Abstract
The increasing prevalence of obesity in developed nations has far-reaching implications for medical toxicology. The management of obese patients is complicated by comorbid illnesses, changes in cardiovascular and respiratory physiology, alterations in pharmacokinetics, and a lack of studies to identify appropriate dosing for current therapeutics and antidotes. In this review article, we examine obesity-associated physiologic and pharmacokinetic changes that may increase the vulnerability of obese patients to overdose. Further research is needed to characterize the relationship between drug toxicity and obesity.
Keywords: Obesity, Toxicology, Pharmacokinetics, Antidotes
Introduction
In the past decade, the USA has seen record rates of obesity, with associated increases in diabetes, heart disease, and cerebrovascular disease [1, 2]. Historically, special populations have been described in medical toxicology, due to unique physiologic and pharmacokinetic considerations (e.g., pediatric, pregnant, and geriatric populations). We propose obese patients as another special population to be considered in medical toxicology. In this paper, we provide definitions of essential terminology related to obesity, describe known physiologic and pharmacokinetic alterations among obese populations, and identify areas for future research on the association between obesity and overdose. This review extrapolates from a variety of fields including pharmacology, psychiatry, gastroenterology, and bariatrics.
Methods
We conducted a literature search of MEDLINE. A combination of the following medical subject headings was used: “obesity,” “toxicology,” “drug overdose,” and “pharmacology.” Reference lists of retrieved articles were reviewed for additional studies not found by the above search method. Manuscripts were limited to the English language. No date range was set in the search engine, and articles were retrieved from 1916 through 2015. Articles were included in this review based on relevancy to the topics of obesity, toxicology, pharmacology, and drug overdose. Articles that did not add to the information within these topic headings were excluded.
Definitions
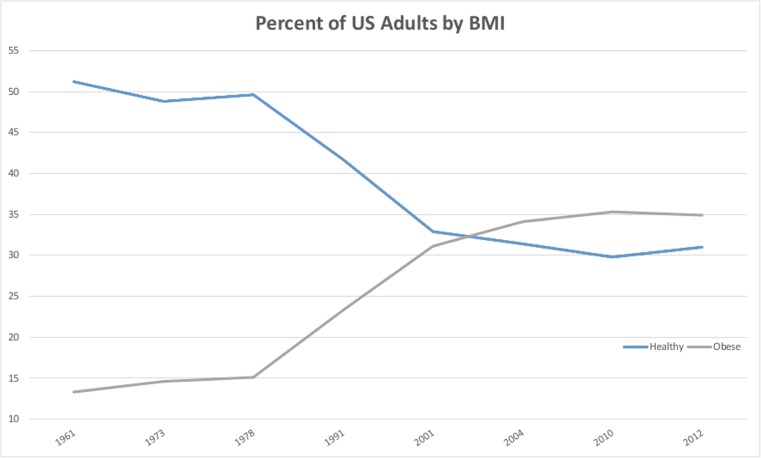
The World Health Organization and the National Institutes of Health define obesity as a BMI of equal to or greater than 30 kg/m2, while overweight adults have a BMI between 25.0 and 29.9 kg/m2 (see Table 1) [4, 5]. As of 2012, 35 % of the American adult population is obese, with an additional 33 % classified as overweight [6]. Over the last five decades, the proportion of healthy weight (BMI 18.5–24.9) Americans has dropped, while the proportion of obese Americans has risen (Fig. 1) [8]. There are currently more obese Americans than Americans of a healthy weight, and the proportion of obese Americans is projected to increase to 51 % by 2030 [9]. While the causes of this increase are many and controversial, there is a growing consensus that societal factors play a large role: increase in portion sizes, higher caloric density of foods, high-calorie beverages, and a drop in physical activity in work and leisure [10–13]. Many of the risks to this population, however, are clear. Obesity has been associated with an increased risk of coronary artery disease, hypertension, impaired cardiac function, diabetes, hypercoagulability, cancer, hypoventilation, chronic pain, and sleep apnea syndromes [14–16].
Table 1.
Definitions of common terms by BMI [3]
| BMI (body mass index) | Category |
|---|---|
| 18.5–24.9 kg/m2 | Normal weight |
| 25.0–29.9 kg/m2 | Overweight |
| ≥30.0 kg/m2 | Obesity |
| ≥40.0 kg/m2 | Extreme obesity |
Fig. 1.
Prevalence of obesity and normal weight adults as percent of US population. Data from [6, 7]
Overall, obesity increases risk of death; in a meta-analysis examining more than 2.8 million people, all grades of obesity were associated with an increase in all-cause mortality (hazard ratio 1.18, 95 % CI 1.12–1.25) [17]. At this time, estimates of morbidity and mortality related to drug overdose in obese patients are not available. Extrapolating from the current 35 % prevalence of obesity in the US adult population and recent reports of 16,651 drug-induced deaths in the USA annually, there may be approximately 5800 drug-induced deaths in obese patients in the USA per year [1, 18]. As both populations are heterogeneous, such an approximation is unlikely to be correct, but it serves as a starting place to indicate the scale of the problem and the potential for interactions. The authors believe that comorbid conditions as well as physiologic and pharmacologic changes in obese patients may place obese patients at greater risk of drug-related death.
Physiologic Changes in Obesity
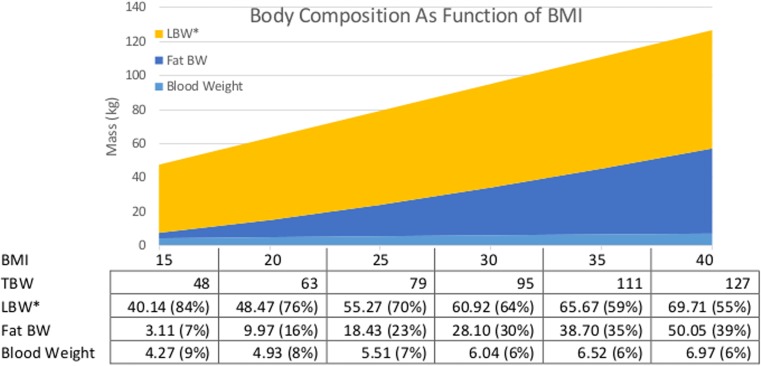
Obesity is more than a biometric measure; obese patients have altered anatomy and physiology that affects their response to pharmacologic exposures (Table 2). While providers might assume that a 100-kg man is equivalent to a 70-kg man with an additional 30 kg of adipose, this is incorrect. As a patient’s weight increases, their body composition also changes (illustrated in Fig. 2). A “standard” lean 70-kg man is composed primarily of lean mass with some adipose and blood. As this individual increases in size, lean mass and adipose increase, but adipose tissue increases disproportionately. Circulating blood volume also increases. The same man has a higher lean body weight and circulating blood volume at 100 kg (BMI 31.6) than at 70 kg (BMI 22.1). As discussed below, this is important because factors such as apparent volume of distribution and hepatic and renal clearance correlate with changes in body composition. Brief descriptions of the changes that occur are described in the following sections.
Table 2.
Obesity related factors that may affect drug toxicity
| Physiologic changes |
| Increased circulating blood volumes and hemodynamic changes |
| Increased risk of cardiomyopathy and atherosclerosis |
| Alterations in pulmonary function |
| Changes in large airway anatomy associated with obstructive sleep apnea |
| Increased rate of daytime hypoxemia and sleep disordered breathing |
| Increased risk of aspiration |
| Psychiatric changes |
| Association with mood disorders |
| Disordered dopamine mediated reward mechanisms (which regulate both substance abuse behaviors and binge eating behaviors) |
| Pharmacologic changes |
| Lack of consensus regarding dose adjustment in obese patients |
| Altered absorption following gastric bypass surgery |
| Altered volume of distribution of lipophilic drugs in obese patients |
| Altered drug metabolism due to enzyme levels and activity |
| Generally obese patients have a higher GFR which may increase drug elimination |
| Renal disease is more common in obese patients which may decrease drug elimination |
Fig. 2.
Body composition as a function of BMI. Estimated mass (kg) of various body compartments in an average 1.78-m male. Fat body weight is the difference between TBW and LBW calculated by Jamahasatians formulae. Blood weight is calculated [19]. LBW* LBW calculated by Janmahasatians formulae minus blood weight to give a blood-free LBW
Cardiovascular
On average, increased adipose tissue and body mass lead to an increase in circulating blood volume and a compensatory increase in cardiac output at rest [20]. Obesity itself is associated with cardiomyopathy, and obese patients with left ventricular hypertrophy may have an impaired ability to increase stroke volumes during times of stress, leading to a greater reliance on heart rate to improve cardiac output [21]. Many cardioactive medications, such as beta-blockers and calcium channel blockers, may blunt such a compensatory increase.
Obese patients have higher baseline heart rates and decreased tolerance for orthostatic states, necessitating higher rises in heart rate [22]. Hypertension occurs six times more often in obese patients than lean men and women [23]. One of the purported mechanisms for this is underlying endothelial dysfunction, with decreased nitric oxide production [24]. Obese patients with long-standing hypertension may not tolerate acute episodes of hypotension, leading to cerebral hypoperfusion, resulting in visual changes and stroke [25, 26].
Respiratory
The effects of obesity on respiratory function are well characterized. The anatomic and physiologic changes that occur in obesity impair the respiratory system at every level, including compliance, reserve capacity, airway resistance, ventilatory drive, and work of breathing [27]. Obese patients are at increased risk for obstructive sleep apnea [27]. Central nervous system and respiratory depressants (such as opioids, benzodiazepines, and alcohol) may accentuate sleep-disordered breathing, resulting in hypoxemia and hypercarbia [28, 29]. For this reason, such agents should be avoided by patients with sleep apnea. Obese patients may also suffer from obesity hypoventilation syndrome, which results in daytime hypoxemia and a blunted respiratory response to acidemia [30]. There are conflicting reports on the mechanism of these effects that often fail to differentiate between central and anatomic contributions.
Opioids are at the center of the current epidemic of drug overdose deaths [8]. Although the number of opioid overdose deaths in obese patients is not known, opioids may worsen the already impaired respiratory physiology in obese patients. Opioids can produce a measurable, dose-dependent decline in all phases of respiration. Accordingly, both obesity and sleep-disordered breathing are implicated as risk factors for opioid overdose [31].
Gastrointestinal
Though not often emphasized, obese patients can have alterations in gastrointestinal functioning. Obese patients present an increased risk of aspiration due to increased incidence of hiatal hernia, increased intra-abdominal pressure, and decreased lower esophageal sphincter tone [32]. There is no consensus over changes in gastric emptying time among obese patients. Some authors have identified faster gastric emptying time among the obese, mainly with solid foods, which could reduce the effectiveness of decontamination [33–35]. However, in other studies, obese patients were found to have delayed gastric emptying time [36, 37]. Studies of intestinal transit time are limited but suggest an enhanced upper intestinal motility and a delayed distal small intestine and colonic motility [32].
Obesity is the most significant risk factor for the development of hepatic steatosis [38, 39]. Differential expression of metabolic and transporter enzymes have been described in patients with hepatic steatosis [40].
Psychiatric
Obesity has a bidirectional and reinforcing relationship with mood disorders. The Nurses’ Health Study, one of the largest longitudinal studies of its kind, demonstrated that obese women are more likely to be depressed when followed up several years later [41]. An even greater risk is found between depression and subsequent development of obesity. This does not demonstrate that obesity causes depression; however, the societal stigma and comorbidities associated with obesity can create psychosocial stress in at-risk persons, conceivably leading to the development or unmasking of depression [42].
Conversely, dysregulation of the hypothalamic-pituitary axis, found in depressed patients, may result in general anhedonia and unhealthy focus on reward-seeking behaviors such as binge eating and drug addiction [43, 44]. Positron emission tomography studies demonstrate altered dopamine receptor expression in the brains of obese patients and drug-addicted patients [45, 46]. This reward motivation for overeating may lead to obesity. As similar dopamine-mediated reward mechanisms lead to substance abuse and binge eating, patients with obesity may be at a higher risk of substance abuse. Surprisingly, current research does not support this theory and suggests that higher BMI may be associated with a lower incidence of substance abuse [47, 48]. Current explanations include competition for the same reward pathways.
Pain
Obesity has been associated with chronic non-cancer pain, specifically knee, hip, back, and overall body pain [49]. While this may be explained by increased pain and osteoarthritis in weight-bearing joints or peripheral neuropathy due to comorbid diabetes, reports of other types of pain including headache, oral pain, arm pain, stomach pain, and whole body pain also increase in obesity. This suggests a complex relationship that goes beyond arthritis and may be mediated by central pain pathways. The effect is robust and proportional, with the incidence of pain related complaints increasing as BMI increases. Obese patients receive 1.8 times as many defined daily doses of non-steroidal anti-inflammatory pain medications as age and sex matched normal weight controls [50]. This trend is also concerning as the use of opioid pain medications for chronic pain, especially at higher doses, has been associated with overdose and death [51, 52]. While plausible, an association between opioid use in obese patients and risk of overdose has not been demonstrated.
Pharmacokinetic Changes in Obesity
Dosing Scalars
Changes in body composition and physiology have important implications for dosing medications. Dosing calculations in obese patients can be made via several metrics, including total body weight (TBW), body surface area (BSA), ideal body weight (IBW), and lean body weight (LBW) (Table 3).
Table 3.
| Measurement | Calculation |
|---|---|
| Body mass index (BMI) | |
| Body surface area (BSA) | TBW(kg)0.425 × height(cm)0.725 × 0.007184 |
| Ideal body weight (IBW) | Devine’s estimation = 45.4 + 0.89 × (height(cm) − 152.4) + 4.5 (if male) May also be determined via standardized tables according to height |
| Lean body weight (LBW) | Jame’s equations: Janmahasatian’s formulae: |
TBW total body weight
There is a lack of consensus on dose adjustments for specific drugs in obese patients, as this population is excluded from most clinical trials [53, 54]. This deficiency in research trials has led some to advocate a research agenda that “recognizes obese patients as a separate entity with characteristics that differ from the rest of the population” and “special dosing instructions in drug labels for all classes of obesity” [55].
TBW is commonly used in pediatrics and is convenient for adult dosing, but it fails to account for changes in body composition that occur with increasing body mass. Total body weight is perhaps the most commonly employed metric, and it is easily measured using a scale. However, the use of TBW can lead to gross errors, such as overdosing.
Historically, agents with a narrow therapeutic index, such as chemotherapeutics, have been dosed according to BSA. BSA is used as a proxy for drug clearance, and the purpose of BSA-based dosing is to decrease toxicity while maintaining chemotherapeutic efficacy [56, 57]. The relationship between BSA and drug clearance is not universally reliable or accurate, though, and there is concern that BSA-based dosing may lead to toxicity in obese patients with BSA greater than 2.2 m2. A maximum BSA of 2.2 m2 is often used for dosing, effectively under dosing tall and obese patients [58, 59].
There is concern that dosing by TBW and BSA may lead to toxicity. Rather than an arbitrary maximum dose, some have argued for dosing drugs with weak liphophilicity (e.g., lithium and vecuronium) according to IBW [60]. IBW can be derived from empiric tables based on patient height or calculated via Devine’s estimation IBW = (45.4 + 0.89 × (height (cm) − 152.4) + 4.5 [if male]) [61]. The IBW is the metric used in the United States Food and Drug Administration approval process. However, the IBW makes assumptions that patients of the same sex and height have the same lean body mass (LBM).
The LBW is conceptually easiest to understand, as it attempts to subtract fat mass from total body weight to measure the non-adipose component of weight [62]. The traditional equations for LBW derived by James tend to lose validity at extremes of body size, paradoxically predicting lower LBW as BMI increases [1]. For this reason, Janmahasatian’s formulae have largely supplanted them for calculating LBW in obese patients [63].
Numerous reviews have explored changes in pharmacokinetics for commonly administered anesthetics and recommend body scalars to use for obese patients [19, 55, 59–61, 64–70]. What follows is an attempt to summarize these changes in absorption, distribution, and elimination.
Absorption
Oral absorption is not significantly altered in obesity; however, parenteral absorption can be affected [71]. Canine studies have demonstrated that obesity is associated with a decreased diffusion of insulin into the interstitial space and decreased enhancement of skeletal muscle glucose uptake [72]. Human studies have demonstrated increasing subcutaneous tissue thickness as well as higher BMI is associated with delayed absorption of subcutaneous injection of insulin [73, 74]. Additionally, the increased subcutaneous fat associated with obesity may lead to inadvertent subcutaneous injection of medications intended to be administered intramuscularly, altering pharmacokinetics [75]. Similarly, decreased absorption of subcutaneously injected enoxaparin has been associated with decreased systemic absorption and effect [76].
Obesity is a common indication for bariatric surgery, and anatomic alterations that occur with such surgery can affect oral absorption. This is especially important given the 13-fold increase in bariatric surgeries from 1993 to 2006 [77]. Gastric bypass typically facilitates weight loss through both restriction and malabsorption. Decreased gastric mixing, diminished mucosal absorption, and alterations in pH can impair drug absorption. It is believed that absorption is likely to be affected for those drugs which require a more acidic environment (for absorption, uncoating, or activation) and drugs that rely on intestinal transporters located in the duodenum [78]. Additionally, after sleeve gastrectomy, emptying of both liquids and solids is accelerated [79]. Evidence of diminished absorption following gastric bypass has been identified in multiple case reports for cyclosporine, tacrolimus, thyroxine, phenytoin, and rifampin [80]. At the very least, when treating patients who have undergone bariatric surgery, care must be taken to understand possible variations in drug absorption.
Distribution
The apparent volume of distribution (Vd) of a drug is a composite of distribution to various body compartments (and thus body composition), regional blood flow, and binding to plasma proteins [60]. While distribution occurs between various tissue compartments, the complex equilibration constants, which describe the rate of this change and the concentration at equilibrium, are often modeled using two compartments. This simplification makes assumptions about the volumes of body tissues as well as rates of drug exchange within them.
Variations in body composition and blood flow, that occur in obese patients, may lead to inaccurate pharmacokinetic modeling. The effect on Vd can vary depending on particular drug characteristics such as hydrophilicity and protein binding. Hydrophilic drugs, such as lithium, remain in circulation and have slower distribution to tissues resulting in a low volume of distribution (Vd 0.7–1.4 L/kg) [81]. Dosing such drugs according to TBW may lead to higher peak serum levels and increased toxicity in obese patients [82]. Lipophilic drugs, such as chloroquine, may partition into adipose tissues resulting in higher volumes of distribution (Vd 115–285 L/kg) making achievement of therapeutic serum concentrations difficult in the obese. Drugs with high protein binding, such as warfarin, also tend to remain in circulation and, thus, have a low volume of distribution (0.15 L/kg in the case of warfarin) [81].
Obesity can affect Vd and, thus, initial dosing. This is due to distribution of the drug in the adipose compartment as well as blood supply to various body tissues. A drug’s affinity for lipophilic body compartments such as the brain and fat is modeled using the octanol-water partition coefficient (represented as logP). Charged, hydrophilic xenobiotics (such as lithium) predominate in the water portion, while lipophilic, uncharged xenobiotics (such as propofol) predominate in octanol. How does this relate to distribution in obese patients? Lithium, with a low volume of distribution and logP of −0.42, is largely intravascular and is not significantly affected by changes in adipose tissue. Studies looking at lithium in obese and non-obese patients have demonstrated that the volume of distribution correlates significantly with IBW not TBW [15].
Lipophilic molecules such as anesthetics and analgesics would be expected to have more deposition in adipose compartments and be affected by increased TBW. Such is the case in sufentanil, where Vd correlates well with TBW rather than LBW [83]. However, this finding cannot be generalized to all lipophilic anesthetics. The volume of distribution of remifentanil does not correlate with TBW and is usually dosed according to IBW [84]. This may be related to remifentanil’s unique ester-linkages which allow for rapid metabolism by plasma and tissue esterase, limiting adipose redistribution.
Distribution to adipose tissue may explain the delayed time of onset for cisatracurium in obese patients dosed according to IBW [85]. As previously mentioned, ideal body weight does not account for increased adiposity in obesity and may lead to underdosing. Dosing cisatracurium based on TBW overcomes this limitation, resulting in similar time of onset in obese and non-obese patients. Conversely, time to onset of rocuronium was similar in obese and non-obese patients, but the duration of action was prolonged when dosing was based on TBW [86]. This finding may be related to the higher lipophilicity of cisatracurium (logP 0.5) vs rocuronium (logP −2.12).
In comparison to other body tissues, adipose tissue is a metabolically inactive third space allowing for deposition of drug without elimination. Over time, as drug levels in other body compartments drop, drug redistribution from peripheral to central compartments may prolong clinical effects. This may explain why both cisatracurium and rocuronium have significantly longer durations of effect in obese patients dosed according to TBW [85, 86].
An analysis of pharmacokinetic data from adult patients being treated with chemotherapeutic agents found that obesity greatly affects distribution for specific agents [87]. The Vd in obese and non-obese individuals did not vary significantly in the case of doxorubicin, topotecan, irinotecan, carboplatin, or paclitaxel; however, there was a significant increase in Vd in cisplatin and docetaxel in obese patients. Similarly, while TBW is recommended for dosing of vancomycin, the Vd of ciprofloxacin varies inversely with BMI [88]. Thus, it appears that ciprofloxacin distributes less in adipose tissue than in other tissues. For this reason, ciprofloxacin dosing in obese patients is based on factoring in IBW and a portion of excess body weight (TBW − IBW) [88].
Obese patients may have alterations that affect the Vd and drug concentration within target organ systems. Obese patients generally have smaller CSF volumes due to elevated abdominal pressure, requiring less local anesthetic to achieve spinal anesthesia [55, 89, 90]. This may have clinical significance for drugs that distribute in this space either by direct injection or distribution from plasma.
Metabolism
Changes in drug metabolism in obese patients are affected by a relatively higher proportion of drug in metabolically inactive adipose tissue, as well as variations in enzymatic activity. Obesity is associated with increased rates of hepatic steatosis which may alter drug metabolism [40].
A variety of enzymatic levels and activities are altered in obese patients. Table 4 summarizes some of these changes. It is important to remember that individuals may have enzymatic variability due to genetic, environmental, and demographic factors other than obesity; metabolic studies look at the obese patient population as a group. The clinical relevance of these changes is unclear; however, upregulation of CYP2E1 in alcoholics has been linked to increased acetaminophen- and isoniazid-induced hepatotoxicity [99, 100].
Table 4.
Alterations in drug clearance in obesity according to primary CYP involved and clinical relevance
| Enzyme | Pharmacokinetic changes | Clinical relevance |
|---|---|---|
| CYP1B1, CYP2U1 [54, 91] | Normal levels found in adipose tissue | Increased clearance of hormone contraceptives |
| CYP3A4 [92] | Decreased clearance | Decreased clearance of alprazolam, midazolam, fentanyl, carbamazepine, cyclosporine |
| CYP2E1 [92–94] | Upregulated | Increased clearance of chlorzoxazone, enflurane, sevoflurance, and halothane; increased conversion of acetaminophen to NAPQI |
| CYP2D6, CYP1A2 [92] | No significant change | |
| CYP2C9 [92, 95] | Increased rate of clearance, no change when normalized by TBW | Increased clearance of ibuprofen and phenytoin |
| UGT [92, 96, 97] | Increased glucuronidation | Increased glucuronidation of acetaminophen, oxazepam, lorazepam |
| Pseudocholinesterase [98] | Increased levels | Increased clearance of succinylcholine |
Elimination
The primary organs of elimination, the liver and kidneys, rely on the circulatory system for xenobiotic delivery. Much of the liver’s contribution to elimination of xenobiotics is via enzyme-mediated metabolism, as discussed in the previous section. Therefore, this section focuses on the non-enzymatic renal elimination and excretion of xenobiotics. Obese patients have higher cardiac output and GFR, generally leading to increased renal clearance of both toxins and antidotes [101, 102]. Conversely, long-standing obesity has been associated with renal dysfunction and heart failure, leading to decreased GFR and renal clearance [103–105]. Specifically, obesity has been associated with focal and segmental glomerulosclerosis and a progression towards renal failure independent of the presence or absence of other risk factors, such as diabetes and hypertension [106, 107].
Additionally, the increased volume of distribution in obese patients, discussed previously, can lead to changes in elimination. Drugs with higher volumes of distribution will have lower serum concentrations and, thus, slower elimination rates, independent of changes in hepatic and renal function [108].
Creatinine clearance is a commonly used surrogate for renal function, and numerous methods exist for direct measurement as well as calculated estimates based on age, height, and weight. The Cockcroft-Gault equation for estimating creatinine clearance is notably less accurate in obese patients, overestimating clearance by almost 40 % [109, 110]. This is likely because the increases in GFR relate to renal blood flow and are not directly proportional to TBW. Adjustments to Cockcroft-Gault using fat-free body weight or ideal body weight have been proposed and give creatinine clearances that better model measured values [109, 111]. As ideal body weight is solely based on height, it ignores the increased clearance associated with higher circulating blood volumes in obese patients. Winters-modified formula, which adds 40 % of actual body weight to ideal body weight (ABW0.4), has been shown to provide the most accurate modeling of creatinine clearance in obese patients with the least amount of bias [111].
Numerous studies have attempted to evaluate the effect of body size on clearance of particular drugs [54, 92, 101, 112]. Usually, these studies were focused on drugs with a narrow therapeutic index where serum levels are routinely monitored to allow for convenient pharmacokinetic calculations. This has created a skewed data set as immunology and oncology medications make up a third of a recent meta-analysis of such studies, while representing a minority of drugs on the market [113]. In contrast, analgesic medications, which make up a large portion of prescribed medications, make up less than 5 % of the studies.
Changes in clearance due to GFR can be studied by looking at clearance of drugs that are excreted largely unchanged in the urine. These drugs include vancomycin, daptomycin, carboplatin, low-molecular-weight heparins, and cimetidine; their clearance correlates with creatinine clearance [92]. Clearance of vancomycin increased from 77 mL/min in non-obese patients to 197 mL/min in obese patients; this discrepancy appeared to be directly correlated with total body weight (1.13 vs 1.19 mL/min/kg, respectively) [114]. Studies of daptomycin have found conflicting effects of obesity on drug clearance; however, this may be due to the relatively small (n = 7–12) and heterogeneous study groups [115, 116]. Studies of low molecular weight heparins (enoxaparin & dalteparin) demonstrate increased renal clearance [117, 118]; however, numerous studies do not report drug clearance, instead focusing on factor Xa levels in obese patients [119]. Studies of cimetidine, similar to studies of daptomycin, are small (n = 12–13) and conflicting about the effect of obesity on renal drug clearance [120, 121].
To summarize, obese patients may have markedly altered pharmacokinetics of commonly prescribed drugs; however, this relationship varies within and between drug classes. The changes discussed should provide a framework for understanding the many ways in which drug absorption, distribution, and elimination are altered in obese patients. Medical comorbidities and weight loss surgeries further complicate things. After reviewing 458 studies, McLeay et al. concluded that drug clearance increases along with total body weight, but this increase is not linear [113].
Implications for Toxicology
Alterations in Decontamination
We were unable to find any studies of gastrointestinal decontamination focusing on obese patients. Literature to support the use of activated charcoal in acute overdose comes from healthy volunteer studies [122]. These studies usually report demographic information including weight but not height, which makes calculating BMI impossible. In one such study, charcoal and acetaminophen were administered to ten healthy volunteers with an average weight of 76 kg (range, 66 to 86 kg) [123]. The efficacy of charcoal decontamination varies by agent, patient, and time since exposure, with unknown implications for obese patients.
The increased risk of aspiration in obese patients (as a result of relaxed esophageal sphincter and increased intra-abdominal pressure) may increase risk of charcoal aspiration [32].
Multidose activated charcoal (MDAC) has been recommended following ingestion of carbamazepine, dapsone, phenobarbital, quinine, and theophylline [124]. The rationale for MDAC following overdose involves decreasing serum drug levels by reducing the enteroenteric and enterohepatic circulation of xenobiotics. Recommendations for MDAC are based on healthy volunteer studies, which suffer the same limitations as those for single-dose charcoal. The effect of obesity on enteroenteric and enterohepatic transit has not been reported; however, patients who have undergone bariatric surgery—which is known to disrupt the enterohepatic circulation of bile acids—may have similarly impaired enterohepatic circulation of drugs [125]. It is unclear if such disruption is clinically relevant.
Prior bariatric surgery should be considered a contraindication to gastric lavage. In addition to a lack of data to support its use, there is reason to believe that the reduced gastric volume in addition to the presence of anastomotic sites would lead to an increased risk of perforation. One such case involved a woman who underwent gastric lavage and charcoal decontamination 8 weeks following Roux-en-Y gastric bypass [126]. This led to disruption of the post-operative gastric pouch, spilling of charcoal into the peritoneum, and peritonitis requiring laparotomy and wash-out. Even distant history of Roux-en-Y gastric bypass has been associated with gastric perforations and peritonitis; however, these do not appear to be frequent [127]. Anecdotally, patients are often told to avoid nasogastric tubes in the immediate period following bariatric surgery; however, this does not appear to be an official recommendation from bodies such as the American College of Surgeons.
Commonly Used Antidotes
Drug monographs for FDA-approved antidotes commonly used in overdose (e.g., naloxone, flumazenil, digoxin immune fab, deferoxamine) do not usually address dose adjustments in obese patients [117, 118, 120, 121]. Safety and efficacy studies generally recruit healthy volunteers and exclude patients in special populations (e.g., children, elderly, pregnant women). Thus, the clinical relevance of these findings for the treatment of obese patients is unclear. Dosing based on TBW, such as in N-acetylcysteine administration, results in higher doses in obese patients. The first reported fatality following administration of intravenous N-acetylcysteine administration was in 2002 in an obese woman with asthma [128]. An FDA review makes note of this and indicates a risk of anaphylactoid reactions in patients with allergies and asthma, but does not comment on whether the higher total dose administered contributed to toxicity [129]. Table 5 lists common weight-based antidotes and recommended dosing in obese patients, when available.
Table 5.
Common weight-based dosing of antidotes and adjustments in obesity
| Agent | Typical dosing | Weight adjustments |
|---|---|---|
| N-acetylcysteine IV | 150 mg/kg ABW [119] | 150 mg/kg IBW [81]; 150 mg/kg ABW; 22.5 g maximum [130] |
| Digoxin immune fAb | [120] | None reported; maximum dosing weight calculated in package insert is 100 kg. No max dose specified |
| DMSA | 10 mg/kg ABW [121] | None reported |
| Fomepizole | 15 mg/kg ABW [117] | None reported |
| Insulin | 1 unit/kg ABW [81] | None |
| Lipid emulsion | 1.5 mL/kg ABW [81] | 1.5 mL/kg LBW [131] |
| Methylene blue | 1–2 mg/kg ABW [132] | None |
Prognosis
While there are many factors that affect prognosis, obesity has been associated with longer hospital length of stay, longer ICU length of stay, and longer time on mechanical ventilation [133, 134]. There are conflicting data about the association of obesity with in-hospital mortality, although several studies do find an association between mortality and obesity in ICU patients [133, 135]. Mortality rates increased by approximately two-fold in the obese study groups in comparison to ideal body weight groups. It has been suggested that improved nutritional reserve, specialized care of obese patients, and adipose-related hormonal changes may mitigate hospital-associated complications, resulting in lower than predicted mortality [134, 136].
Future Directions
Available literature (i.e., case series, ad hoc data analysis, pharmacokinetic studies) regarding obesity and drug overdose is limited by reliance on incomplete data that does not identify patient BMI or is restricted to narrow classes of drugs (e.g., antibiotics, chemotherapeutics). A broad expansion of current research is required to explore the relationship between BMI and drug toxicity. This includes the following:
Drug trials must include obese subjects, rather than excluding them. Post-marketing surveillance of drug toxicity should include patient BMI.
Current datasets that record drug toxicity (such as the American Association of Poison Control Centers National Poison Data System, the American College of Medical Toxicology Toxicology Investigators Consortium, FDA MedSafe, and the RADARS System) which do not regularly record patient BMI should make efforts to do so.
Clinical studies evaluating the effect of obesity on drug pharmacokinetics before and after bariatric surgery may also be useful.
Further research in obese patients is important to evaluate the risk and benefit of gastric decontamination, common antidotes, and extracorporeal elimination.
Conclusions
Obese patients represent a special population in medical toxicology. They may have alterations in physiology and mental health risk factors (such as mood disorder) that increase their risk of drug overdose, as well as affecting their prognosis following overdose. Alterations in physiology and comorbidities may increase the risk of cardiovascular and respiratory complications, as well as utilization of critical care and antidotal therapies. All of these factors suggest a poorer prognosis for toxicology patients with obesity. Medical toxicologists must utilize and produce evidence-based literature that guides the management of patients with obesity. Further research is needed to explore the complex relationship between the overlapping obesity and drug overdose epidemics that are threatening the public health.
Acknowledgments
Funding
The authors have no funding to report in relation to this study.
Conflict of Interest
The authors declare that they have no competing interests.
References
- 1.Freedman DS, CDC Obesity—United States, 1988–2008. MMWR Surveill Summ. 2011;60(Suppl):73–7. [PubMed] [Google Scholar]
- 2.May AL, Freedman D, Sherry B, Blanck HM, CDC Obesity—United States, 1999–2010. MMWR Surveill Summ. 2013;62(Suppl 3):120–8. [PubMed] [Google Scholar]
- 3.Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol. 2014;63(25 Pt B):2985–3023. doi: 10.1016/j.jacc.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i–xii, 1–253. [PubMed]
- 5.Expert Panel on the Identification, Evaluation, and Treatment of Overweight in Adults Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: executive summary. Am J Clin Nutr. 1998;68(4):899–917. doi: 10.1093/ajcn/68.4.899. [DOI] [PubMed] [Google Scholar]
- 6.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311(8):806–14. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity in the United States, 2009–2010. NCHS Data Brief. 2012;(82):1–8. [PubMed]
- 8.NCHS. Health, United States, 2012: with special feature on emergency care. Hyattsville, Maryland; 2013. [PubMed]
- 9.Finkelstein EA, Khavjou OA, Thompson H, Trogdon JG, Pan L, Sherry B, et al. Obesity and severe obesity forecasts through 2030. Am J Prev Med. 2012;42(6):563–70. doi: 10.1016/j.amepre.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 10.Hill JO. Environmental contributions to the obesity epidemic. Science. 1998;280(5368):1371–4. doi: 10.1126/science.280.5368.1371. [DOI] [PubMed] [Google Scholar]
- 11.Cutler D, Glaeser E, Shapiro J. Why have Americans become more obese? J Econ Perspect. 2003;17(3):93–118. doi: 10.1257/089533003769204371. [DOI] [Google Scholar]
- 12.Du H, Feskens E. Dietary determinants of obesity. Acta Cardiol. 2010;65(4):377–86. doi: 10.2143/AC.65.4.2053895. [DOI] [PubMed] [Google Scholar]
- 13.Mitchell JA, Byun W. Sedentary behavior and health outcomes in children and adolescents. Am J Lifestyle Med. 2013 [Google Scholar]
- 14.McVinnie DS. Obesity and pain. Br J Pain. 2013;7(4):163–70. doi: 10.1177/2049463713484296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Health implications of obesity. National Institutes of Health Consensus Development Conference Statement. Ann Intern Med. 1985;103(1):147–51. [PubMed]
- 16.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348(17):1625–38. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 17.Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013;309(1):71–82. doi: 10.1001/jama.2012.113905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mack KA, CDC Drug-induced deaths—United States, 1999–2010. MMWR Surveill Summ. 2013;62(Suppl 3):161–3. [PubMed] [Google Scholar]
- 19.Lemmens HJ, Bernstein DP, Brodsky JB. Estimating blood volume in obese and morbidly obese patients. Obes Surg. 2006;16(6):773–6. doi: 10.1381/096089206777346673. [DOI] [PubMed] [Google Scholar]
- 20.Alpert MA. Obesity cardiomyopathy: pathophysiology and evolution of the clinical syndrome. Am J Med Sci. 2001;321(4):225–36. doi: 10.1097/00000441-200104000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Ferraro S, Perrone-Filardi P, Desiderio A, Betocchi S, D’Alto M, Liguori L, et al. Left ventricular systolic and diastolic function in severe obesity: a radionuclide study. Cardiology. 1996;87(4):347–53. doi: 10.1159/000177118. [DOI] [PubMed] [Google Scholar]
- 22.Lee JF, Harrison ML, Christmas KM, Kim K, Hurr C, Brothers RM. Elevated resting heart rate and reduced orthostatic tolerance in obese humans. Clin Auton Res. 2013 doi: 10.1007/s10286-013-0222-x. [DOI] [PubMed] [Google Scholar]
- 23.Stamler R, Stamler J, Riedlinger WF, Algera G, Roberts RH. Weight and blood pressure. Findings in hypertension screening of 1 million Americans. JAMA. 1978;40(15):1607–10. doi: 10.1001/jama.1978.03290150053024. [DOI] [PubMed] [Google Scholar]
- 24.Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, et al. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113(6):898–918. doi: 10.1161/CIRCULATIONAHA.106.171016. [DOI] [PubMed] [Google Scholar]
- 25.Strandgaard S, Andersen GS, Ahlgreen P, Nielsen PE. Visual disturbances and occipital brain infarct following acute, transient hypotension in hypertensive patients. Acta Med Scand. 1984;216(4):417–22. doi: 10.1111/j.0954-6820.1984.tb03826.x. [DOI] [PubMed] [Google Scholar]
- 26.McLaren GD, Danta G. Cerebral infarction due to presumed haemodynamic factors in ambulant hypertensive patients. Clin Exp Neurol. 1987;23:55–66. [PubMed] [Google Scholar]
- 27.Honiden S. Caring for the critically ill, obese patient. PCCSU; 2011.
- 28.Wang D, Teichtahl H. Opioids, sleep architecture and sleep-disordered breathing. Sleep Med Rev. 2007;11(1):35–46. doi: 10.1016/j.smrv.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 29.Mogri M, Desai H, Webster L, Grant BJ, Mador MJ. Hypoxemia in patients on chronic opiate therapy with and without sleep apnea. Sleep Breath. 2009;13(1):49–57. doi: 10.1007/s11325-008-0208-4. [DOI] [PubMed] [Google Scholar]
- 30.Harris AT, Morell D, Bajaj Y, Martin-Hirsch DP. A discussion of airway and respiratory complications along with general considerations in obese patients. Int J Clin Pract. 2010;64(6):802–6. doi: 10.1111/j.1742-1241.2010.02350.x. [DOI] [PubMed] [Google Scholar]
- 31.Ramachandran SK, Haider N, Saran KA, Mathis M, Kim J, Morris M, et al. Life-threatening critical respiratory events: a retrospective study of postoperative patients found unresponsive during analgesic therapy. J Clin Anesth. 2011;23(3):207–13. doi: 10.1016/j.jclinane.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 32.Mushref MA, Srinivasan S. Effect of high fat-diet and obesity on gastrointestinal motility. Ann Transl Med. 2013;1(2):14. doi: 10.3978/j.issn.2305-5839.2012.11.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cardoso-Junior A, Coelho LG, Savassi-Rocha PR, Vignolo MC, Abrantes MM, de Almeida AM, et al. Gastric emptying of solids and semi-solids in morbidly obese and non-obese subjects: an assessment using the 13C-octanoic acid and 13C-acetic acid breath tests. Obes Surg. 2007;17(2):236–41. doi: 10.1007/s11695-007-9031-4. [DOI] [PubMed] [Google Scholar]
- 34.Acosta A, Camilleri M, Shin A, Vazquez-Roque MI, Iturrino J, Burton D, et al. Quantitative gastrointestinal and psychological traits associated with obesity and response to weight-loss therapy. Gastroenterology. 2015;148(3):537–46 e4. doi:10.1053/j.gastro.2014.11.020. [DOI] [PMC free article] [PubMed]
- 35.Wright RA, Krinsky S, Fleeman C, Trujillo J, Teague E. Gastric emptying and obesity. Gastroenterology. 1983;84(4):747–51. [PubMed] [Google Scholar]
- 36.Jackson SJ, Leahy FE, McGowan AA, Bluck LJ, Coward WA, Jebb SA. Delayed gastric emptying in the obese: an assessment using the non-invasive (13)C-octanoic acid breath test. Diabetes Obes Metab. 2004;6(4):264–70. doi: 10.1111/j.1462-8902.2004.0344.x. [DOI] [PubMed] [Google Scholar]
- 37.Horowitz M, Collins PJ, Cook DJ, Harding PE, Shearman DJ. Abnormalities of gastric emptying in obese patients. Int J Obes. 1983;7(5):415–21. [PubMed] [Google Scholar]
- 38.Festi D, Colecchia A, Sacco T, Bondi M, Roda E, Marchesini G. Hepatic steatosis in obese patients: clinical aspects and prognostic significance. Obes Rev. 2004;5(1):27–42. doi: 10.1111/j.1467-789X.2004.00126.x. [DOI] [PubMed] [Google Scholar]
- 39.Cikim AS, Topal E, Harputluoglu M, Keskin L, Zengin Z, Cikim K, et al. Epicardial adipose tissue, hepatic steatosis and obesity. J Endocrinol Investig. 2007;30(6):459–64. doi: 10.1007/BF03346328. [DOI] [PubMed] [Google Scholar]
- 40.Buechler C, Weiss TS. Does hepatic steatosis affect drug metabolizing enzymes in the liver? Curr Drug Metab. 2011;12(1):24–34. doi: 10.2174/138920011794520035. [DOI] [PubMed] [Google Scholar]
- 41.Pan A, Sun Q, Czernichow S, Kivimaki M, Okereke OI, Lucas M, et al. Bidirectional association between depression and obesity in middle-aged and older women. Int J Obes (Lond) 2012;36(4):595–602. doi: 10.1038/ijo.2011.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dixon JB, Dixon ME, O’Brien PE. Depression in association with severe obesity: changes with weight loss. Arch Intern Med. 2003;163(17):2058–65. doi: 10.1001/archinte.163.17.2058. [DOI] [PubMed] [Google Scholar]
- 43.McElroy SL, Kotwal R, Malhotra S, Nelson EB, Keck PE, Nemeroff CB. Are mood disorders and obesity related? A review for the mental health professional. J Clin Psychiatry. 2004;65(5):634–51, quiz 730. [DOI] [PubMed]
- 44.Alcaro A, Panksepp J. The SEEKING mind: primal neuro-affective substrates for appetitive incentive states and their pathological dynamics in addictions and depression. Neurosci Biobehav Rev. 2011;35(9):1805–20. doi: 10.1016/j.neubiorev.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 45.Wang GJ, Volkow ND, Thanos PK, Fowler JS. Imaging of brain dopamine pathways: implications for understanding obesity. J Addict Med. 2009;3(1):8–18. doi: 10.1097/ADM.0b013e31819a86f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baik JH. Dopamine signaling in reward-related behaviors. Front Neural Circ. 2013;7:152. doi: 10.3389/fncir.2013.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bluml V, Kapusta N, Vyssoki B, Kogoj D, Walter H, Lesch OM. Relationship between substance use and body mass index in young males. Am J Addict. 2012;21(1):72–7. doi: 10.1111/j.1521-0391.2011.00192.x. [DOI] [PubMed] [Google Scholar]
- 48.Gearhardt AN, Harrison EL, McKee SA. Does co-morbid depression alter the inverse relationship between obesity and substance use disorders? Drug Alcohol Depend. 2012;124(1–2):185–8. doi: 10.1016/j.drugalcdep.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hitt HC, McMillen RC, Thornton-Neaves T, Koch K, Cosby AG. Comorbidity of obesity and pain in a general population: results from the Southern Pain Prevalence Study. J Pain. 2007;8(5):430–6. doi: 10.1016/j.jpain.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 50.Counterweight Project Team The impact of obesity on drug prescribing in primary care. Br J Gen Pract. 2005;55(519):743–9. [PMC free article] [PubMed] [Google Scholar]
- 51.Dunn KM, Saunders KW, Rutter CM, Banta-Green CJ, Merrill JO, Sullivan MD, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152(2):85–92. doi: 10.7326/0003-4819-152-2-201001190-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Manchikanti L, Helm S, Fellows B, Janata JW, Pampati V, Grider JS, et al. Opioid epidemic in the United States. Pain Phys. 2012;15(3 Suppl):ES9–38. [PubMed] [Google Scholar]
- 53.Han PY, Duffull SB, Kirkpatrick CM, Green B. Dosing in obesity: a simple solution to a big problem. Clin Pharmacol Ther. 2007;82(5):505–8. doi: 10.1038/sj.clpt.6100381. [DOI] [PubMed] [Google Scholar]
- 54.Hanley MJ, Abernethy DR, Greenblatt DJ. Effect of obesity on the pharmacokinetics of drugs in humans. Clin Pharmacokinet. 2010;49(2):71–87. doi: 10.2165/11318100-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 55.Leykin Y, Miotto L, Pellis T. Pharmacokinetic considerations in the obese. Best Pract Res Clin Anaesthesiol. 2011;25(1):27–36. doi: 10.1016/j.bpa.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 56.Du BD, Du BE. Clinical calorimetry: tenth paper a formula to estimate the approximate surface area if height and weight be known. Arch Intern Med. 1916;XVII(6_2):863–71. doi: 10.1001/archinte.1916.00080130010002. [DOI] [Google Scholar]
- 57.Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987;317(17):1098. doi: 10.1056/NEJM198710223171717. [DOI] [PubMed] [Google Scholar]
- 58.Lopes-Serrao MD, Ussery SM, Hall RG, Shah SR. Evaluation of chemotherapy-induced severe myelosuppression incidence in obese patients with capped dosing. J Oncol Pract. 2011;7(1):13–7. doi: 10.1200/JOP.2010.000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Griggs JJ, Mangu PB, Anderson H, Balaban EP, Dignam JJ, Hryniuk WM, et al. Appropriate chemotherapy dosing for obese adult patients with cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2012;30(13):1553–61. doi: 10.1200/JCO.2011.39.9436. [DOI] [PubMed] [Google Scholar]
- 60.Cheymol G. Effects of obesity on pharmacokinetics implications for drug therapy. Clin Pharmacokinet. 2000;39(3):215–31. doi: 10.2165/00003088-200039030-00004. [DOI] [PubMed] [Google Scholar]
- 61.Green B, Duffull SB. What is the best size descriptor to use for pharmacokinetic studies in the obese? Br J Clin Pharmacol. 2004;58(2):119–33. doi: 10.1111/j.1365-2125.2004.02157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Morgan DJ, Bray KM. Lean body mass as a predictor of drug dosage. Implications for drug therapy. Clin Pharmacokinet. 1994;26(4):292–307. doi: 10.2165/00003088-199426040-00005. [DOI] [PubMed] [Google Scholar]
- 63.Janmahasatian S, Duffull SB, Ash S, Ward LC, Byrne NM, Green B. Quantification of lean bodyweight. Clin Pharmacokinet. 2005;44(10):1051–65. doi: 10.2165/00003088-200544100-00004. [DOI] [PubMed] [Google Scholar]
- 64.De Baerdemaeker LE, Mortier EP, Struys MM. Pharmacokinetics in obese patients. Contin Educ Anaesth Crit Care Pain. 2004;4(5):152–5. doi: 10.1093/bjaceaccp/mkh042. [DOI] [Google Scholar]
- 65.Casati A, Putzu M. Anesthesia in the obese patient: pharmacokinetic considerations. J Clin Anesth. 2005;17(2):134–45. doi: 10.1016/j.jclinane.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 66.Lloret Linares C, Decleves X, Oppert JM, Basdevant A, Clement K, Bardin C, et al. Pharmacology of morphine in obese patients: clinical implications. Clin Pharmacokinet. 2009;48(10):635–51. doi: 10.2165/11317150-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 67.Ingrande J, Lemmens HJ. Dose adjustment of anaesthetics in the morbidly obese. Br J Anaesth. 2010;105(Suppl 1):i16–23. doi: 10.1093/bja/aeq312. [DOI] [PubMed] [Google Scholar]
- 68.Alsio A, Rembeck K, Askarieh G, Christensen PB, Farkkila M, Langeland N, et al. Impact of obesity on the bioavailability of peginterferon-alpha2a and ribavirin and treatment outcome for chronic hepatitis C genotype 2 or 3. PLoS ONE. 2012;7(5) doi: 10.1371/journal.pone.0037521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Diepstraten J, Chidambaran V, Sadhasivam S, Esslinger HR, Cox SL, Inge TH, et al. Propofol clearance in morbidly obese children and adolescents: influence of age and body size. Clin Pharmacokinet. 2012;51(8):543–51. doi: 10.1007/BF03261930. [DOI] [PubMed] [Google Scholar]
- 70.Mornar S, Chan LN, Mistretta S, Neustadt A, Martins S, Gilliam M. Pharmacokinetics of the etonogestrel contraceptive implant in obese women. Am J Obstet Gynecol. 2012;207(2):110 e1-6. doi:10.1016/j.ajog.2012.05.002. [DOI] [PubMed]
- 71.Blouin RA, Warren GW. Pharmacokinetic considerations in obesity. J Pharm Sci. 1999;88(1):1–7. doi: 10.1021/js980173a. [DOI] [PubMed] [Google Scholar]
- 72.Kolka CM, Harrison LN, Lottati M, Chiu JD, Kirkman EL, Bergman RN. Diet-induced obesity prevents interstitial dispersion of insulin in skeletal muscle. Diabetes. 2010;59(3):619–26. doi: 10.2337/db09-0839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sindelka G, Heinemann L, Berger M, Frenck W, Chantelau E. Effect of insulin concentration, subcutaneous fat thickness and skin temperature on subcutaneous insulin absorption in healthy subjects. Diabetologia. 1994;37(4):377–80. doi: 10.1007/BF00408474. [DOI] [PubMed] [Google Scholar]
- 74.Vora JP, Burch A, Peters JR, Owens DR. Relationship between absorption of radiolabeled soluble insulin, subcutaneous blood flow, and anthropometry. Diabetes Care. 1992;15(11):1484–93. doi: 10.2337/diacare.15.11.1484. [DOI] [PubMed] [Google Scholar]
- 75.Dayananda L, Belaval VV, Raina A, Chandana R. Intended intramuscular gluteal injections: are they truly intramuscular? J Postgrad Med. 2014;60(2):175–8. doi: 10.4103/0022-3859.132334. [DOI] [PubMed] [Google Scholar]
- 76.Chantel S, Martin P, Petit P, Massignon D, Dode X, Maire P, et al. Pharmacokinetic variability of the absorption of enoxaparin used subcutaneously for venous thromboembolism prophylaxis. Population Approach Group Europe; 2006.
- 77.Livingston EH. The incidence of bariatric surgery has plateaued in the U.S. Am J Surg. 2010;200(3):378–85. doi: 10.1016/j.amjsurg.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Edwards A, Ensom MH. Pharmacokinetic effects of bariatric surgery. Ann Pharmacother. 2012;46(1):130–6. doi: 10.1345/aph.1Q414. [DOI] [PubMed] [Google Scholar]
- 79.Braghetto I, Davanzo C, Korn O, Csendes A, Valladares H, Herrera E, et al. Scintigraphic evaluation of gastric emptying in obese patients submitted to sleeve gastrectomy compared to normal subjects. Obes Surg. 2009;19(11):1515–21. doi: 10.1007/s11695-009-9954-z. [DOI] [PubMed] [Google Scholar]
- 80.Padwal R, Brocks D, Sharma AM. A systematic review of drug absorption following bariatric surgery and its theoretical implications. Obes Rev. 2010;11(1):41–50. doi: 10.1111/j.1467-789X.2009.00614.x. [DOI] [PubMed] [Google Scholar]
- 81.Olson K. Poisoning and drug overdose. 6. New York: McGraw-Hill Education; 2007. [Google Scholar]
- 82.Corcoran GB, Salazar DE. Obesity as a risk factor in drug-induced organ injury. IV. Increased gentamicin nephrotoxicity in the obese overfed rat. J Pharmacol Exp Ther. 1989;248(1):17–22. [PubMed] [Google Scholar]
- 83.Schwartz AE, Matteo RS, Ornstein E, Young WL, Myers KJ. Pharmacokinetics of sufentanil in obese patients. Anesth Analg. 1991;73(6):790–3. [PubMed] [Google Scholar]
- 84.Egan TD, Huizinga B, Gupta SK, Jaarsma RL, Sperry RJ, Yee JB, et al. Remifentanil pharmacokinetics in obese versus lean patients. Anesthesiology. 1998;89(3):562–73. doi: 10.1097/00000542-199809000-00004. [DOI] [PubMed] [Google Scholar]
- 85.Leykin Y, Pellis T, Lucca M, Lomangino G, Marzano B, Gullo A. The effects of cisatracurium on morbidly obese women. Anesth Analg. 2004;99(4):1090–4. doi: 10.1213/01.ANE.0000132781.62934.37. [DOI] [PubMed] [Google Scholar]
- 86.Leykin Y, Pellis T, Lucca M, Lomangino G, Marzano B, Gullo A. The pharmacodynamic effects of rocuronium when dosed according to real body weight or ideal body weight in morbidly obese patients. Anesth Analg. 2004;99(4):1086–9. doi: 10.1213/01.ANE.0000120081.99080.C2. [DOI] [PubMed] [Google Scholar]
- 87.Sparreboom A, Wolff AC, Mathijssen RH, Chatelut E, Rowinsky EK, Verweij J, et al. Evaluation of alternate size descriptors for dose calculation of anticancer drugs in the obese. J Clin Oncol. 2007;25(30):4707–13. doi: 10.1200/JCO.2007.11.2938. [DOI] [PubMed] [Google Scholar]
- 88.Allard S, Kinzig M, Boivin G, Sorgel F, LeBel M. Intravenous ciprofloxacin disposition in obesity. Clin Pharmacol Ther. 1993;54(4):368–73. doi: 10.1038/clpt.1993.162. [DOI] [PubMed] [Google Scholar]
- 89.Taivainen T, Tuominen M, Rosenberg PH. Influence of obesity on the spread of spinal analgesia after injection of plain 0.5% bupivacaine at the L3-4 or L4-5 interspace. Br J Anaesth. 1990;64(5):542–6. doi: 10.1093/bja/64.5.542. [DOI] [PubMed] [Google Scholar]
- 90.Hogan QH, Prost R, Kulier A, Taylor ML, Liu S, Mark L. Magnetic resonance imaging of cerebrospinal fluid volume and the influence of body habitus and abdominal pressure. Anesthesiology. 1996;84(6):1341–9. doi: 10.1097/00000542-199606000-00010. [DOI] [PubMed] [Google Scholar]
- 91.Ellero S, Chakhtoura G, Barreau C, Langouet S, Benelli C, Penicaud L, et al. Xenobiotic-metabolizing cytochromes p450 in human white adipose tissue: expression and induction. Drug Metab Dispos. 2010;38(4):679–86. doi: 10.1124/dmd.109.029249. [DOI] [PubMed] [Google Scholar]
- 92.Brill MJ, Diepstraten J, van Rongen A, van Kralingen S, van den Anker JN, Knibbe CA. Impact of obesity on drug metabolism and elimination in adults and children. Clin Pharmacokinet. 2012;51(5):277–304. doi: 10.2165/11599410-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 93.Snawder JE, Roe AL, Benson RW, Roberts DW. Loss of CYP2E1 and CYP1A2 activity as a function of acetaminophen dose: relation to toxicity. Biochem Biophys Res Commun. 1994;203(1):532–9. doi: 10.1006/bbrc.1994.2215. [DOI] [PubMed] [Google Scholar]
- 94.Murphy R, Swartz R, Watkins PB. Severe acetaminophen toxicity in a patient receiving isoniazid. Ann Intern Med. 1990;113(10):799–800. doi: 10.7326/0003-4819-113-10-799. [DOI] [PubMed] [Google Scholar]
- 95.Abernethy DR, Greenblatt DJ. Ibuprofen disposition in obese individuals. Arthritis Rheum. 1985;28(10):1117–21. doi: 10.1002/art.1780281006. [DOI] [PubMed] [Google Scholar]
- 96.Abernethy DR, Divoll M, Greenblatt DJ, Ameer B. Obesity, sex, and acetaminophen disposition. Clin Pharmacol Ther. 1982;31(6):783–90. doi: 10.1038/clpt.1982.111. [DOI] [PubMed] [Google Scholar]
- 97.Abernethy DR, Greenblatt DJ, Divoll M, Shader RI. Enhanced glucuronide conjugation of drugs in obesity: studies of lorazepam, oxazepam, and acetaminophen. J Lab Clin Med. 1983;101(6):873–80. [PubMed] [Google Scholar]
- 98.Bentley JB, Borel JD, Vaughan RW, Gandolfi AJ. Weight, pseudocholinesterase activity, and succinylcholine requirement. Anesthesiology. 1982;57(1):48–9. doi: 10.1097/00000542-198207000-00014. [DOI] [PubMed] [Google Scholar]
- 99.Tanaka E, Yamazaki K, Misawa S. Update: the clinical importance of acetaminophen hepatotoxicity in non-alcoholic and alcoholic subjects. J Clin Pharm Ther. 2000;25(5):325–32. doi: 10.1046/j.1365-2710.2000.00301.x. [DOI] [PubMed] [Google Scholar]
- 100.Vuilleumier N, Rossier MF, Chiappe A, Degoumois F, Dayer P, Mermillod B, et al. CYP2E1 genotype and isoniazid-induced hepatotoxicity in patients treated for latent tuberculosis. Eur J Clin Pharmacol. 2006;62(6):423–9. doi: 10.1007/s00228-006-0111-5. [DOI] [PubMed] [Google Scholar]
- 101.Salazar DE, Schentag JJ, Corcoran GB. Obesity as a risk factor in drug-induced organ injury. V. Toxicokinetics of gentamicin in the obese overfed rat. Drug Metab Dispos. 1992;20(3):402–6. [PubMed] [Google Scholar]
- 102.Jesudason DR, Clifton P. Interpreting different measures of glomerular filtration rate in obesity and weight loss: pitfalls for the clinician. Int J Obes (Lond) 2012;36:1421–7. doi: 10.1038/ijo.2011.242. [DOI] [PubMed] [Google Scholar]
- 103.Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, et al. Obesity and the risk of heart failure. N Engl J Med. 2002;347(5):305–13. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 104.Abrass CK. Overview: Obesity: what does it have to do with kidney disease? J Am Soc Nephrol. 2004;15(11):2768–72. doi: 10.1097/01.ASN.0000141963.04540.3E. [DOI] [PubMed] [Google Scholar]
- 105.Lavie CJ, Mehra MR, Milani RV. Obesity and heart failure prognosis: paradox or reverse epidemiology? Eur Heart J. 2005;26(1):5–7. doi: 10.1093/eurheartj/ehi055. [DOI] [PubMed] [Google Scholar]
- 106.Praga M. Obesity—a neglected culprit in renal disease. Nephrol Dial Transplant. 2002;17:1157–9. doi: 10.1093/ndt/17.7.1157. [DOI] [PubMed] [Google Scholar]
- 107.Amann K, Benz K. Structural renal changes in obesity and diabetes. Semin Nephrol. 2013;33:23–33. doi: 10.1016/j.semnephrol.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 108.Penzak SR, Gubbins PO, Rodvold KA, Hickerson SL. Therapeutic drug monitoring of vancomycin in a morbidly obese patient. Ther Drug Monit. 1998;20:261–5. doi: 10.1097/00007691-199806000-00005. [DOI] [PubMed] [Google Scholar]
- 109.Salazar DE, Corcoran GB. Predicting creatinine clearance and renal drug clearance in obese patients from estimated fat-free body mass. Am J Med. 1988;84(6):1053–60. doi: 10.1016/0002-9343(88)90310-5. [DOI] [PubMed] [Google Scholar]
- 110.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 111.Winter MA, Guhr KN, Berg GM. Impact of various body weights and serum creatinine concentrations on the bias and accuracy of the Cockcroft-Gault equation. Pharmacotherapy. 2012;32(7):604–12. doi: 10.1002/j.1875-9114.2012.01098.x. [DOI] [PubMed] [Google Scholar]
- 112.Cho S-J, Yoon I-S, Kim D-D. Obesity-related physiological changes and their pharmacokinetic consequences. J Pharm Investig. 2013;43(3):161–9. doi: 10.1007/s40005-013-0073-4. [DOI] [Google Scholar]
- 113.McLeay SC, Morrish GA, Kirkpatrick CM, Green B. The relationship between drug clearance and body size: systematic review and meta-analysis of the literature published from 2000 to 2007. Clin Pharmacokinet. 2012;51(5):319–30. doi: 10.2165/11598930-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 114.Bauer LA, Black DJ, Lill JS. Vancomycin dosing in morbidly obese patients. Eur J Clin Pharmacol. 1998;54:621–5. doi: 10.1007/s002280050524. [DOI] [PubMed] [Google Scholar]
- 115.Dvorchik BH, Damphousse D. The pharmacokinetics of daptomycin in moderately obese, morbidly obese, and matched nonobese subjects. J Clin Pharmacol. 2005;45:48–56. doi: 10.1177/0091270004269562. [DOI] [PubMed] [Google Scholar]
- 116.Pai MP, Norenberg JP, Anderson T, Goade DW, Rodvold KA, Telepak RA, et al. Influence of morbid obesity on the single-dose pharmacokinetics of daptomycin. Antimicrob Agents Chemother. 2007;51:2741–7. doi: 10.1128/AAC.00059-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yee JY, Duffull SB. The effect of body weight on dalteparin pharmacokinetics. A preliminary study. Eur J Clin Pharmacol. 2000;56:293–7. doi: 10.1007/s002280000141. [DOI] [PubMed] [Google Scholar]
- 118.Barras MA, Duffull SB, Atherton JJ, Green B. Modelling the occurrence and severity of enoxaparin-induced bleeding and bruising events. Br J Clin Pharmacol. 2009;68:700–11. doi: 10.1111/j.1365-2125.2009.03518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Willett KC, Alsharhan M, Durand C, Cooper MR. Dosing of enoxaparin for venous thromboembolism prophylaxis in obese patients. Ann Pharmacother. 2013;47:1717–20. doi: 10.1177/1060028013507902. [DOI] [PubMed] [Google Scholar]
- 120.Bauer LA, Wareing-Tran C, Edwards WA, Raisys V, Ferreri L, Jack R, et al. Cimetidine clearance in the obese. Clin Pharmacol Ther. 1985;37:425–30. doi: 10.1038/clpt.1985.66. [DOI] [PubMed] [Google Scholar]
- 121.Abernethy DR, Greenblatt DJ, Matlis R, Gugler R. Cimetidine disposition in obesity. Am J Gastroenterol. 1984;79:91–4. [PubMed] [Google Scholar]
- 122.Chyka PA, Seger D, Krenzelok EP, Vale JA, AACT. EAPCCT Position paper: single-dose activated charcoal. Clin Toxicol (Phila) 2005;43(2):61–87. doi: 10.1081/CLT-51867. [DOI] [PubMed] [Google Scholar]
- 123.McNamara RM, Aaron CK, Gemborys M, Davidheiser S. Efficacy of charcoal cathartic versus ipecac in reducing serum acetaminophen in a simulated overdose. Ann Emerg Med. 1989;18(9):934–8. doi: 10.1016/S0196-0644(89)80456-1. [DOI] [PubMed] [Google Scholar]
- 124.Position statement and practice guidelines on the use of multi-dose activated charcoal in the treatment of acute poisoning. American Academy of Clinical Toxicology; European Association of Poisons Centres and Clinical Toxicologists. J Toxicol Clin Toxicol. 1999;37(6):731–51. [DOI] [PubMed]
- 125.Krag E, Hojgaard L. Bile acid metabolism after intestinal bypass operations. Int J Obes. 1981;5(5):519–25. [PubMed] [Google Scholar]
- 126.Dunning K, Plymyer MR. Charcoal peritonitis causing chronic pelvic pain: a unique complication following bariatric surgery. Obes Surg. 2006;16(9):1238–42. doi: 10.1381/096089206778392121. [DOI] [PubMed] [Google Scholar]
- 127.Van Dinter TG, Jr, John L, Guileyardo JM, John SF. Intestinal perforation caused by insertion of a nasogastric tube late after gastric bypass. Proc (Baylor Univ Med Cent) 2013;26(1):11–5. doi: 10.1080/08998280.2013.11928900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Appelboam AV, Dargan PI, Knighton J. Fatal anaphylactoid reaction to N-acetylcysteine: caution in patients with asthma. Emerg Med J. 2002;19(6):594–5. doi: 10.1136/emj.19.6.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Prizont R. Medical officer review: acetadote. FDA Center for Drug Evaluation and Research: Office of Drug Evaluation. 2003. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2004/21-539_Acetadote_Medr.pdf. Accessed 16 May 2015.
- 130.Duncan R, Cantlay G, Paterson B. New recommendation for N-acetylcystiene dosing may reduce incidence of adverse effects. Emerg Med J. 2006;23(7):584. doi: 10.1136/emj.2005.031104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Intralipid [package insert]. Deerfield: Baxter Healthare Corporation; 2007.
- 132.Methylene blue [package insert]. Lake Forest: Akorn, Inc.; 2008.
- 133.El-Solh A, Sikka P, Bozkanat E, Jaafar W, Davies J. Morbid obesity in the medical ICU. Chest. 2001;120(6):1989–97. doi: 10.1378/chest.120.6.1989. [DOI] [PubMed] [Google Scholar]
- 134.Akinnusi ME, Pineda LA, El Solh AA. Effect of obesity on intensive care morbidity and mortality: a meta-analysis. Crit Care Med. 2008;36(1):151–8. doi: 10.1097/01.CCM.0000297885.60037.6E. [DOI] [PubMed] [Google Scholar]
- 135.Bercault N, Boulain T, Kuteifan K, Wolf M, Runge I, Fleury JC. Obesity-related excess mortality rate in an adult intensive care unit: a risk-adjusted matched cohort study. Crit Care Med. 2004;32(4):998–1003. doi: 10.1097/01.CCM.0000119422.93413.08. [DOI] [PubMed] [Google Scholar]
- 136.Tremblay A, Bandi V. Impact of body mass index on outcomes following critical care. Chest. 2003;123(4):1202–7. doi: 10.1378/chest.123.4.1202. [DOI] [PubMed] [Google Scholar]