Abstract
Many anti-diabetic drugs with different mechanisms of action are now available for treatment of type 2 diabetes mellitus. Sulfonylureas have been extensively used for treatment of type 2 diabetes for nearly 50 years and, even in our times, are widely used for treatment of this devastating chronic illness. Here, we review some of the available data on sulfonylureas, evaluating their mechanism of action and their effects on glycemic control. We can conclude that sulfonylureas are still the most used anti-diabetic agents: maybe this is due to their lower cost, to the possibility of mono-dosing and to the presence of an association with metformin in the same tablet. However, sulfonylureas, especially the older ones, are linked to a greater prevalence of hypoglycemia, and cardiovascular risk; newer prolonged-release preparations of sulfonylureas are undoubtedly safer, mainly due to reducing hypoglycemia, and for this reason should be preferred.
Keywords: glycemic control, hypoglycemia, sulfonylureas
Introduction
Many anti-diabetic drugs with different mechanisms of action are now available to treat type 2 diabetes mellitus, including sulfonylureas, glinides, thiazolidinediones [1, 2], biguanides [3], and α-glucosidase inhibitors [4, 5]. Recently, incretin-related drugs, such as dipeptidyl peptidase-4 (DPP-4) inhibitors [6, 7], and glucagon-like peptide-1 (GLP-1) receptor agonists [8, 9], have been developed. Despite the large number of anti-diabetic agents available, however, sulfonylureas remain the most widely used drugs for treating patients with type 2 diabetes [10].
Sulfonylureas were discovered in 1942, when Janbon et al. observed that some sulfonamides generated hypoglycemia in experimental animals. From this observation carbutamide (1-butyl-3-sulfonylurea) was synthesized. Carbutamide was the first sulfonylurea used to treat diabetes, but was subsequently withdrawn from the market because of its adverse effects on bone marrow.
By the 1960s several sulfonylureas became available; they are traditionally classified into 2 groups (or generations). Gliclazide, glipizide, glibenclamide and glimepiride are second-generation sulfonylureas, currently used, while first-generation drugs (such as tolbutamide and chlorpropamide) are no longer used. Second-generation drugs are equally effective in lowering blood glucose concentrations, but there are differences in absorption, metabolism and dosing (Table I).
Table I.
Various generations of sulfonylureas
| Molecules | Gen. | Dose [mg] | Duration of action* T1/2 | Activity of metabolites T1/2 | Elimination | Structure |
|---|---|---|---|---|---|---|
| Tolbutamide | I | 500–2000 | Short 4.5 to 6.5 h |
Inactive | Urine ≈ 100% | 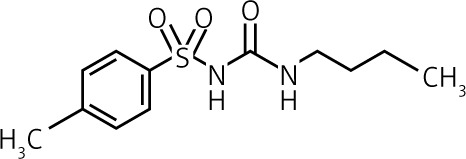 |
| Glibenclamide | II | 2.5–15 | Intermediate to long 5 to 7 h |
Active 10 h |
Bile ≈ 50% | 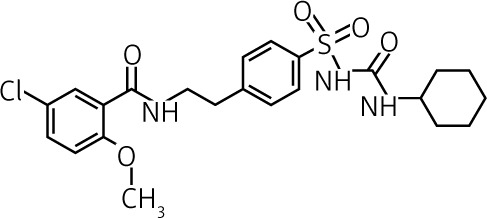 |
| Glimepiride | II | 1–6 | Intermediate 5 to 8 h |
Active 3 to 6 h |
Urine ≈ 80% | 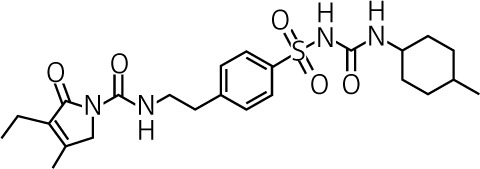 |
| Glipizide | II | 2.5–20 | Short to intermediate 2 to 4 h |
Inactive | Urine ≈ 70% | 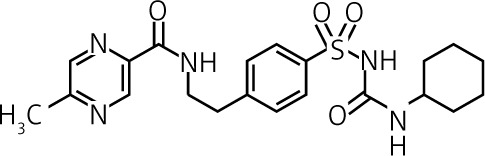 |
| Gliclazide | II | 40–320 | Intermediate 10 h |
Inactive | Urine ≈ 65% | 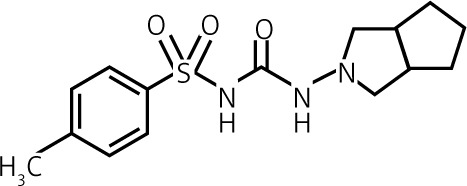 |
| Gliquidone | II | 15–180 | Short to intermediate 3 to 4 h |
Inactive | Bile ≈ 95% | 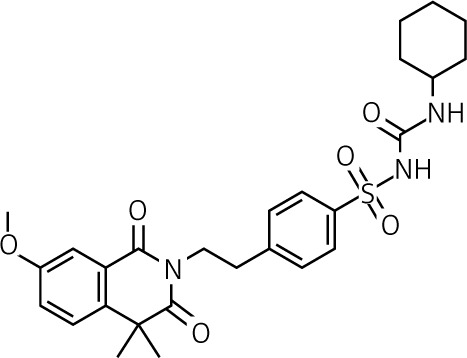 |
Short duration of activity means < 12 h, intermediate 12–24 h, long over 24 h.
Sulfonylureas should be considered for diabetic patients who are not overweight or those for whom metformin is contraindicated or is not enough to achieve adequate glycemic control [11].
Mechanism of action
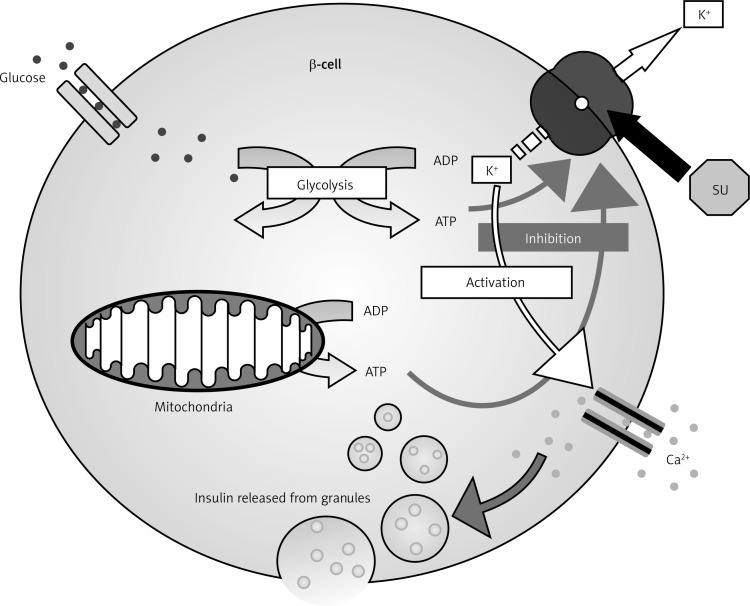
The main effect of sulfonylureas is the rise in plasma insulin concentrations; consequently they are effective only when residual pancreatic β-cells are present. The rise in plasma insulin levels occurs for two reasons. Firstly, there is stimulation of insulin secretion by pancreatic β-cells, and secondly, there is a decrease in hepatic clearance of insulin. In particular, this second effect appears mainly after the increase of insulin secretion has taken place. In fact, in the first month of treatment, the levels of insulin and insulin response to glucose rise rapidly, resulting in lowered blood glucose. After this period, baseline and stimulated insulin levels become lower compared to those measured at the beginning of treatment; however, blood glucose values remain unchanged. The reason for this observation is not clear. With regard to the secretory activity of sulfonylureas, the mechanism is now known. They act by binding to the specific receptor for sulfonylureas on β-pancreatic cells, blocking the inflow of potassium (K+) through the ATP-dependent channel: the flow of K+ within the β-cell goes to zero, the cell membrane becomes depolarized, thus removing the electric screen which prevents the diffusion of calcium into the cytosol. The increased flow of calcium into β-cells causes the contraction of the filaments of actomyosin responsible for the exocytosis of insulin, which is therefore promptly secreted in large amounts (Figure 1).
Figure 1.
Mechanism of action of sulfonylureas
On the top right corner is represented the SUR, while octagon is sulfonylurea (SU). When the SU binds SUR, the flow of K (arrows) stopped, so the cell membrane is depolarized. An increased flow of calcium cause the contraction of the filaments of actomyosin responsible for the exocytosis of insulin.
In particular, the sulfonylureas receptor (SUR1), a 1581-amino acid protein, has high affinity for glibenclamide. SUR1 is a member of the ATP-binding cassette (ABC) super-family that has two nucleotide binding folds (NBF-1 and NBF-2). Each nucleotide binding fold contains the Walker A and B motifs and the SGGQ ABC signature, and it is important in nucleotide regulation of the functional activities of ABC proteins. SUR1 has three transmembrane domains (TMD), TMD0, TMD1 and TMD2, which consist respectively of 5, 6 and 6 transmembrane (TM) segments that are numerated progressively. TMD0 contains the TM segments from 1 to 5, TMD1 contains the TM segments from 6 to 11, and TMD2 contains the TM segments from 12 to 17. SUR1 is expressed at higher levels in pancreatic islets. SUR1 is also present in the brain. Also a second type of sulfonylureas receptor exists; it is named SUR2A (formerly called SUR2), and it is an isoform of SUR1. SUR2A is a protein of 1545 amino acids sharing 68% amino acid identity with SUR1. SUR2A has a low affinity for glibenclamide. Several variants of SUR2A have also been identified. One of them, SUR2B, differs from SUR2A by 42 amino acids in the C-terminus, where it is, instead, similar to SUR1. Although SUR2A is expressed predominantly in heart and skeletal muscle, SUR2B is expressed widely in other tissues. In the past a two-site model (A site and B site) had been proposed for the interaction between sulfonylureas, glinides and SUR. The A site is located on the eighth (between TM segments 15 and 16) cytosolic loop, which is specific for SUR1. Instead the B site involves the third (between TM segments 5 and 6) cytosolic loop, which is very similar in all SURs. According to these different sites of interaction, sulfonylureas and glinides can be divided into three groups. The first of these includes nateglinide, tolbutamide and gliclazide, which are molecules that bind specifically the A site of SUR1, while the second group, which includes glimepiride and glibenclamide, binds non-specifically the B sites of both SUR1 and SUR2A as well as the A site of SUR1; finally, the third group (which includes meglitinide and repaglinide) binds to the B site of SUR1 and SUR2A.
Beside the “first phase”, sulfonylureas also increase the “second phase” of insulin secretion that begins 10 min later as insulin granules are translocated to the membrane of the β-cell. This second phase involves the progressive formation of new insulin granules, and it is possible only if β-cell function is preserved. It is important to underline that the release of insulin induced by sulfonylureas is independent of glucose levels, and this can increase the risk of hypoglycemia.
The impairment of the effect on insulin secretion that occurs during chronic administration of sulfonylureas is due to the down-regulation of the receptor for sulfonylureas on the surface of β-cells. This phenomenon disappears after discontinuing treatment for a period of time. In fact, resuming the administration of these drugs, the first administration effect reappears. Through a similar mechanism sulfonylureas can stimulate the secretion of somatostatin and suppress the secretion of glucagon in δ-cells and α-cells [12, 13]. In addition to the β-cells of the pancreas, sulfonylureas exert their effects on other cells. As an example, an increase of receptors for insulin present on monocytes, adipocytes and erythrocytes has been demonstrated in patients chronically treated with sulfonylureas [14]. Moreover, sulfonylureas seem to exert other effects as well: they increase peripheral glucose utilization by two mechanisms of action, by stimulating hepatic gluconeogenesis, and by increasing the number and sensitivity of insulin receptors [15]. However, their main effect is an increased responsiveness of β-cells to both glucose and non-glucose secretagogues (such as amino acids), resulting in more insulin released at any blood glucose concentration. Moreover, and this fact should not be underrated, they may cause suppression, sometimes significant, of overnight hepatic glucose output, thus further lowering the fasting blood glucose concentration [16].
Extra-pancreatic action of sulfonylureas
Over the years, a number of interesting extra-pancreatic actions of sulfonylurea drugs have been described. Many of these actions have required concentrations of sulfonylureas far in excess of the therapeutic levels usually attained in the plasma.
The extra-pancreatic effects of sulfonylurea drugs can be divided into different groups:•
Effects probably related to anti-diabetic action: enhancement of insulin stimulation of carbohydrate transport in skeletal muscle; enhancement of insulin action on the liver.
-
Effects possibly related to anti-diabetic action:
Direct effects on the liver: inhibition of triglyceride lipase; limitation of anionic substrate movement across the inner membrane of hepatic mitochondria; inhibition of ketosis; inhibition of glucose output.
Direct effects on adipose tissue: inhibition of lipolysis; inhibition of triglyceride lipase; increased uptake and oxidation of glucose.
Effects unlikely related to anti-diabetic action: activation of adenylate cyclase; inhibition of adenosine 3’,5’ monophosphate diesterase; inhibition of catecholamine release in vitro; alteration of rate of amino acid incorporation into protein; inhibition of transaminase activity; inhibition of the ratio of bound to free insulin; reduction of intestinal glucose absorption; inhibition in insulinases.
Effects not related to anti-diabetic action: increased cardiac contractility; effects on water balance (either diuretic or anti-diuretic); inhibition of platelet aggregation [17].
Pharmacokinetics
Although with time and different quantities, all sulfonylureas are absorbed by the intestine after oral intake, each one with its specific absorption time and bioavailability. Hyperglycemia can reduce the absorption of sulfonylureas as it impairs intestinal motility, thereby reducing the absorption of all orally administered drugs. This same phenomenon occurs for food intake as well. For this reason, to optimize their absorption, sulfonylureas should be taken 30 min before meals, and their dosage should be increased every 2 weeks if glycemic control has not been reached. The typical starting dose should be low (for example glibenclamide 2.5 mg or glimepiride 2 mg). Higher doses (for example, more than 10 mg of glibenclamide) rarely further improve glycemic control and should be avoided [18]. Due to their prolonged biological effect, sulfonylureas are given once or twice daily. After absorption, sulfonylureas bind almost completely to plasma proteins, especially albumin (on average 95%, ranging from 90% for chlorpropamide to 99% for glibenclamide). The volume of distribution is about 0.2 l/kg.
The biological effect of sulfonylureas often lasts much longer than their plasma half-life, because of receptor interaction and formation of active metabolites [19, 20], persisting 24 h or more. Moreover, their half-life is prolonged in the presence of renal failure. Beside possible alterations of absorption and metabolism, genetic differences can also change the response to sulfonylureas: in recent years genetic failure of β-cells has been demonstrated. These genetic variants obviously alter the effectiveness of sulfonylureas. Some of these gene polymorphisms were identified in the genes encoding the K+ ATP channel (KCNJ11 and ABCC8). These mutations cause a change in insulin secretion and insulin response to sulfonylurea treatment. Other polymorphisms were found on genes encoding enzymes or transcription factors [21].
For all of the above mentioned reasons, sulfonylureas are not all alike: they differ in their dosage, rate of absorption, duration of action, route of elimination and binding site on their target pancreatic β-cell receptor. The pharmacokinetic properties, therefore, are the determinant of these differences (Table I).
As indicated in Table I, it seems worthwhile to note that, unlike most of the sulfonylureas characterized by a prevalent renal excretion, gliclazide and, above all, gliquidone, show a predominant biliary clearance (= 95%). This can be useful in clinical practice, especially when treating diabetic patients with renal impairment.
Sulfonylureas lower blood glucose concentrations by about 20% and HbA1c by 1 to 2% [22]: they exert effects on HbA1c similar to those of metformin, but their use entails a greater risk of hypoglycemia and of undesired weight gain, averaging approximately 2 kg.
Side effects
Sulfonylureas are usually well tolerated. The most common side effect is hypoglycemia, more common with long-acting sulfonylureas such as chlorpropamide and glibenclamide [23–25]. However, all sulfonylureas may cause hypoglycemia, usually due to an excessive dosage. It is important to remember that hypoglycemia may persist for many hours and require in-hospital treatment.
A 4-year retrospective study of 14,000 patients, 65 years or older, with type 2 diabetes, treated with different sulfonylurea drugs, showed that episodes of serious hypoglycemia were rare [26]. The incidence was higher in those patients taking glibenclamide, and lower among those taking tolbutamide (19.9 vs. 3.5 episodes per 1000 person-years, respectively). Other shorter-acting drugs, such as tolazamide and glipizide, were also associated with a lower incidence, while the incidence with chlorpropamide was similar to that with glibenclamide [16]. Patients recently discharged from hospital were at the highest risk (4.5 episodes per 100 person-years) [26].
Patients should be cautioned about those situations in which hypoglycemia is most likely to occur: after exercise or a missed meal, or when taking an excessive dosage. In addition to the use of longer-acting drugs such as glibenclamide or chlorpropamide, it is necessary to recognize other situations at risk of hypoglycemia. For example, sulfonylureas should be used with caution in patients who are undernourished or alcohol abusers, in patients with impaired renal or cardiac function or gastrointestinal disease, in patients concurrently treated with salicylates, sulfonamides, fibric acid derivatives (such as gemfibrozil), and warfarin [27], and during hospitalization [28].
A good way to prevent hypoglycemia is to start therapy with sulfonylureas at a low dose. The dosage may be increased at intervals of 2–4 weeks until the glycemic target is reached. Obviously, self-monitoring of blood glucose by the patient may be helpful. Nevertheless, in general, patients who have managed less fasting hyperglycemia after a trial of diet and exercise are more likely to develop hypoglycemia.
The reported frequency of sulfonylurea-related hypoglycemia in the elderly is variable, and frequently underestimated (usually only severe hypoglycemias are considered). Even though this risk is lower with the newer sulfonylureas (glipizide, glimepiride) [24], these episodes, more frequent and dangerous in the elderly, can limit their use. In fact, in these patients, autonomic failure, secondary to aging and to longer duration of diabetes, is responsible for asymptomatic and occult hypoglycemias, while difficulty in communication may complicate its management. The concept of the “frail elderly”, in which the glycated haemoglobin (HbA1c) target was raised up to 8.6%, allows less aggressive therapeutic strategies to be followed, and does not justify the use of drugs that carry a risk of prolonged hypoglycemia. Thus, old age (> 75 years), renal impairment and liver disease are conditions in which sulfonylureas should not be used as first line therapy, but as second or third line agents in type 2 diabetes mellitus.
Sulfonylureas act directly on β-cells, leading to progressive dysfunction and worsening of insulin secretion. Thus, despite better glycemic control in the short term, diabetes could worsen in the long term. The clinical result of this phenomenon is known as “secondary failure”, and it represents the inevitable fate of all oral hypoglycemic agents, especially older sulfonylureas. In fact, patients with previous higher dosages and longer treatment with sulfonylureas display a worse response to insulin after changing therapy: sulfonylurea dosage is independently associated with inadequate response to insulin analogues in patients with secondary failure [29].
Weight gain is an almost constant counterpart of treatment with sulfonylureas, even though to a lesser degree than that recorded with insulin [30]. This certainly constitutes a deleterious effect, especially in reference to a chronic illness such as diabetes mellitus, where the control of body weight represents, perhaps, the main target of treatment. Fortunately, the weight gain is usually mitigated by the concurrent administration of metformin.
Other infrequent side effects that may occur with all sulfonylureas include nausea, skin reactions such as erythema multiforme, exfoliative dermatitis and also, more rarely, photosensitivity. Occasionally, they can cause abnormalities in liver function tests, which may rarely lead to cholestatic jaundice, hepatitis and hepatic failure. It seems especially important to recall some disturbing side effects of chlorpropamide, fortunately no longer used, mainly because of its very prolonged duration of action. It may cause, in fact, a flushing skin reaction after alcohol ingestion by inhibiting the metabolism of acetaldehyde [31]; it also may lead to hyponatremia by increasing the secretion of anti-diuretic hormone [32]. This effect has also been described with the use of glimepiride and glipizide.
Some studies suggest that sulfonylureas may affect cardiac function and also may be associated with poorer outcomes after myocardial infarction [33–35]. An increased mortality from cardiovascular disease in diabetic patients taking tolbutamide was reported in the past decades (University Group Diabetes Study) [36].
Subsequently, several studies were designed to shed light on this alarming association. In the Mayo Clinic, in 185 consecutive diabetic patients undergoing percutaneous coronary intervention after myocardial infarction, the odds ratio for death was 2.77 for patients treated with a sulfonylurea at the time of the myocardial infarction [37].
In the DIGAMI (Diabetes Mellitus, Insulin Glucose Infusion in Acute Myocardial Infarction) trial, the patients treated with a sulfonylurea at the time of myocardial infarction were those with the poorest outcome [38].
Finally, in a retrospective Canadian study using pharmaceutical data for 5795 subjects who received initial monotherapy with either a sulfonylurea or metformin, deaths per 1000 person-years during the follow-up period (mean 4.8 years) were 67.6 for first-generation sulfonylurea medications, 61.4 for glibenclamide, and 39.6 for metformin [39]. The risk of death or of an acute ischemic event was greater for subjects exposed to higher amounts of the sulfonylurea, but not to metformin [39]. The greatest risk was for subjects treated with tolbutamide or chlorpropamide (hazard ratio (HR) 2.1, 95% CI: 1.0–4.7).
An explanation of these results may be found in the interaction of sulfonylureas with cardiomyocytes. Indeed, there are sulfonylurea receptor isoforms on cardiac myocytes and vascular smooth muscle. Insulin secretagogues display different tissue selectivity characteristics at therapeutic concentrations, and this may translate into different levels of cardiovascular risk [40].
Since ATP-dependent K+ channels are present on cardiac cells and coronary vessels, sulfonylureas, if present at the time of a myocardial infarction, may impair adequate coronary vasodilatation, thus resulting in a larger area of myocardial damage. Other hypotheses for the effect of sulfonylurea medications on cardiovascular events and mortality are founded on interference with ischemic preconditioning, or possible arrhythmogenic effects, and on the inhibitory effect of sulfonylureas on the reverse cholesterol transport mediated by HDL [41].
The treatment with sulfonylureas also carries other implications. In fact, there is evidence suggesting that the activation of mitochondrial K+ ATP channels plays an important role in the mechanical protection that results from ischemic preconditioning [41]. Glibenclamide prevents cardioprotection induced by ischemia with the interaction with mitochondrial K+ ATP channels, whereas gliclazide does not. In skeletal muscle, activation of K+ ATP channels during fatigue prevents muscle dysfunction by reducing resting tension and improving force recovery. However, blocking K+ ATP channels of skeletal muscles by glibenclamide does not affect the fall in force during fatigue. K+ ATP channels can mediate counter-regulatory responses to hypoglycemia, at the level of the central nervous system [42]. The opening of K+ ATP channels during hypoxia also reduces membrane excitability and protects neurons from seizure activity. It has to be underlined that newer sulfonylureas, such as gliclazide, are selective for the pancreatic sulfonylurea receptors over the cardiac receptors and do not appear to be associated with increased cardiovascular mortality compared with metformin or other anti-diabetic medications [43], although direct controlled clinical trials have not been performed [44, 45]. In a study of 1310 French patients with diabetes who were hospitalized for myocardial infarction, in-hospital mortality rates were significantly lower in patients previously treated with sulfonylureas compared with other oral medications, insulin, or no medication (3.9%, 6.4%, 9.4%, and 8.4%, respectively, odds ratio for patients receiving sulfonylureas before admission compared with no sulfonylureas 0.50, 95% CI: 0.27–0.94) [44]. Among the sulfonylurea-treated patients, mortality was significantly lower in patients receiving gliclazide or glimepiride rather than glibenclamide, which is not selective for the pancreatic sulfonylurea receptors.
In addition to these findings, the results of clinical trials, particularly ADVANCE [46], using newer sulfonylureas such as gliclazide, are somewhat reassuring, although they were not specifically designed to address this issue. Moreover, it has been demonstrated that the use of sulfonylureas can increase the risk of developing a neoplastic disease [47].
Regarding the relation between sulfonylurea use and cancer, the evidence is weak: Bowker et al. [47] found that cancer-related mortality among residents of the province of Saskatchewan in Canada was greater for diabetics treated with insulin and sulfonylureas than for metformin users. However, from this study the relationship between sulfonylurea use and cancer development was inconsistent. In fact, many factors must be considered when analyzing the findings from observational studies that examine the association between diabetes therapies and cancer, for example sample size of the population examined, type and duration of diabetes, the level of metabolic control, comorbidities, and duration of follow-up. We know that sulfonylureas are a heterogeneous group of drugs, and the effects of single drugs are drug-specific rather than class-specific. Looking at the study performed by Monami et al. [48], it seems that cancer and its related mortality in patients with type 2 diabetes mellitus using glibenclamide was significantly higher than in those using gliclazide. Furthermore, regarding colorectal cancer, there is no evidence that using sulfonylureas in type 2 diabetes leads to cancer: rather, the risk of developing colorectal cancer in these patients is reduced [49].
Conclusions
Despite the great number of anti-diabetic agents currently available in clinical practice, sulfonylureas are still frequently used: maybe this is due to their lower cost, to the possibility of mono-dosing and to the presence of an association with metformin in the same tablet.
In patients suffering from inadequate glycemic control, sulfonylureas can rapidly achieve significant improvement when added to metformin [50–53]. According to recent guidelines, they can also be associated with glitazones [54–57], GPL-1 analogues, DPP-4 inhibitors [58] or long acting insulin when the association with metformin alone fails to achieve the glycemic target. Considering adverse events, sulfonylureas, especially the older ones, are linked to a greater prevalence of hypoglycemia, and cardiovascular risk; in this respect, newer prolonged-release preparations of sulfonylureas are undoubtedly safer, mainly due to reducing hypoglycemia, and for this reason should be preferred.
The European GUIDE study [59], for example, compared two sulfonylureas designed for once-daily administration used under conditions of everyday clinical practice. Patients were randomized to either gliclazide modified release (MR) 30–120 mg daily or glimepiride 1–6 mg daily as monotherapy or in combination with their current treatment for 27 weeks. Even if gliclazide MR and glimepiride were similarly effective in improving blood glucose control, gliclazide MR gave approximately 50% fewer hypoglycemic episodes in comparison with glimepiride.
We can conclude that sulfonylureas should be used in relatively young patients for a limited period of time, maybe 3–6 months, in order to rapidly achieve adequate glycemic control. In this way we can avoid starting insulin, improving the patient's quality of life and his/her compliance and reducing the cost of anti-diabetic therapy. After 3–6 months, if adequate glycemic control has not been achieved, we should start insulin treatment. On the other hand, if we have reached a lower HbA1c, we should replace sulfonylureas with other oral anti-diabetic agents, according to the guidelines.
Conflict of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties. No writing assistance was utilized in the production of this manuscript.
The authors declare no conflict of interest.
References
- 1.Derosa G, Maffioli P. Thiazolidinediones plus metformin association on body weight in patients with type 2 diabetes. Diabetes Res Clin Pract. 2011;91:265–13. doi: 10.1016/j.diabres.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Derosa G, Tinelli C, Maffioli P. Effects of pioglitazone and rosiglitazone combined with metformin on body weight in people with diabetes. Diabetes Obes Metab. 2009;11:1091–9. doi: 10.1111/j.1463-1326.2009.01087.x. [DOI] [PubMed] [Google Scholar]
- 3.Cicero AFG, Tartagni E, Ertek S. Metformin and its clinical use: new insights for an old drug in clinical practice. Arch Med Sci. 2012;8:907–17. doi: 10.5114/aoms.2012.31622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Derosa G, Maffioli P. Efficacy and safety profile evaluation of acarbose alone and in association with other antidiabetic drugs: a systematic review. Clin Ther. 2012;34:1221–36. doi: 10.1016/j.clinthera.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 5.Derosa G, Maffioli P. Alpha-glucosidase inhibitors and their use in clinical practice. Arch Med Sci. 2012;8:899–906. doi: 10.5114/aoms.2012.31621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Derosa G, Maffioli P. Dipeptidyl peptidase-4 inhibitors: 3 years of experience. Diabetes Technol Ther. 2012;14:350–64. doi: 10.1089/dia.2011.0204. [DOI] [PubMed] [Google Scholar]
- 7.Derosa G, Maffioli P. Patient considerations and clinical utility of a fixed dose combination of saxagliptin/metformin in the treatment of type 2 diabetes. Diabetes Metab Syndr Obes. 2011;4:263–71. doi: 10.2147/DMSO.S16361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Derosa G, Maffioli P. GLP-1 agonists exenatide and liraglutide: a review about their safety and efficacy. Curr Clin Pharmacol. 2012;7:214–28. doi: 10.2174/157488412800958686. [DOI] [PubMed] [Google Scholar]
- 9.Derosa G, Maffioli P. Optimizing glycemic control: clinical utility of exenatide prolonged release injection. Res Rep Endocr Disord. 2012;2:41–51. [Google Scholar]
- 10.Bressler R, Johnson DG. Pharmacological regulation of blood glucose levels in non-insulin-dependent diabetes mellitus. Arch Intern Med. 1997;157:836–48. [PubMed] [Google Scholar]
- 11.American Diabetes Association. Standards of Medical Care in Diabetes-2013. Diabetes Care. 2013;36(Suppl. 1):S11–66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramracheya R, Ward C, Shigeto M, et al. Membrane potential-dependent inactivation of voltage-gated ion channels in alpha-cells inhibits glucagon secretion from human islets. Diabetes. 2010;59:2198–208. doi: 10.2337/db09-1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Braun M, Ramracheya R, Amisten S, et al. Somatostatin release, electrical activity, membrane currents and exocytosis in human pancreatic delta cells. Diabetologia. 2009;52:1566–78. doi: 10.1007/s00125-009-1382-z. [DOI] [PubMed] [Google Scholar]
- 14.Olefsky JM, Reaven GM. Effects of sulphonylurea therapy on insulin binding to mononuclear leukocytes of diabetic patients. Am J Med. 1976;60:89–95. doi: 10.1016/0002-9343(76)90537-4. [DOI] [PubMed] [Google Scholar]
- 15.Blumenthal SA. Potentiation of the hepatic action of insulin by chlorpropamide. Diabetes. 1977;26:485–9. doi: 10.2337/diab.26.5.485. [DOI] [PubMed] [Google Scholar]
- 16.Shorr RI, Ray WA, Daugherty JR, Griffin MR. Individual sulfonylureas and serious hypoglycemia in older people. J Am Geriatr Soc. 1996;44:751–5. doi: 10.1111/j.1532-5415.1996.tb03729.x. [DOI] [PubMed] [Google Scholar]
- 17.Lebovitz HE, Feinglos MN. Sulfonylurea drugs: mechanism of antidiabetic action and therapeutic usefulness. Diabetes Care. 1978;1:189–98. doi: 10.2337/diacare.1.3.189. [DOI] [PubMed] [Google Scholar]
- 18.Stenman S, Melander A, Groop PH, Groop LC. What is the benefit of increasing the sulphonylurea dose? Ann Intern Med. 1993;118:169–72. doi: 10.7326/0003-4819-118-3-199302010-00002. [DOI] [PubMed] [Google Scholar]
- 19.Jönsson A, Rydberg T, Ekberg G, Hallengren B, Melander A. Slow elimination of glyburide in NIDDM subjects. Diabetes Care. 1994;17:142–5. doi: 10.2337/diacare.17.2.142. [DOI] [PubMed] [Google Scholar]
- 20.Rydberg T, Jönsson A, Røder M, Melander A. Hypoglycemic activity of glyburide (glibenclamide) metabolites in humans. Diabetes Care. 1994;17:1026–30. doi: 10.2337/diacare.17.9.1026. [DOI] [PubMed] [Google Scholar]
- 21.Winkler G, Gerô L. Pharmacogenetics of insulin secretagogue antidiabetics. Orv Hetil. 2011;152:1651–60. doi: 10.1556/OH.2011.29175. [DOI] [PubMed] [Google Scholar]
- 22.Hermann LS, Scherstén B, Bitzén PO, Kjellström T, Lindgärde F, Melander A. Therapeutic comparison of metformin and sulphonylurea, alone and in various combinations. A double-blind controlled study. Diabetes Care. 1994;17:1100–9. doi: 10.2337/diacare.17.10.1100. [DOI] [PubMed] [Google Scholar]
- 23.Tessier D, Dawson K, Tétrault JP, Bravo G, Meneilly GS. Glibenclamide vs gliclazide in type 2 diabetes of the elderly. Diabet Med. 1994;11:974–80. doi: 10.1111/j.1464-5491.1994.tb00256.x. [DOI] [PubMed] [Google Scholar]
- 24.Holstein A, Plaschke A, Egberts EH. Lower incidence of severe hypoglycemia in patients with type 2 diabetes treated with glimepiride versus glibenclamide. Diabetes Metab Res Rev. 2001;17:467–73. doi: 10.1002/dmrr.235. [DOI] [PubMed] [Google Scholar]
- 25.Gangji AS, Cukierman T, Gerstein HC, Goldsmith CH, Clase CM. A systematic review and meta-analysis of hypoglycemia and cardiovascular events: a comparison of glyburide with other secretagogues and with insulin. Diabetes Care. 2007;30:389–94. doi: 10.2337/dc06-1789. [DOI] [PubMed] [Google Scholar]
- 26.Shorr RI, Ray WA, Daugherty JR, Griffin MR. Incidence and risk factors for serious hypoglycemia in older persons using insulin or sulfonylureas. Arch Intern Med. 1997;157:1681–6. [PubMed] [Google Scholar]
- 27.Bressler P, DeFronzo RA. Drugs and diabetes. Diabetes Rev. 1994;2:53–84. [Google Scholar]
- 28.Shorr RI, Ray WA, Daugherty JR, Griffin MR. Incidence and risk factors for serious hypoglycemia in older persons using insulin or sulfonylureas. Arch Intern Med. 1997;157:1681–6. [PubMed] [Google Scholar]
- 29.Lee YH, Lee BW, Chun SW, Cha BS, Lee HC. Predictive characteristics of patients achieving glycemic control with insulin after sulphonylurea failure. Int J Clin Pract. 2011;65:1076–84. doi: 10.1111/j.1742-1241.2011.02755.x. [DOI] [PubMed] [Google Scholar]
- 30.Derosa G, Limas CP, Macías PC, Estrella A, Maffioli P. Dietary and nutraceutical approach to type 2 diabetes. Arch Med Sci. 2014;10:336–44. doi: 10.5114/aoms.2014.42587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Groop L, Eriksson CJ, Huupponen R, Ylikahri R, Pelkonen R. Roles of chlorpropamide, alcohol and acetaldehyde in determining the chlorpropamide-alcohol flush. Diabetologia. 1984;26:34–8. doi: 10.1007/BF00252260. [DOI] [PubMed] [Google Scholar]
- 32.Kadowaki T, Hagura R, Kajinuma H, Kuzuya N, Yoshida S. Chlorpropamide-induced hyponatremia: incidence and risk factors. Diabetes Care. 1983;6:468–71. doi: 10.2337/diacare.6.5.468. [DOI] [PubMed] [Google Scholar]
- 33.Rao AD, Kuhadiya N, Reynolds K, Fonseca VA. Is the combination of sulfonylureas and metformin associated with an increased risk of cardiovascular disease or all-cause mortality? A meta-analysis of observational studies. Diabetes Care. 2008;31:1672–8. doi: 10.2337/dc08-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morgan CL, Poole CD, Evans M, Barnett AH, Jenkins-Jones S, Currie CJ. What next after metformin? A retrospective evaluation of the outcome of second-line, glucose-lowering therapies in people with type 2 diabetes. J Clin Endocrinol Metab. 2012;97:4605–12. doi: 10.1210/jc.2012-3034. [DOI] [PubMed] [Google Scholar]
- 35.Kasznicki J, Drzewoski J. Heart failure in the diabetic population – pathophysiology, diagnosis and management. Arch Med Sci. 2014;10:546–56. doi: 10.5114/aoms.2014.43748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.A study of the effects of hypoglycemia agents on vascular complications in patients with adult-onset diabetes. VI. Supplementary report on nonfatal events in patients treated with tolbutamide. Diabetes. 1976;25:1129–53. doi: 10.2337/diab.25.12.1129. [DOI] [PubMed] [Google Scholar]
- 37.Garratt KN, Brady PA, Hassinger NL, Grill DE, Terzic A, Holmes DR., Jr Sulphonylurea drugs increase early mortality in patients with diabetes mellitus after direct angioplasty for acute myocardial infarction. J Am Coll Cardiol. 1999;33:119–24. doi: 10.1016/s0735-1097(98)00557-9. [DOI] [PubMed] [Google Scholar]
- 38.Malmberg K. Prospective randomised study of intensive insulin treatment on long term survival after acute myocardial infarction in patients with diabetes mellitus. DIGAMI (Diabetes Mellitus, Insulin Glucose Infusion in Acute Myocardial Infarction) Study Group. BMJ. 1997;314:1512–5. doi: 10.1136/bmj.314.7093.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simpson SH, Majumdar SR, Tsuyuki RT, Eurich DT, Johnson JA. Dose-response relation between sulphonylurea drugs and mortality in type 2 diabetes mellitus: a population-based cohort study. CMAJ. 2006;174:169–74. doi: 10.1503/cmaj.050748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abdelmoneim AS, Hasenbank SE, Seubert JM, Brocks DR, Light PE, Simpson SH. Variations in tissue selectivity amongst insulin secretagogues: a systematic review. Diabetes Obes Metab. 2012;14:130–8. doi: 10.1111/j.1463-1326.2011.01496.x. [DOI] [PubMed] [Google Scholar]
- 41.Terao Y, Ayaori M, Ogura M, et al. Effect of sulphonylurea agents on reverse cholesterol transport in vitro and vivo. Diab Vasc Dis Res. 2011;18:513–30. doi: 10.5551/jat.7641. [DOI] [PubMed] [Google Scholar]
- 42.Billman GE. The cardiac sarcolemmal ATP-sensitive potassium channel as a novel target for anti-arrhythmic therapy. Pharmacol Ther. 2008;120:54–70. doi: 10.1016/j.pharmthera.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 43.Miki T, Liss B, Minami K, et al. ATP-sensitive K+ channels in the hypothalamus are essential for the maintenance of glucose homeostasis. Nat Neurosci. 2001;4:507–12. doi: 10.1038/87455. [DOI] [PubMed] [Google Scholar]
- 44.Zeller M, Danchin N, Simon D, et al. French Registry of Acute ST-Elevation and Non-ST-Elevation Myocardial Infarction investigators. Impact of type of preadmission sulfonylureas on mortality and cardiovascular outcomes in diabetic patients with acute myocardial infarction. J Clin Endocrinol Metab. 2010;95:4993–5002. doi: 10.1210/jc.2010-0449. [DOI] [PubMed] [Google Scholar]
- 45.Schramm TK, Gislason GH, Vaag A, et al. Mortality and cardiovascular risk associated with different insulin secretagogues compared with metformin in type 2 diabetes, with or without a previous myocardial infarction: a nationwide study. Eur Heart J. 2011;32:1900–8. doi: 10.1093/eurheartj/ehr077. [DOI] [PubMed] [Google Scholar]
- 46.ADVANCE Collaborative Group. Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–72. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 47.Bowker SL, Majumdar SR, Veugelers P, Johnson JA. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care. 2006;29:254–8. doi: 10.2337/diacare.29.02.06.dc05-1558. [DOI] [PubMed] [Google Scholar]
- 48.Monami M, Balzi D, Lamanna C, et al. Are sulfonylureas all the same? A cohort study on cardiovascular and cancer-related mortality. Diabetes Metab Res Rev. 2007;23:479–84. doi: 10.1002/dmrr.736. [DOI] [PubMed] [Google Scholar]
- 49.Gerich J, Raskin P, Jean-Louis L, Purkayastha D, Baron MA. PRESERVE-beta: two-year efficacy and safety of initial combination therapy with nateglinide or glyburide plus metformin. Diabetes Care. 2005;28:2093–9. doi: 10.2337/diacare.28.9.2093. [DOI] [PubMed] [Google Scholar]
- 50.Nestler JE. Metformin in the treatment of infertility in polycystic ovarian syndrome: an alternative perspective. Fertil Steril. 2008;90:14–6. doi: 10.1016/j.fertnstert.2008.04.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Derosa G, Putignano P, Bossi AC, et al. Exenatide or glimepiride added to metformin on metabolic control and on insulin resistance in type 2 diabetic patients. Eur J Pharmacol. 2011;666:251–6. doi: 10.1016/j.ejphar.2011.05.051. [DOI] [PubMed] [Google Scholar]
- 52.Derosa G, Maffioli P, Salvadeo SA, et al. Exenatide versus glibenclamide in patients with diabetes. Diabetes Technol Ther. 2010;12:233–40. doi: 10.1089/dia.2009.0141. [DOI] [PubMed] [Google Scholar]
- 53.Hirst JA, Farmer AJ, Dyar A, Lung TW, Stevens RJ. Estimating the effect of sulfonylurea on HbA1c in diabetes: a systematic review and meta-analysis. Diabetologia. 2013;56:973–84. doi: 10.1007/s00125-013-2856-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Derosa G, Cicero AF, Fogari E, D'Angelo A, Bianchi L, Maffioli P. Pioglitazone compared to glibenclamide on lipid profile and inflammation markers in type 2 diabetic patients during an oral fat load. Horm Metab Res. 2011;43:505–12. doi: 10.1055/s-0031-1275704. [DOI] [PubMed] [Google Scholar]
- 55.Derosa G, Maffioli P. Effects of thiazolidinediones and sulfonylureas in patients with diabetes. Diabetes Technol Ther. 2010;12:491–501. doi: 10.1089/dia.2009.0172. [DOI] [PubMed] [Google Scholar]
- 56.Derosa G. Pioglitazone plus glimepiride: a promising alternative in metabolic control. Int J Clin Pract Suppl. 2007;153:28–36. doi: 10.1111/j.1742-1241.2007.01362.x. [DOI] [PubMed] [Google Scholar]
- 57.Derosa G, Cicero AF, D'Angelo A, et al. Effects of 1 year of treatment with pioglitazone or rosiglitazone added to glimepiride on lipoprotein (a) and homocysteine concentrations in patients with type 2 diabetes mellitus and metabolic syndrome: a multicenter, randomized, double-blind, controlled clinical trial. Clin Ther. 2006;28:679–88. doi: 10.1016/j.clinthera.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 58.Derosa G, Maffioli P, Ferrari I, et al. Effects of one year treatment of vildagliptin added to pioglitazone or glimepiride in poorly controlled type 2 diabetic patients. Horm Metab Res. 2010;42:663–9. doi: 10.1055/s-0030-1255036. [DOI] [PubMed] [Google Scholar]
- 59.Schernthaner G, Grimaldi A, Di Mario U, et al. GUIDE study: double-blind comparison of once-daily gliclazide MR and glimepiride in type 2 diabetic patients. Eur J Clin Invest. 2004;34:535–42. doi: 10.1111/j.1365-2362.2004.01381.x. [DOI] [PubMed] [Google Scholar]