Abstract
Substantial ongoing research continues to explore the contribution of genetics and environment to the onset, extent and severity of periodontal disease(s). Existing evidence supports that periodontal disease appears to have an increased prevalence in family units with a member having aggressive periodontitis. We have been using the nonhuman primate as a model of periodontal disease for over 25 years with these species demonstrating naturally-occurring periodontal disease that increases with age. This report details our findings from evaluation of periodontal disease in skulls from 97 animals (5–31 years of age) derived from the skeletons of the rhesus monkeys (Macaca mulatta) on Cayo Santiago. Periodontal disease was evaluated by determining the distance from the base of the alveolar bone defect to the cemento-enamel junction on 1st/2nd premolars and 1st/2nd molars from all 4 quadrants. The results demonstrated an increasing extent and severity of periodontitis with aging across the population of animals beyond only compensatory eruption. Importantly, irrespective of age, extensive heterogeneity in disease expression was observed among the animals. Linking these variations to multi-generational matriarchal family units supported familial susceptibility of periodontitis. As the current generations of animals that are descendants from these matrilines are alive, studies can be conducted to explore an array of underlying factors that could account for susceptibility or resistance to periodontal disease.
Keywords: nonhuman primates, periodontitis, familial risk
INTRODUCTION
Substantial ongoing research continues to explore the contribution of genetics and environment to the onset, extent and severity of periodontal disease(s). Existing evidence supports that periodontal disease appears to have an increased prevalence in family units with a member having aggressive periodontitis [Demmer and Papapanou, 2010; Genco and Borgnakke, 2013; Kulkarni and Kinane, 2014]. In addition, various studies have indicated the presence of single nucleotide polymorphisms or specifically mutated genes that occur in patients and populations with increased incidence of periodontitis [Yoshie et al., 2007; Nibali et al., 2009; Divaris et al., 2013]. However, these studies have generally been limited to one or 2 generations in attempting to delineate the heritability of this chronic disease.
We have been using the nonhuman primate as a model of periodontal disease for over 25 years. There is clear evidence that the various species of primates (e.g. M. fascicularis, M. mulatta, M. nemestrina, S. scuireus, P. anubis) all demonstrate naturally-occurring periodontal disease that increases with aging [Schou et al., 1993; Madden and Caton, 1994; Miller et al., 1995; Struillou et al., 2010; Oz and Puleo, 2011]. These species demonstrate clinical, microbiological, and immunologic similarities to the disease in humans. Most recently we have developed a collaborative arrangement with the Caribbean Primate Research Center in Sabana Seca, PR to evaluate the ontogeny of the immune system in gingival tissues across the lifespan [Ebersole et al., 2008; Gonzalez et al., 2011; Gonzalez et al., 2013]. This cohort of animals was derived from a large, free-ranging colony of rhesus monkeys that was created in 1938 by seeding 400 Indian rhesus monkeys onto the isolated island of “Cayo Santiago” off the southeast coast of Puerto Rico. This colony currently houses approximately 900 animals from newborns to aged animals naturally organized into hierarchical groups with a specific hierarchical order within and between groups [Kessler and Berard, 1989; Whitehair, 1989]. Of particular importance to this investigation is that: (1) the existence of the colony for 75 years has enabled the creation of extensive pedigrees for all animals over 8 generations [Rothschild et al., 1999; Hernandez-Pacheco et al., 2013]; and (2) natural death of the animals in this predator-free environment has led to the creation of a collection of full skeletons from over 2000 animals from this colony with defined matriarchal lineage [Burr et al., 1989; Rothschild et al., 1999; Wang et al., 2006a; Wang et al., 2006b; Wang et al., 2007]. These characteristics enabled us to develop the hypothesis that periodontitis will be increased in specific nonhuman primate pedigrees demonstrating susceptibility/heritability of the expression of this disease. This report provides an initial evaluation of the distribution of periodontitis in this large nonhuman primate colony and the relationship of this distribution to the matrilineage of the cohort.
METHODS
Skull collection and matrilines
The CPRC Skeletal collection contains 397 specimens derived from 3 founding mothers, 065, 22, and 116. Matrilines were obtained based upon matriarchal lineage through 6–8 generations. Based upon preliminary findings, 97 skulls were selected among animals ranging from 5 years to 31 years of age. Twenty-one skulls were derived from 6 generations of 065, 25 skulls from 8 generations of 22, and 51 skulls from 6 generations of 116. Table I summarizes the demographics of the population that was examined.
Table I.
Demographics of the population
| Matriline | N | Generations | Gender (M:F) | Age in years mean (range) |
|---|---|---|---|---|
| 22 | 25 | 8 | 9:12 | 13.07 (5.12–23.50) |
| 065 | 21 | 6 | 13:8 | 15.42 (5.10–31.44) |
| 116 | 51 | 6 | 24:27 | 13.86 (5.81–24.92) |
An extensive pedigree history of the nonhuman primates enabled determination of the approximate age of each animal at the time of death. Thus, the “population” could be stratified based upon age of the sample. Detailed analysis of the alveolar bone characteristics were acquired throughout the maxillary and mandibular quadrants. These periodontal characteristics were plotted with age and specifically related to the pedigree of the animal.
No animals were sacrificed for this study. Rather, material from a pre-existing skeletal collection served as the database. Thus, IACUC review as not required. This research adhered to the American Society of Primatologists Principles for the Ethical Treatment of Nonhuman primates.
Periodontal disease measures
Periodontal disease was evaluated using a standard William’s color-coded periodontal probe by determining the distance from the deepest extent of the vertical bone defect to the cemento-enamel junction (CEJ) on 1stand 2nd premolars and 1st and 2nd molars from all 4 quadrants. The alveolar bone levels were measured on both mesiobuccal and distobuccal sites for each tooth since periodontitis is frequently developed in these sites by non-human primates. Data was assessed for total bone loss defects (mm; BD), as well as frequency of sites with loss that was greater than 4 mm and 5 mm. Missing teeth were assigned a value of 10 based upon the maximal magnitude of bone loss that was detected across the population for existing diseased teeth.
Statistical analyses
Differences among the matrilines in bone defect levels and frequency of sites with bone defects were evaluated using a ANOVA with post hoc testing of groups using Tukey’s HSD method (SigmaStat, Systat Software, Inc., Richmond, CA). Differences in frequency of affected animals used a χ2 test for 2×2 contingency analysis with Yates correction. Data with an alpha of less than or equal to 0.05 (after being adjusted for the multiple comparisons) were accepted as statistically significant using a two-tailed analysis.
RESULTS
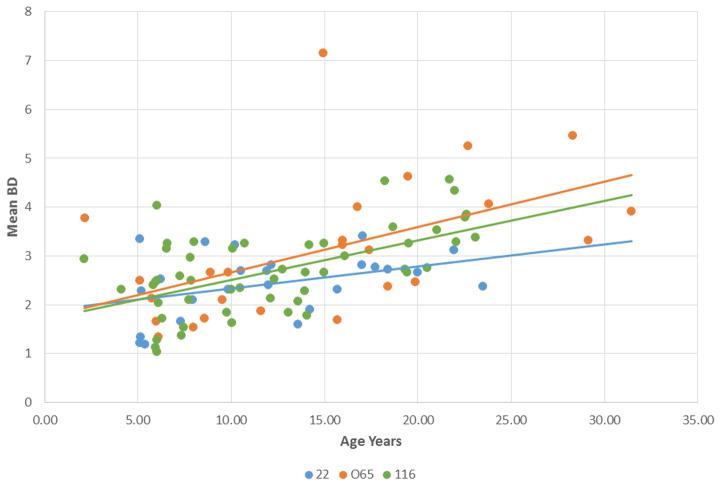
Figure 1 provides a schematic of the teeth and sites that were analyzed by probing. This provided up to 32 individual measurements to quantify the alveolar bone height related to the CEJ of the teeth. Figure 2 summarizes the relationship of the bone loss data depicting mean bone defect (mm) for individual animals across the entire age range in each of the 3 matrilines. As was expected, there was a clear increase in disease with aging in each of the family units. Interestingly that rate of this disease expression differed, with the 22 matriline showing a substantially lower rate of disease, and the 065 matriline demonstrating the greatest rate of bone loss, albeit, early in life (i.e. 5 years; comparable to approximately 20 human years) no difference were noted.
Figure 1.
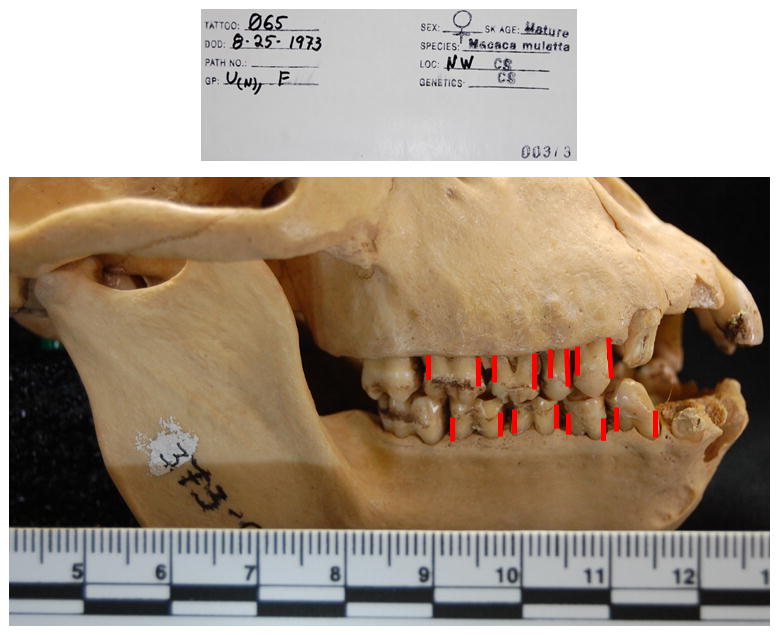
Extraoral photograph of approach to evaluating periodontal disease in the skulls. Animal is 065, that died at 20.7 years of age in 1973. The red lines denote the measures obtained from the cementoenamel junction to the bone height on the distal and mesial buccal surfaces at mandibular and maxillary 1st and 2nd premolar and molar teeth. Measures were obtained from all four quadrants. The ruler is in centimeters.
Figure 2.
Regression analysis of expression of the extent of periodontitis [Mean bone defect (mm)] related to age of animals in the 3 matrilines. Each point denotes an animal within the family and the matching colored lines describe a linear regression of the relationships.
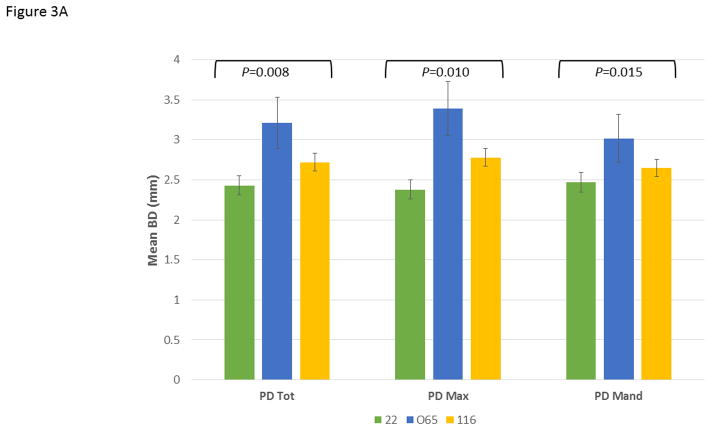
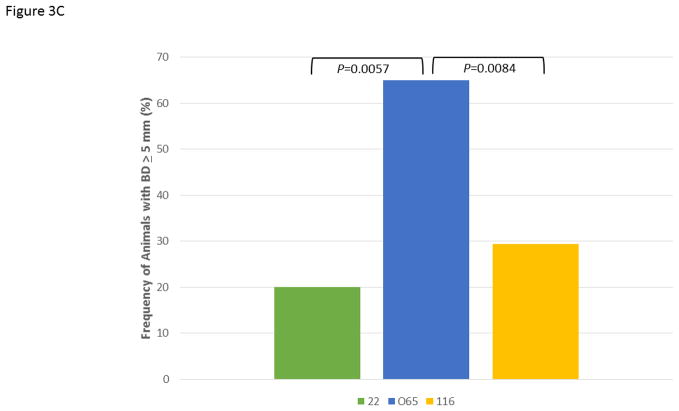
Figure 3A demonstrates the mean bone defect for the entire mouth (BD Tot), for the maxillary quadrants (BD Max) and mandibular quadrants (BD Mand). These data show, as was suggested by the age progression data, that the 065 matriline animals exhibited significantly greater disease than 22 and increased levels of disease in both quadrants compared to the 116 matriline animals. Extending this information, Figure 3B shows the severity of disease in the animals as a percentage of sites with greater levels of alveolar bone loss. The 065 animals demonstrated a greater frequency of sites with bone defects >4 mm and >5 mm compared to the other groups. Additionally, while the mean levels of disease (i.e. extent) were somewhat greater in the 116 versus 22 animals, the measures of severity were quite similar. Finally, Figure 3C summarizes the frequency of more severe disease identified at the animal level across all animals in each of the 3 matrilines and demonstrates the heightened level of disease in a greater number of animals in the 065 matriline.
Figure 3.
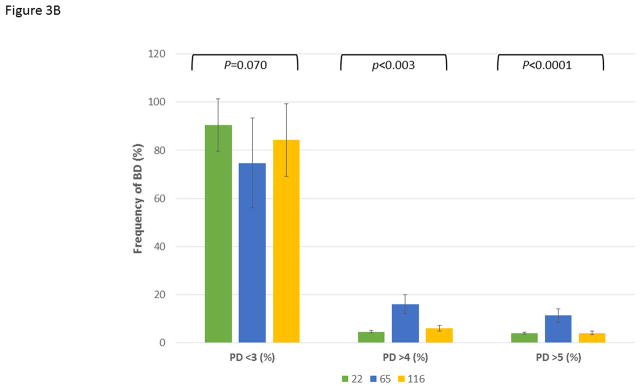
Comparison of disease expression across all ages in each of the family units using ANOVA with Tukey HSD. The bars are group means and the vertical brackets enclose 1 SE. (A) Extent of disease described by mouth mean bone defects (BD Tot; F=5.12, df1, P=0.0077), as well as similar measures limited to the maxillary (BD Max; F=4.79, df1, P=0.0104) and mandibular (BD Mand; F=4.42, df1, P=0.0145) quadrants. (B) Severity of disease described by frequency of sites with bone defects less than (<)3 mm (F=2.73, df1, P=0.0701), greater than (>)4 mm (F=6.12, df1, P=0.0031) and 5 mm (F=12.34, df1, P<0.0001). (C) Frequency of animals across the age groups in each family unit demonstrating 1 or more bone defects greater than or equal to 5 mm using Chi-square analysis with Yates correction (22 vs. 065; χ2=7.641, df1, P=0.0057; 065 vs. 116; χ2=6.940, df1, P=0.0084).
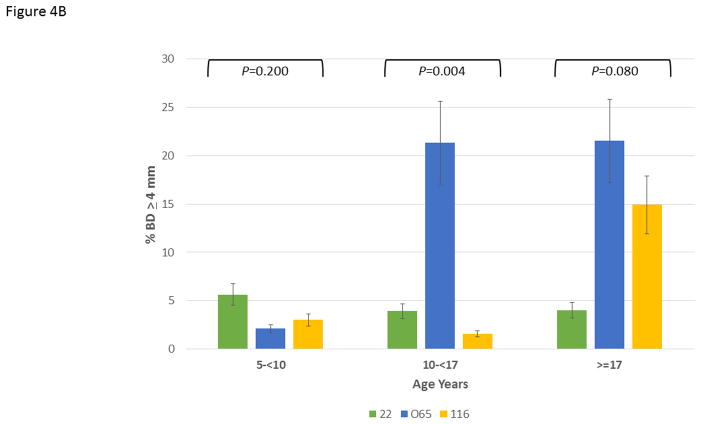
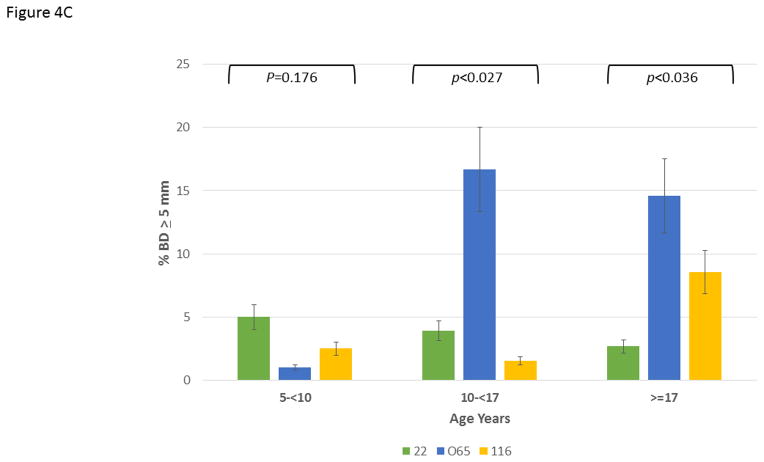
Figure 4A depicts the data exploring the expression of disease stratified by age category and analyzed within the 3 matrilines. In younger animals, no differences were observed in mean bone defect across all sites evaluated. However, a significant increase in disease was noted in the 10–17 year old animals from the 065 matriline compared to each of the other groups. Within the population greater than17 years of age, similar disease levels were seen in the 065 and 116 matrilines, with both greater than that noted in 22 matriline. Figure 4B summarizes the severity data stratified with age and similarly highlights the more extensive disease expression in the 065 matriline in the 10–17 year old animals. Similar results are seen in Figure 4C quantifying disease severity as bone defects >5 mm. Of interest was the observation that the 065 animals also showed increases in disease severity in the greater than17 year old animals compared to both of the other matriline units.
Figure 4.
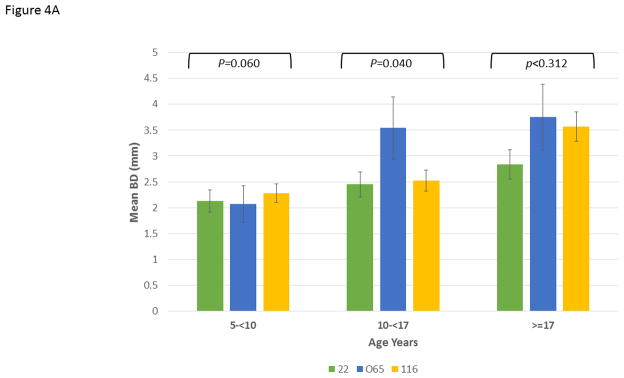
Disease expression in the family units stratified based upon age categories of the members evaluated using ANOVA with Tukey HSD. The bars are group means and the vertical brackets enclose 1 SE. (A) mean bone defects (BD) in 5-less than (<)10 years (F=3.56, df1, P=0.0395); 10-less than (<)17 years (F=2.84, df1, P=0.0753); greater than (>)17 years (F=1.22, df1, P=0.312), (B) frequency of sites with bone defects greater than or equal to 4 mm in 5-less than (<)10 years (F=1.69, df1, P=0.1997); 10-less than (<)17 years (F=6.85, df1, P=0.0038); greater than (>)17 years (F=2.72, df1, P=0.084), and (C) frequency of sites with bone defects greater than or equal to 5 mm in 5-less than (<)10 years (F=1.83, df1, P=0.1759); 10-less than (<)17 years (F=4.13, df1, P=0.0270); greater than (>)17 years (F=3.84, df1, P=0.0360).
DISCUSSION
Periodontitis is a major global disease that affects primarily adults leading to extensive individual quality of life issues expressed both societally and economically [Marcenes et al., 2013; Richards, 2013]. It is the primary reason for tooth loss in adults [Armitage and Robertson, 2009; Armitage and Cullinan, 2010]. This polymicrobial infection results in a chronic inflammatory response resulting in soft and hard tissue damage that can lead to exfoliation of the teeth.
In humans, clinical evidence has supported a familial tendency for this disease, with multiple affected individuals occurring in families, particularly where rapidly progressive or more generalized severe disease is expressed by one or more members of the family. [Baab et al., 1985; Page et al., 1985; van der Velden, 1991; Marazita et al., 1994; Hodge et al., 2000; Modeer and Wondimu, 2000; Kinane et al., 2005; Carlsson et al., 2006; Armitage and Cullinan, 2010]. Additionally, data clearly demonstrate the familial distribution of periodontitis that affects younger individuals, termed localized or generalized periodontitis, early onset periodontitis or aggressive periodontitis [Baab et al., 1985; Page et al., 1985; Schenkein and Van Dyke, 1994; Hart and Kornman, 1997; Diehl et al., 1999; Kinane et al., 1999]. Some of these clinical presentations, particularly leukocyte adhesion deficiency (LAD)-associated periodontitis, have been documented to be related to genetic modifications of neutrophil functions [Van Dyke et al., 1984; Baab et al., 1985; Page et al., 1985; Waldrop et al., 1987; Schenkein and Van Dyke, 1994; Hart and Kornman, 1997; Diehl et al., 1999; Kinane et al., 1999; Gronert et al., 2004; Moutsopoulos et al., 2014] polymorphisms in Cathepsin C [Hart et al., 2000], or have an autosomal recessive gene pattern in specific families [Marazita et al., 1994]. However, these potential genetic regulators are also interfaced with experimental evidence documenting the transmission of a particular periodontal pathogen, Aggregatibacter actinomycetemcomitans within the affected individuals in these families [Zambon et al., 1983; Schenkein and Van Dyke, 1994; Papapanou, 2002; Kilian et al., 2006; Teixeira et al., 2006; Dogan et al., 2008]. Thus, the expression of disease appears to require both a genetic predisposition plus environmental interactions for disease to be expressed within the families.
In contrast, the genetic underpinning of chronic adult periodontitis, which represents approximately 95% of the disease, globally, remains ill-defined. An array of studies has attempted to identify polymorphisms associated with genes of innate immunity and inflammation that could identify an increased relative risk for periodontitis susceptibility [Kinane and Hart, 2003; Yoshie et al., 2007]. These studies have targeted a priori numerous biomolecules and examined a wide array of ethnic and racial populations. Generally, these approaches have been less than enlightening regarding predicting risk of chronic adult periodontitis. More recently, a number of genome-wide association studies (GWAS) have been conducted to attempt to identify “risk” genes in a more unbiased approach. These population studies have, at this point, been rather small in number, been challenged with the issue of determination of affected individuals, and complicated by a complex disease that clearly has an aging component related to expression of the clinical symptoms [Schaefer et al., 2010; Divaris et al., 2012; Divaris et al., 2013; Teumer et al., 2013; Feng et al., 2014; Shaffer et al., 2014; Vaithilingam et al., 2014]. While there has been some early suggestion of potential genes of interest, with some having biological plausibility in contributing to the disease process, the fundamental concept of heritability of this disease remains to be determined. To initiate examination of this concept, this investigation developed an hypothesis that periodontitis will be increased in specific nonhuman primate pedigrees demonstrating susceptibility/heritability to the expression of this disease. The results demonstrated a clear familial relationship of the onset and severity of disease expression in the matrilines of nonhuman primates comprising the population of animals at Cayo Santiago.
Numerous disease phenotypes, including periodontitis, are quantitative under natural conditions, with a complex etiology, encompassing multiple environmental and genetic triggers. Observations that complex disease traits cluster in relation to genetic relatedness do suggest an underlying heritability. Enormous progress in mapping complex traits in humans has been made with some early success for prevalent diseases with complex phenotypes. Generally the findings demonstrate that with these common disorders the predominant genetic pattern is that of many loci contributing individually with small effects on the expressed phenotype. Moreover, for most complex traits evaluated in human populations, the sum of the identified genetic effects generally comprise less than ½ of the estimated trait heritability. Various hypotheses have been proposed to explain these investigational findings, including untested rare variants, and gene–gene and gene–environment interactions [Stranger et al., 2011]. Nevertheless, as noted by Qin and colleagues [2012; 157] “the genetic architecture of human complex disease remains elusive, the clinical application of current genetic knowledge remains a formidable challenge”.
Within this context, we had the ability to evaluate a large closed population of rhesus monkeys spanning many generations. This allowed us to address a question regarding the familial relationship of periodontitis, and identify potential heritability of the disease within the population. We evaluated skulls from 6–8 generations among 3 matrilines in animals aged 5–31 years. The results demonstrated that within this population, animals derived from the 065 matriline, had a higher frequency of periodontitis [70% vs. 36% (matriline 22) and 55% (matriline 116)] and elevated occurrence of more severely disease sites [65% vs. 20% (matriline 22) and 29% (matriline 116)]. Evaluation of rates of disease expression also demonstrated that members of the 065 lineage, while similar to the other matrilines at a younger age, had a more rapid increase in disease extent and severity with aging. The data showed that in the 10–17 year old group (equivalent to approximately 35–60 year old humans), the 065 matriline members developed more generalized and severe disease than the other matrilines. Even in the older animals (greater than 17 years), the 065 monkeys had a greater extent and severity, albeit, matriline 116 members greater than 17 years demonstrated a substantial increase in both extent and severity of disease, which suggests that there could be differences in the age-related onset of disease between matrilines. However, a single study by Dean and colleagues (1992) reported on examination of dentition from about 180 wild-shot great apes. The results indicated that there was no change in alveolar bone height from young adulthood to old age. Total tooth height above the alveolar bone crest was generally constant in all teeth through stages of wear. The amount of root exposed above the level of the alveolar bone over time suggested that with increasing tooth wear there was some compensatory eruption to maintain a constant height of tooth tissue. Their findings indicated that combined chronic pulpo/periodontal infections were the basis of final vertical alveolar bone and tooth loss in these great apes, although, our measures could have been impacted somewhat due to compensatory eruption.
As this represents a preliminary retrospective study provided by access to a unique portfolio of specimens tracking nearly 5 decades, the underlying etiology for these differences cannot be discerned. The net outcome of periodontal disease extent and severity appears dependent upon the characteristics of the oral microbial ecology. These include colonization/emergence of some opportunistic pathogens that appear to alter the local environment affecting the overall microbiome attributes and burden, and act in concert with host responses that are regulated by genetics and modified by the environment in humans [Kinane and Bartold, 2007; Hajishengallis et al., 2012; Armitage, 2013; Ebersole et al., 2013; Wade, 2013; Kornman and Polverini, 2014; Duran-Pinedo et al., 2014]. Since descendants of each of these matrilines still exist within the population and preliminary data suggests an increased risk of demonstrating periodontitis in living animals within the 065 matriline. We plan to develop prospective studies of animals from the various matrilines with predictions of greater susceptibility to periodontitis and the ability to link the clinical outcomes to unique local and systemic responses, as well as related to variations in the microbial ecology as an explanatory variable for differences in disease extent and severity.
Acknowledgments
This work was supported by P20 GM103538 from the National Institute of General Medical Sciences, the National Center for Research Resources (NCRR) and the Office of Research Infrastructure Programs (ORIP) of the National Institutes of Health (NIH) through Grant Number 5P40OD012217 to the Caribbean Primate Research Center. Infrastructure support was provided, in part, by grants from the National Center for Research Resources G12RR003051 (National Center for Research Resources) andG12MD007600 (National Institute on Minority Health and Health Disparities) from the National Institutes of Health.
Footnotes
The authors acknowledge no conflict of interest with the support for this research project.
References
- Armitage GC. Learned and unlearned concepts in periodontal diagnostics: a 50-year perspective. Periodontology 2000. 2013;62:20–36. doi: 10.1111/prd.12006. [DOI] [PubMed] [Google Scholar]
- Armitage GC, Cullinan MP. Comparison of the clinical features of chronic and aggressive periodontitis. Periodontology 2000. 2010;53:12–27. doi: 10.1111/j.1600-0757.2010.00353.x. [DOI] [PubMed] [Google Scholar]
- Armitage GC, Robertson PB. The biology, prevention, diagnosis and treatment of periodontal diseases: scientific advances in the United States. Journal of the American Dental Association. 2009;140(Suppl 1):36S–43S. doi: 10.14219/jada.archive.2009.0356. [DOI] [PubMed] [Google Scholar]
- Baab DA, Page RC, Morton T. Studies of a family manifesting premature exfoliation of deciduous teeth. Journal of Periodontology. 1985;56:403–409. doi: 10.1902/jop.1985.56.7.403. [DOI] [PubMed] [Google Scholar]
- Burr DB, Nishikawa RY, Van Gerven D. Bone growth and remodeling in Cayo Santiago--derived Macaca mulatta. Puerto Rican Health Sciences Journal. 1989;8:191–196. [PubMed] [Google Scholar]
- Carlsson G, Wahlin YB, Johansson A, Olsson A, Eriksson T, Claesson R, Hanstrom L, Henter JI. Periodontal disease in patients from the original Kostmann family with severe congenital neutropenia. J Periodontol. 2006;77:744–51. doi: 10.1902/jop.2006.050191. [DOI] [PubMed] [Google Scholar]
- Dean M, Jones ME, Pilley JR. The natural history of tooth wear, continuous eruption and periodontal disease in wild shot great apes. Journal of Human Evolution. 1992;22:23–39. [Google Scholar]
- Demmer RT, Papapanou PN. Epidemiologic patterns of chronic and aggressive periodontitis. Periodontol 2000. 2010;53:28–44. doi: 10.1111/j.1600-0757.2009.00326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl SR, Wang Y, Brooks CN, Burmeister JA, Califano JV, Wang S, Schenkein HA. Linkage disequilibrium of interleukin-1 genetic polymorphisms with early-onset periodontitis. J Periodontol. 1999;70:418–30. doi: 10.1902/jop.1999.70.4.418. [DOI] [PubMed] [Google Scholar]
- Divaris K, Monda KL, North KE, Olshan AF, Lange EM, Moss K, Barros SP, Beck JD, Offenbacher S. Genome-wide association study of periodontal pathogen colonization. Journal of Dental Research. 2012;91:21S–28S. doi: 10.1177/0022034512447951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divaris K, Monda KL, North KE, Olshan AF, Reynolds LM, Hsueh WC, Lange EM, Moss K, Barros SP, Weyant RJ, Liu Y, Newman AB, Beck JD, Offenbacher S. Exploring the genetic basis of chronic periodontitis: a genome-wide association study. Human Molecular Genetics. 2013;22:2312–2324. doi: 10.1093/hmg/ddt065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogan B, Kipalev AS, Okte E, Sultan N, Asikainen SE. Consistent intrafamilial transmission of Actinobacillus actinomycetemcomitans despite clonal diversity. Journal of Periodontology. 2008;79:307–15. doi: 10.1902/jop.2008.070270. [DOI] [PubMed] [Google Scholar]
- Duran-Pinedo AE, Chen T, Teles R, Starr JR, Wang X, Krishnan K, Frias-Lopez J. Community-wide transcriptome of the oral microbiome in subjects with and without periodontitis. International Society for Microbial Ecology Journal. 2014;8:1659–1672. doi: 10.1038/ismej.2014.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebersole JL, Dawson DR, 3rd, Morford LA, Peyyala R, Miller CS, Gonzalez OA. Periodontal disease immunology: ‘double indemnity’ in protecting the host. Periodontology 2000. 2013;62:163–202. doi: 10.1111/prd.12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebersole JL, Steffen MJ, Gonzalez-Martinez J, Novak MJ. Effects of age and oral disease on systemic inflammatory and immune parameters in nonhuman primates. Clinical Vaccine and Immunology. 2008;15:1067–75. doi: 10.1128/CVI.00258-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng P, Wang X, Casado PL, Kuchler EC, Deeley K, Noel J, Kimm H, Kim JH, Haas AN, Quinelato V, Bonato LL, Granjeiro JM, Susin C, Vieira AR. Genome wide association scan for chronic periodontitis implicates novel locus. BMC Oral Health. 2014;14:84. doi: 10.1186/1472-6831-14-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genco RJ, Borgnakke WS. Risk factors for periodontal disease. Periodontology 2000. 2013;62:59–94. doi: 10.1111/j.1600-0757.2012.00457.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez OA, Novak MJ, Kirakodu S, Orraca L, Chen KC, Stromberg A, Gonzalez-Martinez J, Ebersole JL. Comparative analysis of gingival tissue antigen presentation pathways in ageing and periodontitis. Journal of Clinical Periodontology. 2014;41:327–339. doi: 10.1111/jcpe.12212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez OA, Stromberg AJ, Huggins PM, Gonzalez-Martinez J, Novak MJ, Ebersole JL. Apoptotic genes are differentially expressed in aged gingival tissue. Journal of Dental Research. 2011;90:880–886. doi: 10.1177/0022034511403744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronert K, Kantarci A, Levy BD, Clish CB, Odparlik S, Hasturk H, Badwey JA, Colgan SP, Van Dyke TE, Serhan CN. A molecular defect in intracellular lipid signaling in human neutrophils in localized aggressive periodontal tissue damage. Journal of Immunology. 2004;172:1856–1861. doi: 10.4049/jimmunol.172.3.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G, Krauss JL, Liang S, McIntosh ML, Lambris JD. Pathogenic microbes and community service through manipulation of innate immunity. Advances in Experimental Medicine and Biology. 2012;946:69–85. doi: 10.1007/978-1-4614-0106-3_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart TC, Hart PS, Michalec MD, Zhang Y, Marazita ML, Cooper M, Yassin OM, Nusier M, Walker S. Localisation of a gene for prepubertal periodontitis to chromosome 11q14 and identification of a cathepsin C gene mutation. Journal of Medical Genetics. 2000;37:95–101. doi: 10.1136/jmg.37.2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart TC, Kornman KS. Genetic factors in the pathogenesis of periodontitis. Periodontology 2000. 1997;14:202–15. doi: 10.1111/j.1600-0757.1997.tb00198.x. [DOI] [PubMed] [Google Scholar]
- Hernandez-Pacheco R, Rawlins RG, Kessler MJ, Williams LE, Ruiz-Maldonado TM, Gonzalez-Martinez J, Ruiz-Lambides AV, Sabat AM. Demographic variability and density-dependent dynamics of a free-ranging rhesus macaque population. American Journal of Primatology. 2013;75:1152–1164. doi: 10.1002/ajp.22177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge PJ, Teague PW, Wright AF, Kinane DF. Clinical and genetic analysis of a large North European Caucasian family affected by early-onset periodontitis. Journal of Dental Research. 2000;79:857–863. doi: 10.1177/00220345000790031201. [DOI] [PubMed] [Google Scholar]
- Kessler MJ, Berard JD. A brief description of the Cayo Santiago rhesus monkey colony. Puerto Rican Health Sciences Journal. 1989;8:55–59. [PubMed] [Google Scholar]
- Kilian M, Frandsen EV, Haubek D, Poulsen K. The etiology of periodontal disease revisited by population genetic analysis. Periodontology 2000. 2006;42:158–179. doi: 10.1111/j.1600-0757.2006.00159.x. [DOI] [PubMed] [Google Scholar]
- Kinane DF, Bartold PM. Clinical relevance of the host responses of periodontitis. Periodontology 2000. 2007;43:278–293. doi: 10.1111/j.1600-0757.2006.00169.x. [DOI] [PubMed] [Google Scholar]
- Kinane DF, Hart TC. Genes and gene polymorphisms associated with periodontal disease. Critical Reviews in Oral Biology and Medicine. 2003;14:430–449. doi: 10.1177/154411130301400605. [DOI] [PubMed] [Google Scholar]
- Kinane DF, Hodge P, Eskdale J, Ellis R, Gallagher G. Analysis of genetic polymorphisms at the interleukin-10 and tumour necrosis factor loci in early-onset periodontitis. Journal of Periodontal Research. 1999;34:379–386. doi: 10.1111/j.1600-0765.1999.tb02270.x. [DOI] [PubMed] [Google Scholar]
- Kinane DF, Shiba H, Hart TC. The genetic basis of periodontitis. Periodontology 2000. 2005;39:91–117. doi: 10.1111/j.1600-0757.2005.00118.x. [DOI] [PubMed] [Google Scholar]
- Kornman KS, Polverini PJ. Clinical application of genetics to guide prevention and treatment of oral diseases. Clinical Genetics. 2014;86:44–49. doi: 10.1111/cge.12396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni C, Kinane DF. Host response in aggressive periodontitis. Periodontology 2000. 2014;65:79–91. doi: 10.1111/prd.12017. [DOI] [PubMed] [Google Scholar]
- Madden TE, Caton JG. Animal models for periodontal disease. Methods in Enzymology. 1994;235:106–119. doi: 10.1016/0076-6879(94)35135-x. [DOI] [PubMed] [Google Scholar]
- Marazita ML, Burmeister JA, Gunsolley JC, Koertge TE, Lake K, Schenkein HA. Evidence for autosomal dominant inheritance and race-specific heterogeneity in early-onset periodontitis. Journal of Periodontology. 1994;65:623–630. doi: 10.1902/jop.1994.65.6.623. [DOI] [PubMed] [Google Scholar]
- Marcenes W, Kassebaum NJ, Bernabe E, Flaxman A, Naghavi M, Lopez A, Murray CJ. Global burden of oral conditions in 1990–2010: a systematic analysis. Journal of Dental Research. 2013;92:592–597. doi: 10.1177/0022034513490168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DR, Aufdemorte TB, Fox WC, Waldrop TC, Mealey BL, Brunsvold MA. Periodontitis in the baboon: a potential model for human disease. Journal of Periodontal Research. 1995;30:404–409. doi: 10.1111/j.1600-0765.1995.tb01294.x. [DOI] [PubMed] [Google Scholar]
- Modeer T, Wondimu B. Periodontal diseases in children and adolescents. Dental Clinics of North America. 2000;44:633–658. [PubMed] [Google Scholar]
- Moutsopoulos NM, Konkel J, Sarmadi M, Eskan MA, Wild T, Dutzan N, Abusleme L, Zenobia C, Hosur KB, Abe T, Uzel G, Chen W, Chavakis T, Holland SM, Hajishengallis G. Defective neutrophil recruitment in leukocyte adhesion deficiency type I disease causes local IL-17-driven inflammatory bone loss. Science Translational Medicine. 2014;6:229–240. doi: 10.1126/scitranslmed.3007696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibali L, D’Aiuto F, Donos N, Griffiths GS, Parkar M, Tonetti MS, Humphries SE, Brett PM. Association between periodontitis and common variants in the promoter of the interleukin-6 gene. Cytokine. 2009;45:50–54. doi: 10.1016/j.cyto.2008.10.016. [DOI] [PubMed] [Google Scholar]
- Oz HS, Puleo DA. Animal models for periodontal disease. Journal of Biomedicine and Biotechnology. 2011;2011:754857. doi: 10.1155/2011/754857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page RC, Vandesteen GE, Ebersole JL, Williams BL, Dixon IL, Altman LC. Clinical and laboratory studies of a family with a high prevalence of juvenile periodontitis. Journal of Periodontology. 1985;56:602–610. doi: 10.1902/jop.1985.56.10.602. [DOI] [PubMed] [Google Scholar]
- Papapanou PN. Population studies of microbial ecology in periodontal health and disease. Annals of Periodontology. 2002;7:54–61. doi: 10.1902/annals.2002.7.1.54. [DOI] [PubMed] [Google Scholar]
- Qin H-D, Scott A, Wang H, Shugart Y. From GWAS to next-generation sequencing of human complex diseases: The implications for translational medicine and therapeutics. Applied Computational Genomics and Translational Bioinformatics. 2012;1:157–179. [Google Scholar]
- Richards D. Oral diseases affect some 3.9 billion people. Evidence Based Dentistry. 2013;14:35. doi: 10.1038/sj.ebd.6400925. [DOI] [PubMed] [Google Scholar]
- Rothschild BM, Hong N, Turnquist JE. Skeletal survey of Cayo Santiago rhesus macaques: osteoarthritis and articular plate excrescences. Seminars in Arthritis and Rheumatology. 1999;29:100–111. doi: 10.1016/s0049-0172(99)80041-9. [DOI] [PubMed] [Google Scholar]
- Schaefer AS, Richter GM, Nothnagel M, Manke T, Dommisch H, Jacobs G, Arlt A, Rosenstiel P, Noack B, Groessner-Schreiber B, Jepsen S, Loos BG, Schreiber S. A genome-wide association study identifies GLT6D1 as a susceptibility locus for periodontitis. Human Molecular Genetics. 2010;19:553–562. doi: 10.1093/hmg/ddp508. [DOI] [PubMed] [Google Scholar]
- Schenkein HA, Van Dyke TE. Early-onset periodontitis: systemic aspects of etiology and pathogenesis. Periodontology 2000. 1994;6:7–25. doi: 10.1111/j.1600-0757.1994.tb00023.x. [DOI] [PubMed] [Google Scholar]
- Schou S, Holmstrup P, Kornman KS. Non-human primates used in studies of periodontal disease pathogenesis: a review of the literature. Journal of Periodontology. 1993;64:497–508. doi: 10.1902/jop.1993.64.6.497. [DOI] [PubMed] [Google Scholar]
- Shaffer JR, Polk DE, Wang X, Feingold E, Weeks DE, Lee MK, Cuenco KT, Weyant RJ, Crout RJ, McNeil DW, Marazita ML. Genome-wide association study of periodontal health measured by probing depth in adults ages 18–49 years. G3 (Bethesda) 2014;4:307–314. doi: 10.1534/g3.113.008755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranger BE, Stahl EA, Raj T. Progress and promise of genome-wide association studies for human complex trait genetics. Genetics. 2011;187:367–383. doi: 10.1534/genetics.110.120907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struillou X, Boutigny H, Soueidan A, Layrolle P. Experimental animal models in periodontology: a review. Open Dentistry Journal. 2010;4:37–47. doi: 10.2174/1874210601004010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira RE, Mendes EN, Roque de Carvalho MA, Nicoli JR, de Farias LM, Magalhaes PP. Actinobacillus actinomycetemcomitans serotype-specific genotypes and periodontal status in Brazilian subjects. Canadien Journal of Microbiology. 2006;52:182–188. doi: 10.1139/w05-121. [DOI] [PubMed] [Google Scholar]
- Teumer A, Holtfreter B, Volker U, Petersmann A, Nauck M, Biffar R, Volzke H, Kroemer HK, Meisel P, Homuth G, Kocher T. Genome-wide association study of chronic periodontitis in a general German population. Journal of Clinical Periodontology. 2013;40:977–985. doi: 10.1111/jcpe.12154. [DOI] [PubMed] [Google Scholar]
- Vaithilingam RD, Safii SH, Baharuddin NA, Ng CC, Cheong SC, Bartold PM, Schaefer AS, Loos BG. Moving into a new era of periodontal genetic studies: relevance of large case-control samples using severe phenotypes for genome-wide association studies. Journal of Periodontal Research. 2014;49:683–695. doi: 10.1111/jre.12167. [DOI] [PubMed] [Google Scholar]
- van der Velden U. The onset age of periodontal destruction. J Clin Periodontol. 1991;18:380–3. doi: 10.1111/j.1600-051x.1991.tb02304.x. [DOI] [PubMed] [Google Scholar]
- Van Dyke TE, Taubman MA, Ebersole JL, Haffajee AD, Socransky SS, Smith DJ, Genco RJ. The Papillon-Lefevre syndrome: neutrophil dysfunction with severe periodontal disease. Clinical Immunology and Immunopathology. 1984;31:419–429. doi: 10.1016/0090-1229(84)90094-1. [DOI] [PubMed] [Google Scholar]
- Wade WG. The oral microbiome in health and disease. Pharmacologic Research. 2013;69:137–143. doi: 10.1016/j.phrs.2012.11.006. [DOI] [PubMed] [Google Scholar]
- Waldrop TC, Anderson DC, Hallmon WW, Schmalstieg FC, Jacobs RL. Periodontal manifestations of the heritable Mac-1, LFA-1, deficiency syndrome. Clinical, histopathologic and molecular characteristics. Journal of Periodontology. 1987;58:400–416. doi: 10.1902/jop.1987.58.6.400. [DOI] [PubMed] [Google Scholar]
- Wang Q, Dechow PC, Hens SM. Ontogeny and diachronic changes in sexual dimorphism in the craniofacial skeleton of rhesus macaques from Cayo Santiago, Puerto Rico. Journal of Human Evolution. 2007;53:350–361. doi: 10.1016/j.jhevol.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Wang Q, Opperman LA, Havill LM, Carlson DS, Dechow PC. Inheritance of sutural pattern at the pterion in Rhesus monkey skulls. Anatomical Record A Discovery of Molecular and Cellular Evolutionary Biology. 2006a;288:1042–1049. doi: 10.1002/ar.a.20373. [DOI] [PubMed] [Google Scholar]
- Wang Q, Strait DS, Dechow PC. Fusion patterns of craniofacial sutures in rhesus monkey skulls of known age and sex from Cayo Santiago. American Journal of Physical Anthropology. 2006b;131:469–485. doi: 10.1002/ajpa.20481. [DOI] [PubMed] [Google Scholar]
- Whitehair LA. NIH support for the Caribbean Primate Research Center (1975 to present) Puerto Rican Health Sciences Journal. 1989;8:43–44. [PubMed] [Google Scholar]
- Yoshie H, Kobayashi T, Tai H, Galicia JC. The role of genetic polymorphisms in periodontitis. Periodontology 2000. 2007;43:102–132. doi: 10.1111/j.1600-0757.2006.00164.x. [DOI] [PubMed] [Google Scholar]
- Zambon JJ, Christersson LA, Slots J. Actinobacillus actinomycetemcomitans in human periodontal disease. Prevalence in patient groups and distribution of biotypes and serotypes within families. Journal of Periodontology. 1983;54:707–711. doi: 10.1902/jop.1983.54.12.707. [DOI] [PubMed] [Google Scholar]