Abstract
Introduction
Renal cell carcinoma is a type of malignant tumor often diagnosed in the urinary system. The aim of this study is to evaluate the anti-tumor effects and mechanisms of a polypeptide, Haishengsu (TG-1), with different concentrations (125, 250, 500 mg/kg) on renal metastatic tumor OS-RC-2 cells.
Material and methods
We first established the renal metastatic tumor model. After being administered with TG-1, the weight of tumors was measured and the microstructural changes of renal carcinoma OS-RC-2 cells were compared using transmission electron microscopy before and after the therapy. The Ki67 expression in renal carcinoma OS-RC-2 cells was analyzed by RT-PCR and downstream signal molecule caspase-3 was measured by Western blot assay.
Results
After treatment with different doses of TG-1, the tumor weights in the positive control group and experimental groups were smaller than those in the untreated control group, suggesting that TG-1 could effectively inhibit tumor growth in mice. The transmission electron microscopy and flow cytometry results indicated that TG-1 induces tumor cell apoptosis (p < 0.05). The tumor cells exhibited polymorphism changes and chromatin edge clustering. TG-1 also inhibited Ki67 expression and promoted caspase-3 expression in the tumor significantly (p < 0.05).
Conclusions
TG-1 inhibits the growth of the tumor and induces apoptosis of the tumor cells by inducing caspase-3 expression. The results provide a basis for clinical application of TG-1 in the future.
Keywords: polypeptide, renal tumor OS-RC-2 cell, Ki67 mRNA, anti-tumor effect
Introduction
Renal cell carcinoma (RCC) is a common malignant tumor of the urinary system, which represents about 2–3% of adult malignant tumors [1, 2]. Every year about 100,000 patients die of RCC in the world. About one in three patients with RCC has metastasis [3]. Moreover, there are still 20–30% patients having metastasis after radical surgery [4, 5]. Renal carcinoma cells are not sensitive to radiotherapy and chemotherapy. Haishengsu (TG-1) is an anti-tumor polypeptide extracted from Tegillarca granosa Linnaeus. Previous studies revealed that TG-1 could induce apoptosis of K562 leukemia cells and had a clear anti-tumor effect on ascitic tumor cells [6, 7]. The combined use of TG-1 with conventional immunotherapy elevated remission rates in patients with advanced renal cell cancer and improved the physical functionality of patients [8].
Ki67, a kind of nuclear antigen which is closely related to the cell cycle, is an indicator for the proliferation of cells and has abnormal expression in many tumor cells [9, 10]. As a DNA binding protein, Ki67 is expressed in G1, S, G2 and M phases and is a key component during proliferation of tumor cells. Previous research revealed that the expression of Ki67 is closely related to the grading, staging and prognosis of many tumors. Poor prognosis is very common among patients with high expression of Ki67 [11–13]. In this study, the effects of TG-1 on renal cell carcinoma OS-RC-2 cells were studied and the Ki67 expression in renal carcinoma OS-RC-2 cells was analyzed. The differentiation and apoptosis of the OS-RC-2 cells induced by TG-1 were measured, which could provide a theoretical basis for the clinical application of TG-1.
Material and methods
Cells and experimental animals
Human renal carcinoma cells (OS-RC-2) were purchased from Shanghai Life Science Research Institute of the Chinese Academy of Sciences. SPF nude mice, male and female mice were supplied by Shanghai Slack Experimental Animals Limited Liability Company, and the mean weight of the animals was 20 ±3 g. All animal experiments were conducted according to the ethical guidelines of Qingdao University.
Reagents
TG-1 was supplied by the manufacturing laboratory of Qingdao Haisheng Tumor Hospital. New-born calf serum was purchased from Gibco (USA). Fetal calf serum and DMEM medium were purchased from Hyclone. The PR-PCR kit and Annexin V-PE/7-ADD apoptosis test kit were supplied by Promega and Becton Dickinson, respectively. Ki67 primer and the GADPH primer were synthesized by Shanghai Bioengineering Limited Company and Wuhan Boster Bioengineering Limited Company, respectively. The antibody was supplied by Beijing Zhongshan Medicine Preparation Company. FT-207 was supplied by Qilu pharmaceutical factory.
Animal model
Suspensions of mouse renal carcinoma OS-RC-2 cells (107 cells/l) were injected into the mouse by intraperitoneal injection. The ascitic fluid was extracted and diluted with saline, and then was injected into the enterocoella of the mouse again for another passage. The ascitic fluid was extracted again. After being centrifuged and washed by normal saline, the ascetic fluid was inoculated into the forelimb armpit (109/l, 0.2 ml per animal). The animals were then randomly divided into a negative control group, a positive control group (FT-207) and experimental groups (anti-tumor polypeptide), respectively. The experimental groups were further divided into 3 groups by administering different concentrations of TG-1 (125 mg/kg, 250 mg/kg and 500 mg/kg) respectively. There were 8 mice in each group. The animals in the positive control group were administered Tegafur (FT-207, 110 mg/kg), and those in the negative control group were administered normal saline. Animals in all groups were administered treatments for 25 days [14].
Tumor inhibitory rate
After 25 days of administration, the tumors were isolated and weighed. Then the tumor inhibitory rate was measured. Tumor inhibitory rate = (weight of tumors in negative control group-weight of tumors in experimental group)/weight of tumors in negative control group.
Transmission electron microscopy (TEM)
The tumors were cut into pieces 1.5 mm × 2.0 mm. The specimens were fixed in 40 g/l glutaraldehyde and then in 10 g/l osmium tetroxide solution. The fixed specimens were dehydrated in a series of alcohol solutions, and then were embedded with epoxy resin. The samples were stained by uranyl acetate and lead citrate before observation.
Flow cytometry
Suspensions of mouse renal carcinoma OS-RC-2 cells were centrifuged and washed by cold PBS 2 times. The 7-ADD dye liquor (5 µl) was mixed with binding buffer (5 µl), and then added into the suspensions of mouse renal carcinoma OS-RC-2 cells (106 cells/l). The reaction lasted for 5-15 min in the dark at room temperature. The mixture of binding buffer (450 µl) and Annexin V-PE (1 µl) was then added and kept for 5–15 min. Finally, the apoptosis of renal carcinoma OS-RC-2 cells was measured using flow cytometry at 488 nm.
Real-time polymerase chain reaction
Total RNA of the tumor was extracted using Trizol, and the RT-PCR experiment was performed according to the requirement of the kit. The products were electrophoresed in 15 g/l Sepharose gel and scanned for absorbance with Quantity-one. The expression of the Ki67 gene was showed by Aki67/AGADPH. Ki67 primers were 5’-ACTTGCCTCCTAATACGCC-3’ and 5’-TTACTACATCTGCCCATGA-3’; primers of the internal standard (GADPH) were 5′-ACTGCCACCCAGAAGACT-3′ and 5′-GCTCAGTGTAGCCCAGGAT-3′.
Immunohistochemistry
Sections cut from the paraffin blocks were deparaffinized and treated in Tris-EDTA solution at pH 9.0 to retrieve antigens. After peroxidase blocking reagent treatment, the anti-mouse primary antibodies were applied and incubated at 4°C for 12 h. After rinsing PBS at the end of incubation, goat anti-rabbit secondary antibodies were applied. The slides were incubated at room temperature for 30 min before being treated with SABC chromogen at room temperature for 5 min. The slides were incubated at room temperature for another 30 min before being treated with DAB chromogen at room temperature for 5 min. Finally, the slides were rinsed again and stained with hematoxylin.
Statistical analysis
SPSS 17.0 software was used for data analysis and statistics. The data are shown as ± SD. Analysis of variance was used to evaluate statistical differences between groups. The χ2 test was used to evaluate the difference between the enumeration data of different groups. A statistically significant difference was assumed for p < 0.05.
Results
Effects of TG-1 with different concentrations on the growth inhibition rate of tumor OS-RC-2 cells
To determine the effect of TG-1 on the renal metastatic OS-RC-2 cells, the cells were treated with increasing dosages of TG-1 (125 mg/kg, 250 mg/kg, or 500 mg/kg, respectively). The cells treated with medium only served as the control. FT-207, a clinical anti-cancer drug, was used as a positive control in this study. After being treated with different doses of TG-1, the tumor weights in the FT-207 group and experimental groups (TG-1 125 mg/kg, TG-1 250 mg/kg, or TG-1 500 mg/kg) were smaller than those in the negative medium control group. The anti-tumor effects of TG-1 using 250 mg/kg were the best in the experimental group (Figure 1, Table I). These results suggest that TG-1 could inhibit the growth of the renal metastatic cells, with a best working concentration of 250 mg/kg.
Figure 1.
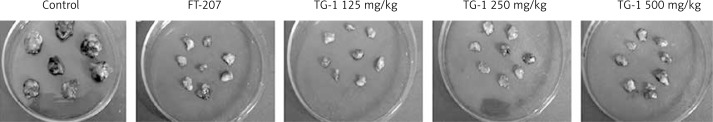
Tumor weight of each group with different concentrations of the polypeptide. Control, the negative control in which the cells were treated with DMEM only. The cells were treated with increasing dosages of TG-1 (125 mg/kg, 250 mg/kg, or 500 mg/kg, respectively). FT-207, a clinical anti-cancer drug, was used as a positive control in this study. The tumor weights of animals in each group were measured
Table I.
Inhibitory and apoptosis rate of renal metastatic tumor OS-RC-2 with different concentrations of the protein polypeptide (X ± SD, n = 8)
| Groups | Tumor quality [g] | Tumor inhibition rates | Apoptosis rates |
|---|---|---|---|
| Control | 1.60 ±0.38 | – | 0.09 ±0.02 |
| FT-207 | 0.56 ±0.10** | 0.65 | 0.53 ±0.04 |
| TG-1 (125 mg/kg) | 0.88 ±0.15** | 0.45 | 0.16 ±0.04* |
| TG-1 (250 mg/kg) | 0.71 ±0.12** | 0.56 | 0.31 ±0.07** |
| TG-1 (500 mg/kg) | 0.76 ±0.08** | 0.52 | 0.22 ±0.08* |
p < 0.05, p < 0.01 vs. the control group (cells treated with DMEM).
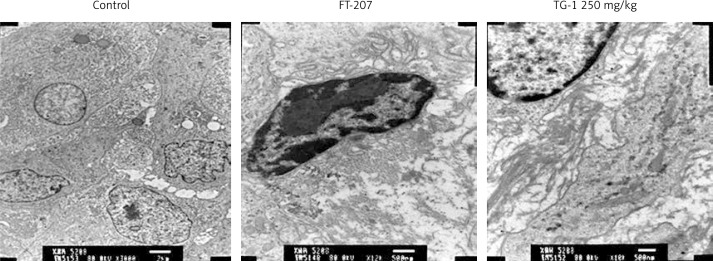
TEM examination of renal carcinoma OS-RC-2 cells
To determine the changes in cytoplasm and nucleus of the renal metastatic cells, we performed TEM examination. The cells treated with medium only served as the control group. In the control group, the tumor cells had a large volume, light electron density, oval nucleus, chromatin without edge clustering and many mitochondria (Figure 2). In the groups administered FT-207 at 250 mg/kg, the volume of the tumor cells was much smaller, surrounded by a large amount of gelatinous fibers. The electron density of the cells became stronger. The nucleus was atypical and the pyknotic chromatin exhibited the edge clustering phenomenon. Some mitochondria exhibited empty bubbles. These results suggest that TG-1 could promote apoptosis of renal metastatic cells.
Figure 2.
Apoptosis of renal carcinoma OS-RC-2 by TEM. Control, cells treated with DMEM only. FT-207, a clinical anti-cancer drug, was used as a positive control in this study. The cells were also treated with TG-1 (250 mg/kg)
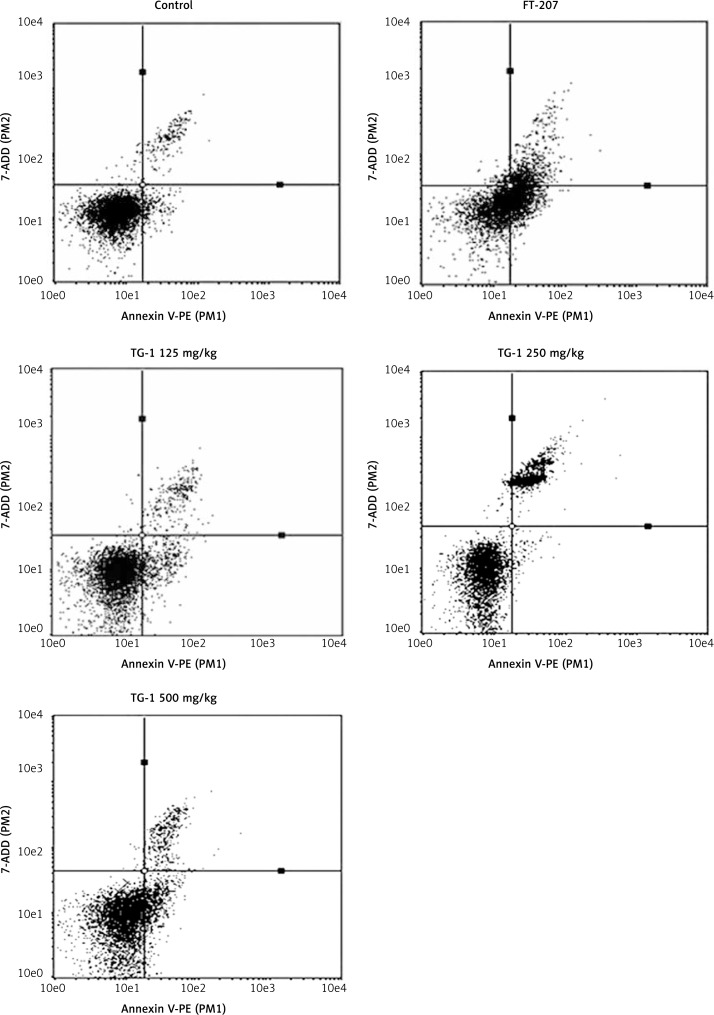
Apoptotic effects of TG-1 with different dosages in renal metastatic cells
To determine the apoptosis rate of TG-1 with different dosages in renal metastatic cells, we performed flow cytometry. As shown in Figure 3 and Table I, the effects of the FT-207 group and experimental groups (TG-1 125 mg/kg, TG-1 250 mg/kg, or TG-1 500 mg/kg) with different concentrations of TG-1 on the apoptosis of renal carcinoma OS-RC-2 cells were all significantly better than those of the negative control group (control) (p < 0.05), and the group administered 250 mg/kg TG-1 was the best (p < 0.01). These results suggest that TG-1 could promote the apoptosis of renal metastatic cells.
Figure 3.
Apoptosis rate of transplant tumor OS-RC-2 with the polypeptide by flow cytometry. Control, cells treated with DMEM only. FT-207, a clinical anti-cancer drug, was used as a positive control to treat cells in this study. The cells were treated with TG-1 (125 mg/kg, 250 mg/kg, or 500 mg/kg, respectively)
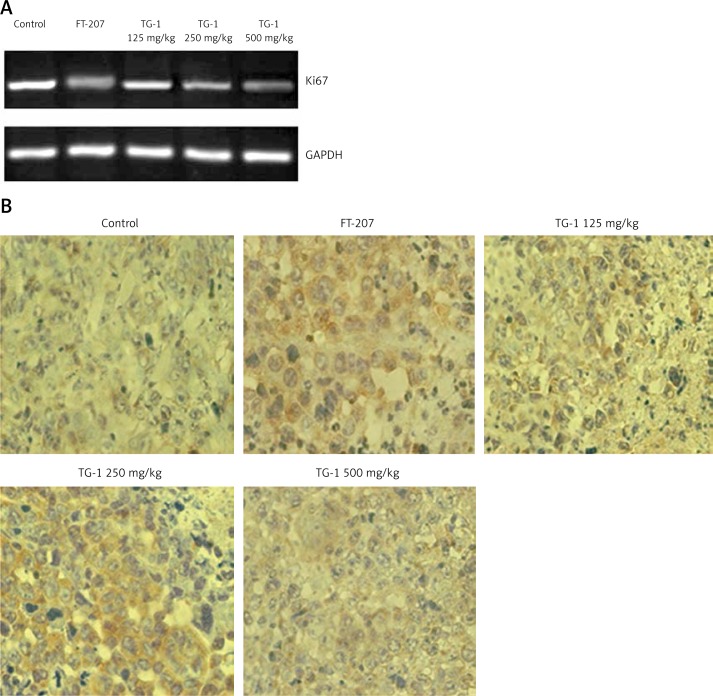
Effects of TG-1 on expression of Ki67 mRNA
To determine the expression of Ki67 mRNA, we performed RT-PCR to determine the changes. The expression of Ki67 mRNA in the FT-207 group and the experimental groups (TG-1 125 mg/kg, TG-1 250 mg/kg, or TG-1 500 mg/kg) was less than that of the negative control group (control) (p < 0.01), as shown in Figure 4 A and Table II. These results suggest that TG-1 could inhibit the expression of Ki67 mRNA.
Figure 4.
RT-PCR detection of Ki67 mRNA and immunohistochemical staining for caspase-3 of the samples. A – Ki67 mRNA expression of transplant tumor OS-RC-2 treated with DMEM, FT-207, TG-1 (125 mg/kg, 250 mg/kg, or 500 mg/kg, respectively). Ki67 mRNA levels were measured by PT-PCR. GAPDH served as a control. B – Immunohistochemical staining for caspase-3 of the samples (400×). The positive reaction of the caspase-3 antigen showed brown particles in the nucleus and/or cytoplasm
Table II.
Ki67 mRNA levels and caspase-3 protein expression of transplant tumor OS-RC-2 with the protein polypeptide (± SD, n = 8)
| Groups | Ki67 mRNA levels | Positive rates of caspase-3 |
|---|---|---|
| Control | 0.985 ±0.024 | 0.396 ±0.324 |
| FT-207 | 0.417 ±0.038** | 0.864 ±0.441** |
| TG-1 (125 mg/kg) | 0.775 ±0.054** | 0.454 ±0.374* |
| TG-1 (250 mg/kg) | 0.521 ±0.027** | 0.639 ±0.425** |
| TG-1 (500 mg/kg) | 0.539 ±0.034** | 0.545 ±0.361* |
p < 0.05,
p < 0.01 vs. the control group.
Expression of caspase-3 in renal carcinoma OS-RC-2 cells after treatment with TG-1
To determine the expression of caspase-3 in the renal carcinoma OS-RC-2 cells, we performed immunohistochemistry. The positive reaction of the caspase-3 antigen showed brown particles in the nucleus and/or cytoplasm. Compared with the negative control group (control), there was excessive expression of caspase-3 in the experimental group using 250 mg/kg TG-1 (p < 0.01), and expression of caspase-3 was also enhanced in the experimental groups using 125 mg/kg and 500 mg/kg TG-1, and the FT-207 group (p < 0.05) (Figure 4 B and Table II). These results revealed that TG-1 could induce apoptosis of the tumor cells by inducing the expression of caspase-3.
Discussion
A previous study indicated that the combined use of TG-1 with conventional immunotherapy can increase the remission rates in RCC patients [8]. In this study, we first established an in vitro mouse renal metastatic tumor model. By using this animal model, we further analyzed the effects of TG-1 with increasing dosages on RCC tumors and compared the effects of TG-1 with a control FT-207. FT-207 is a currently available anti-cancer drug in clinics. By measuring the weight of tumors in animals treated with different dosages and FT-207, we found that a dose of 250 mg/kg might be the best dosage. Such a finding of the TG-1 dosage might be helpful for the following clinical trials. The reason that 500 mg/kg was less effective than 250 mg/kg might be that a high dosage such as 500 mg/kg may affect the unknown cellular signaling pathway and thus induce more side-effects. All dosages of TG-1 and FT-207 treatments resulted in more obvious effects than the medium control treatment, which suggested that our animal model and the treatments are suitable for this and future research. These results were also supported by the microstructural changes of renal carcinoma OS-RC-2 cells by using transmission electron microscopy.
Apoptosis is the independent and orderly death of cells under regulation of a series of genes, and is also known as programmed cell death (PCD). There are mainly two apoptotic pathways: one is to activate various caspases through extracellular signals; the other is to activate caspase through the release of activating factors in mitochondria. Activated caspases (mainly caspase-3) could degrade related proteins and then induce apoptosis of the cells. In the caspase family, caspase-2, 8, 9 and 10 belong to the upstream, and caspase-3, 6 and 7 belong to the downstream. Caspase-3/7 [15, 16] could activate the common pathway of cell apoptosis by activating DNase, which digests DNA into small fragments. Caspase-3 protease is a core enzyme of apoptosis, participating in apoptosis. Caspase-3 protease also plays a pivotal role in the midstream and downstream of the apoptosis signaling pathways mediated by Fas. The quantity of activated caspase-3 is crucial to the apoptosis of the cells. Caspase-3 could digest poly-ADP-ribose polymerase (PARP), leading to fragmentation of DNA, promoting degradation of cells and forming the apoptotic body [17–19]. Observation of the expression of caspase-3 could help understand the apoptotic degree of the cells. In this study, in the TG-1 treatment groups, the caspase-3 protein expression was significantly increased. Obvious nuclear condensation and nuclear chromatin condensation were shown by the electron microscopy. And flow cytometry showed that TG-1 treatment group was significantly apoptosis, which was confirmed by its promotion of the expression of the caspase-3 protein.
Li et al. [20] reported that the expression of Ki67 and caspase-3 protein presents a negative correlation with each other in carcinoma of the vulva. The same relationship of the expression of Ki67 and caspase-3 protein in prostatic tumor was also reported [21, 22]. The appearance of the tumors is closely related with the extensive expression of the Ki67 protein and the inhibition of the caspase-3 protein. Poor prognosis is very common among patients with high expression of Ki67 [11–13]. In this study, we found that TG-1 inhibited the expression levels of Ki67 mRNA in RCC cells when compared with the FT-207 treated cells. However, the relationship between the decreased Ki67 mRNA expression and the increased caspase-3 protein expression in the TG-1-treated cells is still not clear, which will be studied in the future. In addition to the Ki67 and caspase-3 determined in the present study, it was recently reported that some patient behavior factors such as alcohol intake and valproic acid usage also affect the risk of RCC in patients [23, 24].
In conclusion, in this study, it was found that the polypeptide TG-1 induces high expression of the caspase-3 protein and induces apoptosis of tumor cells. TG-1 also inhibited expression of the Ki67 protein, which may be related to decreased proliferation of the tumor cells. This study provides a theoretical basis for the clinical application of the anti-tumor polypeptide TG-1.
Acknowledgments
This work was supported by Qingdao Science and Technology Bureau (grant no. 10-3-3-1-5-NSH) and the National Natural Science Fund (grant no. 31071014).
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Jemal A, Siegel R, Xu J, et al. Cancer statistics. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Xu W, Chang Z, Liu X, et al. Antitumor effects of a polypeptide isolated from Tegillarca granosa Linnaeus and the related molecular mechanism. Pak J Pharm Sci. 2014;27:565–70. [PubMed] [Google Scholar]
- 3.Herrmann E, Bierer S, Wülfing C. Update on systemic therapies of metastatic renal cell carcinoma. World J Urol. 2010;28:303–9. doi: 10.1007/s00345-010-0519-5. [DOI] [PubMed] [Google Scholar]
- 4.Kirchner H, Strumberg D, Bahl A, et al. Patient-based strategy for systemic treatment of metastatic renal cell carcinoma. Expert Rev Anticancer Thera. 2010;10:585–96. doi: 10.1586/era.10.25. [DOI] [PubMed] [Google Scholar]
- 5.Sun X, Huang H, Xu Z, et al. Renal cell carcinoma presents as pleural metastasis without pulmonary involvement. Chin Med J. 2012;125:3193–4. [PubMed] [Google Scholar]
- 6.Li GY, Liu JZ, Yu XM, et al. Effect of a seashell protein Haishengsu on cell growth and expression of apoptosis genes in leukemia K562 cells. Clin Invest Med. 2008;31:E218–21. doi: 10.25011/cim.v31i4.4783. [DOI] [PubMed] [Google Scholar]
- 7.Liu JZ, Chen SG, Zhang B, Wang CB, Li GY, Wang LX. Antitumor effect of the seashell protein Haishengsu on Ehrlich ascites tumor: all experimental study. J Nat Med. 2009;63:459–62. doi: 10.1007/s11418-009-0346-4. [DOI] [PubMed] [Google Scholar]
- 8.Liu JZ, Chen SG, Zhang B, et al. Effect of haishengsu as an adjunct therapy for patients with advanced renal cell cancer: a randomized and placebo-controlled clinical trial. J Altern Complement Med. 2009;15:1127–30. doi: 10.1089/acm.2009.0260. [DOI] [PubMed] [Google Scholar]
- 9.Wang H, Zhao K, Wang J, et al. Expression of cyclooxygenase-2, epidermal growth factor receptor and Ki67 in colorectal cancer and its significance. Shandong Med J. 2008;48:1–2. [Google Scholar]
- 10.Wu Z, Zhang B, Yuan Q. Expressions of cyclin E and Ki67 in carcinoma of large intestine and its clinicopathological meaning. Modern Journal of Integrated Traditional Chinese and Western Medicine. 2008;17:4058–61. [Google Scholar]
- 11.Stefansson IM, Salvesen HB, lmmervoll H, et al. Prognostic impact of histological grade and vascular invasion compared with tumour cell proliferation in endometrial carcinoma of endometrioid type. Histopathology. 2004;44:472–9. doi: 10.1111/j.1365-2559.2004.01882.x. [DOI] [PubMed] [Google Scholar]
- 12.Bongiovanni L, Suter MM, Malatesta D, et al. Nuclear survivin expression as a potentially useful tool for the diagnosis of canine cutaneous sebaceous lesions. Vet Dermatol. 2012;23:394–e73. doi: 10.1111/j.1365-3164.2012.01065.x. [DOI] [PubMed] [Google Scholar]
- 13.Gudlaugsson E, Skaland I, Janssen EA, et al. Comparison of the effect of different techniques for measurement of Ki67 proliferation on reproducibility and prognosis prediction accuracy in breast cancer. Histopathology. 2012;61:1134–44. doi: 10.1111/j.1365-2559.2012.04329.x. [DOI] [PubMed] [Google Scholar]
- 14.Wang XB, Liu QH, Wang P, et al. Study of cell killing effect on renal carcinoma OS-RC-2 by ultrasound activating protoporphyrin IX. Ultrasonics. 2008;48:135–40. doi: 10.1016/j.ultras.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid a, Bcl-2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94:481–90. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- 16.Galvan V, Chen S, Lu D, et al. Caspase cleavage of members of the amyloid precursor family of proteins. Neurochem. 2002;82:283–94. doi: 10.1046/j.1471-4159.2002.00970.x. [DOI] [PubMed] [Google Scholar]
- 17.Ravi R, Mookergee B, Bhujwalla Z, et al. Regulation of tumor angiogenesis by p53-induced degradation of hypoxia-inducible factor 1alpha. Genes Dev. 2000;14:31–44. [PMC free article] [PubMed] [Google Scholar]
- 18.Brodsky SV, Mendelev N, Melamed M, et al. Vascular density and VEGF expression in hepatic lesions. J Gastroint Liver Dis. 2007;16:373–7. [PubMed] [Google Scholar]
- 19.Robertson GS, Crocker SJ, Nicholson DW, et al. Neuroprotection by the inhibition of apoptosis. Brain Pathol. 2000;10:283–92. doi: 10.1111/j.1750-3639.2000.tb00262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y, Zhang L, Wu X. Expression and signifiance of Ki67 and caspase-3 in vulvar neoplasia. Chin J Pract Gynecol Obstetrics. 2008;24:348–50. [Google Scholar]
- 21.Sun P, Jin R. Expression of Ki-67/caspase-3 cocktail double staining in prostatic adenocarcinoma. Chin J Clin Exp Pathol. 2010;26:438–41. [Google Scholar]
- 22.Preusser M, Hoeftberger R, Woehrer A, et al. Prognostic value of Ki67 index in anaplastic oligodendroglial tumours: a translational study of the European Organization for Research and Treatment of Cancer Brain Tumor Group. Histopathology. 2012;60:885–94. doi: 10.1111/j.1365-2559.2011.04134.x. [DOI] [PubMed] [Google Scholar]
- 23.Cheng G, Xie L. Alcohol intake and risk of renal cell carcinoma: a meta-analysis of published case-control studies. Arch Med Sci. 2011;7:648–57. doi: 10.5114/aoms.2011.24135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang F, Shao Y, Yang F, et al. Valproic acid upregulates NKG2D ligand expression and enhances susceptibility of human renal carcinoma cells to NK cell-mediated cytotoxicity. Arch Med Sci. 2013;9:323–31. doi: 10.5114/aoms.2013.34413. [DOI] [PMC free article] [PubMed] [Google Scholar]