Abstract
Lack of physical activity, smoking and/or inappropriate diet can contribute to the increase of oxidative stress, in turn affecting the pathophysiology of cardiovascular diseases. Strong anti-oxidant properties of plant polyphenolic compounds might underlie their cardioprotective activity. This paper reviews recent findings on the anti-oxidant activity of plant leaf extracts and emphasizes their effects on blood platelets, leukocytes and endothelial cells – the targets orchestrating the development and progression of cardiovascular diseases. We also review the evidence linking supplementation with plant leaf extracts and the risk factors defining the metabolic syndrome. The data point to the importance of leaves as an alternative source of polyphenolic compounds in the human diet and their role in the prevention of cardiovascular diseases.
Keywords: anti-oxidants, cardiovascular diseases, leaf extracts, polyphenols
Introduction
Cardiovascular disease (CVD) is one of the leading causes of mortality in developed countries. Cardiovascular disease may be a result of heart and/or blood vessels abnormalities, such as atherosclerosis, coronary heart disease, cerebrovascular disease, peripheral artery disease, congenital heart disease, rheumatic heart disease, pulmonary embolism and deep vein thrombosis. The main cause of CVD is inappropriate lifestyle, such as unhealthy diet, smoking, and the lack of physical activity. These factors contribute to the development of oxidative stress, atherosclerosis, chronic inflammation and metabolic syndrome (MS) [1]. Epidemiological studies indicate that a diet rich in polyphenols may reduce the risk of CVD without changes in lifestyle [2, 3]. Among patients with established CVD, polyphenols can diminish the effects of risk factors and improve parameters disturbed by the development of disease [4]. In vitro studies suggest that the mechanism of action of phenolic compounds is a result of their anti-oxidative properties and their ability to interfere with blood platelets, the immune system and endothelial cell signalling [5–7]. Polyphenols are secondary metabolites distributed in edible as well as inedible parts of plants. Leaves, flowers and woody parts, such as stems or bark, are a rich source of flavonoids, phenolic acids, stilbenes, tannins and lignans. Fruits, especially berries, are the most popular source of polyphenols beneficial to the vascular system, including anthocyanins, proanthocyanidins, flavonols, catechins and hydroxylated derivates of benzoic and cinnamic acid [8]. However, there is evidence that extracts from other parts of plants, e.g. leaves, are also a rich source of phenolic compounds, which may have cardioprotective potential due to their strong free-radical scavenging, anti-oxidant and/or anti-peroxidative properties towards lipids. Leaves from strawberry, red and black raspberry, as well as thornless blackberry, appeared to have even higher total polyphenol content and exhibit higher antioxidant capacity than their fruits [9]. Also, blackcurrant and apple leaves have higher content of polyphenols than their fruits and demonstrate elevated antioxidant activity [10, 11]. Leaves have been widely used in traditional medicine, e.g. Rubus spp. (Rosaceae) leaves have been used as antimicrobial, anticonvulsant and muscle-relaxing agents. Morus alba (Moraceae) leaves have been applied in Chinese medicine as a remedy for fever, as a hepatoprotective agent and as an agent lowering blood pressure [12]. These examples clearly demonstrate that leaves can be at least equally interesting as fruits or other parts of plants. Importantly, they are also a much more accessible source of polyphenols than fruits.
Literature search strategy
We searched for the papers cited in this review in May to November 2013, using the electronic databases PubMed, Google Scholar and Research Gate. The main search keyword phrases were: “leaves” AND “polyphenols”, “leaves” AND “platelet”, “leaves” AND “metabolic syndrome”, “leaves” AND “inflammation”, “leaves” AND “endothelium”, “leaves” AND “atherosclerosis”, “leaves” AND “cardiovascular disease”, “leaves” AND “anti-oxidant”. When the essential pieces of information were cited in the relevant papers (e.g. in the Discussion) found according to the keywords mentioned above, we additionally used the particular Latin names of the eligible plants as search keywords.
Anti-oxidative properties of leaf extracts
Consequences of radical production in vivo
The imbalance between free-radical production during metabolic reactions and their removal from cells by anti-oxidative systems causes oxidative stress, which underlies the pathogenesis of numerous chronic disease, including atherosclerosis, diabetes, Alzheimer disease, carcinogenesis and inflammation [13–15]. Excessive reactive oxygen species (ROS) are released during activation of membrane NADPH oxidase, arachidonic acid metabolism, cyclooxygenase and lipoxygenase pathways [16]. The most significant oxygen radical is the anion superoxide () and derivative products of its conversion, such as hydroxyl radical (HO–), hydrogen peroxide (H2O2), and peroxynitrite (ONOO–) [17, 18]. In small quantities, ROS are important for physiological processes, but they become toxic for cells and lead to their death at higher concentrations [19]. This pathogenic effect of ROS on cells is a result of changes in cellular compounds, i.e. protein oxidation, lipid peroxidation and nucleic acid damage. Changes in amino acid residues, splitting of the polypeptide chain, creation of protein dimers and aggregates are some consequences of protein oxidation. These processes lead to inactivation of membrane transporters, enzymes and regulatory proteins [20, 21]. Major damage to proteins is caused by , but the action of H2O2 oxidizes –SH residues and ONOO– decreases the activity of some enzymes [22, 23]. Hydroxyl radical and singlet oxygen are the main factors responsible for strand breaks in DNA and RNA, as they destabilize phosphodiester and hydrogen bonds and damage nitrogenous bases [24]. Lipid peroxidation is initiated by anion superoxide and the hydroxyl radical, resulting in disintegration of polyunsaturated fatty acids and inactivation of membrane enzymes and transporting proteins [25]. Compounds with anti-oxidative activity, including natural plant extracts, have the ability to eliminate free radicals, protect cells from ROS, prevent lipid and protein oxidation, and reduce DNA damage.
Methods for the detection of anti-oxidant capacity
Anti-oxidative activity of various compounds in vitro can generally be measured using 2 approaches, as a hydrogen atom transfer (HAT) and a single electron transfer (SET).
Oxygen radical absorbance capacity (ORAC) is a commonly applied HAT method based on the monitoring of linearly decreased fluorescence of a molecular probe (usually β-phycoerythrin or fluorescein), caused by free radicals, and referred to as the Trolox equivalent anti-oxidant capacity (TEAC) [26]. Trolox is a synthetic, water soluble, vitamin E derivative with strong anti-oxidant properties, commonly applied as a standard in anti-oxidant activity assays [27]. Most frequently, the applied SET method measures the ability of anti-oxidant to scavenge the stable synthetic radical 1,1-diphenyl-2-picrylhydrazyl radical (DPPH). Upon reduction, DPPH changes colour from purple to yellow, which can be measured spectrophotometrically. The interpretation of results is based on the calculation of EC50 (the concentration of anti-oxidative agent that decreases the initial DPPH concentration by 50%). The main disadvantage is that DPPH as a hydrophobic agent cannot be used to examine water soluble anti-oxidants [28]. The alternative method that allows for examining not only hydrophobic substances, but also those dissolved in water, involves the ABTS (2, 2’-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid)) radical. The ABTS assay depends on reaction time, anti-oxidant concentration and its activity. Its reduction in a solution is observed as the fading of a blue-green colour proportionally to anti-oxidant concentration. Anti-oxidant content is often expressed as TEAC per capacity or weight unit [29]. The FRAP (ferric reducing ability of plasma) assay directly measures anti-oxidative properties of substances. This method is based on measuring the formation of intense blue ferrous tripyridyl triazine complex reduced from its colourless ferric form and read at 593 nm. Anti-oxidative properties of a given substance are determined as a change in sample absorbance in comparison to the change in an Fe (II) standard, which is directly proportional to anti-oxidant concentration. One FRAP unit is equivalent to the reduction of 1 mol/l Fe3+ to Fe2+ [27, 30].
Anti-oxidant activity of plant extracts in biological systems: focus on leaf extracts
Since polyphenols became known as strong reducing agents, hydrogen donors, singlet oxygen quenchers and metal chelators, they have become widely applied in dietary supplements in order to protect cells against the consequences of oxidative stress.
In many studies using DPPH and ABTS assays, polyphenolic compounds present in leaves have proved to exhibit significant anti-oxidant potential, revealing them as a source of natural anti-oxidants. Withania somnifera (Solanaceae), which is commonly used in Indian traditional medicine as a remedy for several diseases including gastrointestinal and neurological disorders, is an abundant source of polyphenols with antimicrobial properties. Withania also demonstrated some cardioprotective action in vivo, when studied in the model ischaemia and reperfusion injury induced in Wistar rats [31, 32]. Alam et al. found that among extracts obtained from different parts of W. somnifera (fruits, roots and leaves), leaf extract was the most abundant source of polyphenols and flavonoids, whereas root extract had the lowest concentrations. The anti-oxidative properties also differed significantly among extracts. Leaf extract was found to be the strongest DPPH radical inhibitor (92%), while the root extract was the weakest (56%). In addition, leaf extract was a better lipid peroxidation inhibitor and more powerful Fe (III) reducer than root extract. HPLC analysis of all extracts allowed 6 compounds to be identified in leaf extract (catechin, gallic, syringic, benzoic, p-coumaric and vanillic acids), 3 in fruit extract (catechin, naringenin, kaempferol), and 2 in root extract (catechin, benzoic acid). Better anti-oxidative properties of leaf extract were explained by a higher content of polyphenols and stronger ability to inhibit the DPPH radical [33, 34].
The leaves and seeds of Abelmoschus moschatus belonging to the Malvaceae family have been applied in traditional medicine for the treatment of digestive system disorders or skin diseases. Seeds are also used as antispasmodic and cardiotonic agents. Gul et al. compared anti-oxidative potential and polyphenolic content between seeds and leaf extracts of this species. The data indicated that extracts from leaves were a more abundant source of polyphenols (9.5–13.8 mg expressed as gallic acid content) and significantly stronger anti-oxidants (total anti-oxidant activity was in the range 13.3–21.5 ascorbic acid equivalent (AAE)/g dry weight) than those obtained from seeds. Polyphenolic content of seed extract ranged from 1. 6 to 3.7 mg, whereas total anti-oxidant activity was 8.1–10.8 AAE/g dry weight. Further experiments showed that phenolic compounds contained in leaves were stronger DPPH radical scavengers (IC50 43–176 µg of gallic acid equivalent (GAE)/ml) than those from seeds (IC50 38–39 µg of GAE/ml). What is more, aqueous leaf extract inhibited superoxide radical formation by up to 67% and hydroxyl radical-mediated deoxyribose degradation by up to 99% [35].
The plant family Fabaceae comprises > 18,000 species, among which some plants are used in traditional medicine and have anti-cancer, anti-inflammatory, antimicrobial and antidiabetic bioactivity [36–38]. For instance, Glycyrrhiza uralensis has been shown to induce apoptosis and G1 cell cycle arrest in human breast cancer cells [36]. Isoflavonoids isolated from Erythrina variegata appear to possess antimicrobial properties against methicillin-resistant Staphylococcus aureus [37]. Among the plants of the Fabaceae family there are also some species with cardioprotective properties, e.g. Trifolium pallidum and Trifolium scabrum have been shown to reduce thrombin-induced platelet adhesion to fibrinogen and platelet aggregation, whereas black soybean (Glycine max) extract has revealed inhibitory activity on collagen-induced platelet aggregation in isolated human platelets [39, 40]. Chew et al. assessed total polyphenolic content (TPC) and anti-oxidant activity among leaf and flower extracts obtained from 9 Fabaceae species: Acacia auriculiformis, Bauhinia kockiana, Bauhinia purpurea, Caesalpinia pulcherrima, Calliandra tergemina, Cassia surattensis, Leucaena leucocephala, Peltophorum pterocarpum, and Samanea saman. Leaf extracts from B. purpurea, C. pulcherrima, C. tergemina, P. pterocarpum and S. saman had significantly higher TPC than extracts of flowers of the same species. The highest TPC was observed in C. pulcherrima leaf extract (5,030 ±602 mg GAE/100 g), whereas the lowest was observed in B. purpurea (1310 ±124 mg GAE/100 g). TPC of these extracts was also positively correlated with its free-radical scavenging activity, which varied from 7690 ±618 mgAA/100 γ (IC50 = 50 µg/ml) for C. pulcherrima to 1010 ±122 mgAA/100 g for B. purpurea (IC50 = 384 µg/ml) [41].
Another species from Fabaceae, Desmodium adscendens, which grows in the Amazonian rainforest of Peru, other South American countries and the West Coast of Africa, is also worth mentioning. Leaves of this plant are widely used in traditional medicine to treat leucorrhoea, body aches, pain, ovarian inflammation, excessive urination, gonorrhoea, diarrhoea, asthma, fever and epilepsy. A positive effect of D. adscendens on hepatic infections was also proven in vivo, but still relatively little is known about its cardioprotective action [42].Desmodium adscendens leaves are a rich source of polyphenols and contain 11.2 mg/g of phenolic compounds (GAE), 12.8 mg/g of flavonoid compounds, 0.018 mg/g of anthocyanins and 0.39 mg/g of condensed tannins. HPLC analysis of water extract from D. adscendens leaves showed the presence of gallic acid, protocatechuic acid, catechin, rutin, quercetin glucoside and dihydrate, and cinnamic acid. Methanolic extracts did not contain gallic and protocatechuic acid, but chlorogenic acid was detected. ABTS and DPPH tests showed that the extract from D. adscendens leaves exhibited scavenging anti-oxidant activity, which was relevant to 12.8 and 8.5 mg of vitamin C equivalent per g dry weight (VCE/g) for ABTS for DPPH tests, respectively (the IC50 value for D. adscendens leaves extract was 4 µg/ml). Anti-oxidative properties of D. adscendens leaf extract were also examined in cell tests with the fluorescent probe 2,7-diacetate dichlorofluorescein. The extract significantly inhibited ROS generation in murine neutrophils treated with exogenous H2O2 by up to 83% [43].
Helichrysum longifolium is a species in the Asteraceae family, the leaves of which are used as dressings for wounds after circumcision, bruises, cuts and also to cure stress-related diseases, but experimental studies focused on this plant are scarce [44]. Photochemical analysis showed that H. longifolium extract is a source of tannins, flavonoids, steroids and saponins. Total phenolic content of aqueous leaf extract was 0.5 mg GAE/g dry weight, whereas the total flavonoid and proanthocyanidin content was respectively 0.71 and 0.005 mg GAE/g dry weight. It was found that bioactive compounds encountered in H. longifolium leaves are potent free-radical scavengers; aqueous extract significantly reduced ABTS concentration to 75%, hydrogen peroxide concentration to 72%, superoxide anion radical to 76%, DPPH radical to 65% and nitric oxide radical to 67% [45].
Overall the above data indicate that there are some significant differences in the distribution of polyphenols between leaves and other parts of plants. Some polyphenols are synthesized only in leaves, e.g. rutin and chlorogenic acid, which was detected in Bauhinia kockiana and Cassia surattensis. The differences in phenolic distribution are reflected in extracts with stronger anti-oxidant activity obtained from leaves. This divergence in anti-oxidative potency of polyphenols contained in leaves may have an evolutionary background and be due to high oxidative stress, which these parts of a plant experience in the course of photosynthesis [46]. Absorption of excessive light energy by the leaf tissue and transformation of absorbed light energy in chlorophyll is connected with the reduction of highly reactive chemical species. Such conditions cause the need for agents which are able to quench and remove ROS and to minimize damage related to oxidative stress [47]. Thus, strong anti-oxidative properties of leaf extracts make them promising natural agents for CVD prevention and treatment by effective reduction of oxidative stress.
Anti-inflammatory and immunomodulatory properties of polyphenolic extracts
Inflammation is an essential process for tissue protection and homeostasis. It is a defensive host response to infection, injury and irritation. Inflammation in a healthy organism is a self-limiting process that enables affected tissue to return to homeostasis. When immune cells are unable to manage with their inflammatory factors, they produce excessive amounts of cytokines and free radicals that result in acute inflammation or chronic disease [48]. Atherosclerosis is a slowly progressing pathological change in arteries that underlies coronary artery disease; it has its origin in endothelium injuries and low-density lipoprotein deposition in the arterial wall. These factors cause plaque formation and activation of an innate as well as an adaptive immune response, which leads to chronic inflammation. Immune cells influence the initiation and progression of atherosclerotic lesions via infiltration of the arterial wall, and cytokine and free-radical production [49–51]. Cytokines act in 2 ways, as pro-inflammatory factors (interleukins: 1, 2, 6, 7, 8, tumor necrosis factor α (TNF-α) and interferon γ (IFN-γ)) and anti-inflammatory agents (interleukins: 4, 10, 13, IFN-α, transforming growth factor β (TGF-β)). In coronary artery disease (CAD), the levels of both anti- and pro-inflammatory factors, i.e. IL-10, IL-2, and TNF-α, are raised [52]. Pro-inflammatory factors, such as TNF-α and IL-1β, enhance adhesion molecule expression and endothelial permeability, which causes LDL deposition in the arterial wall, monocyte and lymphocyte infiltration, elevation of the inflammatory response, disintegration of fibrin filaments, and finally plaque rupture, which can cause for example a stroke [53]. The main cytokine in atherosclerosis progression is IL-6, released by the majority of cells which build up plaque, such as macrophages, foam cells, smooth muscle cells and activated endothelial cells [54]. IL-6 promotes early plaque formation and destabilization by up-regulation of IL-1β, TNF-α level, leukocyte infiltration and activation, lipid deposition, smooth muscle cell proliferation and down-regulation of enzymes involved in collagen synthesis enzymes [55–59]. An atherosclerotic plaque is stabilized by balanced collagen synthesis and decomposition in a fibrous cap. TNF-α destabilization is due to intensification of LDL oxidation; in cooperation with IL-6, it stimulates liver production of C-reactive protein (CRP) [60]. Other factors released in significant quantities by immune cells, including free radicals, intensify the immune response, adhesive molecule expression, platelet activation and tissue damage. The T lymphocyte major cytokine INF-γ also increases the immune response and destabilizes plaque by decreasing collagen synthesis in the fibrous cap [61].
Polyphenols not only limit excessive inflammation by quenching oxidative stress, but may also decrease inflammatory cytokine production, immune cell activation and inflammatory gene expression [62].
Leaf extracts as immunomodulators
The effects of polyphenolic compounds on immune and inflammatory cell function have been investigated in in vitro studies, animal models and clinical trials.
Young leaves of Abelmoschus esculentus (Malvaceae), Hibiscus acetosella (Malvaceae), Manihot esculenta (Euphorbiaceae) and Pteridium aquilinum (Dennstaedtiaceae) are common polyphenolic sources in Western and Central Africa. They are essential dietary components, also widely applied in folk medicine; e.g. Abelmoschus, Hibiscus and Manihot leaves are used to treat fever, headache, rheumatism, haemorrhoids, tumours, conjunctivitis, sores and abscesses. Abelmoschus esculentus also has a beneficial impact on the cardiovascular system. Sabitha et al. demonstrated that A. esculentus peel and seed powder reduced the blood glucose level and improved the lipid profile level in diabetic male Wistar rats [63]. Aqueous extracts prepared from young leaves of these plants were shown by Tsumbu et al. to influence neutrophils and monocytes under conditions pivotal for the first line of host immune defence. After 10 min incubation, the extracts not only decreased production of reactive nitrogen species by phorbol myristate acetate (PMA) stimulated equine neutrophils in a concentration-dependent manner (1–10 µg/ml), but also diminished neutrophil degranulation and myeloperoxidase (MPO) release into the extracellular milieu. The most powerful ROS scavengers were extracts obtained from Pteridium and Hibiscus, which also contained the highest amounts of polyphenol, phenolic acid and flavonoid. Abelmoschus and Pteridium extracts at 10 µg/ml were the most efficient inhibitors of MPO release. Extracts from Pteridium and Manihot at 10 µg/ml also significantly inhibited nitration-peroxidase activity of MPO [64]. Moreover, all these extracts decreased ROS production via HL-60 monocytes activated with PMA in a concentration-dependent manner. Manihot and Pteridium were the strongest inhibitors [65].
Tea (Camellia sinensis, Theaceae), the most important non-alcoholic beverage in the world, has been extensively studied for its putative disease preventive effects. Tea leaves are well known as an abundant source of polyphenols with strong anti-oxidant properties [66]. Regular tea intake prevents cancer and vascular disorders, and regulates the digestive system [67–69]. Animal as well as human studies point to the cardioprotective effect of black tea by lowering of cholesterol level. Also, the ability of black tea to decrease some inflammatory markers and mediators expressed by endothelium clearly points to the beneficial prosperities of this plant towards the vascular system [70]. Polyphenols contained in green tea, mainly catechins and flavonols, influence the immune system. Lymphocytes isolated from IL-2-deficient mice with inflammatory bowel disease have lower INF-γ and TNF-α production after 6 weeks’ oral ingestion of water with green tea polyphenol extract [71]. Green tea extract also reduces the secondary response in an experimental model of spinal cord trauma. Administration of 24 mg/kg at 1 and 6 h after injury caused IκBα degradation and decreased the level of nuclear factor κB (NF-κB) and its phosphorylation at the site of injury. Severe neutrophil infiltration is associated with spinal cord injury. Green tea extract decreased TNF-α and IL-1β concentration and MPO activity compared to the control group, which suggests that the extract restricted neutrophil infiltration. Western blot and immunohistochemical analysis showed that the extract prevented inducible nitric oxide synthase (iNOS) expression and attenuated oxidative stress, which was measured as nitrotyrosine formation, lipid peroxidation and protease-activated receptor (PAR) formation [72].
Immunomodulatory effects of leaf extracts were also shown in vivo in a randomised crossover study on purple sweet potato leaves (Ipomoea batatas, Convolvulaceae), which are common diet constituents in Asian cuisine. Because of the wide tolerance under severe environmental conditions (diseases, pest infestation, flooding), high polyphenolic content (2–14 g/100 g dry weight) and anti-oxidative activity, sweet potato leaves can be an important source of nutrients for people living in poorly resourced areas [73]. Some findings suggest that peripheral blood mononuclear cells (PBMC) isolated from blood of healthy humans who consumed daily 200 g of purple sweet potato leaves (PSPL) for 2 weeks showed increased proliferative responsiveness and elevated secretion of IL-2 and IL-4. Dietary supplementation with sweet potato leaves increased the lytic properties of natural killer (NK) cells and salivary IgA secretion [74]. The data contradict in vitro outcomes which indicate that flavonoids have immunosuppressive activity and decrease cytokine secretion, NK lytic function and lymphocyte proliferation [75]. Purple sweet potato leaves are also a promising supplement for athletes. Meals prepared from these leaves modulated release of inflammatory cytokines during exercise-induced oxidative stress. Analysis of blood taken from healthy individuals after 1 h of running revealed that those on a 1-week PSPL diet had a lower level of lipid peroxidation products and the inflammatory cytokine IL-6 compared to the control group [76].
In this regard, polyphenols contained in leaves modulate production of cytokines (IL-1β, TNF-α, IL-6, INF-γ), adhesive molecule expression and neutrophil infiltration and down-regulate oxidative stress by reducing ROS release and iNOS expression. As this is important in the progression and pathogenesis of arteriosclerosis, this leaf extract is a promising agent in the prevention of CVD and heart failure.
Beneficial effects of polyphenolic extracts in relation to metabolic syndrome
Metabolic syndrome is a group of concurrent risk factors including insulin resistance, hyperinsulinaemia, impaired glucose tolerance, centrally distributed obesity, high levels of triglycerides, low levels of HDL cholesterol, elevated blood pressure, and pro-inflammatory and prothrombotic states [77, 78]. Occurrence of MS is associated with the development of cardiovascular disease, type 2 diabetes, non-alcoholic fatty liver disease, obstructive sleep apnoea, renal disease and cancer [79–83]. Conditions that underlie MS remain unclear, but this syndrome is associated with physical inactivity, ageing and hormonal imbalance, such as polycystic ovary syndrome and testosterone insufficiency [84–87]. Nuclear peroxisome proliferator-activated receptors (PPAR) can be involved in the development of MS; they participate in β-oxidation of fatty acids, adipogenesis, glucose homeostasis and lipid metabolism, which is why their activation can improve some metabolic parameters, such as glucose and lipid levels [88, 89].
Activity of leaf extracts in metabolic syndrome
The therapeutic potential of polyphenols from leaves to treat many of the symptoms of MS has been seen in suitable animal models. Olive (Olea europaea, Oleaceae) leaves have been known for their medicinal properties since ancient times; tea made from them have been used to heal malaria and associated fevers. Extracts of olive leaves have strong antimicrobial, anti-oxidant and hypoglycaemic activity and cardioprotective properties [90–92]. Olive leaf extract increased the proportion of living cells, protected insulin secretion and not only reduced ROS production but also facilitated the excessive antioxidant defence in an insulin-producing β-cell line after pre-incubation with cytokines inducing toxicity [93]. Olive oil leaf extract has also demonstrated strong antimicrobial activity against Campylobacter jejuni, Helicobacter pylori and Staphylococcus aureus (including methicillin-resistant S. aureus) [92]. HPLC analysis showed that an ethanolic olive leaf extract (OLE) is rich in oleuropein (13.0 g/l) and hydroxytyrosol (2.7 g/l). Other polyphenols present in the extract are tyrosol, aesculin, hydroxypinoresinol-glycoside, luteolin 7-glucoside, and oleoside. Oleuropein and hydroxytyrosol are particularly important components of OLE that can reverse both chronic inflammation and oxidative stress, both contributing to cardiovascular, hepatic, and metabolic symptoms in a rat model of diet-induced obesity and diabetes. Male Wistar rats fed with a high fat (high-cholesterol high fat (HCHF)) or carbohydrate cornstarch diet for 8 weeks demonstrated attenuated fat deposition after supplementation with 3% OLE for a further 8 weeks in comparison with those fed for 16 week with a HCHF or cornstarch diet without OLE. Rats on a HCHF diet supplemented with OLE have also shown lower plasma total cholesterol, triglycerides, oxidative stress markers and improved oral glucose tolerance compared to those without supplementation. A high fat diet led to pathological changes in Wistar rat heart (left ventricle inflammation, interstitial collagen deposition), liver (inflammatory cell infiltration, lipid accumulation and portal fibrosis), and coronary vessels (decreased vasorelaxation). Olive leaf extract supplementation markedly reduced these symptoms [91].
In traditional Chinese medicine, leaves of mulberry (Morus sp., Moraceae) have been used to cure diabetes and inflammation. The extract of mulberry possesses anti-oxidant activity, suppresses lipoxygenase, is cytotoxic to cancer cells and inhibits their migration [94, 95]. It was found that some mulberry anthocyanins, cyanidin 3-rutinoside and cyanidin 3-glucoside inhibited migration and invasion of human lung cancer cells. Prenylflavonoids, cudraflavone B, cudraflavone C and oxyresveratrol extracted from Morus alba exhibited a DPPH free radical scavenging effect and hepatoprotective effects on tacrine-induced cytotoxicity in human liver-derived Hep G2 cells. Mulberry polyphenols also act cardioprotectively, e.g. the extract of mulberry root bark significantly inhibited collagen- and arachidonic acid-induced platelet aggregation and thromboxane formation in cultured platelets [96, 97]. Morus leaves are rich in polyphenolic constituents such as quercetin and kaempferol, which have anti-diabetic action and can ameliorate hyperglycaemia and dyslipidaemia [98]. In vivo experiments showed that an extract from Morus leaves ameliorated hyperlipidaemia in high fat diet male Wistar rats. The extract significantly decreased plasma triglycerides and non-esterified fatty acid levels. Rats given Morus leaf extract had an up-regulated PPAR signalling pathway and down-regulated androgen, oestrogen and butanoate metabolism, bile acid biosynthesis and synthesis, and degradation of ketone bodies. Lipid metabolism and B-oxidation of fatty acids were up-regulated in mulberry-treated rats in contrast to lipid and steroid biosynthetic processes, which were down-regulated. Morus leaf extract not only regulated the genes responsible for lipid and fatty acid metabolism, but also up-regulated genes involved in the response to oxidative stress [99].
Sasa quelpaertensis (Poaceae) leaves have been used in traditional medicine as tea with anti-diabetic, diuretic and anti-inflammatory properties, but scientific data concerning the molecular basis underlying the possible benefits of S. quelpaertensis for health are scarce. Ryou et al. studied ovariectomised Sprague-Dawley rats fed on a Sasa quelpaertensis leaf powder diet (the leaf powder comprised 10% of the diet) and found that these animals were characterized by significantly lower daily weight gain, although the effect of such powder is not clearly associated with cholesterol, triglyceride or glucose levels, or with aggregation of blood platelets, compared to sham-operated controls [100]. Otherwise, the leaves of persimmon (Diospyros kaki; Ebenaceae), commonly consumed as a tea, possess an evident anti-diabetic activity. Kawakami et al. found that the addition of the powder concentrate of persimmon leaves, rich in proanthocyanidin oligomers, to the diet of male Wistar rats resulted in a decreased blood glucose level in a concentration-dependent manner [101]. In folk medicine, dandelion (Taraxacum officinale; Asteraceae) has been used to treat hepatic disorders and inflammation with its choleretic, diuretic and anti-rheumatic properties. Dandelion is a source of flavonoids, caffeic acid, chlorogenic acid, luteolin, and luteolin 7-glucoside [102]. Oral administration of dandelion leaves improved parameters in metabolic syndrome of high-cholesterol fed male New Zealand white rabbits. Administration of T. officinale leaf extract for 4 weeks significantly increased HDL cholesterol, and lowered levels of triglycerides and LDL in comparison to the control group. Dandelion supplementation increased activity of the hepatic anti-oxidant enzymes superoxide dismutase (SOD) and glutathione peroxidase (GPx). Haematoxylin and eosin staining of representative aortic sections showed that supplementation with dandelion leaves limited lipid deposition and formation of atherosclerotic lesions within the aortic intima [103]. Cardioprotective action of fruits of Vitis vinifera is broadly described in the literature, e.g. polyphenolic fractions obtained from grape skin have been shown many times to inhibit platelet aggregation and LDL oxidation in vitro [104]. In traditional medicine, also Vitis vinifera leaves are known as a remedy for hypertension, haemorrhages and inflammatory disorders. Extract from V. labrusca leaves had hepatoprotective, cardioprotective, and renal protective effects in Wistar rats. The major phenolic compounds in its leaves are flavonoids and hydroxycinnamic acids. Polyphenols from V. labrusca leaves restored liver and kidney superoxide dismutase and heart catalase activity, and decreased lipid and protein damage in Wistar rat tissues treated with H2O2 [105].
Polyphenols modulate platelet and endothelial function
Platelet and endothelial dysfunction are among the leading factors responsible for CVD. In normal physiological conditions, the endothelium prevents adhesion and activation of platelets through secretion of nitric oxide (NO) and prostacyclin (PGI2) [106, 107]. Pathological changes (diabetes, hyperlipidaemia and hypertension) diminish production of anti-aggregatory factors, increase the release of vasoconstrictors (endothelin-1), and lead to collagen exposure. Platelet adherence to the exposed collagen is connected with their activation and secretion of pro-coagulatory factors, e.g. ADP, calcium, thromboxane A2 [108, 109].
Effect of leaf extracts on platelet function
Polyphenolic compounds contained in plant leaf extracts can attenuate platelet hyper-reactivity and reverse endothelial dysfunction by modulating cellular signalling. In traditional medicine, Urtica dioica (Urticaceae) roots and leaves are a remedy for hypertension, diabetes, prostate hyperplasia and cancer [110–112]. Urtica dioica possesses anti-inflammatory, anti-hyperglycaemic, antimicrobial, anti-oxidant, anti-ulcer and analgesic activity [113–115]. Urtica dioica leaf extract, when administered before glucose loading, has demonstrated strong ability to decrease glucose level in alloxan-induced diabetic rats [113]. Extract from U. dioica also appears to be an effective scavenger of free radicals, including superoxide anion radicals and hydrogen peroxide. Moreover, Urtica leaf extract has revealed antimicrobial activity against nine different microorganisms, antiulcer activity against ethanol-induced ulcerogenesis and an analgesic effect on acetic acid-induced stretching [115]. This herb also demonstrates hypotensive and diuretic actions [116]. The cardioprotective effect of U. dioica has been demonstrated in male Wistar rats fed on a high-cholesterol diet, as significantly decreased levels of total cholesterol, low-density lipoprotein cholesterol, liver enzymes and body weight [117]. Investigating the influence of 3 different U. dioica leaf extracts (in water, methanol or ethyl acetate) on thrombin-induced aggregation of washed platelets in Wistar rats showed that only the ethyl acetate extract possessed significant anti-platelet activity. Further investigation indicated that this distinction in the action of the extract was a result of higher concentrations of flavonoids in ethyl acetate extract [118]. Artemisia dracunculus (Asteraceae) is commonly used in Iranian folk medicine as an anti-coagulant and anti-hyperlipidaemic agent. In vitro studies on A. dracunculus methanolic leaf extract indicated its ability to significantly inhibit thrombin-induced platelet aggregation by 60%, platelet adhesion to laminin coated plates by 50%, and protein secretion from thrombin-activated platelets by 50% [119]. Numerous studies indicate that garlic Allium sativum (Amaryllidaceae), known since ancient times for its healing properties, may be a beneficial agent for the treatment of CVD [120, 121]. Allium sativum can normalize plasma lipids, enhance fibrinolytic activity, inhibit platelet aggregation, and reduce blood pressure and blood glucose level. In experiments on platelet aggregation evoked by ADP, collagen or arachidonic acid, Hiyasat et al. compared the anti-platelet activity of methanolic and aqueous extracts isolated from the leaves of A. ursinum and sativum. Alcoholic extracts of both species and an aqueous extract of A. sativum most efficiently inhibited ADP-induced platelet aggregation, while an aqueous extract of A. ursinum inhibited platelet aggregation, but the effect did not depend on the type of platelet agonist [122]. Olive leaf extract has an anti-aggregatory influence on platelets, seen in a randomised single-blinded study involving healthy male volunteers given supplements of OLE containing 5.4 mg/ml of oleuropein. In vitro, OLE used at 5.4–54 µg/ml inhibited blood platelet aggregation and ADP release in a dose-dependent manner, but significant changes occurred only at the highest concentration of OLE [123].
Effect of leaf extracts on endothelial cells
Numerous reports deal with the influence of leaf extracts on endothelial cells. The main action is the improvement of NO-dependent vasorelaxation, an effect achieved with leaf extracts of Fragaria vesca (Rosaceae), Tanacetum vulgare (Asteraceae) and Mansoa hirsuta (Bignoniaceae) [124–126]. Among leaves, those from Ginkgo (Ginkgoaceae) and Morus species seem to be well confirmed. Extracts of the leaves of Ginkgo biloba are a source of flavonoids (ginkgo flavone glycosides, bioflavonoids) and terpenoids (ginkgolides and bilobalide) and have anti-tumour, anti-aging, hepatoprotective and cardioprotective properties [127–130]. Ginkgo extract decreases the activities of serum marker enzymes and lipid peroxidation in carbon tetrachloride-induced hepatotoxicity in male Wistar rats. Such a hepatoprotective effect has been ascribed to anti-oxidative properties of this extract, which have been associated with increased levels of glutathione, as well as increased activities of superoxide dismutase, catalase, glutathione peroxidase and glutathione reductase [129]. Ginkgo extract also demonstrates cardioprotective activity, which has been demonstrated in an experiment with HgCl2-induced oxidative damage in Wistar albino male and female rats. While HgCl2 has been shown to significantly increase thromboplastic activity and malondialdehyde levels or decrease glutathione levels in serum and tissue samples, this effect has been effectively reversed by Ginkgo leaf extract [130]. It has also been used to treat dementia and vaso-occlusive and cochleovestibular disorders [128, 131]. Ou et al. found that Ginkgo biloba leaf extract (GbE) attenuated endothelial cell dysfunction induced by oxidized low-density lipoprotein (oxLDL). Pre-treatment of human umbilical vein endothelial cells (HUVECs) with GbE before exposure to oxLDL dramatically decreased the level of ROS generation (96% inhibition at 100 µg/ml) in comparison to Trolox (104% inhibition at 2.5 µg/ml). HUVECs treated with oxLDL for 24 h had reduced endothelial nitric oxide synthase (eNOS) protein expression, which stimulated THP-1 cells to increasingly adhere to HUVECs and show enhanced expression of adhesion molecules; however, incubation of HUVECs with GbE for 2 h significantly reduced these tendencies. Moreover, GbE inhibited oxLDL-induced cytotoxicity of HUVECs [132]. The effect of Ginkgo leaf extract has also been investigated in randomised clinical trials in patients with early stage diabetic nephropathy. After 8 weeks of Ginkgo supplementation, patients had less von Willebrand factor and increased NO plasma levels [133].
Adhesion molecules and cytokines are well-recognized markers and mediators of endothelial dysfunction. Therefore, they are often the targets for studying vascular protective activity of plant extracts. Using Western blot analysis, water extract from another commonly investigated species (Morus alba leaves) may suppress expression of vascular cell adhesion molecule-1 (VCAM-1), intracellular adhesion molecule-1 (ICAM-1) and E-selectin after 6 weeks of supplementation in rats fed an atherogenic diet [134]. Also, in experiments in vitro, Morus alba leaf extract decreased expression of the adhesion molecule resistin. It is a cytokine that increases the expression of P-selectin and monocyte adhesion to human endothelial cells. Methanolic extract from M. alba leaves significantly reduces P-selectin expression and inhibits monocyte adhesion to endothelium previously exposed to resistin [135]. Dicksonia sellowiana (Dicksoniaceae) is a common tree in Central and South America; its leaves are used in a folk medicine to treat scabies, pruritus, parasitic diseases and asthma. Hydroalcoholic extract of D. sellowiana (HEDS) decreases hypertension and induces endothelium-dependent relaxation in spontaneously hypertensive rat (SHR) aortic rings. Hydroalcoholic extract of D. sellowiana induces aortic relaxation by activation of muscarinic receptors and stimulation of the NO pathway in SHR rat aortic endothelium. In porcine coronary artery rings, HEDS also causes endothelium-dependent relaxation via redox-sensitive activation of the endothelial PI3-kinase/Akt pathway, which leads to eNOS phosphorylation [136].
Chemical structure, bioavailability and functionality of polyphenols contained in leaf extracts
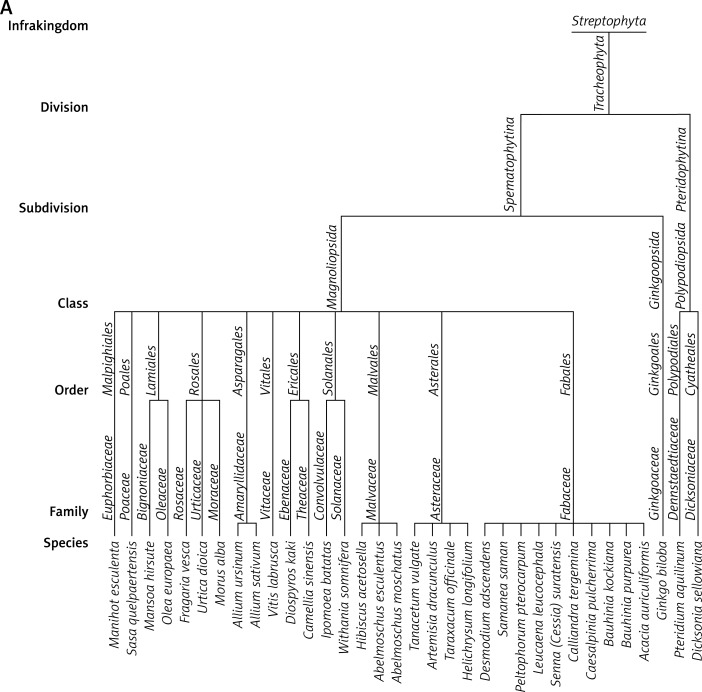
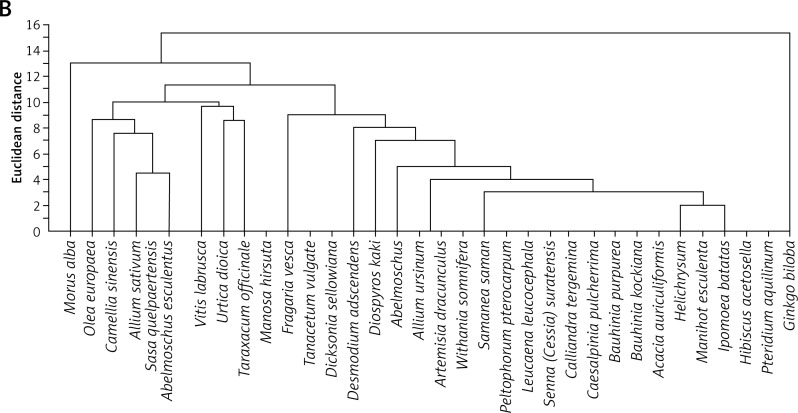
Data presented in this paper suggest that the main acting agents in the investigated leaf extracts are phenolic acids and flavonoids (Table I). However, numerous pieces of evidence indicate that also tannins, terpenoids, saponins and steroids, commonly occurring components in leaf extracts, may exhibit a significant pharmacological influence, very often overlapping with that attributed to polyphenolic agents. Derivatives of hydroxybenzoic acid (e.g. gallic acid, protocatechuic acid) and hydroxycinnamic acid (e.g. caffeic acid, chlorogenic acid) are among the phenolic acids most commonly detected in leaves. Their chemical structures include a single aromatic ring containing the functional groups of either hydroxybenzoic or hydroxycinnamic acid, and possible substitutions at the positions of R1, R2 and R3 include hydrogen, hydroxyl or methoxy residues (Table II). Flavonoids detected in leaf extracts belong mainly to anthocyanins, proanthocyanidins (procyanidin B1, procyanidin B2), flavanols (catechin and epigallocatechin), flavones (luteolin) and flavonols (kaempferol, quercetin) (Tables I and II). Their chemical structures consist of two aromatic rings (C6), one of which is fused with a heterocyclic pyran or hydropyran, whereas the second is substituted at positions 3, 4 and 5 with hydrogen, hydroxyl or methoxy residues. In general, biological and chemical properties of polyphenols are largely determined by their aromatic chemical structure, as well as the number, type and locations of functional groups in a molecule [137]. On the other hand, polyphenolic content of plant extracts depends on environmental factors, such as pedoclimatic (soil type, sun exposure, rainfall) and agronomic conditions (cultures in greenhouses or fields, biological cultures, hydroponic cultures). Also, the exposure to light, and the degree of ripeness (fruits) or maturity (leaves) may influence concentrations and proportions of various polyphenols [138, 139]. Biological activities of extracts from plant specimens belonging to the same species may significantly differ, mainly because they are determined by the chemical composition of the plant tissue (which is dependent on cultivation conditions) or the method of its extraction/preparation [140–142]. The discrepancies between degree of relationship and biological activity are indicated in Figure 1, illustrating the clustering of the discussed species in agglomerates showing the highest similarities. Interestingly, the plant extracts discussed in this review are ascribed to three main agglomerates concerning their biological activity (Figure 1 B), which do not correspond to agglomerates created on the basis of their taxonomic relationships (Figure 1 A). It would indicate that various related plant species may demonstrate different or even divergent biological properties of their extracts originating from differentiated chemical compositions of the extracts. It seems likely that environmental conditions (including plant habitats) may matter much more in determining such biological properties of the extracts than the taxonomic relationships between the plants themselves. Biological activity in vivo is also strongly dependent on bioavailability of consumed polyphenolic compounds. Plasma concentrations of polyphenol metabolites may vary greatly, from 0 to 4 µmol/l. Of the polyphenols commonly occurring in leaf extracts, gallic acid and isoflavones are most efficiently absorbed. Also catechins, flavanones and quercetin glucosides demonstrate bioavailability more favourable than other polyphenols. On the other hand, polyphenols with particularly low availability are proanthocyanidins, galloylated tea catechins and anthocyanins. Following their absorption, these compounds are further metabolized in vivo mainly via the glucuronic acid pathway [143]. Low bioavailability is the cause of much reduced in-organism effectiveness of these flavonoids, as validated by the revealed discrepancies between the activities of certain polyphenolic compounds demonstrated under in vitro and in vivo conditions. The reason for relatively low bioavailability is that polyphenols occur in plants in the form of esters or glycosides that cannot be absorbed without the contribution of intestinal enzymes or colonic gastrointestinal microflora [143]. Moreover, diet in general, as well as regional dietary habits in particular, may considerably influence bioavailability of polyphenols. An example that nicely illustrates this phenomenon refers to the differences between concentrations of catechins and flavonols in black tea brew and black tea customarily served with milk in the UK [70].
Table I.
Characteristics of leaf extracts from 34 selected plants described in the review
| Latin scientific name (and common/ abbreviated name) | Family | Types of common extracts | Class of active ingredients | Active agent | Mode of action |
|---|---|---|---|---|---|
| Abelmoschus esculentus [64, 65] | Malvaceae | Aqueous | Phenolics | Not defined | Anti-radical |
| Flavonoids | Anti-inflammatory | ||||
| Tannins | Modulatory | ||||
| Abelmoschus moschatus [35] | Malvaceae | Aqueous | Phenolics | Not defined | Anti-oxidative |
| Ethanolic | Flavonoids | Antimicrobial | |||
| Anti-proliferative | |||||
| Acacia auriculiformis [41] | Fabaceae | Methanolic | Terpenoids | Not defined | Anti-oxidative |
| Dichloromethane: methanol | Saponins | Antimicrobial | |||
| Steroids | |||||
| Allium sativum [122] | Amaryllidaceae | Aqueous | Alliins | Not defined | Antiplatelet |
| Methanolic | Allicins | ||||
| Saponosides | |||||
| Allium ursinum [122] | Amaryllidaceae | Aqueous | Alliins | Not defined | Antiplatelet |
| Methanolic | Allicins | ||||
| Saponosides | |||||
| Artemisia dracunculus [119] | Asteraceae | Methanolic | Phenolics | Not defined | Antiplatelet |
| Flavonoids | |||||
| Coumarins | |||||
| Bauhinia kockiana [41] | Fabaceae | Methanolic | Flavonoids | Not defined | Anti-oxidative |
| Dichloromethane: methanol | Steroids | Antimicrobial | |||
| Tannins | |||||
| Bauhinia purpurea [41] | Fabaceae | Methanolic | Terpenoids | Not defined | Anti-oxidative |
| Dichloromethane: methanol | Saponins | Antimicrobial | |||
| Steroids | |||||
| Caesalpinia pulcherrima [41] | Fabaceae | Methanolic | Flavonoids | Not defined | Anti-oxidative |
| Dichloromethane: methanol | Terpenoids | Antimicrobial | |||
| Tannins | |||||
| Calliandra tergemina [41] | Fabaceae | Methanolic Dichloromethane: methanol | Flavonoids | Not defined | Anti-oxidative |
| Terpenoids | Antimicrobial | ||||
| Tannins | |||||
| Saponins | |||||
| Camellia sinensis [66, 70, 71] | Theaceae | Aqueous | Phenolics | Epigallocatechin-3-gallate | Anti-oxidative |
| Flavonoids | Epicatechin- 3-gallate | Antimicrobial | |||
| Epigallocatechin | Anti-inflammatory | ||||
| Epicatechin | |||||
| Desmodium adscendens [43] | Fabaceae | Aqueous | Phenolics | Gallic acid | Anti-oxidative |
| Methanolic | Flavonoids | Protocatechuic acid | |||
| Anthocyanins | Catechin | ||||
| Condensed tannins | Rutin | ||||
| Hydroxycinnamic acids | Quercetin glucoside | ||||
| Quercetin dihydrate | |||||
| Chlorogenic acid | |||||
| Cinnamic acid | |||||
| Dicksonia sellowiana (HEDS) [43] | Dicksoniaceae | Ethanolic | Phenolics | Gallic acid | Activation of NO pathway |
| Hydroxycinnamic acids | Protocatechuic acid | ||||
| Chlorogenic acid | |||||
| Coumaric acid | |||||
| Ferulic acid | |||||
| Sinapic acid | |||||
| Cinnamic acid | |||||
| Diospyros kaki [101] | Ebenaceae | Aqueous | Phenolics | Catechin | α-Amylase inhibition |
| Ethyl acetate | Flavonoids | Epigallocatechin | |||
| Proanthocyanidins | Epigallocatechin-3-O-gallate | ||||
| Epicatechin | |||||
| Epicatechin-3-O-gallate | |||||
| Prodelphinidin | |||||
| Fragaria vesca [124] | Rosaceae | Aqueous | Phenolics | Catechin | Improvement of NO-dependent vasorelaxation |
| Flavonoids | Epicatechin | ||||
| Procyanidins | Epigallocatechin | ||||
| Stilbenoids | Epicatechin-3-gallate | ||||
| Quercetin-4’-glucoside | |||||
| Procyanidin B1 | |||||
| Procyanidin B2 | |||||
| Piceid | |||||
| Astringin | |||||
| Trans-resveratrol | |||||
| Ginkgo biloba (GbE) [127–133] | Ginkgoaceae | Commercial | Flavonoids | Ginkgo flavones | Anti-tumour |
| Terpenoids | Glycosides | Anti-aging | |||
| Bioflavonoids | Hepatoprotective | ||||
| Ginkgolides | Cardioprotective | ||||
| Bilobalide | |||||
| Helichrysum longifolium [45] | Asteraceae | Aqueous | Phenolics | Not defined | Anti-oxidative |
| Flavonoids | |||||
| Tannins | |||||
| Steroids | |||||
| Saponins | |||||
| Proanthocyanidins | |||||
| Hibiscus acetosella [64, 65] | Malvaceae | Aqueous | Phenolics | Phenolic acid | Anti-radical |
| Flavonoids | Anti-inflammatory | ||||
| Tannins | Modulatory | ||||
| Ipomoea batatas (PSPL) [73] | Convolvulaceae | Fresh leaf | Phenolics | Not defined | Immuno-modulatory |
| Flavonoids | |||||
| Leucaena leucocephala [41] | Fabaceae | Methanolic | Flavonoids | Not defined | Anti-oxidative |
| Dichloromethane: methanol | Tannins | Antimicrobial | |||
| Steroids | |||||
| Saponins | |||||
| Manihot esculenta [64, 65] | Euphorbiaceae | Aqueous | Flavonoids | Not defined | Anti-radical |
| Anti-inflammatory | |||||
| Modulatory | |||||
| Mansoa hirsuta [126] | Bignoniaceae | Ethanolic | Phenolics | Proanthocyanidin B2 | Improvement of NO-dependent vasorelaxation |
| Tannins | |||||
| Proanthocyanidins | |||||
| Morus alba [94–99] | Moraceae | Aqueous | Phenolics | Epicatechin | Anti-inflammatory |
| Ethanolic | Flavonoids | Myricetin | Anti-diabetic | ||
| Quercetin hydrate | Anti-oxidative | ||||
| Luteolin | |||||
| Kaempferol | |||||
| Olea europaea(OLE) [90–93] | Oleaceae | Ethanolic | Phenolics | Tyrosol | Antimicrobial |
| Flavonoids | Hydroxytyrosol | Anti-oxidant | |||
| Hydroxycinnamic acids | Ligstroside | Hypoglycaemic activity | |||
| Dimethyl oleuropein | Cardioprotective properties | ||||
| Oleoside | |||||
| Oleuropein | |||||
| Apigenin | |||||
| Kaempferol | |||||
| Luteolin | |||||
| Caffeic acid | |||||
| Peltophorum pterocarpum [41] | Fabaceae | Methanolic | Tannins | Not defined | Anti-oxidative |
| Dichloromethane: methanol | Terpenoids | Antimicrobial | |||
| Saponins | |||||
| Steroids | |||||
| Pteridium aquilinum [64, 65] | Dennstaedtiaceae | Aqueous | Polyphenols | Phenolic acid | Anti-radical |
| Flavonoids | Anti-inflammatory | ||||
| Tannins | Modulatory | ||||
| Samanea saman [41] | Fabaceae | Methanolic | Terpenoids | Not defined | Anti-oxidative |
| Dichloromethane: methanol | Steroids | Antimicrobial | |||
| Sasa quelpaertensis [100] | Poaceae | Leaf powder | Flavonoids | Tricin | Tyrosine |
| Hydroxycinnamic acids | Isoorientin | Hydroxylase inhibitor | |||
| P-coumaric acid | |||||
| Chlorogenic acid | |||||
| Senna suratensis (Cessia suratensis) [41] | Fabaceae | Methanolic | Flavonoids | Not defined | Anti-oxidative |
| Dichloromethane: methanol | Tannins | Antimicrobial | |||
| Tanacetum vulgare [125] | Asteraceae | Aqueous | Terpenoids | Not defined | Improvement of no-dependent vasorelaxation |
| Tannins | |||||
| Steroids | |||||
| Taraxacum officinale [102, 103] | Asteraceae | Fresh leaf | Flavonoids | Luteolin | Anti-diabetic |
| Hydroxycinnamic acids | Luteolin 7-glucoside | Diuretic | |||
| Caffeic acid | Anti-inflammatory | ||||
| Chlorogenic acid | |||||
| Urtica dioica [113–118] | Urticaceae | Aqueous | Flavonoids | Genins | Antiplatelet |
| Heteroside | |||||
| Flavonoids | |||||
| Vitis labrusca [105] | Vitaceae | Ethanolic | Phenolics | Resveratrol | Hepatoprotective |
| Flavonoids | Cardioprotective | ||||
| Stilbenoids | Renal-protective | ||||
| Anti-oxidative | |||||
| Withania somnifera [31–34] | Solanaceae | Methanolic | Phenolics | Gallic acid | Anti-oxidant |
| Flavonoids | Syringic acid | Antimicrobial | |||
| Hydroxycinnamic acids | Benzoic acid | ||||
| Catechin | |||||
| Vanillic acid | |||||
| P-coumaric |
Table II.
Basic structures of the typical active components of polyphenols most commonly occurring in leaf extracts
| Group | Structure | Common residues and representatives | |
|---|---|---|---|
| 1 | Phenolic acids | ||
| 1.1 | Hydroxybenzoic acids | 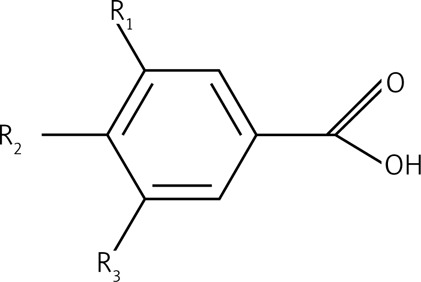 |
R1 = R2 = OH, R3 = H Protocatechuic acid R1 = R2 = OH = R3 = OH Gallic acid |
| 1.2 | Hydroxycinnamic acids | 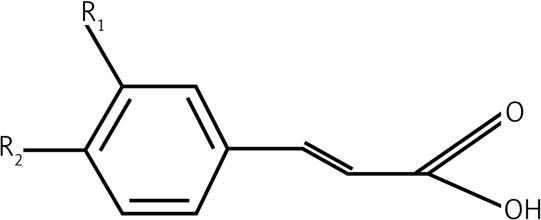 |
R1 = OH Coumaric acid R1 = R2 = OH Caffeic acid R1 = OCH3, R2 = OH |
| 2 | Flavonoids | ||
| 2.1 | Anthocyanins | 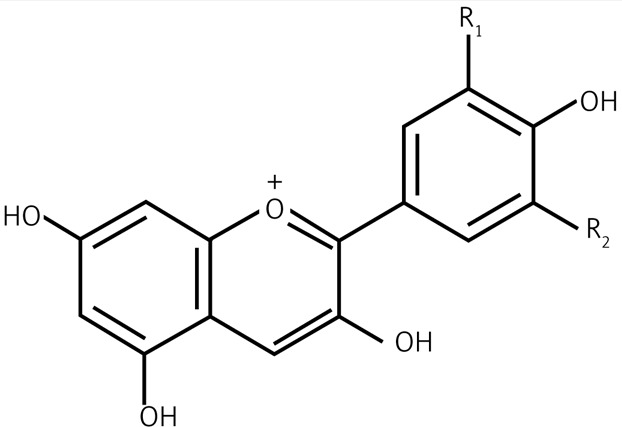 |
|
| 2.2 | Flavanols | 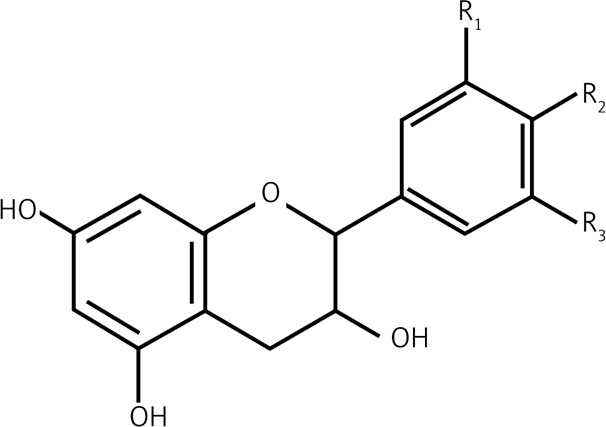 |
R1 = R2 = OH, R3 = H Catechin R1 = R2 = R3 = OH Gallocatechin |
| 2.3 | Flavones | 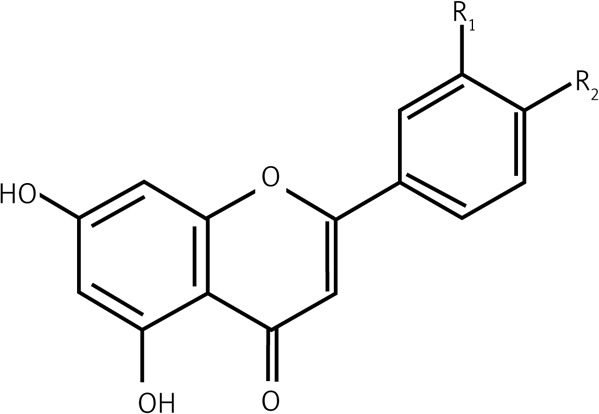 |
R1 = R2 = OH Luteolin |
| 2.4 | Flavonols | 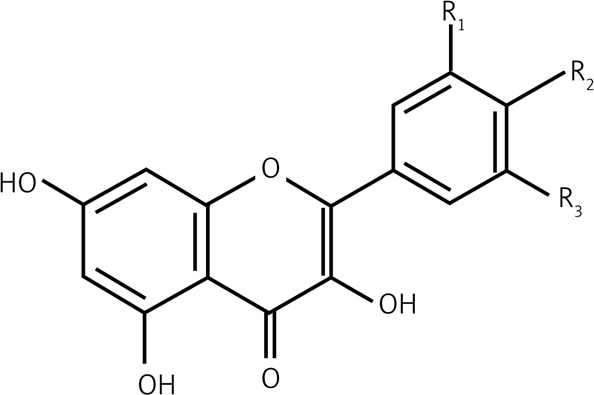 |
R1 = R2 = OH, R3 = H Quercetin R2 = OH, R1 = R3 = H Kaempferol |
| 2.5 | Proanthocyanidins | 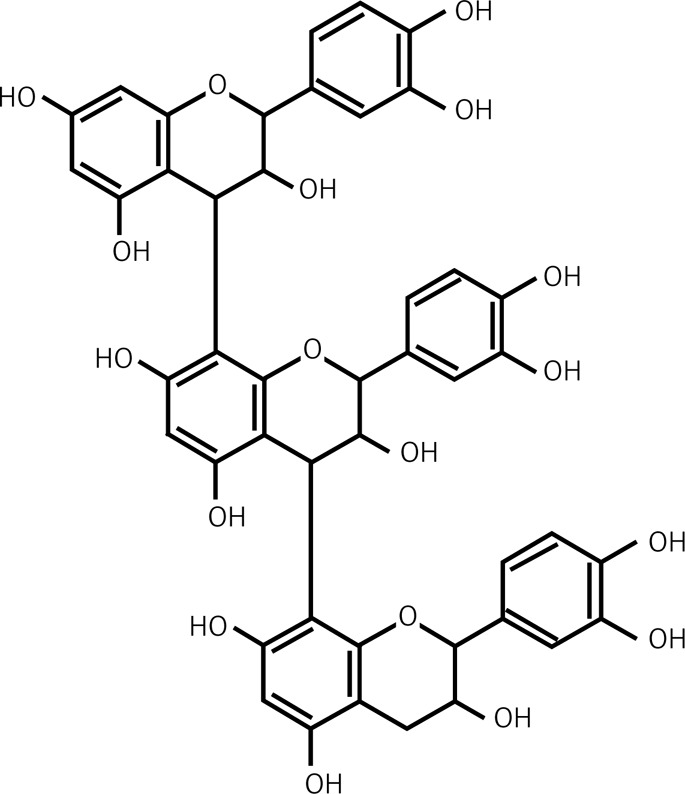 |
Trimeric Procyanidin |
Figure 1 A.
Taxonomic relationships between plant species and similarities among them based on their biological activities. – The plant species discussed in this review were agglomerated in clusters using a single linkage method based on Euclidean distances estimated according to plants belonging to the following taxa: species, family, order, class, subdivision, division, infrakingdom (Source: http://www.itis.gov).
Figure 1 B.
Taxonomic relationships between plant species and similarities among them based on their biological activities. – The plants were agglomerated according to their biological activity including: anti-oxidative, hepatoprotective, renal-protective, anti-inflammatory, antimicrobial, antiplatelet, anti-cancer, anti-aging, anti-diabetic, NO release-propagating, lipid profile improving and adhesion molecule expression lowering properties. Agglomeration analysis was performed using the single linkage method based on Euclidean distances estimated according to distributions of plant leaf extract properties (Source: all essential information is included in Table I)
It is also worth mentioning that general cardioprotective effects of all kinds of polyphenols seem to be largely based on their anti-oxidative action, which results in quenching of blood ROS and preventing their formation by inhibition of enzymes responsible for oxidative stress, such as cyclooxygenases, lipoxygenases or NADPH oxidases [96].
Conclusions
In this review, we have focused on the actions of leaf extracts on various pathophysiological phenomena involving impairments in the cardiovascular system. Apart from their anti-oxidative and anti-inflammatory activities, other beneficial effects of leaf extracts have also been discussed in relation to the metabolic syndrome. In vivo and in vitro studies have clearly indicated that polyphenolic extracts from leaves exert a plethora of effects on cellular functions due to their strong anti-inflammatory, anti-oxidative, anti-aggregatory, vasorelaxant, hypolipaemic and hypoglycaemic properties. Thus, polyphenolic extracts from edible and inedible leaves are promising dietary supplements in preventing and treating cardiovascular disease, and probably deserve to be considered with not lesser enthusiasm than extracts obtained from other parts of plants.
Acknowledgments
This work was supported by the project “FLAWOPIRYNA”, UDA-POIG.01.03.01-10-129/08-00, financed by the European Regional Development Fund within the framework of the Innovative Economy Programme 2007-2013.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Erdman JW, Jr, Balentine D, Arab L, et al. Flavonoids and heart health: proceedings of the ILSI North America Flavonoids Workshop, May 31-June 1, 2005, Washington, DC. J Nutr. 2007;137:718S–37S. doi: 10.1093/jn/137.3.718S. [DOI] [PubMed] [Google Scholar]
- 2.Nothlings U, Schulze MB, Weikert C, et al. Intake of vegetables, legumes, and fruit, and risk for all-cause, cardiovascular, and cancer mortality in a European diabetic population. J Nutr. 2008;138:775–81. doi: 10.1093/jn/138.4.775. [DOI] [PubMed] [Google Scholar]
- 3.Mink PJ, Scrafford CG, Barraj LM, et al. Flavonoid intake and cardiovascular disease mortality: a prospective study in postmenopausal women. Am J Clin Nutr. 2007;85:895–909. doi: 10.1093/ajcn/85.3.895. [DOI] [PubMed] [Google Scholar]
- 4.Keli SO, Hertog MG, Feskens EJ, Kromhout D. Dietary flavonoids, antioxidant vitamins, and incidence of stroke: the Zutphen study. Arch Intern Med. 1996;156:637–42. [PubMed] [Google Scholar]
- 5.Wahyudi S, Sargowo D. Green tea polyphenols inhibit oxidized LDL-induced NF-KB activation in human umbilical vein endothelial cells. Acta Med Indones. 2007;39:66–70. [PubMed] [Google Scholar]
- 6.Olas B, Wachowicz B, Nowak P, et al. Studies on antioxidant properties of polyphenol-rich extract from berries of Aronia melanocarpa in blood platelets. J Physiol Pharmacol. 2008;59:823–35. [PubMed] [Google Scholar]
- 7.Harizi H, Chaabane F, Ghedira K, Chekir-Ghedira L. Inhibition of proinflammatory macrophage responses and lymphocyte proliferation in vitro by ethyl acetate leaf extract from Daphne gnidium. Cell Immunol. 2011;267:94–101. doi: 10.1016/j.cellimm.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Kahkonen MP, Hopia AI, Vuorela HJ, et al. Antioxidant activity of plant extracts containing phenolic compounds. J Agric Food Chem. 1999;47:3954–62. doi: 10.1021/jf990146l. [DOI] [PubMed] [Google Scholar]
- 9.Wang SY, Lin HS. Antioxidant activity in fruits and leaves of blackberry, raspberry, and strawberry varies with cultivar and developmental stage. J Agric Food Chem. 2000;48:140–6. doi: 10.1021/jf9908345. [DOI] [PubMed] [Google Scholar]
- 10.Tabart J, Kevers C, Pincemail J, Defraigne JO, Dommes J. Antioxidant capacity of black currant varies with organ, season, and cultivar. J Agric Food Chem. 2006;54:6271–6. doi: 10.1021/jf061112y. [DOI] [PubMed] [Google Scholar]
- 11.Renard CM, Dupont N, Guillermin P. Concentrations and characteristics of procyanidins and other phenolics in apples during fruit growth. Phytochemistry. 2007;68:1128–38. doi: 10.1016/j.phytochem.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 12.Jia Zhishen TMWJ. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chemistry. 1995;64:555–9. [Google Scholar]
- 13.Pappolla MA, Omar RA, Kim KS, Robakis NK. Immunohistochemical evidence of oxidative [corrected] stress in Alzheimer's disease. Am J Pathol. 1992;140:621–8. [PMC free article] [PubMed] [Google Scholar]
- 14.Bankson DD, Kestin M, Rifai N. Role of free radicals in cancer and atherosclerosis. Clin Lab Med. 1993;13:463–80. [PubMed] [Google Scholar]
- 15.Matkovics B, Varga SI, Szabo L, Witas H. The effect of diabetes on the activities of the peroxide metabolism enzymes. Horm Metab Res. 1982;14:77–9. doi: 10.1055/s-2007-1018928. [DOI] [PubMed] [Google Scholar]
- 16.Sies H. Oxidative stress: oxidants and antioxidants. Exp Physiol. 1997;82:291–5. doi: 10.1113/expphysiol.1997.sp004024. [DOI] [PubMed] [Google Scholar]
- 17.Gow AJ, Duran D, Malcolm S, Ischiropoulos H. Effects of peroxynitrite-induced protein modifications on tyrosine phosphorylation and degradation. FEBS Lett. 1996;385:63–6. doi: 10.1016/0014-5793(96)00347-x. [DOI] [PubMed] [Google Scholar]
- 18.Stadtman ER, Levine RL. Protein oxidation. Ann N Y Acad Sci. 2000;899:191–208. doi: 10.1111/j.1749-6632.2000.tb06187.x. [DOI] [PubMed] [Google Scholar]
- 19.Valko M, Leibfritz D, Moncol J, et al. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Butterfield DA, Kanski J. Brain protein oxidation in age-related neurodegenerative disorders that are associated with aggregated proteins. Mech Ageing Dev. 2001;122:945–62. doi: 10.1016/s0047-6374(01)00249-4. [DOI] [PubMed] [Google Scholar]
- 21.Hensley K, Robinson KA, Gabbita SP, Salsman S, Floyd RA. Reactive oxygen species, cell signaling, and cell injury. Free Radic Biol Med. 2000;28:1456–62. doi: 10.1016/s0891-5849(00)00252-5. [DOI] [PubMed] [Google Scholar]
- 22.Alvarez B, Radi R. Peroxynitrite reactivity with amino acids and proteins. Amino Acids. 2003;25:295–311. doi: 10.1007/s00726-003-0018-8. [DOI] [PubMed] [Google Scholar]
- 23.Ischiropoulos H. Biological selectivity and functional aspects of protein tyrosine nitration. Biochem Biophys Res Commun. 2003;305:776–83. doi: 10.1016/s0006-291x(03)00814-3. [DOI] [PubMed] [Google Scholar]
- 24.Simandan T, Sun J, Dix TA. Oxidation of DNA bases, deoxyribonucleosides and homopolymers by peroxyl radicals. Biochem J. 1998;335:233–40. doi: 10.1042/bj3350233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumagai T, Matsukawa N, Kaneko Y, Kusumi Y, Mitsumata M, Uchida K. A lipid peroxidation-derived inflammatory mediator: identification of 4-hydroxy-2-nonenal as a potential inducer of cyclooxygenase-2 in macrophages. J Biol Chem. 2004;279:48389–96. doi: 10.1074/jbc.M409935200. [DOI] [PubMed] [Google Scholar]
- 26.Ou B, Huang D, Hampsch-Woodill M, Flanagan JA, Deemer EK. Analysis of antioxidant activities of common vegetables employing oxygen radical absorbance capacity (ORAC) and ferric reducing antioxidant power (FRAP) assays: a comparative study. J Agric Food Chem. 2002;50:3122–8. doi: 10.1021/jf0116606. [DOI] [PubMed] [Google Scholar]
- 27.Arts MJ, Haenen GR, Voss HP, Bast A. Antioxidant capacity of reaction products limits the applicability of the Trolox Equivalent Antioxidant Capacity (TEAC) assay. Food Chem Toxicol. 2004;42:45–9. doi: 10.1016/j.fct.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 28.Kedare SB, Singh RP. Genesis and development of DPPH method of antioxidant assay. J Food Sci Technol. 2011;48:412–22. doi: 10.1007/s13197-011-0251-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Osman AM, Wong KK, Fernyhough A. ABTS radical-driven oxidation of polyphenols: isolation and structural elucidation of covalent adducts. Biochem Biophys Res Commun. 2006;346:321–9. doi: 10.1016/j.bbrc.2006.05.118. [DOI] [PubMed] [Google Scholar]
- 30.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70–6. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 31.Gupta SK, Mohanty I, Talwar KK, et al. Cardioprotection from ischemia and reperfusion injury by Withania somnifera: a hemodynamic, biochemical and histopathological assessment. Mol Cell Biochem. 2004;260:39–47. doi: 10.1023/b:mcbi.0000026051.16803.03. [DOI] [PubMed] [Google Scholar]
- 32.Mohanty IR, Arya DS, Gupta SK. Withania somnifera provides cardioprotection and attenuates ischemia-reperfusion induced apoptosis. Clin Nutr. 2008;27:635–42. doi: 10.1016/j.clnu.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 33.Alam N, Hossain M, Khalil MI, Moniruzzaman M, Sulaiman SA, Gan SH. High catechin concentrations detected in Withania somnifera (ashwagandha) by high performance liquid chromatography analysis. BMC Complement Altern Med. 2011;11:65. doi: 10.1186/1472-6882-11-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alam N, Hossain M, Mottalib MA, Sulaiman SA, Gan SH, Khalil MI. Methanolic extracts of Withania somnifera leaves, fruits and roots possess antioxidant properties and antibacterial activities. BMC Complement Altern Med. 2012;12:175. doi: 10.1186/1472-6882-12-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gul MZ, Bhakshu LM, Ahmad F, Kondapi AK, Qureshi IA, Ghazi IA. Evaluation of Abelmoschus moschatus extracts for antioxidant, free radical scavenging, antimicrobial and antiproliferative activities using in vitro assays. BMC Complement Altern Med. 2011;11:64. doi: 10.1186/1472-6882-11-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jo EH, Kim SH, Ra JC, et al. Chemopreventive properties of the ethanol extract of chinese licorice (Glycyrrhiza uralensis) root: induction of apoptosis and G1 cell cycle arrest in MCF-7 human breast cancer cells. Cancer Lett. 2005;230:239–47. doi: 10.1016/j.canlet.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 37.Tanaka H, Sato M, Fujiwara S, Hirata M, Etoh H, Takeuchi H. Antibacterial activity of isoflavonoids isolated from Erythrina variegata against methicillin-resistant Staphylococcus aureus. Lett Appl Microbiol. 2002;35:494–8. doi: 10.1046/j.1472-765x.2002.01222.x. [DOI] [PubMed] [Google Scholar]
- 38.Xue WL, Li XS, Zhang J, Liu YH, Wang ZL, Zhang RJ. Effect of Trigonella foenum-graecum (fenugreek) extract on blood glucose, blood lipid and hemorheological properties in streptozotocin-induced diabetic rats. Asia Pac J Clin Nutr. 2007;16:422–6. [PubMed] [Google Scholar]
- 39.Kim K, Lim KM, Kim CW, et al. Black soybean extract can attenuate thrombosis through inhibition of collagen-induced platelet activation. J Nutr Biochem. 2011;22:964–70. doi: 10.1016/j.jnutbio.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 40.Kolodziejczyk-Czepas J, Olas B, Malinowska J, et al. Extracts from Trifolium pallidum and Trifolium scabrum aerial parts as modulators of blood platelet adhesion and aggregation. Platelets. 2013;24:136–44. doi: 10.3109/09537104.2012.676221. [DOI] [PubMed] [Google Scholar]
- 41.Chew YL, Chan EW, Tan PL, Lim YY, Stanslas J, Goh JK. Assessment of phytochemical content, polyphenolic composition, antioxidant and antibacterial activities of Leguminosae medicinal plants in Peninsular Malaysia. BMC Complement Altern Med. 2011;11:12. doi: 10.1186/1472-6882-11-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gyamfi MA, Yonamine M, Aniya Y. Free-radical scavenging action of medicinal herbs from Ghana: Thonningia sanguinea on experimentally-induced liver injuries. Gen Pharmacol. 1999;32:661–7. doi: 10.1016/s0306-3623(98)00238-9. [DOI] [PubMed] [Google Scholar]
- 43.Muanda FN, Bouayed J, Djilani A, Yao C, Soulimani R, Dicko A. Chemical composition and cellular evaluation of the antioxidant activity of Desmodium adscendens leaves. Evid Based Complement Alternat Med. 2011;2011:620862. doi: 10.1155/2011/620862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aiyegoro OA, Afolayan AJ, Okoh AI. Interactions of antibiotics and extracts of Helichrysum pedunculatum against bacteria implicated in wound infections. Folia Microbiol (Praha) 2010;55:176–80. doi: 10.1007/s12223-010-0026-5. [DOI] [PubMed] [Google Scholar]
- 45.Aiyegoro OA, Okoh AI. Preliminary phytochemical screening and in vitro antioxidant activities of the aqueous extract of Helichrysum longifolium DC. BMC Complement Altern Med. 2010;10:21. doi: 10.1186/1472-6882-10-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chew YL, Goh JK, Lim YY. Assessment of in vitro antioxidant capacity and polyphenolic composition of selected medicinal herbs from Leguminosae family in Peninsular Malaysia. Food Chem. 2009;119:373–8. [Google Scholar]
- 47.Bhattacharyaa S, Kamatb JP, Bandyopadhyaya SK, Chattopadhyay S. Comparative inhibitory properties of some Indian medicinal plant extracts against photosensitization-induced lipid damage. Food Chem. 2009;113:975–9. [Google Scholar]
- 48.Isobe Y, Kato T, Arita M. Emerging roles of eosinophils and eosinophil-derived lipid mediators in the resolution of inflammation. Front Immunol. 2012;3:270. doi: 10.3389/fimmu.2012.00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ross R. Atherosclerosis is an inflammatory disease. Am Heart J. 1999;138:S419–20. doi: 10.1016/s0002-8703(99)70266-8. [DOI] [PubMed] [Google Scholar]
- 50.van der Wal AC, Das PK, Bentz vdB, van der Loos CM, Becker AE. Atherosclerotic lesions in humans. In situ immunophenotypic analysis suggesting an immune mediated response. Lab Invest. 1989;61:166–70. [PubMed] [Google Scholar]
- 51.Libby P. Current concepts of the pathogenesis of the acute coronary syndromes. Circulation. 2001;104:365–72. doi: 10.1161/01.cir.104.3.365. [DOI] [PubMed] [Google Scholar]
- 52.Zubelewicz-Szkodzinska B, Szkodzinski J, Danikiewicz A, et al. Effects of simvastatin on pro-inflammatory cytokines in patients with hypercholesterolemia. Kardiol Pol. 2003;59:465–74. [PubMed] [Google Scholar]
- 53.Marciniak A, Gierblinski I, Stefanski R, et al. Predictive value of plasma interleukin 1, interleukin 6, interleukin 8 and C-reactive protein (CRP) in patients with myocardial infarction. Pol Arch Med Wewn. 2003;109:15–22. [PubMed] [Google Scholar]
- 54.Barton BE. The biological effects of interleukin 6. Med Res Rev. 1996;16:87–109. doi: 10.1002/(SICI)1098-1128(199601)16:1<87::AID-MED3>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 55.Sukovich DA, Kauser K, Shirley FD, DelVecchio V, Halks-Miller M, Rubanyi GM. Expression of interleukin-6 in atherosclerotic lesions of male ApoE-knockout mice: inhibition by 17beta-estradiol. Arterioscler Thromb Vasc Biol. 1998;18:1498–505. doi: 10.1161/01.atv.18.9.1498. [DOI] [PubMed] [Google Scholar]
- 56.Huber SA, Sakkinen P, Conze D, Hardin N, Tracy R. Interleukin-6 exacerbates early atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 1999;19:2364–7. doi: 10.1161/01.atv.19.10.2364. [DOI] [PubMed] [Google Scholar]
- 57.Cochran FR, Finch-Arietta MB. Interleukin-6 can prime THP-1 macrophages for enhanced production of tumor necrosis factor-alpha in response to LPS. Immunopharmacology. 1992;23:97–103. doi: 10.1016/0162-3109(92)90033-9. [DOI] [PubMed] [Google Scholar]
- 58.Biswas P, Delfanti F, Bernasconi S, et al. Interleukin-6 induces monocyte chemotactic protein-1 in peripheral blood mononuclear cells and in the U937 cell line. Blood. 1998;91:258–65. [PubMed] [Google Scholar]
- 59.Nabata T, Morimoto S, Koh E, Shiraishi T, Ogihara T. Interleukin-6 stimulates c-myc expression and proliferation of cultured vascular smooth muscle cells. Biochem Int. 1990;20:445–53. [PubMed] [Google Scholar]
- 60.Kubica J, Kozinski M, Krzewina-Kowalska A, et al. Combined periprocedural evaluation of CRP and TNF-alpha enhances the prediction of clinical restenosis and major adverse cardiac events in patients undergoing percutaneous coronary interventions. Int J Mol Med. 2005;16:173–80. [PubMed] [Google Scholar]
- 61.Libby P. Molecular bases of the acute coronary syndromes. Circulation. 1995;91:2844–50. doi: 10.1161/01.cir.91.11.2844. [DOI] [PubMed] [Google Scholar]
- 62.Gonzalez-Gallego J, Garcia-Mediavilla MV, Sanchez-Campos S, Tunon MJ. Fruit polyphenols, immunity and inflammation. Br J Nutr. 2010;104(Suppl 3):S15–27. doi: 10.1017/S0007114510003910. [DOI] [PubMed] [Google Scholar]
- 63.Sabitha V, Ramachandran S, Naveen KR, Panneerselvam K. Antidiabetic and antihyperlipidemic potential of Abelmoschus esculentus (L.) Moench. in streptozotocin-induced diabetic rats. J Pharm Bioallied Sci. 2011;3:397–402. doi: 10.4103/0975-7406.84447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tsumbu CN, Deby-Dupont G, Tits M, et al. Polyphenol content and modulatory activities of some tropical dietary plant extracts on the oxidant activities of neutrophils and myeloperoxidase. Int J Mol Sci. 2012;13:628–50. doi: 10.3390/ijms13010628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tsumbu CN, Deby-Dupont G, Tits M, et al. Antioxidant and antiradical activities of Manihot esculenta Crantz (Euphorbiaceae) leaves and other selected tropical green vegetables investigated on lipoperoxidation and phorbol-12-myristate-13-acetate (PMA) activated monocytes. Nutrients. 2011;3:818–38. doi: 10.3390/nu3090818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Samanidou V, Tsagiannidis A, Sarakatsianos I. Simultaneous determination of polyphenols and major purine alkaloids in Greek Sideritis species, herbal extracts, green tea, black tea, and coffee by high-performance liquid chromatography-diode array detection. J Sep Sci. 2012;35:608–15. doi: 10.1002/jssc.201100894. [DOI] [PubMed] [Google Scholar]
- 67.Wang X, Lin YW, Wang S, et al. A meta-analysis of tea consumption and the risk of bladder cancer. Urol Int. 2012;90:10–6. doi: 10.1159/000342804. [DOI] [PubMed] [Google Scholar]
- 68.Turek IA, Kozinska J, Drygas W. Green tea as a protective factor in prophylaxis and treatment of selected cardiovascular diseases. Kardiol Pol. 2012;70:848–52. [PubMed] [Google Scholar]
- 69.Yasui K, Paeng N, Miyoshi N, et al. Effects of a catechin-free fraction derived from green tea on gene expression of enzymes related to lipid metabolism in the mouse liver. Biomed Res. 2012;33:9–13. doi: 10.2220/biomedres.33.9. [DOI] [PubMed] [Google Scholar]
- 70.Skotnicka M, Chorostowska-Wynimko J, Jankun J, Skrzypczak-Jankun E. The black tea bioactivity: an overview. Centr Eur J Immunol. 2011;36:284–92. [Google Scholar]
- 71.Varilek GW, Yang F, Lee EY, et al. Green tea polyphenol extract attenuates inflammation in interleukin-2-deficient mice, a model of autoimmunity. J Nutr. 2001;131:2034–9. doi: 10.1093/jn/131.7.2034. [DOI] [PubMed] [Google Scholar]
- 72.Paterniti I, Genovese T, Crisafulli C, et al. Treatment with green tea extract attenuates secondary inflammatory response in an experimental model of spinal cord trauma. Naunyn Schmiedebergs Arch Pharmacol. 2009;380:179–92. doi: 10.1007/s00210-009-0414-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chu YH, Chang ChL, Hsu HF. Flavonoid content of several vegetables and their antioxidant activity. J Sci Food Agr. 2000;80:561–6. [Google Scholar]
- 74.Chen CM, Li SC, Lin YL, Hsu CY, Shieh MJ, Liu JF. Consumption of purple sweet potato leaves modulates human immune response: T-lymphocyte functions, lytic activity of natural killer cell and antibody production. World J Gastroenterol. 2005;11:5777–81. doi: 10.3748/wjg.v11.i37.5777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Middleton E., Jr Effect of plant flavonoids on immune and inflammatory cell function. Adv Exp Med Biol. 1998;439:175–82. doi: 10.1007/978-1-4615-5335-9_13. [DOI] [PubMed] [Google Scholar]
- 76.Chang WH, Hu SP, Huang YF, Yeh TS, Liu JF. Effect of purple sweet potato leaves consumption on exercise-induced oxidative stress and IL-6 and HSP72 levels. J Appl Physiol. 2010;109:1710–5. doi: 10.1152/japplphysiol.00205.2010. [DOI] [PubMed] [Google Scholar]
- 77.Jeppesen J, Hein HO, Suadicani P, Gyntelberg F. Low triglicerydes, high high-density lipoprotein cholesterol and risk of ischaemic heart disease. Arch Intern Med. 2001;161:361–6. doi: 10.1001/archinte.161.3.361. [DOI] [PubMed] [Google Scholar]
- 78.Kaplan NM. The deadly quartet: upper-body obesity, glucose intolerance, hypertriglicerydemia and hypertension. Arch Intern Med. 1989;149:1514–20. doi: 10.1001/archinte.149.7.1514. [DOI] [PubMed] [Google Scholar]
- 79.Anty R, Gual P, Huet PM, Marchand-Brustel YL, Tran A. Metabolic fatty liver diseases: hepatic consequences of the metabolic syndrome. Gastroenterol Clin Biol. 2007;31:1127–34. doi: 10.1016/s0399-8320(07)78350-1. [DOI] [PubMed] [Google Scholar]
- 80.Vegas-Valle JM, Garcia-Ruiz JM, Hernandez-Martin E, de la Hera JM. Metabolic syndrome, diabetes, and coronary artery disease: a very common association. Rev Esp Cardiol (Engl.) 2012;65:108–9. doi: 10.1016/j.recesp.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 81.Tanaka H, Shiohira Y, Uezu Y, Higa A, Iseki K. Metabolic syndrome and chronic kidney disease in Okinawa, Japan. Kidney Int. 2006;69:369–74. doi: 10.1038/sj.ki.5000050. [DOI] [PubMed] [Google Scholar]
- 82.Drager LF, Queiroz EL, Lopes HF, Genta PR, Krieger EM, Lorenzi-Filho G. Obstructive sleep apnea is highly prevalent and correlates with impaired glycemic control in consecutive patients with the metabolic syndrome. J Cardiometab Syndr. 2009;4:89–95. doi: 10.1111/j.1559-4572.2008.00046.x. [DOI] [PubMed] [Google Scholar]
- 83.Hursting SD, Hursting MJ. Growth signals, inflammation, and vascular perturbations: mechanistic links between obesity, metabolic syndrome, and cancer. Arterioscler Thromb Vasc Biol. 2012;32:1766–70. doi: 10.1161/ATVBAHA.111.241927. [DOI] [PubMed] [Google Scholar]
- 84.Pedroso DC, Melo AS, Carolo AL, Vieira CS, Silva AC, Reis RM. Frequency and risk factors for metabolic syndrome in adolescents and adults women with polycystic ovary syndrome. Rev Bras Ginecol Obstet. 2012;34:357–61. doi: 10.1590/s0100-72032012000800003. [DOI] [PubMed] [Google Scholar]
- 85.Countryman AJ, Saab PG, Llabre MM, Penedo FJ, McCalla JR, Schneiderman N. Cardiometabolic risk in adolescents: associations with physical activity, fitness, and sleep. Ann Behav Med. 2012;45:121–31. doi: 10.1007/s12160-012-9428-8. [DOI] [PubMed] [Google Scholar]
- 86.Yuan JQ, Xu T, Zhang XW, et al. Metabolic syndrome and androgen deprivation therapy in metabolic complications of prostate cancer patients. Chin Med J (Engl.) 2012;125:3725–9. [PubMed] [Google Scholar]
- 87.Sun K, Liu J, Ning G. Active smoking and risk of metabolic syndrome: a meta-analysis of prospective studies. PLoS One. 2012;7:e47791. doi: 10.1371/journal.pone.0047791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bishop-Bailey D. Peroxisome proliferator-activated receptors in the cardiovascular system. Br J Pharmacol. 2000;129:823–34. doi: 10.1038/sj.bjp.0703149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Barroso I, Gurnell M, Crowley VE, et al. Dominant negative mutations in human PPARgamma associated with severe insulin resistance, diabetes mellitus and hypertension. Nature. 1999;402:880–3. doi: 10.1038/47254. [DOI] [PubMed] [Google Scholar]
- 90.Turkez H, Togar B. Olive (Olea europaea L.) leaf extract counteracts genotoxicity and oxidative stress of permethrin in human lymphocytes. J Toxicol Sci. 2011;36:531–7. doi: 10.2131/jts.36.531. [DOI] [PubMed] [Google Scholar]
- 91.Poudyal H, Campbell F, Brown L. Olive leaf extract attenuates cardiac, hepatic, and metabolic changes in high carbohydrate-, high fat-fed rats. J Nutr. 2010;140:946–53. doi: 10.3945/jn.109.117812. [DOI] [PubMed] [Google Scholar]
- 92.Sudjana AN, D'Orazio C, Ryan V, et al. Antimicrobial activity of commercial Olea europaea (olive) leaf extract. Int J Antimicrob Agents. 2009;33:461–3. doi: 10.1016/j.ijantimicag.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 93.Cumaoglu A, Ari N, Kartal M, Karasu C. Polyphenolic extracts from Olea europea L. protect against cytokine-induced beta-cell damage through maintenance of redox homeostasis. Rejuvenation Res. 2011;14:325–34. doi: 10.1089/rej.2010.1111. [DOI] [PubMed] [Google Scholar]
- 94.Chen PN, Chu SC, Chiou HL, Kuo WH, Chiang CL, Hsieh YS. Mulberry anthocyanins, cyanidin 3-rutinoside and cyanidin 3-glucoside, exhibited an inhibitory effect on the migration and invasion of a human lung cancer cell line. Cancer Lett. 2006;235:248–59. doi: 10.1016/j.canlet.2005.04.033. [DOI] [PubMed] [Google Scholar]
- 95.Oh H, Ko EK, Jun JY, et al. Hepatoprotective and free radical scavenging activities of prenylflavonoids, coumarin, and stilbene from Morus alba. Planta Med. 2002;68:932–4. doi: 10.1055/s-2002-34930. [DOI] [PubMed] [Google Scholar]
- 96.Michalska M, Gluba A, Mikhailidis DP, et al. The role of polyphenols in cardiovascular disease. Med Sci Monit. 2010;16:RA110–9. [PubMed] [Google Scholar]
- 97.Lee JJ, Yang H, Yoo YM, et al. Morusinol extracted from Morus alba inhibits arterial thrombosis and modulates platelet activation for the treatment of cardiovascular disease. J Atheroscler Thromb. 2012;19:516–22. doi: 10.5551/jat.10058. [DOI] [PubMed] [Google Scholar]
- 98.Andallu B, Vinay Kumar AV, Varadacharyulu NC. Lipid abnormalities in streptozotocin-diabetes: amelioration by Morus indica L. cv Suguna leaves. Int J Diabetes Dev Ctries. 2009;29:123–8. doi: 10.4103/0973-3930.54289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kobayashi Y, Miyazawa M, Kamei A, Abe K, Kojima T. Ameliorative effects of mulberry (Morus alba L.) leaves on hyperlipidemia in rats fed a high-fat diet: induction of fatty acid oxidation, inhibition of lipogenesis, and suppression of oxidative stress. Biosci Biotechnol Biochem. 2010;74:2385–95. doi: 10.1271/bbb.100392. [DOI] [PubMed] [Google Scholar]
- 100.Ryou SH, Kang MS, Kim KI, Kang YH, Kang JS. Effects of green tea or Sasa quelpaertensis bamboo leaves on plasma and liver lipids, erythrocyte Na efflux, and platelet aggregation in ovariectomized rats. Nutr Res Pract. 2012;6:106–12. doi: 10.4162/nrp.2012.6.2.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kawakami K, Aketa S, Nakanami M, Lizuka S, Hirayama M. Major water-soluble polyphenols, proanthocyanidins, in leaves of persimmon (Diospyros kaki) and their alpha-amylase inhibitory activity. Biosci Biotechnol Biochem. 2010;74:1380–5. doi: 10.1271/bbb.100056. [DOI] [PubMed] [Google Scholar]
- 102.Williams CA, Goldstone F, Greenham J. Flavonoids, cinnamic acids and coumarins from the different tissues and medicinal preparations of Taraxacum officinale. Phytochemistry. 1996;42:121–7. doi: 10.1016/0031-9422(95)00865-9. [DOI] [PubMed] [Google Scholar]
- 103.Choi UK, Lee OH, Yim JH, et al. Hypolipidemic and antioxidant effects of dandelion (Taraxacum officinale) root and leaf on cholesterol-fed rabbits. Int J Mol Sci. 2010;11:67–78. doi: 10.3390/ijms11010067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shanmuganayagam D, Beahm MR, Kuhns MA, Krueger CG, Reed JD, Folts JD. Differential effects of grape (Vitis vinifera) skin polyphenolics on human platelet aggregation and low-density lipoprotein oxidation. J Agric Food Chem. 2012;60:5787–94. doi: 10.1021/jf203487g. [DOI] [PubMed] [Google Scholar]
- 105.Oliboni LS, Dani C, Funchal C, Henriques JA, Salvador M. Hepatoprotective, cardioprotective, and renal-protective effects of organic and conventional grapevine leaf extracts on Wistar rat tissues. An Acad Bras Cienc. 2011;83:1403–11. doi: 10.1590/s0001-37652011000400027. [DOI] [PubMed] [Google Scholar]
- 106.de Graaf JC, Banga JD, Moncada S, Palmer RM, de Groot PG, Sixma JJ. Nitric oxide functions as an inhibitor of platelet adhesion under flow conditions. Circulation. 1992;85:2284–90. doi: 10.1161/01.cir.85.6.2284. [DOI] [PubMed] [Google Scholar]
- 107.Radomski MW, Palmer RM, Moncada S. Endogenous nitric oxide inhibits human platelet adhesion to vascular endothelium. Lancet. 1987;2:1057–8. doi: 10.1016/s0140-6736(87)91481-4. [DOI] [PubMed] [Google Scholar]
- 108.Brunner H, Cockcroft JR, Deanfield J, et al. Endothelial function and dysfunction. Part II: Association with cardiovascular risk factors and diseases. A statement by the Working Group on Endothelins and Endothelial Factors of the European Society of Hypertension. J Hypertens. 2005;23:233–46. doi: 10.1097/00004872-200502000-00001. [DOI] [PubMed] [Google Scholar]
- 109.Deanfield J, Donald A, Ferri C, et al. Endothelial function and dysfunction. Part I: Methodological issues for assessment in the different vascular beds: a statement by the Working Group on Endothelin and Endothelial Factors of the European Society of Hypertension. J Hypertens. 2005;23:7–17. doi: 10.1097/00004872-200501000-00004. [DOI] [PubMed] [Google Scholar]
- 110.Ziyyat A, Legssyer A, Mekhfi H, Dassouli A, Serhrouchni M, Benjelloun W. Phytotherapy of hypertension and diabetes in oriental Morocco. J Ethnopharmacol. 1997;58:45–54. doi: 10.1016/s0378-8741(97)00077-9. [DOI] [PubMed] [Google Scholar]
- 111.Lichius JJ, Renneberg H, Blaschek W, Aumuller G, Muth C. The inhibiting effects of components of stinging nettle roots on experimentally induced prostatic hyperplasia in mice. Planta Med. 1999;65:666–8. doi: 10.1055/s-2006-960844. [DOI] [PubMed] [Google Scholar]
- 112.Konrad L, Muller HH, Lenz C, Laubinger H, Aumuller G, Lichius JJ. Antiproliferative effect on human prostate cancer cells by a stinging nettle root (Urtica dioica) extract. Planta Med. 2000;66:44–7. doi: 10.1055/s-2000-11117. [DOI] [PubMed] [Google Scholar]
- 113.Bnouham M, Merhfour FZ, Ziyyat A, Mekhfi H, Aziz M, Legssyer A. Antihyperglycemic activity of the aqueous extract of Urtica dioica. Fitoterapia. 2003;74:677–81. doi: 10.1016/s0367-326x(03)00182-5. [DOI] [PubMed] [Google Scholar]
- 114.Gulcin I, Kufrevioglu OI, Oktay M, Buyukokuroglu ME. Antioxidant, antimicrobial, antiulcer and analgesic activities of nettle (Urtica dioica L.) J Ethnopharmacol. 2004;90:205–15. doi: 10.1016/j.jep.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 115.Riehemann K, Behnke B, Schulze-Osthoff K. Plant extracts from stinging nettle (Urtica dioica), an antirheumatic remedy, inhibit the proinflammatory transcription factor NF-kappaB. FEBS Lett. 1999;442:89–94. doi: 10.1016/s0014-5793(98)01622-6. [DOI] [PubMed] [Google Scholar]
- 116.Tahri A, Yamani S, Legssyer A, et al. Acute diuretic, natriuretic and hypotensive effects of a continuous perfusion of aqueous extract of Urtica dioica in the rat. J Ethnopharmacol. 2000;73:95–100. doi: 10.1016/s0378-8741(00)00270-1. [DOI] [PubMed] [Google Scholar]
- 117.Nassiri-Asl M, Zamansoltani F, Abbasi E, Daneshi MM, Zangivand AA. Effects of Urtica dioica extract on lipid profile in hypercholesterolemic rats. Zhong Xi Yi Jie He Xue Bao. 2009;7:428–33. doi: 10.3736/jcim20090506. [DOI] [PubMed] [Google Scholar]
- 118.El HM, Bnouham M, Bendahou M, et al. Inhibition of rat platelet aggregation by Urtica dioica leaves extracts. Phytother Res. 2006;20:568–72. doi: 10.1002/ptr.1906. [DOI] [PubMed] [Google Scholar]
- 119.Shahriyary L, Yazdanparast R. Inhibition of blood platelet adhesion, aggregation and secretion by Artemisia dracunculus leaves extracts. J Ethnopharmacol. 2007;114:194–8. doi: 10.1016/j.jep.2007.07.029. [DOI] [PubMed] [Google Scholar]
- 120.Agarwal KC. Therapeutic actions of garlic constituents. Med Res Rev. 1996;16:111–24. doi: 10.1002/(SICI)1098-1128(199601)16:1<111::AID-MED4>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 121.Neil HA, Silagy CA, Lancaster T, et al. Garlic powder in the treatment of moderate hyperlipidaemia: a controlled trial and meta-analysis. J R Coll Physicians Lond. 1996;30:329–34. [PMC free article] [PubMed] [Google Scholar]
- 122.Hiyasat B, Sabha D, Grotzinger K, et al. Antiplatelet activity of Allium ursinum and Allium sativum. Pharmacology. 2009;83:197–204. doi: 10.1159/000196811. [DOI] [PubMed] [Google Scholar]
- 123.Singh I, Mok M, Christensen AM, Turner AH, Hawley JA. The effects of polyphenols in olive leaves on platelet function. Nutr Metab Cardiovasc Dis. 2008;18:127–32. doi: 10.1016/j.numecd.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 124.Mudnic I, Modun D, Brizic I, et al. Cardiovascular effects in vitro of aqueous extract of wild strawberry (Fragaria vesca, L.) leaves. Phytomedicine. 2009;16:462–9. doi: 10.1016/j.phymed.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 125.Campana PR, Braga FC, Cortes SF. Endothelium-dependent vasorelaxation in rat thoracic aorta by Mansoa hirsuta D.C. Phytomedicine. 2009;16:456–61. doi: 10.1016/j.phymed.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 126.Lahlou S, Tangi KC, Lyoussi B, Morel N. Vascular effects of Tanacetum vulgare L. leaf extract: in vitro pharmacological study. J Ethnopharmacol. 2008;120:98–102. doi: 10.1016/j.jep.2008.07.041. [DOI] [PubMed] [Google Scholar]
- 127.Zhang L, Rui YC, Yang PY, Qiu Y, Li TJ, Liu HC. Inhibitory effects of Ginkgo biloba extract on vascular endothelial growth factor in rat aortic endothelial cells. Acta Pharmacol Sin. 2002;23:919–23. [PubMed] [Google Scholar]
- 128.Diamond BJ, Shiflett SC, Feiwel N, et al. Ginkgo biloba extract: mechanisms and clinical indications. Arch Phys Med Rehabil. 2000;81:668–78. doi: 10.1016/s0003-9993(00)90052-2. [DOI] [PubMed] [Google Scholar]
- 129.Naik SR, Panda VS. Antioxidant and hepatoprotective effects of Ginkgo biloba phytosomes in carbon tetrachloride-induced liver injury in rodents. Liver Int. 2007;27:393–9. doi: 10.1111/j.1478-3231.2007.01463.x. [DOI] [PubMed] [Google Scholar]
- 130.Tunali-Akbay T, Sener G, Salvarli H, Sehirli O, Yarat A. Protective effects of Ginkgo biloba extract against mercury(II)-induced cardiovascular oxidative damage in rats. Phytother Res. 2007;21:26–31. doi: 10.1002/ptr.2007. [DOI] [PubMed] [Google Scholar]
- 131.Le Bars PL, Katz MM, Berman N, Itil TM, Freedman AM, Schatzberg AF. A placebo-controlled, double-blind, randomized trial of an extract of Ginkgo biloba for dementia. North American EGb Study Group. JAMA. 1997;278:1327–32. doi: 10.1001/jama.278.16.1327. [DOI] [PubMed] [Google Scholar]
- 132.Ou HC, Lee WJ, Lee IT, et al. Ginkgo biloba extract attenuates oxLDL-induced oxidative functional damages in endothelial cells. J Appl Physiol. 2009;106:1674–85. doi: 10.1152/japplphysiol.91415.2008. [DOI] [PubMed] [Google Scholar]
- 133.Li XS, Zheng WY, Lou SX, Lu XW, Ye SH. Effect of Ginkgo leaf extract on vascular endothelial function in patients with early stage diabetic nephropathy. Chin J Integr Med. 2009;15:26–9. doi: 10.1007/s11655-009-0026-8. [DOI] [PubMed] [Google Scholar]
- 134.Lee YJ, Choi DH, Kim EJ, et al. Hypotensive, hypolipidemic, and vascular protective effects of Morus alba L. in rats fed an atherogenic diet. Am J Chin Med. 2011;39:39–52. doi: 10.1142/S0192415X11008634. [DOI] [PubMed] [Google Scholar]
- 135.Pirvulescu MM, Gan AM, Stan D, et al. Curcumin and a Morus alba extract reduce pro-inflammatory effects of resistin in human endothelial cells. Phytother Res. 2011;25:1737–42. doi: 10.1002/ptr.3463. [DOI] [PubMed] [Google Scholar]
- 136.Rattmann YD, Anselm E, Kim JH, et al. Natural product extract of Dicksonia sellowiana induces endothelium-dependent relaxations by a redox-sensitive Src- and Akt-dependent activation of eNOS in porcine coronary arteries. J Vasc Res. 2012;49:284–98. doi: 10.1159/000336647. [DOI] [PubMed] [Google Scholar]
- 137.Michalska M, Gluba A, Mikhailidis DP, et al. The role of polyphenols in cardiovascular disease. Med Sci Monit. 2010;16:RA110–9. [PubMed] [Google Scholar]
- 138.Manach C, Scalbert A, Morand C, Remesy C, Jimenez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004;79:727–47. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 139.Unachukwu UJ, Ahmed S, Kavalier A, Lyles JT, Kennelly EJ. White and green teas (Camellia sinensis var. sinensis): variation in phenolic, methylxanthine, and antioxidant profiles. J Food Sci. 2010;75:C541–8. doi: 10.1111/j.1750-3841.2010.01705.x. [DOI] [PubMed] [Google Scholar]
- 140.Fabisiak A, Sheng L, Stawczyk J, Witrowa-Rajchert D. The influence of method and apples drying temperature on the antioxidant activity of extracts produced from those dried apples. Zywność Nauka Technologia Jakość. 2005;2:318–27. [Google Scholar]
- 141.Soong YY, Soong BP. Antioxidant activity and phenolic content of selected fruit seeds. Food Chem. 2004;88:411–7. [Google Scholar]
- 142.Velioglu YS, Mazza G, Gao L, Oomah BD. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J Agric Food Chem. 1998;46:4113–7. [Google Scholar]
- 143.Manach C, Williamson G, Morand C, Scalbert A, Remesy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr. 2005;81:230S–2S. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]