Buck et al. discuss the role of lymphocyte metabolism on immune cell development and function.
Abstract
Lymphocytes must adapt to a wide array of environmental stressors as part of their normal development, during which they undergo a dramatic metabolic remodeling process. Research in this area has yielded surprising findings on the roles of diverse metabolic pathways and metabolites, which have been found to regulate lymphocyte signaling and influence differentiation, function and fate. In this review, we integrate the latest findings in the field to provide an up-to-date resource on lymphocyte metabolism.
“Part of the secret of success in life is to eat what you like and let the food fight it out inside.”
– Mark Twain
Simply stated, we are what we eat. Our genetics, coupled with environmental influences, dictate how we metabolize the nutrients that we consume and how this shapes our growth, function, and overall health. The same principles hold true at the cellular level. Just as a track runner quickly engages their muscles to propel themselves from rest to sprint in response to a starting gun, pathogen-derived or inflammatory signals drive T cells out of quiescence, resulting in rapid modulation of gene expression and the acquisition of new functions. These changes range from increased production of cytokines and cytolytic molecules to the ability to undergo cell division and migration. Intimately integrated into this program of activation is the regulation of cellular metabolism.
The engagement of specific metabolic pathways profoundly affects cell differentiation and function. Metabolic reprogramming is controlled by key receptor signaling events and growth factor cytokines, as well as availability of nutrients. In addition, metabolic products provide substrates that can alter the functional fate of a cell through posttranslational modifications (PTMs) or epigenetic remodeling. Several recent articles have covered these and other emerging topics in T cell metabolism (Chapman and Chi, 2014; Bird, 2015; Lochner et al., 2015; O’Sullivan and Pearce, 2015; Palmer et al., 2015; Ramsay and Cantrell, 2015; Ron-Harel et al., 2015). In this review, we provide a general but comprehensive overview of lymphocyte metabolism integrated with current research. Our focus will be on data and concepts derived primarily from T cell studies, with tie-ins from other fields when relevant.
T cell development and quiescence
Although the bulk of T cell metabolism research centers on mature T cells, even at their inception and throughout their development, T cells cycle through states of metabolic quiescence and activation. Hematopoietic stem cell progenitors that are double negative (DN) for CD4 and CD8 co-receptors migrate from the bone marrow and seed the thymus where they rearrange their antigen receptor gene loci to produce a functional TCR. Signals from the receptor Notch1 maintain cell survival and promote T cell lineage commitment (Pui et al., 1999; Radtke et al., 1999; Maillard et al., 2006). Induced deletion of Notch1 during neonatal development results in arrest at the most immature DN1 (CD44+CD25−) stage (Radtke et al., 1999), whereas enforced expression of constitutively active Notch1 in bone marrow cells blocks B cell differentiation and instead causes the ectopic development of CD4+CD8+ double-positive (DP) T cells (Pui et al., 1999).
Successful expression of TCRβ with pTα and CD3 forms the pre-TCR, which signals with Notch1 to drive cells out of quiescence as they enter β selection (Saint-Ruf et al., 1994; Ciofani et al., 2004; Ciofani and Zúñiga-Pflücker, 2005). RAG recombinase expression declines and expression of the transferrin receptor CD71 and other nutrient transporters are induced as the cells proliferate (Ciofani and Zúñiga-Pflücker, 2005; Kelly et al., 2007). Signaling from the pre-TCR, Notch1, and the chemokine receptor CXCR4 converge to activate phosphatidylinositol 3-kinase (PI3K); this stimulates the switch to anabolic metabolism (metabolic pathways that create biomass from smaller molecules; Ciofani and Zúñiga-Pflücker, 2005; Janas et al., 2010). Increased expression of the glucose transporter Glut1 is required during this stage, and its expression is dependent on activation of the kinase Akt by PI3K (Swainson et al., 2005; Juntilla et al., 2007; Wieman et al., 2007). PI3K–Akt signaling also activates the mechanistic target of rapamycin (mTOR), and signals from this kinase augment the glycolytic metabolism used to support cell growth and proliferation (MacIver et al., 2013).
Disruptions in PI3K signaling also affect the transition of DP thymocytes to single-positive CD4 and CD8 T cells and the development of NKT cells, which require sustained signaling to join tcra Vα to distal Jα gene segments that define their invariant TCR (D’Cruz et al., 2010; Rodríguez-Borlado et al., 2003). PTEN (phosphatase and tensin homologue) is the principal negative regulator of the PI3K pathway. Thymocytes from mice that lack the microRNA cluster miR-181a1b1 have altered cellular metabolism caused by a significant increase in PTEN expression (Henao-Mejia et al., 2013). Glucose uptake, measured by acquisition of the fluorescent glucose analogue 2-NBDG, and glycolytic rate are reduced in these cells, and nutrient transporter expression is diminished. As a result of dysregulated PI3K signals, these mice have deficiencies in DP cells and completely lack NKT cells (Henao-Mejia et al., 2013).
The cytokine IL-7 has a pivotal role in ensuring the survival of developing and quiescent naive T cells by increasing expression of the antiapoptotic factor Bcl-2 (B cell lymphoma 2; Akashi et al., 1997; Maraskovsky et al., 1997; Tan et al., 2001; Yu et al., 2003). Mice deficient in IL-7 or the IL-7Rα chain have defects in T cell development (Peschon et al., 1994; von Freeden-Jeffry et al., 1995). IL-7 signals through the JAK3–STAT5 pathway but can also activate PI3K (Pallard et al., 1999; Wofford et al., 2008). A recent study suggests that in addition to maintaining the survival of developing lymphocytes, IL-7 signaling promotes the growth and proliferation of DN4 cells by increasing levels of trophic receptors, such as CD71 and the amino acid transporter CD98 (Pearson et al., 2012; Boudil et al., 2015), activities that were previously attributed mainly to Notch1 signaling. However, Notch1 can induce IL-7Rα expression and therefore its effects could be downstream of IL-7 signals (González-García et al., 2009; Magri et al., 2009).
Mature naive T cells exit from the thymus into the periphery. As quiescent cells, they primarily oxidize glucose-derived pyruvate in their mitochondria via oxidative phosphorylation (OXPHOS), or they use fatty acid oxidation (FAO) to generate ATP (Fig. 1; Fox et al., 2005; Wang et al., 2011; van der Windt and Pearce, 2012; Pearce and Pearce, 2013; Pearce et al., 2013). A balance between tonic TCR signals and IL-7 is needed to sustain naive T cells. Homeostatic proliferation of naive T cells is supported by TCR ligation with self-peptides presented on MHC molecules in the periphery (Ernst et al., 1999; Goldrath and Bevan, 1999; Muranski et al., 2000). However, unrestrained Akt activation, or deletion of negative regulators of TCR stimulation, leads to loss of quiescence (Yang et al., 2011). T cells defective in tuberous sclerosis complex 1 (TSC1), a negative regulator of mTOR signaling, prematurely exit from quiescence and have increased rates of apoptosis and hyperactive responses to TCR stimulation (Yang et al., 2011). In addition, TCR-mediated PI3K-Akt activation down-regulates IL-7Rα (Cekic et al., 2013), but, as discussed in the previous paragraph, IL-7 signaling is essential to prevent apoptosis and ensure survival of the naive T cell pool (Rathmell et al., 2001; Surh and Sprent, 2008). A recent study showed that the metabolite adenosine, which is a byproduct of metabolic activity, suppresses TCR signaling in a dose dependent manner (Cekic et al., 2013). The G-protein–coupled adenosine receptor subtype A2AR is predominantly expressed in T cells. Binding with adenosine activates cAMP-dependent protein kinase A (PKA), which suppresses TCR-mediated activation of the PI3K pathway and prevents IL-7Rα down-regulation (Cekic et al., 2013).
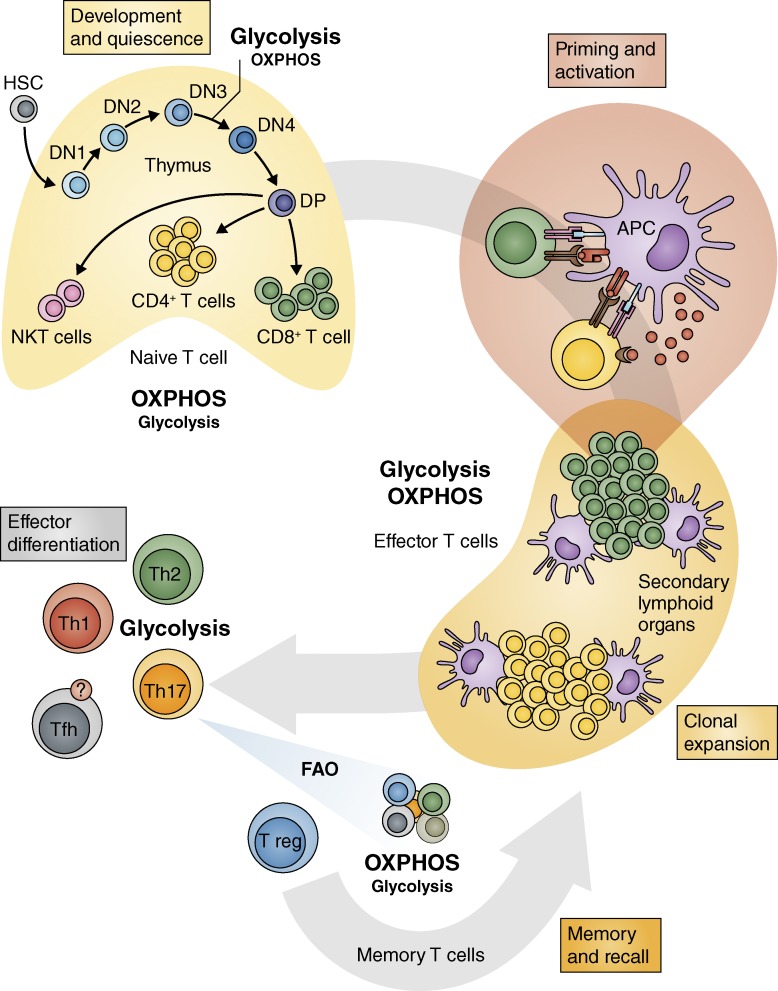
Figure 1.
Metabolism drives the life cycle of T cells. T cells engage specific metabolic pathways during development that underpin their differentiation and function. Naive T cells mature and exit from the thymus primarily relying on OXPHOS for their metabolic needs, although they augment with glycolytic metabolism during times of proliferation that follow TCR gene rearrangements. In secondary lymphoid organs, TCR ligation, costimulation, and growth factor cytokine signals induce clonal expansion and metabolic reprogramming of an antigen-specific T cell. This conversion to an activated effector T cell is marked by the engagement of aerobic glycolysis and increased OXPHOS activity. Glycolytic metabolism differentiates CD4 Th1, Th2, and Th17 effector cells (and possibly Tfh cells) from T reg cells. Promoting FAO and catabolic metabolism enhances T reg and memory T cell development (blue arrow). Memory T cells are a quiescent population of cells that primarily use OXPHOS, but both OXPHOS and glycolysis increase rapidly after antigen rechallenge and facilitate their recall responses.
Activation and effector T cell differentiation
Metabolic reprogramming during T cell activation.
Once in the periphery, a mature naive T cell is like a bomb, lying dormant in the lymphoid organs and circulation until it is triggered to activate and explode in a proliferative chain reaction. T cell activation stimulated by TCR ligation and binding with costimulatory molecules induces metabolic remodeling of the naive T cell to a program of anabolic growth and biomass accumulation; this is marked by the engagement of aerobic glycolysis, a process in which glucose is converted into lactate even though sufficient oxygen is present to support glucose catabolism via the tricarboxylic acid (TCA) cycle and OXPHOS (Fig. 1; Vander Heiden et al., 2009; MacIver et al., 2013). Although aerobic glycolysis is less efficient than OXPHOS at yielding an abundance of ATP per molecule of glucose, aerobic glycolysis can generate metabolic intermediates important for cell growth and proliferation, and provides a way to maintain redox balance (NAD+/NADH) in the cell (Fig. 2; Vander Heiden et al., 2009; Anastasiou et al., 2011; Macintyre and Rathmell, 2013). For example, glucose-6-phosphate and 3-phosphoglycerate (3PG) produced during glycolysis can be metabolized in the pentose phosphate and serine biosynthesis pathways, respectively, donating important precursors for nucleotide and amino acid synthesis (Wang et al., 2011; Pearce et al., 2013). Glucose can also enter the mitochondria as pyruvate, where it is converted to acetyl-CoA and joins the TCA cycle by condensing with oxaloacetate to form citrate. Breakdown of substrates in the TCA cycle not only provides reducing equivalents for OXPHOS, but also precursors for biosynthesis. Glucose-derived citrate can be exported into the cytosol to generate acetyl-CoA by ATP citrate lyase (ACL) for use in lipid synthesis (Bauer et al., 2005; Hatzivassiliou et al., 2005; DeBerardinis et al., 2008). Similarly, oxaloacetate can be used to produce aspartate, an additional precursor for generating nucleotides (Fig. 2; DeBerardinis et al., 2007).
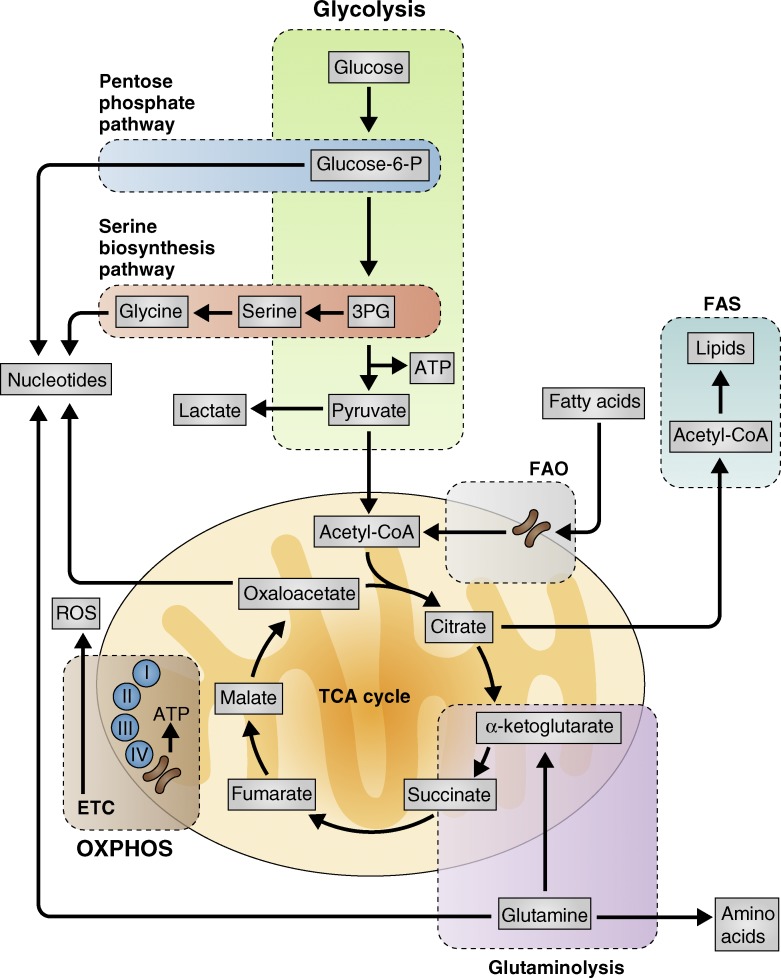
Figure 2.
Metabolic pathways that support T cells. ATP is the molecular currency of energy in the cell. It can be derived from glucose through two integrated pathways. The first of these, glycolysis (green), involves the enzymatic breakdown of glucose to pyruvate in the cytoplasm. The TCA cycle (orange) encompasses the second pathway, where pyruvate is converted to acetyl-CoA in the mitochondria and shuttled through several enzymatic reactions to generate reducing equivalents to fuel OXPHOS (brown). Other substrates can also be metabolized in the TCA cycle, such as glutamine via glutaminolysis (purple) or fatty acids via β-oxidation (FAO; gray). These connected biochemical pathways can also provide metabolic precursors for biosynthesis. Intermediates from glucose catabolism during glycolysis can shuttle through the pentose phosphate (dark blue) and serine biosynthesis pathways (red) to fuel nucleotide and amino acid production. Oxaloacetate from the TCA cycle can similarly be used to generate aspartate for use in nucleotide synthesis. Precursors for amino acid and nucleotide biosynthesis can be obtained from glutamine. Citrate from the TCA cycle can be exported from the mitochondria and converted to acetyl-CoA for FAS (light blue). ROS generated from the ETC during OXPHOS can also act as secondary signaling molecules.
Several transcription factors and signaling pathways coordinately support and regulate this change in T cell metabolic programs after activation. Growth factor cytokines such as IL-2 and ligation of costimulatory molecules promote the switch to glycolysis through the enhancement of nutrient transporter expression and activation of the key metabolic regulator mTOR (Fig. 1; Frauwirth et al., 2002; Jones and Thompson, 2007; Wieman et al., 2007; Kolev et al., 2015). mTOR exists as two complexes, mTORC1 and mTORC2, and integrates extrinsic and intrinsic signals related to nutrient levels, energy status, and stress to induce changes in cellular metabolism, growth, and proliferation (Laplante and Sabatini, 2012). CD28 ligation enhances PI3K activity, which recruits 3-phosphoinositide–dependent protein kinase-1 (PDPK1) and Akt. PDPK1, together with mTORC2, phosphorylates Akt, which in turn activates mTORC1. Both Akt and mTOR promote aerobic glycolysis and support effector T cell differentiation, growth, and function (Delgoffe et al., 2011; Pollizzi et al., 2015). Akt regulates nutrient transporter expression and can phosphorylate the glycolytic enzyme hexokinase II, promoting its localization to the mitochondria and augmenting its enzymatic activity (Miyamoto et al., 2008; John et al., 2011). mTORC1 activation increases protein translation via phosphorylation of 4E-BP1 and p70S6 kinase (Laplante and Sabatini, 2012) and promotes lipid synthesis by activating SREBP2 (sterol regulatory element-binding protein 2; Porstmann et al., 2008).
The up-regulation of transcription factors c-Myc, estrogen-related receptor α (ERRα), and hypoxia inducible factor-1α (HIF-1α) coordinately drives the expression of genes involved in intermediary metabolism that fuel the rapid proliferation of effector T cells during clonal expansion (Michalek et al., 2011b; Wang et al., 2011; Doedens et al., 2013). First discovered as an oncogene important for cell growth and proliferation (Sheiness et al., 1978; Cole, 1986), c-Myc has been shown to be a critical regulator of metabolic reprogramming after T cell activation (Wang et al., 2011). c-Myc drives the expression of enzymes that promote aerobic glycolysis and glutaminolysis and coordinates these metabolic pathways with lipid, amino acid, and nucleic acid synthesis. However, c-Myc expression is not continually sustained after T cell activation (Nie et al., 2012; Best et al., 2013). A recent study suggests that c-Myc induces the transcription factor AP4, which maintains the glycolytic transcriptional program initiated by c-Myc to support T cell population expansion (Chou et al., 2014; Karmaus and Chi, 2014). HIF-1α, a transcription factor that responds to oxygen levels, also increases glucose uptake and catabolism through glycolysis (Kim et al., 2006; Finlay et al., 2012). Deletion of its negative regulator, von Hippel-Lindau (VHL), enhances HIF-1α–mediated CD8 T cell glycolysis and effector responses to persistent viral infection (Doedens et al., 2013).
ROS signaling.
Although much of the attention on metabolic reprogramming in activated T cells has focused on the engagement of aerobic glycolysis, recent research has revealed the importance of mitochondrial-driven activities in this process. In addition to energy production, the electron transport chain (ETC) is a major source of reactive oxygen species (ROS; Turrens, 2003), which are important for T cell responses (Fig. 2; Chaudhri et al., 1988; Devadas et al., 2002; Jones et al., 2007). T cells deficient for Rieske iron sulfur protein (RISP), a subunit of mitochondrial complex III, have impaired activation and antigen-specific T cell expansion in vitro and in vivo due to defects in mitochondrial-derived ROS signaling (Sena et al., 2013). More recently, using a forward genetic screen, another group identified mice with enhanced CD8 T cell responses to viral and tumor challenge (Okoye et al., 2015). The source of the heightened immunity gained after germline mutagenesis was the increased expression of an orphan protein, identified as lymphocyte expansion molecule (LEM). Interestingly, augmented OXPHOS and mitochondrial ROS levels were detected in CD8 T cells isolated from LEM-deficient mice after infection, whereas heterozygous LEM-deficient CD8 T cells had reduced OXPHOS and mitochondrial ROS levels. LEM helps stabilize a protein involved in inserting ETC complex proteins in the mitochondrial membrane, which may account for the increased ROS and enhanced proliferation evident in CD8 T cells from these mice (Okoye et al., 2015).
Although ROS is produced as a general byproduct of mitochondrial metabolism, new studies have specifically linked the metabolite succinate to both the generation of ROS and activation of HIF-1α in settings of inflammation or injury (Tannahill et al., 2013; Chouchani et al., 2014). Innate immune receptor activation increases intracellular succinate from glutamine via glutamine-dependent anerplerosis and the γ-aminobutyric acid shunt pathway, and this leads to HIF-1α stabilization and activation (Tannahill et al., 2013). During ischemia reperfusion injury, which happens when blood supply to a tissue is disrupted and then restored, succinate accumulates from reverse activity of the enzyme succinate dehydrogenase (SDH) and is rapidly oxidized upon reperfusion. This leads to overreduction of the electron carrier coenzyme Q, causing reverse electron transport through mitochondrial complex I and, subsequently, excessive ROS production (Chouchani et al., 2014; O’Neill, 2014). Given that mitochondrial ROS and HIF-1α activity are important for the metabolic reprogramming of naive T cells after activation, it is interesting to speculate that the metabolite succinate may also support the transition from a naive to an activated effector T cell.
Metabolic programming of T helper cell differentiation.
Activation of T cells is intimately tied to the engagement of specific metabolic pathways, so it is no surprise that distinct metabolic programs also support the differentiation of CD4 T helper (Th) cells into their separate lineages. Initial studies found that suppression of mTOR with rapamycin promoted the generation of FoxP3+ regulatory T (T reg) cells even in the presence of Th17-polarizing cytokines in vitro (Kopf et al., 2007), and genetic deletion of mTOR in T cells augmented production of T reg cells upon activation, but not Th1, Th2, or Th17 cells (Delgoffe et al., 2009). These results are consistent with the metabolic profiles of these cells: Th1, Th2, and Th17 cells strongly engage glycolysis via mTOR signaling, whereas T reg cells depend more on the oxidation of lipids (Fig. 1; Michalek et al., 2011a). Th17 cells in particular have been found to heavily rely on glycolysis for their development and maintenance, stimulated by HIF-1α activity downstream of mTOR. Mice deficient in HIF-1α have increased generation of T reg cells, and blocking glycolysis with 2-Deoxy-d-glucose (2-DG) inhibits Th17 cell differentiation (Dang et al., 2011; Shi et al., 2011). T reg cell homeostasis and survival depends on the delicate balance between mTORC1 activation from PI3K-Akt and regulation from PTEN (Zeng et al., 2013; Huynh et al., 2015; Shrestha et al., 2015). Signaling through mTORC1 versus mTORC2 also selectively differentiates CD4 T cells into the Th1 and Th2 lineages, respectively (Lee et al., 2010; Delgoffe et al., 2011), although activation of mTORC1 and its component Raptor is still required for T cell exit from quiescence to begin the transition into Th2 cells (Yang et al., 2013). Less is known about T follicular helper (Tfh) cell metabolism compared with other T cell subsets, but their lineage-defining transcription factor Bcl6 has been shown to suppress glycolysis potentiated by c-Myc and HIF-1α (Johnston et al., 2009; Nurieva et al., 2009; Oestreich et al., 2014).
Substrate utilization in activated T cells
Glucose is a key metabolic substrate for T cells.
Upon T cell activation, Glut1 traffics to the cell surface from intracellular vesicles (Rathmell et al., 2000; Frauwirth et al., 2002; Wieman et al., 2007). Overexpression of Glut1 in mice results in larger naive T cells and an increased number of CD44hi T cells, suggesting that glucose acquisition mediates early steps in T cell activation, such as promoting the expression of activation markers and increasing cell size (Jacobs et al., 2008). Consistent with these observations, T cell specific deletion of Glut1 impairs CD4 T cell activation, clonal expansion, and survival (Macintyre et al., 2014). When deprived of glucose, CD8 T cells display defects in functional capacity with reduced IFN-γ, granzyme, and perforin production (Cham and Gajewski, 2005; Cham et al., 2008; Jacobs et al., 2008). More recently, it was shown that T cells can become activated and proliferate when glucose catabolism through aerobic glycolysis is limited, as they can rely on OXPHOS (Chang et al., 2013; Sena et al., 2013). However, in this case, effector function is compromised, with impaired cytokine production caused by posttranscriptional regulation by the glycolytic enzyme GAPDH. When disengaged from glycolysis, GAPDH can function as a RNA-binding protein (RBP) and prevent the translation of cytokine messenger RNAs containing AU-rich elements in their 3′-UTRs (Chang et al., 2013). Therefore, in addition to providing precursors for biomass, augmenting aerobic glycolysis in activated T cells allows for the acquisition of full effector function.
Amino acids.
Although glucose is a critical substrate for T cells, glutamine is also essential during T cell activation (Frauwirth et al., 2002; Carr et al., 2010; Wang et al., 2011). T cells increase the expression of glutamine transporters, and their deletion impairs the transition to an effector T cell (Carr et al., 2010; Sinclair et al., 2013). Clear differences in concentrations of other amino acids also exist in quiescent compared with activated T cells, corresponding to their distinct metabolic requirements (Pearson et al., 2012; Ananieva et al., 2014). New research has begun to uncover the vast array of additional amino acid transporters and catabolizing enzymes that regulate amino acid levels, revealing previously unappreciated roles for amino acids in T cell metabolism and function.
Deficiency in the neutral amino acid transporter Slc7a5 (LAT1), which transports leucine, prevents the metabolic reprogramming, clonal expansion, and/or effector function of both CD4 and CD8 T cells (Hayashi et al., 2013; Sinclair et al., 2013). These cells had impaired mTORC1 activation and were unable to induce key metabolic processes, such as enhancing glutamine and glucose uptake (Sinclair et al., 2013). This deficiency, however, did not impair the ability of CD4 T cells to differentiate into T reg cells. Leucine can activate mTOR via leucyl-tRNA synthetase, and thus it is not surprising that reduced leucine uptake impairs mTOR activation (Han et al., 2012). However, the effects of Slc7a5 deficiency were more severe than those induced by mTOR inhibition using rapamycin (Sinclair et al., 2013), suggesting either that rapamycin may not have completely blocked mTOR activation, or that leucine deficiency has effects over and above limiting mTOR activation (Thoreen et al., 2009; Powell, 2013). Additionally, although no overt decrease in global protein expression occurred in Slc7a5-deficient cells, protein expression of the key metabolic transcription factor, c-Myc, was diminished, despite its increased mRNA expression upon activation (Sinclair et al., 2013). This raises the intriguing question of whether leucine deficiency results in posttranslational regulation of c-Myc expression. Alternatively, this effect could simply result from a limitation in the supply of amino acids in Slc7a5-deficient cells, which is not sufficient to keep up with the demands of synthesizing proteins such as c-Myc, which have a short half-life (Sinclair et al., 2013).
Results from another study suggest that modulation of intracellular leucine concentrations can be used to regulate metabolic reprogramming. It was found that the expression of the cytosolic branched chain aminotransferase (BCATc), which can reduce intracellular leucine concentrations through a transamination reaction, limited mTORC1 activation (Ananieva et al., 2014). BCATc expression was up-regulated upon CD4 T cell activation, and T cells that lacked BCATc had increased intracellular leucine, which correlated with enhanced activation of mTORC1 and glycolytic phenotype. Increased BCATc expression has been observed in anergic T cells, which have impaired metabolic function (Zheng et al., 2009; Ananieva et al., 2014). These data could suggest that leucine depletion by BCATc contributes to T cell anergy through suppression of mTOR activity.
The alanine serine and cysteine transporter system (ASCT2/Slc1a5), which also transports glutamine, is another solute carrier whose expression increases after T cell activation (Levring et al., 2012). It was recently found that loss of ASCT2 decreased glutamine import and impaired OXPHOS and glucose metabolism in activated CD4 T cells (Nakaya et al., 2014). Surprisingly, the loss of ASCT2 did not inhibit proliferation or IL-2 production. However, ASCT2-deficient cells cultured in vitro had a decreased ability to differentiate into Th1 and Th17 cells, but not Th2 or T reg cells. Interestingly, glutamine transport into cells can substantially enhance leucine transport via Slc7a5, as increased intracellular glutamine levels result in glutamine export and concomitant import of leucine by this transporter (Nicklin et al., 2009). Supporting this additional role for glutamine in T cell activation, addition of leucine to T cells lacking ASCT2 helps rescue their polarization defects (Nakaya et al., 2014).
Depletion of extracellular arginine has been found to impair T cell proliferation and aerobic glycolysis, but not mitochondrial OXPHOS (Fletcher et al., 2015). However, provided that extracellular concentrations of citrulline are sufficient, T cells can partially compensate by synthesizing arginine de novo via an argininosuccinate 1 (ASS1)–dependent process (Qualls et al., 2012; Fletcher et al., 2015; Tarasenko et al., 2015). One study suggested that ASS1 activity may contribute to T cell function in ways beyond simple synthesis of arginine, as deletion of ASS1 can negatively impact in vitro Th1 and Th17 cell polarization, even in the presence of extracellular arginine (Tarasenko et al., 2015). Arginine metabolism also has a role in macrophage polarization and dictating metabolic phenotype (Galván-Pena and O’Neill, 2014; Rath et al., 2014), but whether arginine metabolism also has such roles in T cells remains to be determined.
A recent study suggests that intracellular recycling of amino acids also contributes to T cell amino acid homeostasis. Deficiency in cytosolic protease tripeptidyl peptidase II (TPPII), which digests proteins for the recycling of amino acids, lead to increased sensitivity to perturbations in intracellular amino acids concentrations, impaired IFN-γ production, and a susceptibility to viral infections (Lu et al., 2014). Lack of TPPII activity in both human and murine T cells resulted in impaired glycolysis caused by enhanced degradation of the key glycolytic enzyme hexokinase II, an effect that likely contributed to their impaired cytokine production (Lu et al., 2014). Another study also found that TPPII deficiency caused susceptibility to viral infections, although under these particular experimental conditions, defects in cytokine production were not observed and the T cell dysfunction was attributed to premature immunosenescence (Stepensky et al., 2015). Although it remains to be tested, this premature cell senescence could be linked to reduced glycolytic flux. In a model of oncogenic stress-induced senescence, inhibition of hexokinase II or glucose uptake induces senescence in human epithelial cells (Gitenay et al., 2014).
Many products of amino acid catabolism also have important nonanaplerotic roles that can alter cell signaling and function. The metabolic byproduct of tryptophan catabolism, kynurenine, can ligate the aryl hydrocarbon receptor and enhance polarization of CD4 T cells to a T reg phenotype (Mezrich et al., 2010; Opitz et al., 2011). Another example of this is catabolism of phenylalanine by IL-4–induced gene 1 protein (IL4I1). When highly expressed by tumors or APCs, IL4I1 can inhibit T cell proliferation (Boulland et al., 2007; Lasoudris et al., 2011). This effect appears to be caused by the production of H2O2, a product of phenylalanine catabolism. IL4I1 is also expressed in Th17 and T reg cells (Santarlasci et al., 2012; Scarlata et al., 2015). The specific purpose for IL4l1 expression in these T cell subsets remains ambiguous, although its expression in Th17 cells was speculated to have a self-regulatory role, where its induction led to diminished proliferation (Santarlasci et al., 2012). However given that low concentrations of H2O2 can act as a signaling molecule (Veal et al., 2007), IL4I1 might also play a role in cell signaling pathways independent of mechanisms that inhibit proliferation.
Fluctuations in environmental amino acid concentrations, as well as metabolic products from amino acid catabolism, can dramatically alter T cell activity and polarization. A well-documented example of this is indoleamine-2,3-dioxygenase (IDO)–mediated tryptophan catabolism. IDO, which is often expressed at high levels by APCs or tumor cells, can deplete tryptophan within a tissue microenvironment, and this in turn can lead to inhibition of effector T cell proliferation and induction of anergy (Munn et al., 2002; Uyttenhove et al., 2003). Depletion of tryptophan causes activation of the integrated stress response inducer general control nonderepressible 2 (GCN2) kinase, which results in the inhibition of translation initiation and metabolic remodeling (Munn et al., 2005; Guo and Cavener, 2007; Castilho et al., 2014).
Studies into the interactions of APCs with T cells have highlighted multiple pathways through which APCs modulate extracellular concentrations of amino acids, or their catabolic products, to regulate T cell responses. In a tumor, TGF-β–producing DCs can enhance expression of transporters for histidine, leucine, valine, and tryptophan, depleting these amino acids from the extracellular microenvironment and directly impairing T cell proliferation (Angelini et al., 2002). T reg cells can also enhance expression of particular amino acid catabolizing enzymes, including arginase 1, histidine decarboxylase, threonine dehydrogenase, and IL4I1, in skin grafts and bone marrow–derived DCs (Cobbold et al., 2009). Limitations in these amino acids, singularly or in combination, enhanced T reg cell polarization when T cells were activated in vitro (Cobbold et al., 2009). Although depletion of amino acids from the microenvironment appears to be a way in which APCs can negatively regulate T cell activity, the opposite also occurs, whereby APCs can support T cell activation through supplementing a microenvironment. For example, DCs and monocytes can release cysteine, which is thought to support T cell activation and function (Sido et al., 2000; Angelini et al., 2002). Cysteine supply is a limiting factor in T cell proliferation and is used extensively for protein and glutathione synthesis, as well as providing beneficial catabolic products, such as taurine, which may support T cell function through regulating osmolality (Kaesler et al., 2012; Sikalidis, 2015).
Lipid metabolism.
Lipids or fatty acids encompass another critical substrate group for T cells. They are a vital component of cell membranes, provide a high yielding energy source, and can also supply substrates for cell signaling and PTMs (Lochner et al., 2015; Thurnher and Gruenbacher, 2015). After T cell activation, the demand for lipids rapidly increases. Within 24 h, in vitro–activated T cells augment fatty acid synthesis (FAS), while concomitantly decreasing FAO, thus enhancing the accumulation of fatty acid metabolites needed for membrane synthesis (Wang et al., 2011). c-Myc and mTOR have important roles in coordinating these metabolic changes (Wang et al., 2011; Yang et al., 2013), and SREBP transcription factors are critical for reprogramming lipid metabolism (Kidani et al., 2013). SREBPs induce expression of genes involved in FAS and mevalonate pathways, which supply de novo synthesized fatty acids and cholesterol, respectively (Thurnher and Gruenbacher, 2015). CD4 T cells deficient in Raptor, and thus mTORC1 signaling, have impaired de novo FAS, most likely caused by reduced expression of SREBP1 and SREBP2 protein (Yang et al., 2013).
Loss of SREBP function in CD8 T cells results in a failure to induce metabolic pathways needed for clonal expansion during a viral infection (Kidani et al., 2013). Exogenous cholesterol rescues the defects in SREBP-deficient T cells, suggesting that at least in this context, a lack of cholesterol is the main limiting factor. This requirement for cholesterol synthesis is consistent with results showing that perturbing sterol homeostasis in activated T cells—by activating the liver X receptor (LXR), which targets genes that are involved in cholesterol cellular export—impairs T cell proliferation. The inhibitory effect of LXR activation can be overcome through the addition of mevalonate, a cholesterol precursor (Bensinger et al., 2008). Inhibition of 3-hydroxy-3-methylgutaryl-coenzymeA (HMG-CoA) reductase, an enzyme in the mevalonate pathway, results in a Th2 cell bias in the experimental autoimmune encephalomyelitis (EAE) disease model, due to impaired biosynthesis of isoprenoids and a subsequent reduction in prenylation of Ras and RhoA GTPases (Youssef et al., 2002). These data suggest that in addition to cholesterol homeostasis, other products of the mevalonate pathway can also influence T cell differentiation. The impact of commonly used drugs that lower cholesterol by inhibiting HMG-CoA reductase can affect both prenylation and cholesterol synthesis, and thus it is plausible these drugs have multiple effects on activated T cells.
The synthesis of fatty acids is also important for effector T cell function. Although activated T cells readily acquire and use extracellular fatty acids, it appears that there may also be cell-intrinsic requirements for de novo–synthesized fatty acids (Berod et al., 2014; Lee et al., 2014; O’Sullivan and Pearce, 2014; O’Sullivan et al., 2014). Inhibition of acetyl-CoA carboxylase 1 (ACC1), an enzyme in FAS, was shown to limit Th17 cell differentiation and promote the development of T reg cells. This effect translated into improved disease outcomes in EAE (Berod et al., 2014). Inhibition of ACC1 impaired phospholipid synthesis in Th17 cells while also impairing glycolytic flux, both through aerobic glycolysis and the TCA cycle. In contrast, T reg cells were able to sustain their requirements for fatty acids through acquisition from extracellular sources (Berod et al., 2014). ACC1 deficiency also impairs Th1 and Th2 development, suggesting that CD4 effector T cells have a common requirement for FAS (Berod et al., 2014). In contrast, T cell–specific deletion of ACC1 does not impair CD8 effector T cell development after infection, although effector T cell expansion is diminished due to increased cell death, indicating that FAS is required for the persistence of CD8 effector T cells (Lee et al., 2014). Collectively, these findings suggest that there are varying requirements for de novo–synthesized fatty acids between different T cell subsets. Interestingly, defects after ACC1 inhibition in either Th17 cells or CD8 effector T cells can be rescued through the addition of excess free fatty acids to the media (Berod et al., 2014; Lee et al., 2014), indicating that these cells can compensate for the lack of FAS if the extracellular fatty acid supply is plentiful. Addition of extracellular fatty acids can also enhance T cell proliferation (Gorjão et al., 2007). It is plausible that the demand for fatty acids is so substantial in these highly proliferative populations that de novo FAS can be supplemented through extracellular uptake. This concept would be consistent with a recent study suggesting that lipid released from adipose tissue may enhance T cell proliferation in vivo (Kim et al., 2015b). Responses to TNF-mediated signaling in the hypothalamus induced B and T cell proliferation in the spleen, an effect mediated by an induction of lipolysis through sympathetic nervous system signaling to adipose tissue and a resultant increase in circulating leptin and free fatty acids (Kim et al., 2015b).
Although in general the balance of FAS to FAO within effector T cell populations is weighted heavily toward FAS, effector T cells can use FAO (Byersdorfer et al., 2013; O’Sullivan et al., 2014). Given that the demand for energy is high in these cells, it is likely that they need some metabolic flexibility in their fuel sources, an idea that is consistent with recent work highlighting the importance of adenosine monophosphate-activated protein kinase (AMPK) in effector T cell function (Blagih et al., 2015). The extent to which FAO occurs in effector T cells is likely to be highly context dependent, in part due to the heterogeneity of this population of cells during an immune response. Studies using animal models of graft versus host disease (GvHD) have found that alloreactive T cells increase fatty acid uptake and enhance FAO compared with other effector T cells (Gatza et al., 2011; Byersdorfer et al., 2013; Glick et al., 2014). Suppressing Akt during activation can induce a metabolic profile suggestive of FAO utilization (Crompton et al., 2015), and culturing CD8 effector T cells in low glucose enhances FAO (O’Sullivan et al., 2014). A recent report also found that inhibition of T cell signaling through ligation of PD-1 induces changes in the metabolic profile of activated T cells, including decreased aerobic glycolysis and enhanced FAO (Patsoukis et al., 2015). Collectively, these data suggest that the utilization of FAO in effector T cells may be influenced by several factors, such as activation state, exposure to antigen, inflammatory signals, and microenvironmental nutrient availability.
Memory T cell metabolism
Effector T cell populations contract after pathogen clearance and undergo apoptosis, leaving behind a small population of long-lived memory T cells that can respond vigorously upon antigen rechallenge (Williams and Bevan, 2007). Although both naive and memory T cells acquire effector functions upon activation, memory T cells have an accelerated response to antigen, proliferate faster, and produce more cytokines than their naive counterparts. Work from our laboratory and others has shown that changes in metabolism also drive memory T cell development (Fig. 1; Araki et al., 2009; Pearce et al., 2009; Rao et al., 2010).
AMPK and mTOR.
Increases in intracellular AMP-to-ATP concentrations activate the energy stress sensor AMPK, a signal that also promotes FAO (Jones and Thompson, 2007). AMPK is important for the development of memory T cells, and administration of the metabolic stressor and AMPK activator metformin enhances the generation of memory T cells after infection (Pearce et al., 2009; Rolf et al., 2013). In addition, AMPK allows for effector T cells to metabolically adapt during nutrient stress and modulates T cell effector function through suppression of mTOR (Tamás et al., 2006; MacIver et al., 2011; Blagih et al., 2015). Inhibiting mTOR with rapamycin boosts memory T cell development in vivo (Araki et al., 2009; Pearce et al., 2009; Rao et al., 2010). Loss of the mTORC1-negative regulator TSC1 compromises formation of memory T cell precursors that are present during the primary effector response (Kaech et al., 2003; Shrestha et al., 2014). Similarly, suppressing mTORC2 fosters memory T cell generation (Pollizzi et al., 2015). Inhibition of mTOR and activation of AMPK also strongly stimulate the catabolic process of autophagy. Autophagy has been shown to support T cell viability and bioenergetics after activation (Pua et al., 2007; Hubbard et al., 2010). Consistent with the idea that activation of catabolic pathways promotes the development of memory T cells, a recent study found that deletion of the autophagy molecules Atg5 or Atg7 compromised the formation of CD8 memory T cells after viral infection (Xu et al., 2014).
FAO and mitochondria.
Work from our laboratory has shown that CD8 memory T cells are dependent on FAO for their development, long-term persistence, and ability to robustly respond to antigen stimulation (Fig. 1; Pearce et al., 2009; van der Windt et al., 2012, 2013). Enhancing FAO in memory T cells through increased expression of carnitine palmitoyltransferase 1a (CPT1a)—a critical mitochondrial transporter of long-chain fatty acids and rate limiting step to β-oxidation— increases CD8 memory T cell numbers after infection (van der Windt et al., 2012). During an immune response, common γ chain cytokines like IL-15 and IL-7 have an essential role in supporting catabolic metabolism by promoting mitochondrial biogenesis, CPT1a expression, and FAO (van der Windt et al., 2012). As a result, memory T cells have increased mitochondrial mass and greater spare respiratory capacity (SRC) compared with naive and effector T cells, which endows them with a bioenergetic advantage for survival and recall after antigen rechallenge (van der Windt et al., 2012, 2013; Gubser et al., 2013). Interestingly, a study investigating heterologous prime-boost vaccination found that memory T cell differentiation is hastened and enhanced after secondary or tertiary immunization, including acquisition of substantially greater mitochondrial mass and SRC (Fraser et al., 2013). In a sense, memory T cells are metabolically primed and ready to respond to secondary infections. This idea has recently been extended to innate immune cells under the novel concept of trained immunity, whereby metabolic priming confers superior immunity to a secondary pathogenic insult (Cheng et al., 2014). Previous engagement of aerobic glycolysis in monocytes driven by mTOR and HIF-1α was found to induce epigenetic modifications that endowed them with enhanced function against other infections (Cheng et al., 2014).
The specific role of FAO in promoting memory T cell development and survival remains to be elucidated, but it appears that metabolic reprogramming associated with FAO enhances mitochondria-associated processes. Induction of FAO in memory T cells enhances SRC, which is the reserve capacity of mitochondria to produce energy over and above normal energy outputs (van der Windt et al., 2012). This parameter is probably important for the longevity of memory T cells, especially in times of stress or nutrient restriction, conditions that may present themselves when infection is resolved and growth factor signals are scarce. Surprisingly, endothelial cells, unlike most other cell types, use carbon derived from FAO for nucleotide synthesis and proliferation (Schoors et al., 2015), providing another way in which FAO supports cell function.
Another unexpected discovery was that memory T cells preferentially use de novo FAS to fuel FAO (O’Sullivan et al., 2014). Specifically, CD8 memory T cells use glucose to produce triacylglycerides (TAGs) that are subsequently hydrolyzed by lysosomal acid lipase (LAL) to support mitochondrial FAO (O’Sullivan et al., 2014). It was also recently shown that glucose metabolism is critical for CD4 memory T cell survival, and this is controlled by Notch signaling (Maekawa et al., 2015). The requirement for FAS in CD8 memory T cells is supported by a recent study showing that glycerol import into the cell via IL-7–induced aquaporin-mediated transport is required for memory T cell longevity (Cui et al., 2015). Glycerol is the molecular backbone for TAGs. Aquaporin 9 (AQP9)-deficient T cells had reduced glycerol import and TAG synthesis and impaired memory T cell survival after viral infection (Cui et al., 2015).
The reasons why CD8 memory T cells synthesize and then catabolize fatty acids in an apparently futile cycle rather than simply acquire extracellular fatty acids are not understood. However, this synthesis/catabolism cycle has also been shown to occur in muscle and adipose tissues (Dulloo et al., 2004; Yu et al., 2002). If viewed on a purely energetic level, this process appears counter-productive, as there would be no net gain in ATP. It is possible that building and burning fatty acids allows memory T cells to sustain their glycolytic and lipogenic machinery while maintaining mitochondrial health during times of quiescence, allowing for the rapid recall ability that is characteristic of memory T cells after antigen recognition and activation (Gubser et al., 2013; van der Windt et al., 2013). It could also potentially provide a mechanism for balancing redox state or metabolic intermediates. An energetically futile cycle has been described in yeast grown in glucose rich media, whereby trehalose cycling provides a buffer system to maintain intracellular phosphate levels and balance glycolytic intermediates (van Heerden et al., 2014).
Emerging topics and concluding remarks
It is an exciting time for the field of immunometabolism. Although the body of literature surrounding this topic is increasing at an exponential rate, much remains to be explored. We are just beginning to understand the many connections between metabolism and gene regulation in T cells (Lu and Thompson, 2012; Wang and Green, 2012; Kaelin and McKnight, 2013; Öst and Pospisilik, 2015). Acetyl-CoA and NAD+ generated from oxidative metabolism are used for histone acetyltransferase (HAT) and histone deacetylase (HDAC) activity (Imai and Guarente, 2010; Cantó and Auwerx, 2011; Wellen and Thompson, 2012). Protein acetylation is a reversible PTM that influences epigenetic changes mediated by HATs and HDACs and also controls the actions of transcription factors and molecular chaperones (Glozak et al., 2005). T cell metabolic reprogramming during activation increases cytosolic NAD+ and citrate, the precursor of acetyl-CoA, which may direct cell-fate decisions through protein acetylation (Berger et al., 1987). In agreement with this idea, lineage-specific cytokine-encoding genes that affect T cell differentiation undergo dynamic changes in histone acetylation after antigen-driven stimulation (Avni et al., 2002; Fields et al., 2002). The actions of the transcription factor FoxP3 in directing T reg cell development are controlled through opposing activities of the HATs p300 and TIP60, and the HDAC Sirtuin1 (Sirt1; Li et al., 2007; Tao et al., 2007; van Loosdregt et al., 2010; Beier et al., 2011). Acetylation also affects the activity of circadian clock proteins, and reciprocally, the circadian acetylome has been found to regulate the epigenome and mitochondrial metabolic pathways (Koike et al., 2012; Lu and Thompson, 2012; Masri et al., 2013). Organisms rely on the cell autonomous transcription–translation oscillator loop managed by solar time to accommodate physiological changes brought about by the daily pattern of rest, activity, and feeding (Curtis et al., 2014). The circadian clock also in part regulates Th17 cell development (Yu et al., 2013). Circadian rhythm controls nutrient acquisition and metabolic flux, and it will be interesting to see how the body’s internal clock may connect to lymphocyte metabolism, regulation, and function (Rey and Reddy, 2013).
Intermediates from glucose catabolism can be converted into substrates that are needed to support cell growth and proliferation. Recent studies of one-carbon metabolism in cancer research involving the serine and glycine biosynthetic pathways may have implications for T cell metabolism, given the many common features shared between activated T cells and proliferating cancer cells. Although the role of one-carbon metabolism in generating units for nucleic acid synthesis from folate has long been appreciated, it was more recently recognized that this pathway is an important source of NADPH to maintain redox balance and methyl groups for methylation (Locasale, 2013; Fan et al., 2014). For example, depletion of methylenetetrahydrofolate dehydrogenase (MTHFD) in cancer cells results in a decreased cellular NADPH/NADP+ ratio and increased oxidized glutathione, enhancing sensitivity to oxidative stress (Fan et al., 2014). Serine and glycine metabolism also have a vital role in cell survival under harsh environmental conditions of nutrient scarcity and hypoxia (Kim et al., 2015a). Tumors foster these conditions, but tumor cells are able to survive and function under these stressors. A recent study found that increased expression of mitochondrial serine hydroxymethyltransferase and glycine decarboxylase confer a survival advantage for glioma cells by allowing them to lower their oxygen consumption and metabolize toxic molecules in the tumor microenvironment (Kim et al., 2015a). T cells also migrate and travel to sites of infection or tumors and must adapt to these hypoxic or nutrient-depleted environments (Pearce et al., 2013). It remains to be explored whether these metabolic pathways support lymphocyte survival by similar mechanisms or have evolved to serve other purposes.
Another area of interest is how substrate availability affects T cell differentiation and their functional fate. Studies investigating the relationship between the gut microbiome and lymphocytes have found that metabolites produced by commensal bacteria have important implications for maintaining immune cell gastrointestinal homeostasis and defense against pathogens. Short chain fatty acids such as butyrate, acetate, and propionate produced by bacteria induce differentiation of colonic T reg cells (Furusawa et al., 2013; Smith et al., 2013) and also Th17 cells under certain conditions (Park et al., 2015). The vitamin A metabolite retinoic acid can synergize with TGF-β to stimulate T reg conversion (Coombes et al., 2007; Denning et al., 2007; Mucida et al., 2007; Sun et al., 2007; Elias et al., 2008). However, vitamin A metabolite deficiency also abrogates Th1 and Th17 cell immunity (Hall et al., 2011) and, more recently, was found to diminish type 3 innate lymphoid cells (ILC3s), but expand ILC2 cells (Spencer et al., 2014). Lymphocytes can also regulate whole body metabolism by affecting the tissues in which they reside. ILC2s can promote beiging of white adipose tissue and control caloric expenditure through secretion of methionine-enkephaline peptides (Brestoff et al., 2015). Loss of insulin sensitivity as a result of inflammation of adipose tissue in obesity and type 2 diabetes results in part from deficiencies in adipose tissue-specific T reg cell populations controlled by the transcriptional regulator PPAR-γ (Feuerer et al., 2009; Cipolletta et al., 2012). These are just a few examples of what remains to be explored in the interplay between lymphocytes, their environment, and metabolism.
In the development of novel therapeutics for the treatment of human disease, targeting T cell metabolism provides a unique opportunity to manipulate T cell function (O’Sullivan and Pearce, 2015). For example, it has been demonstrated that compared with Th1 and T reg cells, Th17 cells have elevated pyruvate dehydrogenase kinase 1 (PDK1) expression, which promotes aerobic glycolysis through inhibition of pyruvate dehydrogenase (PDH). Inhibition of PDK1 using dichloroacetate (DCA) selectively impairs Th17 proliferation and survival and reduces T cell–mediated inflammation in models of inflammatory bowel disease and EAE (Gerriets et al., 2015). Targeting trophic transporters on T cells may also provide a way in which to manipulate T cell function through altering their nutrient uptake; for example, JPH203, which inhibits amino acid transporter Slc7a5, could be used to inhibit inflammatory T cells without impairing T reg cell function (Hayashi et al., 2013; Sinclair et al., 2013). In the context of cancer, the development of adoptive cellular immunotherapies using in vitro–expanded tumor infiltrating lymphocytes (TILs) could benefit from tailoring culture conditions to optimize TIL metabolism before transfer into the patient (Restifo et al., 2012). Exaggerated glycolysis and cell size resulting from in vitro expansion conditions, such as high glucose, can be detrimental to TIL survival and persistence in vivo, and strategies to limit glycolysis directly or suppress Akt activation in TILs have already shown promising results in this context (Sukumar et al., 2013; Crompton et al., 2015). Inhibiting glycolysis and oxidative metabolism with 2-DG and metformin may also hold therapeutic potential in other disease settings as highlighted by a recent study using models of system lupus erythematosus (Yin et al., 2015).
It is apparent that many diverse processes integrate with lymphocyte signaling, gene regulation, and function to shape T cell metabolism. Understanding the metabolic regulation that dictates T cell fate and how nutrient availability and microenvironmental factors influence T cell function will provide further insight into immune cell biology and could lead to new approaches to treating human diseases.
Acknowledgments
We thank Edward Pearce and members of the Pearce laboratories for their insight and critical reading of the manuscript.
This work was supported by grants from the National Institutes of Health (R01-CA181125 and R01-AI091965 to E.L. Pearce), The Burroughs Wellcome Fund (Investigator in the Pathogenesis of Infectious Disease Award to E.L. Pearce), and the National Science Foundation Graduate Research Fellowship (DGE-1143954 to M.D. Buck).
The authors declare no competing financial interests.
Footnotes
Abbreviations used:
- ACC1
- acetyl-CoA carboxylase 1
- AMPK
- adenosine monophosphate-activated protein kinase
- ASS1
- argininosuccinate 1
- CPT1a
- carnitine palmitoyltransferase 1a
- DN
- double negative
- DP
- double positive
- ERRα
- estrogen-related receptor α
- ETC
- electron transport chain
- FAS
- fatty acid synthesis
- HAT
- histone acetyltransferase
- HDAC
- histone deacetylase
- IDO
- indoleamine-2,3-dioxygenase
- PDK1
- pyruvate dehydrogenase kinase
- PDPK1
- 3-phosphoinositide–dependent protein kinase-1
- PI3K
- phosphatidylinositol 3-kinase
- PKA
- protein kinase A
- PTM
- posttranslational modification
- ROS
- reactive oxygen species
- TCA
- tricarboxylic acid
- TPPII
- tripeptidyl peptidase II
- TSC1
- tuberous sclerosis complex 1
References
- Akashi K., Kondo M., von Freeden-Jeffry U., Murray R., and Weissman I.L.. 1997. Bcl-2 rescues T lymphopoiesis in interleukin-7 receptor-deficient mice. Cell. 89:1033–1041. 10.1016/S0092-8674(00)80291-3 [DOI] [PubMed] [Google Scholar]
- Ananieva E.A., Patel C.H., Drake C.H., Powell J.D., and Hutson S.M.. 2014. Cytosolic branched chain aminotransferase (BCATc) regulates mTORC1 signaling and glycolytic metabolism in CD4+ T cells. J. Biol. Chem. 289:18793–18804. 10.1074/jbc.M114.554113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasiou D., Poulogiannis G., Asara J.M., Boxer M.B., Jiang J.K., Shen M., Bellinger G., Sasaki A.T., Locasale J.W., Auld D.S., et al. 2011. Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science. 334:1278–1283. 10.1126/science.1211485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelini G., Gardella S., Ardy M., Ciriolo M.R., Filomeni G., Di Trapani G., Clarke F., Sitia R., and Rubartelli A.. 2002. Antigen-presenting dendritic cells provide the reducing extracellular microenvironment required for T lymphocyte activation. Proc. Natl. Acad. Sci. USA. 99:1491–1496. 10.1073/pnas.022630299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki K., Turner A.P., Shaffer V.O., Gangappa S., Keller S.A., Bachmann M.F., Larsen C.P., and Ahmed R.. 2009. mTOR regulates memory CD8 T-cell differentiation. Nature. 460:108–112. 10.1038/nature08155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avni O., Lee D., Macian F., Szabo S.J., Glimcher L.H., and Rao A.. 2002. T(H) cell differentiation is accompanied by dynamic changes in histone acetylation of cytokine genes. Nat. Immunol. 3:643–651. [DOI] [PubMed] [Google Scholar]
- Bauer D.E., Hatzivassiliou G., Zhao F., Andreadis C., and Thompson C.B.. 2005. ATP citrate lyase is an important component of cell growth and transformation. Oncogene. 24:6314–6322. 10.1038/sj.onc.1208773 [DOI] [PubMed] [Google Scholar]
- Beier U.H., Wang L., Bhatti T.R., Liu Y., Han R., Ge G., and Hancock W.W.. 2011. Sirtuin-1 targeting promotes Foxp3+ T-regulatory cell function and prolongs allograft survival. Mol. Cell. Biol. 31:1022–1029. 10.1128/MCB.01206-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensinger S.J., Bradley M.N., Joseph S.B., Zelcer N., Janssen E.M., Hausner M.A., Shih R., Parks J.S., Edwards P.A., Jamieson B.D., and Tontonoz P.. 2008. LXR signaling couples sterol metabolism to proliferation in the acquired immune response. Cell. 134:97–111. 10.1016/j.cell.2008.04.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger S.J., Manory I., Sudar D.C., Krothapalli D., and Berger N.A.. 1987. Pyridine nucleotide analog interference with metabolic processes in mitogen-stimulated human T lymphocytes. Exp. Cell Res. 173:379–387. 10.1016/0014-4827(87)90278-3 [DOI] [PubMed] [Google Scholar]
- Berod L., Friedrich C., Nandan A., Freitag J., Hagemann S., Harmrolfs K., Sandouk A., Hesse C., Castro C.N., Bähre H., et al. 2014. De novo fatty acid synthesis controls the fate between regulatory T and T helper 17 cells. Nat. Med. 20:1327–1333. 10.1038/nm.3704 [DOI] [PubMed] [Google Scholar]
- Best J.A., Blair D.A., Knell J., Yang E., Mayya V., Doedens A., Dustin M.L., and Goldrath A.W.. Immunological Genome Project Consortium. 2013. Transcriptional insights into the CD8+ T cell response to infection and memory T cell formation. Nat. Immunol. 14:404–412. 10.1038/ni.2536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird L. 2015. T cells. Endogenous agonists for orphan RORγ. Nat. Rev. Immunol. 15:70–71. 10.1038/nri3812 [DOI] [PubMed] [Google Scholar]
- Blagih J., Coulombe F., Vincent E.E., Dupuy F., Galicia-Vázquez G., Yurchenko E., Raissi T.C., van der Windt G.J., Viollet B., Pearce E.L., et al. 2015. The energy sensor AMPK regulates T cell metabolic adaptation and effector responses in vivo. Immunity. 42:41–54. 10.1016/j.immuni.2014.12.030 [DOI] [PubMed] [Google Scholar]
- Boudil A., Matei I.R., Shih H.Y., Bogdanoski G., Yuan J.S., Chang S.G., Montpellier B., Kowalski P.E., Voisin V., Bashir S., et al. 2015. IL-7 coordinates proliferation, differentiation and Tcra recombination during thymocyte β-selection. Nat. Immunol. 16:397–405. 10.1038/ni.3122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulland M.L., Marquet J., Molinier-Frenkel V., Möller P., Guiter C., Lasoudris F., Copie-Bergman C., Baia M., Gaulard P., Leroy K., and Castellano F.. 2007. Human IL4I1 is a secreted L-phenylalanine oxidase expressed by mature dendritic cells that inhibits T-lymphocyte proliferation. Blood. 110:220–227. 10.1182/blood-2006-07-036210 [DOI] [PubMed] [Google Scholar]
- Brestoff J.R., Kim B.S., Saenz S.A., Stine R.R., Monticelli L.A., Sonnenberg G.F., Thome J.J., Farber D.L., Lutfy K., Seale P., and Artis D.. 2015. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature. 519:242–246. 10.1038/nature14115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byersdorfer C.A., Tkachev V., Opipari A.W., Goodell S., Swanson J., Sandquist S., Glick G.D., and Ferrara J.L.. 2013. Effector T cells require fatty acid metabolism during murine graft-versus-host disease. Blood. 122:3230–3237. 10.1182/blood-2013-04-495515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantó C., and Auwerx J.. 2011. NAD+ as a signaling molecule modulating metabolism. Cold Spring Harb. Symp. Quant. Biol. 76:291–298. 10.1101/sqb.2012.76.010439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr E.L., Kelman A., Wu G.S., Gopaul R., Senkevitch E., Aghvanyan A., Turay A.M., and Frauwirth K.A.. 2010. Glutamine uptake and metabolism are coordinately regulated by ERK/MAPK during T lymphocyte activation. J. Immunol. 185:1037–1044. 10.4049/jimmunol.0903586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castilho B.A., Shanmugam R., Silva R.C., Ramesh R., Himme B.M., and Sattlegger E.. 2014. Keeping the eIF2 alpha kinase Gcn2 in check. Biochim. Biophys. Acta. 1843:1948–1968. 10.1016/j.bbamcr.2014.04.006 [DOI] [PubMed] [Google Scholar]
- Cekic C., Sag D., Day Y.J., and Linden J.. 2013. Extracellular adenosine regulates naive T cell development and peripheral maintenance. J. Exp. Med. 210:2693–2706. 10.1084/jem.20130249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cham C.M., and Gajewski T.F.. 2005. Glucose availability regulates IFN-gamma production and p70S6 kinase activation in CD8+ effector T cells. J. Immunol. 174:4670–4677. 10.4049/jimmunol.174.8.4670 [DOI] [PubMed] [Google Scholar]
- Cham C.M., Driessens G., O’Keefe J.P., and Gajewski T.F.. 2008. Glucose deprivation inhibits multiple key gene expression events and effector functions in CD8+ T cells. Eur. J. Immunol. 38:2438–2450. 10.1002/eji.200838289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C.H., Curtis J.D., Maggi L.B. Jr, Faubert B., Villarino A.V., O’Sullivan D., Huang S.C., van der Windt G.J., Blagih J., Qiu J., et al. 2013. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell. 153:1239–1251. 10.1016/j.cell.2013.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman N.M., and Chi H.. 2014. mTOR links environmental signals to t cell fate decisions. Front. Immunol. 5:686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri G., Hunt N.H., Clark I.A., and Ceredig R.. 1988. Antioxidants inhibit proliferation and cell surface expression of receptors for interleukin-2 and transferrin in T lymphocytes stimulated with phorbol myristate acetate and ionomycin. Cell. Immunol. 115:204–213. 10.1016/0008-8749(88)90174-8 [DOI] [PubMed] [Google Scholar]
- Cheng S.C., Quintin J., Cramer R.A., Shepardson K.M., Saeed S., Kumar V., Giamarellos-Bourboulis E.J., Martens J.H., Rao N.A., Aghajanirefah A., et al. 2014. mTOR- and HIF-1α–mediated aerobic glycolysis as metabolic basis for trained immunity. Science. 345:1250684 10.1126/science.1250684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou C., Pinto A.K., Curtis J.D., Persaud S.P., Cella M., Lin C.C., Edelson B.T., Allen P.M., Colonna M., Pearce E.L., et al. 2014. c-Myc-induced transcription factor AP4 is required for host protection mediated by CD8+ T cells. Nat. Immunol. 15:884–893. 10.1038/ni.2943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouchani E.T., Pell V.R., Gaude E., Aksentijević D., Sundier S.Y., Robb E.L., Logan A., Nadtochiy S.M., Ord E.N., Smith A.C., et al. 2014. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. 515:431–435. 10.1038/nature13909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciofani M., and Zúñiga-Pflücker J.C.. 2005. Notch promotes survival of pre-T cells at the beta-selection checkpoint by regulating cellular metabolism. Nat. Immunol. 6:881–888. 10.1038/ni1234 [DOI] [PubMed] [Google Scholar]
- Ciofani M., Schmitt T.M., Ciofani A., Michie A.M., Cuburu N., Aublin A., Maryanski J.L., and Zúñiga-Pflücker J.C.. 2004. Obligatory role for cooperative signaling by pre-TCR and Notch during thymocyte differentiation. J. Immunol. 172:5230–5239. 10.4049/jimmunol.172.9.5230 [DOI] [PubMed] [Google Scholar]
- Cipolletta D., Feuerer M., Li A., Kamei N., Lee J., Shoelson S.E., Benoist C., and Mathis D.. 2012. PPAR-γ is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature. 486:549–553. 10.1038/nature11132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbold S.P., Adams E., Farquhar C.A., Nolan K.F., Howie D., Lui K.O., Fairchild P.J., Mellor A.L., Ron D., and Waldmann H.. 2009. Infectious tolerance via the consumption of essential amino acids and mTOR signaling. Proc. Natl. Acad. Sci. USA. 106:12055–12060. 10.1073/pnas.0903919106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole M.D. 1986. The myc oncogene: its role in transformation and differentiation. Annu. Rev. Genet. 20:361–384. 10.1146/annurev.ge.20.120186.002045 [DOI] [PubMed] [Google Scholar]
- Coombes J.L., Siddiqui K.R., Arancibia-Cárcamo C.V., Hall J., Sun C.M., Belkaid Y., and Powrie F.. 2007. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β and retinoic acid–dependent mechanism. J. Exp. Med. 204:1757–1764. 10.1084/jem.20070590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crompton J.G., Sukumar M., Roychoudhuri R., Clever D., Gros A., Eil R.L., Tran E., Hanada K., Yu Z., Palmer D.C., et al. 2015. Akt inhibition enhances expansion of potent tumor-specific lymphocytes with memory cell characteristics. Cancer Res. 75:296–305. 10.1158/0008-5472.CAN-14-2277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui G., Staron M.M., Gray S.M., Ho P.C., Amezquita R.A., Wu J., and Kaech S.M.. 2015. IL-7-induced glycerol transport and TAG synthesis promotes memory CD8+ T cell longevity. Cell. 161:750–761. 10.1016/j.cell.2015.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis A.M., Bellet M.M., Sassone-Corsi P., and O’Neill L.A.. 2014. Circadian clock proteins and immunity. Immunity. 40:178–186. 10.1016/j.immuni.2014.02.002 [DOI] [PubMed] [Google Scholar]
- D’Cruz L.M., Knell J., Fujimoto J.K., and Goldrath A.W.. 2010. An essential role for the transcription factor HEB in thymocyte survival, Tcra rearrangement and the development of natural killer T cells. Nat. Immunol. 11:240–249. 10.1038/ni.1845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang E.V., Barbi J., Yang H.Y., Jinasena D., Yu H., Zheng Y., Bordman Z., Fu J., Kim Y., Yen H.R., et al. 2011. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell. 146:772–784. 10.1016/j.cell.2011.07.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBerardinis R.J., Mancuso A., Daikhin E., Nissim I., Yudkoff M., Wehrli S., and Thompson C.B.. 2007. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc. Natl. Acad. Sci. USA. 104:19345–19350. 10.1073/pnas.0709747104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBerardinis R.J., Lum J.J., Hatzivassiliou G., and Thompson C.B.. 2008. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 7:11–20. 10.1016/j.cmet.2007.10.002 [DOI] [PubMed] [Google Scholar]
- Delgoffe G.M., Kole T.P., Zheng Y., Zarek P.E., Matthews K.L., Xiao B., Worley P.F., Kozma S.C., and Powell J.D.. 2009. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 30:832–844. 10.1016/j.immuni.2009.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgoffe G.M., Pollizzi K.N., Waickman A.T., Heikamp E., Meyers D.J., Horton M.R., Xiao B., Worley P.F., and Powell J.D.. 2011. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat. Immunol. 12:295–303. 10.1038/ni.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denning T.L., Wang Y.C., Patel S.R., Williams I.R., and Pulendran B.. 2007. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat. Immunol. 8:1086–1094. 10.1038/ni1511 [DOI] [PubMed] [Google Scholar]
- Devadas S., Zaritskaya L., Rhee S.G., Oberley L., and Williams M.S.. 2002. Discrete generation of superoxide and hydrogen peroxide by T cell receptor stimulation: selective regulation of mitogen-activated protein kinase activation and fas ligand expression. J. Exp. Med. 195:59–70. 10.1084/jem.20010659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doedens A.L., Phan A.T., Stradner M.H., Fujimoto J.K., Nguyen J.V., Yang E., Johnson R.S., and Goldrath A.W.. 2013. Hypoxia-inducible factors enhance the effector responses of CD8+ T cells to persistent antigen. Nat. Immunol. 14:1173–1182. 10.1038/ni.2714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulloo A.G., Gubler M., Montani J.P., Seydoux J., and Solinas G.. 2004. Substrate cycling between de novo lipogenesis and lipid oxidation: a thermogenic mechanism against skeletal muscle lipotoxicity and glucolipotoxicity. Int. J. Obes. Relat. Metab. Disord. 28:S29–S37. 10.1038/sj.ijo.0802861 [DOI] [PubMed] [Google Scholar]
- Elias K.M., Laurence A., Davidson T.S., Stephens G., Kanno Y., Shevach E.M., and O’Shea J.J.. 2008. Retinoic acid inhibits Th17 polarization and enhances FoxP3 expression through a Stat-3/Stat-5 independent signaling pathway. Blood. 111:1013–1020. 10.1182/blood-2007-06-096438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst B., Lee D.S., Chang J.M., Sprent J., and Surh C.D.. 1999. The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the periphery. Immunity. 11:173–181. 10.1016/S1074-7613(00)80092-8 [DOI] [PubMed] [Google Scholar]
- Fan J., Ye J., Kamphorst J.J., Shlomi T., Thompson C.B., and Rabinowitz J.D.. 2014. Quantitative flux analysis reveals folate-dependent NADPH production. Nature. 510:298–302. 10.1038/nature13236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuerer M., Herrero L., Cipolletta D., Naaz A., Wong J., Nayer A., Lee J., Goldfine A.B., Benoist C., Shoelson S., and Mathis D.. 2009. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat. Med. 15:930–939. 10.1038/nm.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields P.E., Kim S.T., and Flavell R.A.. 2002. Cutting edge: changes in histone acetylation at the IL-4 and IFN-γ loci accompany Th1/Th2 differentiation. J. Immunol. 169:647–650. 10.4049/jimmunol.169.2.647 [DOI] [PubMed] [Google Scholar]
- Finlay D.K., Rosenzweig E., Sinclair L.V., Feijoo-Carnero C., Hukelmann J.L., Rolf J., Panteleyev A.A., Okkenhaug K., and Cantrell D.A.. 2012. PDK1 regulation of mTOR and hypoxia-inducible factor 1 integrate metabolism and migration of CD8+ T cells. J. Exp. Med. 209:2441–2453. 10.1084/jem.20112607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher M., Ramirez M.E., Sierra R.A., Raber P., Thevenot P., Al-Khami A.A., Sanchez-Pino D., Hernandez C., Wyczechowska D.D., Ochoa A.C., and Rodriguez P.C.. 2015. L-Arginine depletion blunts antitumor T-cell responses by inducing myeloid-derived suppressor cells. Cancer Res. 75:275–283. 10.1158/0008-5472.CAN-14-1491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox C.J., Hammerman P.S., and Thompson C.B.. 2005. Fuel feeds function: energy metabolism and the T-cell response. Nat. Rev. Immunol. 5:844–852. 10.1038/nri1710 [DOI] [PubMed] [Google Scholar]
- Fraser K.A., Schenkel J.M., Jameson S.C., Vezys V., and Masopust D.. 2013. Preexisting high frequencies of memory CD8+ T cells favor rapid memory differentiation and preservation of proliferative potential upon boosting. Immunity. 39:171–183. 10.1016/j.immuni.2013.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frauwirth K.A., Riley J.L., Harris M.H., Parry R.V., Rathmell J.C., Plas D.R., Elstrom R.L., June C.H., and Thompson C.B.. 2002. The CD28 signaling pathway regulates glucose metabolism. Immunity. 16:769–777. 10.1016/S1074-7613(02)00323-0 [DOI] [PubMed] [Google Scholar]
- Furusawa Y., Obata Y., Fukuda S., Endo T.A., Nakato G., Takahashi D., Nakanishi Y., Uetake C., Kato K., Kato T., et al. 2013. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 504:446–450. 10.1038/nature12721 [DOI] [PubMed] [Google Scholar]
- Galván-Peña S., and O’Neill L.A.. 2014. Metabolic reprograming in macrophage polarization. Front. Immunol. 5:420 10.3389/fimmu.2014.00420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatza E., Wahl D.R., Opipari A.W., Sundberg T.B., Reddy P., Liu C., Glick G.D., and Ferrara J.L.. 2011. Manipulating the bioenergetics of alloreactive T cells causes their selective apoptosis and arrests graft-versus-host disease. Sci. Transl. Med. 3:ra8 10.1126/scitranslmed.3001975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerriets V.A., Kishton R.J., Nichols A.G., Macintyre A.N., Inoue M., Ilkayeva O., Winter P.S., Liu X., Priyadharshini B., Slawinska M.E., et al. 2015. Metabolic programming and PDHK1 control CD4+ T cell subsets and inflammation. J. Clin. Invest. 125:194–207. 10.1172/JCI76012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitenay D., Wiel C., Lallet-Daher H., Vindrieux D., Aubert S., Payen L., Simonnet H., and Bernard D.. 2014. Glucose metabolism and hexosamine pathway regulate oncogene-induced senescence. Cell Death Dis. 5:e1089 10.1038/cddis.2014.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick G.D., Rossignol R., Lyssiotis C.A., Wahl D., Lesch C., Sanchez B., Liu X., Hao L.Y., Taylor C., Hurd A., et al. 2014. Anaplerotic metabolism of alloreactive T cells provides a metabolic approach to treat graft-versus-host disease. J. Pharmacol. Exp. Ther. 351:298–307. 10.1124/jpet.114.218099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glozak M.A., Sengupta N., Zhang X., and Seto E.. 2005. Acetylation and deacetylation of non-histone proteins. Gene. 363:15–23. 10.1016/j.gene.2005.09.010 [DOI] [PubMed] [Google Scholar]
- Goldrath A.W., and Bevan M.J.. 1999. Low-affinity ligands for the TCR drive proliferation of mature CD8+ T cells in lymphopenic hosts. Immunity. 11:183–190. 10.1016/S1074-7613(00)80093-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-García S., García-Peydró M., Martín-Gayo E., Ballestar E., Esteller M., Bornstein R., de la Pompa J.L., Ferrando A.A., and Toribio M.L.. 2009. CSL-MAML–dependent Notch1 signaling controls T lineage–specific IL-7Rα gene expression in early human thymopoiesis and leukemia. J. Exp. Med. 206:779–791. 10.1084/jem.20081922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorjão R., Cury-Boaventura M.F., de Lima T.M., and Curi R.. 2007. Regulation of human lymphocyte proliferation by fatty acids. Cell Biochem. Funct. 25:305–315. 10.1002/cbf.1388 [DOI] [PubMed] [Google Scholar]
- Gubser P.M., Bantug G.R., Razik L., Fischer M., Dimeloe S., Hoenger G., Durovic B., Jauch A., and Hess C.. 2013. Rapid effector function of memory CD8+ T cells requires an immediate-early glycolytic switch. Nat. Immunol. 14:1064–1072. 10.1038/ni.2687 [DOI] [PubMed] [Google Scholar]
- Guo F., and Cavener D.R.. 2007. The GCN2 eIF2α kinase regulates fatty-acid homeostasis in the liver during deprivation of an essential amino acid. Cell Metab. 5:103–114. 10.1016/j.cmet.2007.01.001 [DOI] [PubMed] [Google Scholar]
- Hall J.A., Cannons J.L., Grainger J.R., Dos Santos L.M., Hand T.W., Naik S., Wohlfert E.A., Chou D.B., Oldenhove G., Robinson M., et al. 2011. Essential role for retinoic acid in the promotion of CD4+ T cell effector responses via retinoic acid receptor alpha. Immunity. 34:435–447. 10.1016/j.immuni.2011.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J.M., Jeong S.J., Park M.C., Kim G., Kwon N.H., Kim H.K., Ha S.H., Ryu S.H., and Kim S.. 2012. Leucyl-tRNA synthetase is an intracellular leucine sensor for the mTORC1-signaling pathway. Cell. 149:410–424. 10.1016/j.cell.2012.02.044 [DOI] [PubMed] [Google Scholar]
- Hatzivassiliou G., Zhao F., Bauer D.E., Andreadis C., Shaw A.N., Dhanak D., Hingorani S.R., Tuveson D.A., and Thompson C.B.. 2005. ATP citrate lyase inhibition can suppress tumor cell growth. Cancer Cell. 8:311–321. 10.1016/j.ccr.2005.09.008 [DOI] [PubMed] [Google Scholar]
- Hayashi K., Jutabha P., Endou H., Sagara H., and Anzai N.. 2013. LAT1 is a critical transporter of essential amino acids for immune reactions in activated human T cells. J. Immunol. 191:4080–4085. 10.4049/jimmunol.1300923 [DOI] [PubMed] [Google Scholar]
- Henao-Mejia J., Williams A., Goff L.A., Staron M., Licona-Limón P., Kaech S.M., Nakayama M., Rinn J.L., and Flavell R.A.. 2013. The microRNA miR-181 is a critical cellular metabolic rheostat essential for NKT cell ontogenesis and lymphocyte development and homeostasis. Immunity. 38:984–997. 10.1016/j.immuni.2013.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard V.M., Valdor R., Patel B., Singh R., Cuervo A.M., and Macian F.. 2010. Macroautophagy regulates energy metabolism during effector T cell activation. J. Immunol. 185:7349–7357. 10.4049/jimmunol.1000576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh A., DuPage M., Priyadharshini B., Sage P.T., Quiros J., Borges C.M., Townamchai N., Gerriets V.A., Rathmell J.C., Sharpe A.H., et al. 2015. Control of PI(3) kinase in Treg cells maintains homeostasis and lineage stability. Nat. Immunol. 16:188–196. 10.1038/ni.3077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai S., and Guarente L.. 2010. Ten years of NAD-dependent SIR2 family deacetylases: implications for metabolic diseases. Trends Pharmacol. Sci. 31:212–220. 10.1016/j.tips.2010.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs S.R., Herman C.E., Maciver N.J., Wofford J.A., Wieman H.L., Hammen J.J., and Rathmell J.C.. 2008. Glucose uptake is limiting in T cell activation and requires CD28-mediated Akt-dependent and independent pathways. J. Immunol. 180:4476–4486. 10.4049/jimmunol.180.7.4476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janas M.L., Varano G., Gudmundsson K., Noda M., Nagasawa T., and Turner M.. 2010. Thymic development beyond beta-selection requires phosphatidylinositol 3-kinase activation by CXCR4. J. Exp. Med. 207:247–261. 10.1084/jem.20091430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- John S., Weiss J.N., and Ribalet B.. 2011. Subcellular localization of hexokinases I and II directs the metabolic fate of glucose. PLoS ONE. 6:e17674 10.1371/journal.pone.0017674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R.J., Poholek A.C., DiToro D., Yusuf I., Eto D., Barnett B., Dent A.L., Craft J., and Crotty S.. 2009. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 325:1006–1010. 10.1126/science.1175870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R.G., and Thompson C.B.. 2007. Revving the engine: signal transduction fuels T cell activation. Immunity. 27:173–178. 10.1016/j.immuni.2007.07.008 [DOI] [PubMed] [Google Scholar]
- Jones R.G., Bui T., White C., Madesh M., Krawczyk C.M., Lindsten T., Hawkins B.J., Kubek S., Frauwirth K.A., Wang Y.L., et al. 2007. The proapoptotic factors Bax and Bak regulate T Cell proliferation through control of endoplasmic reticulum Ca2+ homeostasis. Immunity. 27:268–280. 10.1016/j.immuni.2007.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juntilla M.M., Wofford J.A., Birnbaum M.J., Rathmell J.C., and Koretzky G.A.. 2007. Akt1 and Akt2 are required for αβ thymocyte survival and differentiation. Proc. Natl. Acad. Sci. USA. 104:12105–12110. 10.1073/pnas.0705285104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech S.M., Tan J.T., Wherry E.J., Konieczny B.T., Surh C.D., and Ahmed R.. 2003. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 4:1191–1198. 10.1038/ni1009 [DOI] [PubMed] [Google Scholar]
- Kaelin W.G. Jr, and McKnight S.L.. 2013. Influence of metabolism on epigenetics and disease. Cell. 153:56–69. 10.1016/j.cell.2013.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaesler S., Sobiesiak M., Kneilling M., Volz T., Kempf W.E., Lang P.A., Lang K.S., Wieder T., Heller-Stilb B., Warskulat U., et al. 2012. Effective T-cell recall responses require the taurine transporter Taut. Eur. J. Immunol. 42:831–841. 10.1002/eji.201141690 [DOI] [PubMed] [Google Scholar]
- Karmaus P.W., and Chi H.. 2014. c-Myc and AP4: a relay team for metabolic reprogramming of CD8+ T cells. Nat. Immunol. 15:828–829. 10.1038/ni.2962 [DOI] [PubMed] [Google Scholar]
- Kelly A.P., Finlay D.K., Hinton H.J., Clarke R.G., Fiorini E., Radtke F., and Cantrell D.A.. 2007. Notch-induced T cell development requires phosphoinositide-dependent kinase 1. EMBO J. 26:3441–3450. 10.1038/sj.emboj.7601761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidani Y., Elsaesser H., Hock M.B., Vergnes L., Williams K.J., Argus J.P., Marbois B.N., Komisopoulou E., Wilson E.B., Osborne T.F., et al. 2013. Sterol regulatory element-binding proteins are essential for the metabolic programming of effector T cells and adaptive immunity. Nat. Immunol. 14:489–499. 10.1038/ni.2570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.W., Tchernyshyov I., Semenza G.L., and Dang C.V.. 2006. HIF-1–mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 3:177–185. 10.1016/j.cmet.2006.02.002 [DOI] [PubMed] [Google Scholar]
- Kim D., Fiske B.P., Birsoy K., Freinkman E., Kami K., Possemato R.L., Chudnovsky Y., Pacold M.E., Chen W.W., Cantor J.R., et al. 2015a. SHMT2 drives glioma cell survival in ischaemia but imposes a dependence on glycine clearance. Nature. 520:363–367. 10.1038/nature14363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M.S., Yan J., Wu W., Zhang G., Zhang Y., and Cai D.. 2015b. Rapid linkage of innate immunological signals to adaptive immunity by the brain-fat axis. Nat. Immunol. 16:525–533. 10.1038/ni.3133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike N., Yoo S.H., Huang H.C., Kumar V., Lee C., Kim T.K., and Takahashi J.S.. 2012. Transcriptional architecture and chromatin landscape of the core circadian clock in mammals. Science. 338:349–354. 10.1126/science.1226339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolev M., Dimeloe S., Le Friec G., Navarini A., Arbore G., Povoleri G.A., Fischer M., Belle R., Loeliger J., Develioglu L., et al. 2015. Complement regulates nutrient influx and metabolic reprogramming during Th1 cell responses. Immunity. 42:1033–1047. 10.1016/j.immuni.2015.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopf H., de la Rosa G.M., Howard O.M., and Chen X.. 2007. Rapamycin inhibits differentiation of Th17 cells and promotes generation of FoxP3+ T regulatory cells. Int. Immunopharmacol. 7:1819–1824. 10.1016/j.intimp.2007.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplante M., and Sabatini D.M.. 2012. mTOR signaling in growth control and disease. Cell. 149:274–293. 10.1016/j.cell.2012.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasoudris F., Cousin C., Prevost-Blondel A., Martin-Garcia N., Abd-Alsamad I., Ortonne N., Farcet J.P., Castellano F., and Molinier-Frenkel V.. 2011. IL4I1: an inhibitor of the CD8+ antitumor T-cell response in vivo. Eur. J. Immunol. 41:1629–1638. 10.1002/eji.201041119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K., Gudapati P., Dragovic S., Spencer C., Joyce S., Killeen N., Magnuson M.A., and Boothby M.. 2010. Mammalian target of rapamycin protein complex 2 regulates differentiation of Th1 and Th2 cell subsets via distinct signaling pathways. Immunity. 32:743–753. 10.1016/j.immuni.2010.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Walsh M.C., Hoehn K.L., James D.E., Wherry E.J., and Choi Y.. 2014. Regulator of fatty acid metabolism, acetyl coenzyme a carboxylase 1, controls T cell immunity. J. Immunol. 192:3190–3199. 10.4049/jimmunol.1302985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levring T.B., Hansen A.K., Nielsen B.L., Kongsbak M., von Essen M.R., Woetmann A., Odum N., Bonefeld C.M., and Geisler C.. 2012. Activated human CD4+ T cells express transporters for both cysteine and cystine. Sci. Rep. 2:266 10.1038/srep00266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B., Samanta A., Song X., Iacono K.T., Bembas K., Tao R., Basu S., Riley J.L., Hancock W.W., Shen Y., et al. 2007. FOXP3 interactions with histone acetyltransferase and class II histone deacetylases are required for repression. Proc. Natl. Acad. Sci. USA. 104:4571–4576. 10.1073/pnas.0700298104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locasale J.W. 2013. Serine, glycine and one-carbon units: cancer metabolism in full circle. Nat. Rev. Cancer. 13:572–583. 10.1038/nrc3557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochner M., Berod L., and Sparwasser T.. 2015. Fatty acid metabolism in the regulation of T cell function. Trends Immunol. 36:81–91. 10.1016/j.it.2014.12.005 [DOI] [PubMed] [Google Scholar]
- Lu C., and Thompson C.B.. 2012. Metabolic regulation of epigenetics. Cell Metab. 16:9–17. 10.1016/j.cmet.2012.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W., Zhang Y., McDonald D.O., Jing H., Carroll B., Robertson N., Zhang Q., Griffin H., Sanderson S., Lakey J.H., et al. 2014. Dual proteolytic pathways govern glycolysis and immune competence. Cell. 159:1578–1590. 10.1016/j.cell.2014.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macintyre A.N., and Rathmell J.C.. 2013. Activated lymphocytes as a metabolic model for carcinogenesis. Cancer Metab. 1:5 10.1186/2049-3002-1-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macintyre A.N., Gerriets V.A., Nichols A.G., Michalek R.D., Rudolph M.C., Deoliveira D., Anderson S.M., Abel E.D., Chen B.J., Hale L.P., and Rathmell J.C.. 2014. The glucose transporter Glut1 is selectively essential for CD4 T cell activation and effector function. Cell Metab. 20:61–72. 10.1016/j.cmet.2014.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIver N.J., Blagih J., Saucillo D.C., Tonelli L., Griss T., Rathmell J.C., and Jones R.G.. 2011. The liver kinase B1 is a central regulator of T cell development, activation, and metabolism. J. Immunol. 187:4187–4198. 10.4049/jimmunol.1100367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIver N.J., Michalek R.D., and Rathmell J.C.. 2013. Metabolic regulation of T lymphocytes. Annu. Rev. Immunol. 31:259–283. 10.1146/annurev-immunol-032712-095956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maekawa Y., Ishifune C., Tsukumo S., Hozumi K., Yagita H., and Yasutomo K.. 2015. Notch controls the survival of memory CD4+ T cells by regulating glucose uptake. Nat. Med. 21:55–61. 10.1038/nm.3758 [DOI] [PubMed] [Google Scholar]
- Magri M., Yatim A., Benne C., Balbo M., Henry A., Serraf A., Sakano S., Gazzolo L., Lévy Y., and Lelièvre J.D.. 2009. Notch ligands potentiate IL-7-driven proliferation and survival of human thymocyte precursors. Eur. J. Immunol. 39:1231–1240. 10.1002/eji.200838765 [DOI] [PubMed] [Google Scholar]
- Maillard I., Tu L., Sambandam A., Yashiro-Ohtani Y., Millholland J., Keeshan K., Shestova O., Xu L., Bhandoola A., and Pear W.S.. 2006. The requirement for Notch signaling at the beta-selection checkpoint in vivo is absolute and independent of the pre-T cell receptor. J. Exp. Med. 203:2239–2245. 10.1084/jem.20061020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maraskovsky E., O’Reilly L.A., Teepe M., Corcoran L.M., Peschon J.J., and Strasser A.. 1997. Bcl-2 can rescue T lymphocyte development in interleukin-7 receptor-deficient mice but not in mutant rag-1−/− mice. Cell. 89:1011–1019. 10.1016/S0092-8674(00)80289-5 [DOI] [PubMed] [Google Scholar]
- Masri S., Patel V.R., Eckel-Mahan K.L., Peleg S., Forne I., Ladurner A.G., Baldi P., Imhof A., and Sassone-Corsi P.. 2013. Circadian acetylome reveals regulation of mitochondrial metabolic pathways. Proc. Natl. Acad. Sci. USA. 110:3339–3344. 10.1073/pnas.1217632110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezrich J.D., Fechner J.H., Zhang X., Johnson B.P., Burlingham W.J., and Bradfield C.A.. 2010. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J. Immunol. 185:3190–3198. 10.4049/jimmunol.0903670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalek R.D., Gerriets V.A., Jacobs S.R., Macintyre A.N., MacIver N.J., Mason E.F., Sullivan S.A., Nichols A.G., and Rathmell J.C.. 2011a. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J. Immunol. 186:3299–3303. 10.4049/jimmunol.1003613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalek R.D., Gerriets V.A., Nichols A.G., Inoue M., Kazmin D., Chang C.Y., Dwyer M.A., Nelson E.R., Pollizzi K.N., Ilkayeva O., et al. 2011b. Estrogen-related receptor-α is a metabolic regulator of effector T-cell activation and differentiation. Proc. Natl. Acad. Sci. USA. 108:18348–18353. 10.1073/pnas.1108856108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto S., Murphy A.N., and Brown J.H.. 2008. Akt mediates mitochondrial protection in cardiomyocytes through phosphorylation of mitochondrial hexokinase-II. Cell Death Differ. 15:521–529. 10.1038/sj.cdd.4402285 [DOI] [PubMed] [Google Scholar]
- Mucida D., Park Y., Kim G., Turovskaya O., Scott I., Kronenberg M., and Cheroutre H.. 2007. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 317:256–260. 10.1126/science.1145697 [DOI] [PubMed] [Google Scholar]
- Munn D.H., Sharma M.D., Lee J.R., Jhaver K.G., Johnson T.S., Keskin D.B., Marshall B., Chandler P., Antonia S.J., Burgess R., et al. 2002. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science. 297:1867–1870. 10.1126/science.1073514 [DOI] [PubMed] [Google Scholar]
- Munn D.H., Sharma M.D., Baban B., Harding H.P., Zhang Y., Ron D., and Mellor A.L.. 2005. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity. 22:633–642. 10.1016/j.immuni.2005.03.013 [DOI] [PubMed] [Google Scholar]
- Muranski P., Chmielowski B., and Ignatowicz L.. 2000. Mature CD4+ T cells perceive a positively selecting class II MHC/peptide complex in the periphery. J. Immunol. 164:3087–3094. 10.4049/jimmunol.164.6.3087 [DOI] [PubMed] [Google Scholar]
- Nakaya M., Xiao Y., Zhou X., Chang J.H., Chang M., Cheng X., Blonska M., Lin X., and Sun S.C.. 2014. Inflammatory T cell responses rely on amino acid transporter ASCT2 facilitation of glutamine uptake and mTORC1 kinase activation. Immunity. 40:692–705. 10.1016/j.immuni.2014.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicklin P., Bergman P., Zhang B., Triantafellow E., Wang H., Nyfeler B., Yang H., Hild M., Kung C., Wilson C., et al. 2009. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell. 136:521–534. 10.1016/j.cell.2008.11.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie Z., Hu G., Wei G., Cui K., Yamane A., Resch W., Wang R., Green D.R., Tessarollo L., Casellas R., et al. 2012. c-Myc is a universal amplifier of expressed genes in lymphocytes and embryonic stem cells. Cell. 151:68–79. 10.1016/j.cell.2012.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurieva R.I., Chung Y., Martinez G.J., Yang X.O., Tanaka S., Matskevitch T.D., Wang Y.H., and Dong C.. 2009. Bcl6 mediates the development of T follicular helper cells. Science. 325:1001–1005. 10.1126/science.1176676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill L.A. 2014. Biochemistry: succinate strikes. Nature. 515:350–351. 10.1038/nature13941 [DOI] [PubMed] [Google Scholar]
- O’Sullivan D., and Pearce E.L.. 2014. Fatty acid synthesis tips the TH17-Treg cell balance. Nat. Med. 20:1235–1236. 10.1038/nm.3744 [DOI] [PubMed] [Google Scholar]
- O’Sullivan D., and Pearce E.L.. 2015. Targeting T cell metabolism for therapy. Trends Immunol. 36:71–80. 10.1016/j.it.2014.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan D., van der Windt G.J., Huang S.C., Curtis J.D., Chang C.H., Buck M.D., Qiu J., Smith A.M., Lam W.Y., DiPlato L.M., et al. 2014. Memory CD8+ T cells use cell-intrinsic lipolysis to support the metabolic programming necessary for development. Immunity. 41:75–88. 10.1016/j.immuni.2014.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oestreich K.J., Read K.A., Gilbertson S.E., Hough K.P., McDonald P.W., Krishnamoorthy V., and Weinmann A.S.. 2014. Bcl-6 directly represses the gene program of the glycolysis pathway. Nat. Immunol. 15:957–964. 10.1038/ni.2985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okoye I., Wang L., Pallmer K., Richter K., Ichimura T., Haas R., Crouse J., Choi O., Heathcote D., Lovo E., et al. 2015. T cell metabolism. The protein LEM promotes CD8+ T cell immunity through effects on mitochondrial respiration. Science. 348:995–1001. 10.1126/science.aaa7516 [DOI] [PubMed] [Google Scholar]
- Opitz C.A., Litzenburger U.M., Sahm F., Ott M., Tritschler I., Trump S., Schumacher T., Jestaedt L., Schrenk D., Weller M., et al. 2011. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature. 478:197–203. 10.1038/nature10491 [DOI] [PubMed] [Google Scholar]
- Öst A., and Pospisilik J.A.. 2015. Epigenetic modulation of metabolic decisions. Curr. Opin. Cell Biol. 33:88–94. 10.1016/j.ceb.2014.12.005 [DOI] [PubMed] [Google Scholar]
- Pallard C., Stegmann A.P., van Kleffens T., Smart F., Venkitaraman A., and Spits H.. 1999. Distinct roles of the phosphatidylinositol 3-kinase and STAT5 pathways in IL-7-mediated development of human thymocyte precursors. Immunity. 10:525–535. 10.1016/S1074-7613(00)80052-7 [DOI] [PubMed] [Google Scholar]
- Palmer C.S., Ostrowski M., Balderson B., Christian N., and Crowe S.M.. 2015. Glucose metabolism regulates T cell activation, differentiation, and functions. Front. Immunol. 6:1 10.3389/fimmu.2015.00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J., Kim M., Kang S.G., Jannasch A.H., Cooper B., Patterson J., and Kim C.H.. 2015. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol. 8:80–93. 10.1038/mi.2014.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patsoukis N., Bardhan K., Chatterjee P., Sari D., Liu B., Bell L.N., Karoly E.D., Freeman G.J., Petkova V., Seth P., et al. 2015. PD-1 alters T-cell metabolic reprogramming by inhibiting glycolysis and promoting lipolysis and fatty acid oxidation. Nat. Commun. 6:6692 10.1038/ncomms7692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce E.L., and Pearce E.J.. 2013. Metabolic pathways in immune cell activation and quiescence. Immunity. 38:633–643. 10.1016/j.immuni.2013.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce E.L., Walsh M.C., Cejas P.J., Harms G.M., Shen H., Wang L.S., Jones R.G., and Choi Y.. 2009. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 460:103–107. 10.1038/nature08097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce E.L., Poffenberger M.C., Chang C.H., and Jones R.G.. 2013. Fueling immunity: insights into metabolism and lymphocyte function. Science. 342:1242454 10.1126/science.1242454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson C., Silva A., and Seddon B.. 2012. Exogenous amino acids are essential for interleukin-7 induced CD8 T cell growth. [corrected]. PLoS ONE. 7:e33998 (corrected) 10.1371/journal.pone.0033998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peschon J.J., Morrissey P.J., Grabstein K.H., Ramsdell F.J., Maraskovsky E., Gliniak B.C., Park L.S., Ziegler S.F., Williams D.E., Ware C.B., et al. 1994. Early lymphocyte expansion is severely impaired in interleukin 7 receptor–deficient mice. J. Exp. Med. 180:1955–1960. 10.1084/jem.180.5.1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollizzi K.N., Patel C.H., Sun I.H., Oh M.H., Waickman A.T., Wen J., Delgoffe G.M., and Powell J.D.. 2015. mTORC1 and mTORC2 selectively regulate CD8+ T cell differentiation. J. Clin. Invest. 125:2090–2108. 10.1172/JCI77746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porstmann T., Santos C.R., Griffiths B., Cully M., Wu M., Leevers S., Griffiths J.R., Chung Y.L., and Schulze A.. 2008. SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab. 8:224–236. 10.1016/j.cmet.2008.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell J.D. 2013. Slc7a5 helps T cells get with the program. Nat. Immunol. 14:422–424. 10.1038/ni.2594 [DOI] [PubMed] [Google Scholar]
- Pua H.H., Dzhagalov I., Chuck M., Mizushima N., and He Y.W.. 2007. A critical role for the autophagy gene Atg5 in T cell survival and proliferation. J. Exp. Med. 204:25–31. 10.1084/jem.20061303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pui J.C., Allman D., Xu L., DeRocco S., Karnell F.G., Bakkour S., Lee J.Y., Kadesch T., Hardy R.R., Aster J.C., and Pear W.S.. 1999. Notch1 expression in early lymphopoiesis influences B versus T lineage determination. Immunity. 11:299–308. 10.1016/S1074-7613(00)80105-3 [DOI] [PubMed] [Google Scholar]
- Qualls J.E., Subramanian C., Rafi W., Smith A.M., Balouzian L., DeFreitas A.A., Shirey K.A., Reutterer B., Kernbauer E., Stockinger S., et al. 2012. Sustained generation of nitric oxide and control of mycobacterial infection requires argininosuccinate synthase 1. Cell Host Microbe. 12:313–323. 10.1016/j.chom.2012.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radtke F., Wilson A., Stark G., Bauer M., van Meerwijk J., MacDonald H.R., and Aguet M.. 1999. Deficient T cell fate specification in mice with an induced inactivation of Notch1. Immunity. 10:547–558. 10.1016/S1074-7613(00)80054-0 [DOI] [PubMed] [Google Scholar]
- Ramsay G., and Cantrell D.. 2015. Environmental and metabolic sensors that control T cell biology. Front. Immunol. 6:99 10.3389/fimmu.2015.00099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao R.R., Li Q., Odunsi K., and Shrikant P.A.. 2010. The mTOR kinase determines effector versus memory CD8+ T cell fate by regulating the expression of transcription factors T-bet and Eomesodermin. Immunity. 32:67–78. 10.1016/j.immuni.2009.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rath M., Müller I., Kropf P., Closs E.I., and Munder M.. 2014. Metabolism via arginase or nitric oxide synthase: two competing arginine pathways in macrophages. Front. Immunol. 5:532 10.3389/fimmu.2014.00532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathmell J.C., Vander Heiden M.G., Harris M.H., Frauwirth K.A., and Thompson C.B.. 2000. In the absence of extrinsic signals, nutrient utilization by lymphocytes is insufficient to maintain either cell size or viability. Mol. Cell. 6:683–692. 10.1016/S1097-2765(00)00066-6 [DOI] [PubMed] [Google Scholar]
- Rathmell J.C., Farkash E.A., Gao W., and Thompson C.B.. 2001. IL-7 enhances the survival and maintains the size of naive T cells. J. Immunol. 167:6869–6876. 10.4049/jimmunol.167.12.6869 [DOI] [PubMed] [Google Scholar]
- Restifo N.P., Dudley M.E., and Rosenberg S.A.. 2012. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat. Rev. Immunol. 12:269–281. 10.1038/nri3191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey G., and Reddy A.B.. 2013. Protein acetylation links the circadian clock to mitochondrial function. Proc. Natl. Acad. Sci. USA. 110:3210–3211. 10.1073/pnas.1300419110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Borlado L., Barber D.F., Hernández C., Rodríguez-Marcos M.A., Sánchez A., Hirsch E., Wymann M., Martínez-A C., and Carrera A.C.. 2003. Phosphatidylinositol 3-kinase regulates the CD4/CD8 T cell differentiation ratio. J. Immunol. 170:4475–4482. 10.4049/jimmunol.170.9.4475 [DOI] [PubMed] [Google Scholar]
- Rolf J., Zarrouk M., Finlay D.K., Foretz M., Viollet B., and Cantrell D.A.. 2013. AMPKα1: a glucose sensor that controls CD8 T-cell memory. Eur. J. Immunol. 43:889–896. 10.1002/eji.201243008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron-Harel N., Sharpe A.H., and Haigis M.C.. 2015. Mitochondrial metabolism in T cell activation and senescence: a mini-review. Gerontology. 61:131–138. 10.1159/000362502 [DOI] [PubMed] [Google Scholar]
- Saint-Ruf C., Ungewiss K., Groettrup M., Bruno L., Fehling H.J., and von Boehmer H.. 1994. Analysis and expression of a cloned pre-T cell receptor gene. Science. 266:1208–1212. 10.1126/science.7973703 [DOI] [PubMed] [Google Scholar]
- Santarlasci V., Maggi L., Capone M., Querci V., Beltrame L., Cavalieri D., D’Aiuto E., Cimaz R., Nebbioso A., Liotta F., et al. 2012. Rarity of human T helper 17 cells is due to retinoic acid orphan receptor-dependent mechanisms that limit their expansion. Immunity. 36:201–214. 10.1016/j.immuni.2011.12.013 [DOI] [PubMed] [Google Scholar]
- Scarlata C.M., Celse C., Pignon P., Ayyoub M., and Valmori D.. 2015. Differential expression of the immunosuppressive enzyme IL4I1 in human induced Aiolos+, but not natural Helios+, FOXP3+ Treg cells. Eur. J. Immunol. 45:474–479. 10.1002/eji.201444897 [DOI] [PubMed] [Google Scholar]
- Schoors S., Bruning U., Missiaen R., Queiroz K.C., Borgers G., Elia I., Zecchin A., Cantelmo A.R., Christen S., Goveia J., et al. 2015. Fatty acid carbon is essential for dNTP synthesis in endothelial cells. Nature. 520:192–197. 10.1038/nature14362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sena L.A., Li S., Jairaman A., Prakriya M., Ezponda T., Hildeman D.A., Wang C.R., Schumacker P.T., Licht J.D., Perlman H., et al. 2013. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity. 38:225–236. 10.1016/j.immuni.2012.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheiness D., Fanshier L., and Bishop J.M.. 1978. Identification of nucleotide sequences which may encode the oncogenic capacity of avian retrovirus MC29. J. Virol. 28:600–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L.Z., Wang R., Huang G., Vogel P., Neale G., Green D.R., and Chi H.. 2011. HIF1α-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J. Exp. Med. 208:1367–1376. 10.1084/jem.20110278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha S., Yang K., Wei J., Karmaus P.W., Neale G., and Chi H.. 2014. Tsc1 promotes the differentiation of memory CD8+ T cells via orchestrating the transcriptional and metabolic programs. Proc. Natl. Acad. Sci. USA. 111:14858–14863. 10.1073/pnas.1404264111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha S., Yang K., Guy C., Vogel P., Neale G., and Chi H.. 2015. Treg cells require the phosphatase PTEN to restrain TH1 and TFH cell responses. Nat. Immunol. 16:178–187. 10.1038/ni.3076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sido B., Braunstein J., Breitkreutz R., Herfarth C., and Meuer S.C.. 2000. Thiol-mediated redox regulation of intestinal lamina propria T lymphocytes. J. Exp. Med. 192:907–912. 10.1084/jem.192.6.907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikalidis A.K. 2015. Amino acids and immune response: a role for cysteine, glutamine, phenylalanine, tryptophan and arginine in T-cell function and cancer? Pathol. Oncol. Res. 21:9–17. 10.1007/s12253-014-9860-0 [DOI] [PubMed] [Google Scholar]
- Sinclair L.V., Rolf J., Emslie E., Shi Y.B., Taylor P.M., and Cantrell D.A.. 2013. Control of amino-acid transport by antigen receptors coordinates the metabolic reprogramming essential for T cell differentiation. Nat. Immunol. 14:500–508. 10.1038/ni.2556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P.M., Howitt M.R., Panikov N., Michaud M., Gallini C.A., Bohlooly-Y M., Glickman J.N., and Garrett W.S.. 2013. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 341:569–573. 10.1126/science.1241165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer S.P., Wilhelm C., Yang Q., Hall J.A., Bouladoux N., Boyd A., Nutman T.B., Urban J.F. Jr, Wang J., Ramalingam T.R., et al. 2014. Adaptation of innate lymphoid cells to a micronutrient deficiency promotes type 2 barrier immunity. Science. 343:432–437. 10.1126/science.1247606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepensky P., Rensing-Ehl A., Gather R., Revel-Vilk S., Fischer U., Nabhani S., Beier F., Brümmendorf T.H., Fuchs S., Zenke S., et al. 2015. Early-onset Evans syndrome, immunodeficiency, and premature immunosenescence associated with tripeptidyl-peptidase II deficiency. Blood. 125:753–761. 10.1182/blood-2014-08-593202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukumar M., Liu J., Ji Y., Subramanian M., Crompton J.G., Yu Z., Roychoudhuri R., Palmer D.C., Muranski P., Karoly E.D., et al. 2013. Inhibiting glycolytic metabolism enhances CD8+ T cell memory and antitumor function. J. Clin. Invest. 123:4479–4488. 10.1172/JCI69589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C.M., Hall J.A., Blank R.B., Bouladoux N., Oukka M., Mora J.R., and Belkaid Y.. 2007. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J. Exp. Med. 204:1775–1785. 10.1084/jem.20070602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surh C.D., and Sprent J.. 2008. Homeostasis of naive and memory T cells. Immunity. 29:848–862. 10.1016/j.immuni.2008.11.002 [DOI] [PubMed] [Google Scholar]
- Swainson L., Kinet S., Manel N., Battini J.L., Sitbon M., and Taylor N.. 2005. Glucose transporter 1 expression identifies a population of cycling CD4+ CD8+ human thymocytes with high CXCR4-induced chemotaxis. Proc. Natl. Acad. Sci. USA. 102:12867–12872. 10.1073/pnas.0503603102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamás P., Hawley S.A., Clarke R.G., Mustard K.J., Green K., Hardie D.G., and Cantrell D.A.. 2006. Regulation of the energy sensor AMP-activated protein kinase by antigen receptor and Ca2+ in T lymphocytes. J. Exp. Med. 203:1665–1670. 10.1084/jem.20052469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J.T., Dudl E., LeRoy E., Murray R., Sprent J., Weinberg K.I., and Surh C.D.. 2001. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc. Natl. Acad. Sci. USA. 98:8732–8737. 10.1073/pnas.161126098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannahill G.M., Curtis A.M., Adamik J., Palsson-McDermott E.M., McGettrick A.F., Goel G., Frezza C., Bernard N.J., Kelly B., Foley N.H., et al. 2013. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature. 496:238–242. 10.1038/nature11986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao R., de Zoeten E.F., Ozkaynak E., Chen C., Wang L., Porrett P.M., Li B., Turka L.A., Olson E.N., Greene M.I., et al. 2007. Deacetylase inhibition promotes the generation and function of regulatory T cells. Nat. Med. 13:1299–1307. 10.1038/nm1652 [DOI] [PubMed] [Google Scholar]
- Tarasenko T.N., Gomez-Rodriguez J., and McGuire P.J.. 2015. Impaired T cell function in argininosuccinate synthetase deficiency. J. Leukoc. Biol. 97:273–278. 10.1189/jlb.1AB0714-365R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoreen C.C., Kang S.A., Chang J.W., Liu Q., Zhang J., Gao Y., Reichling L.J., Sim T., Sabatini D.M., and Gray N.S.. 2009. An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J. Biol. Chem. 284:8023–8032. 10.1074/jbc.M900301200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurnher M., and Gruenbacher G.. 2015. T lymphocyte regulation by evalonate metabolism. Sci. Signal. 8:re4 10.1126/scisignal.2005970 [DOI] [PubMed] [Google Scholar]
- Turrens J.F. 2003. Mitochondrial formation of reactive oxygen species. J. Physiol. 552:335–344. 10.1113/jphysiol.2003.049478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyttenhove C., Pilotte L., Théate I., Stroobant V., Colau D., Parmentier N., Boon T., and Van den Eynde B.J.. 2003. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat. Med. 9:1269–1274. 10.1038/nm934 [DOI] [PubMed] [Google Scholar]
- van der Windt G.J., and Pearce E.L.. 2012. Metabolic switching and fuel choice during T-cell differentiation and memory development. Immunol. Rev. 249:27–42. 10.1111/j.1600-065X.2012.01150.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Windt G.J., Everts B., Chang C.H., Curtis J.D., Freitas T.C., Amiel E., Pearce E.J., and Pearce E.L.. 2012. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity. 36:68–78. 10.1016/j.immuni.2011.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Windt G.J., O’Sullivan D., Everts B., Huang S.C., Buck M.D., Curtis J.D., Chang C.H., Smith A.M., Ai T., Faubert B., et al. 2013. CD8 memory T cells have a bioenergetic advantage that underlies their rapid recall ability. Proc. Natl. Acad. Sci. USA. 110:14336–14341. 10.1073/pnas.1221740110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Heerden J.H., Wortel M.T., Bruggeman F.J., Heijnen J.J., Bollen Y.J., Planqué R., Hulshof J., O’Toole T.G., Wahl S.A., and Teusink B.. 2014. Lost in transition: start-up of glycolysis yields subpopulations of nongrowing cells. Science. 343:1245114 10.1126/science.1245114 [DOI] [PubMed] [Google Scholar]
- van Loosdregt J., Vercoulen Y., Guichelaar T., Gent Y.Y., Beekman J.M., van Beekum O., Brenkman A.B., Hijnen D.J., Mutis T., Kalkhoven E., et al. 2010. Regulation of Treg functionality by acetylation-mediated Foxp3 protein stabilization. Blood. 115:965–974. 10.1182/blood-2009-02-207118 [DOI] [PubMed] [Google Scholar]
- Vander Heiden M.G., Cantley L.C., and Thompson C.B.. 2009. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 324:1029–1033. 10.1126/science.1160809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veal E.A., Day A.M., and Morgan B.A.. 2007. Hydrogen peroxide sensing and signaling. Mol. Cell. 26:1–14. 10.1016/j.molcel.2007.03.016 [DOI] [PubMed] [Google Scholar]
- von Freeden-Jeffry U., Vieira P., Lucian L.A., McNeil T., Burdach S.E., and Murray R.. 1995. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J. Exp. Med. 181:1519–1526. 10.1084/jem.181.4.1519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R., and Green D.R.. 2012. Metabolic checkpoints in activated T cells. Nat. Immunol. 13:907–915. 10.1038/ni.2386 [DOI] [PubMed] [Google Scholar]
- Wang R., Dillon C.P., Shi L.Z., Milasta S., Carter R., Finkelstein D., McCormick L.L., Fitzgerald P., Chi H., Munger J., and Green D.R.. 2011. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity. 35:871–882. 10.1016/j.immuni.2011.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellen K.E., and Thompson C.B.. 2012. A two-way street: reciprocal regulation of metabolism and signalling. Nat. Rev. Mol. Cell Biol. 13:270–276. [DOI] [PubMed] [Google Scholar]
- Wieman H.L., Wofford J.A., and Rathmell J.C.. 2007. Cytokine stimulation promotes glucose uptake via phosphatidylinositol-3 kinase/Akt regulation of Glut1 activity and trafficking. Mol. Biol. Cell. 18:1437–1446. 10.1091/mbc.E06-07-0593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M.A., and Bevan M.J.. 2007. Effector and memory CTL differentiation. Annu. Rev. Immunol. 25:171–192. 10.1146/annurev.immunol.25.022106.141548 [DOI] [PubMed] [Google Scholar]
- Wofford J.A., Wieman H.L., Jacobs S.R., Zhao Y., and Rathmell J.C.. 2008. IL-7 promotes Glut1 trafficking and glucose uptake via STAT5-mediated activation of Akt to support T-cell survival. Blood. 111:2101–2111. 10.1182/blood-2007-06-096297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Araki K., Li S., Han J.H., Ye L., Tan W.G., Konieczny B.T., Bruinsma M.W., Martinez J., Pearce E.L., et al. 2014. Autophagy is essential for effector CD8+ T cell survival and memory formation. Nat. Immunol. 15:1152–1161. 10.1038/ni.3025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K., Neale G., Green D.R., He W., and Chi H.. 2011. The tumor suppressor Tsc1 enforces quiescence of naive T cells to promote immune homeostasis and function. Nat. Immunol. 12:888–897. 10.1038/ni.2068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K., Shrestha S., Zeng H., Karmaus P.W., Neale G., Vogel P., Guertin D.A., Lamb R.F., and Chi H.. 2013. T cell exit from quiescence and differentiation into Th2 cells depend on Raptor-mTORC1-mediated metabolic reprogramming. Immunity. 39:1043–1056. 10.1016/j.immuni.2013.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y., Choi S.C., Xu Z., Perry D.J., Seay H., Croker B.P., Sobel E.S., Brusko T.M., and Morel L.. 2015. Normalization of CD4+ T cell metabolism reverses lupus. Sci. Transl. Med. 7:274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssef S., Stüve O., Patarroyo J.C., Ruiz P.J., Radosevich J.L., Hur E.M., Bravo M., Mitchell D.J., Sobel R.A., Steinman L., and Zamvil S.S.. 2002. The HMG-CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature. 420:78–84. 10.1038/nature01158 [DOI] [PubMed] [Google Scholar]
- Yu X.X., Lewin D.A., Forrest W., and Adams S.H.. 2002. Cold elicits the simultaneous induction of fatty acid synthesis and beta-oxidation in murine brown adipose tissue: prediction from differential gene expression and confirmation in vivo. FASEB J. 16:155–168. 10.1096/fj.01-0568com [DOI] [PubMed] [Google Scholar]
- Yu Q., Erman B., Bhandoola A., Sharrow S.O., and Singer A.. 2003. In vitro evidence that cytokine receptor signals are required for differentiation of double-positive thymocytes into functionally mature CD8+ T cells. J. Exp. Med. 197:475–487. 10.1084/jem.20021765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X., Rollins D., Ruhn K.A., Stubblefield J.J., Green C.B., Kashiwada M., Rothman P.B., Takahashi J.S., and Hooper L.V.. 2013. TH17 cell differentiation is regulated by the circadian clock. Science. 342:727–730. 10.1126/science.1243884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng H., Yang K., Cloer C., Neale G., Vogel P., and Chi H.. 2013. mTORC1 couples immune signals and metabolic programming to establish T(reg)-cell function. Nature. 499:485–490. 10.1038/nature12297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y., Delgoffe G.M., Meyer C.F., Chan W., and Powell J.D.. 2009. Anergic T cells are metabolically anergic. J. Immunol. 183:6095–6101. 10.4049/jimmunol.0803510 [DOI] [PMC free article] [PubMed] [Google Scholar]