Abstract
Cathepsin B, a lysosomal cysteine protease of the papain family, has recently been implicated in the quality and developmental competence of bovine preimplantation embryos. In this study, to determine whether inhibition of cathepsin B activity can improve porcine oocyte maturation and early embryo developmental competence, we supplemented in vitro maturation or embryo culture media with E-64, a cathepsin B inhibitor. Cathepsin B activity was high in poor quality germinal vesicle stage oocytes, but no differences in mRNA expression or protein localization were observed between good and poor quality oocytes, which were categorized based on morphology. Following treatment with 1 μM E-64, cathepsin B activity sharply decreased in 4-cell and blastocyst stage embryos. E-64 had no effect on cell number but significantly (P < 0.05) increased blastocyst formation and decreased the number of apoptotic cells in blastocysts. It also significantly (P < 0.05) enhanced mitochondrial membrane potential in blastocysts, reducing the release of cytochrome c and resulting in decreased expression of Caspase-3 and Caspase-9. In conclusion, inhibition of cathepsin B activity in porcine parthenotes using 1 μM E-64 resulted in attenuation of apoptosis via a reduction in the release of cytochrome c from mitochondria.
Keywords: Cathepsin B, Cytochrome c, Embryo development, Oocyte maturation
Animal reproductive technology has proved to be very important in the last three decades, and the development of efficient procedures for assisted reproduction in livestock has generated multiple opportunities for commercialization. Oocytes subjected to in vitro maturation (IVM) are used in the vast majority of laboratories producing embryos by somatic cell nuclear transfer, in vitro fertilization and parthenogenetic activation (PA); however, the efficiency of development is lower than that of oocytes matured in vivo [1, 2]. In assisted reproductive technology, the capacity of development is determined by the quality of the oocytes and blastocysts produced following a long period of in vitro maturation and development, with high quality oocytes and blastocysts showing the capacity for successful development [3]. Thus, it is necessary to improve IVM and culture systems to produce embryos of good quality and high developmental competence [4].
In the early stages of embryonic development, apoptosis is closely related to embryo quality. Apoptosis, or programmed cell death, is a widespread biological phenomenon and is typically characterized by membrane blebbing, chromatin condensation, and DNA fragmentation [5]. Apoptosis involves a number of membrane receptors and a signal transduction cascade, resulting in the activation of several cysteine proteases known as caspases [6, 7]. In mammalian cells, the release of caspase activators from mitochondria regulates apoptosis [8]. In vivo, apoptosis first appears in 32- to 64-cell embryos and is subsequently observed throughout embryogenesis [5]. Although apoptosis occurs in mammalian blastocysts and is in fact crucial for further development, alterations to this process in the blastocyst compromise development and may lead to early embryonic death or fetal anomalies [9]. Apoptosis has been shown to compromise the developmental competence of mammalian oocytes [10, 11] and has been related to embryonic arrest and fragmentation in early stage embryos [12].
In recent years, cathepsins, and in particular cathepsin B, have been found to influence bovine oocyte developmental competence by regulating apoptosis [10, 13, 14]. Cathepsin B is a lysosomal cysteine protease of the papain enzyme family involved in inducing apoptosis, degrading the extracellular matrix, catabolizing intracellular proteins; and processing prohormones into active forms [15]. Cathepsin B can activate caspases indirectly through induction of mitochondrial membrane degradation, leading to translocation of apoptosis-inducing components from the mitochondria to the cytosol [16].
The function of cathepsin B has been well established in bovine cumulus-oocyte complexes (COCs), and an increase in its activity has been observed in poor quality and heat-shocked oocytes [10, 13]. Artificially inhibiting cathepsin B with the specific inhibitor E-64 greatly improves the developmental competence of preimplantation embryos and increases the number of good quality embryos by attenuating apoptosis [14].
Until now, success rates for in vitro development of porcine embryos have been very low, partly due to poor culture conditions and apoptosis during embryonic development [17, 18]. Although the role of cathepsin B has been elucidated in bovine oocytes, very little information exists regarding its function in porcine oocytes and early stage embryos. In the present study, we investigated the activity of cathepsin B in both porcine GV stage oocytes and PA embryos; and evaluated the consequences of its inhibition using E-64. Furthermore, mitochondrial membrane potential, apoptosis in blastocysts; and cytochrome c release were analyzed.
Materials and Methods
Unless otherwise indicated, all chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Oocyte collection and sorting
Prepubertal porcine ovaries were obtained from a local slaughterhouse. Oocytes of good and poor quality were separated based on a previously published method [19]. In brief, COCs with more than three layers of cumulus cells were collected and defined as the good quality group, while denuded oocytes or COCs with dark cumulus cells were separated and considered the poor quality group. For evaluation of cathepsin B activity, all COCs were denuded by repeated pipetting in 0.1% hyaluronidase. The denuded oocytes were then washed three times in IVM medium prior to use in a cathepsin B activity assay.
IVM, PA; and culture of embryos
After collection, oocytes were cultured for 44 h in IVM media; consisting of tissue culture medium 199 (Medium 199, Gibco, Grand Island, NY, USA) supplemented with 0.57 mM cysteine, 10 ng/ml epidermal growth factor, 0.5 IU/ml luteinizing hormone; and 0.5 IU/ml follicle stimulating hormone. To evaluate the influence of E-64 on their maturation, porcine oocytes were cultured in IVM medium in the presence of 0, 1, 10; or 100 μM E-64. After maturation, COCs were isolated and cumulus cells were removed by repeated pipetting in the presence of 0.1% hyaluronidase for 2 to 3 min. Oocytes that extruded the first polar body were sorted into matured oocytes. To establish the effect of E-64 on embryo development, oocytes matured in IVM medium in the absence of E-64 were parthenogenetically activated with calcium ionophore A23187 (50 µM) for 5 min, followed by incubation for 3 h in in vitro culture (IVC) medium (PZM-5 medium [20] supplemented with 0.4% (w/v) bovine serum albumin, BSA) albumin, BSA) containing 7.5 µg/ml cytochalasin B. Finally, embryos were cultured in IVC medium and 0, 1, 10, or 100 μM E-64, under light mineral oil for 7 days at 38.5 C in 5% (v/v) CO2. Blastocysts used for additional experiments were obtained from the 1 µM E-64 treatment group.
Reverse transcription quantitative polymerase chain reaction (RT-qPCR)
Extraction of mRNA and cDNA synthesis were performed as previously described [21]. Briefly, for each independent experiment, mRNA was extracted from 10 oocytes or 10 embryos of each stage with a Dynabeads mRNA DIRECT Kit (Dynal Biotech ASA, Oslo, Norway), and cDNA was synthesized by reverse transcription of RNA using oligo (dT)12–18 primers and SuperScript Reverse Transcriptase (Invitrogen, Grand Island, NY, USA), according to the manufacturer’s instructions.
RT-qPCR was performed in a Bio-Rad qPCR system (CFX ConnectTM, Bio-Rad, Singapore, Singapore). The cycling conditions were as follows: 95 C for 10 min; 40 cycles of 95 C for 30 sec, 60 C for 30 sec, and 72 C for 25 sec; and then a final extension at 72 C for 5 min. For each PCR reaction, the amount of cDNA corresponded to that of 0.4 oocytes or embryos. Relative mRNA expression was analyzed using the 2–ΔΔCT method [22]. Gapdh mRNA was used as an internal control. Three independent experiments were performed, with each containing samples in triplicate. The primers used to amplify each gene are given in Table 1.
Table 1. Primer sequences and product sizes used for RT-qPCR.
| Gene | GeneBank accession number | Primer sequence (5'–3') | Length (bp) |
| Cathepsin B | NM_001097458 | F:GGCCTCTATGACTCGCATGT | 150 |
| R:TTTGTAGGACGGGGTGTAGC | |||
| Caspase 3 | NM_214131 | F:GGATTGAGACGGACAGTGGG | 124 |
| R:CCGTCCTTTGAATTTCGCCA | |||
| Caspase 9 | XM_003127618 | F:GTCTGCCCACACCTAGTGAC | 264 |
| R:AGGGGTCCCAGCCTCATTAT | |||
| Gapdh | NM_001206359 | F:CGTGTCGGTTGTGGATCTGA | 209 |
| R:TGACGAAGTGGTCGTTGAGG |
Immunofluorescence and confocal laser scanning microscopy
To determine the localization of cathepsin B, oocytes or embryos were washed twice in Dulbecco’s phosphate buffered saline containing 0.1% polyvinyl alcohol (PVA-PBS). Then the cells were fixed in 3.7% paraformaldehyde for 30 min at room temperature. Next, oocytes and embryos were permeabilized using PVA-PBS containing 1% Triton X-100 at 37 C for at least 1 h. Following this, oocytes and embryos were incubated with a blocking solution of PVA-PBS containing 3.0% BSA at 37 C for at least 1.5 h and subsequently incubated overnight at 4 C with an anti-cathepsin B antibody (diluted 1:100; ab58802, Abcam, Cambridge, MA, USA). To investigate the colocalization of mitochondria and cytochrome c, blastocysts were incubated with 5 μM MitoTracker Red at 37 C for 45 min, and after three washes with IVC medium, they were fixed in 3.7% paraformaldehyde for 30 min at room temperature. Next, the blastocysts were permeabilized as described above and incubated with blocking solution at 37 C for 1 h. The samples were then incubated with an anti-cytochrome c antibody (diluted 1:100; ab110325, Abcam) at 37 C for 2 h. After incubation with primary antibodies, all samples were exposed to a fluorescein isothiocyanate-labeled secondary antibody for 1 h at 37 C. Thereafter, they were washed twice with PVA-PBS, treated with 5 μg/ml Hoechst 33342 for 10 min, washed twice more, and then mounted on glass slides. Microscopy was performed using a Zeiss LSM 710 inverted confocal laser scanning microscope with a 63× oil immersion objective (Zeiss MicroImaging, Jena, Germany). Images were processed using the ZEN software (Zeiss). The colocalization of mitochondria and cytochrome c was analyzed using Image-Pro Plus (version 6.0; Media Cybernetics, Rockville, MD, USA).
TdT-mediated dUTP nick-end labeling (TUNEL) assay
Approximately ten blastocysts were washed three times in PVA-PBS, and fixed in 3.7% (w/v) paraformaldehyde in PVA-PBS for 1 h at room temperature. After fixation, the embryos were washed with PVA-PBS and permeabilized with 0.5% (v/v) Triton X-100 for 1 h at room temperature. The embryos were then washed twice with PVA-PBS and incubated with fluorescein-conjugated dUTP and terminal deoxynucleotidyl transferase (In Situ Cell Death Detection Kit, Roche, Mannheim, Germany) in the dark for 1 h at 37 C. The embryos were then washed in PVA-PBS, mounted beneath a coverslip, and examined under a fluorescence microscope (Eclipse Ti, Nikon, Tokyo, Japan).
Detection of intracellular cathepsin B activity
Detection of cathepsin B activity in oocytes and embryos was carried out using a Magic Red Cathepsin B Assay Kit (ImmunoChemistry Technologies, Bloomington, MN, USA) according to a previously published method [14]. In brief, germinal vesicle (GV) stage oocytes of good or poor quality, and 4-cell and blastocyst stage embryos were incubated in 500 ml PVA-PBS containing 2 ml of reaction mix in a humidified atmosphere of 5% CO2 at 38.5 C for 20 min. After rinsing in PBS with 3 mg/ml polyvinylpyrrolidone, the freshly stained embryos were mounted onto a glass slide and observed under a fluorescence microscope (Nikon). Excitation filters of 365 nm and 550 nm were used for observing the nuclei and detecting intracellular cathepsin B activity, respectively. All images of cathepsin B activity were captured using the same exposure time and analyzed using Image-Pro Plus. In brief, embryos were selected, and the red fluorescence of cathepsin B was distinguished from the red cytoplasmic background by altering the threshold values (30–255). The average total fluorescence emitted (pixels) was used to compare good and poor quality groups.
Mitochondrial membrane potential assay
To measure mitochondrial membrane potential (Δψm), blastocysts were washed three times with PVA-PBS and incubated in a culture medium containing 0.5 μM 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-imidacarbocyanine iodide (JC-1) (Invitrogen, Grand Island, NY, USA) at 37 C in 5% CO2 for 30 min. Membrane potential was calculated as the ratio of red florescence, which corresponded to activated mitochondria (J-aggregates), to green fluorescence, which corresponded to less-activated mitochondria (J-monomers)[23]. Fluorescence was visualized with a Zeiss inverted confocal microscope equipped with a 40× oil immersion objective. Images were processed with the ZEN software. The fluorescence intensity in the control group was arbitrarily set to 1, and the relative fluorescence intensity in the treatment groups was then measured. Three separate experiments were performed with 10–15 blastocysts in each.
Statistical analysis
Data are representative of at least three independent experiments. The general linear models procedure in the SAS program (SAS Institute, Cary, NC, USA) was used for data analysis. Significant differences were determined using Tukey’s multiple range test, and P-values less than 0.05 were considered significant.
Results
Detection of intracellular cathepsin B in oocytes classified by quality
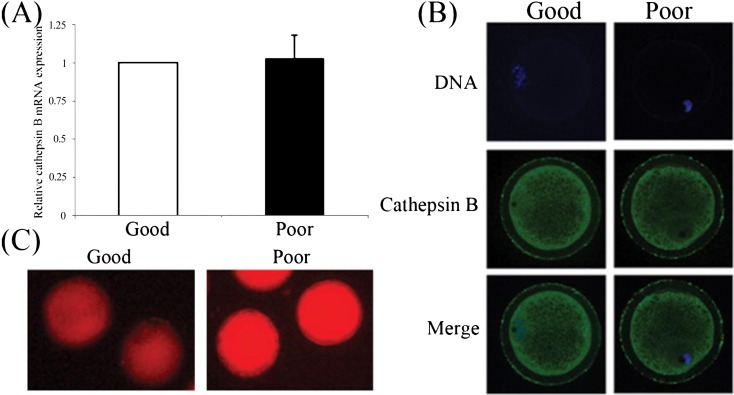
To determine the relationship between cathepsin B and COC quality, the mRNA expression, localization; and activity of cathepsin B were measured in denuded oocytes classified as being of good and poor quality. There were no obvious differences in mRNA level (Fig. 1A) and protein distribution (Fig. 1B) in GV stage oocytes. Cathepsin B was clearly observed to be localized in the cytoplasm, and its activity was detected in the cytosol of both good and poor quality oocytes. The activity assay revealed that the red fluorescence of hydrolyzed cresyl violet (Arginine-Arginine)2 appeared stronger (P < 0.05) in poor quality oocytes than in those of good quality (Fig. 1C).
Fig. 1.
Cathepsin B in porcine GV stage oocytes of good and poor quality. (A) Cathepsin B expression was measured by RT-qPCR. (B) Cathepsin B was localized in the cytoplasm in both good and poor quality oocytes. (C) High cathepsin B activity was observed in GV stage oocytes of poor quality. Values represent the means ± SEMs of three independent experiments.
Expression and localization of cathepsin B in porcine PA embryos
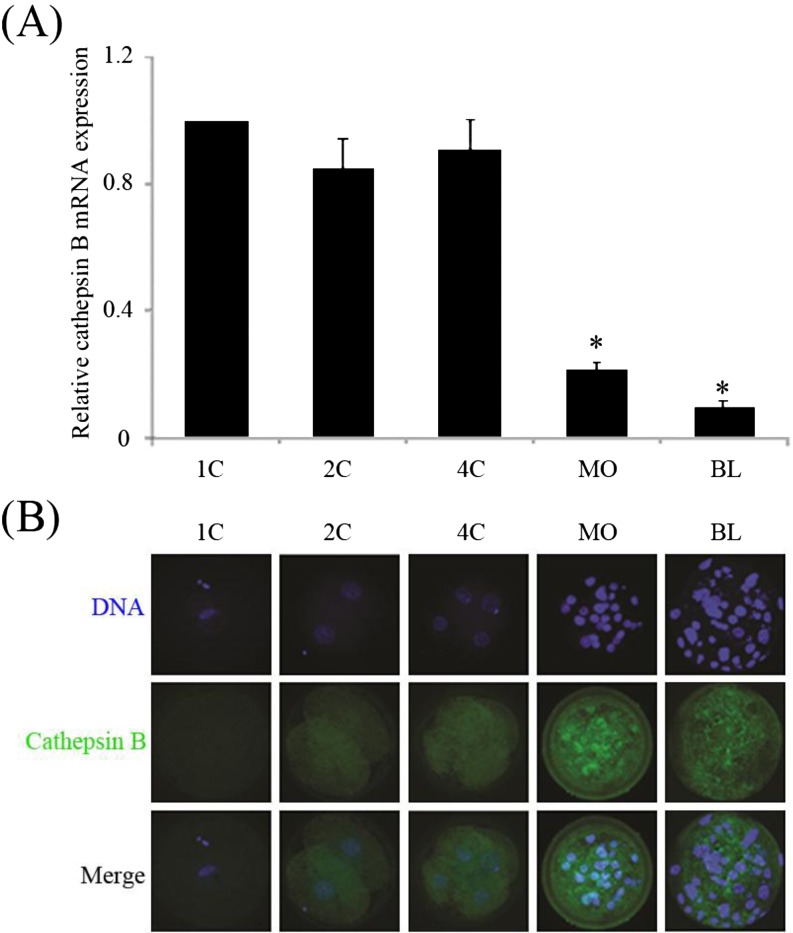
To investigate the expression and localization of cathepsin B, porcine embryos of various stages, from the 1-cell to blastocyst stage, were used for RT-qPCR and immunostaining. As shown in Fig. 2A, compared with the 1-cell stage, similar cathepsin B mRNA levels (P > 0.05) were observed in 2-; and 4-cell stage embryos. However, expression was dramatically reduced (P < 0.05) in the morula and blastocyst stages compared with the 1-cell stage. During earlier embryonic stages, cathepsin B protein displayed a uniform distribution in the cytoplasm, but was detected as strong dots in the cytoplasms of morulae and blastocysts (Fig. 2B).
Fig. 2.
Expression and localization of cathepsin B at various stages of development were measured by RT-qPCR (A) and immunofluorescence (B), respectively. *P < 0.05 compared with 1-cell stage. Values represent the means ± SEMs of three or four independent experiments.
Effect of E-64 on oocyte maturation
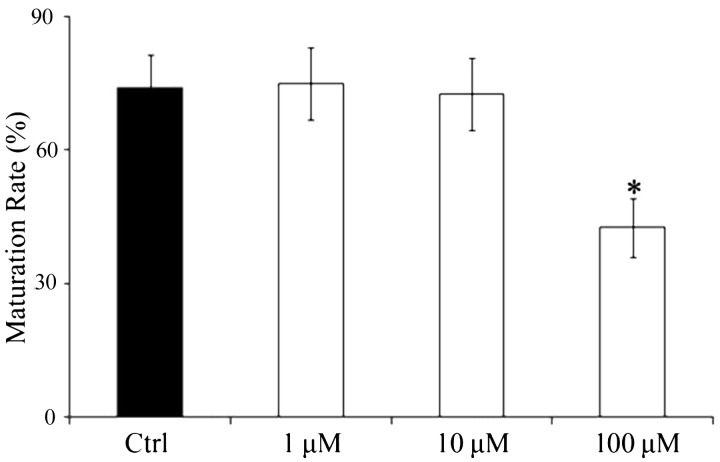
To assess the inhibitory effect of E-64 on oocyte maturation, COCs were matured with 0, 1, 10, or 100 μM E-64. There were no significant differences in maturation rate using concentrations of 0, 1, or 10 μM, but 100 μM of E-64 significantly reduced this rate (42.51 ± 6.19%) compared with the control group (74.71 ± 6.60%, P < 0.05, Fig. 3).
Fig. 3.
Effect of E-64 on IVM of porcine oocytes. Oocytes were cultured in medium in the presence of 0, 1, 10; or 100 μM E-64. Black bar, control group; white bar, E-64 treatment. *P < 0.05, significant difference from control group. Values represent the means ± SEMs of three or four independent experiments.
Effect of E-64 on embryo development and apoptosis
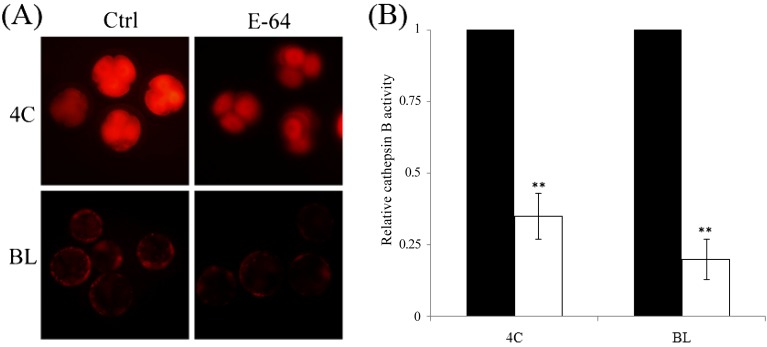
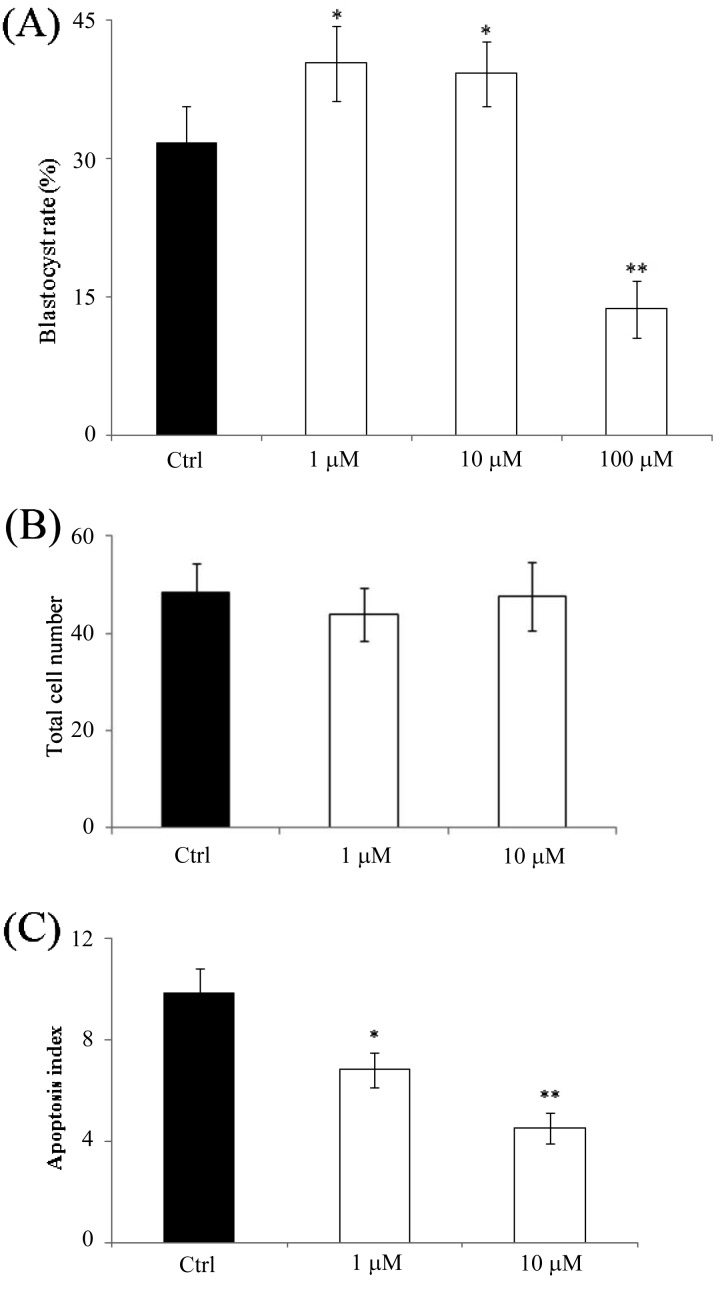
To investigate whether the inhibition of cathepsin B can increase blastocyst formation and reduce apoptosis, activated oocytes were cultured in medium containing 0, 1, 10, or 100 μM E-64. After treatment, the activity of cathepsin B was significantly reduced in both 4-cell and blastocyst stage embryos (Figs. 4A and 4B). Embryos treated with 1 μM (40.3 ± 4.06%) or 10 μM E-64 (39.2 ± 3.33%) displayed increased blastocyst formation compared with the control group (31.6 ± 3.91%). However, formation of blastocysts at a concentration of 100 μM (3.7 ± 3.04%) was lower than that of the control group (P < 0.01, Fig. 5A). Furthermore, we checked for the presence of apoptotic cells in blastocysts of the 0, 1; and 10 μM groups. Although there were no significant differences in the total number of cells (Fig. 5B), the number of apoptotic cells was notably lower in the 1 μM (6.8 ± 0.67, P < 0.05) and 10 μM groups (4.5 ± 0.58, P < 0.01) compared with the control group (9.8 ± 0.99, Fig. 5C).
Fig. 4.
Effect of E-64 treatment on cathepsin B activity of porcine parthenotes. (A) E-64 treatment reduced the activity of cathepsin B in both the 4-cell and blastocyst stages. (B) Fluorescence intensities calculated using Image-Pro Plus. Black bar, control group; white bar, E-64 treatment group. *P < 0.05 and **P < 0.01. Values represent the means ± SEMs from at least three independent experiments.
Fig. 5.
Effect of E-64 treatment on development and quality of porcine parthenotes. (A) Blastocyst formation of porcine parthenotes cultured in the presence of E-64. (B) Total cell number was determined using Hoechst 33342 staining. (C) The number of apoptotic cells was determined by TUNEL assay. Black bar, control group; white bar, E-64 treatment group. *P < 0.05 and **P < 0.01 compared with control group, respectively. Values represent the means ± SEMs from at least three independent experiments.
Effect of E-64 on the expression of apoptosis-related genes
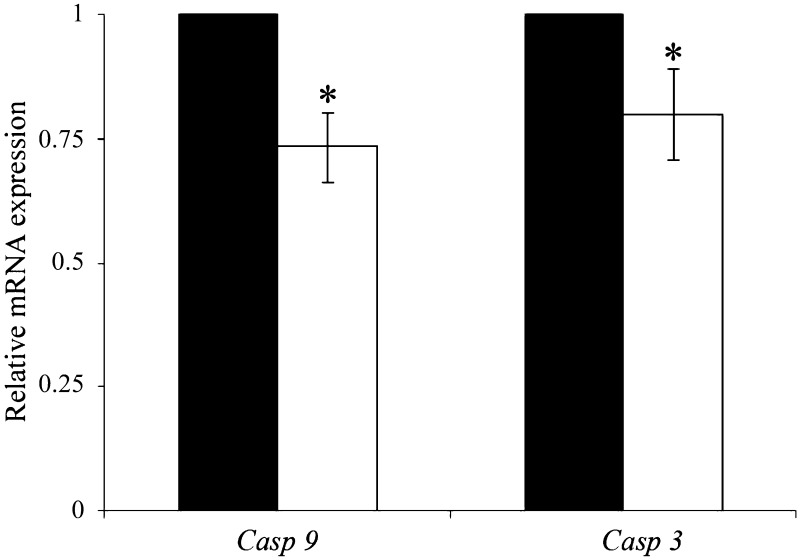
To explore the effect of cathepsin B inhibition on the expression of apoptotic genes, blastocysts cultured in the presence or absence of 1 μM E-64 were analyzed by RT-qPCR. The results demonstrated that cathepsin B inhibition markedly reduced the expression of caspase-9 and caspase-3 in blastocysts (P < 0.05, Fig. 6).
Fig. 6.
Expression of apoptosis-related genes in porcine blastocysts cultured for 7 days. The expression of mRNA was analyzed by SYBR Green RT-qPCR. Black bar, control group; white bar, E-64 treatment group. *P < 0.05. Values represent the means ± SEMs of three or four independent experiments.
Effect of E-64 on colocalization of mitochondria and cytochrome c
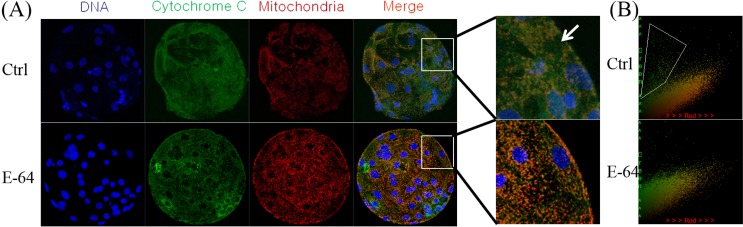
To determine the manner in which inhibition of cathepsin B reduces apoptosis, the colocalization of mitochondria and cytochrome c was analyzed, as shown in Fig. 7. In untreated blastocysts, cytochrome c was dispersed throughout the cytoplasm, while it displayed a strong blot-like distribution in the E-64 treatment group, and was distinctly colocalized with mitochondria. Pearson’s correlation showed that E-64 treatment resulted in obvious colocalization compared with the control group, and that cytochrome c was weakly present in the cytoplasm in the treated group.
Fig. 7.
Colocalization of cytochrome c and mitochondria in blastocysts cultured for 7 days in the absence or presence of E-64. (A) Blastocysts were labeled with a cytochrome c-specific antibody (green) and MitoTracker Red (red). (B) Colocalization was analyzed using Image-Pro Plus, Arrow and frame, released cytochrome c.
Effect of E-64 on mitochondrial membrane potential in blastocysts
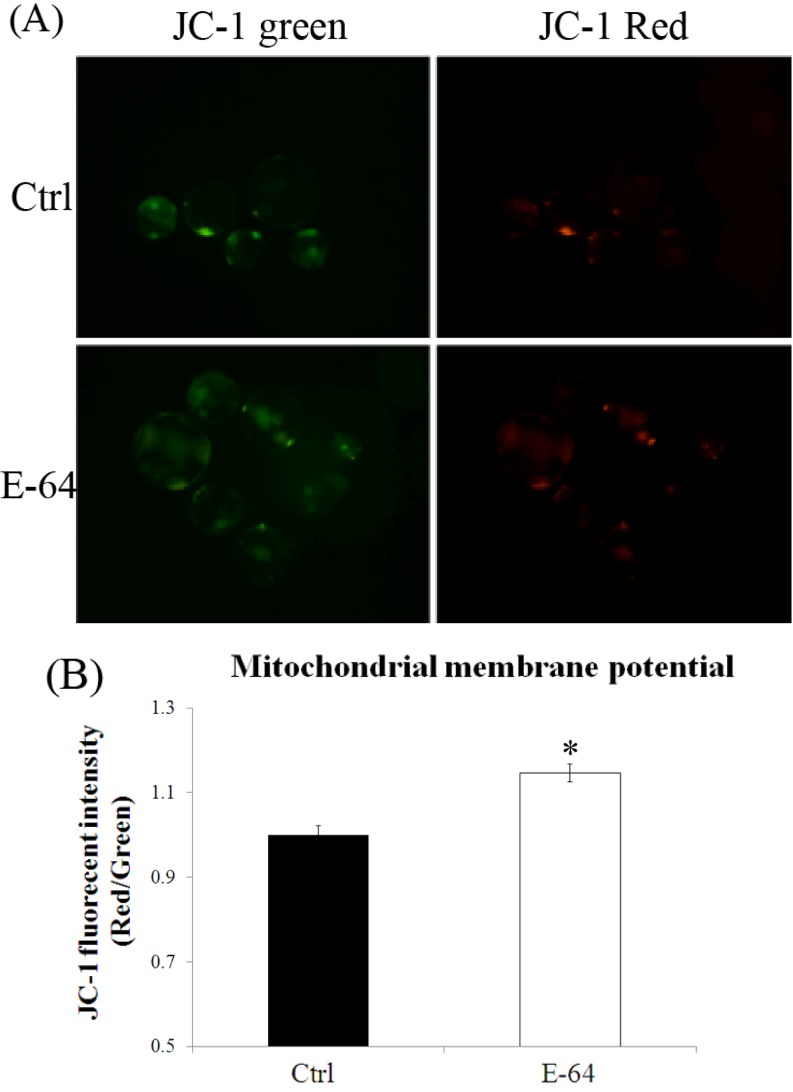
To explore the mechanism by which E-64 affects the development of porcine parthenotes, mitochondrial membrane potential in blastocysts was measured. The results are shown in Fig. 8. E-64 treatment resulted in significantly higher mitochondrial membrane potential (P < 0.05) compared with the control group.
Fig. 8.
Mitochondrial membrane potentials in blastocysts cultured in the absence or presence of E-64. (A) Membrane potential was calculated as the ratio of red fluorescence, which corresponds to activated mitochondria (J-aggregates), to green fluorescence, which corresponds to less-activated mitochondria (J-monomers). (B) Fluorescence emitted from each blastocyst was analyzed using the Image-Pro Plus software with the control arbitrarily set as 1. Black bar, control group; white bar, E-64 treatment group. *P < 0.05. Values represent the means ± SEMs from at least three separate experiments.
Discussion
In the present study, we investigated the expression, subcellular distribution, and activity of cathepsin B in porcine oocytes of poor and good quality. Inhibition of cathepsin B dramatically enhanced the development of PA embryos by protecting mitochondrial membranes and thus preventing cytochrome c release and apoptosis in blastocysts.
Follicular atresia is a key component of oogenesis and consisting of the removal of follicles not selected for ovulation. More than 99% of follicles undergo atresia in mammalian ovaries [24]; thus only very few oocytes of the highest quality are available for IVM following aspiration from the ovaries. Cathepsin-induced apoptosis is one mechanism by which follicular atresia is induced in mammalians [25, 26]. We therefore hypothesized that cathepsin B activity reduces the quality of both oocytes and early stage embryos in pigs.
To test this hypothesis, we first investigated the mRNA expression, protein distribution, and activity of cathepsin B in GV stage oocytes. Although no difference in expression and distribution were observed, higher activity was seen in oocytes of poor quality. These results agree with those of similar studies using bovine cells [13]. In addition, it is generally accepted that a low oocyte quality compromises development following activation [19]. These findings demonstrate that cathepsin B activity is negatively correlated with oocyte quality, and that such activity can be used as an indicator of oocyte quality.
We subsequently investigated the distribution of cathepsin B in embryos. A very weak signal was seen in embryos of 1 to 4 cells, while a clustered localization pattern was present in morulae and blastocysts. This result corresponds well with the observation that apoptosis first appears at the 32- to 64-cell stages [5] and strongly indicates that cathepsin B is closely related to this process in embryos.
To test whether cathepsin B induces apoptosis in blastocysts, E-64 was employed to inhibit its activity. When treated with E-64, 4-cell and blastocyst stage embryos showed reduced cathepsin B activity, and the blastocyst formation rate increased as a result of cathepsin B inhibition. Furthermore, the results of our TUNEL assay clearly indicate that inhibiting cathepsin B activity protects blastocysts from apoptosis.
The mechanism by which cathepsin B induces apoptosis in porcine embryos has, until now, been unclear. Cathepsin B is a lysosomal cysteine protease that plays an important role in the degradation of intracellular proteins in lysosomes [27]. It is a glycoprotein that, in its active form, consists of disulfide-linked heavy and light chain subunits and has a molecular weight of 30 kDa [28]. In several cell types, cathepsin B has been shown to lyse mitochondrial membranes, promoting cytochrome c release into the cytosol [29, 30]. As a result, a series of biochemical reactions are induced that ultimately result in caspase activation and subsequent cell death [31]. To establish whether this same apoptotic mechanism also occurs in porcine embryos, we tested mitochondrial membrane potential and the colocalization of mitochondria and cytochrome c. Our results clearly reveal that E-64 increased mitochondrial membrane potentials, while the absence of colocalization of mitochondria and cytochrome c in the control group indicates that cytochrome c was released into the cytosol. This should activate the cysteine protease caspase-9, which in turn should go on to activate caspase-3. Indeed, mRNA levels of caspase-3 and caspase-9 were lower in blastocysts treated with E-64, signifying that cathepsin B induces apoptosis in porcine embryos via a cytochrome c-related pathway.
In conclusion, we found that cathepsin B induces apoptosis during the early stages of embryo development by degrading mitochondrial membranes, causing the release of cytochrome c into the cytosol; and resulting in the activation of caspases-9 and caspase-3. In addition, we established that cathepsin B activity can be used as a marker to evaluate the quality of porcine oocytes.
Acknowledgments
This work was supported by grants from the Next-Generation BioGreen 21 Program (PJ011126), Rural Development Administration, Republic of Korea.
References
- 1.Rath D, Niemann H, Torres CR. In vitro development to blastocysts of early porcine embryos produced in vivo or in vitro. Theriogenology 1995; 43: 913–926. [DOI] [PubMed] [Google Scholar]
- 2.Macháty Z, Day BN, Prather RS. Development of early porcine embryos in vitro and in vivo. Biol Reprod 1998; 59: 451–455. [DOI] [PubMed] [Google Scholar]
- 3.Lindner GM, Wright RW., Jr Bovine embryo morphology and evaluation. Theriogenology 1983; 20: 407–416. [DOI] [PubMed] [Google Scholar]
- 4.Pawlak P, Renska N, Pers-Kamczyc E, Warzych E, Lechniak D. The quality of porcine oocytes is affected by sexual maturity of the donor gilt. Reprod Biol 2011; 11: 1–18. [DOI] [PubMed] [Google Scholar]
- 5.Brill A, Torchinsky A, Carp H, Toder V. The role of apoptosis in normal and abnormal embryonic development. J Assist Reprod Genet 1999; 16: 512–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thornberry NA. The caspase family of cysteine proteases. Br Med Bull 1997; 53: 478–490. [DOI] [PubMed] [Google Scholar]
- 7.Turk B, Stoka V. Protease signalling in cell death: caspases versus cysteine cathepsins. FEBS Lett 2007; 581: 2761–2767. [DOI] [PubMed] [Google Scholar]
- 8.Estaquier J, Vallette F, Vayssiere JL, Mignotte B. The mitochondrial pathways of apoptosis. Adv Exp Med Biol 2012; 942: 157–183. [DOI] [PubMed] [Google Scholar]
- 9.Brison DR, Schultz RM. Apoptosis during mouse blastocyst formation: evidence for a role for survival factors including transforming growth factor alpha. Biol Reprod 1997; 56: 1088–1096. [DOI] [PubMed] [Google Scholar]
- 10.Balboula AZ, Yamanaka K, Sakatani M, Kawahara M, Hegab AO, Zaabel SM, Takahashi M. Cathepsin B activity has a crucial role in the developmental competence of bovine cumulus-oocyte complexes exposed to heat shock during in vitro maturation. Reproduction 2013; 146: 407–417. [DOI] [PubMed] [Google Scholar]
- 11.Cui XS, Jeong YJ, Lee HY, Cheon SH, Kim NH. Fetal bovine serum influences apoptosis and apoptosis-related gene expression in porcine parthenotes developing in vitro. Reproduction 2004; 127: 125–130. [DOI] [PubMed] [Google Scholar]
- 12.Yu M, Qiu ZL, Li H, Zeng WS, Chen LN, Li QH, Quan S. [Association between cell apoptosis and the quality of early mouse embryos]. Nan Fang Yi Ke Da Xue Xue Bao 2011; 31: 409–413 (in Chinese). [PubMed] [Google Scholar]
- 13.Balboula AZ, Yamanaka K, Sakatani M, Hegab AO, Zaabel SM, Takahashi M. Cathepsin B activity is related to the quality of bovine cumulus oocyte complexes and its inhibition can improve their developmental competence. Mol Reprod Dev 2010; 77: 439–448. [DOI] [PubMed] [Google Scholar]
- 14.Balboula AZ, Yamanaka K, Sakatani M, Hegab AO, Zaabel SM, Takahashi M. Intracellular cathepsin B activity is inversely correlated with the quality and developmental competence of bovine preimplantation embryos. Mol Reprod Dev 2010; 77: 1031–1039. [DOI] [PubMed] [Google Scholar]
- 15.Stoka V, Turk B, Turk V. Lysosomal cysteine proteases: structural features and their role in apoptosis. IUBMB Life 2005; 57: 347–353. [DOI] [PubMed] [Google Scholar]
- 16.Bossy-Wetzel E, Newmeyer DD, Green DR. Mitochondrial cytochrome c release in apoptosis occurs upstream of DEVD-specific caspase activation and independently of mitochondrial transmembrane depolarization. EMBO J 1998; 17: 37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mateusen B, Van Soom A, Maes DG, Donnay I, Duchateau L, Lequarre AS. Porcine embryo development and fragmentation and their relation to apoptotic markers: a cinematographic and confocal laser scanning microscopic study. Reproduction 2005; 129: 443–452. [DOI] [PubMed] [Google Scholar]
- 18.Peters JK, Milliken G, Davis DL. Development of porcine embryos in vitro: effects of culture medium and donor age. J Anim Sci 2001; 79: 1578–1583. [DOI] [PubMed] [Google Scholar]
- 19.Alvarez GM, Dalvit GC, Achi MV, Miguez MS, Cetica PD. Immature oocyte quality and maturational competence of porcine cumulus-oocyte complexes subpopulations. Biocell 2009; 33: 167–177. [PubMed] [Google Scholar]
- 20.Suzuki C, Iwamura S, Yoshioka K. Birth of piglets through the non-surgical transfer of blastocysts produced in vitro. J Reprod Dev 2004; 50: 487–491. [DOI] [PubMed] [Google Scholar]
- 21.Cui XS, Li XY, Jeong YJ, Jun JH, Kim NH. Gene expression of cox5a, 5b, or 6b1 and their roles in preimplantation mouse embryos. Biol Reprod 2006; 74: 601–610. [DOI] [PubMed] [Google Scholar]
- 22.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 23.Ge H, Tollner TL, Hu Z, Dai M, Li X, Guan H, Shan D, Zhang X, Lv J, Huang C, Dong Q. The importance of mitochondrial metabolic activity and mitochondrial DNA replication during oocyte maturation in vitro on oocyte quality and subsequent embryo developmental competence. Mol Reprod Dev 2012; 79: 392–401. [DOI] [PubMed] [Google Scholar]
- 24.Hirshfield AN. Development of follicles in the mammalian ovary. Int Rev Cytol 1991; 124: 43–101. [DOI] [PubMed] [Google Scholar]
- 25.Sriraman V, Richards JS. Cathepsin L gene expression and promoter activation in rodent granulosa cells. Endocrinology 2004; 145: 582–591. [DOI] [PubMed] [Google Scholar]
- 26.Dhanasekaran N, Sheela Rani CS, Moudgal NR. Studies on follicular atresia: lysosomal enzyme activity and gonadotropin receptors of granulosa cells following administration or withdrawal of gonadotropins in the rat. Mol Cell Endocrinol 1983; 33: 97–112. [DOI] [PubMed] [Google Scholar]
- 27.Barrett AJ, Kirschke H. Cathepsin B, Cathepsin H, and cathepsin L. Methods Enzymol 1981; 80: 535–561. [DOI] [PubMed] [Google Scholar]
- 28.Klose A, Zigrino P, Dennhöfer R, Mauch C, Hunzelmann N. Identification and discrimination of extracellularly active cathepsins B and L in high-invasive melanoma cells. Anal Biochem 2006; 353: 57–62. [DOI] [PubMed] [Google Scholar]
- 29.Joy B, Sivadasan R, Abraham T E, John M, Sobhan PK, Seervi M, T R S. Lysosomal destabilization and cathepsin B contributes for cytochrome c release and caspase activation in embelin-induced apoptosis. Mol Carcinog 2010; 49: 324–336. [DOI] [PubMed] [Google Scholar]
- 30.Guicciardi ME, Deussing J, Miyoshi H, Bronk SF, Svingen PA, Peters C, Kaufmann SH, Gores GJ. Cathepsin B contributes to TNF-alpha-mediated hepatocyte apoptosis by promoting mitochondrial release of cytochrome c. J Clin Invest 2000; 106: 1127–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang X, Wang X. Cytochrome C-mediated apoptosis. Annu Rev Biochem 2004; 73: 87–106. [DOI] [PubMed] [Google Scholar]