Abstract
Microglia are a specialized population of myeloid cells that mediate CNS innate immune responses. Efforts to identify the cellular and molecular mechanisms that regulate microglia behaviors have been hampered by the lack of effective tools for manipulating gene expression. Cultured microglia are refractory to most chemical and electrical transfection methods, yielding little or no gene delivery and causing toxicity and/or inflammatory activation. Recombinant adeno-associated viral (rAAVs) vectors are non-enveloped, single-stranded DNA vectors commonly used to transduce many primary cell types and tissues. In this study, we evaluated the feasibility and efficiency of utilizing rAAV serotype 2 (rAAV2) to modulate gene expression in cultured microglia. rAAV2 yields high transduction and causes minimal toxicity or inflammatory response in both neonatal and adult microglia. To demonstrate that rAAV transduction can induce functional protein expression, we used rAAV2 expressing Cre-recombinase to successfully excise a LoxP-flanked miR155 gene in cultured microglia. We further evaluated rAAV serotypes 5, 6, 8, and 9, and observed that all efficiently transduced cultured microglia to varying degrees of success and caused little or no alteration in inflammatory gene expression. These results provide strong encouragement for the application of rAAV-mediated gene expression in microglia for mechanistic and therapeutic purposes.
Keywords: Recombinant Adeno-associated Viral (rAAV) vector, Microglia, In Vitro, Heparin
Introduction
Microglia are a specialized population of resident myeloid cells in the central nervous system (CNS) that comprise 5–15% of CNS cells (Carson et al., 1998). Microglia participate in the regulation of diverse physiological processes, influencing the development, growth, and function of other brain cells and serving as a key immune surveillance system. Their diverse repertoire of activities in the inflammatory response include destroying infectious pathogens, removing cell debris, and promoting brain tissue repair (Garden et al., 2004).
Understanding the molecular signals responsible for determining specific microglia behaviors has been slow to develop compared to other CNS cell types. One reason for this is that microglia are strongly refractory to various means of gene transfer, both in vitro and in vivo. In the past, attempts to genetically manipulate primary hematopoietic cells have principally focused on transferring genes into pluripotent stem cell populations (Cucchiarini et al., 2003). Some significant steps have been taken to genetically modify cultured microglia as therapeutic tools. For example, implantation of ex vivo engineered microglial cell lines, or precursor cells, has been successfully performed in several animal models (Benninger et al., 2000; Sawada et al., 1998). In vitro engineered microglia that retain the nuclear content of olfactory ensheathing cells were also successfully delivered into brain as a method of somatic cell nuclear transfer, in hope of reprogramming somatic cells to treat neurodegenerative diseases caused by mitochondrial DNA mutations (Baig, 2014). However, techniques to reliably manipulate gene expression in cultured microglia without toxicity or inflammatory activation have been extremely difficult to develop. Methods involving chemical or electrical transfection have shown little or no delivery of vector and cause moderate toxicity and inflammatory gene expression (Lungwitz et al., 2005).
Previous studies with lentiviral vectors have shown effective gene transfer into microglia cell lines and neonatal microglia (Balcaitis et al., 2005; Tun et al., 2007). However, we observed that in primary neonatal microglia cultures, lentiviral infection was associated with modest toxicity and mild inflammatory activation, limiting the experimental utility of these vectors. Here, we developed a novel methodology for the culture of microglia from adult mouse brain and observed that toxicity following lentiviral infection was significantly higher than that in neonatal microglia cultures. Therefore, we sought to develop a new approach to modify gene expression in cultured microglia that would be amenable for use in both adult and neonatal preparations. To approach this task, we elected to use recombinant adeno-associated viral (rAAV) vectors as gene transfer vehicles. In recent years, rAAV vectors have become increasingly valuable for in vivo studies in animals, and are also currently being tested in human clinical trials. rAAV vectors are nonenveloped, single-stranded DNA vectors that reside episomally with exceedingly rare integration events, and are able to establish stable long-term gene expression in both dividing and non-dividing cells (de Backer et al., 2011) with little to no immunogenicity (Fisher et al., 1997). Studies using AAV initially involved rAAV serotype 2 (rAAV2), the first characterized AAV serotype, where the majority of clinical trials currently underway involve delivery of rAAV2 into the brain, a relatively immunologically privileged organ. To date, rAAV2-based recombinant genomes have been packaged in dozens of different capsid types, resulting in a wide array of “pseudotyped vectors” that constitute a rich resource for the development of gene therapy clinical trials (Gao et al., 2011b).
While the efficiency of rAAV-mediated gene transfer varies widely between different cell types, this class of vector has been used successfully to transduce several primary cell types and tissues that are refractory to most viral vectors, including striated muscle, liver hepatocytes, and vessel endothelium (Flotte et al., 1993; Gregorevic et al., 2004; Inouye et al., 1997; Snyder et al., 1999; Xiao et al., 1996). It was recently reported that rAAV2 was successfully utilized to express Follistatin in ovine myoblast cells (Nazari et al., 2014). However, the use of rAAV vectors to modulate gene expression in cultured microglia has not yet been well documented.
The majority of in vitro microglia studies utilize rodent neonatal microglial cultures (Santambrogio et al., 2001). Although these cells have been useful to study several signal transduction and transcriptional response systems (Przanowski et al., 2014; Su et al., 2014), neonatal microglia are likely to be functionally distinct from adult microglial cells. Analysis on microglia isolated from different postnatal ages shows that microglia undergo developmental reorganization by exhibiting changes in gene expression profile and varied cellular responses following TLR4 stimulation (Scheffel et al., 2012). Moreover, neonatal microglia exhibit a partially activated phenotype in vitro, as indicated by an intermediate expression level of MHC class II and co-stimulatory molecules not observed in adult microglia in situ (Aloisi, 2001; Carson et al., 1998). Cumulatively, this evidence suggests that neonatal microglia are not functionally mature. To date, a small number of studies have utilized freshly isolated, ex vivo adult microglia to study changes in gene expression induced by in vivo disease or injury models (Floden and Combs, 2006; Moussaud and Draheim, 2010). Unfortunately, early studies with adult microglia in vitro reported that they undergo cell death within several days of culture. It was observed that mouse adult microglia do not proliferate in vitro, while they could be differentiated into dendritic-like cells in the presence of GM-CSF (Fischer and Reichmann, 2001). Astrocyte feeder layers from neonatal culture have been used to sustain adult microglia culture (Scheffel et al., 2012), but the complex interaction between microglia and astrocytes makes it difficult to specifically assay microglia behaviors and/or modulate microglia gene expression. A study utilized Percoll gradient to isolate microglia and macrophage colony-stimulating factor (M-CSF) to aid cell proliferation (Ponomarev et al., 2005). Traditional isolation method using Percoll-based gradient is time-consuming, and resulted in a mixed population of microglia, astrocytes, and oligodendrocytes. Additionally, Percoll is toxic to microglia and the cell yield is usually not sufficient for immediate cellular analysis (Banker and Goslin, 1998).
In this study, we developed a modified method for adult microglia culture and evaluated the feasibility and efficiency utilizing rAAV serotype 2 as a transfer vector to modulate gene expression in cultured microglia. We compared 4 different transfection reagents as well as two viral vectors on cultured microglia, and found that rAAV-mediated gene transfer resulted in best transduction efficiency with least cellular toxicity. In addition, rAAV2 transduction yielded no inflammatory responses or toxicity in both neonatal and adult microglia. Using a combination of rAAV and Cre-LoxP systems, we were able to successfully excise a LoxP flanked miR155 gene in cultured microglia, demonstrating that a functional protein was generated from the rAAV vector. To our knowledge, this is the first demonstration of selective gene transfer in cultured adult microglia using rAAV-derived vectors. Additionally, our survey of rAAV serotypes determined that rAAV6 displayed the highest capacity for the transduction of neonatal microglial cells in vitro, approaching a 2-log increase in reporter gene mRNA levels relative to rAAV2. These results provide strong encouragement for the application of this approach for manipulating gene expression in cultured microglia for mechanistic and therapeutic purposes.
Materials and Methods
Animals
C57/BL6 mice (The Jackson Laboratory, Bar Harbor, Maine) were referred as “wild type (WT)” in this study. The floxed miR-155 mice, referred as “flx miR-155” in this study, possess loxP sites flanking exon 2 of the Mir 155 gene on both alleles. All mice were maintained in a specific pathogen-free facility and all procedures were performed in accordance with an IACUC-approved protocol.
Microglia Preparation and Cell Culture
Mixed neonatal glia cultures were generated from cortical tissue dissected on postnatal day 3 or 4, using previously published methods (Jayadev et al., 2011). Pups of both sexes were used. The mixed glia were cultured in DMEM (Dulbecco’s Modified Eagle Medium) high glucose (Gibco, Life Technologies, Grand Island, NY) supplemented with 10% heat inactivated horse serum (Gibco, Life Technologies), 10% nutrient mixture F-12 ham (Sigma-Aldrich, St. Louis, MO), 2mM L-glutamine (Sigma-Aldrich), 10mM HEPES (Sigma-Aldrich), and 20% L929 conditioned medium (Möller et al., 2000). Microglia were isolated from the cultures 7–10 days post-dissection by collecting floating cells. Primary microglia were plated on poly-d-lysine-coated (Sigma-Aldrich) plates at a density of 1×106 per 60mm well, 5×105 per 35mm well, or 2.5×105 per 15 mm well in D10C media supplemented with 10 ng/ml Macrophage Colony-Stimulating Factor (MCSF, R&D Systems, Minneapolis, MN).
Adult microglia were generated using a new protocol modified from a previously published method (Ponomarev et al., 2005) and our published method for ex vivo isolation of adult microglia (Su et al., 2014). For each adult microglia culture, adult mice of both sexes (12–22 wks., n=3 mice per genotype) were intracardially perfused with cold HBSS (No Ca2+/Mg2+)/1mM HEPES (Gibco). Cortical tissue was separated from whole brain and dissociated using the MACS Neural Dissociation Kit (Miltenyi). Dissociated tissues were filtered through a sterile 70 µm filter (Fisher Scientific, Grand Island, NY) and then incubated with myelin removal beads at 4 °C for 15 min (Miltenyi). After incubation, the mixture was washed, and then loaded onto a pre-rinsed LS column (Miltenyi) and the unbound flow through (myelin depleted) was collected and incubated with 80 µl CD11b microbeads at 4 °C for 15 min (Miltenyi), from which the CD11b+ population was collected using LS column. Finally, the collected cells were centrifuged at 300 g for 10 min at 4 °C, resuspended in D10C media supplemented with 10 ng/µl MCSF, and seeded onto poly-d-lysine-coated 4-well plates at a density of 0.5×105 per well. Medium was changed after 3 days in vitro, and cells were used for experimental purposes after 7 days in vitro. For flow cytometry analysis, cells were collected, stained with microglial specific antibodies, and analyzed as previously described (Su et al., 2014).
The HEK293D (kindly provided by Dusty Miller, Fred Hutchinson Cancer Research Center) and BV2 cell line were grown in high glucose DMEM supplemented with 10% FBS (Gibco), 25 U/ml penicillin and 25 mg/ml streptomycin and incubated in a 37 °C cell incubator with 5% CO2 supplemented.
For experiments testing different transfection reagents, neonatal microglia were harvested and plated at a density of 2.5×105 per 15 mm well. For MACSfectin (Miltenyi) transfections, 1 µg of CMV-eGFP plasmids diluted in 50 µl of serum-free medium (SFM) were added into 2 µl or 4 µl of MACSfectin transfection reagent diluted in 50 µl of SFM. The mixtures were incubated at room temperature (RT) for 20min before added into cell medium. For Lipofectamine 2000 (Life Technologies) transfection, 1 µg of CMV-eGFP plasmids diluted in 50 µl of OPTI-MEM® Reduced Serum Medium were added into 2 µl or 4 µl of Lipofectamine 2000 transfection reagent diluted with 50 µl of OPTI-MEM® Reduced Serum Medium. The mixtures were incubated at RT for 5 min before added into cell medium. For Lipofectamine 3000 (Life Technologies) transfection, 0.5 µg of CMV-eGFP plasmids diluted in 25 µl of OPTI-MEM® Reduced Serum Medium and supplemented with 1 µl of P3000 Reagent were added into 0.75 µl or 1.5 µl of Lipofectamine 3000 transfection reagent diluted in 25 µl of OPTI-MEM® Reduced Serum Medium. The mixtures were incubated at RT for 5 min before added into cell medium. For GeneIn (Global Stem, Gaithersburg, MD) transfection, 2 µl or 4 µl of Red GeneIn reagent were added into 0.5 µg or 1 µg of CMV-eGFP plasmids diluted in 50 µl of OPTI-MEM® Reduced Serum Medium and the mixtures were incubated at RT for 5 min. 2 ul of Blue GeneIn reagent was then added into each mixture and incubated at RT for additional 10–15 min before added into the cell medium. For Lentiviral infections, serial dilutions of Lenti-CMV-eGFP vectors (Core Center for Excellence in Hematology at Fred-Hutchinson Cancer Center, Seattle, WA) at 0.5, 1, 2, 4, 6, 8, and 10 vector genome per cell (vg/cell) were added into the cell medium. For rAAV infections, serial dilutions of rAAV2-CMV-GFP vectors at 0.875 × 103, 1.75 × 103, 3.5 × 103 and 7 × 103 vg/cell were added into the cell medium. For all of the treatments, cell medium was changed 12 hr after transfection or infection to reduce toxicity. Cell morphology and GFP expression were examined under the microscope 24 hr and 48 hr post transfection. Cells incubated with Lenti-CMV-eGFP and rAAV2-CMV-eGFP were fixed 72 hr after addition of virus.
rAAV production, purification, and infection conditions
Recombinant AAV vector serotypes (2, 5, 6, 8, 9) that contain eGFP driven by the cytomegalovirus (CMV) immediately early enhancer/promoter were generated using previously published methods (Ayuso et al., 2010). rAAV2 and rAAV6 containing mCherry, firefly luciferase, and Cre-recombinase - driven by cytomegalovirus (CMV) promoter - were prepared by calcium phosphate transfection (CaPO4 co-precipitation) into HEK293D cells with the corresponding shuttle plasmids and pDG2 packaging capsids (for rAAV2) or pDGM6 (for rAAV6) according to previously published methods with several modifications (Allen et al., 2000). Briefly, co-transfection of HEK293D cells with the calcium phosphate method was carried out using pDG(*)-encoding the appropriate capsid (CAP) gene for packaging viral genome and AAV vector plasmids (two plasmid system) to produce high-titer infectious AAV (Grimm et al., 2003). 6 hr post-transfection, medium was changed to serum-free media and cultured for 48–60 hours. Cells and virus-containing medium were harvested by scraping cells off the plate and intracellular vectors were released by performing 3 freeze-thaw cycles (dry ice/ethanol − 37 °C water bath). rAAV vector serotype 2 and 6 were purified by affinity chromatography using a heparin column (McClure et al., 2011), while other serotypes were purified using CsCl gradient ultracentrifugation as previously described (Halbert et al., 2001), eluent was concentrated using 100K Amicon Ultra-4 centrifugal filters (Millipore, Billerica, MA) and titered by qRT-PCR as previously described (Halbert et al., 2001). Stock solutions of virus were diluted in PBS. Microglia were initially incubated with serial dilutions of rAAV2-CMV-mCherry and a titer of 1.75 × 103 vg/cell in 500 µl culture medium resulted in maximal infectivity with no apparent cell death. In the following experiments, neonatal or adult microglia medium was replaced with virus-containing media at 1.75 × 103 vg/cell, and were harvested for either RNA extraction, microscopy, or flow cytometry analysis 7 days post infection. For comparison of rAAV serotype 2, 5, 6, 8 and 9, neonatal microglia were replaced in the same density as above and infected with different serotype at 1.75 × 103 vg/cell, RNAs were then extracted 7 days post infection. Similar high titers of rAAV being used in vitro on different cultured cell types were previously reported by other groups (Arbetman et al., 2005; Ellis et al., 2013).
Immunocytochemistry
Cultured neonatal and adult microglia were incubated in 4% Paraformaldehyde (PFA, Electron Microscopy Sciences, Hatfield, PA) for 10 min at RT, washed with cold PBS for 3 times at 5 min each, and permeabilized with 0.5% TritonX-100 in PBS for 10 min at RT. Cells were then washed with cold PBS 3 times at 5 min each, blocked with 2.5% BSA/PBS for 1hr at RT, and incubated with primary antibody in 2.5% BSA/PBS overnight at 4 °C with gentle agitation. On the second day, cells were washed with cold PBS 3 times at 5 min each and then incubated with secondary antibodies for 1 hr. at RT. The primary antibodies used in this study were: rabbit anti Iba1 (ionized calcium binding adaptor molecule 1, 019-19741, WAKO, Richmond, VA) 1:200 dilution, mouse anti GFAP (Glial Fibrillary Acidic Protein, G3893, Sigma-Aldrich) 1:400, rabbit anti RFP (ab34771, Abcam, Cambridge, MA) 1:300. The secondary antibodies used in this study were Alexa Fluor dye-conjugated (1:400, Invitrogen).Nuclei were labeled with Hoechst 33258 (2.5 µg/ml) for 10 min at RT.
Real-time Quantitative Reverse Transcriptase PCR
Total RNA was extracted using the miRNeasy kit (Qiagen, Valencia, CA) and/or Direct-zol RNA mini-prep (Zymo Research, Irvine, CA). cDNAs were generated using the High Capacity cDNA Reverse Transcriptase Kit (Applied Biosystems, Life Technologies). Primers were designed using the ProbeFinder software provided on the Roche Universal Probe Library Website (http://lifescience.roche.com/shop/en/mx/overviews/brand/universal-probe-library). qPCR was performed using the StepOnePlus Real Time PCR Instrument (Applied Biosciences) with the Roche Primer/Probe sets as previously described (Su et al., 2014). New primers were designed for FIZZ1 (forward: TTCCCTTCTCATCTGCATCTC, reverse: GTTACAGTGGAGGGATAGTTAGCTG, probe #78) and YM1 (forward: TCTGGGTACAAGATCCCTGAA, reverse: TCATATGGAGATTTATAGAGGGGACT, probe #47).
Phagocytosis Assay
Labeled apoptotic bodies were generated by fluorescently labeling BV2 cells with PKH26 Red Fluorescent Cell Linker Kit (Sigma, St. Louis, MO), followed by UV treatment for 90 min. UV treated cells were incubated at 37°C for 48 to 60 hr. Rounded apoptotic bodies in complete BV2 media were isolated, washed, and diluted two-fold. These diluted apoptotic bodies were then added to PKH2 Green Fluorescent Cell Linker (Sigma, St. Louis, MO) labeled adult or neonatal primary microglia pretreated with 10 ng/ mL TNF-α (R&D Systems), 10 U/mL IFN-γ (R&D Systems), or 10 ng/mL IL-4 (R&D Systems) for 24 hr. After 6 hr. incubation at 37°C, the cultures were washed to remove excess apoptotic cells, trypsinized (0.05% trypsin EDTA) to lift cells and resuspended in FM medium (HBSS with no Ca2+/Mg2+, 10% Fetal Bovine Serum, 10mM HEPES, pH 7.3) in preparation for flow cytometry. Samples were stained with DAPI for live/dead cell exclusion and analyzed using the yellow-green, blue, and UV lasers (561 nm, 488 nm, and 355 nm for PE, FITC-A and DAPI detection respectively) on the LSR II flow cytometer (BD Biosciences, San Jose, CA) at the University of Washington Pathology Flow Cytometry Core Facility. The population of microglia that internalized apoptotic bodies was determined by calculating the percent of events detected to be both PE and FITC-A positive relative to the number of FITC-A only positive cells in each sample. Compensation was performed when appropriate during sample collection utilizing PE and FITC-A positive and negative populations, or by the data analysis software FlowJo® (Ashland, OR) after data was fully acquired.
Statistical Analysis
All data from this study, except the flow cytometry, are given as mean±SEM. Statistical evaluations were carried out using PRISM software (GraphPad, San Diego, CA). Comparisons were made by analysis of variance (ANOVA) or unpaired t-test, as stated in the Figure Legends for each specific experiment.
Results
Isolation and In vitro culture of microglia from adult mouse brain
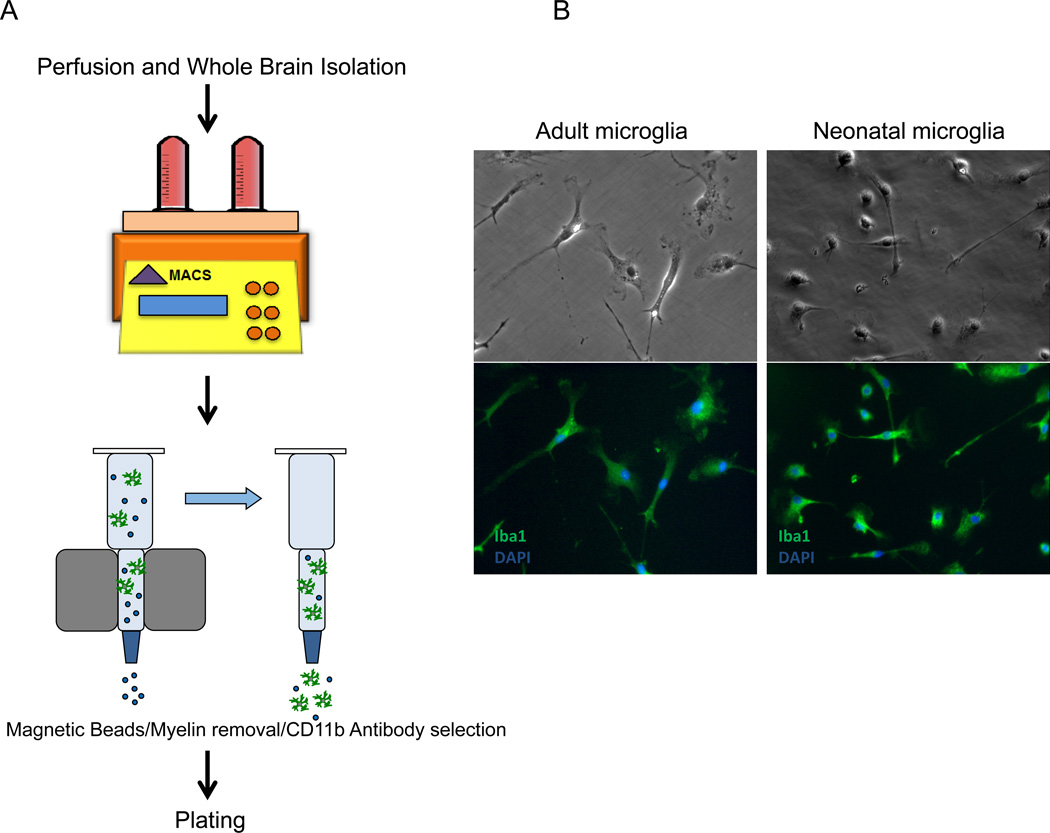
We sought to develop a protocol to culture microglia from adult mouse brain as they are functionally different from neonatal microglia cultures that are commonly used. Protocols for microglia isolation using Percoll gradients to separate monocytes from myelin have been previously reported (Lee and Tansey, 2013; Ponomarev et al., 2005). However, we observed that the Percoll gradient approach to separation results in a mixed population of microglia, astrocytes, and oligodendrocytes with very low yield of microglia. We therefore developed a modified method based on MACS® Technology (Miltenyi Biotec GmbH) to achieve a higher purity and recovery of adult microglia. As demonstrated in Figure 1A, adult mice that are 12–22 wks. old were perfused, whole brain was isolated and dissociated using the optimized program on GentleMACS™ Dissociator and MACS Neural Dissociation Kit. Dissociated tissues were then processed through myelin removal and CD11b selection steps, from which the CD11b positive population was collected and plated. On day 3 post isolation, unattached debris was washed off and recovered cells were replaced with fresh culture medium. These ex vivo isolated cells looked phase bright and round with no significant microglial morphology. Starting from day 4, cells extended processes and began to display the unique microglial ramified shape. By 7 days post isolation, flow cytometry analysis demonstrated that 99% of cultured cells were microglia (CD45intermediate, CD11bpositive, 1A8negative, F4/80positive, data not shown). Interestingly, compared to neonatal microglia, these adult cells looked larger with a relatively bigger soma and longer processes (Figure 1B). Immunocytochemical staining demonstrated that 97.5% of cultured CD11b+ cells were Iba-1 positive. Cultured adult microglia were maintained for up to 8 weeks without any significant morphological change.
Figure 1. Adult microglia isolated by magnetic bead separation survive in culture.
(A): Diagram of microglia isolation from adult mice brain. Adult mice (12–22 wks., n=3 mice per genotype) were intracardially perfused, whole brain was isolated and dissociated by GentleMACS® tissue dissociator (Miltenyi). The dissociated tissues were incubated with Miltenyi magnetic myelin beads to remove myelin, followed by positive selection with CD11b microbeads. The CD11b positive population of cells was collected and plated for culture. (B): Immunofluorescence demonstrated that >98% of cultured CD11b+ cells from adult brain cultured with M-CSF expressed the microglia marker protein Iba-1.
rAAV2 is an effective approach to gene delivery for cultured neonatal and adult microglia
Microglia are strongly refractory to most means of gene transfer, and we observed that adult microglia were not amenable to infection by lentiviral vectors. We therefore evaluated the feasibility of utilizing rAAV as a transfer vector to modulate gene expression in cultured microglia. For a given application and target cell, many possible pseudotyped vectors are available. rAAV2 and rAAV5 vectors are the only serotypes previously reported to drive gene expression in microglia (Cucchiarini et al., 2003) and little information on toxicity or inflammatory activation was included in this report. Since most rAAV generated to date have been derived from serotype 2, and rAAV2 was reported with the longest history of usage and the least immunogenicity (Chamberlin et al., 1998; Kaplitt et al., 1994; Riviere et al., 2006), we first utilized rAAV2 as our primary gene transfer vehicle.
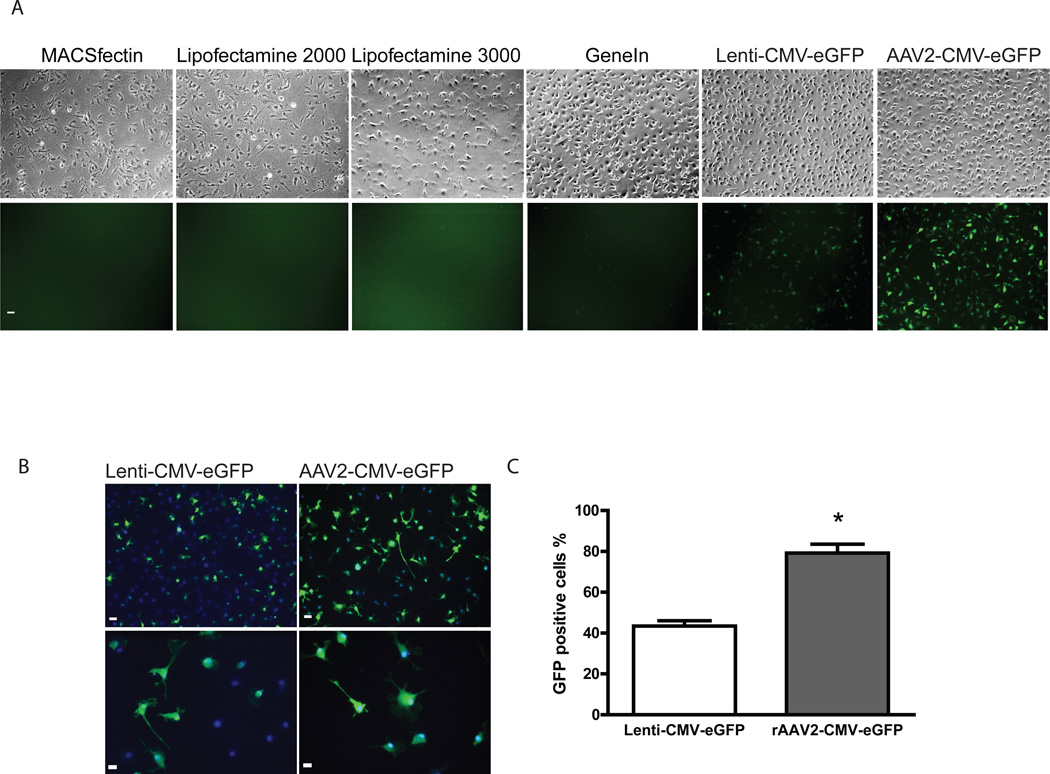
We performed a direct comparison for gene transduction efficiency in cultured microglia utilizing different transfection reagents as well as viral vectors. We picked four transfection reagents: MACSfectin, Lipofectamine 2000, Lipofectamine 3000, and GeneIn. Lenti-CMV-eGFP was also included as a comparison. Wild type neonatal microglia were harvested from neuronal-glial co-culture 7 days after dissection and re-plated onto coated 4-well plates at density of 2.5 × 105 /well. CMV-eGFP plasmids were mixed with transfection reagent at different lipid/cationic polymer to DNA ratio as described in Materials and Methods. As shown in Figure 2A, we found that all four transfection reagents had very poor transduction efficiencies, consistent with what we have observed in the past. Cells incubated with MACSfectin and lipofectamine 2000 started to look unhealthy as early as 24hr post transfection. At 48hr, over 80% of cell death was observed in MACSfectin and Lipofectamine 2000 infected cells. Lipofectamine 3000 caused less toxicity, however approximately 20~30% of cell death could be seen at all lipid/cationic polymer to DNA ratios tested. Out of all four transfection methods, GeneIn showed the least microglia toxicity, however the degree of GFP expression was low (Figure 2A). Compared to transfection reagents, cells incubated with viral vectors had better transduction efficiency. Cells were incubated with serial dilutions of Lenti-CMV-eGFP or rAAV2-CMV-eGFP vectors and the wells with maximal infectivity/minimum cell death were selected for comparison. With the similar cell survival rate, we observed a much greater percentage of GFP positive cells with rAAV2 infection (Figure 2A). We then stained the cells with anti-GFP antibody and were able to calculate the percentage of GFP positive cells (Figure 2B and 2C). We observed about 80% of gene transduction efficiency for rAAV2-CMV-eGFP while only 43% for Lenti-CMV-eGFP.
Figure 2. rAAV is the most effective and least toxic means of gene transduction in cultured microglia.
(A): Phase images and GFP expression of the neonatal microglia incubated with different transfection reagents and viral vectors. Neonatal microglia were plated and incubated with transfection reagents or viral vectors as described in Materials and Methods. Phase and fluorescent images were obtained 48 hr post transfection/viral infection. Images of cells with best survival and least toxicity from each method are shown. All images were taken under 10× magnification. (B): Comparison of transduction efficiency in cells incubated with Lenti-CMV-eGFP or rAAV2-CMV-eGFP vectors. 72 hr post infection, cells were fixed and stained with anti-GFP antibodies. Images under either 10× or 32× magnification were taken immediately after labeling. Scale bar represents 10 µm in the field. (C) Infectivity of cells incubated with Lentivirus or rAAV was calculated as percentage of eGFP positive cells (green) versus Hoechst positive cells (blue). 80% of rAAV2-CMV-eGFP treated cells and 43% of Lenti-CMV-eGFP treated cells were eGFP positive (n=2 separate culture experiments, and 5 images were taken from each treatment in each culture experiment, p<0.001 by unpaired t-test).
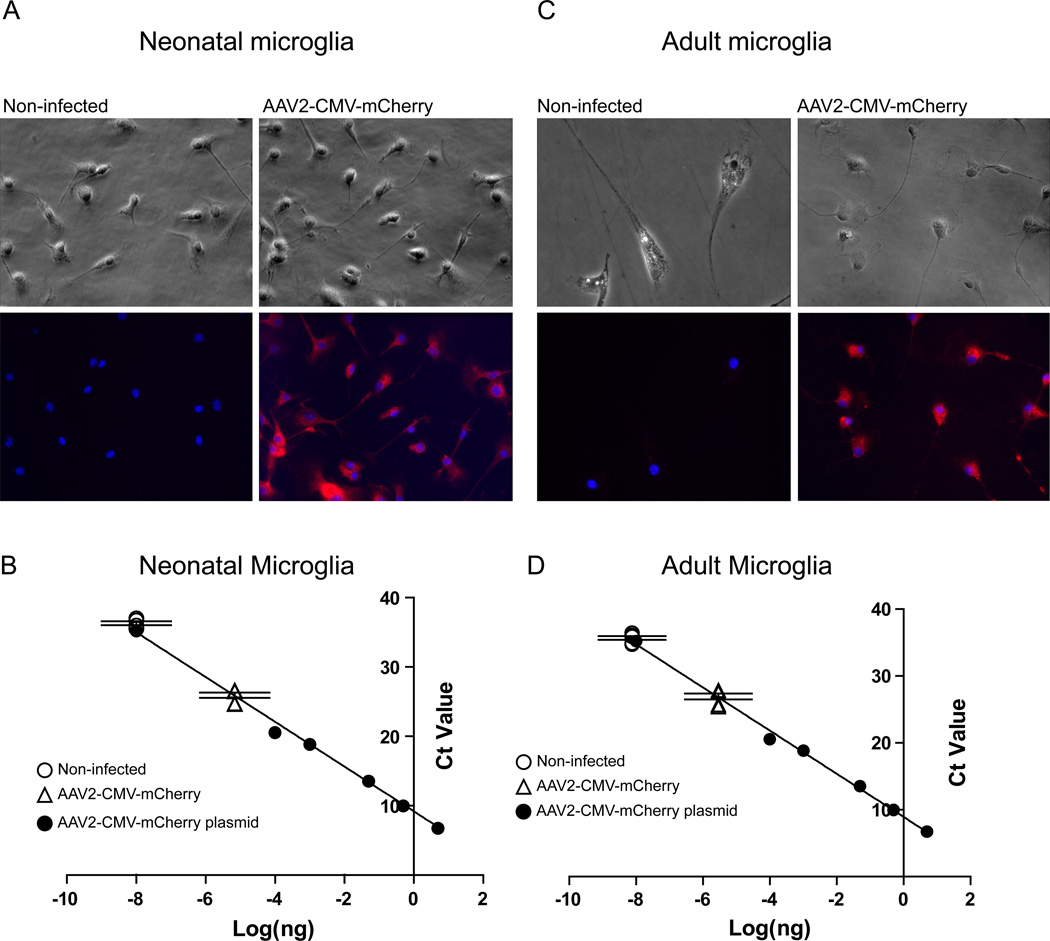
We next tested the feasibility of rAAV on microglia isolated from adult mice brain. Adult microglia were used for viral infection starting from day 7 after isolation. rAAV2-CMV-mCherry vectors were diluted and added into the medium at 1.75 × 103 vg/cell. 7 days after vector addition cultured microglia were fixed and stained with anti-red fluorescence protein (RFP) antibody and subject to microscopy for RFP expression (Figure 3A). We observed >98% of neonatal microglia expressed RFP with no obvious morphological change compared to non-infected cells. The amount of RFP expression was plotted onto a standard curve generated by titrating serial amount of rAAV2-CMV-mCherry DNA into the total purified cellular RNA (Figure 3B). The average Ct value for RFP detected from non-infected cells was 36.3, while the one from rAAV2-CMV-mCherry infected cells was 25.84 (Figure 3B). Similarly, we observed >99% of adult microglia successfully expressed RFP 7 days post infection with no obvious morphological change or reduction in cell number (Figure 3C). Average Ct value for RFP mRNA was 36.04 for non-infected cells and 27.59 for rAAV2-CMV-mCherry infected cells (Figure 3D). Taken together, these results demonstrated that both neonatal and adult microglia can be transduced to express exogenous genes without morphological evidence for inflammatory activation or toxicity using rAAV2.
Figure 3. rAAV2 is an effective vector for gene delivery in cultured neonatal and adult microglia.
(A): Wild type neonatal microglia were harvested and re-plated as stated in materials and methods. rAAV2-CMV-mCherry vectors were added into the medium at 1.75 × 103 vg/cell. Cells were fixed and stained with anti-RFP antibody and subject to microscopy for RFP expression. (B): Standard curve to titrate the amount of CMV-mCherry was generated by titrating serial dilution of DNA into total cellular cDNAs and plotted as Ct value against log[mCherry DNA(ng)]. Equation generated by linear fitting is y=−3.226x+8.9322, R2=0.9959. On top of the standard curve, absolute Ct values of mCherry expression in non-infected cells and rAAV2-CMV-mCherry infected cells were plotted. Compared to non-infected cells, infection with rAAV2-CMV-mCherry induced RFP expression by 682 fold, measured by qRT-PCR. (C): Adult microglia were treated with rAAV2-CMV-mCherry with the same titer and expressed RFP 7 days post infection with no obvious change in morphology. (D): mRNA from adult microglia was analyzed for mCherry expression using qRT-PCR as in (B), transduction of RFP mRNA was 502 fold higher in infected cells than non-infected cells. (n=3 from separate culture experiments and q-PCR assays).
rAAV infection does not alter microglial behavior
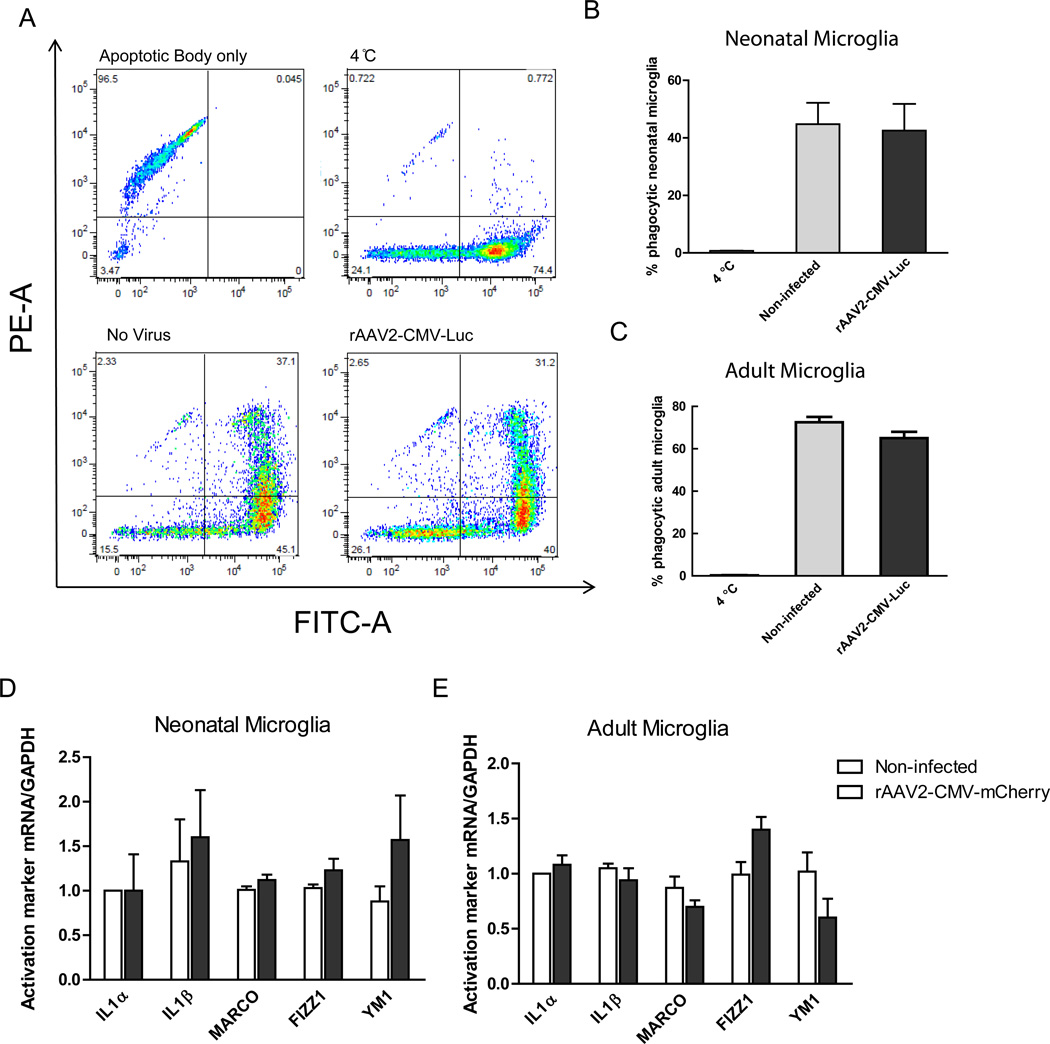
As the primary effectors of innate immunity, microglia actively internalize material from damaged or dying neurons, present antigens at their surface, and secrete a variety of pro-inflammatory mediators that influence the survival of surrounding neurons (Zhang et al., 2010). To determine if rAAV infection alone induced inflammatory activation of microglia, we first examined whether rAAV infection would alter microglia phagocytic activity by assaying for the internalization of apoptotic cells. To generate apoptotic bodies, BV2 cells were labeled with PKH26 Red Fluorescent cell linker kit and apoptosis induced by UV light exposure. Neonatal or adult microglia were infected with rAAV2 containing firefly Luciferase sequence (rAAV2-CMV-Luc) 7 days prior to the phagocytosis assay. rAAV2-CMV-Luc was employed to avoid the impact of RFP expression on the fluorescence based phagocytosis assay. Adult and neonatal microglia cultures were labeled with PKH2 Green Fluorescent Cell Linker kit approximately 24 hours prior to the phagocytosis assay. On the day of experiment, PKH-Red labeled apoptotic bodies were added onto PKH2 labeled microglia at an approximate ratio of ~1:1 and incubated for 6 hours. Un-phagocytosed apoptotic bodies were removed by serial washes, and then microglia were trypsinized and analyzed by flow cytometry. PKH26 red fluorescence linker has a maximum excitation at 551 nm and emission at 567 nm which overlaps with PE channel, while PKH2 linker has maximum excitation at 490 nm and emission at 504 nm which overlaps with FITC channel. All experiments included a control culture maintained at 4 °C. Apoptotic bodies appeared as PE positive (Figure 4A, upper left panel), microglia unexposed to apoptotic bodies were exclusively FITC positive (Figure 4A, upper right panel). Microglia that ingested apoptotic bodies became double positive (Figure 4A, lower two panels). For neonatal microglia, we observed 45% of non-infected microglia population were double positive, while 42.6% of rAAV infected cells were double positive (Figure 4B). For adult microglia, we observed 72.5% of non-infected microglia population were double positive, while 65% of rAAV infected cells were double positive (Figure 4C). As a result, compared to non-infected cells, treatment with rAAV2-CMV-Luc did not significantly alter the percentage of microglia undergoing phagocytosis in both neonatal and adult microglia, suggesting neither the rAAV capsid nor the transgene inadvertently altered phagocytosis events.
Figure 4. rAAV2-mediated viral infection does not influence microglia behavior.
(A): Neonatal microglia phagocytosis of apoptotic bodies analyzed by flow cytometry. The population of apoptotic bodies is shown as PE positive. Microglia that did not phagocytize any apoptotic bodies (4 °C control) are shown as FITC positive only. Microglia that ingested apoptotic bodies are shown as double positive in the upper right quadrant. (B): The percent of neonatal microglia that internalized labeled apoptotic cells was not altered by rAAV-2 infection. (n=3 separate experiments, between non-infected vs. infected group, p>0.05 by one-way ANOVA). (C): For adult microglia, there was no significant impact of rAAV-2 infection on the percent of microglia that internalized labeled apoptotic bodies. (n=3 separate experiments, between non-infected vs. infected group, p>0.05 by one-way ANOVA) (D): M1 and M2 activation markers of microglia in response to rAAV2 treatment were examined by RT-PCR in wild-type neonatal microglia. (E): M1 and M2 activation markers of microglia in response to rAAV2 treatment were examined by RT-PCR in wild-type adult microglia as in (D). (n=3 separate experiments, between non-infected vs. infected group, p>0.05 by two-way ANOVA).
Microglia, like macrophages, are capable of a variety of inflammatory responses to altered environmental stimuli (Rickard and Young, 2009). The classical (M1) activation state of myeloid cells has been linked with promoting inflammation (Weber et al., 2007), whereas the alternative (M2) phenotype is anti-inflammatory and promotes tissue repair (Kigerl et al., 2009). Although host immune response towards rAAV infection are reported minimal in vivo, microglia are innate immune sensors and could be activated by sensing of rAAV particles in vitro or infection with foreign DNA. Therefore, we evaluated M1 and M2 activation markers of microglia in response to rAAV2 infection. Neonatal and adult microglia cultured from wild type mice were harvested and treated 1.75 × 103 vg/cell for 7 days and mRNAs associated with classical or alternative activation were evaluated by RT-PCR. We examined three previously reported genes that are associated with a type I inflammatory response: the cytokines IL-1α, IL-1β and a scavenger receptor upregulated during classical activation, MARCO (Mukhopadhyay et al., 2006).We observed that rAAV infection did not alter expression of these M1 response genes in both neonatal and adult microglia (Figure 4D and Figure 4E). We also observed no induction of FIZZ1 and YM1 expression (Figure 4D and Figure 4E), two frequently used markers of alternative activation (Gordon, 2003; Jayadev et al., 2011). These data suggest that infection with rAAV2 virus does not cause inflammatory activation in cultured neonatal or adult microglia.
Cre recombinase expressed by infection with rAAV2-CMV-Cre successfully excises a floxed gene in neonatal and adult microglia
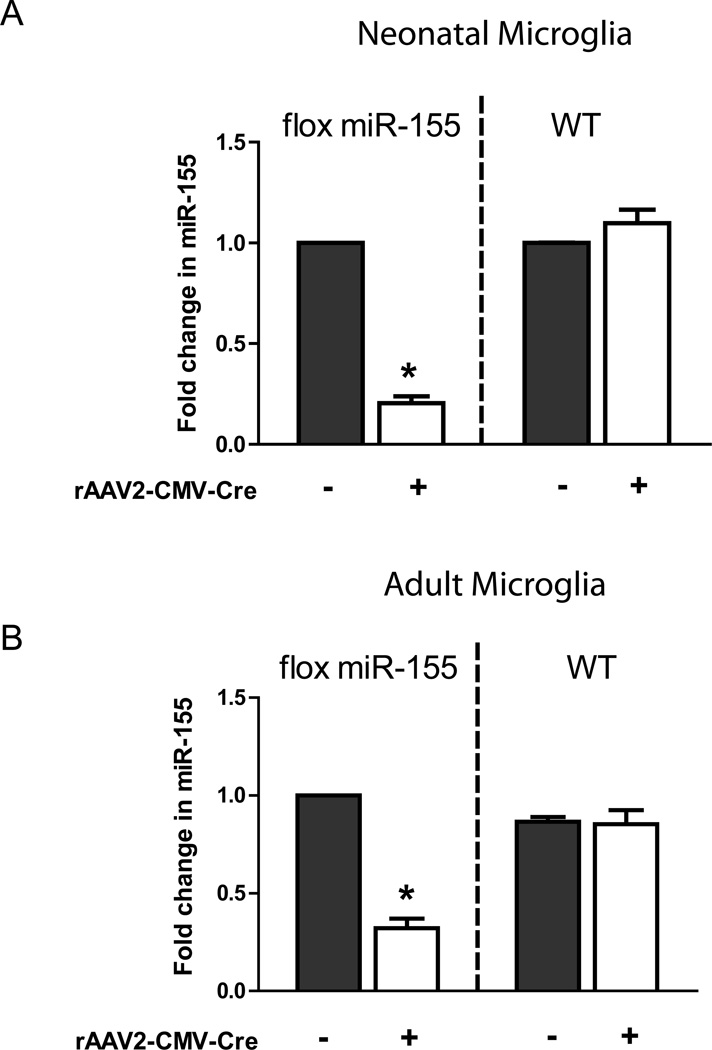
One purpose to develop in vitro culture of microglia and rAAV-mediated gene delivery is to enable the study of molecular mechanisms that regulate microglia behaviors, e.g. microglial activation in response to neuroinflammation. Previously, we have reported that p53 plays an important role in regulation of microglial inflammatory responses by promoting induction of miR-155, a pro-inflammatory microRNA (Su et al., 2014). As a continuation of the study, we generated mice with loxP sites flanking exon 2 of Mir155 to further explore the role of miR-155 in regulation of CNS inflammation (Hu et al., 2014; Lopez-Ramirez et al., 2014; Su et al., 2014). Neonatal and adult microglia were isolated from floxed miR-155 homozygotic mice or wild type mice and infected with rAAV2-CMV-Cre for 7 days. Total RNA was extracted and assayed for miR-155 expression by quantitative RT-PCR. As shown in Figure 5, miR-155 was significantly reduced in flox miR-155 cell infected with rAAV2-CMV-Cre 7 days post infection, to 20% in neonatal (Figure 5A) and 32% in adult microglia (Figure 5B). These results demonstrate that Cre recombinase delivered by rAAV2 is successfully expressed and functions to excise a loxP flanked site.
Figure 5. Infection with rAAV2-CMV-Cre successfully excises a floxed gene in cultured microglia.
rAAV2-CMV-Cre plasmid was constructed and packaged with pDG2 vectors. Neonatal and adult microglia were isolated from floxed miR-155 homozygotic mice and wild type mice (as control group), and treated with rAAV2-CMV-Cre for 7 days. Total RNAs were assayed for miR-155 expression by quantitative RT-PCR. (A): miR-155 was successfully excised in floxed miR-155 neonatal microglia infected with rAAV2-CMV-Cre 7 days post infection. (B): miR-155 was successfully excised in flox miR-155 adult microglia infected with rAAV2-CMV-Cre 7 days post infection. (n=3 from separate isolation of microglia and q-PCR assays, *p<0.001 by two-way ANOVA).
Additional rAAV serotypes can be employed to modulate gene expression in cultured microglia
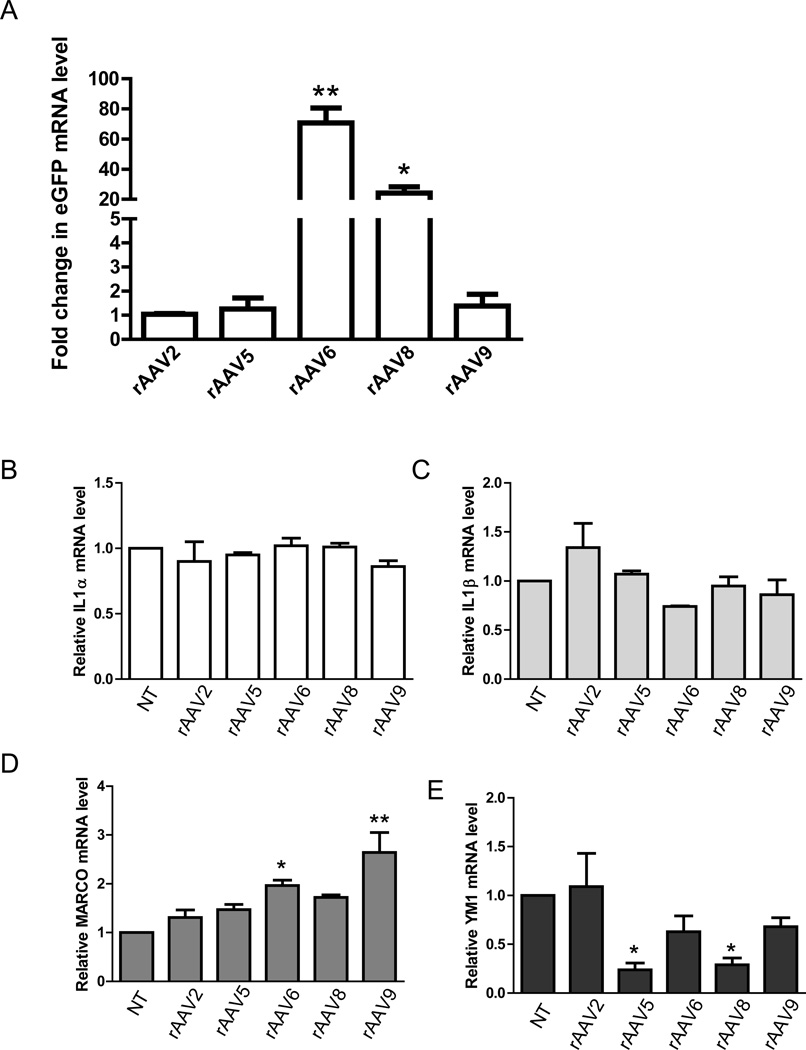
While the efficiency of rAAV-mediated gene transfer varies widely between different cell types, different serotypes of vector have been used successfully to transduce several primary cell types and tissues that have been refractory to infection by alternate rAAV viral vectors. To determine if microglia demonstrate serotype selectivity, we compared several established serotypes of rAAV in microglial culture, including rAAV2, rAAV5, rAAV6, rAAV8 and rAAV9. Cultured neonatal microglia were infected with 1.75 × 103 vg/cell in a volume of 500 µl using 4-well plates with the rAAV-CMV-eGFP from each serotype for 7 days and the level of eGFP expression was assessed by RT-PCR. Surprisingly, rAAV6-CMV-eGFP gave the highest gene expression (80 fold increase) compared to rAAV2-CMV-eGFP (Figure 6A). rAAV8 also resulted in a 25 fold increase in eGFP expression compared to rAAV2-CMV-eGFP. No difference was observed for gene expression driven by rAAV5 and rAAV9. We then asked whether microglial activation is altered among treatment of different serotypes. We measured expression of microglial M1 and M2 activation markers (IL-1α, IL-1β, MARCO, and YM1) in neonatal microglia in response to treatment of rAAV2, rAAV5, rAAV6, rAAV8 and rAAV9. As shown in Figure 6B and Figure 6C, we observed no difference among all serotypes for IL1α or IL1β in cells treated with any of five serotypes. However, expression of MARCO was elevated in cells treated with rAAV6 and rAAV9 (Figure 6D), while YM1 was down-regulated in cells treated with rAAV5 and rAAV8 (Figure 6F). Taken together, these experiments suggest that while all serotypes are effective at transducing gene expression in cultured microglia, only infection with the rAAV2 serotype enables gene transduction without altering the expression of activation marker genes.
Figure 6. Comparison of rAAV serotype 2, 5, 6, 8, and 9 for gene transduction efficiency and their impact on microglial activation.
(A) Neonatal microglia were harvested and plated at the same density of 2.5×105/well and infected with rAAV2, 5, 6, 8, and 9 containing GFP driven by CMV promoter at the same titer of 17500 vg/cell. 7 days later, mRNAs were extracted and level of GFP expression were evaluated by qRT-PCR, and the Ct values were normalized to the expression of endogenous housekeeping gene beta-Actin. (n=3 from separate isolation of microglia and qPCR runs, *p<0.05, **p<0.01 by one-way ANOVA). (B)–(E): M1 and M2 activation markers of microglia in response to various rAAV serotype treatment were examined by RT-PCR in wild-type neonatal microglia. Cells were infected with rAAV(2, 5, 6, 8, and 9)-CMV-GFP for 7 days, RNAs were extracted and expression of IL-1α, IL-1β, MARCO, and YM1 were evaluated. (n=2 from separate isolation of microglia and qPCR runs, *p<0.05, **p<0.01 by one-way ANOVA).
Discussion
Cultured adult microglia are a powerful tool for understanding microglia biology
In this study, we developed a novel method to isolate primary microglia from adult mouse brain. As adult microglia express high levels of CD11b epitope (Floden and Combs, 2006), the combination of GentleMACS tissue dissociation and utility of CD11b magnetic beads resulted in the highly pure population of microglia. Compared to previously published methods, this procedure is simple, quick and reproducible, with a much higher cell survival rate than previously reported (Ponomarev et al., 2005). We believe that the increased yield of microglia obtained with our method is the result of optimized conditions that maintain viability through the gentle dissociation/selection procedure. Functional validation of the microglial cells obtained by this protocol is crucial to demonstrate the feasibility of a new isolation technique. We probed mRNAs extracted from these cells for microglial/macrophage markers: Iba-1, MRC1 (macrophage mannose receptor1), and CSF1 (Colony-stimulating factor 1) and all were expressed (data not shown). We also tested the microglia obtained by this method using different functional assays. Cultured adult microglia show typical morphological changes associated with IFNγ stimulation and increased mRNA for genes associated with a pro-inflammatory response. Thus, the novel approach for obtaining adult microglia reported here could provide the field with a means to perform reductionist experimentation on functionally mature microglia that reflect the environmental and epigenetic changes associated with the maturation process within the CNS environment.
Gene transduction mediated by rAAV is an effective means of modulating gene expression in microglia
Cross-packaging vectors that use the same vector genome in different capsids have allowed direct comparisons of different serotypes. rAAV2-based recombinant genomes have been packaged into dozens of different capsid types, resulting in a wide array of “pseudotyped vectors” that constitute a valuable resource for the development of gene transduction vehicles (Gao et al., 2011b). Previous studies have shown that the tropism can vary among different tissues in vivo (Aschauer et al., 2013; Rabinowitz et al., 2002). The utility of rAAV is especially beneficial in CNS systems as the viral capsid is primarily targeted by the T-cell and B-cell host-mediated immune responses, which are minimized in the CNS environment (de Backer et al., 2011). However, in the CNS, rAAVs have preferential tropism towards neurons (McCown et al., 1996), and the cause of this specificity is not well understood. The susceptibility of microglia to infection by rAAV2 was originally suggested by Bertlett et al using wild-type virus (Bartlett et al., 1998). The presence of the rAAV was detected in microglia following stereotaxic injection of Cy3-labelled virions into rat brain, although preferential binding and uptake by neurons occurred to a much greater extent and at much earlier times, and they were unable to detect rAAV gene expression in the microglia. Later work done by Aschuer et al reported that rAAV1-9 all have the general ability to transduce all major cell type in the brain (neurons, microglia, astrocytes, and oligodendrocytes), although the expression level of a reporter gene varies significantly for specific cell type/serotype combinations (Aschauer et al., 2013). In addition, Arnett et al compared the closely related rAAV1 and 6 in the CNS and found that rAAV1 preferentially targets neurons, while rAAV6 has preference for non-neuronal cell types (Arnett et al., 2013)
Here we demonstrate that rAAV-mediated gene transduction is highly effective in cultured microglia and the degree of gene expression mediated by different serotypes varies. We confirmed the permissivity of primary microglia to rAAV2 transduction and were able to detect high levels of transgene expression in these cells, using two different reporter genes (mCherry and Luciferase) placed under the control of the strong heterologous CMV-IE promoter. We also compared the transduction properties of rAAV 2, 5, 6, 8, and 9 on microglia cultures and discovered that gene expression driven by rAAV6 was the highest. To some degree, this result was unexpected, since rAAV6 was mostly used in airway (alveolar) cell gene transduction (Halbert et al., 2001) or in striated muscles (Gao et al., 2011a; Gregorevic et al., 2004; Salva et al., 2007). rAAV6 was recently compared with other rAAV serotypes for their tropism towards different CNS cell types in mouse brain (Arnett et al., 2013; Aschauer et al., 2013). In this report, minimal infection of microglia was noted compared to neurons. However, confocal microscopy revealed that hippocampal microglia have a preference for rAAV6 and rAAV8, while microglia in striatum and cortex exhibit different preferences (Aschauer et al., 2013). In our study, rAAV6 and 8 are the most efficient for gene transduction in cultured microglia, but activate M1 or suppress M2 gene expression, respectively. Therefore, rAAV2 may be the preferred vector for mechanistic studies, while rAAV 6 or 8 may be better for gene expression of a functional target (e.g. Cre recombinase), or therapeutically where activation of a microglial phenotype along with increasing specific gene expression is intended or accepted for the study.
Since we employed rAAV2 and rAAV6 that varied only in capsid sequence, but rAAV6 produced much higher gene expression, it is possible that a rAAV6-specific cellular surface receptor is present on microglia. Despite the high degree of sequence homology between rAAV serotype 1–9 (99.2%), comparisons between the rAAV capsid structures suggests that variation in surface topology determines the cell surface attachment and receptor usage, intracellular trafficking pathways, and antigenicity between closely related serotypes (Schultz and Chamberlain, 2008; Wu et al., 2006; Xie et al., 2002). Cell transduction phenotypes for different rAAV serotypes have been shown to be due to the ability of their capsids to use different cell surface glycans for binding. rAAV infection involves a multistep process beginning with virus binding to the cell surface, followed by viral uptake, intracellular trafficking, nuclear localization, uncoating, and second-strand DNA synthesis (Bartlett et al., 2000; Schultz and Chamberlain, 2008; Summerford et al., 1999; Summerford and Samulski, 1998). rAAV2 initiates infection by binding to its primary receptor, heparan sulfate proteoglycans (HSPG) (Summerford and Samulski, 1998). In addition, the efficiency of forming the complementary strand can also significantly impact vector transduction (Ferrari et al., 1996; Fisher et al., 1996). Studies also revealed that internalization is enhanced by interactions with one or more of at least six known co-receptors including αVβ5 integrins (Summerford et al., 1999), fibroblast growth factor receptor 1 (Qing et al., 1999), hepatocyte growth factor receptor (Kashiwakura et al., 2005), αVβ1 integrin (Asokan et al., 2006), and laminin receptor (Akache et al., 2006). Defect in any or all of these stages of viral infection can influence the resulting transduction profiles of recombinant rAAV in different cell types. Although not as thoroughly studied as rAAV2, rAAV6 is known to utilize both heparan sulfate (HS) and sialylated proteoglycans as a unique dual receptor-mediated binding for cell recognition (Ng et al., 2010; Zhang et al., 2013). Any factor, even a single amino acid substitution, can influence viral titer, receptor binding, and tissue tropism in different rAAV serotypes (Arnett et al., 2013; Wu et al., 2006). Therefore, the detailed mechanism for high efficiency of gene transduction mediated by rAAV6 requires further investigation.
Conclusions
Our results provide the first evidence that a rAAV vector can be used for gene transfer in adult microglia cultured in vitro. Moreover, the results showed that rAAV vectors can successfully induce expression of functional Cre recombinase in cultured microglia. Given the increased interest in understanding microglial function in CNS health and disease, particularly in neurodegenerative diseases where age is a critical factor, the combination of adult microglia culture and utility of rAAVs could be of immense utility for researchers studying microglial cell biology.
Acknowledgements
The authors gratefully acknowledge Drs. Richard S. Morrison and Suman Jayadev for helpful discussions during the performance of these experiments, and Eric Finn and Dr. James Allen for training and assistance with rAAV vector transfection and purification. This work was supported by grants from the National Institutes of Health (R01NS073848 and R03NS70141) to G.A.G. with facilities support from P30-HD02274 to the UW Center on Human Development and Disability and (P30-DK56465) to Core Center of Excellence in Hematology at Fred Hutchinson Cancer Center for production of lentivirus.
Abbreviations used
- ANOVA
analysis of variance
- CMV
cytomegalovirus
- eGFP
enahanced green fluorescent protein
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- IL
interleukin
- M-CSF
macrophage colony-stimulating factor
- rAAV
recombinant Adeno-associated virus
- RFP
red fluorescent protein
- RT-PCR
reverse transcribed polymerase chain reaction
- RT
room temperature
- vg
vector genome
References
- Akache B, Grimm D, Pandey K, Yant SR, Xu H, Kay MA. The 37/67-Kilodalton Laminin Receptor Is a Receptor for Adeno-Associated Virus Serotypes 8, 2, 3, and 9. J. Virol. 2006;80:9831–9836. doi: 10.1128/JVI.00878-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JM, Halbert CL, Miller AD. Improved Adeno-Associated Virus Vector Production with Transfection of a Single Helper Adenovirus Gene, E4orf6. Mol. Ther. 2000;1:88–95. doi: 10.1006/mthe.1999.0010. [DOI] [PubMed] [Google Scholar]
- Aloisi F. Immune function of microglia. Glia. 2001;36:165–179. doi: 10.1002/glia.1106. [DOI] [PubMed] [Google Scholar]
- Arbetman AE, Lochrie M, Zhou S, Wellman J, Scallan C, Doroudchi MM, Randlev B, Patarroyo-White S, Liu T, Smith P, et al. Novel Caprine Adeno-Associated Virus (AAV) Capsid (AAV-Go.1) Is Closely Related to the Primate AAV-5 and Has Unique Tropism and Neutralization Properties. J. Virol. 2005;79:15238–15245. doi: 10.1128/JVI.79.24.15238-15245.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett ALH, Beutler LR, Quintana A, Allen J, Finn E, Palmiter RD, Chamberlain JS. Heparin-binding correlates with increased efficiency of AAV1- and AAV6-mediated transduction of striated muscle, but negatively impacts CNS transduction. Gene Ther. 2013;20:497–503. doi: 10.1038/gt.2012.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschauer DF, Kreuz S, Rumpel S. Analysis of Transduction Efficiency, Tropism and Axonal Transport of AAV Serotypes 1, 2, 5, 6, 8 and 9 in the Mouse Brain. PLoS ONE. 2013;8:e76310. doi: 10.1371/journal.pone.0076310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asokan A, Hamra JB, Govindasamy L, Agbandje-McKenna M, Samulski RJ. Adeno-Associated Virus Type 2 Contains an Integrin α5β1 Binding Domain Essential for Viral Cell Entry. J. Virol. 2006;80:8961–8969. doi: 10.1128/JVI.00843-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayuso E, Mingozzi F, Montane J, Leon X, Anguela XM, Haurigot V, Edmonson SA, Africa L, Zhou S, High KA, et al. High AAV vector purity results in serotype- and tissue-independent enhancement of transduction efficiency. Gene Ther. 2010;17:503–510. doi: 10.1038/gt.2009.157. [DOI] [PubMed] [Google Scholar]
- Baig AM. Designer’s microglia with novel delivery system in neurodegenerative diseases. Med. Hypotheses. 2014;83:510–512. doi: 10.1016/j.mehy.2014.08.003. [DOI] [PubMed] [Google Scholar]
- Balcaitis S, Weinstein JR, Li S, Chamberlain JS, Möller T. Lentiviral transduction of microglial cells. Glia. 2005;50:48–55. doi: 10.1002/glia.20146. [DOI] [PubMed] [Google Scholar]
- Banker G, Goslin K. Culturing nerve cells. Cambridge, Mass.: MIT Press; 1998. [Google Scholar]
- Bartlett JS, Samulski RJ, McCown TJ. Selective and Rapid Uptake of Adeno-Associated Virus Type 2 in Brain. Hum. Gene Ther. 1998;9:1181–1186. doi: 10.1089/hum.1998.9.8-1181. [DOI] [PubMed] [Google Scholar]
- Bartlett JS, Wilcher R, Samulski RJ. Infectious Entry Pathway of Adeno-Associated Virus and Adeno-Associated Virus Vectors. J. Virol. 2000;74:2777–2785. doi: 10.1128/jvi.74.6.2777-2785.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benninger Y, Marino S, Hardegger R, Weissmann C, Aguzzi A, Brandner S. Differentiation and Histological Analysis of Embryonic Stem Cell-Derived Neural Transplants in Mice. Brain Pathol. 2000;10:330–341. doi: 10.1111/j.1750-3639.2000.tb00265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson MJ, Reilly CR, Sutcliffe JG, Lo D. Mature microglia resemble immature antigen-presenting cells. Glia. 1998;22:72–85. doi: 10.1002/(sici)1098-1136(199801)22:1<72::aid-glia7>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Chamberlin NL, Du B, de Lacalle S, Saper CB. Recombinant adeno-associated virus vector: use for transgene expression and anterograde tract tracing in the CNS. Brain Res. 1998;793:169–175. doi: 10.1016/s0006-8993(98)00169-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cucchiarini M, Ren XL, Perides G, Terwilliger EF. Selective gene expression in brain microglia mediated via adeno-associated virus type 2 and type 5 vectors. Gene Ther. 2003;10:657–667. doi: 10.1038/sj.gt.3301925. [DOI] [PubMed] [Google Scholar]
- de Backer MA, Garner K, Luijendijk MM, Adan RH. Recombinant Adeno-Associated Viral Vectors. In: Merighi A, editor. Neuropeptides. Humana Press; 2011. pp. 357–376. [DOI] [PubMed] [Google Scholar]
- Ellis B, Hirsch M, Barker J, Connelly J, Steininger R, Porteus M. A survey of ex vivo/in vitro transduction efficiency of mammalian primary cells and cell lines with Nine natural adeno-associated virus (AAV1-9) and one engineered adeno-associated virus serotype. Virology. 2013;10:74. doi: 10.1186/1743-422X-10-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari FK, Samulski T, Shenk T, Samulski RJ. Second-strand synthesis is a rate-limiting step for efficient transduction by recombinant adeno-associated virus vectors. J. Virol. 1996;70:3227–3234. doi: 10.1128/jvi.70.5.3227-3234.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer H-G, Reichmann G. Brain Dendritic Cells and Macrophages/Microglia in Central Nervous System Inflammation. J. Immunol. 2001;166:2717–2726. doi: 10.4049/jimmunol.166.4.2717. [DOI] [PubMed] [Google Scholar]
- Fisher KJ, Gao GP, Weitzman MD, DeMatteo R, Burda JF, Wilson JM. Transduction with recombinant adeno-associated virus for gene therapy is limited by leading-strand synthesis. J. Virol. 1996;70:520–532. doi: 10.1128/jvi.70.1.520-532.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher KJ, Jooss K, Alston J, Yang Y, Haecker SE, High K, Pathak R, Raper SE, Wilson JM. Recombinant adeno-associated virus for muscle directed gene therapy. Nat Med. 1997;3:306–312. doi: 10.1038/nm0397-306. [DOI] [PubMed] [Google Scholar]
- Floden AM, Combs CK. β-Amyloid Stimulates Murine Postnatal and Adult Microglia Cultures in a Unique Manner. J. Neurosci. 2006;26:4644–4648. doi: 10.1523/JNEUROSCI.4822-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flotte TR, Afione SA, Conrad C, McGrath SA, Solow R, Oka H, Zeitlin PL, Guggino WB, Carter BJ. Stable in vivo expression of the cystic fibrosis transmembrane conductance regulator with an adeno-associated virus vector. Proc.Natl. Acad. Sci. U. S. A. 1993;90:10613–10617. doi: 10.1073/pnas.90.22.10613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G, Bish LT, Sleeper MM, Mu X, Sun L, Lou Y, Duan J, Hu C, Wang L, Sweeney HL. Transendocardial Delivery of AAV6 Results in Highly Efficient and Global Cardiac Gene Transfer in Rhesus Macaques. Hum. Gene Ther. 2011a;22:979–984. doi: 10.1089/hum.2011.042. [DOI] [PubMed] [Google Scholar]
- Gao G, Zhong L, Danos O. Exploiting Natural Diversity of AAV for the Design of Vectors with Novel Properties. In: Snyder RO, Moullier P, editors. Adeno-Associated Virus. Humana Press; 2011b. pp. 93–118. [DOI] [PubMed] [Google Scholar]
- Garden GA, Guo W, Jayadev S, Tun C, Balcaitis S, Choi J, Montine TJ, Möller T, Morrison RS. HIV associated neurodegeneration requires p53 in neurons and microglia. FASEB J. 2004 doi: 10.1096/fj.04-1676fje. [DOI] [PubMed] [Google Scholar]
- Gordon S. Alternative activation of macrophages. Nat. Rev. Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- Gregorevic P, Blankinship MJ, Allen JM, Crawford RW, Meuse L, Miller DG, Russell DW, Chamberlain JS. Systemic delivery of genes to striated muscles using adeno-associated viral vectors. Nat. Med. 2004;10:828–834. doi: 10.1038/nm1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm D, Kay MA, Kleinschmidt JA. Helper Virus-Free, Optically Controllable, and Two-Plasmid-Based Production of Adeno-associated Virus Vectors of Serotypes 1 to 6. Mol. Ther. 2003;7:839–850. doi: 10.1016/s1525-0016(03)00095-9. [DOI] [PubMed] [Google Scholar]
- Halbert CL, Allen JM, Miller AD. Adeno-Associated Virus Type 6 (AAV6) Vectors Mediate Efficient Transduction of Airway Epithelial Cells in Mouse Lungs Compared to That of AAV2 Vectors. J. Virol. 2001;75:6615–6624. doi: 10.1128/JVI.75.14.6615-6624.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagele Hu R, Huffaker Dominique A, Runtsch Thomas B, Alexander M Marah C, Liu J, Bake E, Su W, Williams Matthew A, Rao Dinesh S, et al. miR-155 Promotes T Follicular Helper Cell Accumulation during Chronic, Low-Grade Inflammation. Immunity. 2014;41:605–619. doi: 10.1016/j.immuni.2014.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye RT, Du B, Boldt-Houle D, Ferrante A, Park IW, Hammer SM, Duan L, Groopman JE, Pomerantz RJ, Terwilliger EF. Potent inhibition of human immunodeficiency virus type 1 in primary T cells and alveolar macrophages by a combination anti-Rev strategy delivered in an adeno-associated virus vector. J. Virol. 1997;71:4071–4078. doi: 10.1128/jvi.71.5.4071-4078.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayadev S, Nesser NK, Hopkins S, Myers SJ, Case A, Lee RJ, Seaburg LA, Uo T, Murphy SP, Morrison RS, Garden GA. Transcription factor p53 influences microglial activation phenotype. Glia. 2011;59:1402–1413. doi: 10.1002/glia.21178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplitt MG, Leone P, Samulski RJ, Xiao X, Pfaff DW, O'Malley KL, During MJ. Long-term gene expression and phenotypic correction using adeno-associated virus vectors in the mammalian brain. Nat. Genet. 1994;8:148–154. doi: 10.1038/ng1094-148. [DOI] [PubMed] [Google Scholar]
- Kashiwakura Y, Tamayose K, Iwabuchi K, Hirai Y, Shimada T, Matsumoto K, Nakamura T, Watanabe M, Oshimi K, Daida H. Hepatocyte Growth Factor Receptor Is a Coreceptor for Adeno-Associated Virus Type 2 Infection. J. Virol. 2005;79:609–614. doi: 10.1128/JVI.79.1.609-614.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of Two Distinct Macrophage Subsets with Divergent Effects Causing either Neurotoxicity or Regeneration in the Injured Mouse Spinal Cord. J. Neurosci. 2009;29:13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J-K, Tansey M. Microglia Isolation from Adult Mouse Brain. In: Joseph B, Venero JL, editors. Microglia. Humana Press; 2013. pp. 17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Ramirez MA, Wu D, Pryce G, Simpson JE, Reijerkerk A, King-Robson J, Kay O, de Vries HE, Hirst MC, Sharrack B, et al. MicroRNA-155 negatively affects blood–brain barrier function during neuroinflammation. FASEB J. 2014;28:2551–2565. doi: 10.1096/fj.13-248880. [DOI] [PubMed] [Google Scholar]
- Lungwitz U, Breunig M, Blunk T, Göpferich A. Polyethylenimine-based non-viral gene delivery systems. European Journal of Pharmaceutics and Biopharmaceutics. 2005;60:247–266. doi: 10.1016/j.ejpb.2004.11.011. [DOI] [PubMed] [Google Scholar]
- McClure C, Cole KLH, Wulff P, Klugmann M, Murray AJ. Production and Titering of Recombinant Adeno-associated Viral Vectors. J. Vis. Exp. 2011:e3348. doi: 10.3791/3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCown TJ, Xiao X, Li J, Breese GR, Jude Samulski R. Differential and persistent expression patterns of CNS gene transfer by an adeno-associated virus (AAV) vector. Brain Res. 1996;713:99–107. doi: 10.1016/0006-8993(95)01488-8. [DOI] [PubMed] [Google Scholar]
- Möller T, Hanisch U-K, Ransom BR. Thrombin-Induced Activation of Cultured Rodent Microglia. J. Neurochem. 2000;75:1539–1547. doi: 10.1046/j.1471-4159.2000.0751539.x. [DOI] [PubMed] [Google Scholar]
- Moussaud S, Draheim HJ. A new method to isolate microglia from adult mice and culture them for an extended period of time. J. Neurosci. Methods. 2010;187:243–253. doi: 10.1016/j.jneumeth.2010.01.017. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay S, Chen Y, Sankala M, Peiser L, Pikkarainen T, Kraal G, Tryggvason K, Gordon S. MARCO, an innate activation marker of macrophages, is a class A scavenger receptor for Neisseria meningitidis. Eur. J. Immunol. 2006;36:940–949. doi: 10.1002/eji.200535389. [DOI] [PubMed] [Google Scholar]
- Nazari M, Salabi F, Zhang L, Zhao F, Wei C, Du L. AAV2-mediated follistatin overexpression induces ovine primary myoblasts proliferation. BMC Biotechnol. 2014;14:1–13. doi: 10.1186/s12896-014-0087-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng R, Govindasamy L, Gurda BL, McKenna R, Kozyreva OG, Samulski RJ, Parent KN, Baker TS, Agbandje-McKenna M. Structural Characterization of the Dual Glycan Binding Adeno-Associated Virus Serotype 6. J. Virol. 2010;84:12945–12957. doi: 10.1128/JVI.01235-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponomarev ED, Novikova M, Maresz K, Shriver LP, Dittel BN. Development of a culture system that supports adult microglial cell proliferation and maintenance in the resting state. J. Immunol. Methods. 2005;300:32–46. doi: 10.1016/j.jim.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Przanowski P, Dabrowski M, Ellert-Miklaszewska A, Kloss M, Mieczkowski J, Kaza B, Ronowicz A, Hu F, Piotrowski A, Kettenmann H, et al. The signal transducers Stat1 and Stat3 and their novel target Jmjd3 drive the expression of inflammatory genes in microglia. J. Mol. Med. 2014;92:239–254. doi: 10.1007/s00109-013-1090-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qing K, Mah C, Hansen J, Zhou S, Dwarki V, Srivastava A. Human fibroblast growth factor receptor 1 is a co-receptor for infection by adeno-associated virus 2. Nat. Med. 1999;5:71–77. doi: 10.1038/4758. [DOI] [PubMed] [Google Scholar]
- Rabinowitz JE, Rolling F, Li C, Conrath H, Xiao W, Xiao X, Samulski RJ. Cross-Packaging of a Single Adeno-Associated Virus (AAV) Type 2 Vector Genome into Multiple AAV Serotypes Enables Transduction with Broad Specificity. J. Virol. 2002;76:791–801. doi: 10.1128/JVI.76.2.791-801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickard AJ, Young MJ. Corticosteroid receptors, macrophages and cardiovascular disease. J. Mol. Endocrinol. 2009;42:449–459. doi: 10.1677/JME-08-0144. [DOI] [PubMed] [Google Scholar]
- Riviere C, Danos O, Douar AM. Long-term expression and repeated administration of AAV type 1, 2 and 5 vectors in skeletal muscle of immunocompetent adult mice. Gene Ther. 2006;13:1300–1308. doi: 10.1038/sj.gt.3302766. [DOI] [PubMed] [Google Scholar]
- Salva MZ, Himeda CL, Tai PWL, Nishiuchi E, Gregorevic P, Allen JM, Finn EE, Nguyen QG, Blankinship MJ, Meuse L, et al. Design of Tissue-specific Regulatory Cassettes for High-level rAAV-mediated Expression in Skeletal and Cardiac Muscle. Mol. Ther. 2007;15:320–329. doi: 10.1038/sj.mt.6300027. [DOI] [PubMed] [Google Scholar]
- Santambrogio L, Belyanskaya SL, Fischer FR, Cipriani B, Brosnan CF, Ricciardi-Castagnoli P, Stern LJ, Strominger JL, Riese R. Developmental plasticity of CNS microglia. Proc.Natl. Acad. Sci. U. S. A. 2001;98:6295–6300. doi: 10.1073/pnas.111152498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada M, Imai F, Suzuki H, Hayakawa M, Kanno T, Nagatsu T. Brain-specific gene expression by immortalized microglial cell-mediated gene transfer in the mammalian brain. FEBS Lett. 1998;433:37–40. doi: 10.1016/s0014-5793(98)00879-5. [DOI] [PubMed] [Google Scholar]
- Scheffel J, Regen T, Van Rossum D, Seifert S, Ribes S, Nau R, Parsa R, Harris RA, Boddeke HWGM, Chuang H-N, et al. Toll-like receptor activation reveals developmental reorganization and unmasks responder subsets of microglia. Glia. 2012;60:1930–1943. doi: 10.1002/glia.22409. [DOI] [PubMed] [Google Scholar]
- Schultz BR, Chamberlain JS. Recombinant Adeno-associated Virus Transduction and Integration. Mol Ther. 2008;16:1189–1199. doi: 10.1038/mt.2008.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder RO, Miao C, Meuse L, Tubb J, DonahueBrian A, Lin H-F, Stafford DW, Patel S, Thompson AR, Nichols T, et al. Correction of hemophilia B in canine and murine models using recombinant adeno-associated viral vectors. Nat. Med. 1999;5:64–70. doi: 10.1038/4751. [DOI] [PubMed] [Google Scholar]
- Su W, Hopkins S, Nesser NK, Sopher B, Silvestroni A, Ammanuel S, Jayadev S, Möller T, Weinstein J, Garden GA. The p53 Transcription Factor Modulates Microglia Behavior through MicroRNA-Dependent Regulation of c-Maf. J. Immunol. 2014;192:358–366. doi: 10.4049/jimmunol.1301397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerford C, Bartlett JS, Samulski RJ. [alpha]V[beta]5 integrin: a co-receptor for adeno-associated virus type 2 infection. Nat. Med. 1999;5:78–82. doi: 10.1038/4768. [DOI] [PubMed] [Google Scholar]
- Summerford C, Samulski RJ. Membrane-Associated Heparan Sulfate Proteoglycan Is a Receptor for Adeno-Associated Virus Type 2 Virions. J. Virol. 1998;72:1438–1445. doi: 10.1128/jvi.72.2.1438-1445.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tun C, Guo W, Nguyen H, Yun B, Libby RT, Morrison RS, Garden GA. Activation of the extrinsic caspase pathway in cultured cortical neurons requires p53-mediated down-regulation of the X-linked inhibitor of apoptosis protein to induce apoptosis. J. Neurochem. 2007;102:1206–1219. doi: 10.1111/j.1471-4159.2007.04609.x. [DOI] [PubMed] [Google Scholar]
- Weber MS, Prod'homme T, Youssef S, Dunn SE, Rundle CD, Lee L, Patarroyo JC, Stuve O, Sobel RA, Steinman L, Zamvil SS. Type II monocytes modulate T cell-mediated central nervous system autoimmune disease. Nat. Med. 2007;13:935–943. doi: 10.1038/nm1620. [DOI] [PubMed] [Google Scholar]
- Wu Z, Asokan A, Grieger JC, Govindasamy L, Agbandje-McKenna M, Samulski RJ. Single Amino Acid Changes Can Influence Titer, Heparin Binding, and Tissue Tropism in Different Adeno-Associated Virus Serotypes. J. Virol. 2006;80:11393–11397. doi: 10.1128/JVI.01288-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X, Li J, Samulski RJ. Efficient long-term gene transfer into muscle tissue of immunocompetent mice by adeno-associated virus vector. J. Virol. 1996;70:8098–8108. doi: 10.1128/jvi.70.11.8098-8108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Q, Bu W, Bhatia S, Hare J, Somasundaram T, Azzi A, Chapman MS. The atomic structure of adeno-associated virus (AAV-2), a vector for human gene therapy. Proc.Natl. Acad. Sci. U. S. A. 2002;99:10405–10410. doi: 10.1073/pnas.162250899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Hu X, Qian L, O’Callaghan JP, Hong J-S. Astrogliosis in CNS Pathologies: Is There A Role for Microglia? Mol. Neurobiol. 2010;41:232–241. doi: 10.1007/s12035-010-8098-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Aguilera J, Beaudet JM, Xie Q, Lerch TF, Davulcu O, Colón W, Chapman MS, Linhardt RJ. Characterization of Interactions between Heparin/Glycosaminoglycan and Adeno-associated Virus. Biochemistry. 2013;52 doi: 10.1021/bi4008676. 10.1021/bi4008676. [DOI] [PMC free article] [PubMed] [Google Scholar]