Abstract
In order to develop excellent germplasm resources for giant grouper (Epinephelus lanceolatus), cryopreservation of giant grouper sperm was examined in the present study. Firstly, 13 kinds of sperm dilution (ELS1-3, EM1-2, TS-2, MPRS, ELRS0-6) were prepared with physiological salt, sucrose, glucose and fetal bovine serum. The physiological parameters of ELRS3 (ratio of fast motion, ratio of slow motion, time of fast motion, time of slow motion, lifespan and motility) and ELS3 (sperm ratio of slow motion, time of slow motion and motility) were significantly higher than those of the other dilutions (P < 0.05). Secondly, after adding 15% DMSO and 10% FBS to ELRS3 and ELS3, most physiological parameters of frozen sperm were also significantly higher than the other gradients (P < 0.05), and sperm motility was as high as 63.68 ± 4.16% to74.75 ± 12.71% (fresh sperm motility, 80.70 ± 1.37% to 80.71 ± 1.49%). Mixed with the above dilutions, a final volume of 105 ml semen was cryopreserved. Finally, the sperm of giant grouper cryopreserved with cryoprotectants (ELRS3 + 15% DMSO + 10% FBS) was used for electron-microscopic observation and crossbreeding with red-spotted groupers (Epinephelus akaara). The electron-microscopic observation revealed that part of the frozen-thawed sperm was cryodamaged, e.g., flagellum fracturing and mitochondria falling out, while the ultrastructure of sperm membrane, mitochondria and flagellum remained intact. Also, the fertilization and hatchability rates of giant grouper frozen sperm and red-spotted grouper eggs were as high as 94.56% and 75.56%, respectively. Thus, a technique for cryopreservation of giant grouper sperm was successfully developed and applied to crossbreeding with red-spotted grouper eggs.
Keywords: Cryopreservation, Crossbreeding, Epinephelus lanceolatus, Physiological characteristics, Sperm
Groupers belong to the subfamily Epinephelinae of the family Serranidae in the order Perciformes. There are approximately 150 species of groupers in the world [1], 36 of which live in the waters of both the East China Sea and the South China Sea [2]. Giant groupers (Epinephelus lanceolatus) are distributed within the Indian and Pacific Oceans, from the east coast of Africa to Micronesia in the central Pacific and down to Australia in the south [3]. In China, giant groupers are distributed in the South China Sea (the Spratly Islands) in low quantities [4]. Giant groupers grow fast, and the weight of a 1-year-old fish can be as high as 3 kg [5]. Known as the “King of Groupers,” the giant grouper is the largest species in the grouper subfamily. Currently, the giant grouper is quite rare in the natural environment, and it has been listed as a national protected fish. The giant grouper is a protogynous hermaphrodite species like other groupers. They mature only as females and have the ability to change sex after sexual maturity. Given that natural mating and breeding are not possible for the giant grouper in an artificial farmed environment, reproduction is accomplished by artificial spawning and fertilization. Moreover, due to the fast growth speed of the giant grouper, sperm of the giant grouper can be applied to the cultivation of a new fast-growing species [6]. Since the early 20th century, artificial propagation, embryonic development [4], industrial breeding [7], genetic polymorphism [8] and effects of environmental factors on sperm motility [9] have been studied in giant groupers. So far, no research of sperm cryopreservation in the giant grouper has been reported.
Sperm cryopreservation technology has been developed for approximately 30 marine fish species [10], including the black grouper (Epinephelus malabaricus) [11, 12], kelp grouper (Epinephelus moara) [13], seven-band grouper (Epinephelus septemfasciatus) [14], sea perch (Lateolabrax japonicas) [15] and red seabream (Pagrosomus major) [16]. Cryopreserved sperm has been used for artificial reproduction, seed production, sex control, induction of gynogenesis and breeding [17,18,19]. Studies regarding cryopreservation of giant grouper sperm and subsequent application in hybridization could not only provide a way of supplying sperm for giant grouper aquaculture but also break the reproductive and geographic isolation of different groupers, resulting in a new species.
In the present study, an efficient sperm cryopreservation technology for giant grouper was developed, and application of frozen giant grouper sperm to crossbreeding with the red-spotted grouper was investigated.
Materials and Methods
Sperm collection
Giant grouper sperm was obtained from male broodstocks (body length, 1.3–1.6 m; body weight, 34–48 kg) cultured at Hainan Blue Ocean Marine Science and Technology Breeding Company (Lingshui County, Hainan Province) and Laizhou MingBo Aquatic Products (Laizhou, Shandong Province). Three to five male fish were used in each experiment. Milt was collected from individual males anesthetized with 50 ppm Eugenol by abdominal massage, and a separate 10 ml syringe was used for each male. Milt was dispensed into 1000 ml glass bottles and immediately placed on crushed ice. The total volume of milt collected in this experiment was 105 ml. After 20-fold dilution with various diluents, the sperm concentration (4.6 × 109 cells/ml) was determined by a hemocytometer under a microscope. Motility (percentage of swimming spermatozoa) was determined by microscope (× 200) using 1 μl of sperm placed on a glass slide with the addition of 200 μl of seawater (28‰). The sperm samples with motility greater than or equal to 86.67 ± 2.89% were selected for the following cryopreservation test.
Preparation of sperm diluents
Sperm diluents were prepared with a base solution consisting of sucrose, KHCO3, KCl, NaHCO3, MgCl2·6H2O (Sinopharm Chemical Reagent, Shanghai, PR China), NaCl, CaCl2·2H2O (Tianjin Regent Chemicals, Shanghai, PR China), NaH2PO4 (Tianjin BASF Chemical, Tianjin, PR China), glucose, TRIS alkali (Beijing Solarbio Science and Technology, Beijing, PR China) and fetal bovine serum (FBS, Gibco, Life Technologies, Carlsbad, CA, USA). In the present study, cryoprotectants and fetal bovine serum (FBS) were added to basic solutions before use. The following additional diluents were also used: EM1-2 [20]; TS-2 [21]; MPRS [15]; an ELS series, ELS1, ELS2 and ELS3; and an ELRS series, ELRS0, ELRS1, ELRS2, ELRS3, ELRS4, ELRS5 and ELRS6 (Table 1). In addition, dimethyl sulfoxide (DMSO) was added to these diluents with a final concentration of 10% (v/v). The pH and osmolality of sperm dilutions were measured with a PHS-25 pH meter (Shanghai Electronics Scientific Instruments, PR China) and Fiske 210 Micro-Sample Osmometer (Fiske Associates, AI Instruments, Norwood, MA, USA).
Table 1. Sperm diluent formulations for the cryopreservation of giant grouper (E. lanceolatus) sperm.
| Dilution | Sucrose (mM) |
Glucose (mM) |
NaCl (mM) |
NaHCO3
(mM) |
KCl (mM) |
KHCO3
(mM) |
Tris (mM) |
CaCl2·2H2O (mM) |
NaH2PO4
(mM) |
MgCl2·6H2O (mM) |
FBS | Osmolality | pH |
| % | mOsm/l | ||||||||||||
| ELS1 | 277.37 | 171.12 | 5.95 | 10 | 520.45 | 7.09 | |||||||
| ELS2 | 138.68 | 171.12 | 5.95 | 10 | 437.11 | 7.11 | |||||||
| ELS3 | 92.46 | 171.12 | 5.95 | 10 | 409.34 | 7.10 | |||||||
| EM1-2 | 0 | 147.16 | 0 | 99.88 | 10 | 507.69 | 7.11 | ||||||
| TS-2 | 109.99 | 0 | 0 | 0 | 99.98 | 9.99 | 10 | 335.00 | 8.20 | ||||
| MPRS | 101.79 | 60.40 | 2.98 | 5.23 | 1.16 | 1.83 | 1.13 | 10 | 202.00 | 6.98 | |||
| ELRS0 | 0 | 111.23 | 2.38 | 1.88 | 0.82 | 0.08 | 10 | 233.33 | 7.03 | ||||
| ELRS1 | 92.46 | 111.23 | 2.38 | 1.88 | 0.82 | 0.08 | 10 | 288.89 | 7.03 | ||||
| ELRS2 | 138.68 | 111.23 | 2.38 | 1.88 | 0.82 | 0.08 | 10 | 316.66 | 7.05 | ||||
| ELRS3 | 277.37 | 111.23 | 2.38 | 1.88 | 0.82 | 0.082 | 10 | 400.00 | 7.03 | ||||
| ELRS4 | 369.82 | 111.23 | 2.38 | 1.88 | 0.82 | 0.08 | 10 | 455.55 | 7.04 | ||||
| ELRS5 | 462.28 | 111.23 | 2.38 | 1.88 | 0.82 | 0.08 | 10 | 511.11 | 7.03 | ||||
| ELRS6 | 554.73 | 111.23 | 2.38 | 1.88 | 0.82 | 0.08 | 10 | 566.66 | 7.04 |
Sperm cryopreservation and thawing
Dilutions (ELS1, ELS2, ELS3, EM1-2, TS-2, MPRS, ELRS0, ELRS1, ELRS2, ELRS3, ELRS4, ELRS5, or ELRS6) (Table 1) were mixed with DMSO (v/v =4:1) and then mixed with sperm (v/v=1:1). The diluted spermatozoa were dispensed into 2 ml cryovials (1 ml/tube) and equilibrated for 5 min at room temperature (20–25 C). The cryovials were placed into cloth bags (5–6 cryovials/bag), suspended 10 cm above the surface of liquid nitrogen (LN) vapor (–60 to –80 C) for pre-freezing at a rate of –20 C /sec, equilibrated for 10 min and then plunged into LN for 4–5 h. For thawing, cryovials were first equilibrated at 20 cm above the surface of LN vapor for 1–2 min with a thawing rate of 1.69 C /sec and then immediately placed in a water bath at 38 C for 50–60 sec with agitation for thawing at a rate of 2.16 C /sec.
Measurement of frozen sperm parameters
The ratio of fast motion, ratio of slow motion, time of fast motion, time of slow motion, lifespan and motility of the frozen sperm were measured by a computer-assisted sperm analysis (CASA) system (CASAS-QH-Ø, Tsinghua Tongfang, Beijing, PR China). Determination of sperm parameters of each sample was replicated six times, and 500–800 sperm were analyzed. Ratio of fast motion is the percentage of spermatozoa with a swimming speed exceeding 50–60 μm/sec, and ratio of slow motion is the percentage of spermatozoa with a swimming speed between 5 μm/sec and 50–60 μm/sec. Time of fast motion is the time between observation of fast movement and slow movement of sperm, and time of slow motion is the time between observation of slow movement and immobility of sperm. Lifespan is the period of time from activation of movement to immobility, and motility is the percentage of swimming spermatozoa (Table 2).
Table 2. Post-thaw sperm physiology and viability parameters for the giant grouper (E. lanceolatus) with different sperm diluents (10% DMSO).
| Sperm diluents | Ratio of fast motion (%) |
Ratio of slow motion (%) |
Time of fast motion (sec) |
Time of slow motion (sec) |
Lifespan (sec) | Motility (%) |
| ELS1 | 11.66 ± 1.37bc | 26.36 ± 1.38cde | 27.52 ± 5.29ce | 115.35 ± 32.09d | 721.05 ± 87.87de | 38.03 ± 0.94de |
| ELS2 | 5.60 ± 1.66defg | 17.38 ± 2.52e | 65.32 ± 27.54de | 115.33 ± 43.94d | 746.57 ± 121.57de | 22.98 ± 3.29fg |
| ELS3 | 12.78 ± 0.39bc | 38.3183 ± 1.50b | 114.63 ± 3.49bc | 334.58 ± 20.63b | 955.52 ± 37.314bc | 51.10 ± 1.88c |
| EM1-2 | 3.89 ± 0.88fg | 16.22 ± 1.87e | 26.05 ± 4.82e | 89.05 ± 14.66d | 622.23 ± 135.37e | 20.12 ± 2.74g |
| TS-2 | 9.15 ± 1.80cd | 19.81 ± 3.63de | 84.27 ± 18.82cd | 239.17 ± 54.75c | 958.48 ± 38.60bc | 28.97 ± 5.35efg |
| MPRS | 1.62 ± 0.66g | 29.78 ± 8.34bcd | 54.43 ± 5.94de | 155.68 ± 18.49d | 865.50 ± 71.85bcd | 31.40 ± 8.57efg |
| ELRS0 | 8.70 ± 5.39cde | 24.25 ± 9.15de | 45.66 ± 9.05e | 121.43 ± 12.68d | 788.42 ± 67.21cde | 32.95 ± 14.51defg |
| ELRS1 | 4.34 ± 1.03efg | 30.38 ± 9.47bcd | 30.45 ± 3.41e | 115.29 ± 25.86d | 656.28 ± 33.03e | 34.72 ± 10.01def |
| ELRS2 | 6.77 ± 1.91def | 21.40 ± 12.16de | 104.45 ± 16.71c | 234.27 ± 13.29c | 875.32 ± 132.08bcd | 28.17 ± 11.44efg |
| ELRS3 | 15.04 ± 4.95b | 54.40 ± 7.29a | 121.37 ± 10.84b | 336.27 ± 10.65b | 1039.60 ± 72.28b | 69.44 ± 8.06b |
| ELRS4 | 9.60 ± 4.37cd | 26.55 ± 3.61cde | 133.88 ± 9.24b | 232.38 ± 46.48c | 860.97 ± 69.95bcd | 36.15 ± 7.96def |
| ELRS5 | 8.74 ± 2.64cde | 36.23 ± 1.43bc | 42.07 ± 9.31e | 95.73 ± 11.06d | 670.40 ± 101.48e | 44.97 ± 3.61cd |
| ELRS6 | 1.24 ± 0.15g | 21.49 ± 2.62de | 82.31 ± 9.52de | 211.05 ± 24.77c | 904.17 ± 43.96bcd | 22.74 ± 2.73fg |
| Fresh sperm | 30.34 ± 1.17a | 50.37 ± 0.32a | 450.43 ± 105.75a | 1114.67 ± 155.67a | 1919.96 ± 364.52a | 80.71 ± 1.49a |
All motion-related parameters were better for sperm in ELRS3 and ELS3 than those in other solutions, although their values were significantly lower than those of fresh sperm (n = 6, P < 0.05). Values are means ± standard deviation, and means with different letters (a, b, c, d, e, f, g) are significantly different (P < 0.05).
Selection of optimal concentrations of DMSO and FBS
To obtain an appropriate DMSO concentration, five different final concentrations of DMSO (5%, 10%, 15%, 20% and 30%) were added to ELS3 and ELRS3. CASA was used to measure the ratio of fast motion, ratio of slow motion, time of fast motion, time of slow motion, lifespan and motility of sperm (Table 3).
Table 3. Post-thaw sperm physiology and viability parameters for the giant grouper (E. lanceolatus) with different DMSO concentrations in ELS3 and ELRS3.
| Sperm diluents | Concentration of DMSO (%) |
Ratio of fast motion (%) |
Ration of slow motion (%) |
Time of fast motion (sec) |
Time of slow motion (sec) |
Lifespan (sec) | Motility (%) |
| ELS3 | 0 | 0.66 ± 0.48c | 4.20 ± 1.55c | 49.73 ± 8.47d | 155.53 ± 50.03c | 356.60 ± 89.57de | 4.86 ± 1.66c |
| 5 | 6.87 ± 3.20bc | 23.03 ± 4.70bc | 56.93 ± 23.48d | 165.83 ± 43.92c | 673.00 ± 266.38cd | 29.90 ± 3.45c | |
| 10 | 15.74 ± 6.41b | 35.64 ± 5.08ab | 200.37 ± 31.38c | 430.43 ± 132.84b | 868.67 ± 218.65c | 51.38 ± 3.25b | |
| 15 | 28.85 ± 8.99a | 39.51 ± 17.79ab | 331.67 ± 24.34b | 558.97 ± 37.34b | 1373.67 ± 238.34b | 68.37 ± 25.23ab | |
| 20 | 15.90 ± 9.45b | 39.45 ± 16.63ab | 306.10 ± 46.13b | 421.20 ± 84.03b | 1258.90 ± 119.39b | 55.35 ± 16.98b | |
| 30 | 5.75 ± 3.33c | 8.87 ± 1.50c | 202.23 ± 27.79c | 360.13 ± 52.80b | 162.67 ± 32.13e | 14.62 ± 4.17c | |
| ELRS3 | 0 | 3.16 ± 2.29b | 7.35 ± 1.45d | 47.23 ± 15.34c | 106.70 ± 7.07d | 293.50 ± 86.10c | 10.51 ± 3.69c |
| 5 | 7.04 ± 3.49b | 14.86 ± 1.35cd | 91.37 ± 36.83c | 186.23 ± 55.52d | 320.57 ± 103.95c | 28.56 ± 8.12bc | |
| 10 | 20.56 ± 10.81a | 21.52 ± 10.11c | 132.00 ± 43.21c | 268.70 ± 51.52d | 774.47 ± 166.41bc | 35.42 ± 11.92b | |
| 15 | 33.43 ± 11.65a | 41.33 ± 2.68b | 327.47 ± 27.81b | 814.87 ± 31.14b | 1795.13 ± 38.26a | 74.75 ± 12.71a | |
| 20 | 5.47 ± 2.04b | 20.55 ± 1.69c | 156.23 ± 43.35c | 426.40 ± 76.69c | 1221.30 ± 532.15b | 26.02 ± 3.22bc | |
| 30 | 4.90 ± 3.55b | 17.73 ± 6.39c | 90.90 ± 10.09c | 203.23 ± 31.58d | 499.40 ± 38.34cd | 22.63 ± 9.43bc | |
| Fresh sperm | 30.33 ± 1.07a | 50.37 ± 0.31a | 450.23 ± 105.85a | 1114.57 ± 156.67a | 1919.90 ± 367.52a | 80.70 ± 1.37a | |
The highest sperm parameters were obtained using 15% DMSO in ELRS3, and relatively high average values were obtained for sperm parameters using 15% DMSO in ELS3 (n = 3, P < 0.05). Values are means ± standard deviation, and means with different letters (a, b, c, d, e) are significantly different (P < 0.05).
Based on the screened DMSO concentration, FBS was added to ELS3 and ELRS3 at four different final concentrations, 5%, 10%, 15% and 20% (v/v), to determine the optimal FBS concentration. The methods of cryopreservation and thawing and the measures of sperm physiology and viability parameters were the same as above (Table 4).
Table 4. Post-thaw sperm physiology and viability parameters for the giant grouper (E. lanceolatus) with different concentrations of FBS in ELS3 (15% DMSO) and ELRS3 (15% DMSO).
| Sperm diluents | Concentration of FBS (%) |
Ratio of fast motion (%) |
Ratio of slow motion (%) |
Time of fast motion (sec) |
Time of slow motion (sec) |
Lifespan (sec) | Motility (%) |
| ELS3 | 0 | 1.94 ± 0.99c | 20.20 ± 3.49b | 144.20 ± 75.37b | 227.13 ± 53.92c | 663.53 ± 166.47c | 22.14 ± 4.44c |
| 5 | 3.78 ± 1.67bc | 22.75 ± 6.17b | 189.13 ± 78.02b | 315.17 ± 13.71bc | 823.47 ± 196.10c | 26.53 ± 7.83bc | |
| 10 | 29.68 ± 4.17a | 41.49 ± 5.39a | 309.63 ± 31.60ab | 432.70 ± 51.75b | 1319.83 ± 71.91b | 71.16 ± 1.34a | |
| 15 | 10.40 ± 5.23b | 21.83 ± 5.87b | 310.20 ± 153.45ab | 305.10 ± 99.78bc | 825.30 ± 42.80c | 32.22 ± 5.25bc | |
| 20 | 10.57 ± 3.24b | 29.03 ± 8.07b | 126.47 ± 22.76b | 168.87 ± 47.90c | 894.67 ± 106.06c | 39.60 ± 11.29b | |
| ELRS3 | 0 | 4.29 ± 2.63c | 18.99 ± 5.90b | 66.97 ± 30.92c | 227.17 ± 61.07b | 476.93 ± 58.77c | 23.28 ± 3.89d |
| 5 | 6.14 ± 3.15c | 25.85 ± 7.46b | 133.53 ± 54.56bc | 270.50 ± 93.71b | 525.00 ± 189.90c | 31.99 ± 9.97c | |
| 10 | 20.59 ± 2.47b | 43.09 ± 6.51a | 239.83 ± 47.05b | 354.70 ± 58.07b | 1080.67 ± 53.01b | 63.68 ± 4.16b | |
| 15 | 7.25 ± 3.35c | 18.95 ± 1.89b | 132.77 ± 33.97bc | 245.43 ± 36.18b | 573.77 ± 85.64c | 26.20 ± 4.79d | |
| 20 | 10.35 ± 3.61c | 21.74 ± 6.25b | 148.47 ± 52.90bc | 327.90 ± 73.91b | 511.27 ± 13.57c | 32.09 ± 4.63c | |
| Fresh sperm | 30.33 ± 1.07a | 50.37 ± 0.31a | 450.23 ± 105.85a | 1114.57 ± 156.67a | 1919.90 ± 367.52a | 80.70 ± 1.37a | |
The highest sperm parameters were obtained for 10% FBS in ELS3 and ELRS3 (n = 3, P < 0.05). Values are means ± standard deviation, and means with different letters (a, b, c, d) are significant.
Morphology observation
Sperm were cryopreserved with ELRS3 + 15% DMSO + 10% FBS and thawed in a water bath at 38 C. For scanning electron microscopy, the frozen-thawed sperm were fixed with 2.5% glutaraldehyde and then 1% osmium tetroxide, dehydrated with graded ethanol and dried with dry ice. Observation was carried out under a JEOL JSM 840 (JOEL, Tokyo, Japan) scanning electron microscope. For transmission electron microscopy, the frozen sperm was fixed carefully in 2.5% glutaraldehyde with seawater after centrifuging. The fixed sperm sample was washed consecutively with 0.1 M phosphate buffer saline (PBS) and then fixed in 1% osmium tetroxide. When it appeared entirely black, it was washed 2–3 times with 0.1 M PBS, and it was then kept in the PBS overnight at 4 C. Subsequently, the sample was treated with graded acetone (30, 50, 70, 80, 90 and 100% acetone respectively) to make a sample for dehydration. After that, the sample was infiltrated with different concentrations of EPON812 resin (acetone:EPON812 resin ratio of 2:1 and 1:2 and 100% EPON812, respectively). Then it was embedded with EPON812 resin and cut with an LKB slicer. After sectioning, the sperm slices were stained with sodium acetate and lead citrate. Then we observed the sperm slices under a JEOL JEM-1200EX transmission electron microscope [15, 23].
Cryopreservation of a large number of giant grouper sperm, hybridization and fertilization
Spermatozoa of giant grouper from Hainan Blue Ocean Science and Technology Breeding Company were cryopreserved with ELRS3 + 15% DMSO + 10% FBS and ELS3 + 15% DMSO + 10% FBS, shipped to Shandong Laizhou MingBo Aquatic Products and maintained in a LN container. The total volume of cryopreserved sperm from the two companies was 105 ml. Thirty red-spotted groupers were cultivated in a pond (30 l3; S, 28‰; T, 22 C; DO, 7–8 mg/l). Female red-spotted groupers were allowed to mature naturally, and then ovulation was induced. Fresh eggs were collected from the female red-spotted groupers by artificial extrusion during the spawning season. In total, 3800 ml of red-spotted grouper eggs were fertilized with frozen sperm from the giant grouper at a ratio of 1:200 (v/v) by dry fertilization. The final concentration of sperm was 4.6 × 109 cells/ml. As a positive control, 0.5 ml of fresh sperm from a red-spotted grouper was used to fertilize 200 ml of red-spotted grouper eggs 3 times. The fertilized eggs were poured into a nylon net and washed with equal amounts of seawater to remove dead fertilized eggs and mucus. Fertilized eggs were hatched in 22 C seawater. The fertilization rate was calculated as the percentage of fertilized embryos recorded at the blastula stage (5 h post fertilization) under a microscope. The hatching rate was calculated as the percentage of hatched larval fish recorded during the embryoid bodies’ rotation period (33 h post fertilization).
Statistical analyses
All data were analyzed by one-way analysis of variance (ANOVA) using the SPSS 11.5 software. A Student-Newman-Keuls (SNK) test was used to analyze differences. All sperm motility data are presented as means ± SD, and differences are represented by the letter identification method (a, b, c, d, e, f and g). The significance level was P < 0.05.
Results
Selection of the appropriate sperm cryopreserving diluents
To screen appropriate sperm cryoprotective diluents, several types of cryoprotective diluents, including ELS1-3, EM1-2, TS-2, MPRS and ELRS0-6, were used with 10% DMSO and 10% FBS to cryopreserve sperm (Table 2). The thawed sperm ratio of fast motion, ratio of slow motion, time of slow motion and motility were significantly highest in ELRS3 (P < 0.05). Excluding the ratio of slow motion, the parameters of ELRS3 were significantly lower than those of fresh sperm (P < 0.05). The thawed sperm ratio of slow motion, time of slow motion and motility were highest in ELS3 among the various diluents (P < 0.05), although they were significantly lower than those of fresh sperm (P < 0.05). This indicates that sperm in ELRS3 and ELS3 behaved better than other solutions with regard to all motion-related parameters (Table 2). The osmolarity and pH values of ELRS3 and ELS3 were 400.00 mOsm/l and 409.34 mOsm/l and 7.03 and 7.10, respectively (Table 1).
Selection of appropriate DMSO concentration
On the basis of 10% FBS six different concentrations of DMSO were added to ELS3 and ELRS3 (Table 3). The significantly highest ratio of fast motion, ratio of slow motion, time of fast motion, time of slow motion, lifespan and motility were obtained with 15% DMSO in ELRS3 (P < 0.05). The ratio of fast motion, lifespan and motility showed no significant difference compared with the fresh sperm (P > 0.05). The significantly highest sperm ratio of fast motion, ratio of slow motion, time of fast motion, lifespan and motility were obtained with 15% DMSO, 10–20% DMSO, 15–20% DMSO, 15–20% DMSO and 10–20% DMSO, respectively, in ELS3 compared with the other concentrations (P < 0.05). In term of the motion parameters, the sperm performance with 15% DMSO was better compared with the other concentrations (Table 3).
Selection of the appropriate FBS concentration
To select the appropriate concentration of FBS, four concentrations of FBS were added to ELS3 + 15% DMSO and ELRS3 + 15% DMSO (Table 4). The sperm ratio of fast motion, ratio of slow motion, lifespan and motility were significantly highest for 10% FBS in ELS3 and ELRS3 (P < 0.05). The time of fast motion and time of slow motion with 0–20% FBS showed no significant difference (P > 0.05). But the average sperm motility parameters with 10% FBS were higher than those for the other concentrations. The ratio of fast motion, ratio of slow motion and motility with 10% FBS in ELS3 showed no significant difference compared with fresh sperm (P > 0.05), while the parameters for the other concentrations were less than those of fresh sperm (P < 0.05) (Table. 4).
Observation of sperm morphology
The transmission electron microscope observations revealed that, for the majority of frozen-thawed sperm, their nucleus (n), plasma membrane (pm), flagellum (f), mitochondria (m) and basal body (bb) structure remained intact, and typical “9 + 2” axonemal structures (nine peripheral and one central pair of microtubules) of the flagellum (f) could be distinctly observed (Fig. 1, I–III). On the other hand, the morphological abnormalities were observed only in a small number of frozen-thawed sperm, with the plasma membrane shrunken, mitochondria of internal structure unclear and the nucleus dispersed and expanded (Fig. 1, IV). Scanning electron microscopic observation showed that the sperm head, midpiece and tail remained intact in most of the frozen-thawed sperm (Fig. 1, V). For a few frozen-thawed sperm, different degrees of abnormalities were observed, such as a dehydrated head and midpiece, rough plasma membrane surface, or detached midpiece or tail (Fig. 1, VI).
Fig. 1.

Transmission electron microscopic photographs (I, II, III and IV) and scanning electron microscopic photographs of frozen-thawed giant grouper sperm (V and VI). I, II and III: Ultrastructure of a normal frozen-thawed sperm with a clear nucleolus; the ultrastructure of the sperm membrane, mitochondria, flagellum membrane is intact, and typical “9 + 2” axoneme structures are present. IV: Ultrastructure of a frozen-thawed sperm with cryodamage and a dissolved nucleolus; the membrane of the sperm is shrunken and fractured, and the internal ultrastructure of the mitochondria is not clear. V: A frozen-thawed sperm with an intact structure. In IV, 1, 2 and 3 represent sperm with different degrees of cryodamage, respectively. The sperm membrane shrank, and the flagellum fell out. A = axonemal; m = mitochondria; f = flagellum; n = nucleus; pm = plasma membrane; h = sperm head; bb = basal body; t = tail; md = midpiece.
Cryopreservation of a large number of giant grouper sperm, fertilization rates and hatchability
A total of 105 ml of spermatozoa from giant grouper was cryopreserved using the ELRS3 + 15% DMSO + 10% FBS and ELS3 + 15% DMSO + 10% FBS cryoprotectants at Shandong Laizhou MingBo Aquatic Products. Sperm motility reached 63.68 ± 4.16% to 74.75 ± 12.71%, so a giant grouper sperm bank was developed with the above cryoprotectants.
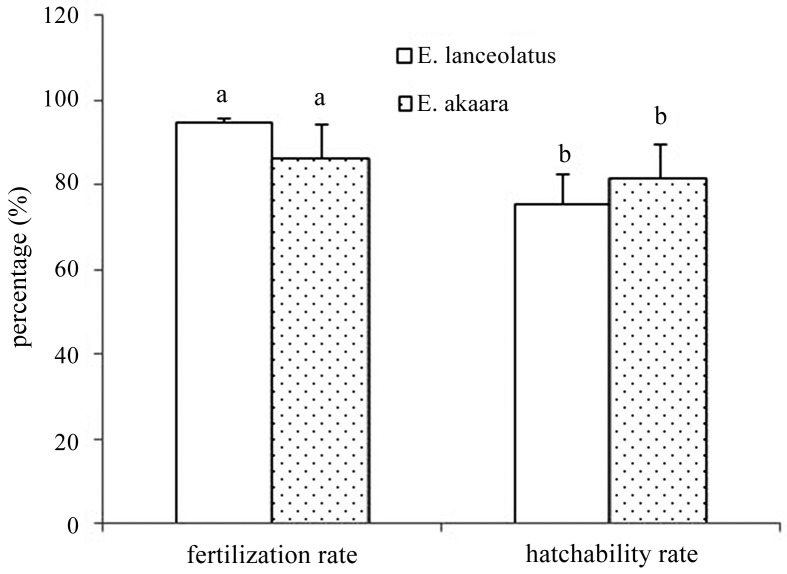
The fertilization and hatching rates of red-spotted grouper eggs and frozen giant grouper sperm were as high as 94.56 ± 1.03% and 75.56 ± 6.94% at the blastula stage, respectively. For red-spotted grouper eggs fertilized with fresh conspecific sperm, the fertilization and hatching rates were 86.44 ± 7.72% and 81.54 ± 8.34%, respectively (Fig. 2). There was no significant difference in fertilization and hatching rates between the frozen giant grouper sperm and fresh red-spotted grouper sperm (P > 0.05). Approximately 800,000 surviving hybrid fry were obtained with normal growth and development (Fig. 3).
Fig. 2.
Fertilization rate and hatchability rate of red-spotted grouper (E. akaara) eggs fertilized with frozen sperm of E. lanceolatus and fresh sperm from red-spotted grouper. There was no significant difference in rates between frozen sperm of the giant grouper and fresh sperm of the red-spotted grouper (P >0.05). Giant grouper sperm cryopreservation using ELRS3 + 15% DMSO + 10% FBS. Values are means ± standard deviation, and means with different letters (a, b) are significantly different (P >0.05).
Fig. 3.

Hybrid fish (E. akaara♀ × E. lanceolatus♂ (cryopreserved sperm)) at 1 day post hatching.
Discussion
It is necessary to screen sperm diluents for different fish sperm cryopreservation solutions due to differences in physiological characteristics, including coelomic fluid component, osmotic pressure and pH. The MPRS, TS-2 and EM1-2 diluents have been proven to be effective for cryopreservation of sperm of the sea perch (Lateolabrax japonicas) [15], kelp grouper (Epinephelus moara) [20], Japanese flounder (Paralichthys olivaceus) [21], turbot (Scophthalmus maximus) [22], and spotted halibut (Verasper variegatus) [24]. The diluents, MPRS, TS-2, T-2 and ES1-3, could be useful for cyroprotection of giant grouper sperm, but the survival rate was too low to meet the requirements for application. In the present study, ELS3 and ELRS3 were screened to successfully cryopreserve spermatozoa of the giant grouper. Also, our experiment indicates that glucose and FBS have important effects on the cryopreservation of spermatozoa, as shown in studies of canine sperm cryopreservation [25, 26].
Due to their function in reducing solution toxicity and buffering osmotic pressure, FBS and bovine serum albumin (BSA) have been often used for sperm cryopreservation [14, 27]. In the present study, 10% FBS, in either ELS3 or ELRS3, could restrain sperm motility before cryopreservation, reduce the toxicity of environmental solutions and cryoprotectants and improve the motility of frozen sperm.
The appropriate concentration of a cryoprotectant, such as DMSO, plays an important role in maintaining the sperm osmotic pressure, protecting sperm cell membrane structures and preventing cell frostbite [10] at a concentration of 5% to 20% [12, 15, 21, 28]. In the present study, 15% DMSO was found to be the optimal concentration for the cryopreservation of sperm from the giant grouper.
Selection of the appropriate osmotic pressure is also one of the key problems for sperm cryopreservation in the giant grouper, as it has been shown in marine fish sperm that a hypertonic solution can activate the motility of sperm [29, 30]. Our study indicates that glucose plays important roles in regulating the osmotic pressure and providing a rich store of sperm energy, which is similar to results reported in mammals [31, 32].
The sperm head and mitochondria are prone to cryodamage during cryopreservation and thawing due to their relatively large sizes [33,34,35,36]. In the present study, an incomplete and deformed sperm plasma membrane, shrunken sperm head and the fractured mitochondria of the midpiece and tail were observed in a small number of frozen-thawed sperm. These morphological abnormalities were likely caused by cryopreservation, dysfunctional spermatogenesis or even the experimental process [34].
The breeding time of the giant grouper in South China and the red-spotted grouper in the East China Sea are primarily summer (28–31 C) [4] and spring (22–23 C), respectively. The genetic basis for hybridization in their same karyotype (2n = 48) [37]. In the future, sperm cryopreservation technology for the giant grouper may make crossbreeding possible and break through the reproductive isolation caused by temporal and geographic isolation.
In conclusion, the appropriate concentrations of glucose, FBS and DMSO for cryopreservation of giant grouper sperm were determined, with ELS3 and ELRS3 selected as the optimal diluents. A large number of giant grouper spermatozoa were cryopreserved and used for crossbreeding with the red-spotted grouper. This study not only established a successful method of sperm cryopreservation for the giant grouper but also supplied a technological approach for crossbreeding groupers experiencing reproductive isolation.
Acknowledgments
This work was supported by grants from the State 863 High-Technology R&D Project of China (2012AA10A408, 2012AA10A402), the National Natural Science Foundation of China (31372510) and the Taishan Scholar Project Fund of Shan-dong Province.
References
- 1.Guo CY, Hang YH, Wei SN, Ouyang ZL, Yan Y, Huang XH, Qin QW. Establishment of a new cell line from the heart of giant grouper, Epinephelus lanceolatus (Bloch), and its application in toxicology and virus susceptibility. J Fisn Dis 2013; doi: . [DOI] [PubMed] [Google Scholar]
- 2.Meng QW, Su JX, Miao XZ. Fishes Taxonomy. Beijing: China Agriculture Press; 1995: 606–622 (In Chinese). [Google Scholar]
- 3.Zeng HS, Ding SX, Wang J, Su YQ. Characterization of eight polymorphic microsatellite loci for the giant grouper (Epinephelus lanceolatus Bloch). Mol Ecol Resour 2008; 8: 805–807. [DOI] [PubMed] [Google Scholar]
- 4.Zhang HF, Wang YX, Liufu YZ, Huang GG, Ou CH, Huang PW, Liang WF. Studies on artificial propagation and embryonic development of Epinephelus lanceolatus. J Guangdong Ocean Univ 2008; 28: 36–41. (In Chinese with English abstract). [Google Scholar]
- 5.Sadovy Y, Donaldson TJ, Graham TR, McGilvray F, Muldoon GJ, Phillips MJ, Rimmer MA, Smith A, Yeeting B. While Stocks Last The Live Reef Food Fish Trade. Manila: Asian Development Bank; 2003: 147. [Google Scholar]
- 6.Yang SS. Orange-spotted Grouper (Epinephelus coioids) ♀×Giant Grouper, Epinephelus lanceolatus ♂ and the genetic analysis of F1. Ph.D. thesis. South China Normal University, Guangzhou, China. 2010. (In Chinese with English abstract).
- 7.Huang ZW, Luo J, Lin B, Guo RX, Yang W, Chen GH. Industrial seed culture of Epinephelus lanceolatus. Mark Sci 2010; 34: 23–30. (In Chinese with English abstract). [Google Scholar]
- 8.Yang S, Wang L, Zhang Y, Liu XC, Lin HR, Meng ZN. Development and characterization of 32 microsatellite loci in the giant grouper Epinephelus lanceolatus (Serranidae). Genet Mol Res 2011; 10: 4006–4011. [DOI] [PubMed] [Google Scholar]
- 9.Liang WF, Zhang HF, Wang YX, Huang GG, Huang PW, Shen NN. Effect of several factors motility of sperm of Eponephelus lanceolatus. J Guangdong Ocean Univ 2009; 29: 29–33. (In Chinese with English abstract). [Google Scholar]
- 10.Suquet M, Dreanno C, Fauvel C, Cosson J, Billard R. Cryopreservation of sperm in marine fish. Aquacult Res 2000; 31: 231–243. [Google Scholar]
- 11.Chao NH, Tsai HP, Liao IC. Short and long-term cryopreservation and sperm suspension of the grouper, Epinephelus malabaricus. Asian Fish Sci 1992; 5: 103–116. [Google Scholar]
- 12.Gwo JC. Cryopreservation of black grouper Epinephelus malabaricus spermatozoa. Theriogenology 1993; 39: 1331–1342. [Google Scholar]
- 13.Miyaki K, Nakano S, Ohta H, Kurokura H. Cryopreservation of kelp grouper Epinephelus moara sperm using only a trehalose solution. Fish Sci 2005; 71: 457–458. [Google Scholar]
- 14.Koh ICC, Yokoi K, Tsuji M, Tsuchihashi Y, Ohta H. Cryopreservation of sperm from seven-band grouper, Epinephelus septemfasciatus. Cryobiology 2010; 61: 263–267. [DOI] [PubMed] [Google Scholar]
- 15.Ji XS, Chen SL, Tian YS, Yu GC, Sha ZX, Xu MY, Zhang SC. Cryopreservation of sea perch (Lateolabrax japonicus) spermatozoa and feasibility for production-scale fertilization. Aquaculture 2004; 241: 517–528. [Google Scholar]
- 16.Li C, Li J, Xue QZ. Cryopreservation of spermatozoa of red sea bream pagrosomus major. Mark Sci 2001; 25: 6–8. [Google Scholar]
- 17.Bart AN. New approaches in cryopreservation of fish embryos. In: Tierch, TR, Mazik, PM (eds.), Cryopreservation in Aquatic Species. Baton Rouge: World Aquacu Soc; 2000; 179–187.
- 18.Chen SL, Tian YS, Yang JF, Shao CW, Ji XS, Zhai JM, Liao XL, Zhuang ZM, Su PZ, Xu JY, Sha ZX, Wu PF, Wang N. Artificial gynogenesis and sex determination in half-smooth tongue sole (Cynoglossus semilaevis). Mar Biotechnol (NY) 2009; 11: 243–251. [DOI] [PubMed] [Google Scholar]
- 19.Gwo JC. Cryopreservation of aquatic invertebrate semen: a review. Aquacult Res 2000; 31: 259–271. [Google Scholar]
- 20.Qi WS, Jiang J, Tian YS, Zhai JM, Chen C, Li B, Chen SL. Sperm cryopreservation of kelp grouper Epinephelus moara. Progress in Fishery Sciences 2014; 35: 26–34. (In Chinese with English abstract). [Google Scholar]
- 21.Ji XS, Chen SL, Zhao Y, Deng H. Cryopreservation of stone flounder and Japanese flounder sperms and its application to the breeding. Mar Fish Res 2005; 26: 13–17. (In Chinese with English abstract). [Google Scholar]
- 22.Chen SL, Ji XS, Yu GC, Tian YS, Sha ZX. Cryopreservation of sperm from turbot (Scophthalmus maximus) and application to large-scale fertilization. Aquaculture 2004; 236: 547–556. [Google Scholar]
- 23.Massar B, Dey S, Dutta K. An electron microscopic analysis on the ultra structural abnormalities in sperm of the common carp Cyprinus carpio L. inhabiting a polluted lake, Umiam (Meghalaya, India). Microsc Res Tech 2011; 74: 998–1005. [DOI] [PubMed] [Google Scholar]
- 24.Tian YS, Chen SL, Ji XS, Zhai JM, Sun LJ, Chen C, Su PZ. Cryopreservation of spotted halibut (Verasper variegatus) sperm. Aquaculture 2008; 284: 268–271. [Google Scholar]
- 25.Rigau T, Rivera M, Palomo MJ, Fernández-Novell JM, Mogas T, Ballester J, Peña A, Otaegui PJ, Guinovart JJ, Rodríguez-Gil JE. Differential effects of glucose and fructose on hexose metabolism in dog spermatozoa. Reproduction 2002; 123: 579–591. [PubMed] [Google Scholar]
- 26.Ponglowhapan S, Essén-Gustavsson B, Linde Forsberg C. Influence of glucose and fructose in the extender during long-term storage of chilled canine semen. Theriogenology 2004; 62: 1498–1517. [DOI] [PubMed] [Google Scholar]
- 27.Cabrita E, Engrola S, Conceição LEC, Pousão-Ferreirac P, Dinisb MT. Successful cryopreservation of sperm from sex-reversed dusky grouper, Epinephelus marginatus. Aquaculture 2009; 287: 152–157. [Google Scholar]
- 28.Imaizumi H, Hotta T, Ota H. Cryopreservation of kelp grouper Epinephelus bruneus sperm and comparison of fertility of fresh and cryopreserved sperm. Suisan Zoshoku 2005; 53: 405–411. [Google Scholar]
- 29.Morisawa M, Suzuki K. Osmolality and potassium ion: their roles in initiation of sperm motility in teleosts. Science 1980; 210: 1145–1147. [DOI] [PubMed] [Google Scholar]
- 30.Oda S, Morisawa M. Rises of intracellular Ca2+ and pH mediate the initiation of sperm motility by hyperosmolality in marine teleosts. Cell Motil Cytoskeleton 1993; 25: 171–178. [DOI] [PubMed] [Google Scholar]
- 31.Reyes-Moreno C, Gagnon A, Sullivan R, Sirard MA. Addition of specific metabolites to bovine epididymal cell culture medium enhances survival and motility of cryopreserved sperm. J Androl 2000; 21: 876–886. [PubMed] [Google Scholar]
- 32.Watson PF. The preservation of semen in mammals. In: Finn CA (ed.), Oxford Rev Rep Biol, vol. 1. Oxford: Clarendon Press; 1979: 283–350.
- 33.Taddei AR, Barbato F, Abelli L, Canese S, Moretti F, Rana KJ, Fausto AM, Mazzini M. Is cryopreservation a homogeneous process—Ultrastructure and motility of untreated, prefreezing, and postthawed spermatozoa of Diplodus puntazzo (Cetti). Cryobiology 2001; 42: 244–255. [DOI] [PubMed] [Google Scholar]
- 34.He S, Woods LC., 3rd Effects of dimethyl sulfoxide and glycine on cryopreservation induced damage of plasma membranes and mitochondria to striped bass (Morone saxatilis) sperm. Cryobiology 2004; 48: 254–262. [DOI] [PubMed] [Google Scholar]
- 35.Lahnsteiner F, Weismann T, Patzner RA. Fine structural changes in spermatozoa of the grayling, Thymallus thymallus (Pisces: Teleostei), during routine cryopreservation. Aquaculture 1992; 103: 73–84. [Google Scholar]
- 36.Gwo JC, Arnold CR. Cryopreservation of Atlantic croaker spermatozoa: evaluation of morphological changes. J Exp Zool 1992; 264: 444–453. [DOI] [PubMed] [Google Scholar]
- 37.Shu H, Wei QL, Luo LJ, Cai XY, Cai WG, Zhang HF. Karyotypes analysis of four grouper fishes from coastal waters of Guangdong. Guangdong Agricult Sci 2012; 39: 124–127. (In Chinese with English abstract). [Google Scholar]