Abstract
Malignant pleural disease (MPD) results in an estimated 150,000 cases of malignant pleural effusions (MPE) annually. The most common malignancies associated with MPD are primary malignant pleural mesothelioma (MPM) and metastatic lung cancer, breast cancer and lymphoma. MPM is a rare, regionally aggressive, malignancy whose incidence is increasing secondary to the latency of disease progression. MPD is characteristic of advanced stage pleural disease and portends a grave clinical prognosis with a median survival between 3 to 12 months. Treatment for MPD is primarily palliative by thoracentesis to drain the effusion or surgical procedures to liberate trapped lung and obliterate cavities in order to limit subsequent collection of pleural effusions. Systemic chemotherapy is typically ineffective due to dose-limiting toxicities and poor intratumoral penetration. Preclinical investigations conducted in flank and intraperitoneal tumor models do not fully recapitulate the pleural tumor microenvironment and the results are not directly translational to the clinically setting. An orthotopic model of pleural malignancy allows investigators to evaluate the efficacy of therapies against novel molecular targets in a microenvironment that mimics the clinical tumor milieu. The protocol described herein provides a mouse model of MPM and MPD from non-hematogenous tumors resulting in reproducible tumor location, tumor progression, animal survival, and histopathology. Pleural tumor growth in this model resembles the regionally aggressive clinical course and tumor microenvironment of human pleural cancers and provides an optimal animal model to investigate MPD biology and therapies.
Keywords: malignant pleural disease, malignant pleural effusion, mesothelioma, breast cancer, orthotopic, mouse model, bioluminescent imaging
INTRODUCTION
Malignant pleural disease (MPD) and can be classified as either primary or metastatic in origin. Malignant pleural mesothelioma (MPM) is a primary neoplasm of the pleura, while lung and breast cancers are the predominant histologies metastatic to the pleura. MPM is a regionally aggressive primary pleural malignancy with a poor prognosis. Lung and breast cancer metastatic to the pleura are a leading causes of cancer-related death. MPD resulting in malignant pleural effusion (MPE) occurs in over 150,000 patients annually (Sahn 2001). In these cases, MPD precludes surgery for curative intent, causes significant respiratory morbidity, and result in a grave prognosis of 3 to 12 month median survival (Antunes, Neville et al. 2003; Mishra and Davies 2010). Regional therapies for MPE, such as pleural drainage or pleurodesis procedures, wherein a sclerosant is injected into the pleural space to cause pleural adherence to the chest wall in order to minimize pleural free space for recurrent fluid build-up, can provide symptomatic relief. However, current systemic chemotherapies are unable to cure patients secondary to dose-limiting toxicities that prevent the drug from achieving effective concentrations within the pleural space (Fenton and Richardson 1995). Advances in therapeutics for MPD are limited due to the lack of a reliable, clinically relevant mouse model accurately reflecting MPD pathobiology. This unit details an orthotopic, intrapleural murine model of MPD, which can be used for preclinical therapy trials. This murine tumor model closely resembles human disease and can be used in conjunction with accurate, quantitative, non-invasive imaging and monitoring of tumor burden.
STRATEGIC PLANNING
Mouse model selection
Selection of the appropriate murine model depends upon the specific experimental aims. To evaluate molecular therapies against human targets, immunodeficient mouse models provide efficacy data and allow for rapid clinical translation. Immunocompetent mouse models permit studying the interactions between the tumor and therapies in an intact immune system. The purchase and housing expenses of immunodeficient mice may cost 2-5 times greater than immunocompetent inbred mouse strains and may be a factor when planning large, long-term, survival studies. Syngeneic or immunocompetent mice require strain-specific tumor lines. Table 1 lists commonly available cell lines used to establish primary and metastatic pleural cancer models in immunocompetent and immunodeficient mice. The number of mice used for efficacy studies are tailored to a statistically significant power based upon expected increases in survival. In our experiments, cohorts of 8 or more mice are powered to detect a ten-day increase in survival. Larger cohorts should be considered if additional planned procedures can induce non-disease related morbidity and mortality. Although MPD in rats is well described and reproducible, due to imitations in available optimized antibodies, the use of rats for these investigations is minimal.
Table 1.
Representative cell lines used to establish MPD models
|
Primary MPD
| ||||
|---|---|---|---|---|
| Cancer Type | Cell line | Lineage | Host | Source/Reference |
| Mesothelioma | MSTO-211H | Human | SCID/bg NOD/SCID/γcnull Nude Athymic | ATCC Differentiation. 1988: 37; 158-171 |
| H226 | Human | SCID/bg NOD/SCID/γcnull Nude Athymic | ATCC J. Cell. Biochem. 1996: 24; suppl. | |
| AB12 | Mouse | BALB/c | CellBank Australia Cancer Immunol Immunother. 1995: 40; 241-50 | |
|
Metastatic MPD
| ||||
|---|---|---|---|---|
| Cancer Type | Cell line | Lineage | Host | Source/Reference |
| Lung | H1299 | Human | SCID/bg NOD/SCID/γcnull Nude Athymic | ATCC Cancer Res. 1992: 52; 2732s-2736s |
| A549 | Human | SCID/bg NOD/SCID/γcnull Nude Athymic | ATCC J. Natl. Cancer Inst. 1973: 51; 1417-1423 | |
| LLC1 | Mouse | C57BL/6 | ATCC Cancer Lett. 1980: 11; 63-73 | |
| Breast | MDA-MB-231 | Human | SCID/bg NOD/SCID/γcnull Nude Athymic | ATCC In Vitro. 1976: 12; 331 |
| 4T1 | Mouse | BALB/c | ATCC Cancer Res. 1992: 52; 1399-1405 | |
Pilot experiments
After choosing the appropriate mouse strain and cell line for investigation, the novice investigator gains familiarity with pre-procedural mouse preparation, positioning, anatomy, and tumor cell injection via small pilot experiments. This allows for troubleshooting on small cohorts and avoids the time and expense of a large, improperly executed experiment resulting in heterogeneous tumor implantation and progression. The objectives of pilot experiments should include: 1) appropriate administration of inhaled anesthesia, 2) adequate subject preparation (i.e., shaving, antiseptic skin preparation, draping), 3) strict adherence to surgical protocol, 4) immediate survival of mice post-injection, 5) proficiency in non-invasive imaging of tumor burden, 6) assessment of tumor burden on necropsy, and 7) the use of assistants (see Critical Parameters & Troubleshooting).
Surgical technique
This protocol employs a minor surgical procedure injecting tumor cells into the pleural space under direct visualization. It requires the maintenance of aseptic surgical technique to prevent infectious complications that not only can affect tumor implantation, development, and progression, but also survival. Several aspects of this procedure are crucial to the maintenance of aseptic conditions. First, an appropriate area for the surgical stage is selected. It must be clean and uncluttered. Second, the surgeon must wear personal protective equipment (PPE) as required by their institution, typically consisting of a cap, gown, mask, and sterile gloves. Finally, the operative site should be prepared in a manner that prevents contamination of the sterile field. This includes disinfecting the skin with povidone-iodine and alcohol, sterilization of surgical instruments, and a sterile drape exposing only the surgical field. A comprehensive treatment of these principles is provided by the NIH Office of the Care and Use of Laboratory Animals (Newell 2007; Hoogstraten-Miller and Brown 2008).
NOTE: All animal experiments must be approved by the institutional animal care and use committee (IACUC) and be performed in accordance with the guidelines set by the NIH Office of Laboratory Animal Welfare and your institution.
BASIC PROTOCOL 1: PREPARATION OF TUMOR CELL SUSPENSION
This protocol describes the steps necessary to generate a single cell suspension for injection into mice using Trypsin-EDTA to remove adherent cells from tissue culture plates.
Materials
Tumor cell line (see Strategic Planning)
10-cm tissue culture plates
Media for cell growth. This includes RPMI-1640 for the MSTO-211H, H1299, and 4T1 cell lines, DMEM for the AB12 and LLC1 cell lines and Leibovitz's L-15 for the MDA-MB-231 cell line.
Sterile Fetal Bovine Serum (FBS)
Penicillin-Streptomycin
1× DPBS
Trypsin-EDTA 0.05%
Tissue culture incubator with 5% CO2 atmosphere
50 ml or 15 ml conical centrifuge tubes
Hemocytometer
0.4% Trypan Blue
1.5 ml micro-centrifuge tubes
Preparation of Cell Suspension
- Culture tumor cells to 90% confluency in 10-cm tissue culture plates containing media supplemented with 10% FBS and 1% Penicillin-Streptomycin in a tissue culture incubator at 37°C with 5% CO2 atmosphere.This protocol employs a tumor cell dose of 1×105 cells in 200 μl of serum-free media per injection and generates reproducible and consistent tumor burden. Two 10-cm tissue culture plates are sufficient for up to 80 injections, as the typical yield is 5-10×106 cells per plate.
- Using pre-warmed solutions (at 37°C), remove the growth media from the plate. Wash with 2 mL of DPBS and remove by aspiration. Pipet 2 mL of trypsin-EDTA onto the tissue culture plate. After ensuring all cells are in contact with trypsin for 30 seconds to 2 minutes (until most of the cells are detached), pipet 5 mL of growth media to collect the cells. Gently agitate the solution to generate a single cell suspension by pipetting. Transfer the cell suspension to a 50 or 15 ml conical centrifuge tube.The time necessary to dislodge cells from tissue culture plates with trypsin is cell line specific, varying from 30 seconds to several minutes. Incubation at 37°C may facilitate release of the particularly adherent cells. To prevent excessive cell death and hydrolytic cleavage of cell-surface antigens, care must be taken to quench the trypsin with media as soon as cells start to roll off the surface and can be dislodged gently tapping the plate against the base of the palm.
Pellet the cells by centrifugation for 5 minutes at 300 × g, 4°C.
- Aspirate supernatant, and resuspend pellet in 5 mL of serum-free media with 1% Penicillin-Streptomycin. Gently agitate the solution by pipetting to generate a single cell suspension. Using a hemocytometer, determine tumor cell concentration and viability.Serum-free media or PBS should be used to resuspend the tumor inoculum in order to minimize any immunogenic response to non-murine serum proteins.When preparing a tumor cell suspension it is advisable to account for possible losses due to spillage and syringe dead space. If possible, inoculum for 15-20% more injections should be prepared in advance.It is not recommended to proceed if the viability of the cells is less than 90% due to variability in tumor cell implantation.
Adjust the tumor cell suspension to a final concentration of 5×105 cells/ml of media (1×105 cells per 200 μl) with serum-free media with 1% Penicillin-Streptomycin
Aliquot the cell suspension into 1.5 ml microcentrifuge tubes and place immediately on ice.
BASIC PROTOCOL 2: PLEURAL TUMOR CELL INJECTION
Orthotopic (i.e., pleural) tumor implantation permits the study of pleural cancer tumor biology and therapy in a more relevant tumor microenvironment. Similar to human MPD, pleural tumor inoculation in mice results in widespread pleural disease with production of malignant pleural effusions. Mice implanted with equal numbers of cells have a predictable, reproducible, disease course, and survival. This model allows for the evaluation of MPD therapies on large cohorts of mice. With the ability to genetically engineer tumor cells expressing bioluminescent reporter genes (e.g., firefly luciferase), bioluminescent imaging (BLI) permits the accurate and quantitative determination of tumor burden (described in Basic Protocol 3).
Materials
Tumor cell suspension (Basic Protocol 1)
Chlorine dioxide solution
Plastic backed absorbent paper
Mice of appropriate strain (6 to 8 week-old pathogen-free mice; see Strategic Planning).
Electric hair clippers
Ophthalmic ointment
Inhalational anesthesia vaporizer system with induction chamber
Additional tubing with nose cone
Oxygen
Isoflurane
Axillary roll (0.8 cm diameter)
Bench-top halogen lamp
10% Povidone-iodine
Sterile alcohol prep pads (70% isopropyl alcohol)
Fenestrated sterile drape
Personal protective equipment (PPE) including cap, gown, mask, and gloves
Dry bead sterilizer (e.g., Germinator 500; SouthPointe Surgical, Inc.)
Autoclave for instrument sterilization
Dissecting scissors - Fine iris scissors (10.5 cm) curved or straight
Adson forceps – toothed 1×2 (12 cm)
Sterile cotton tipped applicators
Tuberculin syringe with 27.5G needle
Counter top vortex
Wound clip applier and 9 mm wound clips (e.g., Autoclip Kit; Braintree scientific, Inc.)
Preparation for Pleural Injection
Disinfect an uncluttered surgical area with chlorine dioxide solution, wipe surfaces, and allow to dry. Cover work area with clean plastic backed absorbent paper.
- Ensure that all surgical supplies and equipment are cleaned in order to remove organic material that may interfere with sterilization. Surgical instruments may be cleaned in an ultrasonic cleaner, or by hand using a stiff bristle brush and a moderately alkaline, low-sudsing detergent in deionized or distilled water. Sterilize instruments and supplies in an autoclave.It is important to recognize that alcohol provides disinfection, and not sterilization, and should not be used to sterilize instruments.
Turn on bead sterilizer and confirm a proper working temperature of 250°C.
Prepare surgical stage with nose cone connected to inhalational anesthesia vaporizer to provide intra-operative inhaled anesthesia.
Shave the right side of the mouse from the axilla to the mid-abdomen to a width of 3 cm centered over the mid-axillary line using electric clippers. Remove loose fur by dusting the surgical site in order to prevent contamination of the incision site. Apply ophthalmic ointment to both eyes to prevent corneal desiccation.
- Place the mice in the induction chamber with an oxygen flow rate of 2-3 L/min and 2-3% vaporized isoflurane.Over- and under-sedation must be avoided (see Critical Parameters & Troubleshooting). The exact flow rate and isoflurane concentration may need to be decreased as mice exhibit the signs and symptoms of pleural disease. Signs of distress include decreased respiratory rate, shallow (agonal) respirations, hypothermia, cyanosis, and pallor. Mice showing these signs should immediately be removed from the induction chamber and recovered.
- Insure adequate depth of anesthesia through observation of slow and steady respirations and an absent withdrawal to noxious stimuli (e.g., toe or tail pinch).Please note that while this procedure can be performed with one operator, we recommend at least one assistant to facilitate the concurrent preparation of cells, syringe loading, mouse preparation, and post-injection recovery.
With anesthesia scavengers in place above the surgical field, open the flow valve to allow the flow of vaporized isoflurane through the nose cone at approximately 2-3 L/min.
Transfer a shaven, anesthetized, mouse to the surgical stage, placing the mouse's head carefully within the nose cone.
- Place the mouse on its left side (left lateral decubitus) with an axillary roll (0.8 cm in diameter) under the left axilla to provide support and position the contralateral chest in the proper anatomical alignment (Figure 1).
Disinfect shaved skin and an area at least 3-cm from the incision site alternating with 10% povidone-iodine and 70% isopropyl prep pad. The area is swabbed in a bull's eye pattern, starting at the incision site and moving outward in a radial fashion. Repeat povidone-iodine and isopropyl scrub for a total of three times. Paint the surgical site with 10% povidone-iodine.
Place a sterile fenestrated drape to expose the surgical field. The draping procedure must be performed using sterile surgical gloves.
- Sterilize instruments by placing the distal 3 cm of the instruments into the bead sterilizer at working temperature for 5 seconds. Remove the instruments and allow to cool for 30 seconds. Do not touch the tips of your instruments any surface other than the surgical field as they will become contaminated and will require re-application to the bead sterilizer. Prior to each new mouse injection, the instruments should be cleaned and re-sterilized.The surgeon should be dressed with personal protective equipment (PPE) including cap, gown, mask, and gloves (see Critical Parameters & Troubleshooting).
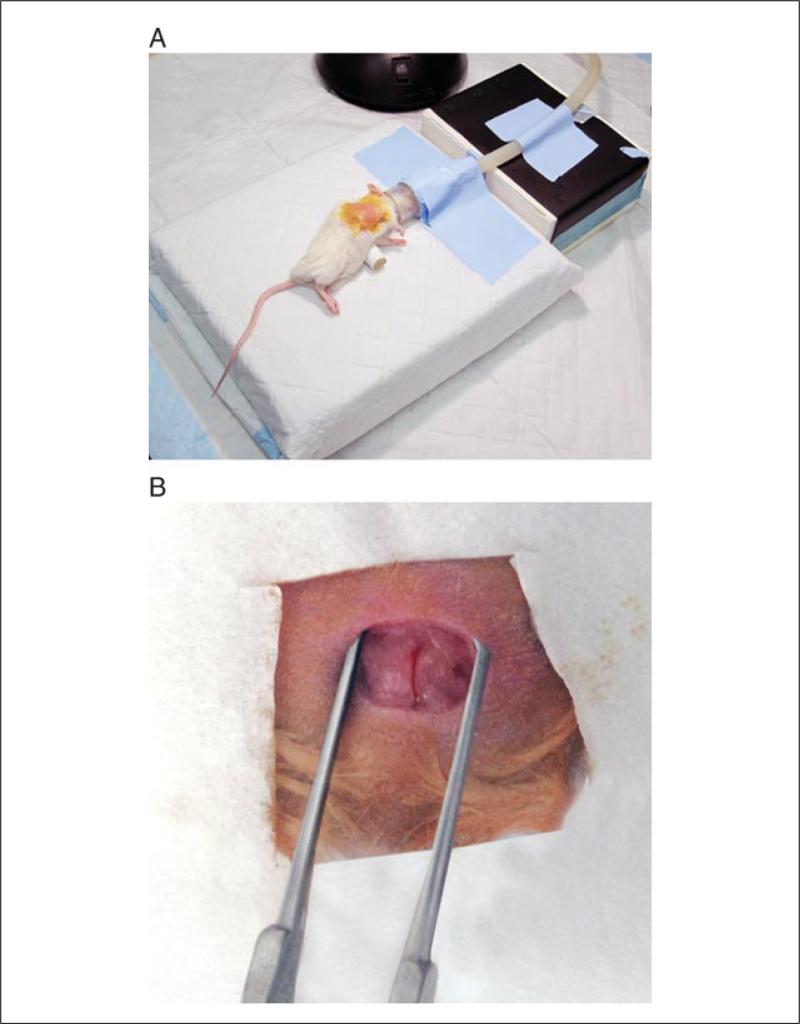
Figure 1.
Surgical stage setup, mouse positioning, and initial surgical exposure. (A) The surgical area consists of a surgical stage, anesthetic tubing and nose cone (taped securely to the stage), and an axillary roll (0.8 cm diameter) positioned underneath the left axilla to maximize exposure of thoracic landmarks. (B) After the initial incision, care must be taken to avoid avulsion of a superficial vein consistently located near the incision site.
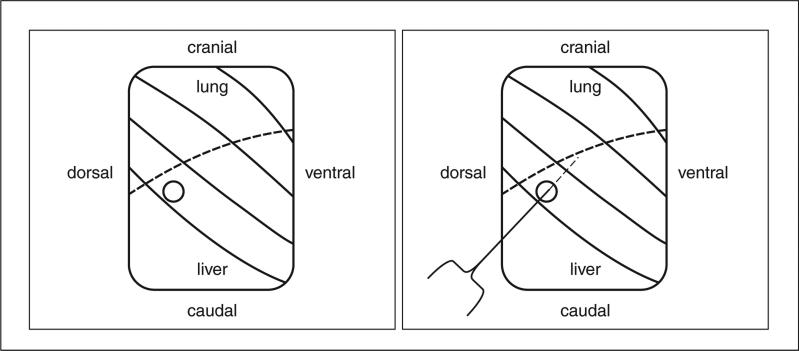
Figure 2.
Schematic view of anatomic landmarks for orthotopic pleural injections. Dissection to the right chest wall exposes the ribs (solid lines), diaphragm (dotted line), lungs (immediately superior to the diaphragm), and liver (inferior to the diaphragm). (A) The injection site is identified by a circle. (B) The needle enters through the circle, between the diaphragm and lung into the pleural space and injects the tumor cell suspension in the pleural cavity.
Pleural Injection of Tumor Cells
-
14.
Grasp the prepped skin midway between the scapula and costal margin approximately 1 cm inferior from the right axilla. While tenting the skin, make a 5-10 mm incision with the scissors. The optimal incision is 5 mm in length.
-
15.Gently grasp and bluntly dissect the subcutaneous tissue with dissecting scissors while providing countertraction with forceps to expose the chest wall. Caution needs to be exercised during dissection as to neither violate the pleural nor peritoneal cavities. Adequate dissection will expose the liver, lungs, and diaphragm (Figure 2).A small obliquely angled vein runs parallel to the dissection plane (Figure 1). This vein can be avulsed with excessive dissection and can result in significant blood loss. This should be avoided with smaller incisions and focused, blunt, dissection down to the chest wall. Should bleeding be encountered, gentle pressure with sterile cotton tipped applicators results in hemostasis (see Critical Parameters & Troubleshooting).
-
16.Vortex tumor cell suspension once the anatomy is clearly identified and hemostasis achieved. Withdraw exactly 200 μl (1×105 tumor cells) of the suspension into a tuberculin syringe.Vortexing the tumor cell suspension and precise syringe loading is a critical step of this procedure to ensure reproducible tumor burden. The syringe should be loaded without the needle in place to prevent excessive shearing forces on the cells. Consistently loading the syringe with an exact volume will promote equivalent tumor burden development. All air bubbles should be removed from the needle and syringe as this decreases the likelihood of pneumothorax, a significant cause of morbidity and mortality associated with this procedure.Since the murine pleural spaces communicate between the left and right sides (in contrast to human thoracic anatomy) the cell suspension will circulate and seed the pleural space evenly.
-
17.Expose the chest wall over the diaphragm visualizing the liver and pleural space (Figure 2). Slowly insert the needle caudad to the intercostal space between the diaphragm and lung at a shallow angle of 15°, needle bevel up, entering the pleural space through the diaphragm.The three right-sided lung lobes are mobile, and move away from the inserted needle when gentle, atraumatic, pressure is applied. This is a sign that the needle tip is in the pleural space and minimizes the possibility of intrapulmonary injection.
-
18.After entering the pleural space, inject the tumor inoculum slowly.Resistance suggests a possible intrapulmonary, intrahepatic, or subpleural injection. Should this occur, note the mouse as a possible failed injection and carefully monitor tumor burden with non-invasive imaging. Exclude mice if intraperitoneal signal is noted.
-
19.Grasp the skin and subcutaneous tissue with the forceps when the injection is completed. Cover the injection site with the tissue and atraumatically withdraw the needle.This is a critical step in minimizing post-operative pneumothorax and mortality.
-
20.
With the skin still held by the forceps, close the incision by applying 2 wound clips.
-
21.
Remove the mouse from the surgical stage, and allow to recover in a warm cage, prepared with moistened food. The mouse is positioned so that the mouse's head, nose, and mouth are not blocked by bedding or food.
-
22.Check tumor cell viability upon completion of the procedure by trypan blue exclusion.Even when injecting large cohorts of mice, decreases in viability should not be observed if cells are properly maintained and injections proceed in a timely fashion. If cell viability decreases, consider the use of additional assistants in future experiments to minimize the time that cells spend on ice.
-
23.A post-operative check should be made 4 hours after the procedure and daily thereafter. Document all post-operative checks.Immediate post-operative mouse death must be accompanied by prompt necropsy to determine cause of death and possible errors in surgical technique. Findings of sanguineous or serosanguineous pleural fluid on necropsy are signs of lung parenchymal injury or inadequate hemostasis.
-
24.
Remove wound clips 10 days after the procedure.
-
25.Physically examine mice daily. Euthanize mice by CO2 asphyxiation if they display signs of compromised health, (e.g., lethargy, hunching, ataxia, ruffled fur, anorexia, pallor, or labored breathing). Non-invasively image mice with BLI (Basic Protocol 3) if tumors expressing are used to establish a pleural cancer model.Imaging does not take the place of physical observation and monitoring of the mice.
BASIC PROTOCOL 3: BIOLUMINESCENT IMAGING (BLI) OF MPD MOUSE MODEL
Xenograft models in immunodeficient mice allow for the use of cell lines transduced to express the firefly luciferase reporter gene. This protocol describes the step-by-step procedure to acquire and analyze bioluminescent images.
Materials
Tumor cell lines expressing a luminescent reporter gene, such as firefly Luciferase. [Any tumor cell line of interest may be utilized (see “Strategic Planning”) transduced to express a luminescent reported gene. Luciferase vectors are available commercially from a number of vendors (e.g., Promega Biosciences, CA)]
d-Luciferin substrate (15 mg/mL; Caliper LifeSciences)
1× DPBS
MPD-bearing mice (see Basic Protocol 2)
Isoflurane/oxygen-based anesthesia system and induction chamber
In vivo bioluminescent imaging system (e.g., Xenogen IVIS 200, Caliper LifeSciences)
1-ml tuberculin syringes
Acquire Bioluminescent Images
- Orthotopically inject recipient mice with luciferase-expressing tumor cells as described in Basic Protocol 2.One week to 10 days after tumor implantation, BLI is used to verify pleural tumor implantation. At this time, signal should be confined to the chest and mice with any peritoneal signal should be excluded from further analysis – representing a failed pleural injection. For drug efficacy studies mice are separated into cohorts with equivalent bioluminescent signals based upon this imaging.
Initialize and calibrate imaging system per manufacturer's instructions.
- With a 1-mL tuberculin syringe loaded with d-luciferin (150 mg/kg per mouse) intraperitoneally (i.p.) inject 200 μl (3mg; 15 mg/mL stock solution).Timing of d-luciferin injection to imaging is critical in achieving consistent, reproducible, and maximal bioluminescent signal. In this model, the optimal incubation time is 20 minutes prior to imaging. The imaging of large numbers of mice is facilitated by an organized, team-based, approach with one user responsible of the injection of d-luciferin and the other for imaging.
- Anesthetize 1 to 5 mice in an induction chamber with a mixture of 2-3% isoflurane at a flow rate of 2-3 L/min O2. When adequate depth of anesthesia is obtained (as described in Basic Protocol 2, step 7), begin anesthetic flow to the imaging system.To prevent motion artifacts, adequate sedation must be maintained. Under-sedation can result in mouse escape or loss within the imaging chamber – potential hazards to both the mice and equipment.
- Transfer mice to imaging system in the supine, ventral, position (i.e., laying on their back).Many imaging systems are designed to image several mice at once. Opaque dividers between mice minimize bioluminescent signal bleed from adjacent mice. For longitudinal imaging studies it is critically important that mice are uniquely identified with an ear clip, toe clip, ear punch, and/or indelible marker.
- Acquire bioluminescent signal 20 minutes after i.p. d-luciferin administration for this pleural cancer model. Save the associated image. Immediately reposition the mice into the prone, dorsal, position (i.e., laying on their stomach), re-image, and save associated image.With the Xenogen IVIS 100 System (Caliper LifeSciences), we use the following acquisition settings: field of view= 20-cm, exposure time= 30 seconds, and binning= medium resolution. For accurate image analysis, it is critical to ensure that the acquired signal does not saturate the CCD camera. Saturated images can be avoided by decreasing exposure time or bin size (see Critical Parameters & Troubleshooting).
Repeat imaging weekly to determine changes in tumor burden.
Analyze Bioluminescent Images
-
8.Using image analysis software accompanying the imaging system (e.g., Living Image Software for IVIS 100), define a region of interest (ROI) encompassing the entire mouse.In this model, most images are optimally viewed with a bioluminescent spectrum between 1×105-1×107 photons/sec.
-
9.Measure bioluminescent signal from dorsal and ventral ROIs. Average the dorsal and ventral signals.Data are subsequently analyzed with the software packages Microsoft Excel 2008 (Microsoft Corporation) and Prism 5.0 (GraphPad Software Incorporated).
REAGENTS AND SOLUTIONS
d-Luciferin
Dissolve 1 mg of d-luciferin (Caliper LifeSciences) in 67 mL of DPBS. Vortex and aliquot into 1.5 mL microfuge polystyrene tubes. Store at −20° to −70°C for up to 3 months. Avoid repeated freeze thaw cycles. When ready to use, thaw the minimally required aliquots of d-luciferin at room temperature. As d-luciferin is light sensitive, all solutions must be protected from light until prior to injection.
COMMENTARY
Background Information
An implantable mouse model of pleural cancers can conveniently provide cohorts of mice with uniform tumor burden to evaluate novel anti-cancer therapies. Animal models used to study these malignancies include xenograft models with intravenous, pseudo-orthotopic (intraperitoneal), or subcutaneous tumor injections. These models do not fully recapitulate the pleural microanatomy and microenvironment. Intravenous tumor cell implantation results in systemic metastases with rare pleural metastases and mice usually succumb to systemic disease burden prior to developing pleural disease or MPE. The pseudo-orthotopic (intraperitoneal) model usually results in disseminated peritoneal carcinomatosis without lymphatic uptake. This is a consequence of the peritoneal and pleural microenvironments differing in their cell biology and ability to recirculate peritoneal fluid (Zocchi 2002; Michailova and Usunoff 2006). Furthermore, intraperitoneal models do not allow radiotherapy investigations due to gastrointestinal toxicity. Flank tumor models lack characteristic pleural space lymphangiogenesis, and mice are typically sacrificed due to large tumor burden without development of MPD.
However, an orthotopic mouse model of MPD can effectively emulate the biology and tumor microenvironment observed in human pleural cancers. The parietal pleural surface contains a lymphatic nexus of stoma. These lymphatics provide access for pleural metastases from distant tumors and an escape for MPM and lung cancers into the systemic circulation (Manac'h, Riquet et al. 2001; Zocchi 2002; Osaki, Nagashima et al. 2004; Shimizu, Yoshida et al. 2005). The sequelae of pleural cancers, such as MPE, result from interactions between the tumor and host which are fully manifested by syngeneic mouse models (Stathopoulos and Kalomenidis 2009). In addition, immunocompetent orthotopic models of pleural cancer facilitate the study of inflammation on tumor progression. However, to study novel molecular therapies targeting human antigens, immunodeficient models must be employed in order to use human cancer cell lines. This protocol is applicable the both immunodeficient and immunocompetent models of pleural cancer and facilitates not only the study of MPD pathophysiology but also the effect of novel therapies (Adusumilli, Eisenberg et al. 2006; Adusumilli, Stiles et al. 2006; Stiles, Adusumilli et al. 2006).
Critical Parameters & Troubleshooting
The procedure described in this unit requires that the user be intimately familiar with not only the protocol but also the surgical techniques need to minimize morbidity and mortality. Novice users are encouraged to perform pilot experiments prior to attempting any large studies. The following section details critical parameters necessary for successful, reproducible implementation of this protocol. Potential problems and troubleshooting are summarized in Table 2.
Table 2.
Troubleshooting
| Problem | Possible Cause | Recommendation |
|---|---|---|
|
Establishing an orthotopic pleural cancer model
| ||
| Pleural tumor does not develop | Cell viability | Check cell viability post-procedure using hemocytometer. Use cells that are in growth phase at the time of harvest. |
| Cell suspension not adequately mixed | Ensure adequate vortexing for proper resuspension of tumor cells when loading syringe. | |
| Tumor dose too low | Increase tumor cell dose. | |
| Non-pleural injection | Recognize anatomic landmarks prior to injecting tumor. Verify tumor location with non-invasive imaging, if possible. |
|
| Mice develop infection | Contaminated instruments or incision site | Make sure to adequately clean and sterilize instruments prior to use. Verify that bead sterilizer reaches its working temperature. |
| Mouse does not recover after surgery | Excessive anesthesia | Carefully monitor respiratory status of mice while on anesthesia. Titrate anesthesia to lowest possible settings. |
| Inadequate thermoregulation | Use heating lamp/pad to adequately maintain normothermia. Decrease operative time with use of assistants. |
|
| Inadequate hemostasis | Ensure hemostasis by applying direct pressure with sterile cotton tip applicator to sites of bleeding. Pilot experiments to improve technique, minimize the length of the incision, and decrease operative time. |
|
| Hemorrhage | Blood loss >0.3ml warrants fluid replacement with 0.14 ml warmed sterile saline injected SQ. Post-operative check 3 hours after surgery to assess hemostasis. |
|
| Possible pneumothorax | Remove all air bubbles from the needle prior to injecting tumor. Minimize trauma of needle insertion by entering the pleural cavity slowly. Gently applying pressure with the needle to move the lung from the chest wall. Decrease the angle of needle insertion (~15°) |
|
|
BLI imaging of pleural cancer model | ||
| No BLI signal, even with long exposure times (>5 min) | Tumors did not form | Check cell viability post-procedure using hemocytometer. Use cells that are in growth phase at the time of harvest. Verify tumor burden with complete necropsy. |
| Tumors formed, with unstable reporter gene expression | Check stability of reporter gene transduction prior to animal experiment. Flow sort cell population for highest expressing cells. |
|
| D-luciferin not injected or inactive | Use freshly reconstituted substrate. Avoid repeated freeze-thaw cycles and protect from light. Test in vitro substrate activity on tumor cells expressing luciferase. |
|
| Saturation of CCD | Long exposure time | Reduce exposure time and/or binning. |
Anesthesia should be titrated to prevent under- or over-sedation. Rapid breathing and continuing extremity movement is a sign of under-sedation. After checking the anesthetic circuit and excluding mechanical obstruction, oxygen flow rate or isoflurane dose should be incrementally increased. Over-sedation results in decreased respiratory rate, shallow respirations, and eventually respiratory arrest. Mice exhibiting any signs of over-sedation must be immediately removed from anesthesia and recovered in a cage, placed in the lateral decubitus position with an unobstructed airway. Mice showing signs of impending respiratory arrest (i.e., cyanosis and agonal respirations) should be recovered with oxygen supplementation and thoracic chest compressions. When fully recovered from anesthesia, the mouse may be placed into the induction chamber with a decreased oxygen flow rate and isoflurane dose.
A key requirement is the adequate exposure of the surgical site and aseptic technique. All members handling mice during this procedure must wear a barrier gown, surgical mask, and cap. Hands should be washed for at least 3 minutes using a disinfectant soap before donning gloves. The incision site is scrubbed alternating between a disinfectant (10% povidone-iodine) and isopropyl alcohol three times, followed by application of sterile surgical drape. Shaving and disinfecting a 3×3 cm area, centered over the right chest, from the inferior costal margin to the scapula will ensure that the disinfected skin remains covered by a surgical drape and resides outside the incision. Strict adherence to aseptic technique and proper surgical attire minimizes the risk of surgical site infections. In addition to adequate exposure, proper lighting with a bench-top halogen lamp is critical for the discrimination of anatomical landmarks. Furthermore, heat generated from the lamp decreases anesthesia induced hypothermia.
A regimented surgical protocol ensures consistent, reproducible, and high rates of tumor engraftment. Amongst the most critical aspects of the procedure is adequate mixing of the tumor cell suspension by thoroughly vortexing, precisely loading 200 μl (1×105 cells) into the syringe, and intrapleurally administering a complete dose. Any variation in these factors will result in heterogeneous tumor implantation. Basic surgical tenets minimize morbidity and mortality. Adequate hemostasis is required to not only maximize survival but also visualization of anatomic structures needed to identify the pleural cavity. Tumor cell suspension should not be injected unless the user is absolutely certain they have entered the pleural space. Upon inserting the needle through the chest wall, the lung lobes should move away from the inserted needle when gentle, atraumatic, pressure is applied. This is a sign that the needle tip is in the pleural space and minimizes the possibility of intrapulmonary injection. Covering the needle with subcutaneous tissue as the needle is withdrawn from the pleural space minimizes the rate of pneumothorax in this procedure.
Post-operative mortality of <3% is a benchmark for adequate technique. All mice dying in the immediate post-operative period must undergo a necropsy to determine the cause of death. Findings of bloody pleural fluid are a sign of bleeding due to improper surgical technique (i.e., lung parenchymal injury) or inadequate hemostasis. Absence of effusions points to potential pneumothorax from parenchymal injury as a cause of death. If mortalities greater than expected are encountered, it is encouraged to review each step of the protocol and determine any errors in technique.
Non-invasive imaging (e.g., BLI or MRI) facilitates tumor monitoring. The protocol for bioluminescent imaging is described in detail in Basic Protocol 3 with added commentary following the protocol. Parameters critically important for reproducible and quantitative BLI are as follows: 1) mice must be imaged 20 minutes after i.p. injection of uniform doses of d-luciferin (3 mg) to ensure that the bioluminescent signal is reflective of tumor burden and not substrate availability, 2) when imaging multiple mice, opaque dividers must be used to minimize bioluminescent signal from adjacent mice, and 3) saturated bioluminescent signal is non-quantitative and is overcome by reducing exposure time or binning. Necropsy is performed to assess the pleural tumor burden and sequelae of MPD in this tumor model. Given that in the murine thoracic cavity the left and right pleural spaces communicate, tumor cells should circulate freely resulting in tumor throughout the chest. Necropsy will confirm subpleural, intrahepatic, pulmonary, peritoneal, and subcutaneous injections. Finally, a team-based approach will minimize time, effort, and significantly improve the success rate. Assistants facilitate the rapid injection of tumors, can identify technical errors not readily apparent, can suggest alternate strategies, and provide effective troubleshooting. With successful completion of these objectives, a reproducible and consistent model of MPD can be achieved.
Anticipated Results
With a regimented approach to this protocol, an experienced investigator can obtain rates of tumor engraftment is >95%. The survival of untreated mice varies according to the mouse model selected. Syngeneic models of MPM and lung cancer (AB12 with BALB/c mice and LLC1 with C57BL/6 mice, respectively) have a median survival of 18-25 days when intrapleurally injected with 1×105 cells. Survival of immunodeficient mouse models (using MSTO-211H, H226, A549, H1299, or MDA-MB-231 in SCID/bg mice) range from 25-35 days. Mice develop symptoms of pleural disease approximately 10-20 days after injection including weight loss, decreased pulmonary reserve to handling, and labored breathing and eventually succumb to pleural tumor burden and pleural effusions. These effusions are typically comprised of blood and tumor and may have a gelatinous consistency. This model has proven useful in the study of pleural tumor pathogenesis and the evaluation of novel therapies for pleural cancers.
Since pleural tumor burden cannot be palpated by virtue of its location, we employ non-invasive MRI or bioluminescence imaging (BLI) to monitor tumor progression and response to therapy (Figure 3). In our laboratory's experience, when tumor cells are transduced to express firefly luciferase and intrapleurally injected, BLI is an accurate, sensitive, and quantitative measure of pleural tumor burden. Alternatively when using cancer cells that do not express a bioluminescent reporter, MRI imaging is performed using a Bruker 4.7T USR scanner (Bruker Biospin Inc., Ettlingen, Germany) equipped with a 400 mT/m gradient coil and a 32 mm ID custom build birdcage resonator. Mice are anesthetized with 1% isoflurane in 20% O2 and imaged. Thoracic axial MRI images are acquired with a RARE fast spin-echo sequence with a repetition time (TR) of 1.7s, echo time (TE) 40 ms and 12 averages. The acquisition is triggered by animal respiration to reduce motion artifacts. The slice thickness is 0.7 mm and the in-plane image resolution was 117 × 156 mm.
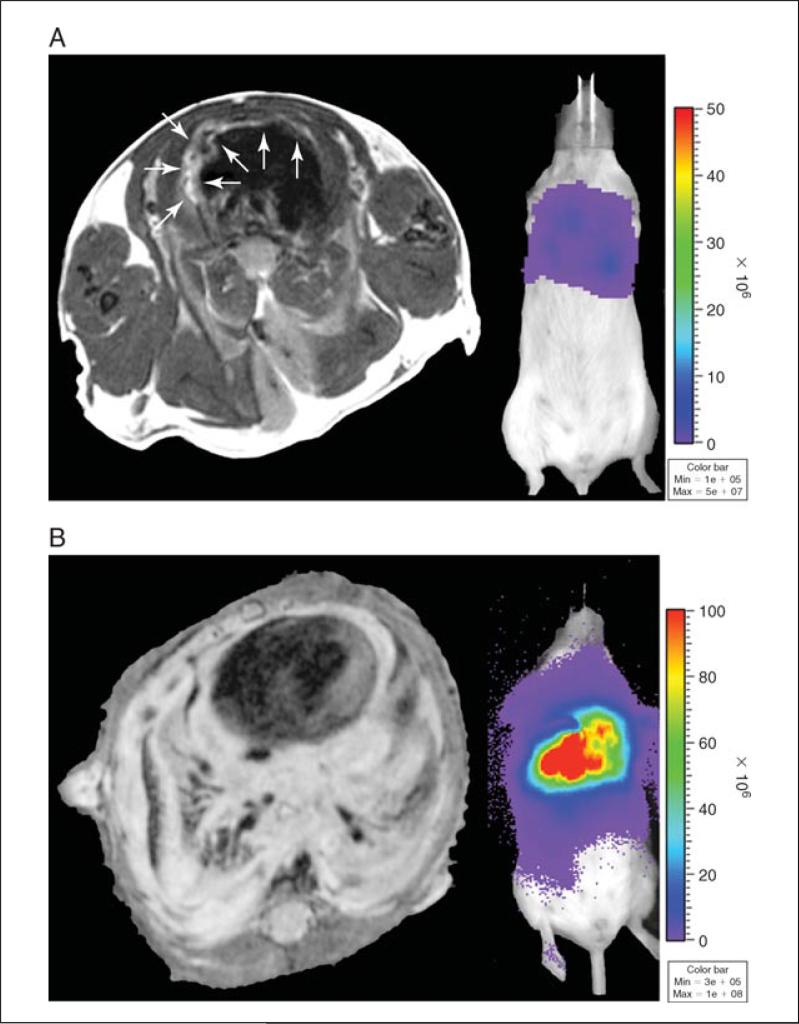
Figure 3.
Pleural cancer model non-invasive imaging, macroscopic and microscopic findings. MRI (left) and BLI (right) of MPM tumor (MSTO-211H, 1×105 cells/200μl), (A) 6 days after implantation showing in pleural involvement. (B) 29 days after tumor implantation showing encasement of mediastinal structures and pleura. (C) Orthotopic injection of LLC1 (1×105 cells/200μl) results in pleural tumor and bloody pleural effusions. (D) H&E section of pleural tumor along the chest wall.
Experimental Study
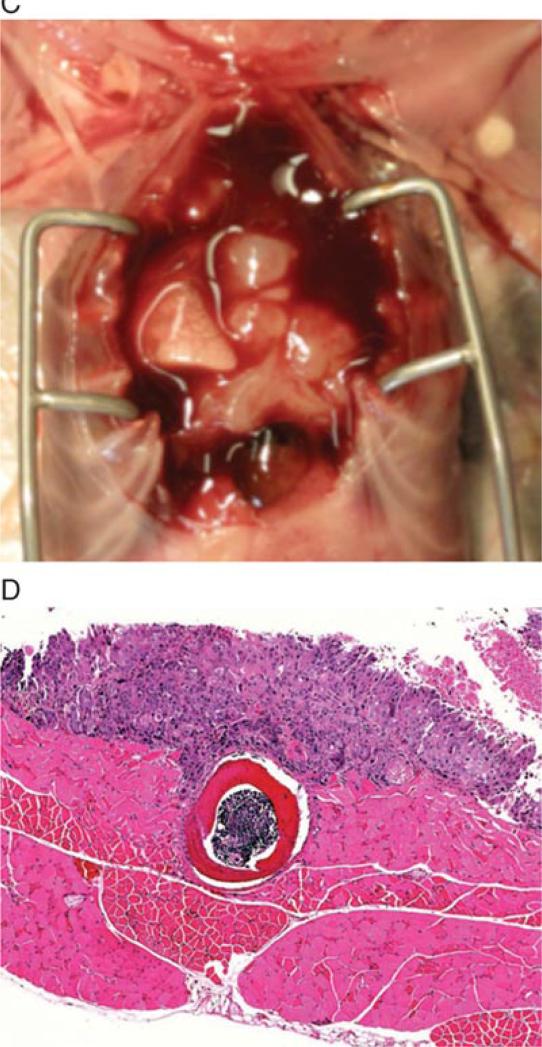
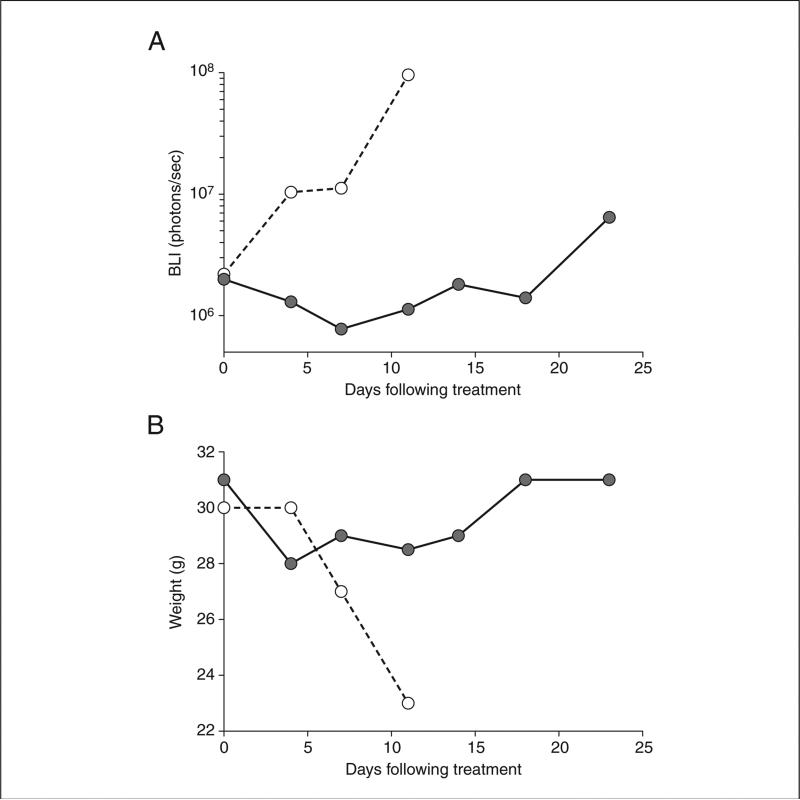
This orthotopic model of pleural malignancy displays the sentinel characteristics of pleural cancers including vascular tumor nodules, local invasion and metastases, and malignant pleural effusions. Based on these observations, this model offers a platform to examine the response of pleural cancer to therapy. In our laboratory we have used this model to assess the effect of intrapleural and systemic therapies and evaluate responses with quantitative non-invasive imaging. To examine if quantitative BLI measured response to therapy, nude mice were inoculated with 1×106 MPM tumor cells and treated with a single dose of cisplatin (4 mg/kg), thoracic radiation therapy (20Gy in 5 fractions), combination chemoradiation therapy, or vehicle. Treated mice showed decreased BLI signal, maintained body weight, and exhibited prolonged survival with best response in those mice treated with chemoradiation (p=0.05). In contrast, untreated mice succumbed to progressive disease and BLI signal increased (Figure 4). These results demonstrate the utility of this orthotopic mouse model as an appropriate platform to investigate clinically relevant treatment regimens for malignant pleural disease.
Figure 4.
Orthotopic model of pleural malignancy permits evaluation of therapies. In an experiment where athymic nude mice were inoculated with MPM cells (1×106 MSTO-211H) intrapleurally and then treated 10 days later. (A) BLI signal from a representative mouse receiving a single dose of cisplatin (4 mg/kg; ●) and an untreated mouse (○ shows disease progression in the absence of treatment. (B) A single dose of chemotherapy prolongs survival and maintains body weight. (○ = untreated; ● = cisplatin 4 mg/kg).
Timing Considerations
Tumor cell suspension preparation requires a minimum of 30 minutes. Preparation of the surgical area, stage, induction chamber, and inhaled anesthesia circuit can be completed in less than 30 minutes. Although not recommended, if this protocol is undertaken by a single investigator, the preparation, injection, and recovery of 10 mice will take approximately 60 minutes depending on the user's level of proficiency. With practice this can be decreased to less than 30 minutes per 10 mice. When aided by one assisting in the post-operative recovery of the mice, this protocol can be efficiently carried out with minimal mortality on large cohort of mice. When assisted by two or more colleagues, experienced users can inject 100 mice in 3 hrs, with less than 3% post-operative mortality.
Literature Cited
- Adusumilli P, Eisenberg D, et al. Intraoperative localization of lymph node metastases with a replication-competent herpes simplex virus. Journal of thoracic and cardiovascular surgery. 2006;132(5):1179–1188. doi: 10.1016/j.jtcvs.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Adusumilli PS, Stiles BM, et al. Imaging and therapy of malignant pleural mesothelioma using replication-competent herpes simplex viruses. J Gene Med. 2006;8(5):603–615. doi: 10.1002/jgm.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antunes G, Neville E, et al. BTS guidelines for the management of malignant pleural effusions. Thorax. 2003;58(Suppl 2):ii29–38. doi: 10.1136/thorax.58.suppl_2.ii29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton KN, Richardson JD. Diagnosis and management of malignant pleural effusions. Am J Surg. 1995;170(1):69–74. doi: 10.1016/s0002-9610(99)80257-8. [DOI] [PubMed] [Google Scholar]
- Hoogstraten-Miller SL, Brown PA. Techniques in aseptic rodent surgery. Curr Protoc Immunol. 2008 doi: 10.1002/0471142735.im0112s82. Chapter 1: Unit 1 12 11-11 12-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manac'h D, Riquet M, et al. Visceral pleura invasion by non-small cell lung cancer: an underrated bad prognostic factor. Ann Thorac Surg. 2001;71(4):1088–1093. doi: 10.1016/s0003-4975(00)02649-7. [DOI] [PubMed] [Google Scholar]
- Michailova KN, Usunoff KG. Serosal membranes (pleura, pericardium, peritoneum). Normal structure, development and experimental pathology. Adv Anat Embryol Cell Biol. 2006;183:i–vii. 1–144. back cover. [PubMed] [Google Scholar]
- Mishra E, Davies RJO. Advances in the investigation and treatment of pleural effusions. Expert Review of Respiratory Medicine. 2010;4(1):123–133. doi: 10.1586/ers.09.67. [DOI] [PubMed] [Google Scholar]
- Newell J. Guidelines for Survival Rodent Surgery, NIH-OACU. 2007 [Google Scholar]
- Osaki T, Nagashima A, et al. Visceral pleural involvement in nonsmall cell lung cancer: prognostic significance. Ann Thorac Surg. 2004;77(5):1769–1773. doi: 10.1016/j.athoracsur.2003.10.058. [DOI] [PubMed] [Google Scholar]
- Sahn SA. Malignant Pleural Effusions. Semin Respir Crit Care Med. 2001;22(06):607–616. doi: 10.1055/s-2001-18796. [DOI] [PubMed] [Google Scholar]
- Shimizu K, Yoshida J, et al. Visceral pleural invasion is an invasive and aggressive indicator of non-small cell lung cancer. Journal of thoracic and cardiovascular surgery. 2005;130(1):160–165. doi: 10.1016/j.jtcvs.2004.11.021. [DOI] [PubMed] [Google Scholar]
- Stathopoulos GT, Kalomenidis I. Animal models of malignant pleural effusion. Curr Opin Pulm Med. 2009;15(4):343–352. doi: 10.1097/MCP.0b013e32832af07c. [DOI] [PubMed] [Google Scholar]
- Stiles BM, Adusumilli PS, et al. Minimally invasive localization of oncolytic herpes simplex viral therapy of metastatic pleural cancer. Cancer Gene Ther. 2006;13(1):53–64. doi: 10.1038/sj.cgt.7700860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zocchi L. Physiology and pathophysiology of pleural fluid turnover. Eur Respir J. 2002;20(6):1545–1558. doi: 10.1183/09031936.02.00062102. [DOI] [PubMed] [Google Scholar]