. doi: 10.1016/j.neuroimage.2010.04.251. [DOI] [PubMed] [Google Scholar]
- Cole DM, Smith SM, Beckmann CF. Advances and pitfalls in the analysis and interpretation of resting-state FMRI data. Front Syst Neurosci. 2010b;4:8. doi: 10.3389/fnsys.2010.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossley NA, Mechelli A, Scott J, Carletti F, Fox PT, McGuire P, Bullmore ET. The hubs of the human connectome are generally implicated in the anatomy of brain disorders. Brain. 2014;137:2382–2395. doi: 10.1093/brain/awu132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X, Lee SW. Changes of functional and effective connectivity in smoking replenishment on deprived heavy smokers: a resting-state FMRI study. PLoS One. 2013;8:e59331. doi: 10.1371/journal.pone.0059331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams J. Williams J. Structured Clinical Interview for DSM-IV Axis I disorders - Patient Edition (SCID-I/P, Version 2.0). Vol., ed.^eds. New York: Biometrics Research Department, New York State Psychiatric Institute; 1996. [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Giessing C, Thiel CM, Alexander-Bloch AF, Patel AX, Bullmore ET. Human brain functional network changes associated with enhanced and impaired attentional task performance. J Neurosci. 2013;33:5903–5914. doi: 10.1523/JNEUROSCI.4854-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossop M, Griffiths P, Powis B, Strang J. Severity of dependence and route of administration of heroin, cocaine and amphetamines. British Journal of Addiction. 1992;87:1527–1536. doi: 10.1111/j.1360-0443.1992.tb02660.x. [DOI] [PubMed] [Google Scholar]
- Grant BF, Hasin DS, Chou SP, Stinson FS, Dawson DA. Nicotine dependence and psychiatric disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry. 2004;61:1107–1115. doi: 10.1001/archpsyc.61.11.1107. [DOI] [PubMed] [Google Scholar]
- Gu H, Salmeron BJ, Ross TJ, Geng X, Zhan W, Stein EA, Yang Y. Mesocorticolimbic circuits are impaired in chronic cocaine users as demonstrated by resting-state functional connectivity. Neuroimage. 2010;53:593–601. doi: 10.1016/j.neuroimage.2010.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick RM, Rottschy C, Miall RC, Eickhoff SB. A quantitative meta-analysis and review of motor learning in the human brain. Neuroimage. 2013;67:283–297. doi: 10.1016/j.neuroimage.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong LE, Gu H, Yang Y, Ross TJ, Salmeron BJ, Buchholz B, Thaker GK, Stein EA. Association of nicotine addiction and nicotine's actions with separate cingulate cortex functional circuits. Arch Gen Psychiatry. 2009;66:431–441. doi: 10.1001/archgenpsychiatry.2009.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakab A, Blanc R, Berenyi EL. Mapping changes of in vivo connectivity patterns in the human mediodorsal thalamus: correlations with higher cognitive and executive functions. Brain Imaging Behav. 2012;6:472–483. doi: 10.1007/s11682-012-9172-5. [DOI] [PubMed] [Google Scholar]
- Jia Z, Worhunsky PD, Carroll KM, Rounsaville BJ, Stevens MC, Pearlson GD, Potenza MN. An initial study of neural responses to monetary incentives as related to treatment outcome in cocaine dependence. Biol Psychiatry. 2011;70:553–560. doi: 10.1016/j.biopsych.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalman D, Morissette SB, George TP. Co-morbidity of smoking in patients with psychiatric and substance use disorders. Am J Addict. 2005;14:106–123. doi: 10.1080/10550490590924728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly C, Zuo XN, Gotimer K, Cox CL, Lynch L, Brock D, Imperati D, Garavan H, Rotrosen J, Castellanos FX, Milham MP. Reduced interhemispheric resting state functional connectivity in cocaine addiction. Biol Psychiatry. 2011;69:684–692. doi: 10.1016/j.biopsych.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konova AB, Moeller SJ, Tomasi D, Volkow ND, Goldstein RZ. Effects of Methylphenidate on Resting-State Functional Connectivity of the Mesocorticolimbic Dopamine Pathways in Cocaine Addiction. Jama Psychiatry. 2013;70:857–868. doi: 10.1001/jamapsychiatry.2013.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. Effects of methylphenidate on extracellular dopamine, serotonin, and norepinephrine: comparison with amphetamine. J Neurochem. 1997;68:2032–2037. doi: 10.1046/j.1471-4159.1997.68052032.x. [DOI] [PubMed] [Google Scholar]
- Lerman C, Gu H, Loughead J, Ruparel K, Yang Y, Stein EA. Large-scale brain network coupling predicts acute nicotine abstinence effects on craving and cognitive function. JAMA Psychiatry. 2014;71:523–530. doi: 10.1001/jamapsychiatry.2013.4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SJ, Biswal B, Li Z, Risinger R, Rainey C, Cho JK, Salmeron BJ, Stein EA. Cocaine administration decreases functional connectivity in human primary visual and motor cortex as detected by functional MRI. Magn Reson Med. 2000;43:45–51. doi: 10.1002/(sici)1522-2594(200001)43:1<45::aid-mrm6>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Liang X, Zou Q, He Y, Yang Y. Coupling of functional connectivity and regional cerebral blood flow reveals a physiological basis for network hubs of the human brain. Proc Natl Acad Sci U S A. 2013;110:1929–1934. doi: 10.1073/pnas.1214900110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma N, Liu Y, Li N, Wang CX, Zhang H, Jiang XF, Xu HS, Fu XM, Hu X, Zhang DR. Addiction related alteration in resting-state brain connectivity. Neuroimage. 2010;49:738–744. doi: 10.1016/j.neuroimage.2009.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma N, Liu Y, Fu XM, Li N, Wang CX, Zhang H, Qian RB, Xu HS, Hu X, Zhang DR. Abnormal brain default-mode network functional connectivity in drug addicts. PLoS One. 2011;6:e16560. doi: 10.1371/journal.pone.0016560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh MJ, Demers CH, Salmeron BJ, Devous MD, Sr, Stein EA, Adinoff B. Cortico-amygdala coupling as a marker of early relapse risk in cocaine-addicted individuals. Front Psychiatry. 2014;5:16. doi: 10.3389/fpsyt.2014.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M. The Fifth Edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Miller GA, Chapman JP. Misunderstanding analysis of covariance. J Abnorm Psychol. 2001;110:40–48. doi: 10.1037//0021-843x.110.1.40. [DOI] [PubMed] [Google Scholar]
- Moeller SJ, Honorio J, Tomasi D, Parvaz MA, Woicik PA, Volkow ND, Goldstein RZ. Methylphenidate enhances executive function and optimizes prefrontal function in both health and cocaine addiction. Cereb Cortex. 2014;24:643–653. doi: 10.1093/cercor/bhs345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauls AM, O'Daly OG, Rubia K, Riedel WJ, Williams SC, Mehta MA. Methylphenidate effects on prefrontal functioning during attentional-capture and response inhibition. Biol Psychiatry. 2012;72:142–149. doi: 10.1016/j.biopsych.2012.03.028. [DOI] [PubMed] [Google Scholar]
- Pisotta I, Molinari M. Cerebellar contribution to feedforward control of locomotion. Front Hum Neurosci. 2014;8:475. doi: 10.3389/fnhum.2014.00475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov M, Bullmore E. Schizophrenia and abnormal brain network hubs. Dialogues Clin Neurosci. 2013;15:339–349. doi: 10.31887/DCNS.2013.15.3/mrubinov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmuel A, Leopold DA. Neuronal correlates of spontaneous fluctuations in fMRI signals in monkey visual cortex: Implications for functional connectivity at rest. Hum Brain Mapp. 2008;29:751–761. doi: 10.1002/hbm.20580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O, Honey CJ, Kotter R. Identification and classification of hubs in brain networks. PLoS One. 2007;2:e1049. doi: 10.1371/journal.pone.0001049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J, Baler RD, Volkow ND. Understanding the effects of stimulant medications on cognition in individuals with attention-deficit hyperactivity disorder: a decade of progress. Neuropsychopharmacology. 2011;36:207–226. doi: 10.1038/npp.2010.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Caparelli EC, Chang L, Ernst T. fMRI-acoustic noise alters brain activation during working memory tasks. Neuroimage. 2005;27:377–386. doi: 10.1016/j.neuroimage.2005.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND. Functional connectivity density mapping. Proc Natl Acad Sci U S A. 2010;107:9885–9890. doi: 10.1073/pnas.1001414107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND. Functional connectivity hubs in the human brain. Neuroimage. 2011;57:908–917. doi: 10.1016/j.neuroimage.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND. Aging and functional brain networks. Mol Psychiatry. 2012;17:471, 549–558. doi: 10.1038/mp.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Wang GJ, Volkow ND. Energetic cost of brain functional connectivity. Proc Natl Acad Sci U S A. 2013;110:13642–13647. doi: 10.1073/pnas.1303346110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay J, Maleki N, Potter J, Elman I, Rudrauf D, Knudsen J, Wallin D, Pendse G, McDonald L, Griffin M, Anderson J, Nutile L, Renshaw P, Weiss R, Becerra L, Borsook D. Alterations in brain structure and functional connectivity in prescription opioid-dependent patients. Brain. 2010;133:2098–2114. doi: 10.1093/brain/awq138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura J, Liberman RP, Green MF, Shaner A, Mintz J. Training and quality assurance with the Structured Clinical Interview for DSM-IV (SCID-I/P) Psychiatry Res. 1998;79:163–173. doi: 10.1016/s0165-1781(98)00038-9. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Gatley SJ, Logan J, Ding YS, Hitzemann R, Pappas N. Dopamine transporter occupancies in the human brain induced by therapeutic doses of oral methylphenidate. Am J Psychiatry. 1998;155:1325–1331. doi: 10.1176/ajp.155.10.1325. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Swanson JM. Dopamine in drug abuse and addiction: results from imaging studies and treatment implications. Mol Psychiatry. 2004;9:557–569. doi: 10.1038/sj.mp.4001507. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Telang F, Logan J, Wong C, Ma J, Pradhan K, Benveniste H, Swanson JM. Methylphenidate decreased the amount of glucose needed by the brain to perform a cognitive task. PLoS ONE. 2008;3:e2017. doi: 10.1371/journal.pone.0002017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger AH, Sofuoglu M. The impact of cigarette smoking on stimulant addiction. Am J Drug Alcohol Abuse. 2009;35:12–17. doi: 10.1080/00952990802326280. [DOI] [PMC free article] [PubMed] [Google Scholar]