Abstract
The oviduct plays a role in successful animal reproduction not only in spermatozoa and ova transport to the fertilization site but also by affording a microenvironment for fertilization and early embryonic development. The sperm reservoir (SR) is restricted in the uterotubal junction (UTJ) and caudal isthmus. Billions of porcine spermatozoa are distributed to the female reproductive tract during/after insemination, and small amounts of them are stored for about 36–40 hours in the SR, which maintains sperm viability in the pre-ovulation period through its surface epithelium and production of fluid. The SR regulates the release of spermatozoa so that only a small population moves towards the fertilization site (ampulla) to decrease polyspermy. This review attempts to provide information about the structure and function of the porcine SR, its intraluminal content (hyaluronan, HA), and the influences of HA on porcine spermatozoa in vivo. In pigs, the spermatozoa are stored in a mucous-like fluid within the UTJ and caudal isthmus in the pre-ovulation period. The oviduct fluid contains sulfated glycosaminoglycans (GAGs) and non-sulfated GAGs, i.e., HA. It is interesting to note that HA is synthesized by hyaluronan synthase-3 (HAS-3), and its receptor, CD44, is found in the epithelium of the porcine SR site. Additionally, sperm capacitation does not occur in vivo in the SR during the pre- and peri-ovulation periods, but spermatozoa in the SR will attempt to capacitate if exposed to bicarbonate. However, capacitation in the SR will rise in the post-ovulation period, indicating the role of HA in modulating sperm capacitation after ovulation. All data support the understanding that the porcine SR ensures the viability of fertile spermatozoa and maintains the non-capacitated status during the pre-ovulation period. This basic knowledge about the SR is believed to be useful to advance sperm preparation procedures for in vitro fertilization (IVF) and improve the preservation process of porcine semen.
Keywords: CD44, Hyaluronan, Morphology, Oviduct, Pig, Sperm reservoir, Viability
During fertilization in pigs, numerous factors such as the appropriate moment to permit mating and the exact time of ovulation, including the quality and quantity of spermatozoa, are essential to accomplish regulation. Nearly four decades ago, the elementary protocols for porcine artificial insemination (AI) were first implemented [1]. Still, at present, the procedures are being improved. The AI industry in swine farms has developed massively, and suitable extenders for boar semen preservation have been explored to obtain defined spermatozoa for traveling in the female reproductive tract [2]. In vitro maturation (IVM), in vitro fertilization (IVF) and in vitro culture (IVC) of pig embryos were not accomplished until the 1990s, by which time these in vitro technologies were raised to a satisfactory level [3]. The biggest difficulties faced in this species are inadequate maturation of oocytes and a high percentage of polyspermy [4]. One solution among the various factors to attain an enhanced outcome is to scrutinize the benefits of supplementation of IVM/IVF media to increase the oocyte maturation rate and reduce the polyspermic fertilization rate, respectively. Consequently, studies involving sperm function and the surrounding microenvironment in the oviduct have contributed to distinguishing sperm, which should enable definite modifications to produce a better IVF medium [5]. During natural mating, billions of porcine spermatozoa are transported to the female, and a few hundred thousand are stored in a sperm reservoir (SR) for at least 36–40 h [6]. The uterotubal junction (UTJ) and posterior part of the isthmus have been confirmed to be the SR location in which the trapped spermatozoa await ovulation and are then unleashed in small amounts to the ampullary-isthmic junction (AIJ) for fertilization [7]. The morphology of the SR is depicted as a very narrow lumen with a sticky intraluminal fluid that is able to confine porcine spermatozoa [8]. Fundamentally, massive numbers of spermatozoa in the SR retain their viability and fertilizing ability as they stay away from assaults by female immune system cells [9]. Studies of the porcine oviduct confirmed that the oviduct fluid influences sperm functions in different manners [10, 11], and the main components of the fluid are glycosaminoglycans (GAGs). Hyaluronan (HA), a non-sulfated GAG, has been reported to modulate sperm capacitation-like alterations and reduce polyspermy by interaction with the sperm plasma membrane [12, 13]. Therefore, the appearance of HA and its association in the porcine SR could be evidence leading to realization of crucial requirements during sperm storage for the preservation of sperm viability and fertilizing ability. This review describes the general morphology and function of the porcine SR with a focus on the presence of HA, its receptors and synthesizing enzymes, including the effect of HA on boar spermatozoa.
General Aspects and Definite Regulations of the Sperm Reservoir
The SR was first reported in hamsters and rabbits in 1963 [14], and almost two decades later, the specific morphology together with other evidence now suggest that the UTJ and caudal part of the isthmus represent the oviductal SR in pigs [6, 7]. These observations describe how massive numbers of spermatozoa stored in the porcine SR are trapped in the mucosal folds by chemotactic attraction and intraluminal secretion and defended from attack by polymorphonuclear leukocytes. Remarkably, the great amounts of spermatozoa with epithelial interaction, especially in the SR crypts, exhibit an intact plasma membrane (Fig. 1) during the pre-ovulation period of the estrous phase [15]. There have been various explanations for the mechanisms in the porcine SR before and after insemination. Firstly, the SR is the furthermost area of the oviduct that the spermatozoa confront [16], and its convoluted lumen becomes narrowest due to the subepithelial edema stimulated by a high estrogen level during the proestrus to estrus phases [7, 17]. These morphological changes could capture the massive numbers of spermatozoa during the early phase of sperm transport. Secondly, the expression of an enzyme, carbonic anhydrase, in the secretory cells, particularly in the deep grooves of the porcine SR [18], is similar to that in the boar cauda epididymis [19]. These findings imply that the secretory fluid in which spermatozoa is immersed may depict the suitable levels of electrolyte and acid-base status for slowing down sperm motility during storage in the SR [20]. Thirdly, the rise in the temperature gradient from the posterior isthmus to the ampulla is caused by the extent and activity of the vascular and lymphatic supplies in the pre-ovulation period and could also be associated with the reduction of sperm motility in the specific porcine SR [21]. Fourthly, the adherence of boar spermatozoa to the surface epithelium of the oviduct has been shown to be necessary for SR construction [22, 23]. Lastly, the intraluminal mucus-like fluid has been found in the oviduct in the estrus period in different species, such as the human [24], rabbit [25], cow [26] and pig [27, 28]. Therefore, these essential observations have to be incorporated into the factors that would contribute to detention of spermatozoa and establishment of an SR in vivo [29]. The principal circumstances taking place in the porcine SR could depress metabolism and subsequently motility during sperm storage [30], leading to a definite status of sperm quiescence for protecting viable spermatozoa and maintaining their capability [17]. In an in vitro experiment, spermatozoa attached to oviductal epithelial cells were revealed to remain viable for longer periods compared with a sperm population incubated in a culture medium alone [31], indicating that the oviduct epithelium and the secretory fluid it produces can preserve sperm viability. Furthermore, spermatozoa co-cultured with membrane secretory vesicles from the apical portions of oviduct epithelial cells have been shown to sustain intracellular Ca2+ at lower levels and thus prolong sperm viability [32]. Previous studies have also emphasized the achievement of in situ intraluminal fluid and cultured medium from oviductal epithelium in the reduction of polyspermic penetration [11, 33]. Consequently, certain mechanisms that modulate suitable sperm functions and maintain sperm survival in the porcine SR might come from component substances enclosed in the oviductal intraluminal fluid.
Fig. 1.
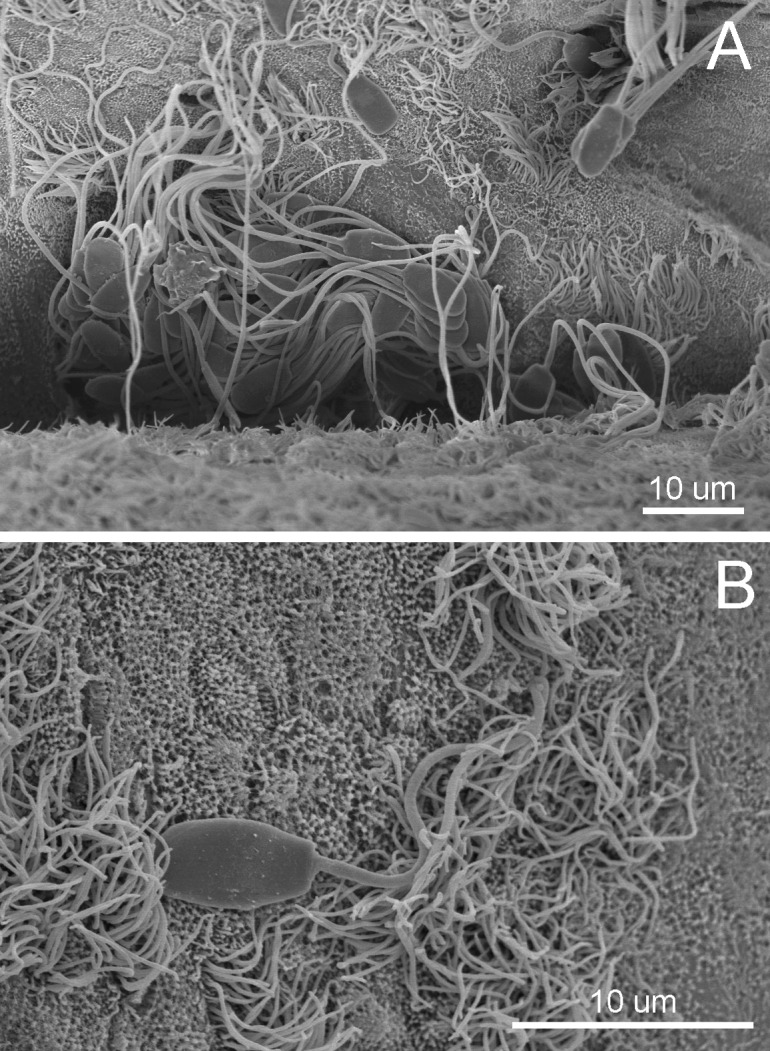
Representative scanning electron micrographs of the porcine sperm reservoir (in the uterotubal junction or UTJ) during the pre-ovulatory period of the estrous cycle demonstrating (A) clusters of the sperm population in the profound furrow and (B) attachment of an intact spermatozoon (notice the morphology of the sperm head) to the microvilli and cilia on the lining epithelium.
Specific Attributes of Hyaluronan and Its Association in the Female Genital Organ
A mucus-like substance appears in the intraluminal secretion of mammal oviducts, particularly in the inferior segment, composed of a diversity of glycoproteins [26, 34] and GAGs [35]. There are two major classes of GAGs; one consists of sulfated GAGs, i.e. keratin sulfate, heparin, heparin sulfate, chondroitin sulfate and dermatan sulfate, and the other consists of non-sulfated GAG, i.e., HA [36]. Considering the fact that HA is present in porcine spermatozoa and oocytes, HA was selected as more important to describe than the other sulfated GAGs in this review. The properties of HA were described almost 80 years ago [37]; it is a constituent of the extracellular matrix, has a high molecular weight and is composed of disaccharide duplicates of N-acetylglucosamine and D-glucuronic acid. HA is an enormous polymer with approximately 25,000 disaccharide units and a molecular weight of about 4×106 kDa [38]. Generally, HA arises from salt formation and is present in high concentrations within continuous networks of various loose connective tissues [39]. Apart from the umbilical cord, vitreous humor and synovial fluid, HA is the main component of the cumulus-oocyte complex (COC) viscous cloud during cumulus expansion [40, 41]. Fundamentally, HA can be naturally synthesized by integral membrane enzymes with hyaluronan synthases recognized as HAS1, HAS2, and HAS3 [42, 43]. Interestingly, HA plays an assortment of vital roles in the extracellular matrix by adhering to cell surfaces and other elements by way of explicit and non-explicit interactions, i.e., HA receptors. Up till now, CD44 has been known as a multifunctional cell surface glycoprotein, and it is distinguished as the major transmembrane hyaluronan receptor appearing in most cell types [44, 45], including the cumulus cells of porcine COCs [46]. Moreover, several studies have indicated the appearance of HA in the female genital tract of various species, for example, rats [47], humans [48], horses [49] and cows [35]. These essential observations signify the involvement of HA in the regulations of spermatozoa, oocytes and early embryos during their journey in the female reproductive organ.
Mechanisms of Hyaluronan and Its Functions in the Porcine Sperm Reservoir
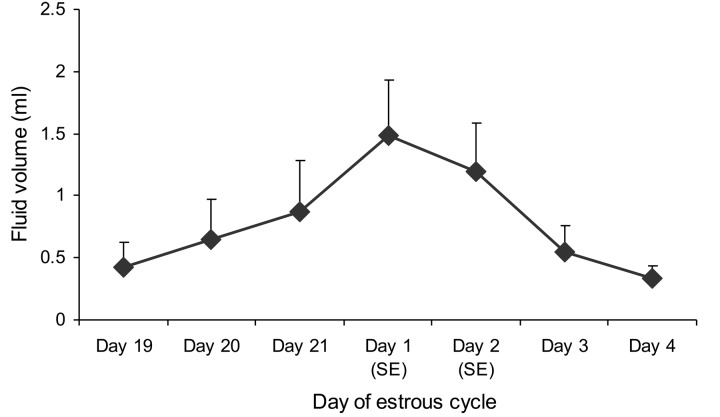
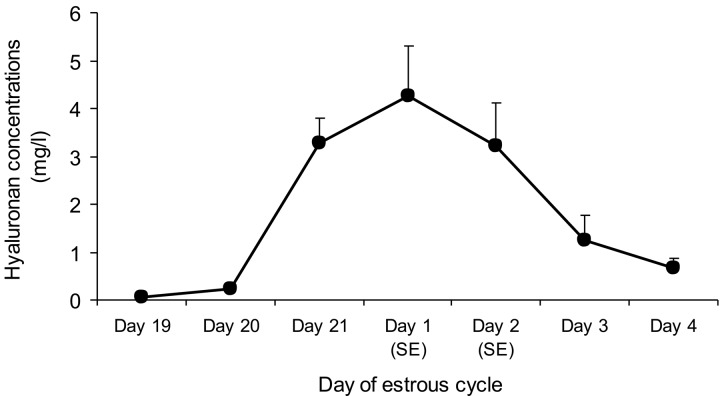
The luminal secretory fluid in the oviduct is a compound assortment originating from two major sources, i.e., selective transudation from blood plasma [50] and specific secretion from epithelial cells [51]. The porcine epithelial lining naturally consists of ciliated cells and secretory cells. The latter cells are present in greater number in the isthmic segment which is comparable to the ampulla or the infundibulum [27], and these cells undertake a series of proliferative and degenerative changes according to hormonal levels during the estrous cycle [52]. It is well known that the secretory intraluminal fluid affects sperm characteristics in different manners, for example, viability, motility and capacitation [53,54,55]. In this review, the collective oviductal fluid of pigs from proestrus to metestrus (Fig. 2) was measured for HA levels and found to a higher HA concentration at standing estrus (Fig. 3). HA was localized by histochemistry to the epithelium of the caudal isthmic portion and the UTJ, mostly in the pre-ovulatory period [28], suggesting that the mucus-like constituent found in various species would be involved in the presence of HA and implying the essential actions of HA in the SR. Furthermore, this research also proved that HA can be produced by porcine epithelial cells through an integral membrane protein, HAS3 [56]. As mentioned previously, the properties of HA are multipurpose, and they include such things as it being extremely hydrophilic, forming gel at low level of concentration and creating viscosity and elasticity [57]. There have been some reports that have implied that HA occupies the protective physiochemical actions that could afford additional protective influences on chondrocytes in articular cartilage [58]. Under these circumstances, HA might capture spermatozoa during their storage and inhibit the uterine and ampullary liquids flowing into the SR [59] during the pre-ovulation period, separating the sperm subpopulation from unsuitable surroundings [17]. It is believed that spermatozoa submerged in the HA-secretory mucus of the SR could antigenically disguise themselves from recognition by phagocytes of the female immune system [9]. Besides its structures and hygroscopic properties, HA is capable of interrelating with specific receptors, causing the stimulation of signaling cascades that affect several cellular mechanisms [60]. The principal HA receptor, CD44 (expression of both protein and mRNA), is noticeably localized both on the membranous surface and in the supranuclear domain of the epithelial cells, especially in the porcine SR in the pre-ovulation period [61]. This could imply that the function of HA binding to CD44 on the membranous surface is accountable for cell-cell and, of course, cell-extracellular milieu [62] and restraint apoptosis [63]. However, the CD44 expression in the supranuclear region of most epithelial cells would indicate the participation of CD44 in indigenous HA uptake from and degradation of porcine oviduct fluid [35]. In porcine oocytes, the HA-CD44 interplay could influence fertility [64] and maturation during cumulus expansion [40]. Consequently, the appearance of CD44 in the efficient porcine SR suggests that the HA-CD44 signaling pathway might occur to reserve this location with the sticky cloud which the spermatozoa are bathed in to lengthen sperm viability, defend spermatozoa from the local female immune system and avoid the early stage of sperm capacitation [5] during the pre-ovulatory phase. In addition, the specific HA-CD44 pathway in the porcine SR could play an important role in oocyte maturation and other collaborations before fertilization [65] as well.
Fig. 2.
Daily volumes (mean ± SEM) of sow intraluminal fluid collected from the isthmus via indwelling specific catheters during proestrus, estrus and metestrus (n = 5). SE = standing estrus.
Fig. 3.
Hyaluronan concentrations (mean ± SEM) in the intraluminal fluid of the non-inseminated sow isthmus analyzed using a Pharmacia hyaluronan test during proestrus, estrus and metestrus (n = 5). SE = standing estrus.
Relationships between Boar Spermatozoa and Hyaluronan in the Sperm Reservoir
Among several phenomena in the female reproductive tract after natural or artificial mating, sperm capacitation is one of the most essential incidents that facilitate successful fertilization. It is well known that sperm capacitation is a gradual step-wise procedure taking place when spermatozoa move along the female genital tube, and this specific mechanism involves sequential reorganization and adjustment of the sperm plasma membrane by exclusion of cholesterol and seminal plasma proteins [66]. These processes are complemented by the relocation of lipid diffusion in the plasma membrane and accomplished with the destabilization of its construction [67]. The molecular changes during capacitation appear without noticeable morphological alterations, but they can be visualized indirectly by examination with the chlortetracycline (CTC) assay [68]. It has been found that the CTC assay can detect the transposition of Ca2+ influx in the plasma membrane, which occurs during the latter step of capacitation and then takes part in the acrosome reaction [69]. However, the preliminary steps of sperm capacitation can be assessed by loading spermatozoa with the membrane fluorescent lipophilic dye Merocyanine 540, since an advanced level of lipid disorganization in the sperm plasma membrane is indicative of the initiation of capacitation [20]. Some studies have suggested that sperm capacitation could be induced and arise in the SR [70, 71]. In in vitro experiments, supplementation of epithelial cells from the oviduct prolongs sperm viability, and if the spermatozoa are persuaded to capacitate, they will be released from oviductal epithelial binding [72] by their concurrently improved hyperactivated movement [73, 74]. The regulation involved in sperm release from the porcine SR is believed to be due to the endocrine hint [75]. The data from the in vivo study specified that a principal subpopulation (73%) of boar spermatozoa collected from the SR in the pre-ovulation period was alive and displayed a non-capacitated pattern [76]. These observations correspond with previous research indicating that 63–70% of boar spermatozoa washed out from the SR exhibited an intact plasma membrane [77]. Furthermore, a group of spermatozoa stored in UTJ crypts revealed a completed plasma membrane under both scanning and transmission electron microscopy [15]. Thus, it can be concluded that the porcine SR at spontaneous standing estrus does not motivate sperm capacitation, whereas this particular location lengthens the viability and fertilizing capacity of the residing spermatozoa until the post-ovulation period [5, 75]. In addition, the HA supplementation (500 µl/ml) in the conditioned medium of IVF seemed to provoke capacitation-like changes in a CTC assay for porcine frozen-thawed spermatozoa not showing an acrosome reaction [13]. Later research added HA at a dose similar to that of spermatozoa flushed from the porcine SR, incubated the spermatozoa in modified Brackett-Oliphant (mBO) medium with bicarbonate (NaHCO3) and performed Merocyanine 540 fluorescence staining, showing high percentages of non-capacitated spermatozoa [75]. Consequently, the HA present in the luminal fluid and on the oviductal epithelium [28] could serve as a defensive substance to preserve stored spermatozoa until occurrence of the modifications of the oviduct microenvironment due to the mechanisms of ovulation. It is important to note that porcine spermatozoa consecutively progress from the SR to AIJ for fertilization which is associated with the ovulation time [15, 76]. However, this advanced motility of boar spermatozoa is not instantaneous, even though it might happen under the influence of female hormones, promoting alterations of the oviduct epithelium and smooth muscle contraction [74]. As a result of the above, the gradual movement of small clusters and subsequent massive migration of spermatozoa from the SR was revealed. Earlier research suggested that the luminal diameter of the porcine SR enlarges in correlation with the amount of mucus-like substances reduced and liquefied [27]. Likewise, the relative concentration of HA both in intraluminal fluid and in the staining revealed by histochemistry tend to decline during the postovulatory stage compared with the preovulatory stage [28]. These changes in luminal size and reduction in the amount of mucus and HA might enable the progression of boar spermatozoa from the SR to their target. Nevertheless, this study also found that a bulky sperm subpopulation still existed in the SR in the post-ovulation period and that most spermatozoa showed a non-capacitated pattern [75]. Thus, this review was able to clarify the characteristics of spermatozoa released from the SR, which are released in limited numbers and how this phenomenon is required to prevent polyspermy. Besides the modifications of intraluminal substances from mucus-like substances to liquid, the boarder lumen and the smooth muscle contraction, restricted amounts of spermatozoa would abandon the SR because the mechanism of capacitation at the initiation stage permits their release from their binding to the oviductal epithelium [71].
Conclusion
HA, the non-sulfated GAG, is present in the intraluminal mucus-like component and on the epithelial surface; it is noticeably present in the grooves of the porcine SR in the pre-ovulation period, and the HA and specific morphological conditions in this site are appropriate for preservation spermatozoa. Additionally, the major HA receptor, CD44, is also localized by the oviductal epithelium, implying that the HA-CD44 signaling pathway might play a vital role during sperm storage. Finally, the spermatozoa collected in vivo from the SR are viable and non-capacitated in the pre- and peri-ovulatory phases, sustaining the HA function in the SR related to arresting sperm capacitation during these periods and then to initiate their capacitation after ovulation.
References
- 1.Riesenbeck A. Review on international trade with boar semen. Reprod Domest Anim 2011; 46(Suppl 2): 1–3. [DOI] [PubMed] [Google Scholar]
- 2.Flowers WL, Esbenshade KL. Optimizing management of natural and artificial matings in swine. J Reprod Fertil Suppl 1993; 48: 217–228. [PubMed] [Google Scholar]
- 3.Grupen CG. The evolution of porcine embryo in vitro production. Theriogenology 2014; 81: 24–37. [DOI] [PubMed] [Google Scholar]
- 4.Wang WH, Niwa K, Okuda K. In-vitro penetration of pig oocytes matured in culture by frozen-thawed ejaculated spermatozoa. J Reprod Fertil 1991; 93: 491–496. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez-Martinez H, Tienthai P, Suzuki K, Funahashi H, Ekwall H, Johannisson A. Involvement of oviduct in sperm capacitation and oocyte development in pigs. Reprod Suppl 2001; 58: 129–145. [PubMed] [Google Scholar]
- 6.Hunter RH. Sperm transport and reservoirs in the pig oviduct in relation to the time of ovulation. J Reprod Fertil 1981; 63: 109–117. [DOI] [PubMed] [Google Scholar]
- 7.Fléchon JE, Hunter RH. Distribution of spermatozoa in the utero-tubal junction and isthmus of pigs, and their relationship with the luminal epithelium after mating: a scanning electron microscope study. Tissue Cell 1981; 13: 127–139. [DOI] [PubMed] [Google Scholar]
- 8.Hunter RH, Fléchon B, Fléchon JE. Distribution, morphology and epithelial interactions of bovine spermatozoa in the oviduct before and after ovulation: a scanning electron microscope study. Tissue Cell 1991; 23: 641–656. [DOI] [PubMed] [Google Scholar]
- 9.Rodríguez-Martínez H, Saravia F, Wallgren M, Tienthai P, Johannisson A, Vázquez JM, Martínez E, Roca J, Sanz L, Calvete JJ. Boar spermatozoa in the oviduct. Theriogenology 2005; 63: 514–535. [DOI] [PubMed] [Google Scholar]
- 10.Kim NH, Funahashi H, Abeydeera LR, Moon SJ, Prather RS, Day BN. Effects of oviductal fluid on sperm penetration and cortical granule exocytosis during fertilization of pig oocytes in vitro. J Reprod Fertil 1996; 107: 79–86. [DOI] [PubMed] [Google Scholar]
- 11.Kim NH, Day BN, Lim JG, Lee HT, Chung KS. Effects of oviductal fluid and heparin on fertility and characteristics of porcine spermatozoa. Zygote 1997; 5: 61–65. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki K, Eriksson B, Shimizu H, Nagai T, Rodriguez-Martinez H. Effect of hyaluronan on monospermic penetration of porcine oocytes fertilized in vitro. Int J Androl 2000; 23: 13–21. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki K, Asano A, Eriksson B, Niwa K, Nagai T, Rodriguez-Martinez H. Capacitation status and in vitro fertility of boar spermatozoa: effects of seminal plasma, cumulus-oocyte-complexes-conditioned medium and hyaluronan. Int J Androl 2002; 25: 84–93. [DOI] [PubMed] [Google Scholar]
- 14.Yanagimachi R, Chang MC. Sperm ascent through the oviduct of the hamster and rabbit in relation to the time of ovulation. J Reprod Fertil 1963; 6: 413–420. [DOI] [PubMed] [Google Scholar]
- 15.Mburu JN, Rodriguez-Martinez H, Einarsson S. Changes in sperm ultrastructure and localisation in the porcine oviduct around ovulation. Anim Reprod Sci 1997; 47: 137–148. [DOI] [PubMed] [Google Scholar]
- 16.Lefebvre R, Chenoweth PJ, Drost M, LeClear CT, MacCubbin M, Dutton JT, Suarez SS. Characterization of the oviductal sperm reservoir in cattle. Biol Reprod 1995; 53: 1066–1074. [DOI] [PubMed] [Google Scholar]
- 17.Hunter RH. Vital aspects of Fallopian tube physiology in pigs. Reprod Domest Anim 2002; 37: 186–190. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez-Martinez H, Ekstedt E, Ridderstråle Y. Histochemical localization of carbonic anhydrase in the female genitalia of pigs during the oestrous cycle. Acta Anat (Basel) 1991; 140: 41–47. [DOI] [PubMed] [Google Scholar]
- 19.Ekstedt E, Ridderstråle Y, Plöen L, Rodriguez-Martinez H. Histochemical localization of carbonic anhydrase in the testis and epididymis of the boar. Acta Anat (Basel) 1991; 141: 257–261. [DOI] [PubMed] [Google Scholar]
- 20.Harrison RA, Ashworth PJ, Miller NG. Bicarbonate/CO2, an effector of capacitation, induces a rapid and reversible change in the lipid architecture of boar sperm plasma membranes. Mol Reprod Dev 1996; 45: 378–391. [DOI] [PubMed] [Google Scholar]
- 21.Hunter RH, Nichol R. A preovulatory temperature gradient between the isthmus and ampulla of pig oviducts during the phase of sperm storage. J Reprod Fertil 1986; 77: 599–606. [DOI] [PubMed] [Google Scholar]
- 22.Verhage HG, Fazleabas AT, Mavrogianis PA, O’Day-Bowman MB, Schmidt A, Arias EB, Jaffe RC. Characteristics of an oviductal glycoprotein and its potential role in fertility control. J Reprod Fertil Suppl 1997; 51: 217–226. [PubMed] [Google Scholar]
- 23.Töpfer-Petersen E. Carbohydrate-based interactions on the route of a spermatozoon to fertilization. Hum Reprod Update 1999; 5: 314–329. [DOI] [PubMed] [Google Scholar]
- 24.Jansen RP. Fallopian tube isthmic mucus and ovum transport. Science 1978; 201: 349–351. [DOI] [PubMed] [Google Scholar]
- 25.Jansen RP, Bajpai VK. Oviduct acid mucus glycoproteins in the estrous rabbit: ultrastructure and histochemistry. Biol Reprod 1982; 26: 155–168. [DOI] [PubMed] [Google Scholar]
- 26.Suarez SS, Brockman K, Lefebvre R. Distribution of mucus and sperm in bovine oviducts after artificial insemination: the physical environment of the oviductal sperm reservoir. Biol Reprod 1997; 56: 447–453. [DOI] [PubMed] [Google Scholar]
- 27.Johansson M, Tienthai P, Rodriguez-Martinez H. Histochemistry and ultrastructure of the intraluminal mucus in the sperm reservoir of the pig oviduct. J Reprod Dev 2000; 46: 183–192. [Google Scholar]
- 28.Tienthai P, Kjellén L, Pertoft H, Suzuki K, Rodriguez-Martinez H. Localization and quantitation of hyaluronan and sulfated glycosaminoglycans in the tissues and intraluminal fluid of the pig oviduct. Reprod Fertil Dev 2000; 12: 173–182. [DOI] [PubMed] [Google Scholar]
- 29.DeMott RP, Lefebvre R, Suarez SS. Carbohydrates mediate the adherence of hamster sperm to oviductal epithelium. Biol Reprod 1995; 52: 1395–1403. [DOI] [PubMed] [Google Scholar]
- 30.Smith TT. The modulation of sperm function by the oviductal epithelium. Biol Reprod 1998; 58: 1102–1104. [DOI] [PubMed] [Google Scholar]
- 31.Ellington JE, Samper JC, Jones AE, Oliver SA, Burnett KM, Wright RW. In vitro interactions of cryopreserved stallion spermatozoa and oviduct (uterine tube) epithelial cells or their secretory products. Anim Reprod Sci 1999; 56: 51–65. [DOI] [PubMed] [Google Scholar]
- 32.Murray SC, Smith TT. Sperm interaction with fallopian tube apical membrane enhances sperm motility and delays capacitation. Fertil Steril 1997; 68: 351–357. [DOI] [PubMed] [Google Scholar]
- 33.Nichol R, Hunter RH, de Lamirande E, Gagnon C, Cooke GM. Motility of spermatozoa in hydrosalpingeal and follicular fluid of pigs. J Reprod Fertil 1997; 110: 79–86. [DOI] [PubMed] [Google Scholar]
- 34.Jansen RP, Bajpai VK. Periovulatory glycoprotein secretion in the macaque fallopian tube. Am J Obstet Gynecol 1983; 147: 598–608. [DOI] [PubMed] [Google Scholar]
- 35.Lee CN, Ax RL. Concentrations and composition of glycosaminoglycans in the female bovine reproductive tract. J Dairy Sci 1984; 67: 2006–2009. [DOI] [PubMed] [Google Scholar]
- 36.Hileman RE, Fromm JR, Weiler JM, Linhardt RJ. Glycosaminoglycan-protein interactions: definition of consensus sites in glycosaminoglycan binding proteins. BioEssays 1998; 20: 156–167. [DOI] [PubMed] [Google Scholar]
- 37.Meyer K, Palmer JW. The polysaccharide of vitreous humor. J Biol Chem 1934; 107: 629–634. [Google Scholar]
- 38.Fessler JH, Fessler LI. Electron microscopic visualization of the polysaccharide hyaluronic acid. Proc Natl Acad Sci USA 1966; 56: 141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Toole BP. Hyaluronan promotes the malignant phenotype. Glycobiology 2002; 12: 37R–42R. [DOI] [PubMed] [Google Scholar]
- 40.Yokoo M, Kimura N, Abe H, Sato E. Influence of hyaluronan accumulation during cumulus expansion on in vitro porcine oocyte maturation. Zygote 2008; 16: 309–314. [DOI] [PubMed] [Google Scholar]
- 41.Yokoo M, Kimura N, Sato E. Induction of oocyte maturation by hyaluronan-CD44 interaction in pigs. J Reprod Dev 2010; 56: 15–19. [DOI] [PubMed] [Google Scholar]
- 42.Weigel PH, Hascall VC, Tammi M. Hyaluronan synthases. J Biol Chem 1997; 272: 13997–14000. [DOI] [PubMed] [Google Scholar]
- 43.Lee JY, Spicer AP. Hyaluronan: a multifunctional, megaDalton, stealth molecule. Curr Opin Cell Biol 2000; 12: 581–586. [DOI] [PubMed] [Google Scholar]
- 44.Alho AM, Underhill CB. The hyaluronate receptor is preferentially expressed on proliferating epithelial cells. J Cell Biol 1989; 108: 1557–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tzircotis G, Thorne RF, Isacke CM. Chemotaxis towards hyaluronan is dependent on CD44 expression and modulated by cell type variation in CD44-hyaluronan binding. J Cell Sci 2005; 118: 5119–5128. [DOI] [PubMed] [Google Scholar]
- 46.Yokoo M, Tientha P, Kimura N, Niwa K, Sato E, Rodriguez-Martinez H. Localisation of the hyaluronan receptor CD44 in porcine cumulus cells during in vivo and in vitro maturation. Zygote 2002; 10: 317–326. [DOI] [PubMed] [Google Scholar]
- 47.Laurent C, Hellström S, Engström-Laurent A, Wells AF, Bergh A. Localization and quantity of hyaluronan in urogenital organs of male and female rats. Cell Tissue Res 1995; 279: 241–248. [DOI] [PubMed] [Google Scholar]
- 48.Edelstam GA, Lundkvist OE, Wells AF, Laurent TC. Localization of hyaluronan in regions of the human female reproductive tract. J Histochem Cytochem 1991; 39: 1131–1135. [DOI] [PubMed] [Google Scholar]
- 49.Varner DD, Forrest DW, Fuentes F, Taylor TS, Hooper RN, Brinsko SP, Blanchard TL. Measurements of glycosaminoglycans in follicular, oviductal and uterine fluids of mares. J Reprod Fertil Suppl 1991; 44: 297–306. [PubMed] [Google Scholar]
- 50.Oliphant G, Bowling A, Eng LA, Keen S, Randall PA. The permeability of rabbit oviduct to proteins present in the serum. Biol Reprod 1978; 18: 516–520. [DOI] [PubMed] [Google Scholar]
- 51.Leese HJ, Tay JI, Reischl J, Downing SJ. Formation of Fallopian tubal fluid: role of a neglected epithelium. Reproduction 2001; 121: 339–346. [DOI] [PubMed] [Google Scholar]
- 52.Iritani A, Sato E, Nishikawa Y. Secretion rates and chemical composition of oviduct and uterine fluids in sows. J Anim Sci 1974; 39: 582–588. [DOI] [PubMed] [Google Scholar]
- 53.Zhu J, Barratt CL, Lippes J, Pacey AA, Lenton EA, Cooke ID. Human oviductal fluid prolongs sperm survival. Fertil Steril 1994; 61: 360–366. [PubMed] [Google Scholar]
- 54.Grippo AA, Way AL, Killian GJ. Effect of bovine ampullary and isthmic oviductal fluid on motility, acrosome reaction and fertility of bull spermatozoa. J Reprod Fertil 1995; 105: 57–64. [DOI] [PubMed] [Google Scholar]
- 55.Parrish JJ, Susko-Parrish JL, Handrow RR, Sims MM, First NL. Capacitation of bovine spermatozoa by oviduct fluid. Biol Reprod 1989; 40: 1020–1025. [DOI] [PubMed] [Google Scholar]
- 56.Tienthai P, Kimura N, Heldin P, Sato E, Rodriguez-Martinez H. Expression of hyaluronan synthase-3 in porcine oviducal epithelium during oestrus. Reprod Fertil Dev 2003a; 15: 99–105. [DOI] [PubMed] [Google Scholar]
- 57.Scott JE, Heatley F. Hyaluronan forms specific stable tertiary structures in aqueous solution: a 13C NMR study. Proc Natl Acad Sci USA 1999; 96: 4850–4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Akmal M, Singh A, Anand A, Kesani A, Aslam N, Goodship A, Bentley G. The effects of hyaluronic acid on articular chondrocytes. J Bone Joint Surg Br 2005; 87: 1143–1149. [DOI] [PubMed] [Google Scholar]
- 59.Laurent TC, Fraser JR. Hyaluronan. FASEB J 1992; 6: 2397–2404. [PubMed] [Google Scholar]
- 60.Taylor KR, Trowbridge JM, Rudisill JA, Termeer CC, Simon JC, Gallo RL. Hyaluronan fragments stimulate endothelial recognition of injury through TLR4. J Biol Chem 2004; 279: 17079–17084. [DOI] [PubMed] [Google Scholar]
- 61.Tienthai P, Yokoo M, Kimura N, Heldin P, Sato E, Rodriguez-Martinez H. Immunohistochemical localization and expression of the hyaluronan receptor CD44 in the epithelium of the pig oviduct during oestrus. Reproduction 2003; 125: 119–132. [PubMed] [Google Scholar]
- 62.Aruffo A. CD44: one ligand, two functions. J Clin Invest 1996; 98: 2191–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kaneko T, Saito H, Toya M, Satio T, Nakahara K, Hiroi M. Hyaluronic acid inhibits apoptosis in granulosa cells via CD44. J Assist Reprod Genet 2000; 17: 162–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ohta N, Saito H, Kuzumaki T, Takahashi T, Ito MM, Saito T, Nakahara K, Hiroi M. Expression of CD44 in human cumulus and mural granulosa cells of individual patients in in-vitro fertilization programmes. Mol Hum Reprod 1999; 5: 22–28. [DOI] [PubMed] [Google Scholar]
- 65.Bergqvist AS, Yokoo M, Heldin P, Frendin J, Sato E, Rodríguez-Martínez H. Hyaluronan and its binding proteins in the epithelium and intraluminal fluid of the bovine oviduct. Zygote 2005; 13: 207–218. [DOI] [PubMed] [Google Scholar]
- 66.Yanagimachi R. Sperm capacitation and gamete interaction. J Reprod Fertil Suppl 1989; 38: 27–33. [PubMed] [Google Scholar]
- 67.de Lamirande E, Leclerc P, Gagnon C. Capacitation as a regulatory event that primes spermatozoa for the acrosome reaction and fertilization. Mol Hum Reprod 1997; 3: 175–194. [DOI] [PubMed] [Google Scholar]
- 68.Wang WH, Abeydeera LR, Fraser LR, Niwa K. Functional analysis using chlortetracycline fluorescence and in vitro fertilization of frozen-thawed ejaculated boar spermatozoa incubated in a protein-free chemically defined medium. J Reprod Fertil 1995; 104: 305–313. [DOI] [PubMed] [Google Scholar]
- 69.Abeydeera LR, Funahashi H, Kim NH, Day BN. Chlortetracycline fluorescence patterns and in vitro fertilisation of frozen-thawed boar spermatozoa incubated under various bicarbonate concentrations. Zygote 1997; 5: 117–125. [DOI] [PubMed] [Google Scholar]
- 70.Ellington JE, Ignotz GG, Varner DD, Marcucio RS, Mathison P, Ball BA. In vitro interaction between oviduct epithelial and equine sperm. Arch Androl 1993; 31: 79–86. [DOI] [PubMed] [Google Scholar]
- 71.Lefebvre R, Suarez SS. Effect of capacitation on bull sperm binding to homologous oviductal epithelium. Biol Reprod 1996; 54: 575–582. [DOI] [PubMed] [Google Scholar]
- 72.Fazeli A, Duncan AE, Watson PF, Holt WV. Sperm-oviduct interaction: induction of capacitation and preferential binding of uncapacitated spermatozoa to oviductal epithelial cells in porcine species. Biol Reprod 1999; 60: 879–886. [DOI] [PubMed] [Google Scholar]
- 73.Suarez SS. Hyperactivated motility in sperm. J Androl 1996; 17: 331–335. [PubMed] [Google Scholar]
- 74.Ho HC, Suarez SS. Hyperactivation of mammalian spermatozoa: function and regulation. Reproduction 2001; 122: 519–526. [DOI] [PubMed] [Google Scholar]
- 75.Hunter RH. Ovarian endocrine control of sperm progression in the Fallopian tubes. Oxf Rev Reprod Biol 1995; 17: 85–124. [Google Scholar]
- 76.Tienthai P, Johannisson A, Rodriguez-Martinez H. Sperm capacitation in the porcine oviduct. Anim Reprod Sci 2004; 80: 131–146. [DOI] [PubMed] [Google Scholar]
- 77.Mburu JN, Einarsson S, Lundeheim N, Rodriguez-Martinez H. Distribution, number and membrane integrity of spermatozoa in the pig oviduct in relation to spontaneous ovulation. Anim Reprod Sci 1996; 45: 109–121. [DOI] [PubMed] [Google Scholar]