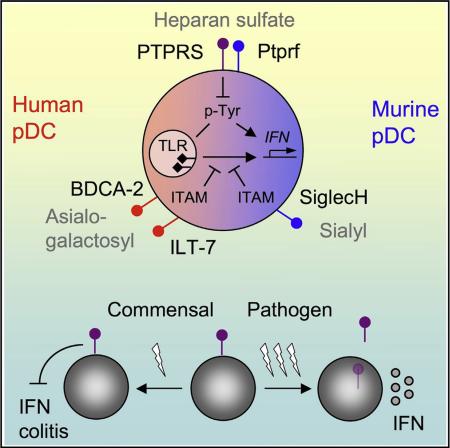
SUMMARY
Plasmacytoid dendritic cells (pDCs) are primary producers of type I interferon (IFN) in response to viruses. The IFN-producing capacity of pDCs is regulated by specific inhibitory receptors, yet none of the known receptors are conserved in evolution. We report that within the human immune system, receptor protein tyrosine phosphatase sigma (PTPRS) is expressed specifically on pDCs. Surface PTPRS was rapidly downregulated after pDC activation, and only PTPRS– pDCs produced IFN-α. Antibody-mediated PTPRS crosslinking inhibited pDC activation, whereas PTPRS knockdown enhanced IFN response in a pDC cell line. Similarly, murine Ptprs and the homologous receptor phosphatase Ptprf were specifically co-expressed in murine pDCs. Haplodeficiency or DC-specific deletion of Ptprs on Ptprf-deficient background were associated with enhanced IFN response of pDCs, leukocyte infiltration in the intestine and mild colitis. Thus, PTPRS represents an evolutionarily conserved pDC-specific inhibitory receptor, and is required to prevent spontaneous IFN production and immune-mediated intestinal inflammation.
Graphical Abstract
INTRODUCTION
Plasmacytoid dendritic cells (pDCs) represent a distinct innate immune cell type whose function, phenotype, and core gene expression program are conserved across mammalian species (Colonna et al., 2004; Liu, 2005). Despite their lymphoid morphology, pDCs are closely related to classical DCs (cDCs) based on their common progenitors, expression profile, and sentinel function in immunity (Merad et al., 2013; Mildner and Jung, 2014). pDCs express endosomal Toll-like receptors TLR7 and TLR9 that recognize their respective nucleic acid ligands single-stranded RNA and unmethylated CpG-containing DNA (CpG). pDCs respond to these stimuli with rapid and abundant secretion of type I interferon (interferon α or β, IFN), producing up to 1,000-fold more IFN than other cell types. This unique IFN-producing capacity of pDCs is important for the control of viral infections, e.g., by facilitating virus-specific T cell responses (Cervantes-Barragan et al., 2012; Swiecki et al., 2010). Conversely, aberrant hyperactivation of pDCs has been proposed as a common effector mechanism in several autoimmune diseases (Ganguly et al., 2013). Thus, IFN production by pDCs is a powerful immune response that must be tightly regulated to maintain immune homeostasis.
The pDCs possess multiple adaptations for their IFN secreting capacity, including secretory plasma cell-like morphology; baseline expression of IFN gene “master regulator” IRF7; the recognition of TLR ligands in early endosomes, facilitated by the AP-3 adaptor complex (Blasius et al., 2010; Sasai et al., 2010); and pDC-specific membrane adaptor molecules such as Pacsin1 (Esashi et al., 2012). On the other hand, the potentially dangerous IFN production by pDCs is restricted by a unique set of pDC-specific receptors (Gilliet et al., 2008). Human pDCs express several specific receptors including BDCA-2 (CD303) and ILT7 (CD85 g), and their ligation by antibodies inhibits pDC function (Cao et al., 2006; Dzionek et al., 2001). ILT7 recognizes Bst2, an IFN-inducible protein that sends a negative feedback signal to IFN-producing pDCs (Cao et al., 2009). In mice, SiglecH is preferentially expressed on pDCs and inhibits IFN production upon antibody-mediated crosslinking (Blasius et al., 2006). All these receptors signal through ITAM-containing adaptor proteins and activate an Src kinase-dependent pathway, which inhibits IFN production by pDCs through unknown mechanisms. Furthermore, the role of these inhibitory receptors in pDC function and immune homeostasis in vivo is still poorly understood. Strikingly, all known pDC-specific inhibitory receptors are unique to their respective species: thus, BDCA-2 and ILT7 have no murine orthologs, whereas SiglecH has no human ortholog. Given the similar function and expression profile of murine and human pDCs, additional conserved receptors would be expected to control pDC function in both species.
Receptor-type protein tyrosine phosphatases are widely expressed on immune cells and often restrict their activation (Rhee and Veillette, 2012). A distinct subfamily of leukocyte common antigen-related (LAR) receptor-type phosphatases is composed of three homologous receptors: LAR (Ptprf), sigma (Ptprs), and delta (Ptprd). Ptprd is brain-specific, whereas Ptprf and Ptprs are expressed more broadly and regulate the development of mammary gland and brain, respectively. Ptprf and Ptprs show partial genetic redundancy in certain murine tissues such as the developing genitourinary tract (Uetani et al., 2009). Expression of Ptprf was reported on immature thymocytes (Kondo et al., 2010; Terszowski et al., 2001); however, Ptprf is entirely dispensable for T cell development and function (Terszowski et al., 2001). The expression or function of Ptprs in the immune system has not been explored. Notably, polymorphisms in the human PTPRS gene have been associated with ulcerative colitis, and the few surviving Ptprs-deficient mice on mixed genetic background develop mild colitis (Muise et al., 2007). This was ascribed to the putative function of Ptprs in the intestinal epithelial barrier (Muise et al., 2007; Murchie et al., 2014), although the colitis’ potential origins within the epithelial or hematopoietic compartment have not been investigated.
Here we report that Ptprs is expressed specifically on pDCs in both human and murine immune systems, whereas Ptprf is similarly pDC-specific in murine immune cells. The expression of PTPRS was inversely correlated with pDC activation, and its crosslinking inhibited cytokine production by pDCs. The reduction of Ptprs and Ptprf in mice enhanced IFN production by pDCs and caused mild intestinal inflammation. These results identify Ptprs as an evolutionarily conserved inhibitory receptor on pDCs and suggest that constitutive pDC hyperactivation might disrupt immune homeostasis in barrier tissues.
RESULTS
PTPRS Is Specifically Expressed on pDCs among Human Immune Cells
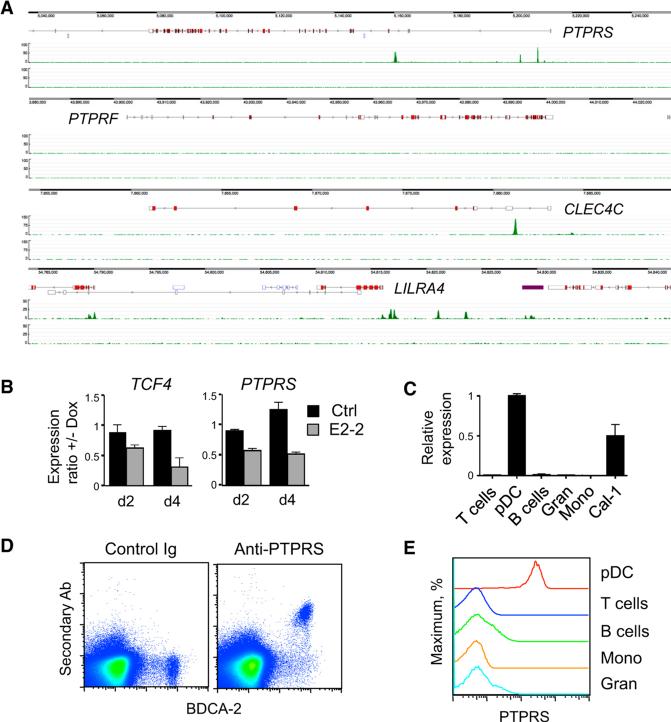
Transcription factor E2-2 (TCF4) controls pDC development and functionality in both mice and humans and specifies a conserved pDC-specific gene-expression program (Cisse et al., 2008). The analysis of E2-2 chromatin targets in human pDCs (Ghosh et al., 2014) revealed prominent binding of E2-2 near the promoter and within the first intron of PTPRS (Figure 1A). As expected, the bound regions contained multiple consensus E boxes (Figure S1A). Similar binding was observed in the CLEC4C and LILRA4 genes encoding BDCA-2 and ILT-7, respectively, but not in the homologous PTPRF gene (Figure 1A). Doxycycline (Dox)-inducible knockdown of E2-2 by short interfering RNAs (shRNA) (Sawai et al., 2013) reduced PTPRS expression in human pDC cell lines Gen2.2 (Figure 1B) and CAL-1 (data not shown), suggesting that the expression of PTPRS is E2-2-dependent. Among major cell types in the human peripheral blood, only pDCs expressed PTPRS transcript (Figure 1C). The expression atlas of human immune cells showed the predominant expression of PTPRS in pDCs, similar to CLEC4C and LILRA4 (Figure S1B). Low expression of all three genes was also apparent in the CD141+ cDCs. These data also reveal that homologous LAR phosphatases PTPRF and PTPRD are not expressed in human immune cells. Deep sequencing of the human transcriptome by the FANTOM5 consortium revealed that the expression of PTPRS in pDCs is among the highest in the body, equaling or exceeding the brain (Figure S1C). These studies also defined potential enhancers in the first intron of PTPRS; notably, these enhancers appear active exclusively in pDCs and overlap with the regions of E2-2 binding (Figure S1D).
Figure 1. PTPRS Is Expressed Specifically in pDCs within the Human Immune System.
(A) The binding of E2-2 to PTPRS locus in the human pDC cell line CAL-1 as determined by ChIP-seq. Shown are enrichment peaks of E2-2-associated chromatin (top track) and total chromatin input (bottom track) across the indicated loci.
(B) The expression of PTPRS after E2-2 knockdown in the human pDC cell line Gen2.2. Cells were treated with Dox to induce the expression of shRNA specific for E2-2-encoding TCF4 gene or a scrambled control (Ctrl) shRNA, and the expression of TCF4 and PTPRS was measured by qRTPCR on days 2 and 4. Shown is the ratio of expression levels with or without Dox (means ± SD of triplicate PCR reactions); representative of two independent experiments with two TCF4-specific shRNAs.
(C) The expression of PTPRS in primary human peripheral blood cells as determined by qRT-PCR (mean ± SD of triplicate reactions). CAL-1 cells were included as a positive control.
(D and E) Cell surface expression of PTPRS on normal human PBMC. Cells were stained with control IgG or a polyclonal antibody to human PTPRS, followed by secondary fluorescent antibody and primary antibodies to cell surface markers. Shown is a representative staining profile of PBMC stained for pDC marker BDCA-2 (D) and PTPRS staining profiles in gated cell types including BDCA2+ CD123hi pDCs, T and B lymphocytes, monocytes (Mono), and granulocytes (Gran) (E). Similar results were obtained with PBMC from multiple donors and with commercial buffy coat samples.
We stained human peripheral blood mononuclear cells (PBMC) with a polyclonal antibody against the extracellular domain of PTPRS. Surface staining for PTPRS was restricted to BDCA-2+ pDCs, and only pDCs, but not other cell types, showed homogeneous PTPRS expression (Figures 1D and 1E). These data demonstrate that within the human immune system, PTPRS is expressed specifically in the pDC lineage.
PTPRS Inversely Correlates with and Inhibits the Activation of Human pDCs
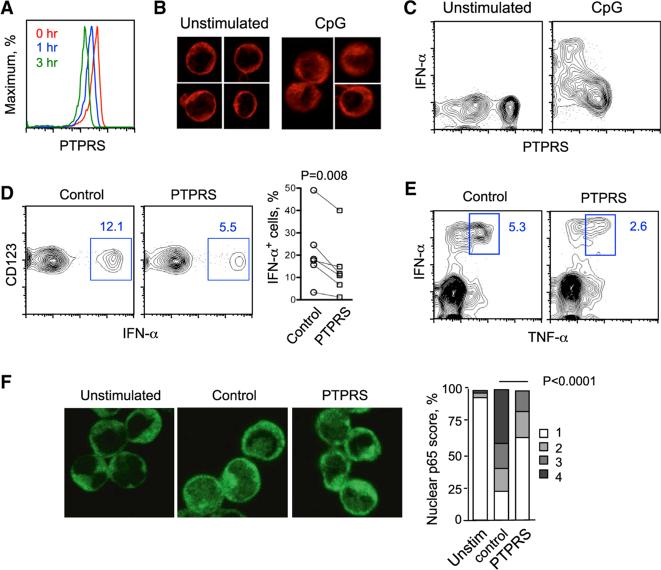
Following the culture of PBMC with TLR9 ligand CpG, the surface expression of PTPRS on pDCs was progressively reduced within several hours (Figure 2A). Immunofluorescent staining for PTPRS revealed diffuse intracellular signal in activated pDCs compared to intense membrane signal in naive pDCs (Figure 2B), suggesting that PTPRS is internalized after activation. In addition, the propensity of LAR phosphatases to undergo activation-induced shedding of their ectodomains (Aicher et al., 1997; Ruhe et al., 2006) is also likely to contribute to the rapid loss of PTPRS. After overnight culture, a sizable fraction of pDCs lost surface PTPRS expression even in medium alone, whereas all pDCs downregulated PTPRS after activation with CpG (Figure 2C). Notably, intracellular staining for IFN-α revealed that only pDCs with the lowest PTPRS surface expression were producing the cytokine (Figure 2C).
Figure 2. PTPRS Inversely Correlates with and Inhibits the Activation of Human pDCs.
(A) The dynamics of PTPRS expresion in primary human pDCs upon activation. Total PBMCs were cultured for 0–3 hr with CpG and stained for cell surface markers and PTPRS. Shown are staining profiles of gated pDCs at the indicated time points (representative of three experiments).
(B) Intracellular distribution of PTPRS in human pDCs upon activation. The pDCs were enriched from PBMC, cultured with or without CpG for 4 hr, fixed, stained for PTPRS and analyzed by immunofluorescence microscopy. Representative of two experiments.
(C) The expression of surface PTPRS in IFN-producing human pDCs. PBMCs were cultured with or without CpG overnight, stained for cell surface markers, fixed, and stained for intracellular IFN-α. Shown is the staining for PTPRS versus IFN-α in gated pDCs (representative of four experiments).
(D) The effect of PTPRS crosslinking on IFN-α production by primary human pDCs. PBMCs were cultured in the presence of control IgG or anti-PTPRS antibody for 1 hr, activated with CpG for 16 hr, and stained for cell surface markers and intracellular IFN-α. Left panel shows representative staining profiles of gated pDCs with the fraction of IFN-α+ cells highlighted. Right panel shows the fractions of IFN-α+ cells within gated pDCs from six individual donors (mean values of two or three independent experiments per donor per condition).
(E) The effect of PTPRS crosslinking on the production of TNF-α by human pDCs. PBMCs were activated and stained as above for cell surface markers and intracellular IFN-α and TNF-α. Shown are staining profiles of gated pDCs with the fraction of IFN-α+ TNF-α+ cells highlighted (representative of three individual donors).
(F) The effect of PTPRS crosslinking on the activation of NF-κB in human pDCs. The pDCs were enriched from PBMC, cultured for 3 hr without (unstim.) or with CpG in the presence of control IgG or anti-PTPRS, fixed, stained for NF-κB p65 and DNA and scored for the degree of p65 nuclear translocation. Shown are representative immunofluorescence images of p65 staining and the percentage of pDCs with translocated p65 on the scale of 1 (full nuclear exclusion) to 4 (prominent nuclear staining), out of >200 cells in each group.
The inverse correlation between PTPRS expression and IFN production suggested that active PTPRS-mediated signaling might inhibit pDC activation. We therefore incubated PBMC with control or anti-PTPRS antibody and analyzed CpG-induced IFN-α production. PTPRS crosslinking reduced the fraction of IFN-α+ activated pDCs in multiple independent donors (Figure 2D). In addition to the unique IRF7-dependent production of IFN, TLR ligation in pDCs induces the activation of NF-κB pathway and production of TNF-α. We found that the fraction of TNF-α+ pDCs was reduced by PTPRS crosslinking in three independent donors (Figure 2E). Accordingly, activation-induced nuclear translocation of NF-κB p65 in purified pDCs was also reduced by PTPRS crosslinking (Figure 2F). Thus, forced induction of PTPRS signaling inhibits the two major outcomes of TLR9-mediated pDC activation.
PTPRS Restricts the Activation of a Human pDC Cell Line
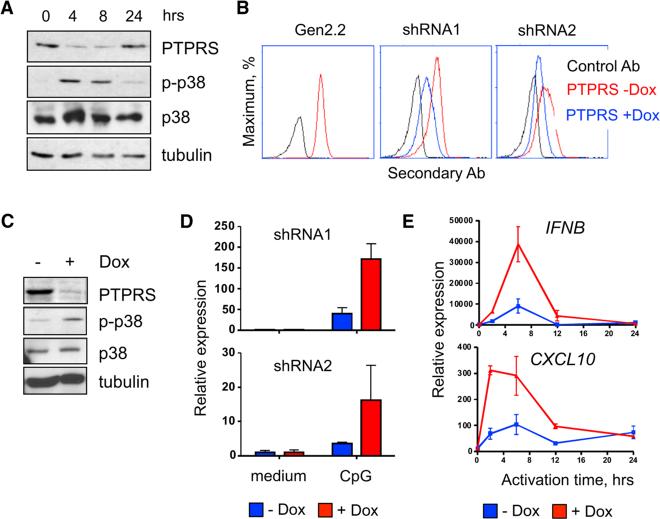
To test the effect of PTPRS reduction on pDC function, we used the human pDC cell line Gen2.2, which retains some activation potential (Chaperot et al., 2006). Gen2.2 cells expressed PTPRS protein, which was downregulated after activation with CpG (Figure 3A). We also observed that the induction of p38 kinase phosphorylation, an important event in pDC activation (Takauji et al., 2002; Zaru et al., 2015), mirrored the decrease of PTPRS (Figure 3A). Furthermore, crosslinking of PTPRS on Gen2.2 cells delayed the characteristic induction of both p-p38 and p-Stat1 (Di Domizio et al., 2009) and inhibited CpG-induced nuclear translocation of IRF7 (Figure S2).
Figure 3. PTPRS Knockdown Enhances the Activation of a Human pDC Cell Line.
(A) Western blot analysis of PTPRS and p38 (total and phosphorylated, p-p38) in the human pDC cell line Gen2.2 after stimulation with CpG.
(B) Inducible knockdown of PTPRS in the Gen2.2 cell line. Gen2.2 cells were transduced with retroviral vectors encoding two independent shRNA for PTPRS (shRNA1 and shRNA2), and treated with Dox to induce shRNA expression. PTPRS expression was measured 2 days later by cell surface staining.
(C) Western blot analysis of p-p38 in Gen2.2 cells that were treated with Dox for 48 hr to induce PTPRS knockdown and activated with CpG for 6 hr.
(D) The expression of IFNB by Gen2.2 cells after Dox-inducible PTPRS knockdown. Cells carrying Dox-inducible shRNAs for PTPRS were treated with Dox for 48 hr, stimulated with type A CpG for 6 hr, and IFNB expression was determined by qRT-PCR (mean ± SD of triplicate reactions, representative of three experiments).
(E) The expression of IFNB and IFN-inducible gene CXCL10 in Gen2.2 cells with Dox-induced PTPRS knockdown (shRNA1) at the indicated time points after stimulation with CpG (mean ± SD of triplicate PCRreactions; representative ofthreeexperiments).
To test the effect of PTPRS reduction on pDC function, we used the Dox-inducible shRNA expression system in Gen2.2 cells. The induction of two independent PTPRS-specific shRNAs reduced surface protein expression of PTPRS (Figure 3B). Furthermore, Dox-induced PTPRS knockdown increased the phosphorylation of p38 in CpG-treated Gen2.2 cells (Figure 3C). Due to multiple rounds of selection during retrofitting and shRNA expression, the resulting Gen2.2 cells had only minimal IFN-α response to CpG but manifested a robust IFN-β transcript induction. As shown in Figure 3D, CpG-induced IFNB expression was increased after PTPRS knockdown by two shRNAs. Moreover, the induction of IFNB and of a canonical IFN-inducible gene CXCL10 was both accelerated and prolonged (Figure 3E). Collectively, the opposite outcomes of PTPRS crosslinking and inducible PTPRS knockdown support the inhibitory role of PTPRS in human pDCs.
Ptprs and Ptprf Are Coexpressed in Murine pDCs and Inhibit Their Function
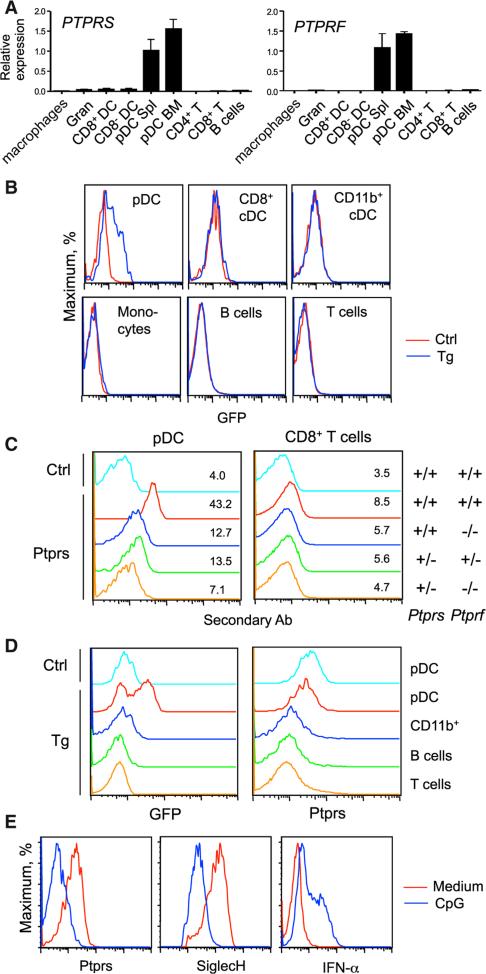
Because LAR phosphatases are highly conserved in vertebrates, we examined whether the murine ortholog of PTPRS is similarly expressed in the pDC lineage. By qRT-PCR, Ptprs transcript was highly enriched in the BM and splenic pDCs, was detected at low levels in cDCs, and was virtually absent from myeloid cells and lymphocytes (Figure 4A). In addition, the homologous LAR phosphatase Ptprf was also specifically expressed in murine pDCs (Figure 4A), in contrast to its absence from human pDCs (Figure S1A). The expression atlas of murine immune cells confirmed the preferential expression of both Ptprs and Ptprf in pDCs (Figure S3A). The expression of Ptprs, but not of Ptprf, was reduced after the deletion of E2-2 from murine pDCs in vivo (Ghosh et al., 2010), confirming Ptprs as a conserved E2-2 target in both humans and mice (Figure S3B). To further analyze the expression of murine Ptprf gene, we used a GFP reporter driven by the entire Ptprf locus in a bacterial artificial chromosome (BAC) transgene. As shown in Figure 4B, only pDCs expressed detectable GFP signal, whereas all other cells were negative in PtprfGFP transgenic mice.
Figure 4. Ptprs and Ptprf Are Specifically Coexpressed in Murine pDCs.
(A) The expression of Ptprs and Ptprf in sorted murine immune cell types as determined by qRT-PCR (mean ± SD of triplicate reactions). Cells included BM granulocytes (Gran), BM and splenic pDCs, and splenic CD8+ and CD8− cDCs, macrophages, and lymphocytes.
(B) The expression of Ptprf-GFP transgenic reporter in the indicated immune cell populations from the spleen. Shown are representative profiles of GFP fluorescence in the transgenic (Tg) and wild-type control (Ctrl) animals.
(C) Cell surface expression of LAR phosphatases in murine pDCs. Splenocytes from mice with the indicated Ptprs and Ptprf genotypes were stained with control (Ctrl) or anti-PTPRS antibodies, followed by secondary fluorescent antibody and antibodies to cell surface markers. Shown are histograms and mean fluorescence intensities of gated pDCs and of CD8+ T cells as a negative control cell type.
(D) The expression of LAR phosphatases in intestinal intraepithelial lymphocytes (IEL). IEL were isolated from Ptprf-GFP transgenic (Tg) or wild-type control (Ctrl) animals and stained for anti-PTPRS and surface markers. Shown are profiles of GFP fluorescence and PTPRS staining in the indicated gated populations.
(E) The expression of LAR phosphatases in murine pDCs after activation. Total BM cells were incubated with medium only or CpG for 16 hr, stained for cell surface markers, fixed, and stained for intracellular IFN-α. Shown are staining intensities of the indicated proteins in gated pDCs.
Anti-human PTPRS antibody (Figures 1 and 2) showed weak but detectable surface staining of pDCs from wild-type mice, which was reduced in Ptprf null (Ptprs+/+ Ptprf−/−) and double-haplodeficient (Ptprs+/− Ptprf+/−) mice (Figure 4C). The staining was further reduced in the Ptprf null, Ptprs-haplodeficient (Ptprs+/− Ptprf−/−) mice, suggesting that the antibody recognizes both LAR phosphatases co-expressed on murine pDCs. Little or no surface expression of Ptprs or Ptprf was observed on T cells and other cell types in the spleen and BM (Figure 4C and data not shown). Within leukocytes in peripheral tissues such as the intestine, the expression of PtprfGFP and surface staining for Ptprs and Ptprf were restricted to pDCs (Figure 4D). Similar to human PTPRS, the surface expression of murine Ptprs and Ptprf was profoundly downregulated in pDCs activated with CpG in vitro (Figure 4E). These results suggest that Ptprs, as well as the homologous LAR phosphatase Ptprf, are specifically coexpressed on naive quiescent pDCs and are downregulated upon activation.
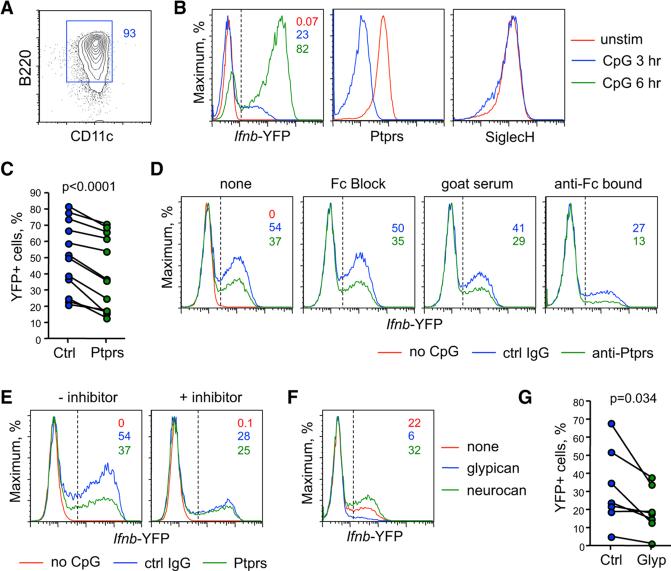
To further explore the role of LAR phosphatases in murine pDCs, we utilized a conditionally transformed progenitor cell line HoxB8-FL that can be differentiated into functional pDCs (Redecke et al., 2013). We derived HoxB8-FL cells expressing yellow fluorescent protein (YFP) reporter from the IFN-β-encoding Ifnb gene and differentiating into pDCs with >90% efficiency (Figure 5A). The differentiated HoxB8-IfnbYFP cells showed rapid induction of YFP in response to CpG, as well as baseline expression and rapid loss of surface LAR phosphatases (Figure 5B). Pre-incubation with plate-bound anti-PTPRS caused a signifi-cant reduction of CpG-induced Ifnb expression (Figures 5C and 5D). This reduction was observed even when Fc receptors were blocked by specific antibodies or excess immunoglobulin G (IgG) in serum, or when anti-PTPRS was bound to the plate via the Fc portion (Figure 5D). These data suggest that the inhibition is due to the engagement of LAR phosphatases rather than a co-engagement of inhibitory Fc receptors, similar to anti-BDCA2 antibodies (Pellerin et al., 2015).
Figure 5. Ptprs and Ptprf Inhibit the Activation of Murine pDCs.
Murine HoxB8-FL cell line carrying the YFP knock-in reporter alleles of Ifnb (HoxB8-IfnbYFP) was differentiated into pDCs, activated with CpG in the indicated conditions, and analyzed for YFP expression.
(A) Surface phenotype of the differentiated HoxB8-IfnbYFP cell clone used for the analysis.
(B) The expression of IfnbYFP reporter and LAR phosphatases in HoxB8-IfnbYFP cells activated with CpG for the indicated time periods.
(C) The effect of LAR phosphatases crosslinking on Ifnb induction in HoxB8-IfnbYFP cells. Shown are the fractions of YFP+ cells within HoxB8-IfnbYFP cells activated with CpG for 3–5 hr in the plates pre-coated with control IgG or anti-PTPRS (each symbol represents an independent experiment).
(D) The effect of Fc receptor blockade on the inhibitory activity of anti-PTPRS. Shown are YFP expression profiles of HoxB8-IfnbYFP cells activated with CpG on plate-bound control IgG or anti-PTPRS without any additional treatments (none), or in the presence of blocking anti-FcR antibody (Fc Block) or normal goat serum. Alternatively, control IgG or anti-PTPRS were bound to the plate via Fc fragments by pre-coating with anti-goat IgG (Fc) secondary antibodies (anti-Fc bound).
(E) The role of tyrosine phosphorylation in the Ifnb expression by HoxB8-IfnbYFP cells. Cells were activated with CpG on plates pre-coated with control IgG or anti-PTPRS, with or without the tyrosine phosphorylation inhibitor.
(F) The effect of known LAR phosphatase ligands on Ifnb expression by HoxB8-IfnbYFP cells. Cells were activated with CpG on plates pre-coated with recombinant glypican or neurocan.
(G) The effect of glypican on Ifnb expression within HoxB8-IfnbYFP cells activated with CpG for 3–5 hr (each symbol represents an independent experiment).
Consistent with prior reports (Balmelli et al., 2011; Fujita et al., 2013; Wang et al., 2014), CpG-induced YFP expression by HoxB8-IfnbYFP cells was reduced by an inhibitor of tyrosine phosphorylation (Figure 5E). The inhibitory effect of anti-PTPRS was minimal in the presence of the inhibitor, suggesting that LAR phosphatases exert their function by reducing tyrosine phosphorylation in pDCs. Indeed, PTPRS crosslinking abolished CpG-induced tyrosine phosphorylation in HoxB8-IfnbYFP and Gen2.2 cells as determined by flow cytometry (data not shown). Heparan sulfate (HS)-containing proteoglycans (HSPG) represent a major class of PTPRS ligands that cross-link PTPRS via their HS moieties; conversely, chondroitin sulfate proteoglycans (CSPG) might antagonize PTPRS by preventing its crosslinking by HS (Coles et al., 2011). We found that the prototypical HSPG ligand of PTPRS, glypican, inhibited Ifnb expression by HoxB8-IfnbYFP cells (Figures 5F and 5G), whereas CSPG neurocan enhanced it (Figure 5F). The inhibitory effect of glypican and of HS on IFN expression was observed both in HoxB8-IfnbYFP cells and in primary BM pDCs (Figure S4). While glypican and other HSPG might engage multiple receptors on pDCs, the inhibitory effects of this known PTPRS ligand are consistent with the proposed inhibitory role of LAR phosphatases.
Ptprs and Ptprf Restrict the Activation of Murine pDCs
We tested the role of Ptprs and Ptprf in the development and function of pDC lineage. Because Ptprs-deficient animals on B6 background die perinatally, we used fetal liver cells from double-deficient Ptprs−/− Ptprf−/− embryos to reconstitute irradiated recipients. The fraction of peripheral pDCs among the donor-derived cells in the resulting LAR double-knockout (LAR-KO) chimeras was slightly increased (Figure S5A). To analyze animals in the steady state without irradiation, we examined the viable Ptprs+/− Ptprf−/− mice, which show a profound reduction of Ptprs and Ptprf surface expression (Figure 4C). The pDC fraction in these “LAR three-quarter knockout” (LAR¾) mice was normal in the BM and spleen but slightly increased in the lymph nodes (Figure S5B). The expression of lineage and activation markers in pDCs from both LAR-KO chimeras and LAR¾ mice was normal, suggesting that LAR phosphatases are generally dispensable for the development and homeostasis of murine pDCs.
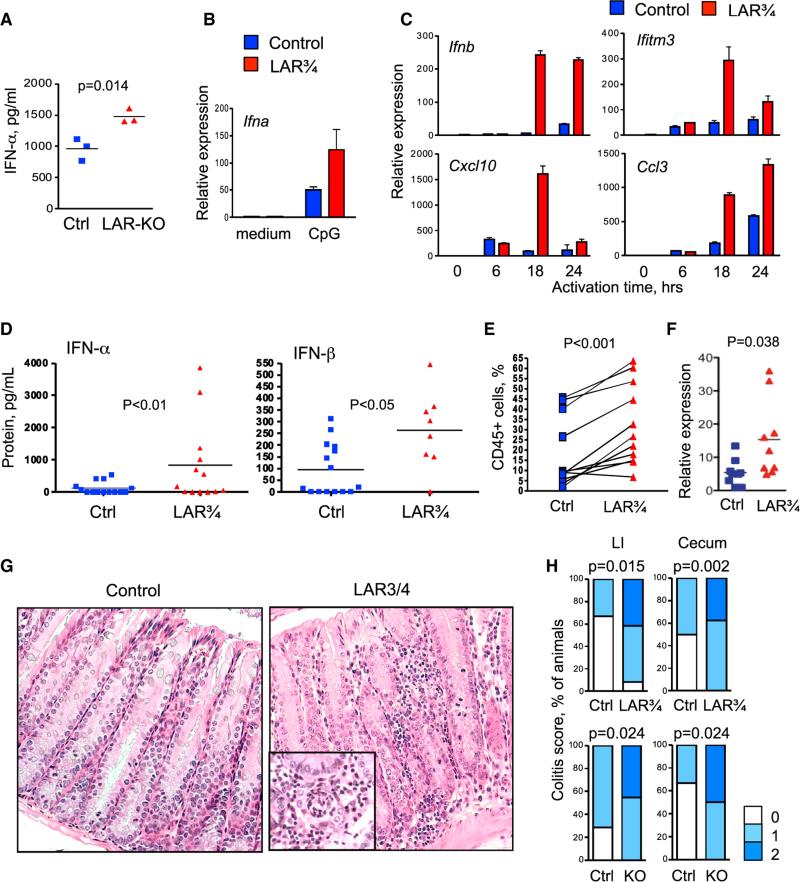
Despite the normal number of pDCs, the CpG-induced secretion of IFN-α was higher in LAR-KO BM, suggesting a higher activity per pDC (Figure 6A). Indeed, sorted LAR¾ pDCs showed higher induction of Ifna at 24 hr after activation (Figure 6B). Furthermore, LAR¾ pDCs showed an earlier and stronger induction of Ifnb and of several IFN-inducible genes at 18–24 hr after activation (Figure 6C). Global CpG-induced IFN production in vivo was comparable among control and LAR¾ mice, likely due to the strong overriding effect of CpG injection (data not shown). However, naive LAR¾ mice showed a significantly increased baseline amounts of serum IFN-α and IFN-β (Figure 6D). Thus, reduced dosage of LAR phosphatases is associated with enhanced IFN expression by pDCs and spontaneous systemic IFN production in the steady state.
Figure 6. The Reduction of LAR Phosphatases Leads to pDC Hyperactivation and Colitis.
(A) CpG-induced IFN-α production in the BM of mice reconstituted with control or Ptprs−/−Ptprf−/− (LAR-KO) hematopoietic cells. Total BM cells from individual control and LAR-KO chimeras were incubated with CpG for 24 hr, and IFN-α concentration in the supernatant was measured by ELISA.
(B and C) The expression of IFN and IFN-inducible genes by pDCs from Ptprs+/−Ptprf−/− (LAR¾) mice. pDCs were sorted from the BM of control and LAR¾ mice, stimulated with CpG and examined by qRT-PCR (presented as mean ± SD of triplicate reactions). (B) shows the expression of Ifna after 24 hr after stimulation (representative of three independent experiments). (C) shows the expression of Ifnb and IFN-inducible genes by the same pDCs at the indicated time points after stimulation.
(D) Concentrations of IFN-α and IFN-β in the sera of naive LAR¾ mice as measured by ELISA.
(E) The fraction of CD45+ hematopoietic cells in the small intestinal intraepithelial lymphocyte preparations from individual control and LAR¾ mice. Each experiment involved one control and one LAR¾ animal; because of large variation between experiments, the results are presented as paired analysis.
(F) The expression of IFN-α in the intestinal tissue of LAR¾ mice. Shown is the expression of Ifna in colon samples from individual control and LAR¾ mice as determined by qRT-PCR relative to a randomly chosen control sample.
(G) Representative sections of the large intestines from LAR¾ mice and controls. Magnification, 2003; inset illustrates crypt abscess.
(H) The frequency of intestinal inflammation as scored by histopathology, with statistical significance indicated. Numbers of analyzed animals were 13 (LAR¾ mice), 6 (LAR¾ controls), 10–11 (LAR-KO chimeras), and 7–9 (control chimeras).
The Loss of LAR Phosphatases in Hematopoietic Cells Causes Colitis
Given the persistent microbial exposure in the gut and the association of PTPRS with colitis, we examined the intestinal immune system of LAR-deficient mice. The fraction of CD45+ hematopoietic cells was significantly increased in the intestinal epithelial preparations of LAR¾ mice, revealing immune cell infiltration (Figure 6E). The relative proportions of various immune cell types in the intestinal epithelium and LP were not significantly changed, although LP lymphocytes showed a trend toward increased IFN and IFN-inducible gene expression (Figure S5C). Importantly, samples of colon tissue showed elevated expression of Ifna transcript (Figure 6F), suggesting that the observed infiltration is accompanied by net increase in local IFN-α production.
Histological analysis of naive adult LAR¾ mice revealed mild colitis and cecal inflammation (typhlitis) with increased leukocyte infiltration in the LP, in the intercryptal spaces and at crypt bases, mild edema and occasional crypt abscesses (Figures 6G and 6H). No leukocyte infiltration or overt colitis has been observed in Ptprf−/− Ptprs+/+ mice (data not shown), suggesting that the combined loss of Ptprs and Ptprf is required for these manifestations. Furthermore, chimeras reconstituted with LAR-KO hematopoietic cells showed similar histological manifestations of colitis and typhlitis (Figure 6H). Thus, the loss of LAR phosphatases Ptprs and Ptprf is associated with intestinal inflammation that is hematopoietic in origin.
Ptprs Expression in Dendritic Cells Restricts pDC Activation and Colitis
To further elucidate the cell type specificity of LAR phosphatase function, we generated a mouse strain for Cre recombinase-mediated conditional targeting of Ptprs (Ptprsflox). Because pDC-specific Cre deleter strains are not available, we used the ItgaxCre strain that deletes genes in all CD11c+ DC subsets including pDCs and cDCs. The resulting mice with DC-specific deletion of Ptprs (Ptprsflox/flox ItgaxCre) were viable, had no abnormalities in the DC lineage composition or function, and showed no signs of colitis (data not shown). We therefore crossed these mice onto Ptprf null background to generate Ptprf−/− Ptprsflox/flox ItgaxCre mice that lack both LAR phosphatases in the DC lineage (DC-specific LAR conditional knockout, LAR-CKO). LAR phosphatase expression on pDCs from LAR-CKO mice was reduced in the BM and nearly abolished in the spleen (Figure S6A). Consistent with the weak expression of Ptprs in human and murine cDCs (Figures S1A, 4A, and S3A), cDCs showed weak staining that was reduced by the loss of Ptprs, but not Ptprf. These data confirm that the co-expression of the two LAR phosphatases is specific to pDCs and has been ablated in LAR-CKO mice, albeit with delayed kinetics.
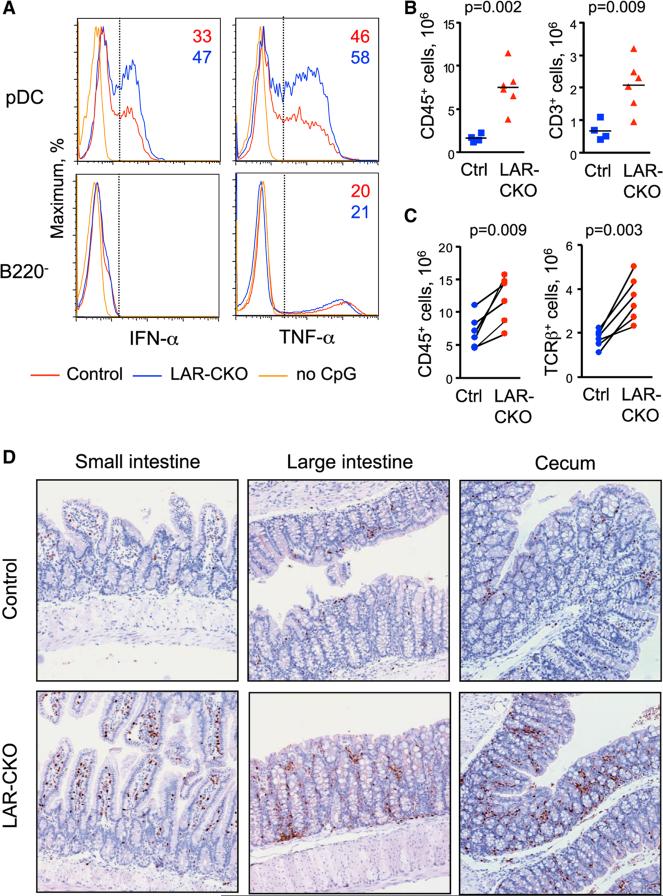
The number and phenotype of pDCs were not significantly changed in LAR-CKO mice, although a trend toward higher pDC fraction in the LN was noted (data not shown). Upon stimulation of total BM with CpG, pDCs from LAR-CKO mice showed higher frequency of IFN-α and TNF-α-producing cells compared to controls (Figure 7A). Consistent with the pDC-specific effect, the fraction of TNF-α-producing cells among non-pDCs was not increased. Intestinal LP cell preparations of LAR-CKO mice showed significantly increased numbers of CD45+ cells and CD3+ T cells (Figure 7B). The same increase was evident in co-housed pairs of LAR-CKO mice and control littermates (Figure 7C). All cell types including B and T cells were increased in numbers, but not in relative proportions (Figure S6B); similarly, no change in the proportion of LP T cells producing IL-17 and/or IFN-γ was detected (Figure S6C). The infiltration of T cells was confirmed by immunochemical staining of large and small intestine (Figure 7D). Finally, LAR-CKO mice exhibited histological colitis that was weaker than in LAR¾ mice but significant compared to controls (Figure S6D). Thus, DC-specific loss of Ptprs on Ptprf null background is associated with hyperresponsiveness of pDCs and mild intestinal inflammation, partially recapitulating the phenotype of global Ptprs/Ptprf reduction. Because the expression of Ptprs and Ptprf within the DC lineage is restricted to pDCs, these results suggest that LAR phosphatases inhibit pDC activation and consequently promote immune homeostasis in the intestine.
Figure 7. The Deletion of LAR Phosphatases in Dendritic Cells Leads to pDC Hyperactivation and Colitis.
Animals with conditional LAR phosphatase deletion (LAR-CKO, Ptprf−/− Ptprsflox/flox ItgaxCre), or controls (Ptprf+/+ Ptprsflox/flox ItgaxCre-negative in all panels except C) were examined.
(A) Cytokine production by LAR-CKO pDCs. Total BM cells were cultured with CpG for 16 hr and stained for cell surface markers and intracellular cytokines. Shown are the histograms of IFN-α or TNF-α staining in gated B220+ SiglecH+ pDCs or in B220− non-pDC myeloid cells. The threshold of positive staining and the fraction of positive cells are indicated. Representative of three experiments.
(B) Leukocyte infiltration in the intestinal LP of LAR-CKO and control mice. The number of total CD45+ leukocytes or CD3+ T cells recovered from the LP preparations of individual mice are shown.
(C) Leukocyte and T cell infiltration in the intestinal LP from co-housed pairs of LAR-CKO and littermate controls (Ptprf+/− Ptprsflox/flox ItgaxCre-negative).
(D) T cell infiltration in the intestine of LAR-CKO and control mice. Sections of the indicated intestinal compartments were stained for CD3 (brown) and counterstained with hematoxylin (blue).
DISCUSSION
LAR-type receptor tyrosine phosphatases PTPRS and PTPRF regulate the development and function of multiple tissues including genitourinary tract, mammary gland, and brain. Here we show that PTPRS is also expressed in the immune system, where it appears specific for the pDC lineage in both humans and mice. This conserved pDC-specific expression is controlled by E2-2, a similarly conserved transcriptional regulator of pDC development and maintenance. In addition, we found that Ptprf is expressed in murine, but not in human pDCs. The expression of PTPRS was prominent on the surface of naive pDCs but was rapidly reduced upon activation, likely through internalization and/or shedding from the membrane. Notably, IFN-α-producing cells were contained in the pDC population with the lowest surface expression of PTPRS, suggesting that a threshold of PTPRS reduction might be necessary for cytokine production by activated pDCs.
Consistent with the inverse relationship between PTPRS expression and IFN production, antibody-mediated crosslinking of PTPRS inhibited pDC activation in primary human pDCs, a human pDC cell line and in vitro-derived murine pDCs. Conversely, inducible knockdown of PTPRS and genetic reduction of Ptprs/Ptprf in primary murine pDCs enhanced TLR-induced pDC activation. These combined data from diverse experimental systems in two species suggest that PTPRS (along with Ptprf in the mouse) is a pDC-specific inhibitory receptor. Indeed, antibodies to known inhibitory receptors including BDCA-2, ILT7, and SiglecH reduce IFN production by pDCs (Blasius et al., 2004; Cao et al., 2006; Dzionek et al., 2001). Furthermore, BDCA-2 and ILT7 are downregulated following TLR-induced pDC activation in vitro (Meyer-Wentrup et al., 2008; Tavano et al., 2013). Unlike all these receptors, however, the expression and function of PTPRS in pDCs appear similar in mice and humans. The identification of a conserved pDC-specific inhibitory receptor strongly supports the genetic and functional conservation of the pDC lineage in mammals. It also highlights the evolutionary pressure for lineage-specific regulatory mechanisms that restrict the powerful cytokine-producing capacity of pDCs.
The crosslinking of PTPRS inhibited both the IRF7-dependent pathway of IFN production and the NF-κB-dependent inflammatory pathway, suggesting that PTPRS might block an upstream tyrosine phosphorylation-dependent mechanism of pDC activation. Tyrosine phosphorylation is required for optimal IFN production by pDCs and involves both the Src family kinase pathway and yet unidentified Src-independent components (Balmelli et al., 2011; Fujita et al., 2013; Wang et al., 2014). Interestingly, the Src pathway also mediates the inhibitory signaling through ITAM-dependent pDC-specific receptors (Gilliet et al., 2008). Thus, PTPRS might restrict tyrosine phosphorylation-dependent pathways downstream of TLR signaling in quiescent pDCs, whereas its rapid loss upon pDC activation might enable subsequent signaling by other inhibitory receptors. Whereas the physiological ligands of PTPRS in pDCs remain to be defined, they are likely to be broadly available in tissues to ensure constitutive signaling in the steady state. In that respect, known PTPRS ligands HSPG are major components of the extracellular matrix and cell membranes, and we here observed their inhibitory effect on pDC activation. Indeed, HSPG were recently proposed to inhibit IFN production by macrophages in the context of atherosclerosis (Gordts et al., 2014). The HSPG that are most relevant for pDC function remain to be identified, and they might signal through PTPRS as well as other surface receptors on pDCs. Of note, BDCA-2 and SiglecH are lectins that appear to bind specific carbohydrate modifications, e.g., asialo-galactosyl oligosaccharides in the case of BDCA-2 (Riboldi et al., 2011). Therefore, carbohydrate components of proteoglycans emerge as major regulators of pDC activity that restrict pDC activation through both conserved (PTPRS) and species-specific (BDCA-2, SiglecH) receptors.
The biological role of pDC-specific inhibitory receptors is still poorly understood. Deletion of SiglecH, the only murine receptor known so far, did not cause overt autoimmune or inflammatory diseases. SiglecH-deficient mice show higher IFN response to murine cytomegalovirus (MCMV), whereas other immune pheno-types in these mice are not pDC-intrinsic (Puttur et al., 2013; Swiecki et al., 2014). We found that Ptprs reduction or DC-specific deletion on Ptprf null background caused mild spontaneous colitis that could be transferred with hematopoietic cells. Although the colitis in Ptprs-deficient mice (Muise et al., 2007) or in compound Ptprs/Ptprf mice (this study) might have complex origins and involve the function of LAR phosphatases in other cell types, the pDC-intrinsic function of Ptprs appears essential. Notably, pDC hyperactivation has been documented in inflammatory bowel disease (Baumgart et al., 2011) and in colitis associated with the Wiskott-Aldrich syndrome (Prete et al., 2013). Consistent with the emerging key role of DCs as regulators of intestinal inflammation (Bar-On et al., 2011; Bogunovic et al., 2012), our results suggest that a primary pDC hyperactivation might be linked to disrupted immune homeostasis in the intestine.
In conclusion, we describe the LAR phosphatase PTPRS as an evolutionarily conserved pDC-specific receptor that restricts pDC activation and thereby maintains immune homeostasis. Due to their important role in autoimmune diseases such as lupus (Rowland et al., 2014; Sisirak et al., 2014), pDCs have emerged as a target of immunotherapies such as antibody-mediated depletion. Given the expression of PTPRS in non-immune tissues such as the brain, other pDC-specific receptors such as BDCA-2 might provide better targets for depleting antibodies (Pellerin et al., 2015). On the other hand, the conservation and potent activity of PTPRS make it an attractive candidate for the modulation of pDC function, e.g., via cross-linking with non-depleting agonist antibodies. Conversely, recently developed peptides that selectively inhibit PTPRS (Lang et al., 2015) might be utilized to boost the insufficient activity of pDCs in conditions like chronic viral infections or tumors.
EXPERIMENTAL PROCEDURES
Primary Human Cells
All human studies were performed according to the investigator's protocol approved by the Institutional Review Board of Columbia University. PBMC were isolated from healthy adult volunteers by Histopaque density gradient centrifugation; where indicated, pDCs were enriched using the Diamond pDC isolation kit (Miltenyi Biotec) to >95% purity. Human PBMC subsets for expression analysis were purchased from Allcells.
For pDC activation, PBMCs were plated at 5 × 3 105 /well of flat-bottom 96 well plates in RPMI medium with 10% FCS in the presence of affinity-purified polyclonal goat IgG antibody to the extracellular domain of human PTPRS (R&D Systems) or of control goat IgG (Santa Cruz Biotechnology). In titration experiments, antibody concentrations >0.01 μg/mL were found to have the same effect. CpG type A (ODN 2216, Invivogen) was added 1 hr later at 5 mM concentration. After 6 hr, protein transport inhibitor (BD Golgi Plug™, BD Biosciences) was added and cells were incubated for additional 10 hr. For the analysis by immunofluorescence, purified pDC were pre-incubated with anti-PTPRS or control IgG and then activated with CpG for 3 hr.
Animals
All mouse studies were performed according to the investigator's protocol approved by the Institutional Animal Care and Use Committee of Columbia University. The Ptprf and Ptprf mutant strains and crosses thereof are described in the Supplemental Experimental Procedures.
Cell Lines
The culture and analysis of human pDC cell lines CAL-1 and Gen2.2 and of the mouse HoxB8-transformed cell line with pDC differentiation potential and IFN-β reporter (HoxB8-IfnbYFP) are described in the Supplemental Experimental Procedures.
Cell and Gene-Expression Analysis
To detect PTPRS expression on human PBMC and pDC cell lines, we incubated cells with 10% normal mouse serum, washed, and stained with 0.5 μg/ml goat anti-PTPRS (R&D Systems) followed by a secondary PE-conjugated F(ab’)2 fragment of donkey anti-goat IgG pre-adsorbed against other species (Jackson ImmunoResearch). For staining mouse cells, the blocking step was omitted. Cells were then stained with directly conjugated monoclonal antibodies against cell surface markers. Cells were acquired using BD LSRII or BD Fortessa (BD Biosciences), and data were analyzed using FlowJo software (Treestar). The detection of intracellular cytokines, immunofluorescence microscopy, and the isolation and analysis of intestinal leukocytes were done as described in the Supplemental Experimental Procedures.
ChIP-seq analysis and shRNA-mediated knockdown of human TCF4 (E2-2) in pDC cell lines has been described (Ghosh et al., 2014; Sawai et al., 2013). For qRT-PCR analysis, stained cell suspensions were sorted directly into Trizol LS reagent (Invitrogen) using FACSAria II cell sorter (BD Biosciences). The isolated total RNA was reverse transcribed and assayed by SYBR green-based real-time PCR with MX3000P instrument (Stratagene). The expression of all genes was normalized to that of Actb or (for intestinal tissue) of Hprt, and expressed relative to the indicated reference sample via the DDCT method. Murine IFN-α and IFN-β were measured in culture supernatants and in sera using sandwich ELISA with primary antibody pairs from PBL Interferon Source.
Statistical Analysis
Statistical significance was estimated using two-tailed Student's t test; un-paired and paired t tests, respectively, were used for pooled groups or matched pairs as indicated. Immunofluorescence parameters were analyzed using the chi-square test. Histological scores were analyzed using Wilcoxon signed-rank test.
Supplementary Material
Highlights.
Human pDCs specifically express receptor protein tyrosine phosphatase PTPRS
Murine pDCs specifically express Ptprs and the homologous phosphatase Ptprf
Ptprs inhibits interferon production by murine and human pDCs
Combined loss of Ptprs and Ptprf cause pDC hyperactivation and mild colitis
ACKNOWLEDGMENTS
We thank Dr. Ivaylo Ivanov for advice, Alexei Kartashov for help with statistical analysis, and T. and E. Reizis for help in scoring IRF7 translocation. Supported by NIH grant AI072571 (B.R.), Irvington Institute Fellowship of the Cancer Research Institute (V.S.), American Society of Hematology (H.S.G.) and NIH training grant CA009503 (C.Y.).
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes six figures and Supplemental Experimental Procedures and can be found with this article online at http://dx.doi.org/10.1016/j.immuni.2015.07.009.
REFERENCES
- Aicher B, Lerch MM, Müller T, Schilling J, Ullrich A. Cellular redistribution of protein tyrosine phosphatases LAR and PTPsigma by inducible proteolytic processing. J. Cell Biol. 1997;138:681–696. doi: 10.1083/jcb.138.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balmelli C, Steiner E, Moulin H, Peduto N, Herrmann B, Summerfield A, McCullough K. Porcine circovirus type 2 DNA influences cytoskeleton rearrangements in plasmacytoid and monocyte-derived dendritic cells. Immunology. 2011;132:57–65. doi: 10.1111/j.1365-2567.2010.03339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-On L, Zigmond E, Jung S. Management of gut inflammation through the manipulation of intestinal dendritic cells and macrophages? Semin. Immunol. 2011;23:58–64. doi: 10.1016/j.smim.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Baumgart DC, Metzke D, Guckelberger O, Pascher A, Grötzinger C, Przesdzing I, Dörffel Y, Schmitz J, Thomas S. Aberrant plasmacytoid dendritic cell distribution and function in patients with Crohn's disease and ulcerative colitis. Clin. Exp. Immunol. 2011;166:46–54. doi: 10.1111/j.1365-2249.2011.04439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasius A, Vermi W, Krug A, Facchetti F, Cella M, Colonna M. A cell-surface molecule selectively expressed on murine natural inter-feron-producing cells that blocks secretion of interferon-alpha. Blood. 2004;103:4201–4206. doi: 10.1182/blood-2003-09-3108. [DOI] [PubMed] [Google Scholar]
- Blasius AL, Cella M, Maldonado J, Takai T, Colonna M. Siglec-H is an IPC-specific receptor that modulates type I IFN secretion through DAP12. Blood. 2006;107:2474–2476. doi: 10.1182/blood-2005-09-3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasius AL, Arnold CN, Georgel P, Rutschmann S, Xia Y, Lin P, Ross C, Li X, Smart NG, Beutler B. Slc15a4, AP-3, and Hermansky-Pudlak syndrome proteins are required for Toll-like receptor signaling in plasmacytoid dendritic cells. Proc. Natl. Acad. Sci. USA. 2010;107:19973–19978. doi: 10.1073/pnas.1014051107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogunovic M, Mortha A, Muller PA, Merad M. Mononuclear phagocyte diversity in the intestine. Immunol. Res. 2012;54:37–49. doi: 10.1007/s12026-012-8323-5. [DOI] [PubMed] [Google Scholar]
- Cao W, Rosen DB, Ito T, Bover L, Bao M, Watanabe G, Yao Z, Zhang L, Lanier LL, Liu YJ. Plasmacytoid dendritic cell-specific receptor ILT7-Fc epsilonRI gamma inhibits Toll-like receptor-induced interferon production. J. Exp. Med. 2006;203:1399–1405. doi: 10.1084/jem.20052454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W, Bover L, Cho M, Wen X, Hanabuchi S, Bao M, Rosen DB, Wang YH, Shaw JL, Du Q, et al. Regulation of TLR7/9 responses in plasmacytoid dendritic cells by BST2 and ILT7 receptor interaction. J. Exp. Med. 2009;206:1603–1614. doi: 10.1084/jem.20090547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervantes-Barragan L, Lewis KL, Firner S, Thiel V, Hugues S, Reith W, Ludewig B, Reizis B. Plasmacytoid dendritic cells control T-cell response to chronic viral infection. Proc. Natl. Acad. Sci. USA. 2012;109:3012–3017. doi: 10.1073/pnas.1117359109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaperot L, Blum A, Manches O, Lui G, Angel J, Molens JP, Plumas J. Virus or TLR agonists induce TRAIL-mediated cytotoxic activity of plasmacytoid dendritic cells. J. Immunol. 2006;176:248–255. doi: 10.4049/jimmunol.176.1.248. [DOI] [PubMed] [Google Scholar]
- Cisse B, Caton ML, Lehner M, Maeda T, Scheu S, Locksley R, Holmberg D, Zweier C, den Hollander NS, Kant SG, et al. Transcription factor E2-2 is an essential and specific regulator of plasmacytoid dendritic cell development. Cell. 2008;135:37–48. doi: 10.1016/j.cell.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles CH, Shen Y, Tenney AP, Siebold C, Sutton GC, Lu W, Gallagher JT, Jones EY, Flanagan JG, Aricescu AR. Proteoglycan-specific molecular switch for RPTPσ clustering and neuronal extension. Science. 2011;332:484–488. doi: 10.1126/science.1200840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nat. Immunol. 2004;5:1219–1226. doi: 10.1038/ni1141. [DOI] [PubMed] [Google Scholar]
- Di Domizio J, Blum A, Gallagher-Gambarelli M, Molens JP, Chaperot L, Plumas J. TLR7 stimulation in human plasmacytoid dendritic cells leads to the induction of early IFN-inducible genes in the absence of type I IFN. Blood. 2009;114:1794–1802. doi: 10.1182/blood-2009-04-216770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzionek A, Sohma Y, Nagafune J, Cella M, Colonna M, Facchetti F, Günther G, Johnston I, Lanzavecchia A, Nagasaka T, et al. BDCA-2, a novel plasmacytoid dendritic cell-specific type II C-type lectin, mediates antigen capture and is a potent inhibitor of interferon alpha/beta induction. J. Exp. Med. 2001;194:1823–1834. doi: 10.1084/jem.194.12.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esashi E, Bao M, Wang YH, Cao W, Liu YJ. PACSIN1 regulates the TLR7/9-mediated type I interferon response in plasmacytoid dendritic cells. Eur. J. Immunol. 2012;42:573–579. doi: 10.1002/eji.201142045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita H, Kitawaki T, Sato T, Maeda T, Kamihira S, Takaori-Kondo A, Kadowaki N. The tyrosine kinase inhibitor dasatinib suppresses cytokine production by plasmacytoid dendritic cells by targeting endosomal transport of CpG DNA. Eur. J. Immunol. 2013;43:93–103. doi: 10.1002/eji.201242699. [DOI] [PubMed] [Google Scholar]
- Ganguly D, Haak S, Sisirak V, Reizis B. The role of dendritic cells in autoimmunity. Nat. Rev. Immunol. 2013;13:566–577. doi: 10.1038/nri3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh HS, Cisse B, Bunin A, Lewis KL, Reizis B. Continuous expression of the transcription factor e2-2 maintains the cell fate of mature plasmacytoid dendritic cells. Immunity. 2010;33:905–916. doi: 10.1016/j.immuni.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh HS, Ceribelli M, Matos I, Lazarovici A, Bussemaker HJ, Lasorella A, Hiebert SW, Liu K, Staudt LM, Reizis B. ETO family protein Mtg16 regulates the balance of dendritic cell subsets by repressing Id2. J. Exp. Med. 2014;211:1623–1635. doi: 10.1084/jem.20132121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliet M, Cao W, Liu YJ. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat. Rev. Immunol. 2008;8:594–606. doi: 10.1038/nri2358. [DOI] [PubMed] [Google Scholar]
- Gordts PL, Foley EM, Lawrence R, Sinha R, Lameda-Diaz C, Deng L, Nock R, Glass CK, Erbilgin A, Lusis AJ, et al. Reducing macrophage proteoglycan sulfation increases atherosclerosis and obesity through enhanced type I interferon signaling. Cell Metab. 2014;20:813–826. doi: 10.1016/j.cmet.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo S, Kishi H, Muraguchi A. Regulatory role of leukocyte-common-antigen-related molecule (LAR) in thymocyte differentiation. Eur. J. Immunol. 2010;40:1296–1302. doi: 10.1002/eji.200939743. [DOI] [PubMed] [Google Scholar]
- Lang BT, Cregg JM, DePaul MA, Tran AP, Xu K, Dyck SM, Madalena KM, Brown BP, Weng YL, Li S, et al. Modulation of the proteoglycan receptor PTPs promotes recovery after spinal cord injury. Nature. 2015;518:404–408. doi: 10.1038/nature13974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YJ. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu. Rev. Immunol. 2005;23:275–306. doi: 10.1146/annurev.immunol.23.021704.115633. [DOI] [PubMed] [Google Scholar]
- Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu. Rev. Immunol. 2013;31:563–604. doi: 10.1146/annurev-immunol-020711-074950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Wentrup F, Benitez-Ribas D, Tacken PJ, Punt CJ, Figdor CG, de Vries IJ, Adema GJ. Targeting DCIR on human plasmacytoid dendritic cells results in antigen presentation and inhibits IFN-alpha production. Blood. 2008;111:4245–4253. doi: 10.1182/blood-2007-03-081398. [DOI] [PubMed] [Google Scholar]
- Mildner A, Jung S. Development and function of dendritic cell subsets. Immunity. 2014;40:642–656. doi: 10.1016/j.immuni.2014.04.016. [DOI] [PubMed] [Google Scholar]
- Muise AM, Walters T, Wine E, Griffiths AM, Turner D, Duerr RH, Regueiro MD, Ngan BY, Xu W, Sherman PM, et al. Protein-tyro-sine phosphatase sigma is associated with ulcerative colitis. Curr. Biol. 2007;17:1212–1218. doi: 10.1016/j.cub.2007.06.013. [DOI] [PubMed] [Google Scholar]
- Murchie R, Guo CH, Persaud A, Muise A, Rotin D. Protein tyrosine phosphatase σ targets apical junction complex proteins in the intestine and regulates epithelial permeability. Proc. Natl. Acad. Sci. USA. 2014;111:693–698. doi: 10.1073/pnas.1315017111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellerin A, Otero K, Czerkowicz JM, Kerns HM, Shapiro RI, Ranger AM, Otipoby KL, Taylor FR, Cameron TO, Viney JL, Rabah D. Anti-BDCA2 monoclonal antibody inhibits plasmacytoid dendritic cell activation through Fc-dependent and Fc-independent mechanisms. EMBO Mol. Med. 2015;7:464–476. doi: 10.15252/emmm.201404719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prete F, Catucci M, Labrada M, Gobessi S, Castiello MC, Bonomi E, Aiuti A, Vermi W, Cancrini C, Metin A, et al. Wiskott-Aldrich syndrome protein-mediated actin dynamics control type-I interferon production in plasmacytoid dendritic cells. J. Exp. Med. 2013;210:355–374. doi: 10.1084/jem.20120363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puttur F, Arnold-Schrauf C, Lahl K, Solmaz G, Lindenberg M, Mayer CT, Gohmert M, Swallow M, van Helt C, Schmitt H, et al. Absence of Siglec-H in MCMV infection elevates interferon alpha production but does not enhance viral clearance. PLoS Pathog. 2013;9:e1003648. doi: 10.1371/journal.ppat.1003648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redecke V, Wu R, Zhou J, Finkelstein D, Chaturvedi V, High AA, Häcker H. Hematopoietic progenitor cell lines with myeloid and lymphoid potential. Nat. Methods. 2013;10:795–803. doi: 10.1038/nmeth.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee I, Veillette A. Protein tyrosine phosphatases in lymphocyte activation and autoimmunity. Nat. Immunol. 2012;13:439–447. doi: 10.1038/ni.2246. [DOI] [PubMed] [Google Scholar]
- Riboldi E, Daniele R, Parola C, Inforzato A, Arnold PL, Bosisio D, Fremont DH, Bastone A, Colonna M, Sozzani S. Human C-type lectin domain family 4, member C (CLEC4C/BDCA-2/CD303) is a receptor for asialo-galactosyl-oligosaccharides. J. Biol. Chem. 2011;286:35329–35333. doi: 10.1074/jbc.C111.290494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland SL, Riggs JM, Gilfillan S, Bugatti M, Vermi W, Kolbeck R, Unanue ER, Sanjuan MA, Colonna M. Early, transient depletion of plasmacytoid dendritic cells ameliorates autoimmunity in a lupus model. J. Exp. Med. 2014;211:1977–1991. doi: 10.1084/jem.20132620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhe JE, Streit S, Hart S, Ullrich A. EGFR signaling leads to downregulation of PTP-LAR via TACE-mediated proteolytic processing. Cell. Signal. 2006;18:1515–1527. doi: 10.1016/j.cellsig.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Sasai M, Linehan MM, Iwasaki A. Bifurcation of Toll-like receptor 9 signaling by adaptor protein 3. Science. 2010;329:1530–1534. doi: 10.1126/science.1187029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawai CM, Sisirak V, Ghosh HS, Hou EZ, Ceribelli M, Staudt LM, Reizis B. Transcription factor Runx2 controls the development and migration of plasmacytoid dendritic cells. J. Exp. Med. 2013;210:2151–2159. doi: 10.1084/jem.20130443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisirak V, Ganguly D, Lewis KL, Couillault C, Tanaka L, Bolland S, D'Agati V, Elkon KB, Reizis B. Genetic evidence for the role of plasmacytoid dendritic cells in systemic lupus erythematosus. J. Exp. Med. 2014;211:1969–1976. doi: 10.1084/jem.20132522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiecki M, Gilfillan S, Vermi W, Wang Y, Colonna M. Plasmacytoid dendritic cell ablation impacts early interferon responses and antiviral NK and CD8(+) T cell accrual. Immunity. 2010;33:955–966. doi: 10.1016/j.immuni.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiecki M, Wang Y, Riboldi E, Kim AH, Dzutsev A, Gilfillan S, Vermi W, Ruedl C, Trinchieri G, Colonna M. Cell depletion in mice that express diphtheria toxin receptor under the control of SiglecH encompasses more than plasmacytoid dendritic cells. J. Immunol. 2014;192:4409–4416. doi: 10.4049/jimmunol.1303135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takauji R, Iho S, Takatsuka H, Yamamoto S, Takahashi T, Kitagawa H, Iwasaki H, Iida R, Yokochi T, Matsuki T. CpG-DNA-induced IFN-alpha production involves p38 MAPK-dependent STAT1 phosphorylation in human plasmacytoid dendritic cell precursors. J. Leukoc. Biol. 2002;72:1011–1019. [PubMed] [Google Scholar]
- Tavano B, Galao RP, Graham DR, Neil SJ, Aquino VN, Fuchs D, Boasso A. Ig-like transcript 7, but not bone marrow stromal cell antigen 2 (also known as HM1.24, tetherin, or CD317), modulates plasmacytoid dendritic cell function in primary human blood leukocytes. J. Immunol. 2013;190:2622–2630. doi: 10.4049/jimmunol.1202391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terszowski G, Jankowski A, Hendriks WJ, Rolink AG, Kisielow P. Within the hemopoietic system, LAR phosphatase is a T cell lineage-specific adhesion receptor-like protein whose phosphatase activity appears dispensable for T cell development, repertoire selection and function. Eur. J. Immunol. 2001;31:832–840. doi: 10.1002/1521-4141(200103)31:3<832::aid-immu832>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Uetani N, Bertozzi K, Chagnon MJ, Hendriks W, Tremblay ML, Bouchard M. Maturation of ureter-bladder connection in mice is controlled by LAR family receptor protein tyrosine phosphatases. J. Clin. Invest. 2009;119:924–935. doi: 10.1172/JCI37196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Lau KY, Jung J, Ravindran P, Barrat FJ. Bruton's tyrosine kinase regulates TLR9 but not TLR7 signaling in human plasmacytoid dendritic cells. Eur. J. Immunol. 2014;44:1130–1136. doi: 10.1002/eji.201344030. [DOI] [PubMed] [Google Scholar]
- Zaru R, Edgar AJ, Hanauer A, Watts C. Structural and functional basis for p38-MK2-activated Rsk signaling in toll-like receptor-stimulated dendritic cells. Mol. Cell. Biol. 2015;35:132–140. doi: 10.1128/MCB.00773-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.