Abstract
We recently demonstrated that luteal cells flow out from the ovary via lymphatic vessels during luteolysis. However, the regulatory mechanisms of the outflow of luteal cells are not known. Matrix metalloproteinases (MMPs) can degrade the extracellular matrix and basal membrane, and tissue inhibitors of matrix metalloproteinases (TIMPs) inhibit the activity of MMPs. To test the hypothesis that MMP expression in luteal cells is regulated by luteolytic factors, we investigated the effects of prostaglandin F2α (PGF), interferon γ (IFNG) and tumor necrosis factor α (TNF) on the mRNA expression of MMPs and TIMPs in cultured luteal cells. Luteal cells obtained from the CL at the mid-luteal stage (days 8–12 after ovulation) were cultured with PGF (0.01, 0.1, 1 μM), IFNG (0.05, 0.5, 5 nM) and TNF (0.05, 0.5, 0.5 nM) alone or in combination for 24 h. PGF and IFNG significantly increased the expression of MMP-1 mRNA. In addition, 1 μM PGF in combination with 5 nM IFNG stimulated MMP-1 and MMP-9 mRNA expression significantly more than either treatment alone. In contrast, IFNG significantly decreased the level of MMP-14 mRNA. The mRNA expression of TIMP-1, which preferentially inhibits MMP-1, was suppressed by 5 nM INFG. One μM PGF and 5 nM IFNG suppressed TIMP-2 mRNA expression. These results suggest a new role of MMPs: luteal MMPs stimulated by PGF and IFNG break down the extracellular matrix surrounding luteal cells, which accelerates detachment from the CL during luteolysis, providing an essential prerequisite for outflow of luteal cells from the CL to lymphatic vessels.
Keywords: Luteal cells, Lymphatic vessel, Matrix metalloproteinases, Structural luteolysis
In mammals, the corpus luteum (CL) is an essential endocrine gland for the establishment and maintenance of pregnancy. If pregnancy is not established, the CL loses its capacity to produce progesterone (functional luteolysis), decreases in volume and disappears from the ovary (structural luteolysis) [1]. During structural luteolysis, luteal cells are eliminated by apoptosis and phagocytosis by macrophages [2,3,4]. We recently found that during structural luteolysis luteal cells flow out of the CL via lymphatic vessels [5], although the mechanisms regulating the outflow are unclear.
Matrix metalloproteinases (MMPs) are a family of structurally-related proteins that degrade the extracellular matrix (ECM) and basal membrane [6]. The ECM, besides providing structural support between cells, has been implicated in modulating cell processes, such as differentiation, migration, gene expression and apoptosis [6, 7]. In cancer metastasis, MMPs are required for cancer cells to degrade the ECM and intravasation at nearby blood vessels or lymphatic vessels [8,9,10]. In luteal cells, ECM degradation by MMPs induces changes in the mRNA expression of luteinizing hormone receptor, low density lipoprotein receptor and 3β-hydroxy steroid dehydrogenase, which are factors required to produce steroid hormones in luteal cells [11]. Hence, although these changes mediated by MMPs have been suggested to be involved in the loss of capacity of luteal tissue to synthesize progesterone during luteolysis, the roles of MMPs in structural luteolysis are unclear.
Luteal cells that flow out to the lymphatic vessels during structural luteolysis must be detached from the CL tissue. In the bovine CL tissue, MMP-1, 2, 9 and 14 increase drastically during luteolysis induced by prostaglandin F2α (PGF) injection [12]. The authors implied that the main source of MMP-1 and MMP-9 in the CL tissue is immune cells invading the CL during luteolysis [12]. However, since luteal cells have been immunohistochemically shown to be the source of MMPs in the CL during luteolysis [12], MMPs secreted by luteal cells may break down the ECM surrounding luteal cells in the same way that MMPs secreted by cancer cells break down the ECM in metastasis. The aim of the present study was to validate possibility of the above idea. Since outflow of luteal cells occurs during luteolysis [5], we hypothesized that luteolytic factors increase MMP expression in luteal cells. To test this hypothesis, we investigated the effects of three factors that are known to induce luteolysis PGF, interferon γ (IFNG) and tumor necrosis factor α (TNF) [4]) on the mRNA expressions of four MMPs involved in degrading collagen and the basal membrane in cultured luteal cells. Since tissue inhibitors of matrix metalloproteinases (TIMPs) are known to inhibit MMP activity [7], in the present study, we also investigated the effects of PGF, IFNG and TNF on mRNA expression of TIMPs in cultured luteal cells.
Materials and Methods
Collection of bovine corpora lutea
In this study, we did not perform any animal experiments. The ovaries were collected from nonpregnant Holstein cows at a local abattoir (Tsuyama Meat Center) in accordance with protocols approved by the local institutional animal care committee. All the samples and data analyzed in the present study were obtained with the permission of the above center.
Ovaries with CLs at the mid-luteal stage were collected at a local abattoir within 10–20 min after exsanguination. The luteal stage was classified by macroscopic observation of the ovary and uterus [13, 14]. For cell culture experiments, ovaries with a mid-luteal CL (days 8–12 of the estrous cycle) were submerged in ice-cold physiological saline and transported to the laboratory.
Cell isolation
Luteal cells were obtained as described previously [15]. Briefly, mid-luteal CL tissue from four cows was enzymatically dissociated, and the resulting cell suspensions were centrifuged (5 min at 50 × g) three times to separate the luteal cells (pellet) from endothelial cells and other types of luteal non-steroidogenic cells (supernatant). The dissociated luteal cells were suspended in culture medium (Dulbecco’s Modified Eagle’s Medium/Nutrient Mixture F-12 Ham ([D/F], 1:1, v/v; no. D8900; Sigma-Aldrich, St. Louis, MO, USA) containing 5% calf serum (no. 16170-078; Life Technologies, Grand Island, NY, USA) and 20 µg/ml gentamicin (no. 15750-060; Life Technologies) and cultured with 5% CO2 in air. Cell viability was greater than 80%, as assessed by trypan blue exclusion. The cells in the cell suspension after centrifugation consisted of about 70% small luteal cells, 20% large luteal cells, 10% endothelial cells or fibrocytes, and no erythrocytes.
Cell culture
The dispersed luteal cells were seeded at 2.0 × 105 viable cells per milliliter in 24-well culture dishes (no. 662160; Greiner Bio-One, Frickenhausen, Germany) to evaluate mRNA expression. The cultures were kept in a humidified atmosphere of 5% CO2 in air at 38 C in an N2–O2–CO2-regulated incubator (no. BNP-110; ESPEC, Osaka, Japan). After 24 h of culture, the culture medium was replaced with fresh medium containing 0.1% BSA (no. 15408; Roche Diagnostics, Mannheim, Germany); 5 ng/ml sodium selenite (no. S5261; Sigma-Aldrich); 5 µg/ml holo-transferrin (no. T3400; Sigma-Aldrich); and 0.01, 0.1 or 1 μM PGF (no. 16010; Cayman chemical, Ann Arbor, MI, USA), 0.05, 0.5 or 5 nM TNF (Dainippon Pharmaceutical, Osaka, Japan) or 0.05, 0.5 or 0.5 nM IFNG (Kindly donated by Dr S Inumaru, NIAH, Ibaraki, Japan) for 24 h. The concentrations of PGF, IFNG and TNF for combination treatment were determined based on those that had the most effect on the expression of MMP mRNA in single treatments with PGF, IFNG and TNF. The luteal cells were also exposed to 1 μM PGF in combination with 5 nM IFNG or 5 nM TNF, 5 nM IFNG in combination with 5 nM TNF, 1 μM PGF in combination with 5 nM IFNG and 5 nM TNF for 24 h.
RNA isolation and cDNA synthesis
Total RNA was extracted from CL tissues and cells using TRIzol regent (no. 15596-026; Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s directions. One microgram of each total RNA was reverse transcribed using a ThermoScript RT-PCR System (no. 11146-016; Invitrogen).
Quantitative PCR (Real-Time PCR)
Ten percent of the reaction mixture was used in each PCR using specific primers for MMPs (Table 1). The expression of mRNA was quantified using iQ SYBR Green Supermix (no. 170-8880; Bio-Rad Laboratories, Hercules, CA, USA) starting with 2 ng of reverse-transcribed total RNA. The PCR conditions were 95 C for 15 min, followed by 45 cycles of 94 C for 15 sec, 55 C for 30 sec and 72 C for 30 sec. Use of a QuantiTectTM SYBR Green PCR system at elevated temperatures resulted in reliable and sensitive quantification of the RT-PCR products with high linearity. The relative level of expression of each mRNA was analyzed by the 2-ΔΔCT method [16, 17].
Table 1. Primers for MMPs and TIMPs used in quantitative RT-PCR.
| Target | Primer | Sequence (5'–3') | Accession no. | Product (bp) |
| MMP-1 | Forward | AGGTGCAGGTATCGGAGGAG | NM174112 | 275 |
| Reverse | CACACACTTCTGGGGTTTGG | |||
| MMP-2 | Forward | GGCATCTCTCAGATCCGTGG | NM174745.2 | 155 |
| Reverse | TGTGGGTCTTCGTACACAGC | |||
| MMP-9 | Forward | CTAGTTGGGATCCGGCAGAC | NM174744 | 128 |
| Reverse | CTAGTTGGGATCCGGCAGAC | |||
| MMP-14 | Forward | GAGTGACAGGCAAGGCTGAT | NM174390.2 | 200 |
| Reverse | AAATGTGGCATACTCGCCCA | |||
| TMP-1 | Forward | CATCTACACCCCTGCCATG | NM174471.3 | 231 |
| Reverse | CAGGGGATGGATGAGCAG | |||
| TMP-2 | Forward | GGGTCTCGCTGGACATTG | NM174472.4 | 256 |
| Reverse | TTGATGTTCTTCTCCGTGACC | |||
| TMP-3 | Forward | ACTTTGGAGACTCGAGCAGC | NM174473.4 | 231 |
| Reverse | GGTGTAGACCATCGTGCCAA | |||
| TMP-4 | Forward | CAGCCCTTTATCCCTGCCTC | NM001045871.2 | 220 |
| Reverse | TACTGTCACCCACGTTCTGC | |||
| ACTB | Forward | CAGCAAGCAGGAGTACGATG | AY141970 | 137 |
| Reverse | AGCCATGCCAATCTCATCTC | |||
Statistical analyses
All experimental data are shown as the mean ± SEM. The statistical significances of differences in the expression of MMP mRNA were assessed by one-way ANOVA followed by Tukey’s multiple comparisons test or the Kruskal-Wallis test followed by Dunn’s multiple comparisons test and by the Student’s t-test or Mann-Whitney test based on a test for homogeneity of variance. The statistical analyses performed for each experiment are described the figure legends.
Results
Effects of a single treatment with PGF, IFNG or TNF on MMP mRNA expression
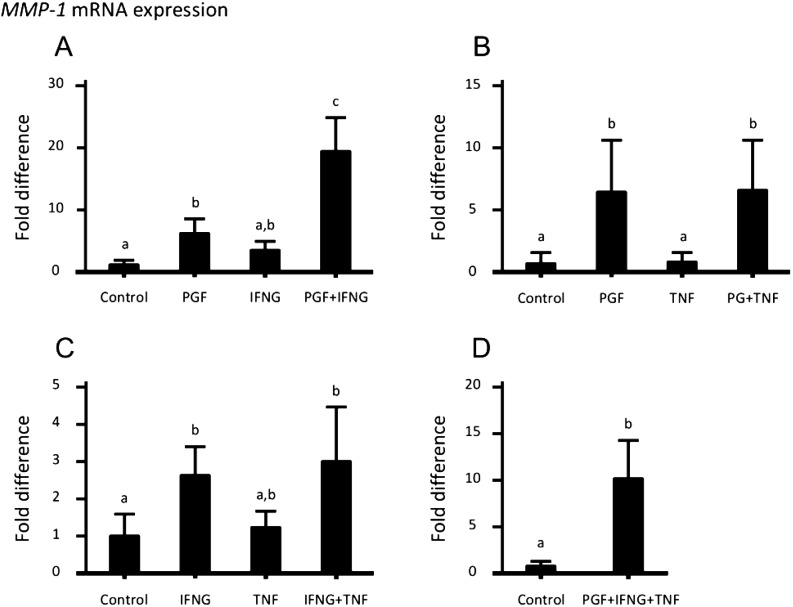
MMP-1 mRNA expression in cultured luteal cells was stimulated by PGF and IFNG (Fig. 1A and B). The levels of MMP-2 and MMP-9 mRNA expression were not affected by PGF, IFNG and TNF (Fig. 1D–I). IFNG suppressed MMP-14 mRNA expression (Fig. 1K).
Fig. 1.
Regulation of MMP mRNA expression in cultured bovine luteal cells following single treatments with different concentrations of PGF, IFNG and TNF for 24 h. (A–C) MMP-1. (D–F) MMP-2. (G–I) MMP-9. (J–L) MMP-14. Different superscript letters indicate significant differences (P < 0.05) compared with other columns as assessed by the Kruskal-Wallis test followed by Dunn’s multiple comparisons test (A and K) or one-way ANOVA followed by Tukey’s multiple comparisons test (B–J and L). Data are the mean ± SEM of 4 experiments.
Effects of combination treatment with PGF, IFNG and/or TNF on MMP and TIMP mRNA expression
Based on the above results, 1 μM PGF, 5 nM IFNG and 5 nM TNF were used in this experiment. MMP-1 mRNA expression was stimulated more by PGF in combination with IFNG than by each treatment alone (Fig. 2A). IFNG in combination with or without PGF and TNF decreased MMP-2 mRNA expression (Figs. 3A and C). PGF in combination with IFNG increased MMP-9 mRNA expression (Fig. 4A), whereas PGF in combination with IFNG and TNF did not affect MMP-9 mRNA expression (Fig. 4D). IFNG in combination with or without PGF and TNF reduced MMP-14 mRNA expression (Figs. 5A, C and D).
Fig. 2.
Regulation of MMP-1 mRNA expression in cultured bovine luteal cells following combination treatment with 1 μM PGF, 5 nM IFNG and 5 nM TNF for 24 h. (A) PGF in combination with IFNG significantly stimulated the expression of MMP-1 mRNA in luteal cells compared with a single treatment with PGF or IFNG. (B, C, D) The other results were the same as those induced by single-dose treatments. Different superscript letters indicate significant differences (P < 0.05) compared with other columns as assessed by the Kruskal-Wallis test followed by Dunn’s multiple comparisons test (A, B, C) and the Mann-Whitney test (D). Data are the mean ± SEM of 4 experiments.
Fig. 3.
Regulation of MMP-2 mRNA expression in cultured bovine luteal cells following combination treatment with 1 μM PGF, 5 nM IFNG and 5 nM TNF for 24 h. (A, B, C, D) All changes in MMP-2 mRNA expression induced by combination treatments were the same as those induced with a single-dose treatment. Different superscript letters indicate significant differences (P < 0.05) compared with other columns as assessed by one-way ANOVA followed by Tukey’s multiple comparisons test (A, B, C) and the Student’s t-test (D). Data are the mean ± SEM of 4 experiments.
Fig. 4.
Regulation of MMP-9 mRNA expression in cultured bovine luteal cells following combination treatment with 1 μM PGF, 5 nM IFNG and 5 nM TNF for 24 h. (A) PGF in combination with IFNG significantly stimulated MMP-9 mRNA expression in luteal cells compared with that in control and single treatments of PGF or IFNG. (B, C) PGF in combination with TNF and IFNG in combination with TNF did not affect the MMP-9 mRNA expression. (D) PGF in combination with IFNG and TNF did not stimulate MMP-9 mRNA expression. Different superscript letters indicate significant differences (P < 0.05) compared with other columns as assessed by one-way ANOVA followed by Tukey’s multiple comparisons test (A, B, C) and the Student’s t-test (D). Data are the mean ± SEM of 4 experiments.
Fig. 5.
Regulation of MMP-14 mRNA expression in cultured bovine luteal cells following combination treatment with 1 μM PGF, 5 nM IFNG and 5 nM TNF for 24 h. (A) PGF in combination with INFG suppressed the expression of MMP-14 mRNA. (B) PGF in combination TNF did not affect MMP-14 mRNA expression. (C) IFNG in combination with TNF suppressed the expression of MMP-14 mRNA. (D) PGF in combination with IFNG and TNF suppressed MMP-14 mRNA expression. Different superscript letters indicate significant differences (P < 0.05) compared with other columns as assessed by the Kruskal-Wallis test followed by Dunn’s multiple comparisons test (A, C) or one-way ANOVA followed by Tukey’s multiple comparisons test (B) and the Mann-Whitney test (D). Data are the mean ± SEM of 4 experiments.
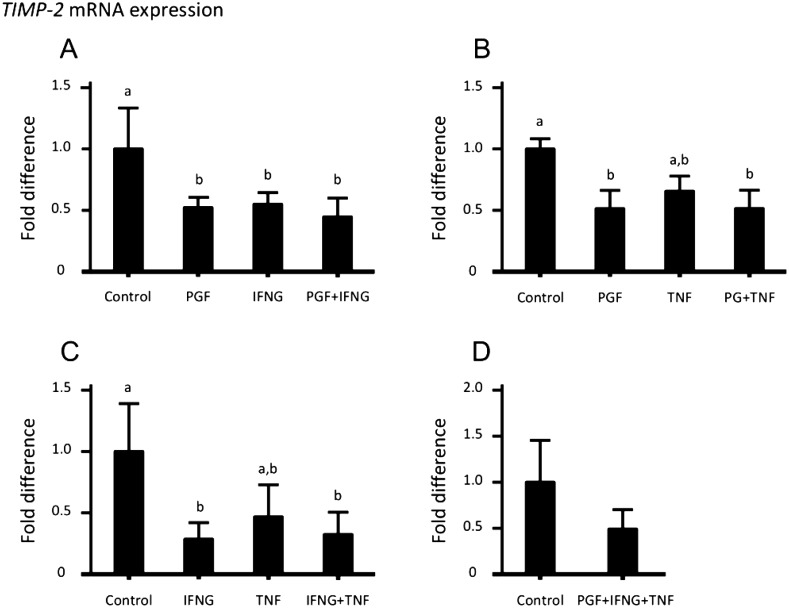
Since luteolytic factors affected MMP mRNA expression, we investigated the effect of luteolytic factors on TIMP mRNA expression in luteal cells. IFNG suppressed TIMP-1 mRNA expression (Figs. 6A and C), while IFNG in combination with PGF and TNF did not affect TIMP-1 mRNA expression. PGF and IFNG suppressed TIMP-2 mRNA expression (Figs. 7A, B and C), while PGF in combination with IFNG and TNF did not affect TIMP-2 mRNA expression (Fig. 7D). PGF, IFNG and TNF did not affect TIMP-3 or TIMP-4 mRNA expression (Figs. 8 and 9).
Fig. 6.
Regulation of TIMP-1 mRNA expression in cultured bovine luteal cells following combination treatment with 1 μM PGF, 5 nM IFNG and 5 nM TNF for 24 h. (A, C) INFG suppressed the expression of TIMP-1 mRNA. (B, C) PGF and TNF did not affect TIMP-1 mRNA expression. (D) IFNG in combination with PGF and TNF did not affect the expression of TIMP-1 mRNA. Different superscript letters indicate significant differences (P < 0.05) compared with other columns as assessed by one-way ANOVA followed by Tukey’s multiple comparisons test (A, C) or the Kruskal-Wallis test followed by Dunn’s multiple comparisons test (B) and the Mann-Whitney test (D). Data are the mean ± SEM of 4 experiments.
Fig. 7.
Regulation of TIMP-2 mRNA expression in cultured bovine luteal cells following combination treatment with 1 μM PGF, 5 nM IFNG and 5 nM TNF for 24 h. (A, C) PGF and IFNG suppressed the expression of TIMP-2 mRNA. (B, C) TNF did not affect TIMP-2 mRNA expression. (D) PGF in combination with IFNG and TNF did not affect the expression of TIMP-2 mRNA. Different superscript letters indicate significant differences (P < 0.05) compared with other columns as assessed by one-way ANOVA followed by Tukey’s multiple comparisons test (A, B, C) and the Student’s t-test (D). Data are the mean ± SEM of 4 experiments.
Fig. 8.
Regulation of TIMP-3 mRNA expression in cultured bovine luteal cells following combination treatment with 1 μM PGF, 5 nM IFNG and 5 nM TNF for 24 h. (A, B, C, D) PGF, IFNG and TNF did not affect the expression of TIMP-3 mRNA. Data are the mean ± SEM of 4 experiments.
Fig. 9.
Regulation of TIMP-4 mRNA expression in cultured bovine luteal cells following combination treatment with 1 μM PGF, 5 nM IFNG and 5 nM TNF for 24 h. (A, B, C, D) PGF, IFNG and TNF did not affect the expression of TIMP-4 mRNA. Data are the mean ± SEM of 4 experiments.
Discussion
The preceding results confirmed that MMP-1, -2, -9 and 14 mRNA are expressed in cultured bovine luteal cells. The expression of MMP-1, 2, 9 and 14 mRNA increased during luteolysis [12]. These MMPs were previously found to be involved in functional luteolysis [11, 18, 19]. PGF secreted by the uterus is a trigger that induces luteolysis in the cow. IFNG and TNF, which are secreted by macrophages that invade the CL, are also involved in inducing luteolysis [4, 20,21,22]. Until now, immune cells infiltrating the regressing CL were considered the main source of MMPs during luteolysis [12]. In the present study, PGF and IFNG stimulated the expression of MMP-1 and MMP-9 mRNA in cultured bovine luteal cells, which suggests that luteal cells are also important sources of MMPs, especially MMP-1, during luteolysis.
During structural luteolysis, luteal cells flow out of the CL via lymphatic vessels drained from the CL [5]. Luteal cells have to be detached from the CL tissue to flow out to the lymphatic vessels. The CL tissue is composed of collagens, which are the main components of the ECM, and the predominant luteal collagen is collagen type I [23, 24]. MMP-1, secreted from cells, and MMP-14, anchored to plasma membrane, have the ability to cleave collagen types I, II and III [25, 26]. In the present study, PGF in combination with IFNG strongly stimulated MMP-1 mRNA expression in cultured luteal cells, which suggests that MMP-1 secreted by luteal cells degrades the ECM surrounding the luteal cells and promotes the detachment of luteal cells from the CL tissue during luteolysis.
Since MMP-14 is expressed on the cell membrane of large luteal cells [12], MMP-14 may assist the outflow of luteal cells by digesting the pericellular ECM. We expected that luteolytic factors such as PGF, IFNG and TNF would upregulate MMP-14 mRNA expression in luteal cells. However, MMP-14 mRNA expression was not changed by any dose of these factors. On the other hand, IFNG strongly suppressed the expression of MMP-14 mRNA in luteal cells. MMP-14 mRNA expression in the CL tissue has been reported to increase during luteolysis [12]. Our results suggest that luteal cells are not involved in the increase of MMP-14 mRNA expression during luteolysis. Since macrophages express MMP-14 [27, 28], the increase of MMP-14 mRNA expression in the CL tissue during luteolysis may be due to the increase of macrophages invading the CL [20]. In cancer cell invasion, MMP-14 is localized at the front of migrating cells and degrades the ECM [29, 30]. Therefore, we expected that luteal cells would be detached by MMP-14 and enter the luteal lymphatic vessels. However, the present results contradict this hypothesis. Another explanation for the movement of luteal cell could be that luteal cells detached from the CL tissue by MMP-1 are transported by interstitial fluids that come from blood plasma and flow into the lymphatic vessels. Further study is needed to clarify how luteal cells move in the CL tissue to the lymphatic vessels.
The basal membrane, which provides structural support to the walls of blood vessels, is degraded by MMP-2 and MMP-9 [26]. The basal membrane has been found surrounding the lymphatic vessels that differentiate from veins [31,32,33]. Immune cells such as lymphocytes and macrophages invading the CL migrate into the lymphatic node from the CL during luteolysis [34]. Therefore, degradation of the basal membrane when immune cells enter the lymphatic vessels is a necessary process. In this study, IFNG suppressed MMP-2 mRNA expression, while PGF in combination with IFNG induced MMP-9 mRNA expression. Interestingly, PGF in combination with IFNG and TNF eliminated the effect of PGF in combination with IFNG on the expression of MMP-9 mRNA. MMP-2 and MMP-9 secreted by luteal cells may support immune cells to enter the lymphatic vessels by degrading the basal membrane, and their expressions in luteal cells may be regulated by macrophages, which have the capacity to secrete IFNG and TNF.
The present study showed that PGF and IFNG reduced TIMP-1 and TIMP-2 mRNA expression in cultured luteal cells. TIMPs form a 1:1 complex with MMPs and inhibit their proteolytic activity [35]. TIMP-1 preferentially inhibits MMP-1, while TIMP-2 is a more effective inhibitor of MMP-2 [36]. TIMP-1 mRNA expression in CL tissue decreases during luteolysis [12, 18]. In the present study, although PGF in combination with IFNG strongly stimulated MMP-1 mRNA expression, the same treatment suppressed TIMP-1 mRNA expression. These findings suggest that MMP-1 breaks down the ECM in the bovine CL during luteolysis. TIMP-2 mRNA expression in the bovine and ovine CL tissue decreases during luteolysis [12, 19]. We showed that PGF and IFNG suppressed TIMP-2 mRNA expression in cultured bovine luteal cells. These results suggest that the decrease of TIMP-2 mRNA expression during luteolysis is caused by luteolytic factors such as PGF and IFNG. During luteolysis, T lymphocytes invade the CL [20, 37]. T lymphocytes secrete MMP-2 and MMP-9, and pass through the basal membrane into extravascular tissue sites [27]. Since IFNG suppresses TIMP-2 expression in luteal cells, IFNG secreted by macrophages appears to conveniently enable T lymphocytes to break down basal membrane with MMP-2 during luteolysis. PGF released by the uterus causes luteolysis in ruminants, and the number of leukocytes in the CL (e.g., T lymphocytes, macrophages) increases at the time of luteolysis [20]. Since leukocytes are known to produce a variety of cytokines including IFNG and TNF [38], the uterus and macrophages seem to be sources of PGF and INFG. In addition, we described an auto-amplification system for PGF in the bovine CL [39]. Luteal PGF may also play a role in regulating MMP-1 and TIMP expression during luteolysis.
MMPs are known to play roles in cell proliferation, migration, differentiation and apoptosis [6]. In the CL, a reduction of P4 secretion and apoptotic cell death of luteal cells are associated with the loss of ECM integrity [40]. Endothelial cells in the ovine CL detach from the ECM [41] and undergo apoptosis during luteolysis [42]. In cattle, capillaries in the CL have been reported to disappear during luteolysis [43]. It is possible that MMPs induce apoptosis of bovine capillary cells by degrading the ECM. During luteolysis, IFNG and TNF induce apoptosis of luteal cells [4, 44]. MMP-1 and MMP-9 have been implicated in the conversion of TNF and Fas ligand, which is a protein homologous with TNF, to active, soluble forms [45, 46]. Therefore, MMP-1 secreted by luteal cells may locally activate TNF, and regulate apoptosis of luteal cells. Interestingly, most of the luteal cells found in the lymphatic fluid were small luteal cells that were alive [5]. MMP-1 produced by luteal cells may induce apoptosis of large luteal cells by activation of TNF and the outflow of small luteal cells by degradation of the ECM surrounding luteal cells.
During luteolysis induced by PGF administration on day 10 post ovulation invivo, the volume of the CL decreases to less than half of its original size in 24 h [47]. Recently, we demonstrated the involvement of lymphatic vessels in this rapid shrinking of the CL [5], and the present findings seem to support this idea. PGF administration seems to upregulate the synthesis of MMP-1 in luteal cells. MMP-1 may degrade the ECM surrounding luteal cells, resulting in detachment of the luteal cells from the CL tissue and their outflow through the lymphatic vessels.
In summary, our results show that luteolytic factors such as PGF and IFNG regulate the expression of MMPs and TIMPs mRNA in cultured luteal cells. PGF and IFNG during luteolysis may be involved in inducing detachment of luteal cells from the CL tissue by increasing the production of MMPs in luteal cells, providing an essential prerequisite for outflow of luteal cells from the CL tissue to lymphatic vessels.
Acknowledgments
This research was supported by Challenging Exploratory Research (No. 25660212) of the Japan Society for the Promotion of Science (JSPS).
References
- 1.Davis JS, Rueda BR. The corpus luteum: an ovarian structure with maternal instincts and suicidal tendencies. Front Biosci 2002; 7: d1949–d1978. [DOI] [PubMed] [Google Scholar]
- 2.Hehnke KE, Christenson LK, Ford SP, Taylor M. Macrophage infiltration into the porcine corpus luteum during prostaglandin F2 α-induced luteolysis. Biol Reprod 1994; 50: 10–15. [DOI] [PubMed] [Google Scholar]
- 3.Pate JL, Landis Keyes P. Immune cells in the corpus luteum: friends or foes? Reproduction 2001; 122: 665–676. [DOI] [PubMed] [Google Scholar]
- 4.Sugino N, Okuda K. Species-related differences in the mechanism of apoptosis during structural luteolysis. J Reprod Dev 2007; 53: 977–986. [DOI] [PubMed] [Google Scholar]
- 5.Abe H, Al-zi’abi MO, Sekizawa F, Acosta TJ, Skarzynski DJ, Okuda K. Lymphatic involvement in the disappearance of steroidogenic cells from the corpus luteum during luteolysis. PLoS ONE 2014; 9: e88953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hulboy DL, Rudolph LA, Matrisian LM. Matrix metalloproteinases as mediators of reproductive function. Mol Hum Reprod 1997; 3: 27–45. [DOI] [PubMed] [Google Scholar]
- 7.Smith MF, McIntush EW, Ricke WA, Kojima FN, Smith GW. Regulation of ovarian extracellular matrix remodelling by metalloproteinases and their tissue inhibitors: effects on follicular development, ovulation and luteal function. J Reprod Fertil Suppl 1999; 54: 367–381. [PubMed] [Google Scholar]
- 8.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer 2002; 2: 161–174. [DOI] [PubMed] [Google Scholar]
- 9.Yang Q, Ye ZY, Zhang JX, Tao HQ, Li SG, Zhao ZS. Expression of matrix metalloproteinase-9 mRNA and vascular endothelial growth factor protein in gastric carcinoma and its relationship to its pathological features and prognosis. Anat Rec (Hoboken) 2010; 293: 2012–2019. [DOI] [PubMed] [Google Scholar]
- 10.Gialeli C, Theocharis AD, Karamanos NK. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J 2011; 278: 16–27. [DOI] [PubMed] [Google Scholar]
- 11.Smith MF, Ricke WA, Bakke LJ, Dow MP, Smith GW. Ovarian tissue remodeling: role of matrix metalloproteinases and their inhibitors. Mol Cell Endocrinol 2002; 191: 45–56. [DOI] [PubMed] [Google Scholar]
- 12.Kliem H, Welter H, Kraetzl WD, Steffl M, Meyer HH, Schams D, Berisha B. Expression and localisation of extracellular matrix degrading proteases and their inhibitors during the oestrous cycle and after induced luteolysis in the bovine corpus luteum. Reproduction 2007; 134: 535–547. [DOI] [PubMed] [Google Scholar]
- 13.Okuda K, Kito S, Sumi N, Sato K. A study of the central cavity in the bovine corpus luteum. Vet Rec 1988; 123: 180–183. [DOI] [PubMed] [Google Scholar]
- 14.Miyamoto Y, Skarzynski DJ, Okuda K. Is tumor necrosis factor α a trigger for the initiation of endometrial prostaglandin F(2α) release at luteolysis in cattle? Biol Reprod 2000; 62: 1109–1115. [DOI] [PubMed] [Google Scholar]
- 15.Okuda K, Miyamoto A, Sauerwein H, Schweigert FJ, Schams D. Evidence for oxytocin receptors in cultured bovine luteal cells. Biol Reprod 1992; 46: 1001–1006. [DOI] [PubMed] [Google Scholar]
- 16.Arai M, Yoshioka S, Tasaki Y, Okuda K. Remodeling of bovine endometrium throughout the estrous cycle. Anim Reprod Sci 2013; 142: 1–9. [DOI] [PubMed] [Google Scholar]
- 17.Yoshioka S, Abe H, Sakumoto R, Okuda K. Proliferation of luteal steroidogenic cells in cattle. PLoS ONE 2013; 8: e84186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ricke WA, Smith GW, McIntush EW, Smith MF. Analysis of luteal tissue inhibitor of metalloproteinase-1, -2, and -3 during prostaglandin F(2α)-induced luteolysis. Biol Reprod 2002; 66: 1387–1394. [DOI] [PubMed] [Google Scholar]
- 19.Towle TA, Tsang PC, Milvae RA, Newbury MK, McCracken JA. Dynamic in vivo changes in tissue inhibitors of metalloproteinases 1 and 2, and matrix metalloproteinases 2 and 9, during prostaglandin F(2α)-induced luteolysis in sheep. Biol Reprod 2002; 66: 1515–1521. [DOI] [PubMed] [Google Scholar]
- 20.Penny LA, Armstrong D, Bramley TA, Webb R, Collins RA, Watson ED. Immune cells and cytokine production in the bovine corpus luteum throughout the oestrous cycle and after induced luteolysis. J Reprod Fertil 1999; 115: 87–96. [DOI] [PubMed] [Google Scholar]
- 21.Townson DH, O’Connor CL, Pru JK. Expression of monocyte chemoattractant protein-1 and distribution of immune cell populations in the bovine corpus luteum throughout the estrous cycle. Biol Reprod 2002; 66: 361–366. [DOI] [PubMed] [Google Scholar]
- 22.Schams D, Berisha B. Regulation of corpus luteum function in cattle—an overview. Reprod Domest Anim 2004; 39: 241–251. [DOI] [PubMed] [Google Scholar]
- 23.Luck MR, Zhao Y. Identification and measurement of collagen in the bovine corpus luteum and its relationship with ascorbic acid and tissue development. J Reprod Fertil 1993; 99: 647–652. [DOI] [PubMed] [Google Scholar]
- 24.Irving-Rodgers HF, Roger J, Luck MR, Rodgers RJ. Extracellular matrix of the corpus luteum. Semin Reprod Med 2006; 24: 242–250. [DOI] [PubMed] [Google Scholar]
- 25.Hua H, Li M, Luo T, Yin Y, Jiang Y. Matrix metalloproteinases in tumorigenesis: an evolving paradigm. Cell Mol Life Sci 2011; 68: 3853–3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klein T, Bischoff R. Physiology and pathophysiology of matrix metalloproteases. Amino Acids 2011; 41: 271–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goetzl EJ, Banda MJ, Leppert D. Matrix metalloproteinases in immunity. J Immunol 1996; 156: 1–4. [PubMed] [Google Scholar]
- 28.Klose A, Zigrino P, Mauch C. Monocyte/macrophage MMP-14 modulates cell infiltration and T-cell attraction in contact dermatitis but not in murine wound healing. Am J Pathol 2013; 182: 755–764. [DOI] [PubMed] [Google Scholar]
- 29.Mori H, Tomari T, Koshikawa N, Kajita M, Itoh Y, Sato H, Tojo H, Yana I, Seiki M. CD44 directs membrane-type 1 matrix metalloproteinase to lamellipodia by associating with its hemopexin-like domain. EMBO J 2002; 21: 3949–3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Itoh Y, Ito N, Nagase H, Evans RD, Bird SA, Seiki M. Cell surface collagenolysis requires homodimerization of the membrane-bound collagenase MT1-MMP. Mol Biol Cell 2006; 17: 5390–5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vainionpää N, Bützow R, Hukkanen M, Jackson DG, Pihlajaniemi T, Sakai LY, Virtanen I. Basement membrane protein distribution in LYVE-1-immunoreactive lymphatic vessels of normal tissues and ovarian carcinomas. Cell Tissue Res 2007; 328: 317–328. [DOI] [PubMed] [Google Scholar]
- 32.Wang Y, Oliver G. Current views on the function of the lymphatic vasculature in health and disease. Genes Dev 2010; 24: 2115–2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kesler CT, Liao S, Munn LL, Padera TP. Lymphatic vessels in health and disease. Wiley Interdiscip Rev Syst Biol Med 2013; 5: 111–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shirasuna K, Shimizu T, Matsui M, Miyamoto A. Emerging roles of immune cells in luteal angiogenesis. Reprod Fertil Dev 2013; 25: 351–361. [DOI] [PubMed] [Google Scholar]
- 35.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res 2003; 92: 827–839. [DOI] [PubMed] [Google Scholar]
- 36.Bourboulia D, Stetler-Stevenson WG. Matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs): Positive and negative regulators in tumor cell adhesion. Semin Cancer Biol 2010; 20: 161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shirasuna K, Nitta A, Sineenard J, Shimizu T, Bollwein H, Miyamoto A. Vascular and immune regulation of corpus luteum development, maintenance, and regression in the cow. Domest Anim Endocrinol 2012; 43: 198–211. [DOI] [PubMed] [Google Scholar]
- 38.Pate JL. Involvement of immune cells in regulation of ovarian function. J Reprod Fertil Suppl 1995; 49: 365–377. [PubMed] [Google Scholar]
- 39.Kumagai A, Yoshioka S, Sakumoto R, Okuda K. Auto-amplification system for prostaglandin F2α in bovine corpus luteum. Mol Reprod Dev 2014; 81: 646–654. [DOI] [PubMed] [Google Scholar]
- 40.McIntush EW, Smith MF. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in ovarian function. Rev Reprod 1998; 3: 23–30. [DOI] [PubMed] [Google Scholar]
- 41.Nett TM, McClellan MC, Niswender GD. Effects of prostaglandins on the ovine corpus luteum: blood flow, secretion of progesterone and morphology. Biol Reprod 1976; 15: 66–78. [DOI] [PubMed] [Google Scholar]
- 42.Modlich U, Kaup FJ, Augustin HG. Cyclic angiogenesis and blood vessel regression in the ovary: blood vessel regression during luteolysis involves endothelial cell detachment and vessel occlusion. Lab Invest 1996; 74: 771–780. [PubMed] [Google Scholar]
- 43.Hojo T, Al-Zi’abi MO, Skarzynski DJ, Acosta TJ, Okuda K. Changes in the vasculature of bovine corpus luteum during the estrous cycle and prostaglandin F2α-induced luteolysis. J Reprod Dev 2009; 55: 512–517. [DOI] [PubMed] [Google Scholar]
- 44.Taniguchi H, Yokomizo Y, Okuda K. Fas-Fas ligand system mediates luteal cell death in bovine corpus luteum. Biol Reprod 2002; 66: 754–759. [DOI] [PubMed] [Google Scholar]
- 45.Gearing AJ, Beckett P, Christodoulou M, Churchill M, Clements J, Davidson AH, Drummond AH, Galloway WA, Gilbert R, Gordon JL.Processing of tumour necrosis factor-α precursor by metalloproteinases. Nature 1994; 370: 555–557. [DOI] [PubMed] [Google Scholar]
- 46.Kayagaki N, Kawasaki A, Ebata T, Ohmoto H, Ikeda S, Inoue S, Yoshino K, Okumura K, Yagita H. Metalloproteinase-mediated release of human Fas ligand. J Exp Med 1995; 182: 1777–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Acosta TJ, Yoshizawa N, Ohtani M, Miyamoto A. Local changes in blood flow within the early and midcycle corpus luteum after prostaglandin F(2 α) injection in the cow. Biol Reprod 2002; 66: 651–658. [DOI] [PubMed] [Google Scholar]