Abstract
The members of the MAF family of transcription factors are homologs of v-Maf –the oncogenic component of the avian retrovirus AS42. The MAF family is subdivided into 2 groups, small and large MAFs. To elucidate the role of the large MAF transcription factors in the endocrine pancreas, we analyzed large MAF gene knockout mice. It has been shown that Mafa−/− mice develop phenotypes including abnormal islet structure soon after birth. This study revealed that Ins1 and Ins2 transcripts and the protein contents were significantly reduced in Mafa−/− mice at embryonic day 18.5. In addition, Mafa−/−;Mafb−/− mice contained less than 10% of the insulin transcript and protein of those of wild-type mice, suggesting that Mafa and Mafb cooperate to maintain insulin levels at the embryonic stage. On the other hand, the number of insulin-positive cells in Mafa−/− mice was comparable to that of wild-type mice, and even under a Mafb-deficient background the number of insulin-positive cells was not decreased, suggesting that Mafb plays a dominant role in embryonic β-cell development. We also found that at 20 weeks of age Mafa−/−;Mafb+/− mice showed a higher fasting blood glucose level than single Mafa−/− mice. In summary, our results indicate that Mafa is necessary for the maintenance of normal insulin levels even in embryos and that Mafb is important for the maintenance of fasting blood glucose levels in the Mafa-deficient background in adults.
Keywords: diabetes mellitus, glucagon, insulin, large MAF, transcription factor
Introduction
The endocrine pancreas is composed of the islets of Langerhans scattered within exocrine tissue. The adult islets contain 4 main cell types and secrete different hormones: α cells secrete glucagon, β cells secrete insulin, δ-cells secrete somatostatin, and PP-cells secrete pancreatic polypeptide [10, 28]. In rodents, β-cells, which occupy the core of the islets, secrete insulin to reduce blood glucose levels, and work as a central determinant of maintaining glucose homeostasis [27].
The proximal insulin promoter, located on chromosome 11 in humans, has been extensively investigated to understand how insulin transcription is controlled in β cells [26]. The promoter, approximately 400 base pairs, contains several regulatory elements including A boxes, GG boxes, CRE elements, C elements, and E boxes [12]. A number of β-cell specific and ubiquitously expressed transcription factors contribute remarkably to proper insulin transcription among species. Specifically, these are Pancreatic Duodenum Homeobox-1 (PDX1) on A3 box, Neurogenic Differentiation 1 (NEUROD1) on E1 element, and v-Maf Avian Musculoaponeurotic Fibrosarcoma Oncogene Homolog A (MAFA) on C1 element [33].
MAFA is a member of the large MAF protein family [14, 25]. The family is comprised of 4 basic-leucine zipper transcription factors, MAFA, MAFB, c-MAF, and NRL. In the endocrine pancreas of embryos, Mafa is expressed only in β cells and Mafb is expressed in both α and β cells. However, in adults, Mafa and Mafb are preferentially expressed in β cells and α cells, respectively [2, 22]. Therefore, this switch from Mafb to Mafa expression in β cells is considered to be crucial for the functional maturation observed in both mouse models and in human embryonic stem cell differentiation [16, 22]. Previously we reported that Mafa-deficient mice exhibit no obvious phenotype at birth, but after birth exhibit impaired glucose-stimulated insulin secretion and structural abnormalities of the Langerhans islets [34]. In contrast, Mafb-deficient mice show a substantial reduction of α and β cells throughout embryogenesis, demonstrating the importance of Mafb in the differentiation of both cell types [1]. Interestingly, whereas Mafb- and c-Maf-deficient mice die within a few hours after birth because of developmental defects, Mafa-deficient mice are born at predicted Mendelian ratios and are fertile [3, 15, 20, 34]. We further reported that Mafa has an intense and sustainable role in β-like cell production in the liver in comparison to Mafb, indicating the difference in reprogramming cell potency between the genes [21].
In the present study, we analyzed insulin and glucagon expressions in compound knockout mice of the large Maf genes. It has been shown that Mafb and Mafa have roles in β cells before and after birth respectively [2, 7, 11]. In addition, our results indicate that Mafa is necessary for the maintenance of normal insulin levels even in embryos and that Mafb contributes to the development of hyperglycemia in the Mafa-deficient background in adults as well as in embryos.
Materials and Methods
Mice
Previous studies have described in detail the Mafa (129Sv;ICR-Mafatm1 (LacZ) Staka/tm1 (LacZ) Staka), Mafb (129Sv;ICR-Mafbtm1 (gfp) Staka/tm1 (gfp) Staka), and c-Maf (129Sv;ICR-Maftm1 (LacZ) Mym/tm1 (LzcZ) Mym) gene knockout mice used in the current study [15, 20, 34]. In this study, the genotypes of the mutants were abbreviated as follows according to the number of alleles: wild-type (WT), Mafa−/− (A0), Mafb+/− (B1), Mafb−/− (B0), Mafa−/−;Mafb+/− (A0B1), Mafa−/−;Mafb−/− (A0B0), c-Maf+/− (C1), and c-Maf−/− (C0) mice. The genetic background of the mice used in this study was mixed with contributions from the 129Sv/J and Jcl:ICR strains. Mice were maintained under pathogen-free conditions in the Laboratory Animal Resource Center, University of Tsukuba. All experiments were performed according to the Guide for the Care and Use of Laboratory Animals at the University of Tsukuba (authorization number 14–050). The day of vaginal plug discovery was designated embryonic day (E) 0.5.
Quantitative analysis of transcripts using real-time RT-PCR
Total RNAs were isolated from whole fetal pancreata and adult pancreatic islets using the Qiagen RNeasy Kit (Qiagen, Hilden, Germany). To obtain the pancreatic islets, pancreata were removed and islets were isolated using the protocol for collagenase digestion as described previously [18]. First-strand cDNAs were synthesized using the QuantiTect Reverse Transcription System (Qiagen). Q-PCR was conducted using SYBR Premix Ex Taq II (Takara, Shiga, Japan) and a TP850 Thermal Cycler Dice Real Time System (Takara). Values were normalized to expression levels of Hprt and shown as amounts relative to those of the wild-type mice. The oligonucleotides used to prime the amplification of products from different cDNA templates included the following: Ins1, 5′-GCCCTCTGGGAGCCCAAA-3′ and 5′-AGAGAGCCTCTACCAGG-3′; Ins2, 5′-GCTTCTTCTACACACCCATGTC-3′ and 5′-AGCACTGATCTACAATGCCAC-3′; Gcg, 5′-AGGGACCTTTACCAGTGATGT-3′ and 5′-AATGGCGACTTCTTCTGGGAA-3′; and Hprt, 5′-TTGTTGTTGGATATGCCCTTGACTA-3′ and 5′-AGGCAGATGGCCACAGGACTA-3′.
Histological analysis
Pancreata were dissected, weighed, and fixed overnight in 4% paraformaldehyde in PBS. Paraffin-embedded sections were incubated in xylene to remove the paraffin and then rehydrated by incubation in a graded series of alcohol solutions. The primary antibodies and concentrations used were: guinea pig anti-insulin (1:1,000; Linco, Billerica, MA, USA); guinea pig anti-glucagon (1:1,000; Linco), rabbit anti-glucagon (1:4,000; Takara), rabbit anti-Somatostatin (1:50; Zymed, San Francisco, CA, USA), rabbit anti-Pancreatic Polypeptide (1:500; Dako, Carpintería, CA, USA), and mouse anti-ISL-1 (40.3A4, 1:300; Developmental Studies Hybridoma Bank, Iowa City, IA, USA). Immunodetection was performed using appropriate secondary antibodies conjugated with fluoresceins (Life Technologies, Carlsbad, CA, USA) and nuclear staining using 4’,6-diamidino-2-phenylindole (DAPI) (Life Technologies). Images were captured and analyzed using a Biorevo BZ-9000 microscope system (Keyence, Osaka, Japan).
Measurement of insulin and glucagon contents
Pancreas tissue samples from E18.5 fetuses and adult mice were collected and homogenized in acid-ethanol. Insulin and glucagon contents were determined using an Ultra Sensitive Mouse Insulin ELISA Kit (Morinaga, Yokohama, Japan) and Glucagon EIA Kit (Yanaihara Institute Inc., Shizuoka, Japan) respectively and normalized to pancreas wet weight as described previously [13].
Blood glucose measurement
Mice that had fasted for 12 h were anesthetized with gaseous isoflurane for venous blood collection from the retro-orbital plexus. Plasma glucose levels were measured using a Fuji DRI-CHEM 3500 (Fuji-Film, Tokyo, Japan).
Statistical analysis
Results are expressed as the mean ± standard error of the mean (SEM). Statistical analyses were performed using one-way ANOVA followed by Dunnett’s procedure or two-way ANOVA followed by Turkey’s procedure. P values are provided in the legend of the figures.
Results
Mafa and Mafb cooperate to maintain normal insulin levels in E18.5 embryos
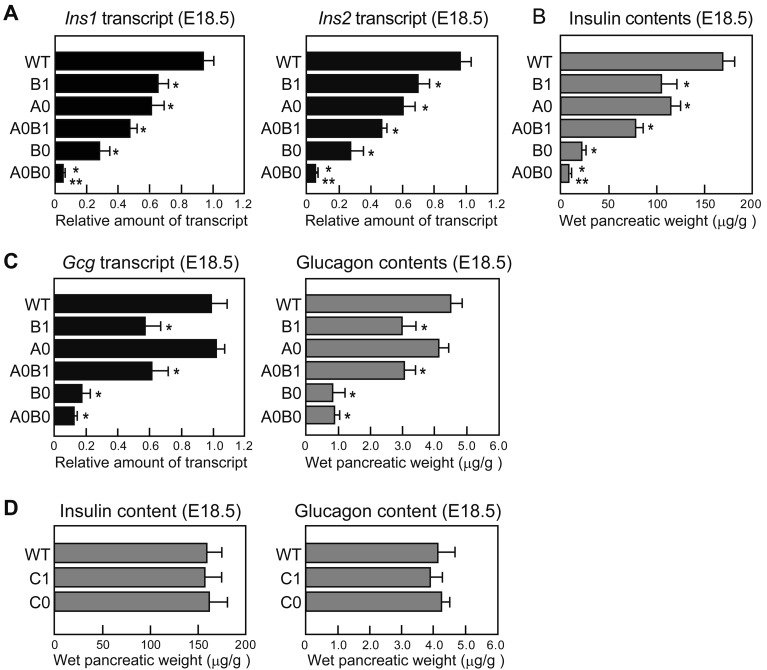
To examine the effect of Mafa and Mafb on the transcript levels of the hormones, we performed quantitative PCR using total RNAs from WT and mutant mice. Ins1 and Ins2 transcript levels of both A0 and B0 mice were decreased at E18.5, and the transcript decline was an additive allele effect of Mafa and Mafb, which resulted in a reduction of less than 10% in A0B0 mice (Fig. 1A). Additionally, the insulin content of crude pancreatic extracts showed a significant decrease that corresponded to the transcript levels of the mutant mice (Fig. 1B). We also found significant decreases in Gcg transcript and content in Mafb-mutant (B1, A0B1, and B0) mice. B1 and A0B1, and B0 and A0B0 mouse pairs showed equivalent amounts of glucagon, suggesting that Mafa was not involved in glucagon production (Fig. 1C). In addition to Mafa and Mafb, c-Maf is also reported to be expressed in adult α and β cells and to activate insulin and glucagon transcriptions in vitro [9, 19]. However, C1 and C0 mutant mice showed comparable levels of the hormones to those of WT mice (Fig. 1D).
Fig. 1.
Contribution of large Maf transcription factors to insulin and glucagon expression during embryogenesis. (A) Quantitative analysis of Ins1 and Ins2 transcripts in WT and mutant mice (n=5−15 per group; *P<0.05, vs. WT; **P<0.05, vs. B0). (B) Quantitative analysis of insulin contents in WT and mutant mice (n=5−15 per group; *P<0.05, vs WT; **P<0.05, vs. B0). (C) Quantitative analysis of Gcg transcript levels and glucagon contents in WT and mutant pancreata at E18.5 (n=4−12 per group; *P<0.05, vs. WT). (D) Insulin and glucagon contents in WT (n=6), C1 (n=5), and C0 (n=4) pancreata at E18.5. Mice genotypes were abbreviated as follows: wild-type (WT), Mafb+/− (B1), Mafa−/− (A0), Mafa−/−; Mafb+/− (A0B1), Mafb−/− (B0), Mafa−/−; Mafb−/− (A0B0), c-Maf+/− (C1), and c-Maf−/− (C0) mice.
Mafb knockout mice develop half the amount of α and β cells of wild-type mice in a Mafa-independent manner
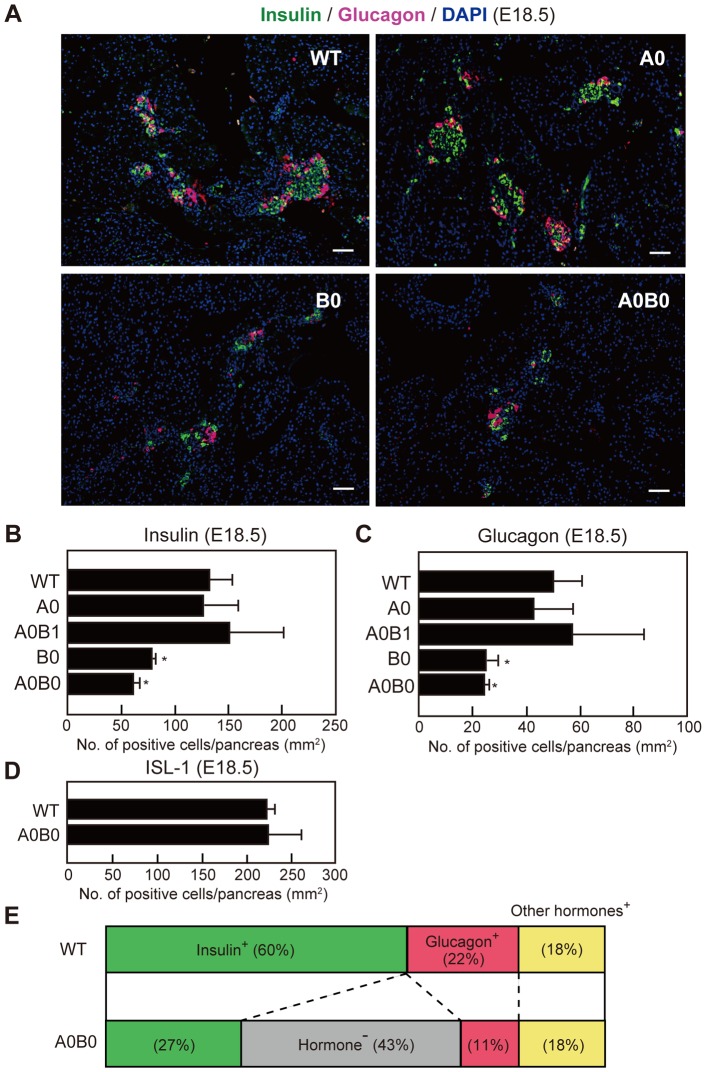
To examine whether the large Maf genes are involved in endocrine cell development, we immunostained WT and large Maf mutant pancreata using anti-insulin and anti-glucagon antibodies at E18.5 (Fig. 2A). Although there were no significant differences in α- and β-cell distribution and numbers between WT and A0 pancreata, B0 and A0B0 mice showed lower α and β cell numbers than WT mice. Quantitative analyses revealed that the numbers of α and β cells in B0 and A0B0 mice were approximately half of those of WT and A0 mice (Figs. 2B and 2C). In addition to the α- and β-cell analyses, we found that the number of somatostatin-positive δ cells and pancreatic polypeptide-positive PP cells showed no differences between the genotypes, suggesting that Large Maf genes are involved only in normal α- and β-cell development. Despite a significant reduction in α- (11% in A0B0 vs. 22% in WT) and β-cell numbers (27% in A0B0 vs. 60% in WT) in A0B0 mice, there was no reduction of the cells expressing panendocrine marker ISL-1, suggesting that approximately 40% of hormone-negative endocrine cells are likely to be found in A0B0 mice (Figs. 2D and 2E). Taking into consideration the lack of reduction in the β-cell number of A0 mice (Fig. 2) and decrease in insulin content in A0 mice (Fig. 1), accumulatively our results suggest that the β cells of A0 mice contain small numbers of insulin transcripts compared with those of WT mice.
Fig. 2.
Numbers of insulin- and glucagon-expressing cells are reduced in B0 and A0B0 embryonic pancreata. (A) Immunostaining of pancreatic sections from WT and mutant embryos at E18.5 with antibodies against insulin (green) and glucagon (magenta). Scale bars: 100 µm. (B, C, D) Numbers of insulin-positive (B), glucagon-positive (C), and ISL-1-positive cells in WT and mutant mice (n=3~4 per group, *P<0.05 between WT vs. mutant embryos). (E) Distribution of hormone-positive and hormone-negative cells in WT and A0B0 pancreata at E18.5. Mouse genotypes were abbreviated as follows: wild-type (WT), Mafa−/− (A0), Mafa−/−;Mafb+/− (A0B1), Mafb−/− (B0), and Mafa−/−; Mafb−/− (A0B0) mice. Values are presented as means ± SEM.
The influence of Mafb on insulin expression gradually decreases during the early postnatal periods
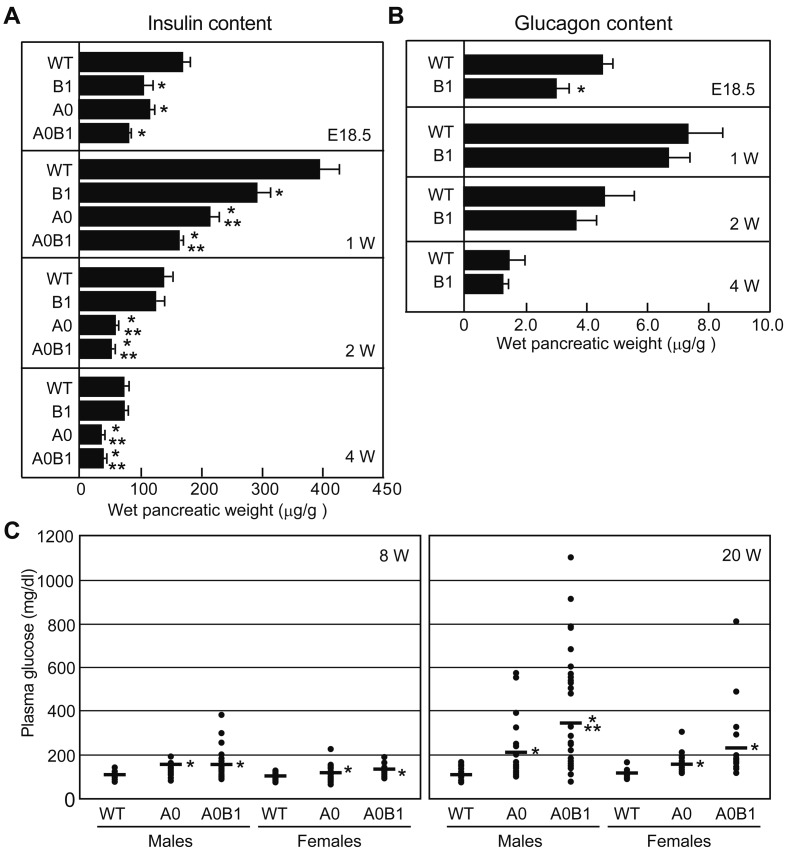
To examine the contributions of Mafa and Mafb in postnatal hormone production, we took extended measurements of insulin and glucagon contents in whole pancreata at 1, 2, and 4 weeks of age (Fig. 3A). Steady-state levels of insulin in A0, B1, and A0B1 pups at 1 week of age were significantly lower than in WT pups. Whereas lower insulin levels persisted in A0 and A0B1 mice, the levels returned to normal in B1 mice before 2 weeks of age. This result indicates that Mafa expression compensates for the insufficient insulin production of B1 mice in the neonatal period. With regard to glucagon contents, B1 embryos at E18.5 showed a significant reduction in glucagon that disappeared during the early neonatal period (Fig. 3B).
Fig. 3.
The postnatal regulation of insulin and glucagon content, and the plasma glucose level are mediated by Mafa and Mafb. (A) Insulin contents in WT, B1, A0, and A0B1 pancreata at E18.5 and 1, 2, and 4 weeks of age. Values are presented as means ± SEM (n=3−9 per group; *P<0.05, vs. WT; **P<0.05, vs. B1). (B) Glucagon contents in WT and B1 pancreata at E18.5 and 1, 2, and 4 weeks of age. Values are presented as means ± SEM (n=5−10 per group; *P<0.05, vs. WT). (C) Fasting blood glucose levels in WT and mutants at 8 and 20 weeks of age (n=10−25 per group; *P<0.05, vs. WT; **P<0.05, vs. A0). Mice genotypes are abbreviated as follows: wild-type (WT), Mafb+/− (B1), Mafa−/− (A0), Mafa−/−; Mafb+/− (A0B1), Mafb−/− (B0), and Mafa−/−; Mafb−/− (A0B0) mice.
Adult A0B1 mice exhibit higher fasting blood glucose levels than A0 mice
In adult mice, Mafb expression is observed only in α cells, and adult B1 mice exhibit normal glucose homeostasis [1]. To examine whether Mafb plays a role in the Mafa-deficient background (A0) we measured fasting blood glucose levels in adult WT, A0, and A0B1 mice at 8 and 20 weeks of age (Fig. 3C). Fasting blood glucose levels in A0 and A0B1 mice were significantly higher than in WT mice at both 8 and 20 weeks. Interestingly, A0B1 male mice showed higher glucose levels than A0 male mice at 20 weeks of age, suggesting that Mafb might contribute to the maintenance of normal glucose homeostasis under specific conditions.
Discussion
In this study we analyzed mice with single and compound knockout of the Mafa, Mafb, and c-Maf genes to determine the roles of the large Mafs in endocrine development and hormonal regulation in the pancreas. For this purpose we measured insulin and glucagon mRNA and protein levels in large Maf-mutant pancreata at strategic points in time. Despite A0 mice pancreata showing no reduction in insulin-positive cell numbers (Fig. 2), steady-state levels of Ins1 and Ins2 transcripts and insulin protein in B1 and A0 pancreata were comparable, but both were significantly lower than those of the WT pancreata (Fig. 1A).
Artner et al. report that Mafa mRNA expression in B0 pancreata is downregulated to 16% of that of WT pancreata, and that insulin-positive cell numbers in B0 pacreata are reduced to 36% of those of the WT panreata. Conversely, Mafb overexpression upregulates Mafa expression in vitro [1, 2]. These results suggest that Mafb regulates insulin expression by upregulating Mafa as well as by directly regulating insulin transcription. They also demonstrate that Mafa∆panc mutant mice, in which the Mafa is inactivated in the developing pancreas, show normal levels of insulin mRNA at E18.5, and ectopic expression of Mafb in adult β cells [2]. Differences in the genetic backgrounds of the lines of mutant mice probably explain this variance among studies.
We previously reported that A0 mice develop impaired glucose-stimulated insulin secretion and diabetes and exhibit immunohistochemical abnormalities of the islets after birth [34]. In this study we compared the phenotypes of A0, B0, and A0B0 mice to test our hypothesis that the normal islet architecture observed in embryonic A0 pancreata may be due to the Mafb-mediated mechanisms in β cells. Our analyses demonstrated that the numbers of insulin-positive and glucagon-positive cells in A0B0 mice were comparable to those observed in B0 mice, suggesting that Mafa had little effect on β-cell development. These results indicate that neither Mafa nor Mafb plays redundant roles in β-cell development, and that Mafb alone has a dominant function in embryonic development of β cells [1, 23]. Although the mechanisms for the formation of abnormal architecture in A0 islets have not been clear, the reduction in β-cell proliferation might be involved in alteration of the β- to α-cell ratio [24]. Similar islet malformation to A0 mice is also observed in cyclin D1 and D2 mutant mice, which exhibit impaired glucose-stimulated insulin secretion along with reduced β-cell proliferation [17].
The contribution of Mafb to insulin production appears to decrease gradually in response to the shift of Mafb expression to α cells during β-cell maturation (Fig. 3A). Although at 8 weeks of age fasting blood glucose levels of A0B1 mice showed comparable levels to those of A0 mice, at 20 weeks, A0B1 mice developed more severe hyperglycemia than A0 mice. This suggests that Mafb also functions in controlling glucose levels in adults with the A0 background. Since Mafb expression is reported to be activated ectopically in β cells during pregnancy, it remains unclear whether embryonic maldevelopment of A0B1 β-cells or ectopic expression of Mafb in A0 mice pancreata contributes to the high blood glucose levels detected in A0B1 mice.
Consistent with a previous report, we found by analyzing B0 mice that only Mafb was involved in α-cell development and glucagon production [1]. The expression of a cascade of different transcription factors including Nkx2.2, Pax6, Foxa2, Mafb, and Arx, stimulates the differentiation into α-cell lineage [4, 5, 8]. Recent advances in diabetes research have revealed that dedifferentiated β cells stressed by high blood glucose levels and oxidative conditions were spontaneously capable of redifferentiating into α cells via molecular mechanisms that were not clearly understood [29,30,31]. The molecular process of α-cell development and their conversion from β cells is not well understood. Thus it is important to elucidate the basis of α-cell genesis from diabetic β cells and also developmental progenitor cells for a fuller understanding of the pathophysiology of the disease [6, 32].
In summary, we analyzed insulin and glucagon levels in compound knockout mice of the large Maf genes. Our results indicate that Mafa is necessary for the maintenance of normal insulin levels even in embryos and that Mafb contributes to the development of hyperglycemia in the Mafa-deficient background in adults as well as in embryos.
Acknowledgments
The authors thank Masami Ojima for technical assistance and Thomas Mayers for grammatical revision of the manuscript. This work was supported in part by the Grants-in-Aid for Scientific Research (KAKENHI no. 26221004 and 26640049) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT).
References
- 1.Artner I., Blanchi B., Raum J.C., Guo M., Kaneko T., Cordes S., Sieweke M., Stein R.2007. MafB is required for islet beta cell maturation. Proc. Natl. Acad. Sci. USA 104: 3853–3858. doi: 10.1073/pnas.0700013104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Artner I., Hang Y., Mazur M., Yamamoto T., Guo M., Lindner J., Magnuson M.A., Stein R.2010. MafA and MafB regulate genes critical to beta-cells in a unique temporal manner. Diabetes 59: 2530–2539. doi: 10.2337/db10-0190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aziz A., Vanhille L., Mohideen P., Kelly L.M., Otto C., Bakri Y., Mossadegh N., Sarrazin S., Sieweke M.H.2006. Development of macrophages with altered actin organization in the absence of MafB. Mol. Cell. Biol. 26: 6808–6818. doi: 10.1128/MCB.00245-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benitez C.M., Goodyer W.R., Kim S.K.2012. Deconstructing pancreas developmental biology. Cold Spring Harb. Perspect. Biol. 4: 4. doi: 10.1101/cshperspect.a012401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collombat P., Mansouri A., Hecksher-Sorensen J., Serup P., Krull J., Gradwohl G., Gruss P.2003. Opposing actions of Arx and Pax4 in endocrine pancreas development. Genes Dev. 17: 2591–2603. doi: 10.1101/gad.269003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dor Y., Glaser B.2013. β-cell dedifferentiation and type 2 diabetes. N. Engl. J. Med. 368: 572–573. doi: 10.1056/NEJMcibr1214034 [DOI] [PubMed] [Google Scholar]
- 7.Eto K., Nishimura W., Oishi H., Udagawa H., Kawaguchi M., Hiramoto M., Fujiwara T., Takahashi S., Yasuda K.2014. MafA is required for postnatal proliferation of pancreatic β-cells. PLoS ONE 9: e104184. doi: 10.1371/journal.pone.0104184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friedman J.R., Kaestner K.H.2006. The Foxa family of transcription factors in development and metabolism. Cell. Mol. Life Sci. 63: 2317–2328. doi: 10.1007/s00018-006-6095-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gosmain Y., Avril I., Mamin A., Philippe J.2007. Pax-6 and c-Maf functionally interact with the alpha-cell-specific DNA element G1 in vivo to promote glucagon gene expression. J. Biol. Chem. 282: 35024–35034. doi: 10.1074/jbc.M702795200 [DOI] [PubMed] [Google Scholar]
- 10.Gradwohl G., Dierich A., LeMeur M., Guillemot F.2000. neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc. Natl. Acad. Sci. USA 97: 1607–1611. doi: 10.1073/pnas.97.4.1607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hang Y., Yamamoto T., Benninger R.K., Brissova M., Guo M., Bush W., Piston D.W., Powers A.C., Magnuson M., Thurmond D.C., Stein R.2014. The MafA transcription factor becomes essential to islet β-cells soon after birth. Diabetes 63: 1994–2005. doi: 10.2337/db13-1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hay C.W., Docherty K.2006. Comparative analysis of insulin gene promoters: implications for diabetes research. Diabetes 55: 3201–3213. doi: 10.2337/db06-0788 [DOI] [PubMed] [Google Scholar]
- 13.im Walde S.S., Dohle C., Schott-Ohly P., Gleichmann H.2002. Molecular target structures in alloxan-induced diabetes in mice. Life Sci. 71: 1681–1694. doi: 10.1016/S0024-3205(02)01918-5 [DOI] [PubMed] [Google Scholar]
- 14.Kataoka K.2007. Multiple mechanisms and functions of maf transcription factors in the regulation of tissue-specific genes. J. Biochem. 141: 775–781. doi: 10.1093/jb/mvm105 [DOI] [PubMed] [Google Scholar]
- 15.Kawauchi S., Takahashi S., Nakajima O., Ogino H., Morita M., Nishizawa M., Yasuda K., Yamamoto M.1999. Regulation of lens fiber cell differentiation by transcription factor c-Maf. J. Biol. Chem. 274: 19254–19260. doi: 10.1074/jbc.274.27.19254 [DOI] [PubMed] [Google Scholar]
- 16.Kroon E., Martinson L.A., Kadoya K., Bang A.G., Kelly O.G., Eliazer S., Young H., Richardson M., Smart N.G., Cunningham J., Agulnick A.D., D’Amour K.A., Carpenter M.K., Baetge E.E.2008. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat. Biotechnol. 26: 443–452. doi: 10.1038/nbt1393 [DOI] [PubMed] [Google Scholar]
- 17.Kushner J.A., Ciemerych M.A., Sicinska E., Wartschow L.M., Teta M., Long S.Y., Sicinski P., White M.F.2005. Cyclins D2 and D1 are essential for postnatal pancreatic beta-cell growth. Mol. Cell. Biol. 25: 3752–3762. doi: 10.1128/MCB.25.9.3752-3762.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lacy P.E., Kostianovsky M.1967. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes 16: 35–39. doi: 10.2337/diab.16.1.35 [DOI] [PubMed] [Google Scholar]
- 19.Matsuoka T.A., Zhao L., Artner I., Jarrett H.W., Friedman D., Means A., Stein R.2003. Members of the large Maf transcription family regulate insulin gene transcription in islet beta cells. Mol. Cell. Biol. 23: 6049–6062. doi: 10.1128/MCB.23.17.6049-6062.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moriguchi T., Hamada M., Morito N., Terunuma T., Hasegawa K., Zhang C., Yokomizo T., Esaki R., Kuroda E., Yoh K., Kudo T., Nagata M., Greaves D.R., Engel J.D., Yamamoto M., Takahashi S.2006. MafB is essential for renal development and F4/80 expression in macrophages. Mol. Cell. Biol. 26: 5715–5727. doi: 10.1128/MCB.00001-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagasaki H., Katsumata T., Oishi H., Tai P.H., Sekiguchi Y., Koshida R., Jung Y., Kudo T., Takahashi S.2014. Generation of insulin-producing cells from the mouse liver using β cell-related gene transfer including Mafa and Mafb. PLoS ONE 9: e113022. doi: 10.1371/journal.pone.0113022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nishimura W., Kondo T., Salameh T., El Khattabi I., Dodge R., Bonner-Weir S., Sharma A.2006. A switch from MafB to MafA expression accompanies differentiation to pancreatic beta-cells. Dev. Biol. 293: 526–539. doi: 10.1016/j.ydbio.2006.02.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nishimura W., Rowan S., Salameh T., Maas R.L., Bonner-Weir S., Sell S.M., Sharma A.2008. Preferential reduction of beta cells derived from Pax6-MafB pathway in MafB deficient mice. Dev. Biol. 314: 443–456. doi: 10.1016/j.ydbio.2007.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishimura W., Takahashi S., Yasuda K.2015. MafA is critical for maintenance of the mature beta cell phenotype in mice. Diabetologia 58: 566–574. doi: 10.1007/s00125-014-3464-9 [DOI] [PubMed] [Google Scholar]
- 25.Nishizawa M., Kataoka K., Goto N., Fujiwara K.T., Kawai S.1989. v-maf, a viral oncogene that encodes a “leucine zipper” motif. Proc. Natl. Acad. Sci. USA 86: 7711–7715. doi: 10.1073/pnas.86.20.7711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohneda K., Ee H., German M.2000. Regulation of insulin gene transcription. Semin. Cell Dev. Biol. 11: 227–233. doi: 10.1006/scdb.2000.0171 [DOI] [PubMed] [Google Scholar]
- 27.Orci L., Unger R.H.1975. Functional subdivision of islets of Langerhans and possible role of D cells. Lancet 2: 1243–1244. doi: 10.1016/S0140-6736(75)92078-4 [DOI] [PubMed] [Google Scholar]
- 28.Slack J.M.1995. Developmental biology of the pancreas. Development 121: 1569–1580. [DOI] [PubMed] [Google Scholar]
- 29.Spijker H.S., Ravelli R.B., Mommaas-Kienhuis A.M., van Apeldoorn A.A., Engelse M.A., Zaldumbide A., Bonner-Weir S., Rabelink T.J., Hoeben R.C., Clevers H., Mummery C.L., Carlotti F., de Koning E.J.2013. Conversion of mature human β-cells into glucagon-producing α-cells. Diabetes 62: 2471–2480. doi: 10.2337/db12-1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Talchai C., Xuan S., Lin H.V., Sussel L., Accili D.2012. Pancreatic β cell dedifferentiation as a mechanism of diabetic β cell failure. Cell 150: 1223–1234. doi: 10.1016/j.cell.2012.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Z., York N.W., Nichols C.G., Remedi M.S.2014. Pancreatic β cell dedifferentiation in diabetes and redifferentiation following insulin therapy. Cell Metab. 19: 872–882. doi: 10.1016/j.cmet.2014.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weir G.C., Aguayo-Mazzucato C., Bonner-Weir S.2013. β-cell dedifferentiation in diabetes is important, but what is it? Islets 5: 233–237. doi: 10.4161/isl.27494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.ZeRuth G.T., Takeda Y., Jetten A.M.2013. The Krüppel-like protein Gli-similar 3 (Glis3) functions as a key regulator of insulin transcription. Mol. Endocrinol. 27: 1692–1705. doi: 10.1210/me.2013-1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang C., Moriguchi T., Kajihara M., Esaki R., Harada A., Shimohata H., Oishi H., Hamada M., Morito N., Hasegawa K., Kudo T., Engel J.D., Yamamoto M., Takahashi S.2005. MafA is a key regulator of glucose-stimulated insulin secretion. Mol. Cell. Biol. 25: 4969–4976. doi: 10.1128/MCB.25.12.4969-4976.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]