Abstract
The elastic modulus of the extracellular matrix is a dynamic property that changes during various biological processes, such as disease progression or wound healing. Most cell culture platforms, however, have traditionally exhibited static properties, making it necessary to replate cells to study the effects of different elastic moduli on cell phenotype. Recently, much progress has been made in the development of substrates with mechanisms for either increasing or decreasing stiffness in situ, but there are fewer examples of substrates that can both stiffen and soften, which may be important for simulating the effects of repeated ECM injury and resolution. In the work presented here, poly(ethylene glycol)-based hydrogels reversibly stiffen and soften with multiple light stimuli via photoisomerization of an azobenzene-containing crosslinker. Upon irradiation with cytocompatible doses of 365 nm light (10 mW/cm2, 5 min), isomerization to the azobenzene cis configuration leads to a softening of the hydrogel up to 100-200 Pa (shear storage modulus, G’). This change in gel properties is maintained over a timescale of several hours due to the long half-life of the cis isomer. The initial modulus of the gel can be recovered upon irradiation with similar doses of visible light. With applications in mechanobiology in mind, cytocompatibility with a mechanoresponsive primary cell type is demonstrated. Porcine aortic valvular interstitial cells were encapsulated in the developed hydrogels and shown to exhibit high levels of survival, as well as a spread morphology. The developed hydrogels enable a route to the noninvasive control of substrate modulus independent of changes in the chemical composition or network connectivity, allowing for investigations of the effect of dynamic matrix stiffness on adhered cell behavior.
Keywords: biomaterials, azobenzene, matrix mechanics, dynamic hydrogel
Introduction
In conjunction with biological ligands and proteins, the mechanical properties of the native extracellular matrix (ECM) environment present powerful cues that direct cell phenotype. To decouple the effects of matrix stiffness from other factors, synthetic hydrogels have emerged as a facile route to presenting cells with a substrate of well-defined elasticity.1, 2 However, the native ECM modulus is a dynamic property that fluctuates as cells remodel their environment during disease progression, wound healing, or development. While many hydrogel platforms can recapitulate the elasticity of a tissue of interest, fewer provide handles to change this elasticity on demand and in the presence of attached cells.
Recently, several strategies have emerged to tune the elastic modulus of a substrate in situ. For example, DNA-crosslinked hydrogels have been shown to modulate reversible changes in stiffness over tens of kilopascals by hybridization with introduced DNA strands that are complementary to the crosslinker.3, 4 Another strategy induces the formation of reversible crosslinks through the addition of divalent cations to a collagen-alginate composite hydrogel.5 Both of these strategies show good reversibility; however, they also both rely on the addition of a component (DNA or cations such as Ca2+) that can be uptaken by cells and may alter cellular signaling or behavior. Changes in protein conformation can also be used to confer dynamic properties to a hydrogel.6, 7 For example, Kong et al. took an innovative approach to reversibly controlling modulus in protein hydrogels by using redox chemistry to fold and unfold the protein, thereby altering the crosslinker length.6 Effects of the changes in oxidation state on cells have yet to be explored. As a complement to these systems, photoreactions are an attractive route to controlling ECM elasticity in a non-invasive way. Kloxin et al. developed poly(ethylene glycol) (PEG) networks with a photodegradable moiety incorporated next to the crosslinking site.8 Using cytocompatible doses of 365 nm light, the user can thus decrease the modulus of a hydrogel over several kilopascals by decreasing the crosslinking density. Light-based chemistries can also be used to stiffen a hydrogel, as demonstrated by Guvendiren et al. in a hyaluronic acid-based system that uses sequential addition and radical polymerization crosslinking reactions.9 While both of these strategies offer spatial and temporal control, the changes they confer to the network structure and matrix mechanics are irreversible.
One of the most enticing platforms for a reversible, photo-based reaction to control matrix mechanics is the use of a photoisomer, especially azobenzene. Azobenzenes have been used to control properties of polymeric networks, such as the gel-sol transition10-12 and the swelling ratio.13 They have emerged as an effective photoswitch for use in biomaterials because they absorb light in a region that is compatible with many biological systems (350-550 nm).14 In small molecule systems, azobenzene converts from the trans isomer to the cis isomer upon irradiation with 360 nm light in a remarkably efficient reaction (~80%-95%). Removal of the light or irradiation in the visible range regenerates the trans isomer almost completely (>99%) because it is the more thermodynamically stable conformation. These properties can be recapitulated in azobenzene-containing hydrogel networks to control matrix properties.
One of the main advantages of controlling hydrogel mechanics with azobenzene is the lack of initiator required to complete photoisomerization. Because this photoreaction proceeds with light alone, no free radicals are created during irradiation. Furthermore, light is a non-invasive stimulus that allows for changes without altering the chemical environment of the cells. Azobenzene undergoes a structural change, and it is therefore anticipated that gel mechanics will be controlled without affecting the overall network connectivity. However, because the reverse isomerization of azobenzene occurs thermally, there is likely to be some reversal of the modulus over time. For this reason, it is critical to choose an azobenzene with stable cis isomer and a long half life.
In the work presented here, azobenzene is incorporated into peptide crosslinkers of PEG hydrogels to modulate the matrix elasticity in a reversible manner with both 365 nm (UV) and 400-500 nm (visible) light. The photoisomerization disrupts hydrogen bonds from the surrounding network, leading to a decrease in matrix elasticity that is fully recovered upon thermal relaxation or the reverse isomerization of the azobenzene. The incorporated photoswitch was carefully chosen to maximize the lifetime of the cis state at 37°C, and cytocompatibility was demonstrated with encapsulated primary porcine aortic valvular interstitial cells (VICs). This work establishes an accessible route to the reversible control of matrix elasticity in the presence of primary cells cultured in three dimensions. Future efforts will focus on maximizing the elastic response to direct cell phenotype.
Materials and Methods
Synthesis of 3-[[4-([[(9H-Fluoren-9-ylmethoxy)carbonyl]amino]methyl)phen-yl]diazenyl]benzoic Acid (3,4’-Fmoc-AMPB)
3,4’-Fmoc-AMPB was synthesized in two steps according to previously published methods.15, 16 Briefly, 3-nitrosobenzoic acid was first synthesized by dissolving 3-aminobenzoic acid (2 g, 14.6 mmol) in DCM (40 mL). Oxone (18 g, 29.3 mmol, 2 equivalents) dissolved in 160 mL water was added to the reaction flask to create a biphasic solution. The solution was stirred under argon at room temperature for 3 hours. The precipitate was separated by first evaporating the DCM, followed by centrifuging. The precipitate was washed two times with water and two times with methanol, then dried in a vacuum oven overnight at 40 °C. 3-nitrosobenzoic acid (Figure S1): 1H NMR (DMSO-d6, 500 MHz): δ= 7.88 (m, 1H), 8.19 (d, 1H), 8.39 (m, 2H), 13.52 (s, br., 1H).
In the second step, the 3-nitrosobenzoic acid (2.0 g, 13.4 mmol) was sonicated in a 1:1 mixture of acetic acid and DMSO (50 mL:50 mL). To this green solution, 4-(N-Fmoc-aminomethyl)aniline (2.3 g, 6.69 mmol, 0.5 equivalents) was added, and the reaction solution was stirred under argon for three days at room temperature. The precipitated orange product was centrifuged to separate it from the reaction solution and then redissolved in ethyl acetate (~ 4L) and washed with water. The organic layers were dried over MgSO4 and the solvent was evaporated. The product was then used without further purification. 3,4’-Fmoc-AMPB (Figure S2): 1H NMR (DMSO-d6, 500 MHz): δ= 4.26 (t, 1H), 4.29 (d, 2H), 4.40 (d, 2H), 7.35 (t, 2H), 7.43 (m, 4H), 7.73 (m, 3H), 7.90 (d, 2H), 7.91 (d, 2H), 7.99 (t, 1H), 8.14 (m, 2H), 8.39 (s, 1H).
Synthesis of azobenzene-containing peptide
An azobenzene-containing peptide crosslinker (KCGGPQG-3,4’-AMPB-IWGQGCK) was synthesized on a solid phase peptide synthesizer (Tribute Protein Synthesizer, Protein Technologies, Inc.) using Rink Amide MBHA resin (Novabiochem, 0.59 mmol/g). Briefly, Fmoc deprotection proceeded via 20% piperidine/2% 1,8-diazabicyclo[5.4.0]undec-7-ene in NMP for 20 minutes. Fmoc-protected amino acids were coupled in 4-fold molar excess in the presence of 0.4M N-methylmorpholine in DMF for 30 minutes, except for the 3,4’-Fmoc-AMPB, which was used in a 2-fold molar excess. The N-terminus of the peptide was acetylated using 5% acetic anhydride and 6% lutidine in NMP, and the peptide was cleaved from the resin using trifluoroacetic acid (TFA), phenol, triisopropylsilane, and water (95/2.5/1.25/1.25 v/v) for at least 2 hours. Crude peptides were precipitated into chilled diethyl ether, then purified by semi-preparative reverse phase HPLC (AutoPurification HPLC, Waters) using a 10 minute gradient of acetonitrile in water (5-50%, 30 mL/min). The peptide eluted at 36.5% acetonitrile, and the expected mass was confirmed by matrix assisted laser desorption ionization time-of-flight mass spectrometry (Figure S3: MALDI-TOF, Applied Biosystems DE Voyager, exp/obs: 1698.9/1697.8). A matrix metalloproteinase (MMP)-degradable sequence (KCG-3,4’-AMPB-GPQGIWGQGCK) was made in the same fashion.
Hydrogel formation
Photoresponsive hydrogels were formed using a Michael-type addition of dithiol peptides (described above) onto multi-arm vinyl sulfone-functionalized poly(ethylene glycol)s (PEG-VS, JenKem Technology USA). PEG-VS was dissolved in either 0.1 M HEPES buffer (pH 7.8) or 0.4 M triethanolamine in PBS (pH 8) at the desired w/v concentration. The peptide crosslinker was dissolved in 3:1 DMSO:H2O at a concentration of 80 mM. Prior to making the peptide stock solutions, the free thiol content of the photoresponsive peptide was measured using an Ellman’s assay. Only peptides with a free thiol content of 70% or greater were used, and adjustment of the functional group concentration with this assay enabled consistent gel formation between peptide batches (Table 1). However, for best results, gels prepared with peptide from the same batch were compared, as in the data presented in Figure 5. The PEG-VS and peptide components were mixed at the desired stoichiometric ratio, quickly vortexed, and reacted in a 0.5 mm thick rubber mold between two SigmaCoted glass slides. The gels were allowed to react at 37°C for 2 hours at pH 8 or 30 minutes at room temperature in the HEPES buffer, as noted. After gelation, the gels were gently removed from the molds and placed into PBS to swell overnight at room temperature.
Table 1.
Properties of swollen photoresponsive hydrogels.
| PEG -VS Macromer |
[PEG] (mM) | ρxl (mM)a | Qexpb | G’ (Pa) | ΔG’ (Pa) c |
|---|---|---|---|---|---|
| 4-arm, 10 kDa | 5.2 | 0.2 | 19.8 | 190 | 7 |
| 11.1 | 2.0 | 15.5 ± 1.5 | 1987 ± 530 | 25 ± 5 | |
| 11.1 | 6.0 | 15.5 ± 1.5 | 6170 ± 410 | 51 ± 1 | |
| 8-arm, 20 kDa | 2.6 | 0.5 | 11.8 ± 2.3 | 500 | 12 |
| 2.6 | 1.0 | 11.8 ± 2.3 | 1170 ± 155 | 48 ± 15 | |
| 2.6 | 2.5 | 11.8 ± 2.3 | 2829 ± 422 | 44 ± 6 | |
| 2.6 | 5.0 | 11.8 ± 2.3 | 5700 ± 200 | 121 ± 20 |
Calculated crosslinking density, which corresponds to the concentration of azobenzenes in the gel
Swelling ratio, calculated by Qexp = 1 + ρpolymer/ρH20*(Mswollen/Mdry−1), where M is the mass of the gel
Change in G’ upon irradiation with 365 nm light, 10 mW/cm2, 5 min
Figure 5.
Encapsulated valvular interstitial cells (VICs) stained with calcein-AM (green, live) and ethidium homodimer (red, dead) after 3 days in (a) 5wt% 8-arm PEG-VS, 100% non-degradable linker (100%nd), (b) 5wt% 8-arm PEG-VS, 38%nd, and (c) 4wt% 8-arm PEG-VS, 38%nd. (d) Quantitative live/dead results at 3 days of culture for 4 wt% and 5 wt% gels with and without MMP-degradable crosslinker (% denotes percentage of non-degradable linker). (e) Immunostaining for αSMA (green), f-actin (red), and nuclei (blue) in 4wt%PEG, 38% non-degradable linker after 5 days of culture. All scale bars = 100 μm.
UV/Vis spectroscopy
Absorbance spectra were collected with a Synergy H1 microplate reader (BioTek) using a wavelength range of 280 nm to 700 nm. For swollen hydrogel measurements, 20 μl hydrogels were directly formed in a clear 96-well plate, and they were allowed to swell overnight in 200 μl of PBS before taking absorbance readings. Two high pressure mercury light sources were used for irradiation experiments: Exfo Omnicure S1000, 365 nm, 10 mW/cm2 and Exfo Acticure 4000, 400-500 nm, 10 mW/cm2.
Rheology
For rheological measurements of swollen hydrogels, the gel was loaded onto a rheometer (TA Instruments, Discovery HR-3) with an 8 mm parallel plate geometry and a UV light guide accessory. A 5 mm liquid light guide was manually switched between two high pressure mercury light sources (Exfo Omnicure S1000, 365 nm, 10 mW/cm2 and Exfo Acticure 4000, 400-500 nm, 10 mW/cm2) at the desired time points while measuring the modulus of the gel at 25°C using an oscillatory strain of 1% and a frequency of 1 rad/s (linear viscoelastic regime). The gel was surrounded with water to maintain equilibrium swelling and avoid drying at the gel-air interface. The starting axial force for all measurements was between 0.2 and 0.4 N.
For in situ gel evolution measurements, 20 μl of the gel precursor solution was quickly vortexed then added to the plate. Modulus measurements were taken immediately after the precursor solution was added to the rheometer, and the formulation was surrounded with dimethylpolysiloxane (Sigma Aldrich) to prevent evaporation of water.
VIC isolation and culture
Using a collagenase digestion, primary VICs were isolated from porcine aortic leaflets, which were obtained from fresh hearts acquired from Hormel within 24 hours of slaughter.17, 18 Aliquots were frozen in a liquid nitrogen cooled tank until needed, then thawed and expanded on tissue culture polystyrene (TCPS) plates. VICs were thawed in Media M199 supplemented with 15% fetal bovine serum (FBS), 100 U/mL penicillin, 100 ug/mL streptomycin, and 1 ug/mL fungizone at 37°C and 5% CO2. VICs were expanded for two days, then passaged once and expanded for an additional day before encapsulation into the hydrogels.
VIC encapsulation
VICs were trypsinized from TCPS plates, pelleted from 15% FBS media, and resuspended in PBS at a concentration of 25 × 106 cells/mL. The appropriate amount of the cell suspension was added to the gel precursor solution to achieve an encapsulation density of 10 × 106 cells/mL. The gel precursor solution contained PEG-VS at a concentration of 10 wt. % in sterile-filtered 0.1 M HEPES buffer at pH 7.8, the peptide crosslinker (80 mM) in 3:1 DMSO:H2O, and the appropriate amount of sterile PBS to achieve the desired final concentration of PEG-VS and peptide. The cell-containing precursor solution was quickly vortexed, and 40 μL were pipetted into a rubber mold (0.5 mm thick, 8 mm diameter) between two SigmaCoted slides. The gels were allowed to form at room temperature for 30 minutes in a sterile tissue culture hood; then they were gently removed from the molds and placed into an ultra-low adhesion well plate with 2 mL of 1% FBS M199 media. Media was changed the next day.
Cell viability analysis
VIC viability was quantified using a Live/Dead membrane integrity assay (Live/Dead, Life Technologies). Samples were incubated in PBS containing 1 μM calcein AM and 4 μM ethidium homodimer for 30 minutes. Cells were imaged on a 710 LSM NLO confocal microscope (Zeiss), and images were stacked using maximum intensity projections. Cells in which the membrane-permeable calcein AM was cleaved by esterases within the cell were counted as live, while cells permeable to ethidium homodimer were counted as dead. Over 100 cells were counted per sample. For each condition, 3 or more biological replicates were performed. Error bars represent standard error of the mean.
Immunostaining for α-smooth muscle actin (αSMA) and f-actin
Samples were fixed in 10% formalin and then washed with PBS. Permeabilization with 0.1% TritonX in PBS was followed by blocking of non-specific staining with 1% BSA in PBS. Mouse anti-αSMA diluted 1:200 in PBS was applied to the samples. Gels were then rinsed with PBS prior to application of AlexaFluor 488 goat-anti-mouse (1:300) and TRITC-phalloidin (1:300) to stain for f-actin. Next, nuclei were stained with 4',6-diamidino-2-phenylindole (0.01 mg/mL). Cells were imaged on a 710 LSM NLO confocal microscope (Zeiss).
Results and Discussion
Here, a three-dimensional hydrogel platform with photoresponsive mechanical properties was developed. Because the elasticity of biological tissues is a dynamic property, a cell culture substrate with reversible properties could lend insight on the effects of changing stiffness on cell phenotype without confounding factors such as replating cells. Furthermore, a three-dimensional geometry better recapitulates the natural extracellular matrix environment compared to a two-dimensional substrate.
Gel Formation
To obtain a hydrogel with photoresponsive mechanical properties, a peptide crosslinker containing a photoisomer (3,4’-AMPB) was first synthesized. Although the photoisomer could also be incorporated at the chain ends of a PEG crosslinker (Figure S4), the use of a peptide crosslinker enabled facile incorporation with solid-phase synthesis methods, as well as site-specific incorporation into the crosslinker. Here, the peptide sequence was based on that of native collagen, and the location of the photoisomer either conferred degradability or non-degradability to the peptide (KCG-3,4’-AMPB-GPQGIWGQGCK or KCGGPQG-3,’4-AMPB-IWGQGCK, respectively). The degradable photoresponsive peptide was only used in the cell encapsulation studies detailed below.
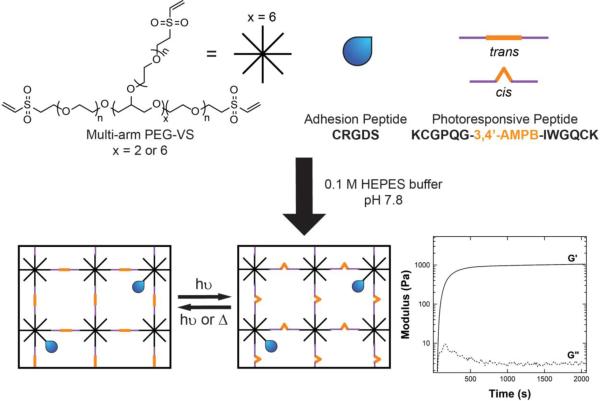
To form the hydrogel, a Michael addition reaction was used under slightly basic conditions with a PEG-VS macromer and the photoresponsive peptide (Figure 1). Previously, Michael addition-type reactions have been shown to be an effective route to encapsulating cells within a hydrogel.19, 20 In addition, the use of Michael addition chemistry avoids the use of a photoinitiator. At 365 nm, the molar absorptivity of the photoresponsive peptide is approximately 2300 M−1cm−1, which is at least an order of magnitude higher than that of other initiators such LAP (218 M−1cm−1)21 or I2959 (4 M−1cm−1)21. The photoresponsive peptide also demonstrates significant absorption in the visible light range (e.g., molar absorptivity at 420 nm ~ 270 M−1cm−1); thus, attempts to photoinitiate gel formation resulted in poor or no gelation with a variety of PEG macromers and photoinitiators. In contrast, the Michael addition reactions allowed for gelation within minutes. Figure 1 illustrates one example of the rapid gelation that occurs for the PEG-VS/peptide gels in 0.1M HEPES buffer at pH 7.8. Crossover of the storage modulus, G’, with the loss modulus, G”, occurs within 2 minutes, and 95% of the final modulus is achieved within 15 minutes. Furthermore, by varying the initial macromer concentration or crosslinking density, a range of initial storage moduli, G’, were achieved that spanned 500 Pa – 8000 Pa.
Figure 1.
Photoresponsive hydrogels are formed using Michael addition reactions between multi-arm PEG vinylsulfones (PEG-VS) and thiol-containing peptides in 0.1 M HEPES buffer at pH 7.8. The schematic above represents an off-stoichiometry formulation. The crossover of the storage (G’) and the loss (G”) modulus occurs within minutes, and the gel evolution plateaus within 15 minutes.
Because the Michael addition reaction occurs at basic pH, the reaction competes with disulfide formation between the peptide crosslinkers. For this reason, the measured moduli of the resulting gels were lower than the theoretical moduli. Although the addition of a reducing agent such as tris(2-carboxyethyl)phosphine (TCEP) would not interfere with the Michael addition and thus facilitate complete gelation, its presence could potentially be detrimental to cellular proteins during encapsulation. For this reason, the gel properties were examined without addition of a reducing agent.
Gel Mechanical Properties
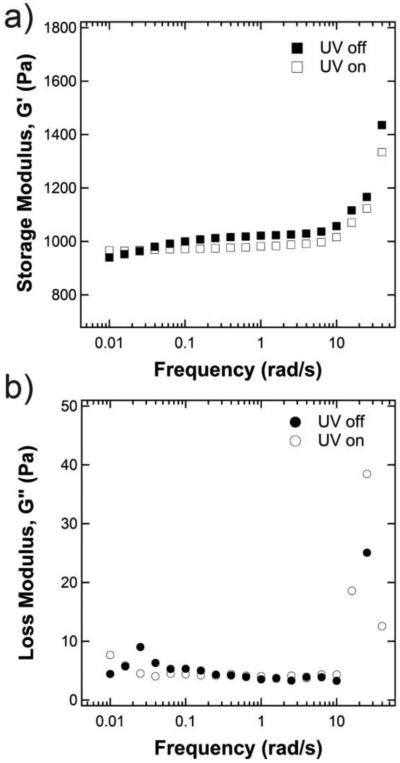
To understand the viscoelastic properties of the hydrogel, the swollen shear modulus was first measured over frequencies ranging from 0.005 rad/s to 100 rad/s at a constant strain of 1% (Figure 2). Over the entire frequency range, G’ (Figure 2a) was approximately two orders of magnitude larger than the loss modulus, G” (Figure 2b), indicating that the elastic properties of the gel dominate over the viscous losses. Furthermore, the hydrogels showed a frequency-independent modulus characteristic of an elastic Hookean solid between frequencies of approximately 0.1 – 10 rad/s. Therefore, a frequency of 1 rad/s was chosen for all subsequent studies. Next, to understand the response of the hydrogel’s mechanical properties to light, the modulus was examined with the UV light either on (open symbols) or off (closed symbols). When the UV light was on, G’ was smaller in the linear viscoelastic range (by approximately 50 Pa for the example shown in Figure 2a), indicating a change in gel structure. Interestingly, at very low frequencies outside of the linear viscoelastic range, there was significant overlap between the irradiated and non-irradiated gels. No significant change was observed in the loss modulus.
Figure 2.
Typical frequency sweeps of photoresponsive hydrogels with and without UV irradiation (365 nm, 10 mW/cm2) showing both the storage (a) and the loss (b) modulus. The gel shown here is formulated with 8-arm, 20 kDa PEG macromer at 5 wt% and 1.0 mM crosslinking density.
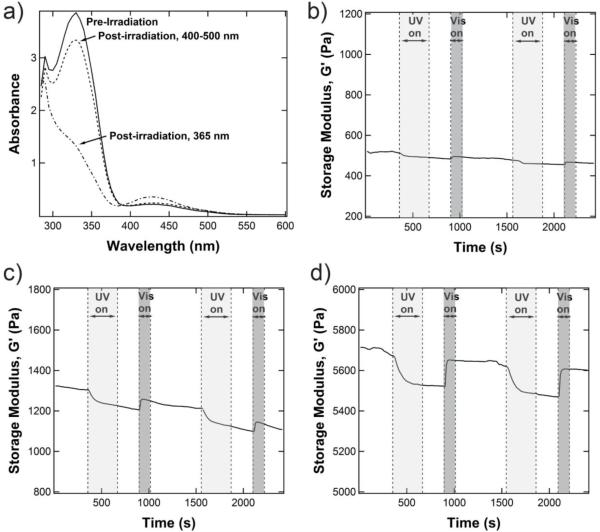
The change in G’ reflects changes in the gel structure conferred by the isomerization of azobenzene between the planar, trans configuration (UV off) and the bent, cis conformation (UV on). Confirmation of the isomeric state of the azobenzene-containing crosslinker was obtained by measuring the absorbance spectrum of the swollen hydrogel (Figure 3a). Azobenzene exhibits two characteristic bands that shift depending on the substituents of the phenyl rings: one in the UV region (~320-360 nm) that corresponds to the π-π* transition and one in the visible region (~420-450 nm) that corresponds to the n-π* transition. Due to symmetry constraints, the n-π* transition is not allowed in the planar trans configuration, therefore making the visible range absorbance very weak. However, upon isomerization, the n-π* transition is allowed, and the absorbance in the visible range increases because the molar extinction coefficient for the cis n-π* transition is larger than that of the trans n-π* transition.22 Simultaneously, the cis π-π* transition in the UV range is weaker than that of the trans configuration. These characteristics are observed in the swollen hydrogels formed here. Before exposure to UV light, the gel displays an absorbance maximum at 330 nm, which is indicative of the π-π* transition and the azobenzene unit in the trans state. Immediately after irradiation at 365 nm (10 mW/cm2, 2 min), the peak at 330 nm largely decreases and a new peak emerges at 425 nm, corresponding to the cis n-π* transition and the emergence of a photostationary state that is predominantly composed of the cis isomer. The trans state can be recovered with minimal hysteresis by irradiation with a visible light source (400-500 nm, 10 mW/cm2, 2 min). These light doses are comparable to those used previously in cytocompatible systems,8, 23, 24 thus allowing for non-invasive manipulation of molecular properties in the presence of cells. At this molecular level, the isomerization is very fast; to probe changes on a bulk level, we turned to rheology.
Figure 3.
a) Representative UV/Vis of an equilibrium swollen 5 wt% 8-arm PEG-VS gel with 5.0 mM azobenzene-containing peptide crosslinker. UV/Vis shows isomerization of the azobenzene-containing crosslinker to the cis form after irradiation with 365 nm light (10 mW/cm2, 2 min) and back to the trans form after irradiation with 400-500 nm light (10 mW/cm2, 2 min). Rheology shows a cyclic modulus change with alternating irradiation at 365 nm (10 mW/cm2, 5 min) and 400-500 nm (10 mW/cm2, 2 min) for an equilibrium-swollen 5 wt% 8-arm PEG-VS hydrogel crosslinked at (b) 0.5 mM, (c) 1.0 mM, and (d) 5.0 mM. The magnitude of the change in modulus increases with increasing azobenzene content. All rheological data here is presented on the same scale for ease of comparison.
The dynamic response of the swollen gel storage modulus G’ to both UV and visible light was measured in situ as shown in Figure 3, for three different gels (Figure 3a corresponds to 0.5 mM crosslinking density, Figure 3b corresponds to 1.0 mM crosslinking density, and Figure 3c corresponds to 5.0 mM crosslinking density). Upon irradiation with UV light (light gray shading, 365 nm, 10 mW/cm2, 5 min), the gel modulus decreased in an exponential fashion. After removal of the light, G’ stayed constant for 4 minutes, after which a visible light cue was introduced (dark gray shading, 400-500 nm, 10 mW/cm2, 2 min) and G’ quickly increased back to the initial modulus. Again, after removal of the light, G’ stayed constant until the next light cue was introduced. Depending on the hydrogel, sometimes a steady decrease in the modulus was observed (as in Figure 3c). This artifact is due to a decreasing axial force during the measurement, which may arise from small changes in the swelling of the gel during isomerization. Subsequent measurements on the same gel showed similar starting moduli, confirming that no degradation had occurred. Although only two cycles are shown in Figure 3, the gel retained its dynamic switching characteristics over numerous cycles. Furthermore, because of its small absolute value, no significant changes were observed in the loss modulus, G”.
One interesting characteristic of these gels is the decrease in modulus upon isomerization of the crosslinker to the cis state. Previously, Petr et al. observed a decrease in modulus as the cis state disrupted liquid crystalline order in a polymeric network.25, 26 In addition, several studies have observed gel-sol transitions upon the disruption of hydrogen bonds between neighboring azobenzene units in supramolecular systems.27, 28 Although the hydrogen bond structure in this system is still under investigation, the planar trans conformation may better be able to stabilize hydrogen bonds between the peptide crosslinkers, thereby rigidifying the substrate. Such a structure would then correspond to the “stiff” trans state and “soft” cis state observed here. Heating the hydrogels confirms the presence of hydrogen bonding, as the modulus sharply decreases at higher temperatures (Figure S5). This result is in direct contrast to that predicted by rubber elasticity theory, which states that the modulus of a swollen network should scale with temperature:
| (1) |
where ρxl is the crosslinking density and Q is the swelling ratio. The magnitude of the switch in modulus was dictated by the crosslinking density, or the content of azobenzenes within the gel (gels without azobenzene showed no switch in modulus, Figure S6). As shown in Figure 3 b-d, the magnitude of the change in modulus increased from 12 Pa for a crosslinking density of 0.5 mM to 121 Pa for a crosslinking density of 5 mM. Table 1 lists the change in modulus for other gel configurations and concentrations. Both the 8-arm, 20 kDa PEG gels and the 4-arm, 10 kDa PEG gels have the same crosslinker length, but the 8-arm, 20 kDa gels have a higher theoretical crosslinking density for the same gel concentration, which corresponds to a greater number of azobenzenes per gel. In addition, the 8-arm configuration increases the local concentration of the azobenzene units, which may allow for more hydrophobic interactions. As a result, the equilibrium swelling for the 8-arm gels is less than that predicted. This difference in swelling may also be related to the observed magnitude change per mmol of azobenzene, which is greater in the 8-arm gels than the 4-arm gels. Interestingly, attempts to further increase the azobenzene content by increasing the concentration of the gel (higher weight %) or decreasing the size of the crosslinker (lower molecular weight) resulted in no switching (Figure S7). Past a critical concentration of azobenzene, the gel structure is likely altered or further stabilized by interactions between neighboring azobenzene units.
Although the change in the hydrogel modulus upon exposure to UV light shows good reversibility, the magnitude is quite modest (in the 10-100 Pa range of shear modulus). Many 2D studies of the effects of matrix elasticity on cellular phenotype use a modulus range that spans 10-100 kPa; however, numerous cell types have demonstrated mechanosensitivity over a much smaller modulus range, especially in 3D geometries.29-34 For instance, dermal fibroblasts exhibited upregulated mediators of inflammation when encapsulated in stiff hydrogels (1200 Pa) compared to soft hydrogels (50 Pa).29 In another example, MMP activity of encapsulated hMSCs increased with stiffness when comparing soft (200 Pa), medium (500 Pa), and stiff (1200 Pa) hydrogels.34 This range of moduli for 3D cell behaviors is similar to that shown by the photoresponsive gels developed here, and current efforts are focused on tuning the parameters of this system to study activation of fibroblasts in 3D.
Gel Relaxation Behavior
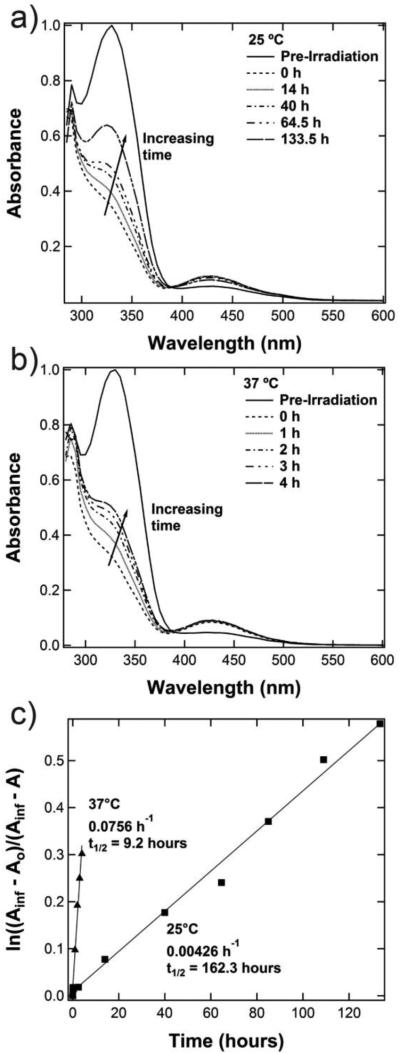
While Figure 3 demonstrates the photoresponsive behavior of the hydrogels on short time scales (minutes), the time scale of interest for many important cell functions is hours or days. After irradiation with UV light, the azobenzene cis isomer should slowly relax back to the more energetically stable trans state. Thus, the thermal relaxation behavior of the photoresponsive gel was studied by monitoring the change in the absorbance spectra over time. After irradiation with 365 nm light (10 mW/cm2, 5 min), the absorbance of the swollen hydrogel was measured over several hours, and the gel was kept in the dark at the desired temperature in between measurements. As shown in Figure 4a for a hydrogel at 25°C, the peak at 330 nm slowly increased over approximately a week, indicating an increasing population of trans isomers. At 37°C (Figure 4b), this relaxation transition occurred much more quickly, which is in agreement with Arrehenius-type behavior.
Figure 4.
a) Thermal relaxation at 25 °C for equilibrium swollen 5 wt% 8-arm PEG-VS hydrogel containing the photoresponsive peptide after irradiation with 365 nm light (10 mW/cm2, 5 min); b) thermal relaxation at 37 °C; c) kinetic plots show that the relaxation behavior follows first-order kinetics and yields a half life of 162.3 hours at 25°C and 9.2 hours at 37°C.
The thermal relaxation behavior follows first order kinetics (Figure 4c), which can be described by Equation 235:
| (2) |
In this equation, A∞ is taken to be the absorbance of the trans isomer at 330 nm before irradiation, A0 is the absorbance at 330 nm for the photostationary state of the gel (t=0 after irradiation), and At is the absorbance at 330 nm at time t. Using this equation, the reaction rate constant for the thermal relaxation to the trans isomer at 37°C is an order of magnitude larger than that at 25°C: 0.0756 h−1 compared to 0.0043 h−1, respectively. The half time of the reaction can therefore be calculated and corresponds to 162.3 hours at 25°C and 9.2 hours at 37°C.
The hydrogels presented here remain in a stiffened state over several hours after only 5 minutes of UV irradiation. The stability of the cis isomer thus enables minimal exposure to UV light and avoids the creation of potentially harmful free radicals. However, it is worthy to note that the stiffening or softening of biological tissues does not happen on the time scale of minutes. One could better approximate a natural timescale by allowing the cis isomer to thermally relax, which proceeds to completion in 18 hours at 37°C. As for stiffening to intermediate moduli, this can be accomplished by irradiation with wavelengths of light that are further away from the absorption maximum of the trans state. Because the efficiency of the photoisomerization depends on the wavelength of light, the cis content of the photostationary state, and therefore the change in modulus, can theoretically be decreased with irradiation by longer wavelengths of UV light.
VIC Viability
To demonstrate cytocompatibility with a mechanoresponsive cell type, valvular interstitial cells (VICs) were encapsulated in the photoresponsive gels at gel compositions of both 4 and 5 wt% using an 8-arm, 20 kDa PEG-VS macromer (Figure 5a-c) with 2 mM RGD as an adhesive ligand (final modulus was between 400-800 Pa). Viability was highest when the VICs could locally degrade their environment and spread, as demonstrated in the gels that contained a fraction of MMP-degradable crosslinker. The degradability was accomplished by simply moving the 3,4’-AMPB monomer out of the peptide cleavage site (KCG-3,4’-AMPB-GPQGIWGQGCK), and the fraction of non-degradable crosslinks was chosen using Flory-Stockmayer theory to calculate the theoretical critical amount of crosslinks needed to form a gel (38%nd = 38% non-degradable crosslinker). Both the degradable and non-degradable versions of the photoresponsive peptides maintained similar reaction efficiencies and therefore affected the mechanical properties of the hydrogels in the same way (Figure S8). As shown in Figure 5d, the VICs maintained high levels of viability (~80%) in 4 wt% hydrogels and were able to achieve a spread fibroblast morphology after three days in the gel. At this time point, cell-laden gels were treated with both 365 nm irradiation (10 mW/cm2, 5 min) and 400-500 nm irradiation (10 mW/cm2, 2 min) and demonstrated high viability 24 hours post-irradiation (Figure S9). Matrix-interactions and spreading are required for VIC survival and are also necessary for VICs to exhibit the activated phenotype of the myofibroblast. With longer culture times, VICs continued to elongate, and after 5 days, many cells exhibited organized f-actin fibers (Figure 5e). Some cells were also positive for αSMA stress fibers, a hallmark of the myofibroblast phenotype. Therefore, in these gels, there is a baseline level of fibroblast activation that may be affected upon changes in gel modulus.
Conclusions
Using a crosslinker that responds to both UV and visible light, a PEG-based hydrogel was designed for mechanically dynamic three-dimensional cell culture. The shear modulus exhibited reversible changes without any alterations in network connectivity or monomer composition. Furthermore, due to the use of a photoisomer, the changes in modulus could be effected without the use of chemical initiators or other additives, making this strategy an attractive route toward controlling the matrix environment in the presence of cells. Current efforts are aimed toward tuning the photoresponsive material properties to maximize changes in modulus for biological studies, such as mechanotransduction of cells in 3D.
Supplementary Material
Acknowledgments
This work was supported by an award from the American Heart Association in the form of a postdoctoral fellowship (A.M. Rosales) and a summer undergraduate fellowship (E.M. Nehls). A.M. Rosales also gratefully acknowledges a Postdoctoral Enrichment Program Award from the Burroughs Wellcome Fund. Finally, this work was also made possible by funding from the Howard Hughes Medical Institute and was supported in part by a grant from the National Science Foundation (CBET 1236662).
Footnotes
Supporting Information
Supporting information includes heating curves for the azobenzene-containing hydrogels, rheology of hydrogels without azobenzene, and additional rheology of various hydrogel formulations. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Kloxin AM, Kloxin CJ, Bowman CN, Anseth KS. Adv. Mater. 2010;22(31):3484–3494. doi: 10.1002/adma.200904179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tibbitt MW, Anseth KS. Biotechnol. Bioeng. 2009;103(4):655–663. doi: 10.1002/bit.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin DC, Yurke B, Langrana NA. J. Mater. Res. 2005;20(06):1456–1464. [Google Scholar]
- 4.Jiang FX, Yurke B, Schloss RS, Firestein BL, Langrana NA. Biomaterials. 2010;31(6):1199–1212. doi: 10.1016/j.biomaterials.2009.10.050. [DOI] [PubMed] [Google Scholar]
- 5.Gillette BM, Jensen JA, Wang M, Tchao J, Sia SK. Adv. Mater. 2010;22(6):686–691. doi: 10.1002/adma.200902265. [DOI] [PubMed] [Google Scholar]
- 6.Kong N, Peng Q, Li H. Adv. Funct. Mater. 2014 n/a-n/a. [Google Scholar]
- 7.Murphy WL, Dillmore WS, Modica J, Mrksich M. Angew. Chem., Int. Ed. 2007;46(17):3066–3069. doi: 10.1002/anie.200604808. [DOI] [PubMed] [Google Scholar]
- 8.Kloxin AM, Kasko AM, Salinas CN, Anseth KS. Science. 2009;324(5923):59–63. doi: 10.1126/science.1169494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guvendiren M, Burdick JA. Nat. Commun. 2012;3:792. doi: 10.1038/ncomms1792. [DOI] [PubMed] [Google Scholar]
- 10.Zhao Y-L, Stoddart JF. Langmuir. 2009;25(15):8442–8446. doi: 10.1021/la804316u. [DOI] [PubMed] [Google Scholar]
- 11.Tamesue S, Takashima Y, Yamaguchi H, Shinkai S, Harada A. Angew. Chem., Int. Ed. 2010;49(41):7461–7464. doi: 10.1002/anie.201003567. [DOI] [PubMed] [Google Scholar]
- 12.Guan Y, Zhao H-B, Yu L-X, Chen S-C, Wang Y-Z. RSC Adv. 2014;4(10):4955–4959. [Google Scholar]
- 13.Peng L, You M, Yuan Q, Wu C, Han D, Chen Y, Zhong Z, Xue J, Tan W. J. Am. Chem. Soc. 2012;134(29):12302–12307. doi: 10.1021/ja305109n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beharry AA, Woolley GA. Chem. Soc. Rev. 2011;40(8):4422–4437. doi: 10.1039/c1cs15023e. [DOI] [PubMed] [Google Scholar]
- 15.Priewisch B, Rück-Braun K. J. Org. Chem. 2005;70(6):2350–2352. doi: 10.1021/jo048544x. [DOI] [PubMed] [Google Scholar]
- 16.Rück-Braun K, Kempa S, Priewisch B, Richter A, Seedorff S, Wallach L. Synthesis. 2009;2009(24):4256–4267. [Google Scholar]
- 17.Benton JA, Fairbanks BD, Anseth KS. Biomaterials. 2009;30(34):6593–6603. doi: 10.1016/j.biomaterials.2009.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson CM, Hanson MN, Helgeson SC. J. Mol. Cell. Cardiol. 1987;19(12):1185–1193. doi: 10.1016/s0022-2828(87)80529-1. [DOI] [PubMed] [Google Scholar]
- 19.Lutolf MP, Hubbell JA. Biomacromolecules. 2003;4(3):713–722. doi: 10.1021/bm025744e. [DOI] [PubMed] [Google Scholar]
- 20.Park Y, Lutolf MP, Hubbell JA, Hunziker EB, Wong M. Tissue Eng. 2004;10(3-4):515–522. doi: 10.1089/107632704323061870. [DOI] [PubMed] [Google Scholar]
- 21.Fairbanks BD, Schwartz MP, Bowman CN, Anseth KS. Biomaterials. 2009;30(35):6702–6707. doi: 10.1016/j.biomaterials.2009.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bandara HMD, Burdette SC. Chem. Soc. Rev. 2012;41(5):1809–1825. doi: 10.1039/c1cs15179g. [DOI] [PubMed] [Google Scholar]
- 23.Bryant SJ, Nuttelman CR, Anseth KS. J. Biomater. Sci., Polym. Ed. 2000;11(5):439–457. doi: 10.1163/156856200743805. [DOI] [PubMed] [Google Scholar]
- 24.Kloxin AM, Benton JA, Anseth KS. Biomaterials. 2010;31(1):1–8. doi: 10.1016/j.biomaterials.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petr M, Hammond PT. Macromolecules. 2011;44(22):8880–8885. [Google Scholar]
- 26.Petr M, Helgeson ME, Soulages J, McKinley GH, Hammond PT. Polymer. 2013;54(12):2850–2856. [Google Scholar]
- 27.Liu G-F, Ji W, Wang W-L, Feng C-L. ACS Appl. Mater. Interfaces. 2014 [Google Scholar]
- 28.Yagai S, Karatsu T, Kitamura A. Langmuir. 2005;21(24):11048–11052. doi: 10.1021/la052076k. [DOI] [PubMed] [Google Scholar]
- 29.Branco da Cunha C, Klumpers DD, Li WA, Koshy ST, Weaver JC, Chaudhuri O, Granja PL, Mooney DJ. Biomaterials. 2014;35(32):8927–8936. doi: 10.1016/j.biomaterials.2014.06.047. [DOI] [PubMed] [Google Scholar]
- 30.Zaman MH, Trapani LM, Sieminski AL, MacKellar D, Gong H, Kamm RD, Wells A, Lauffenburger DA, Matsudaira P. Proc. Natl. Acad. Sci. 2006;103(29):10889–10894. doi: 10.1073/pnas.0604460103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kyburz KA, Anseth KS. Acta Biomater. 2013;9(5):6381–6392. doi: 10.1016/j.actbio.2013.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mason BN, Starchenko A, Williams RM, Bonassar LJ, Reinhart-King CA. Acta Biomater. 2013;9(1):4635–4644. doi: 10.1016/j.actbio.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shen Y-I, Abaci HE, Krupski Y, Weng L-C, Burdick JA, Gerecht S. Biomater. Sci. 2014;2(5):655–665. doi: 10.1039/C3BM60274E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leight JL, Alge DL, Maier AJ, Anseth KS. Biomaterials. 2013;34(30):7344–7352. doi: 10.1016/j.biomaterials.2013.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruslim C, Ichimura K. J. Mater. Chem. 2000;10(12):2704–2707. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.