Abstract
Introduction
The aim of this study was to determine whether baseline right ventricular (RV) function assessed by standard echocardiography may indicate patients who will respond to cardiac resynchronization therapy (CRT).
Material and methods
The data of 57 patients (54 men, 95%), aged 66.4 ±8.7 years with heart failure (HF) having a CRT device implanted were collected. All patients had left ventricular ejection fraction (LVEF) ≤ 35% and QRS complex duration ≥ 120 ms. Echocardiographic examination with tissue Doppler imaging techniques and complex RV evaluation were performed at baseline and three months after CRT onset.
Results
Three months after CRT implantation, patients responding to CRT, defined as a reduction of left ventricle end-systolic volume (LVESV) of at least 10% (n = 34), compared to patients with a reduction of LVESV of less than 10% (n = 23), had at baseline a smaller right atrium diameter (47.85 ±11.33 mm vs. 52.65 ±8.69 mm; p = 0.028), higher TAPSE (14.56 ±2.57 mm vs. 13.04 ±2.93 mm; p = 0.030) and lower grade of tricuspid valve regurgitation (1.82 ±0.97 vs. 2.3 ±0.88; p = 0.033).
Conclusions
This study showed that there are differences in baseline right ventricular function between responders and non-responders to CRT. Yet in our study, none of the baseline RV parameters provided any value in identifying patients who would respond to CRT.
Keywords: cardiac resynchronization therapy, right ventricle, echocardiography, heart failure
Introduction
Cardiac resynchronization therapy (CRT) is a well-established treatment that improves morbidity and mortality in patients with symptomatic, drug refractory heart failure (HF). Cardiac resynchronization therapy reverses left ventricular (LV) remodeling, reduces pathological neurohormonal activation and improves functional status [1–4]. The effects of CRT on LV contractility and synchrony are well-demonstrated, yet little is known about the influence of CRT on right ventricular (RV) function [5]. It seems that the role of the RV in HF has been thus far underestimated, as it was proven to be an independent outcome predictor in patients with moderate to severe HF [6]. Studies have also shown that the LV response to CRT may depend on the baseline RV performance [7, 8]. Moreover, beneficial effects of CRT may be limited by baseline RV function.
The aim of this study was to compare baseline RV echocardiographic parameters between responders and non-responders to CRT, with the hope of identifying parameters that might predict response to CRT.
Material and methods
Patient population
A total of 60 consecutive patients who underwent implantation of a biventricular pacemaker (CRT-P) or defibrillator (CRT-D) for drug-refractory heart failure (71.9% with an ischemic background and 28.1% with dilated cardiomyopathy) were evaluated. All patients fulfilled current inclusion criteria for CRT (New York Heart Association (NYHA) class III–IV despite optimal pharmacological treatment, left ventricular ejection fraction (LVEF) ≤ 35% and QRS duration ≥ 120 ms). Patients in NYHA class IV could not have a history of hospitalization due to HF exacerbation within one month prior to CRT implantation [9]. Inclusion of patients with permanent atrial fibrillation in the study was only possible if more than 95% of ventricular pacing was achieved. If an adequate level of ventricular pacing was impossible to achieve with medication, atrioventricular (AV) junction ablation was performed. All patients provided written informed consent and the study had the approval of a local ethics committee. According to the study protocol, all patients were followed for 12 weeks. During that time three patients died – one due to stroke, one due to myocardial infarction and one of unknown reason. Finally, 57 patients were studied (54 men – 95%, 3 women – 5%, aged 66.4 ±8.7 years). Demographic and clinical variables of the patient group are presented in Table I.
Table I.
Clinical data and pharmacotherapy of the study group (n = 57) – data presented as mean value with standard deviation (SD) or percentage of patients (%)
| Parameter | Results |
|---|---|
| Age [years] | 66.35 ±8.69 |
| Body mass index (BMI) [kg/m2] | 25.82 ±4.15 |
| Male gender, n (%) | 54 (94.7) |
| History of myocardial infarction (%) | 64.9 |
| Diabetes (%) | 40.4 |
| Hypertension (%) | 63.2 |
| Chronic obstructive pulmonary disease (%) | 19.3 |
| Hypercholesterolemia (%) | 77.2 |
| Paroxysmal atrial fibrillation (%) | 22.8 |
| Permanent atrial fibrillation (%) | 17.5 |
| Left bundle branch block (%) | 66.6 |
| Anemia (%) | 3.5 |
| Smoking (%) | 22.8 |
| Chronic kidney disease (%) | 31.6 |
| Ischemic background of heart failure (%) | 71.9 |
| History of coronary artery bypass graft (CABG) (%) | 14.0 |
| History of percutaneous coronary intervention (PCI) (%) | 17.5 |
| B-blockers (%) | 96.5 |
| Angiotensin converting enzyme inhibitors (ACEi)/angiotensin receptor blockers (ARB) (%) | 86.0 |
| Loop diuretics (%) | 87.8 |
| Digoxin (%) | 22.8 |
| Amiodarone (%) | 31.6 |
Device implantation
All CRT implantation procedures were performed under local anesthesia. Leads were placed transvenously via the cephalic and/or subclavian vein. In the presence of sinus rhythm, CRT-P with an atrial lead (setting: DDDR; n = 26) was implanted, while in cases of permanent atrial fibrillation no atrial lead (setting: VVIR; n = 13) was placed. Patients having a history of cardiac arrest and/or malignant ventricular arrhythmias were indicated for placement of CRT-D (n = 18). For patients receiving an atrial lead, placement for each was the right atrial (RA) appendage. In 48 patients the right ventricular lead was placed in the right ventricle outflow tract (RVOT) and in 9 patients in the RV apex. No RV lead implanted de novo was placed in the RV apex. Right ventricular lead apical position was present only in patients in whom CRT was an upgrade of a previously existing device (14 cases of device upgrade: VVI – n = 2, DDD – n = 9, DDD-ICD – n = 3). In each case a coronary sinus venogram was obtained using a balloon catheter followed by insertion of the LV pacing lead. The left ventricular lead was directed to a tributary of the coronary sinus vein and placed in a stable lateral (n = 49) or postero-lateral (n = 8) position, with a < 3.5 V capture threshold. The final position of the LV pacing lead was assessed with cine fluoroscopy. Atrioventricular (AV) delay remained on the standard program (120 ms for non-paced atrial rhythm/150 ms for paced atrial beat) except for the patients with continued ventricular conduction, in whom the AV delay was shortened until the ventricles were consistently paced (4 cases). A nominal value of 5 ms was left for interventricular (VV) timing, with the left ventricle paced first. If no signs of biventricular stimulation in the body surface ECG were observed, VV timing was changed to elicit QRS fusion beats in ECG lead V1.
Study design
Fifty-seven patients were evaluated prior to and 3 months (12–16 weeks) after CRT implantation. Medical history including hospitalizations was taken, NYHA class was assessed and a 6-minute walk test (6-MWT) was performed. Echocardiographic examinations were performed using a GE Vivid 7 device (GE-Vingmed Vivid 7 system; GE Vingmed Ultrasound, Horten, Norway). A routine check-up to assess wound and stimulation parameters took place one month after CRT implantation. In one case it was necessary to perform AV junction ablation, as 95% of ventricular stimulation was not obtained despite maximal pharmacological treatment. Follow-up was than extended to 3 months after successful AV junction ablation. All study participants underwent coronary angiography prior to the CRT procedure. In the presence of at least 50% stenosis of one or more coronary artery branches or a history of coronary artery bypass graft (CABG) or percutaneous coronary intervention (PCI), an ischemic background of congestive heart failure (CHF) was diagnosed.
Echocardiographic assessment
All measurements were obtained following the recommendations of the European Association of Echocardiography and the American Society of Echocardiography [10] at baseline and during follow-up. Echocardiographic studies were performed by the same physician and were reported as an average of three measurements. The RV dimensions and RA dimensions were estimated at end-diastole from a right ventricle-focused apical 4-chamber view. The left parasternal view was used for measuring RV wall thickness. Right ventricular systolic pressure (RVSP) was estimated from tricuspid regurgitation (TR) velocity with the addition of RA pressure. Tricuspid annular plane systolic excursion (TAPSE) was acquired by placing an M-mode cursor through the tricuspid annulus and measuring the amount of longitudinal motion of the annulus at peak systole. S’, E’ and A’ were measured in an apical 4-chamber window with a tissue Doppler pulsed sample volume placed in the tricuspid annulus. To measure right ventricular fractional area change (FAC) the endocardial border was traced in apical 4-chamber views from the tricuspid annulus along the free wall to the apex, then back to the annulus, along the interventricular septum at end-diastole and end-systole. Trabeculation, tricuspid leaflets, and chords were included in the chamber. TR was measured by color Doppler as regurgitation jet area and also as the percentage of right atrium area covered by the regurgitation jet (Table II).
Table II.
Clinical outcomes and echocardiographic parameters after CRT implantation
| Parameter | Directly after implantation (n = 57) | 3 months follow-up (n = 57) | Value of p |
|---|---|---|---|
| NYHA | 3.11 ±0.28 | 2.25 ±0.68 | < 0.001 |
| 6-MWT [m] | 298.04 ±107.42 | 373.12 ±127.15 | < 0.001 |
| QRS [ms] | 184.23 ±28.31 | 152.70 ±19.11 | < 0.001 |
| LVEDd [mm] | 73.33 ±8.94 | 71.54 ±9.87 | 0.005 |
| LVESd [mm] | 62.40 ±10.03 | 60.60 ±11.29 | 0.081 |
| LVEF (%) | 21.70 ±4.81 | 26.05 ±4.86 | < 0.001 |
| LVEDV [ml] | 244.30 ±83.79 | 226.42 ±88.61 | < 0.001 |
| LVESV [ml] | 192.79 ±71.95 | 168.67 ±76.48 | < 0.001 |
| RA minor dimension [mm] | 37.67 ±7.39 | 36.42 ±6.18 | 0.116 |
| RA major dimension [mm] | 49.79 ±10.53 | 48.33 ±9.47 | 0.040 |
| RA end-systolic area [cm2] | 17.49 ±6.30 | 17.06 ±5.91 | 0.155 |
| RV wall thickness [mm] | 5.47 ±1.20 | 5.12 ±1.02 | 0.028 |
| RV longitudinal dimension [mm] | 25.35 ±7.02 | 25.84 ±6.37 | 0.342 |
| RV basal dimension [mm] | 28.74 ±4.47 | 28.32 ±4.24 | 0.266 |
| RV mid cavity dimension [mm] | 24.21 ±4.85 | 23.75 ±5.20 | 0.160 |
| RV end-diastolic area [cm2] | 15.72 ±5.20 | 15.62 ±5.45 | 0.927 |
| RV end-systolic area [cm2] | 10.94 ±4.51 | 10.22 ±4.25 | 0.007 |
| RV fractional area change (%) | 31.35 ±10.32 | 35.40 ±10.51 | < 0.001 |
| TAPSE [mm] | 13.95 ±2.80 | 15.79 ±2.33 | < 0.001 |
| S’ [cm/s] | 8.84 ±3.45 | 11.00 ±3.43 | < 0.001 |
RA – right atrium, RV – right ventricle, TAPSE – tricuspid annular plane systolic excursion, S’ – peak systolic annular velocity, E’ – peak early diastolic annular velocity, A’ – peak late diastolic annular velocity, TR – tricuspid regurgitation, TR%RA – percentage of right atrium area covered by regurgitation jet, RVSP – right ventricle systolic pressure. *Only in patients with sinus rhythm.
Presentations were acquired with General Electric Healthcare Vivid 7 device and included typical long- and short-axis and apical views. Acquired images were analyzed offline using the commercially available software EchoPAC PC version 6.00 (GE Vingmed Ultrasound).
Statistical analysis
Statistical analysis was performed with SAS System 9.1 (SAS Institute Inc., Cary, North Carolina, USA). All parameters were tested for normal distribution with the Shapiro-Wilk test. Student's t-test or Mann-Whitney-U test was applied to determine whether the parameters’ averages before and after the CRT introduction were significantly different. Logistic regression analysis was applied to assess the odds ratios for predictive values of RV parameters using a 95% confidence interval. Statistical significance was considered when p < 0.05. A receiver-operating characteristic (ROC) curve was generated to determine the predictive power of TAPSE for a positive response to CRT.
Results
In the study group, mean NYHA class decreased, 6-MWT distance increased and mean QRS duration decreased. The LV diastolic diameter, LV diastolic and systolic volumes, RA major diameter, RV wall thickness and RV end-systolic area all decreased, whereas EF, RV fractional area change and TAPSE increased. No differences regarding LV end systolic diameter and other RA and RV dimensions were observed.
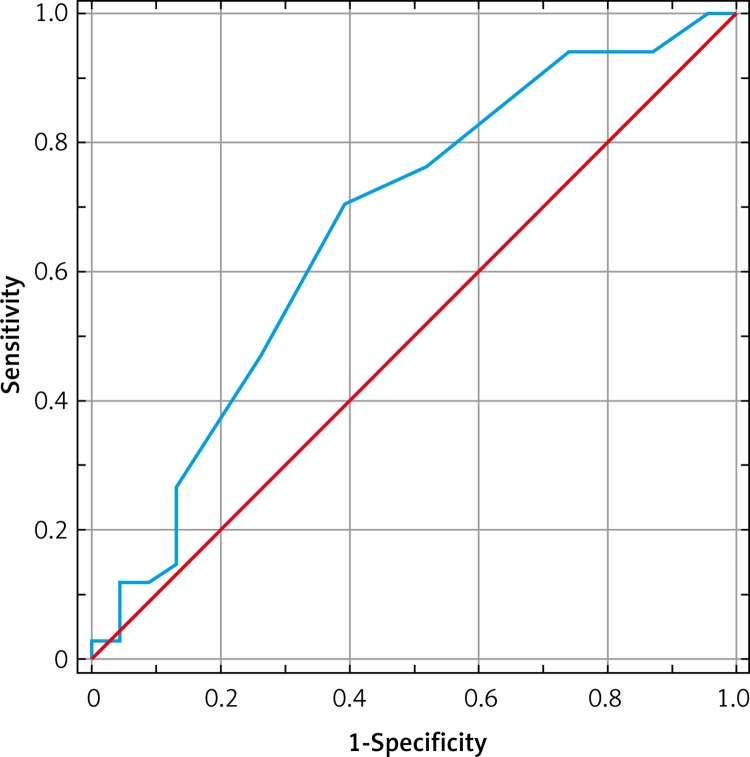
As many as 34 (59.6%) patients responded to CRT according to the applied definition of left ventricle end-systolic volume reduction (LVESV) ≥ 10%. In the group of ischemic HF background the response rate equaled 51.2% (21 out of 41 patients) while in the group of non-ischemic etiology it was 81.3% (13 of 16 patients, p < 0.05). Responders to CRT altogether, as compared to patients with no response to CRT (n = 23), had at baseline a smaller right atrium diameter (47.85 ±11.33 mm vs. 52.65 ±8.69 mm; p = 0.028), higher TAPSE (14.56 ±2.57 mm vs. 13.04 ±2.93 mm; p = 0.030) and a lower grade of tricuspid valve regurgitation (1.82 ±0.97 vs. 2.3 ±0.88; p = 0.033) (Table III). The ROC curve showed that the best cut-off value of TAPSE to predict a good response to CRT was 14 mm with sensitivity of 71%, specificity of 61%, negative predictive value (NPV) of 58% and positive predictive value (PPV) of 73% (Figure 1; Table IV). Of the RV echocardiographic parameters assessed at baseline, no single parameter was able to provide a statistically significant predictive value of response to CRT.
Table III.
Baseline echocardiographic parameters in responders and non-responders to CRT
| Parameter | Non-responders (n = 23) | Responders (n = 34) | Value of p |
|---|---|---|---|
| RA minor dimension [mm] | 38.35 ±7.08 | 37.21 ±7.66 | 0.489 |
| RA major dimension [mm] | 52.65 ±8.69 | 47.85 ±11.33 | 0.028 |
| RA area [cm2] | 19.01 ±5.70 | 16.47 ±6.57 | 0.064 |
| RV wall thickness [mm] | 5.78 ±1.24 | 5.26 ±1.14 | 0.309 |
| RV diastole [mm] | 26.3 ±8.08 | 24.71 ±6.25 | 0.660 |
| RV systole [mm] | 21.74 ±7.96 | 19.85 ±6.56 | 0.499 |
| RV basal minor dimension [mm] | 28.83 ±4.67 | 28.68 ±4.40 | 0.987 |
| RV mid cavity dimension [mm] | 23.83 ±4.74 | 24.47 ±4.97 | 0.858 |
| RV 4CH systolic area [cm2] | 11.07 ±4.61 | 10.86 ±4.51 | 0.776 |
| RV 4CH diastolic area [cm2] | 15.76 ±5.60 | 15.69 ±5.00 | 0.884 |
| RV fractional area change (%) | 30.91 ±8.42 | 31.65 ±11.54 | 0.948 |
| TAPSE [mm] | 13.04 ±2.93 | 14.56 ±2.57 | 0.030 |
| S’ [cm/s] | 8.17 ±3.27 | 9.29 ±3.55 | 0.269 |
| E’ [cm/s]* | 7.52 ±3.33 | 8.24 ±2.94 | 0.323 |
| A’ [cm/s]* | 8.43 ±4.26 | 10.53 ±4.91 | 0.126 |
| TR | 2.30 ±0.88 | 1.82 ±0.97 | 0.033 |
| TR%RA (%) | 23.52 ±11.43 | 18.03 ±11.74 | 0.051 |
| RVSP [mm Hg] | 33.95 ±22.56 | 29.13 ±18.96 | 0.087 |
RA – right atrium, RV – right ventricle, TAPSE – tricuspid annular plane systolic excursion, S’ – peak systolic annular velocity, E’ – peak early diastolic annular velocity, A’ – peak late diastolic annular velocity, TR – tricuspid regurgitation, TR%RA – percentage of right atrium area covered by regurgitation jet, RVSP – right ventricle systolic pressure.
Only in patients with sinus rhythm.
Figure 1.
ROC curve showing best cut-off point of TAPSE for predicting positive response to CRT
Table IV.
Cut-off values of TAPSE for predicting response to CRT
| TAPSE | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|
| 22 | 0.029 | 1.000 | 1.000 | 0.411 |
| 20 | 0.029 | 0.957 | 0.500 | 0.400 |
| 19 | 0.118 | 0.957 | 0.800 | 0.423 |
| 18 | 0.118 | 0.913 | 0.667 | 0.412 |
| 17 | 0.147 | 0.870 | 0.625 | 0.408 |
| 16 | 0.265 | 0.870 | 0.750 | 0.444 |
| 15 | 0.471 | 0.739 | 0.727 | 0.486 |
| 14 | 0.706 | 0.609 | 0.727 | 0.583 |
| 13 | 0.765 | 0.478 | 0.684 | 0.579 |
| 12 | 0.941 | 0.261 | 0.653 | 0.750 |
| 11 | 0.941 | 0.130 | 0.615 | 0.600 |
| 10 | 1.000 | 0.043 | 0.607 | 1.000 |
TAPSE – tricuspid annular plane systolic excursion, PPV – positive predictive value, NPV – negative predictive value.
Discussion
The major finding of the current study is that patients responding to CRT have better baseline RV function, especially systolic function assessed by TAPSE measurements. Yet there is no single RV parameter that could indicate patients who are likely to respond to CRT.
Cardiac resynchronization therapy undoubtedly has a positive effect on RV function [11]. Left ventricular ejection fraction remains the most important echocardiographic parameter in selecting patients for CRT. However, there is a constant need to find additional parameters, as a large percentage of patients still do not benefit from CRT. Right ventricular dysfunction markers may indicate more severe heart disease, and in the presence of low LVEF reflect poorer global function of heart muscle. In this situation improving myocardial synchrony may be of only limited significance.
Response to cardiac resynchronization therapy and baseline right ventricular function
In our study we decided to define response to CRT as having at least a 10% reduction of end-systolic diameter (LVESD). This definition of CRT response is proven to correlate with better long-term survival and all-cause mortality reduction regardless of HF etiology [12]. According to selected criteria, the response rate in our study group was 60%, which is in concordance with the available literature [13].
It is still not yet well established how right ventricle baseline function affects the response to CRT. Data from recent studies demonstrate that some parameters assessing RV function may have potential to indicate patients who will respond to CRT. Among them is the TAPSE. Tricuspid annular plane systolic excursion is simple to measure, does not require special equipment or prolonged examination, is less dependent on optimal image quality and is reproducible [14]. Moreover, it has a good correlation with radionuclide-derived RVEF. Echocardiographic data from CARE-HF show that in patients with baseline TAPSE of ≤ 14 mm, after 18 months of follow-up there is less improvement in LVEF and LVESV. In CARE-HF, unlike intraventricular mechanical delay and etiology of HF, TAPSE was an independent predictor of echocardiographic response. CARE-HF focused only on selected RV parameters, mostly assessing LV function. In our study TAPSE was of borderline significance as an independent predictor of echocardiographic response (Table V).
Table V.
Predictive value of selected RV echocardiographic parameters in responders to CRT
| Parameter | OR | 95% CI |
|---|---|---|
| RA minor dimension [mm] | 0.979 | (0.91–1.05) |
| RA major dimension [mm] | 0.956 | (0.91–1.01) |
| RA area [cm2] | 0.936 | (0.86–1.02) |
| RV wall thickness [mm] | 0.681 | (0.42–1.10) |
| RV diastole [mm] | 0.968 | (0.90–1.05) |
| RV systole [mm] | 0.963 | (0.89–1.04) |
| RV basal minor dimension [mm] | 0.992 | (0.88–1.12) |
| RV mid cavity dimension [mm] | 1.029 | (0.92–1.15) |
| RV 4CH systolic area [cm2] | 0.990 | (0.88–1.11) |
| RV 4CH diastolic area [cm2] | 0.998 | (0.90–1.11) |
| RV FAC (%) | 1.007 | (0.96–1.06) |
| TAPSE [mm] | 1.249 | (1.00–1.57) |
| S’ [cm/s] | 1.105 | (0.94–1.30) |
| E’ [cm/s]* | 1.080 | (0.91–1.29) |
| A’ [cm/s]* | 1.107 | (0.98–1.25) |
| TR | 0.577 | (0.32–1.04) |
| TR%RA (%) | 0.960 | (0.92–1.01) |
| RVSP [mm Hg] | 0.988 | (0.96–1.02) |
RA – right atrium, RV – right ventricle, TAPSE – tricuspid annular plane systolic excursion, TR – tricuspid regurgitation, TR%RA – percentage of right atrium area covered by regurgitation jet, S’ – peak systolic annular velocity, E’ – peak early diastolic annular velocity, A’ – peak late diastolic annular velocity, RVSP – right ventricle systolic pressure.
Only in patients with sinus rhythm.
Patients responding to CRT in our study had higher TAPSE value at baseline. In our study the cut-off point for response to CRT was 14 mm, below the standard echocardiographic cut-off value of 15 mm. Tricuspid annular plane systolic excursion is the most described alternative to right ventricular ejection fraction (RVEF) estimation, which is often challenging due to the RV being a complex structure. There is also growing evidence that TAPSE may help identify patients who would respond to CRT [15–17], with some studies showing this parameter to be even a potential prognostic marker of major adverse events [16]. A low TAPSE value may indicate poor RV performance, which may reflect more extensive and severe heart disease. In such cases, improving myocardial synchrony may not give clinically significant benefits. In our opinion, the clinical value of this parameter requires further investigation.
Our observation also showed that a lower grade of tricuspid regurgitation (TR) may indicate patients with a less favorable response to CRT. In adults, TR is usually a consequence of left-sided valvular lesions or chronic pulmonary disease. In patients with advanced heart failure, there is elevated LV filling pressure and mitral regurgitation (MR) due to ventricle dilatation. Lower grades of TR may reflect less remodeling of LV and indicate patients who will potentially benefit from CRT. Kanzaki et al. found in their study on 26 patients undergoing CRT implantation that resynchronization therapy immediately reduces MR, regurgitant volume and regurgitant fraction. Mitral regurgitation reduction results in decreased pressure in pulmonary arteries and lower RV afterload [18]. This mechanism might be responsible for the improvement of TR observed in our study. Moreover, TR grade and area of regurgitation wave to the right atrium correlated positively in our study with LVESV reduction in the entire study population. Some studies have shown that increased pulmonary artery pressure is associated with non-response to CRT [7]. This observation was not confirmed in our study. While there was a difference in pulmonary pressure at baseline between responders and non-responders to CRT, statistical significance was not reached.
Results from a study to evaluate right ventricle function in patients undergoing cardiac resynchronization therapy conducted by Scuteri et al. showed that all echocardiographic indices of baseline RV function and dimensions were significantly more impaired in non-responders to CRT [7]. This was reported to be true not only for TAPSE, but also for RVSP, RV end-systolic and diastolic areas and RV fractional area change. These results were not confirmed in our observation. Such a difference might be explained not only by different duration of follow-up period, which in our case was 3 months, while it was 6 months in the Italian study. Also, Scuteri et al. chose a different CRT response criterion, which was LVESD reduction of at least 15% after 6 months of biventricular stimulation. The Italian cohort was smaller and only about one third of patients had an ischemic background of HF, while in our group two thirds of patients did.
The clinical predictive value of selected baseline RV parameters was not statistically significant, with TAPSE being the only parameter close to reaching significance. The small difference in baseline TAPSE in responders and non-responders to CRT made even this parameter of no predictive value in any single patient.
The PROSPECT study showed that no single echocardiographic measure of dyssynchrony may be recommended to improve patient selection for CRT. This was reported to be the result of variability from technical and interpretative factors [19]. Cardiac resynchronization therapy procedural factors such as lead location, which may not be optimal in some cases due to coronary venous anatomy, may also limit the predictive value of echocardiography. Taking further into account the limitations of our study (small study group, short observation period), we still believe that some RV parameters, especially TAPSE, might prove to be useful in CRT candidate selection.
In conclusion, this study showed that there are differences in baseline right ventricular function between responders and non-responders to CRT. Yet in our study, none of the individual baseline RV parameters showed any value in identifying patients who would respond to CRT.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Cleland JG, Daubert JC, Erdmann E, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–49. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 2.Wilinski J, Czarnecka D, Wojciechowska W, et al. Baseline tissue Doppler imaging-derived echocardiographic parameters and left ventricle reverse remodelling following cardiac resynchronization therapy introduction. Arch Med Sci. 2011;7:813–22. doi: 10.5114/aoms.2011.25556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Przybyla A, Czarnecka D, Kusiak A, et al. The influence of cardiac resynchronization therapy on selected inflammatory markers and aldosterone levels in patients with chronic heart failure. Przegl Lek. 2011;68:359–61. [PubMed] [Google Scholar]
- 4.Czarnecka D, Kusiak A, Wilinski J, et al. Effects of cardiac resynchronization therapy on sleep apnea, quality of sleep and daytime sleepiness in patients with chronic heart failure. Przegl Lek. 2010;67:1249–52. [PubMed] [Google Scholar]
- 5.Ogunyankin KO, Puthumana JJ. Effect of cardiac resynchronization therapy on right ventricular function. Curr Opin Cardiol. 2010;25:464–8. doi: 10.1097/HCO.0b013e32833c537d. [DOI] [PubMed] [Google Scholar]
- 6.Kjaergaard J, Akkan D, Iversen KK, Kober L, Torp-Pedersen C, Hassager C. Right ventricular dysfunction as an independent predictor of short- and long-term mortality in patients with heart failure. Eur J Heart Failure. 2007;9:610–6. doi: 10.1016/j.ejheart.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Scuteri L, Rordorf R, Marsan NA, et al. Relevance of echocardiographic evaluation of right ventricular function in patients undergoing cardiac resynchronization therapy. Pacing Clin Electrophysiology. 2009;32:1040–9. doi: 10.1111/j.1540-8159.2009.02436.x. [DOI] [PubMed] [Google Scholar]
- 8.Tabereaux PB, Doppalapudi H, Kay GN, McElderry HT, Plumb VJ, Epstein AE. Limited response to cardiac resynchronization therapy in patients with concomitant right ventricular dysfunction. J Cardiovasc Electrophysiol. 2010;21:431–5. doi: 10.1111/j.1540-8167.2009.01634.x. [DOI] [PubMed] [Google Scholar]
- 9.Dickstein K, Vardas PE, Auricchio A, et al. 2010 Focused Update of ESC Guidelines on device therapy in heart failure: an update of the 2008 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure and the 2007 ESC Guidelines for cardiac and resynchronization therapy. Developed with the special contribution of the Heart Failure Association and the European Heart Rhythm Association. Europace. 2010;12:1526–36. doi: 10.1093/europace/euq392. [DOI] [PubMed] [Google Scholar]
- 10.Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiography. 2010;23:685–713. doi: 10.1016/j.echo.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 11.Kusiak A, Wilinski J, Wojciechowska W, et al. Effects of biventricular pacing on right ventricular function assessed by standard echocardiography. Kardiol Pol. 2012;70:883–8. [PubMed] [Google Scholar]
- 12.Di Biase L, Auricchio A, Sorgente A, et al. The magnitude of reverse remodelling irrespective of aetiology predicts outcome of heart failure patients treated with cardiac resynchronization therapy. Eur Heart J. 2008;29:2497–505. doi: 10.1093/eurheartj/ehn221. [DOI] [PubMed] [Google Scholar]
- 13.Fornwalt BK, Sprague WW, BeDell P, et al. Agreement is poor among current criteria used to define response to cardiac resynchronization therapy. Circulation. 2010;121:1985–91. doi: 10.1161/CIRCULATIONAHA.109.910778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghio S, Recusani F, Klersy C, et al. Prognostic usefulness of the tricuspid annular plane systolic excursion in patients with congestive heart failure secondary to idiopathic or ischemic dilated cardiomyopathy. Am J Cardiol. 2000;85:837–42. doi: 10.1016/s0002-9149(99)00877-2. [DOI] [PubMed] [Google Scholar]
- 15.Field ME, Solomon SD, Lewis EF, et al. Right ventricular dysfunction and adverse outcome in patients with advanced heart failure. J Card Fail. 2006;12:616–20. doi: 10.1016/j.cardfail.2006.06.472. [DOI] [PubMed] [Google Scholar]
- 16.Alpendurada F, Guha K, Sharma R, et al. Right ventricular dysfunction is a predictor of non-response and clinical outcome following cardiac resynchronization therapy. J Cardiovasc Magn Reson. 2011;13:68. doi: 10.1186/1532-429X-13-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kjaergaard J, Ghio S, St John Sutton M, Hassager C. Tricuspid annular plane systolic excursion and response to cardiac resynchronization therapy: results from the REVERSE trial. J Card Fail. 2011;17:100–7. doi: 10.1016/j.cardfail.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 18.Kanzaki H, Bazaz R, Schwartzman D, Dohi K, Sade LE, Gorcsan J., 3rd A mechanism for immediate reduction in mitral regurgitation after cardiac resynchronization therapy: insights from mechanical activation strain mapping. J Am Coll Cardiol. 2004;44:1619–25. doi: 10.1016/j.jacc.2004.07.036. [DOI] [PubMed] [Google Scholar]
- 19.Chung ES, Leon AR, Tavazzi L, et al. Results of the predictors of response to CRT (PROSPECT) trial. Circulation. 2008;117:2608–16. doi: 10.1161/CIRCULATIONAHA.107.743120. [DOI] [PubMed] [Google Scholar]