Abstract
The phagocyte–microbe interactions in the immune system is a defense mechanism but when excessively or inappropriately deployed can harm host tissues and participate in the development of different non-immune and immune chronic inflammatory diseases such as autoimmune problems, allergies, some rheumatoid disorders, cancers and others. Immunodrugs include organic synthetics, biological agents such as cytokines and antibodies acting on single targets or pathways have been used to treat immune-related diseases but with limited success. Most of immunostimulants and immunosuppressants in clinical use are the cytotoxic drugs which possess serious side effects. There is a growing interest to use herbal medicines as multi-component agents to modulate the complex immune system in the prevention of infections rather than treating the immune-related diseases. Many therapeutic effects of plant extracts have been suggested to be due to their wide array of immunomodulatory effects and influence on the immune system of the human body. Phytochemicals such as flavonoids, polysaccharides, lactones, alkaloids, diterpenoids and glycosides, present in several plants, have been reported to be responsible for the plants immunomodulating properties. Thus the search for natural products of plant origin as new leads for development of potent and safe immunosuppressant and immunostimulant agents is gaining much major research interest. The present review will give an overview of widely investigated plant-derived compounds (curcumin, resveratrol, epigallocatechol-3-gallate, quercetin, colchicine, capsaicin, andrographolide, and genistein) which have exhibited potent effects on cellular and humoral immune functions in pre-clinical investigations and will highlight their clinical potential.
Keywords: immunomodulation, curcumin, resveratrol, epigallocatechol-3-gallate, quercetin, colchicine, capsaicin
Introduction
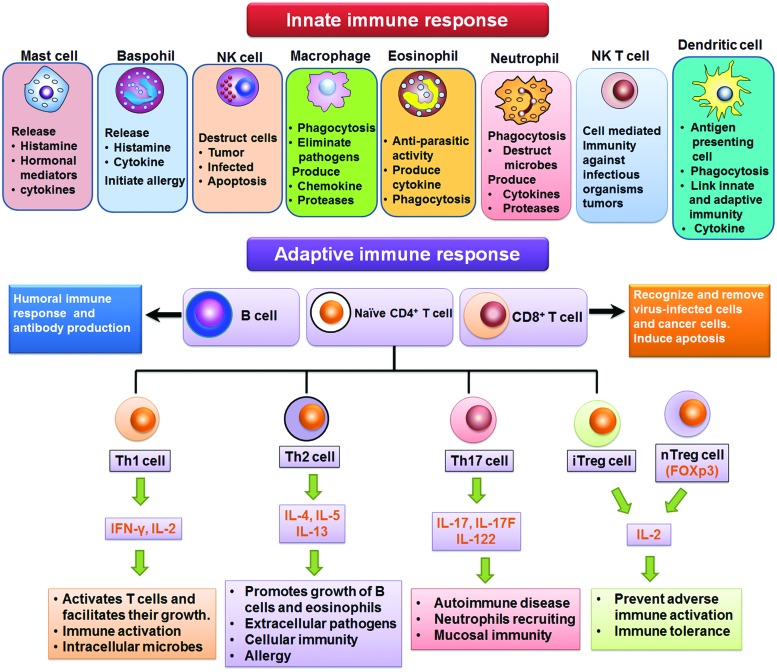
Immunity is the body’s natural defense system against various infectious diseases. The factors which trigger immunity include previous infection, immunization, and various external stimuli (Baxter, 2007). Besides, immunity is capable of discriminating among body’s own proteins/cells and foreign entities. As soon as the foreign particle is identified, the collective and coordinated response of specific cells and mediators against strange substances constitutes the immune response (Baxter, 2007). Based on the function, immune system has been categorized in two broad categories, i.e., innate immune system (non-specific immune system) and adaptive immune system (specific or acquired immune system; Vesely et al., 2011). The microbiological, chemical and physical barriers are also sometimes included in innate immunity, however, the main mediators of immune system which deliver instant defense include cytokines, acute phase proteins, macrophages, monocytes, complement, and neutrophils. Various distinct moieties expressed by pathogens, known as pathogen-associated molecular patterns (PAMPs), are recognized by host to detect presence of a pathogen. The germline-encoded and evolutionarily conserved host sensors known as pattern recognition receptors (PRRs) recognize the PAMPs. Once the PRRs recognize the PAMPs, an array of immune responses are quickly triggered via induction of different type I interferons, chemokines, and cytokines. An important role in host’s defense is played by PRRs families such as DNA receptors (cytosolic sensors for DNA), NOD-like receptors, RIG-I-like receptors and toll-like receptors (Parkin and Cohen, 2001). All phases of non-specific immunity include antigen-presenting cells and macrophages which play pivotal roles in antibody-dependent cell-mediated cytotoxicity, secretion of cytokines, nitric oxide (NO) production and antigen presentation, processing and phagocytosis. Dendritic cells are responsible for the activation of naïve and memory B and naïve T cells. During various phases of dendritic cells’ differentiation, the effectors of innate immunity including natural killer (NK) cells are regulated, which govern specific and natural immune responses by producing tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ) and granulocyte–macrophage colony-stimulating factor (GM-CSF; Moradali et al., 2007). Complement system is the tertiary relevant component of innate immunity. The complement system is the humoral immunity’s main effector among all the physiological systems of host defense (Oh et al., 2000). C3a and C3b (complement system’s components) are activated by C9, and amplify and mediate immune response.
Adaptive immunity is acquired by generating pathogen (antigen)-specific B and T lymphocytes through a gene rearrangement process. The exposure of body to antigen with aim to produce adaptive immune reaction that develops in weeks/months but may last through whole life is called active immunity. The active immunity may either be acquired or natural. The immune system of higher animals is equipped with adaptive immunity. The antigen specific reactions (via B and T lymphoctyes) are involved in adaptive immunity. The strong phagocytic action of myeloid cells and cytotoxic T lymphocytes is enhanced by Th1 lymphocytes which produce TNF-α, IFN-γ, and IL-2. The IL-4, IL-5, and IL-10 are produced by Th2 lymphocytes (which are the mediators of humoral immunity), categorized by B lymphocytes-mediated production of antibodies. The toxins or microorganisms are neutralized after binding with the antibodies. Moreover, antibodies have the ability to opsonize different pathogens, immobilize bacteria, and trigger microorganisms’ destruction by phagocytes via activation of complement proteins (Spellberg and Edwards, 2001). The cell types involved in innate and adaptive immune responses are summarized in Figure 1.
FIGURE 1.
Cells of innate and adaptive immune response.
Immunomodulators
In healthy organism, the immune system maintains homeostasis within the body. The function and efficiency of the immune system are influenced by various exogenous and endogenous factors resulting in either immunosuppression or immunostimulation. Several agents possessing an activity to normalize or modulate pathophysiological processes are called immunomodulators (Puri et al., 1994). The biomolecules of synthetic or biological origin capable of modulating, suppressing and stimulating any components of adaptive or innate immunity are known as immunomodulators, immunorestoratives, immunoaugmentors, or biological response modifiers. Immunomodulators are generally categorized into immunoadjuvants, immunostimulants, and immunosuppressants in clinical practice. Immunoadjuvants are specific immune stimulators which enhance the efficacy of vaccine. Agents that activate or induce the mediators or components of immune system are called as immunostimulants. The resistance against autoimmunity, cancer, allergy, and infection is enhanced by immunostimulants. On the other hand, immunosuppressants are the molecules that inhibit the immune system, can be used to control the pathological immune reaction subsequent to organ transplantation. Additionally, these agents can also be used in the treatment of infection-associated immunopathology, hypersensitivity reactions, and autoimmune diseases. A number of monoclonal antibodies and chemically synthesized compounds are also being used as immunomodulators. However, there are major limitations to the general use of these agents which are summarized in Table 1. Therefore, immunomodulatory entities with additional safety and effectiveness are still in need. Due to the occurrence of chemical drugs-related adverse effects, natural immunomodulators are the potential agents to replace them in therapeutic regimens.
Table 1.
Mechanism of action and side effects of immunomodulatory agents.
| Immunomodulators | Category | Example | Mechanism | Main side effects | Reference |
|---|---|---|---|---|---|
| Immunosuppressant | Inhibitors of lymphocyte gene expression. | Glucocorticoids | Decreased leukocytes extravasation. Reduce pro-inflammatory cytokines expression. | Growth retardation in children, bone necrosis, osteopenia, hyperglycemia, and hypertension. | Golan (2008), Jain (2008) |
| Inhibitors of lymphocyte signaling | Cyclosporine | Antigen-triggered signal transduction constrain in T lymphocytes. Reduce expression of lymphokines and anti-apoptotic proteins. |
Renal dysfunction, gum hyperplasia, hyperuricemia, hyper-cholesterolemia, diabetogenic. | Jain (2008), Tortora and Derrickson (2008) | |
| Tacrolimus | Inhibits T-cell activation by inhibiting calcineurin. | Nephrotoxicity, neurotoxicity hypertension, hyperkalemia, hyperglycemia. | Singhal (2007), Sengupta (2009) | ||
| Sirolimus | Inhibit activation and proliferation of T-lymphocyte. Down regulate T-cell growth factor receptor and IL-2. |
Level of serum cholesterol and triglycerides increase. Impaired renal functions, prolong delayed graft function. |
Katzung and Trevor (2009), Gilman’s (2008) | ||
| Cytotoxic agents | Azathioprine, azathioprine sodium | Inhibit de novo synthesis of purine which leads to inhibit proliferation of lymphocytes. | Leukopenia, thrombocytopenia, hepatotoxicity, alopecia, GI toxicity, pancreatitis. | Katzung and Trevor (2009) | |
| Mycophenolatmofetil | Inhibit de novo synthesis of guanine by inhibiting inosine monophosphate dehydrogenase. | Leukopenia, diarrhea, vomiting, sepsis associated with cytomegalovirus. | Rang and Dale (2007), Richard and Pamela (2009) | ||
| Alkylating agent | Cyclophosphamide | Prevent the cell division and protein synthesis by cross linking in the strands of DNA. | Pancytopenia and hemorrhagic cystitis, graft versus–host disease syndrome, cardiac toxicity, electrolyte disturbances | Mythili et al. (2004) | |
| Cytokine inhibitors | Etanercept, infliximab, adalimumab, anakinra, daclizumab, basiliximab | Bind with tumor necrosis factor-alpha and inhibit TNFα to bind with TNFα receptors. | Reactivation of tuberculosis, psoriasis, invasive fungal infections, hypersensitivity, and anaphylaxis. | Keane et al. (2001), Baudouin et al. (2003), Bartelds et al. (2011) | |
| Antibodies against specific immune cell molecules | Antithymocyte globulin | Reduce circulating lymphocytes by inducing cytotoxicity. To inhibit lymphocytes functions, it binds with cell surface molecules which regulate cell functions. |
Fever, chills, hypotension, serum sickness, glomerulonephritis, leukopenia, and thrombocytopenia. | Hansel et al. (2010) | |
| Muromunab | Prevent subsequent antigen recognition by causing internalization of the T-cell receptor. | Cytokine release syndrome, high fever, chills/rigor, myalgias, arthralgias, aseptic meningitis, cardiovascular collapse, cardiac arrest. | Sgro (1995), Katzung and Trevor (2009), Liu and Li (2014) | ||
| Intercellular adhesion molecules inhibitors | Efalizumab | Inhibit T-cell adhesion and trafficking by inhibiting LFA-1-ICAM interaction. | Bacterial sepsis, viral meningitis, invasive fungal disease, and progressive multifocal leukoencephalopathy. | Berger et al. (2009) | |
| Immunostimu-lants | Imidazothiazole derivative | Levamisole | Repair the suppressed immune function of B and T lymphocytes, monocytes, and macrophages. | Flu-like symptoms, allergic manifestation, nausea, and muscle pain. | Auffenberg et al. (2013) |
| Recombinant cytokines | Aldesleukin, interferon alpha, interferon gamma. | Inhibit cell proliferation and enhance immune cells activities such as increased macrophages phagocytosis and T lymphocytes cytotoxicity. | Hypotension, arrhythmias, cardiomyopathy myocardial infarction, GI distress, and anorexia. | Katzung and Trevor (2009), Strayer and Carter (2012) | |
| Hormonal analog | Isoprinosine | Enhance cytokines (i.e., IL-1, IL-2, and IFN-γ) production. It induces lymphocytes proliferation. | CNS depressant, transient nausea, and increase uric acid level in serum and urine. | Campoli-Richards et al. (1986), Parnham and Nijkamp (2005) |
Presently, majority of research and development still focuses on biochemicals, biologics, or single compounds as lead compounds that aim at particular targets linked with a disease. It is difficult to attain single compound chemicals with high selectivity and potency, and low toxicity for targeted molecular/cellular targets and diseases. Hence, the design and development of drug candidates from numerous conventional or complementary and alternative medicines is gaining interest. The prevention and treatment of disease using plant-based medicines has been reported in human history. In all cultures and through all ages different parts of a huge number of plants were used as drugs against all kinds of ailments. Vinblastine, vincristine and their semi-synthetic derivatives isolated from the Madagascar periwinkle (Catharanthus roseus), capsaicin from chili peppers (Capsicum species), paclitaxel from Pacific yew (Taxus brevifolia) and galantamine from the Caucasian snowdrop (Galanthus caucasicus) are examples of medicines based on plant compounds. The plant-based compounds that served as lead structures and/or were chemically altered are dicoumarol (warfarin), artemisinin (artemether), camptothecin (topotecan and irinotecan), morphine (scores of derivatives), and salicylic acid (acetylsalicylic acid; Oberlies and Kroll, 2004).
Recently the clinical potential of six plant-derived antiinflammatory compounds: curcumin, colchicine, resveratrol, capsaicin, epigallocatechin-3-gallate (EGCG), and quercetin has been highlighted in Fürst and Zündorf (2014). The present review will give an overview of these widely investigated plant-derived compounds including andrographolide and genistein, which have exhibited potent effects on cellular and humoral immune functions in pre-clinical investigations and will highlight their clinical potential.
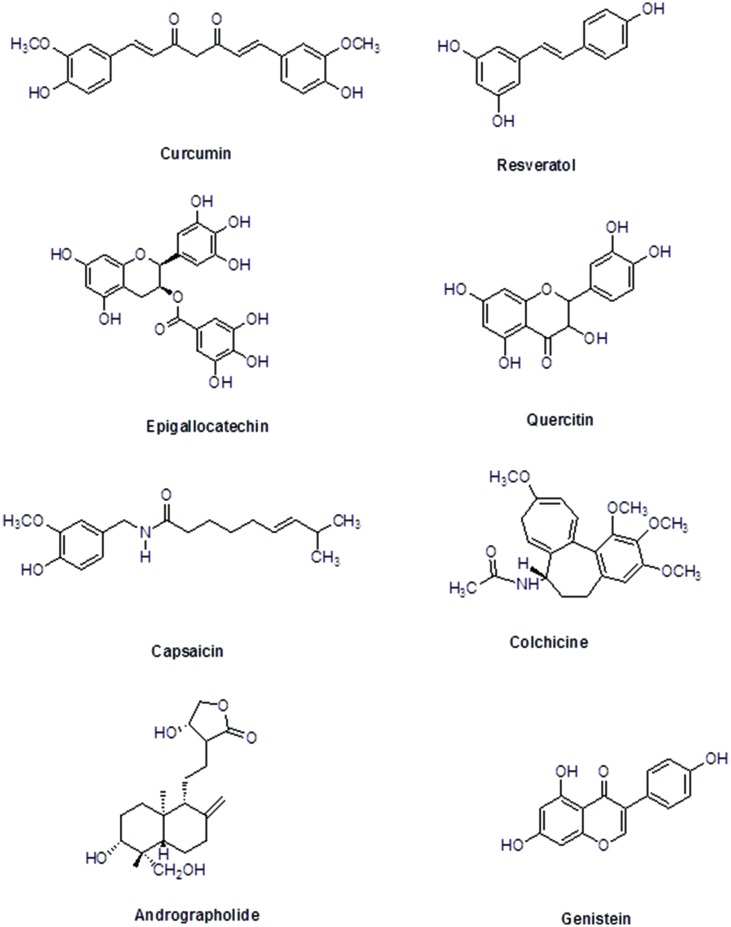
The immunomodulatory characteristics of plant-based therapeutics have gathered attention of researchers. Innovative technologies and the excessive research on immunomodulatory natural products, plants, their extracts, and their active moieties with immunomodulatory potential, may provide us with valuable entities to develop as novel immunomodulatory agents to supplement the present chemotherapies. This review focuses on the plant-based immunomodulatory compounds undergoing clinical trials. Additionally, the potential use as immunomodulatory agents, modes of action and plant origin of numerous important plant-based lead compounds has also been comprehensively discussed (Figure 2). Beside these compounds, other phytochemicals including essential oils, steroids, terpenoids, phenolics, pigments, flavonoids, and alkaloids, etc. have exhibited worth mentioning immunomodulatory effect. Plant-derived compounds showing promising potential as immunomodulatory agents are listed in Table 2.
FIGURE 2.
Chemical structures of selected plant-derived immunomodulators in clinical trials.
Table 2.
Plant derived immunomodulatory agents.
| Chemical class | Plant source | Mechanism | Reference |
|---|---|---|---|
| Alkaloids | |||
| Berberine | Coptis chinensis Franch | Down-regulate T-helper cells cytokines [Th1 (TNF-α, IL-2), and Th2 (IL-4)] production. | Lin and Lin (2011) |
| Chelerythrine | Chelidonium majus L. | Inhibit PGE2 release by regulating cyclooxygenase-2 activity | Niu et al. (2011) |
| Gelselegine | Gelsemium elegans | Inhibited T lymphocyte proliferation. | Xu et al. (2012) |
| Pseudocoptisine | Corydalis turtschaninovii Besser | Suppressing the phosphorylation of ERK and p38 to inhibit NF-κB activation, which resulted in reduction of pro-inflammatory mediators level (iNOS, COX-2, TNF-α, and IL-6). | Yun et al. (2009) |
| Leonurine | Leonurus japonicas Houtt | Downregulate TNF-α, IL-6, iNOS, and COX-2 and upregulate IL-10 by inhibiting the expression of toll like receptors and the activation of NF-κB. Inhibit the ICAM-1, VCAM-1 activity. | Liu et al. (2012), Song et al. (2015) |
| Piperine | Piper longum Linn | Reduce level of pro inflammatory cytokines IL-1β, IL-6, and TNF-α. Down regulate expression of COX-2, NOS-2, and NF-κB. Inhibit eicosanoide generation by inhibiting phospholipase A2 and TXA2 synthase activity. |
Vaibhav et al. (2012), Son et al. (2014) |
| Sinomenine | Sinomenium acutum (Thunb.) Rehd.etWils | Inhibit the production and release of inflammatory cytokine. Inhibit expression of VCAM-1. |
Chen et al. (2011), Oh et al. (2012) |
| Koumine | Gelsemium elegans | Inhibit T lymphocyte proliferation. | Xu et al. (2012) |
| Lycorine | Lycoris radiate | Inhibit iNOS and COX-2 activity. | Kang et al. (2012) |
| Sophocarpine | Sophora alopecuroides L. | Inhibit production of NO and pro inflammatory cytokines TNF-α and IL-6. Inhibit expression of iNOS and COX-2. | Zhang et al. (2008), Gao et al. (2012) |
| Rhynchophylline | Uncaria rhynchophylla (Miq.) Jack | Inhibit phosphorylation of mitogen-activated protein kinases. Inhibit production of pro inflammatory cytokines, NO, PGE2, monocyte chemoattractant protein MCP-1, TNF-α, IL-1β. |
Song et al. (2012) |
| Tetrandrine | Stephania tetrandra | Wu et al. (2011) | |
| Matrine | Sophora flavescens Ait | Reduced production of reactive oxygen species inflammatory mediators. Inhibit myeloperoxidase and maleic dialdehyde activity. |
Zhang et al. (2011) |
| Essential oils | |||
| Z-ligustilide | Angelica sinensis (Oliv.) Diels | Inhibit iNOS and COX-2 induction by regulating the NF-kB and MAPK signal pathways. | Chung et al. (2012) |
| Tetramethylpyra-zine | Ligusticum chuanxiong Hort | Inhibit pro inflammatory cytokines and reactive oxygen species production. Inhibit macrophages chemotaxis, neutrophile infiltration, and nitric oxide synthase activity. Block the phosphorylation of p38 mitogen-activated protein kinase. |
Liu et al. (2010), Hu et al. (2013) |
| Flavonoids | |||
| Chalcone | |||
| Butein | Semecarpus anacardium, Dalbergia odorifera, Toxicodendron vernicifluum | Suppress NO production by attenuating iNOS expression. Inhibit translocation of NF-κB. |
Wang et al. (2014) |
| Xanthohumol | Humulus lupulus | Inhibit NO production which is induced by LPS and INF-γ. | Zhao et al. (2003) |
| Dihydroxanthohumol | Humulus lupulus | Inhibit NO production which is induced by LPS and INF-γ. | Zhao et al. (2003) |
| Mallotophilippens C, D, E | Mallotus, Philippinensis | Inhibit mRNA gene expression of iNOS, COX-2, IL-6, and IL-1β. Inactivate NF-κB. |
Daikonya et al. (2004) |
| Licochalcone E | Glycyrrhiza inflata | Inhibit secretion of pro-inflammatory cytokines IL-1β, IL-6, and TNF-α by inhibiting the activity of NF-κB and activator protein (AP-1). | Lee et al. (2013a) |
| Flavones | |||
| Luteolin |
Lonicera Japonica |
Decreased secretion of inflammatory mediators (INF-γ, IL-6) reduced COX-2, ICAM-1 expression. Block heat shock protein 90 activity. |
Chen et al. (2007, 2014), Ziyan et al. (2007) |
| Apigenin | Cynodon dactylon, Salvia officinalis L., Portulaca oleracea, Mentha longifolia | Downregulate expression of cytokines (IL-1α, TNF-α, IL-8). Decreased response of Th1 and Th17 cells. Downregulate the expression of COX-2 and iNOS. Decreased expression of ICAM and VCAM leading to decreased neutrophile Chemotaxis. |
Nicholas et al. (2007), Kang et al. (2009) |
| Chrysin | Picea crassifolia | Inhibited production of pro-inflammatory cytokine (TNFα, IL-1β, and IL-6). By modulation of intracellular calcium reduce histamine release from mast cells. |
Hougee et al. (2005), Shin et al. (2009), Bae et al. (2011) |
| Nobiletin | Citrus nobilis Lour, Citrus aurantium L. | Inhibit pro-inflammatory mediators, COX-2 and iNOS expression by blocking NF-κB and MAPK signaling pathway. | Kang et al. (2011) |
| Baicalein | Scutellaria altissima L. | Inhibit mRNA expression of iNOS, COX-2, and TNF-α. Inhibit production of NO and inflammatory cytokine (IL-1β, PGE2, and TNF-α) by regulating NF-κB and ER-dependent pathway. Inhibit adhesion and migration of leukocytes by inhibiting cell adhesion molecules expression. |
Chandrashekar et al. (2012), Fan et al. (2013), Lee et al. (2015) |
| Oroxylin A | Scutellariae baicalensis Georgi | Inhibit NO production and iNOS and COX-2 proteins expression of via inhibiting nuclear factor-κB pathway. Enhance antioxidant response element-luciferase reporter activity by increasing the expression of nuclear factor erythroid 2-related factor 2 proteins. |
Chen et al. (2000), Li et al. (2007), Ye et al. (2014) |
| Wogonin | Scutellaria baicalensis Georgi | Inhibit adhesion and migration of leukocytes by inhibiting cell adhesion molecules expression. Reduces allergic airway inflammation by inducing eosinophil apoptosis through activation of caspase-3. |
Guo et al. (2007), Lee et al. (2015), Lucas et al. (2015) |
| Flavonols | |||
| Quercetin | Dysosma veitchii Hemsl. et Wils | Decreased expression of pro-inflammatory cytokines, NF-κB, and iNOS. Reduce expression of VCAM-1 and E-selectin. |
Shaik et al. (2006), Kleemann et al. (2011), Choi et al. (2012) |
| Kaempferol | Found in various fruits and vegetables e.g., tea, tomato, cruciferous vegetables, apple, etc. |
Reduce iNOS and COX-2 activity through suppression of the signaling of STAT-1, NF-kappa B, and AP-1. Decrease the gene expression of ICAM-1, VCAM-1, and monocyte chemotactic protein-1 (MCP-1). | Hamalainen et al. (2007), Kong et al. (2013) |
| Rutin | Ruta graveolens | Inhibit leukocyte migration. Suppress production of TNF-α and IL-6. Inhibit activation of NF-κB and extracellular regulated kinases. |
Yoo et al. (2014) |
| Flavanols | |||
| Epigallocatechin-3-gallate | Camellia sinensis L. | Inhibit reactive oxygen species generation, MAPKs phosphorylation, adhesion molecules expression signal transducers and activators of transcription 3 (STAT-3) and activating transcription factor 2 translocation through induction of heme oxygenase-1 and suppressors of cytokine signaling -3 expression. | Lee et al. (2013b) |
| Isoflavones | |||
| Daidzein | Pueraria mirifica, Pueraria lobata, Glycine max | Decreases TNF-α, IL-1β, MCP-1, NO, and iNOS expression at mRNA level. | Hamalainen et al. (2007) |
| Genistein | Glycine max | Inhibited expression of iNOS and COX-2. Decreased IL-1β and TNF-α production via activation of PPARs. |
Valles et al. (2010) |
| Puerarin | Pueraria lobata (wild) Ohwi | Decrease cytokines level. Inhibit NF-κB and activation of signal transducers and activators of transcription 3 (STAT3). |
Liu et al. (2013) |
| Phloroglucinols | |||
| Myrtucommulone | Myrtus communis L. | Inhibit the PGE2 production by inhibiting the mPGES-1 activity without significantly inhibiting the COX enzymes activity. | Koeberle et al. (2009) |
| Arzanol | Helichrysum italicum | Reduce eicosanoids generation by inhibiting lipooxygenase and cyclooxygenase activity in arachidonic acid metabolism pathway. | Rossi et al. (2009), Bauer et al. (2011) |
| Quinones | |||
| Thymoquinone | Nigella sativa L. | Inhibited LPS-induced fibroblast proliferation and H2O2-induced 4-hydroxynonenal generation. Inhibit IL-1β, TNF-α, MMP-13, COX-2, and PGE2 while blocking phosphorylation of MAPK p38, ERK1/2, and NF-kBp65. |
Vaillancourt et al. (2011) |
| Shikonin | Lithospermum erythrorhyzon Sieb. etZucc. | Inhibit NF-κB activity, inhibit Th1 cytokines expression and induce Th2 cytokines. | Andújar et al. (2010) |
| Emodin-8-O-β-D glucoside | Polygonum amplexicaule D. Don var. sinense Forb. | Stimulate proliferation and differentiation of osteoblasts. Inhibit PGE2 production by increased alkaline phosphatase expression in MC3T3-E1. |
Xiang et al. (2011) |
| Other | |||
| Apocynin | Apocynum cannabinum L. (Canadian hemp), Picrorhiza kurroa Royle ex Benth. | Inhibit NADPH oxidase activity. Suppress pro-inflammatory cytokines and CD4+ and CD8+T cells production. |
Stefanska and Pawliczak (2008), Kim et al. (2012) |
| Stilbenes | |||
| Resveratrol | Fallopia japonica, grape, nuts | Decrease MPO activity and mPGES-1 to basal levels. Inhibit iNOS and COX-2 expression. Reduce the pro-inflammatory cytokines IL-8, TNFα, IFN-γ, and IL-1α. Increase the anti-inflammatory cytokine IL-10 level. |
Youn et al. (2009), Vang et al. (2011) |
| Piceatannol | Fallopia japonica, grape, nuts | Decrease iNOS expression. Inhibit transcription factors activation such as NF-kB, ERK, and STAT3. |
Youn et al. (2009), Vang et al. (2011) |
| Terpenoid | |||
| 14-deoxyandrographolide | Andrographis paniculata | Enhanced proliferation of lymphocytes. Enhanced IL-2 induction in lymphocytes. |
Kumar et al. (2004) |
| 14-deoxy-11,12-didehydroandrographolide | Andrographis paniculata | Enhanced proliferation of lymphocytes. Enhanced IL-2 induction in lymphocytes. |
Kumar et al. (2004) |
| Ginsan | Panax ginseng | Enhances the production of cytokines and reactive oxygen species by macrophages. Stimulates the phagocytic activity of macrophages. |
Shen et al. (2002), Song et al. (2002) |
| Oleanolic acid | Luffa cylindrica, Phytolacca americana | Reduce level of IL-1α, IL-6, and TNF-α, as well as their effect on complement pathway though the inhibition of C3 convertase. Inhibits adenosine deaminase activity. |
Brinker et al. (2007) |
| Echinocystic acid | Luffa cylindrica | Enhance phagocytic index of macrophages in humoral and cell-mediated immune responses. | Khajuria et al. (2007) |
| Triptolide | Tripterygium wilfordii | Inhibits lymphocyte activation and pro-inflammatory cytokines gene expression (IL-2, iNOS, TNF-α, COX-2, and IFN-γ). It also inhibits activation of transcription factors such as NF-kB, NFAT, and STAT3. |
Brinker et al. (2007) |
| Demethylzelasteral | Tripterygium wilfordii | Inhibits proliferation of vascular endothelial cells. | Ramgolam et al. (2000) |
| Celastrol | Tripterygium wilfordii | Inhibit expression of pro-inflammatory cytokines, adhesion molecules, proteasome activity, and topoisomerase II. | Kannaiyan et al. (2011) |
| Asiaticoside | Centella asiatica | Decrease NO production. | Hartog et al. (2009) |
| Madecassoside | Centella asiatica | Reduce spleen cells proliferation. Inhibition of pro-inflammatory mediators such as TNF-α and IL-6. Inhibit production of PGE2, and COX-2. |
Li et al. (2009) |
| 11-keto-β-boswellic acid | Boswellia carteri | Decrease pro-inflammatory cytokines such as IL-1, IL-2, IL-4, IL-6, and IFN-γ through inhibition of NF-kB activation. | Ammon (2006) |
Selected Immunomodulatory Plant-Derived Single Chemical Entities in Clinical Trials
Curcumin
Curcumin is a natural diarylheptanoid compound found in the rhizome of Curcuma longa and related species. The medicinal benefits of curcumin have been known since centuries. A variety of biological and pharmacological properties of curcumin has been reported including anti-cancer, anti-oxidant, anti-angiogenic, anti-proliferative, pro-apoptotic, etc. Curcumin is one of the most extensively studied compound for its immunomodulatory properties. Curcumin lessened inflammatory responses by inhibiting NO production, cyclooxygenase-2 (COX-2), nuclear factor-kappa B (NK-kB), inducible nitric oxide synthase (iNOS), and lipoxygenase in NK cells and IFN-γ, or TNF-α activated macrophages (Bhaumik et al., 2000; Surh et al., 2001). In phorbol 12-myristate 13- acetate (PMA) and H2O2-stimulated human myelomonoblastic cell line, curcumin inhibited NF-κB activation via prevention of degradation and phosphorylation of I kappa B alpha (IκB-α; Singh and Aggarwal, 1995). Protein kinase C, which regulates the proliferation and survival of cell, is activated by PMA. Additionally, LPS and TNF-α also activate protein kinase C, which then activates NF-κB (Holden et al., 2008). Therefore, curcumin might weaken NF-kB activation by the inhibition of protein kinase C. The anti-inflammatory activity of curcumin was partially mediated by inhibiting activator protein (AP)-1 and transcription factor NF-κB. NF-κB and AP-1 act collectively and may enhance tumor development. The treatment of glioma cells with 25 μM curcumin reduced the binding of NF-κB and AP-1 (Dhandapani et al., 2007). The AP-1 activation was also suppressed by curcumin in TNF-activated bovine aortic endothelial cells (Xu et al., 1997). The activated immune cells release pro-inflammatory cytokines that play key role in inflammation. The expression of pro-inflammatory cytokines TNF-α, IL-1, IL-6, and IL-12 was inhibited by curcumin via LPS- or PMA-stimulated monocytes, macrophages, dendritic cells, and splenic lymphocytes (Gao et al., 2004; Kim et al., 2005). The attachment of T cells to endothelial and antigen presenting cells is dependent on cell adhesion molecules. The attachment of monocytes to endothelial cells was blocked by pre-treatment with curcumin. Moreover, the expression of intracellular adhesion molecule (ICAM)-1, vascular cell adhesion molecule (VCAM)-1, and endothelial leukocyte adhesion molecule (ELAM)-1 was also decreased in TNF-α-stimulated human umbilical vein endothelial cells (HUVECs) via inhibition of NF-κB (Kumar et al., 1998).
Jurenka (2009) reviewed pre-clinical and clinical trials of curcumin that were carried out at different places. Presently, 116 studies regarding the diverse actions of curcumin could be found on http://www.clinicaltrials.gov/. Among these 99 studies were based on the anti-inflammatory properties of curcumin. The most noticeable diseases for which trials had been conducted were cancer (e.g., lung, prostate, breast, pancreatic, and colorectal), rheumatoid arthritis and inflammatory bowel diseases (IBD; ulcerative colitis and Crohn’s disease), which reflect the pleiotropic actions of curcumin. This information shows that curcumin is still under extensive clinical investigation. Future clinical trials will predominantly deal with still ongoing various types of cancer and the action of curcumin on cognitive damage. Additionally, the inflammatory conditions will still be studied. Curcumin often acts as an adjunct treatment or dietary supplement to the standard therapy in these trials. The review on effects of curcumin in ulcerative colitis (Kumar et al., 2012) stated that curcumin, when given as adjunct therapy may be effective and safe therapy for the maintenance of remission in quiescent ulcerative colitis. Nonetheless, more operationally thorough randomized controlled trials are required. This conclusion from the authors reflects the overall situation. However, Fürst and Zündorf (2014) suggested that these are preliminary clinical trials which are frequently too weak and of low quality to draw a conclusion due to the low number of enrolled patients, which normally ranges from 10 to 30. As suggested by the authors, more operationally thorough and serious randomized controlled trials are required to evaluate the compound as an effective and safe agent for human use. It is worth mentioning that curcumin suffers from its low bioavailability, though substantial improvement has been made to address this issue via chemical and technological methods (Anand et al., 2007). The future of curcumin as an approved option for the prevention or treatment of the above mentioned indications relies on the findings of high-quality and big cohort studies in the future. Still, from the available literature, a conclusion can be drawn that curcumin exhibits good safety profile; it is nontoxic and well tolerated.
Resveratrol
Resveratrol, chemically (5-[(E)-2-(4-hydroxyphenyl) ethenyl] benzene-1,3-diol), is a derivative of stilbene and phytoalexin. It is found in various dietary products and plants including grapevines, red wine and peanuts. Similar to curcumin, resveratrol was found to exert a variety of pharmacological activities such as antimicrobial, chemopreventive, anticancer/proapoptotic, anti-inflammatory, and antioxidant properties. The inflammatory molecules are strongly inhibited by resveratrol. The immunomodulatory activities of resveratrol include the inhibition of NF-κB in PMA, LPS or TNF-α-mediated macrophages, epithelial (HeLa), Jurkat, myeloid (U-937) and dendritic cells. NF-κB activation was inhibited by resveratrol via inhibition of IκB kinase (Holmes-McNary and Baldwin, 2000; Manna et al., 2000; Gao et al., 2001). The expression of COX-2 and iNOS in cytokine (IFN, IL-1, or TNF-α) stimulated human primary airway epithelial cells was also inhibited by resveratrol (Donnelly et al., 2004) whereas it also blocked the transcription of COX-2 in human mammary epithelial cells stimulated by PMA. The secretion of NO and TNF-α in LPS-activated N9 microglial and cortical microglia cells was also substantially suppressed by resveratrol (Bi et al., 2005), moreover the production of IL-12, IL-6, IL-1, TNF-α, and IFN-γ and by splenic macrophages and lymphocytes was also inhibited (Kowalski et al., 2005). Resveratrol also showed strong inhibition of in vivo C5 anaphylatoxin (C5a)-mediated inflammation. The release of inflammatory cytokines (MIP-1, IL-6, IL-1, and TNF-α) in C5a-activated mouse and human neutrophils was inhibited by the pre-incubation with resveratrol (10–40 M). In addition, ERK-phosphorylation, release of glucuronide and C5a induced oxidative burst (superoxide anion production) were also inhibited by resveratrol. Furthermore, resveratrol inhibited production of inflammatory cytokines and C5a-stimulated neutrophil recruitment/migration in C5a-stimulated acute peritonitis mouse model (Issuree et al., 2009). The expression of cell adhesion molecules was also found to be inhibited by resveratrol. The IL-6-stimulated ICAM-1 expression in endothelial cells was also decreased by resveratrol (Wung et al., 2005), in addition to the inhibition of Porphyromonas gingivalis LPS-induced endothelial dysfunction in human microvascular endothelial cells. Besides, ICAM-1 and VCAM-1 expression on human microvascular endothelial cells was blocked by inhibition of NF-κB activation (Park et al., 2009).
Based on the above-mentioned findings, a lot of emphasis was put into the explanation of the underlying mechanisms of action. Still, this enormous knowledge has yet not been translated into anapproved clinical medicine. More than 40 clinical trials on resveratrol have been listed in PubMed for a variety of diseases like coronary artery disease, obesity, diabetes, and so forth. The main focus of these studies was to either analyze inflammation-related parameters in blood cells (e.g., transcription factors or activated kinases) and in plasma (e.g., IL-6, IL-1β, TNF-α) or to report on functional parameters like the status of the endothelium (Bakker et al., 2010; Tome-Carneiro et al., 2013). Several trials persuasively showed that these parameters are usefully influenced by resveratrol. Though, whether this improved inflammatory status of the patients indeed leads to a clinically relevant improvement of the severity of the diseases or, most significantly, in a decreased incidence of disease-specific life-threatening events has not been studied. Currently 99 studies are listed on http://www.clinicaltrials.gov/, 49 studies have been completed while 28 clinical trials on resveratrol are currently recruiting or planned. Among them 27 studies are linked with the anti-inflammatory properties of resveratrol. The main field of interest is metabolic syndrome/type 2 diabetes, followed by mild cognitive impairment, non-alcoholic fatty liver disease, and polycystic ovary syndrome. Research would benefit by carrying out interventional studies with definite primary outcomes reflecting the stage or/and occurrence of the diseases on a long-term basis.
Epigallocatechin-3-gallate
One of the most active and abundant polyphenol of green tea Camellia sinensis (Theaceae) is epigallocatechin-3-gallate (EGCG), chemically [(2R,3R)-5,7-dihydroxy-2-(3,4, 5-trihydroxyphenyl)-3,4-dihydro-2H-chromen-3-yl]3,4,5-trihydroxybenzoate]. It has been widely reported for its in vitro and in vivo chemopreventive, anti-angiogenic, anti-invasive, anti-proliferative, anti-inflammatory, and anti-oxidant effects (Singh et al., 2011; Yang et al., 2011b). Studies have showed that EGCG blocked the activation of NF-κB by inhibiting the degradation of IκBα (Muraoka et al., 2002) and also inhibited the MAPK pathways (Chung et al., 2001). The down-regulation of iNOS transcription and NO production in macrophages is dependent on the inhibition of NF-κB. In comparison, it was shown that EGCG improved the production of NO to inhibit endothelial exocytosis and leukocyte adherence to endothelial cells (Yamakuchi et al., 2008). Moreover, it was reported that EGCG blocked NF-κB activation in human endothelial cells and thus inhibited the expression of MCP-1 (Hong et al., 2007). By reducing mRNA expression of bax and caspase3 and by decreasing caspase3 activity, EGCG also decreased apoptosis (Hara et al., 2006; Park et al., 2006; Yu et al., 2007). Additionally, it inhibited expression of COX-2, proteasome dependent degradation, the MAPK pathway and growth factor-dependent signaling (e.g., of IGF-I, VEGF, and EGF; Yang et al., 2011a). Likewise, EGCG inhibited DNA methyltransferase 1, topoisomerase II and telomerase, thus affecting the functions of chromatin (Lee et al., 2005; Sadava et al., 2007; Bandele and Osheroff, 2008).
Surprisingly, though, notwithstanding encouraging pre-clinical results and the detailed mechanistic insights, clinical studies related to inflammation are lacking. A small scale study investigated the action of green tea and its extract on biomarkers of inflammation (e.g., ICAM-1, VCAM-1, IL-1β, IL-6, CRP, and adiponectin) in obese patients with metabolic syndrome. After 8 weeks treatment, green tea did not change the biomarker levels (Basu et al., 2011). Another clinical trial reported a useful impact of topical EGCG treatment on acne vulgaris, which may be in part due to anti-inflammatory effects (Yoon et al., 2013). A green tea extract was approved as a prescription drug in 2006 for the topical treatment of anal and genital warts (Condylomata acuminate). This key development fueled additional research to expand the indications of EGCG. Sixty-eight clinical trials are listed on http://www.clinicaltrials.gov/ for upcoming years. Fifteen studies are in recruiting stage while 33 studies have been completed. EGCG will be tested for its effects on albuminuria in diabetic nephropathy along with its action in patients with multiple-system atrophy, Huntington’s disease, fragile X syndrome, Down syndrome, Alzheimer’s disease (early stage), muscular dystrophy of the Duchenne type, and cardiac amyloid light-chain amyloidosis. Furthermore, the trials will study the potential of EGCG on preventing colon polyps in patients at high risk for recurrent colon adenoma and on reactivation of the Epstein–Barr virus in remission patients. More studies will investigate whether topical EGCG exerts an anticarcinogenic potential in patients with superficial basal cell carcinoma, whether gargling with EGCG inhibits influenza infections in teenagers and whether EGCG affects the resistance of insulin. Evidently, the trials will not study classic inflammatory disorder, though some of the mentioned diseases are linked with inflammatory processes (e.g., insulin resistance or Alzheimer’s disease). Nonetheless, it is probable that EGCG will experience an expansion of its indication in future.
Quercetin
The flavonol, quercetin, chemically 2-(3,4-dihydroxyphenyl)- 3,5,7-trihydroxychromen-4-one, belongs to the family of polyphenols representing very extensively spread secondary plant metabolites. Quercetin is found in a variety of food like tea, capers, red onions, broccoli, berries, grapevines, and apples. Quercetin has been found to exert anti-mutagenic, anti-oxidative, anti-inflammatory, anticancer/chemopreventive, neuroprotective, antihypertensive, and blood glucose-lowering activities (Middleton et al., 2000). Quercetin activated various kinases which phosphorylated eukaryotic initiation factor 2, thus inhibiting cell translation (Ito et al., 1999). The mechanisms behind these actions are wide and have been characterized extensively. Quercetin scavenged nitrogen and reactive oxygen species (ROS), targets noticeable pro-inflammatory signaling pathways including MAPK, NF-κB and STAT1, and inhibits replication of many types of viruses and infectivity of target cells (Boots et al., 2008). The activity of COX-2 and iNOS was inhibited by quercitin by the suppression of AP-1, NF-κB and STAT-1 signaling in cytokine- or LPS-induced HUVECs and macrophages (Hamalainen et al., 2007). The expression of pro-inflammatory cytokines in calcium ionophore- and PMA-induced mast cells was attenuated by quercitin. Moreover, the TNF-α-stimulated NF-κB recruitment to pro-inflammatory gene promoters was also suppressed by quercitin in murine intestinal epithelial cells (Ruiz et al., 2007; Park et al., 2008). The TNF-α- or PMA-induced expression of ICAM-1 in human endothelial cells was decreased by quercitin (Kobuchi et al., 1999). The LPS-stimulated NF-κB and nitrite oxide production was also inhibited by quercitin in mice.
A number of studies on inflammatory parameters in humans have been performed in the past few years: one clinical trial estimated the effect of quercetin on biomarkers of inflammation depending on the apo-lipoprotein E genotype of healthy men. Though the risk factors of cardiovascular disease were improved, quercetin exerted a slight pro-inflammatory effect (Pfeuffer et al., 2013). After repeated sprint exercise, quercetin did not affect the levels of the pro-inflammatory cytokine IL-6 (Abbey and Rankin, 2011). Quercetin decreased the markers of inflammation (IL-8 and TNF-α) in sarcoidosis patients (Boots et al., 2011). Quercetin did not change phagocytosis activity, granulocyte oxidative burst or blood leukocyte subsets, TNF-α or IL-6 plasma levels in healthy females (Heinz et al., 2010). It is worth mentioning that no trial reports on the improvement of clinical parameters of inflammatory diseases (incidence and severity). Ten trials are registered on http://www.clinicaltrials.gov/ that will use pure quercetin. Regarding inflammatory disorders, quercetin will be analyzed in two-phase 1–2 trials for its dose-response relationship and safety in chronic obstructive pulmonary disease. For diabetes, quercetin will be tested in a phase 2 trial for its effect on blood vessel function and blood glucose in type 2 diabetes. Quercetin will also be administered in obese patients (with or without type 2 diabetes) to investigate its action on glucose absorption. In study related to cancer, it will be studied whether quercetin can prevent prostate cancer and whether it controls levels of prostate-specific antigen. Additionally, quercetin will be administered in children with Fanconi anemia (pharmacokinetics and safety). Therefore, quercetin will go through interesting studies that may lead to insightful development of knowledge regarding its clinical efficacy. Though, inflammatory diseases are not the main areas of current research.
Colchicine
The tropolone derivative, colchicine, chemically (N-[(7S)-1,2, 3,10-tetramethoxy-9-oxo-6,7-dihydro-5H-benzo(a)heptalen-7] -ylacetamide), is the main alkaloid of Colchicum autumnale (family: Colchicaceae), generally called meadow saffron or autumn crocus. The extracts of this plant have been used against gout attacks for centuries. The US FDA has approved colchicine for the prevention and treatment of familial mediterranean fever and acute gout flares. To obtain this approval, the applying company provided new clinical data and was thus given an exclusive marketing agreement, 7 years for familial Mediterranean fever and 3 years for the indication gout (orphan drug status). The mechanisms of action of colchicine are extensively studied: the molecular target was recognized, the binding spot was accurately characterized, and the biological consequences of damaging microtubule dynamics were examined; complete reviews summarizing these results are available (Bhattacharyya et al., 2008; Nuki, 2008; Stanton et al., 2011). In 1960s, colchicine played a key role for the initial characterization of tubulin subunits and microtubules (Borisy and Taylor, 1967).
In spite of this vast knowledge, researchers have recently conducted numerous trials to expand its fields of application. Several trials in the field of inflammation-associated pathologies with positive outcome: colchicine was tested as an adjunct treatment against acute pericarditis. In an open-label, single-center randomized trial, colchicine addition to glucocorticoids or aspirin traditional treatment reduced the reappearance to half, after early occurrence of acute pericarditis. In a placebo controlled, double-blind, randomized, multicenter trial, colchicine, when added to traditional anti-inflammatory therapy, substantially decreased the rate of recurrent or incessant pericarditis (Imazio et al., 2005a,b, 2011, 2013), to prevent atrial fibrillation after radiofrequency ablation (Deftereos et al., 2012) and for the prevention of post-pericardiotomy syndrome (Imazio et al., 2010). These well-performed and large studies will definitely affect pharmacotherapy guidelines. And the field is still active: 19 clinical trials that are in the recruiting phase, are listed on http://www.clinicaltrials.gov/. They aim to study the action of colchicine primarily in the areas of nephrology and cardiology, for instance, in myocardial infarction, for the prevention of post-pericardiotomy syndrome, or in diabetic nephropathy-all related to inflammatory processes. Colchicine will be investigated to determine its effects on metabolism in adults who are overweight but have not yet developed diabetes. As the number of diseases with an inflammatory component is large, one might wonder that colchicine will further stay an interesting, not yet fully used drug.
Capsaicin
Capsaicin, chemically (E)-N-[(4-hydroxy-3-methoxyphenyl) methyl]-8-methylnon-6-enamide, is a hydrophobic alkaloid found in chili peppers (Capsicum species; Solanaceae) and accounts for the characteristic spiciness/pungency of the fruits of the genus. It has been used in traditional medicine as a counter-irritant and topical rubefacient to relieve pain of joints and muscles. Recently, an 8% capsaicin cutaneous patch has been approved by the EU for use in non-diabetic adults against neuropathic pain and in US against neuropathic pain linked with post-herpetic neuralgia. The research on capsaicin resulted in the discovery of transient receptor potential channel vanilloid subfamily member 1 (TRPV1), which is the direct target of capsaicin (Caterina et al., 1997). TRPV1 is a positively charged indiscriminate channel mainly located in nociceptive neurons with high preference for Ca2+. It is activated by physical and chemical stimuli, like certain inflammatory mediators, capsaicin, low pH and heat (O’Neill et al., 2012). Extended activation of TRPV1 by capsaicin was found to cause desensitization and, therefore, decreased pain sensation (Haanpaa and Treede, 2012). Apart from pain, numerous studies in arthritic rats also discovered that capsaicin could inhibit paw inflammation (Joe et al., 1997; Park et al., 2000) and ethanol-induced inflammation of gastric mucosa in rats. Furthermore, capsaicin was found to inhibit NF-κB pathway, iNOS expression and COX-2 activity in the macrophages in a TRPV1-independent manner (Kim et al., 2003). Regarding inflammation, capsaicin was studied in a latest review by the Cochrane Collaboration: topical capsaicin was discovered to be ineffective against osteoarthritis (Cameron and Chrubasik, 2013). In comparison, one meta-analysis found adequate evidence to report that capsaicin was effective in the management of osteoarthritis, though the authors reported the lacking of randomized clinical trials (De Silva et al., 2011). As far as future therapeutic enhancements are concerned, 24 clinical trials are currently recruiting or are planned, as listed on http://www.clinicaltrials.gov/. Majority of these studies use capsaicin as a model substance to induce pain or as a diagnostic tool. Studies that examine the therapeutic potential of capsaicin study its potential as pre-emptive analgesic in patients going through amputation of a limb and its effect on neuropathic pain from critical ischemia (mainly in feet and hands) and on chronic pain from artificial arteriovenous fistulae in end-stage renal failure patients. Besides, capsaicin will be tested against the decreased swallow response in stroke patients with or opharyngeal dysphagia and against persistent pain after inguinal herniotomy. A study on the mechanism of action of capsaicin against idiopathic rhinitis will be carried out. No study will investigate the immunomodulatory effects of capsaicin. It can be concluded that, in comparison with neuropathic pain, the field of inflammation and capsaicin is not very cutting-edge and will stay on that level in the near future owing to lack of clinical trials.
Andrographolide
Andrographolide is a diterpenoid lactone found in Andrographis paniculata. Chemically, it is (3-[2-[decahydro-6-hydroxy-5-(hydroxymethyl)-5, 8-dimethyl-2-methylene-1-napthalenyl] ethylidene] dihydro- 4-hydroxy-2(3H)-furanone). Diverse biological activities have been exhibited by andrographolide. Numerous immunomodulatory activities of andrographolide have been observed in vitro including reduction of IL-12, TNF-α, PGE2, NO, COX-2 and iNOS in microglia and macrophages (Maiti et al., 2006). The LPS-stimulated iNOS and COX-2 expression in RAW264.7 macrophages was inhibited by andrographolide. It also inhibited Akt and Erk ½ signaling, consequently inhibiting the chemotactic migration of macrophages on the site of inflammation (Tsai et al., 2004; Qin et al., 2006). In neutrophils, the production of ROS was inhibited by andrographolide (Shen et al., 2002). Andrographolide regulated the production of factors such as NK cells, IFN-γ, IL-2, and TNF-α. The administration of andrographolide resulted in improved expression of CD markers and production of TNF-α, therefore increasing the cytotoxic potential of lymphocytes (Rajagopal et al., 2003). Andrographolide inhibited IL-2, IFNγ and IL-6 expression, decreasing the cellular and humoral adaptive immune reaction in T cells. Andrographolide decreased the antigen-presenting potential of dendritic cells to T cells. In an ovalbumin-induced asthma rat model, the andrographolide administration decreased the serum immunoglobulin, IL-13, IL-4, IL-5, and Th2 cytokine. Andrographolide decreased migration and invasion, endothelial cell proliferation and adhesion molecule ICAM-1, signifying its role in angiogenesis (Chiou et al., 2000). The functions of NF-κB were inhibited by a number of immunomodulatory responses and andrographolide inhibited NF-κB binding to DNA and hence decreasing pro-inflammatory proteins expression like iNOS and COX-2 (Wang et al., 2004; Xia et al., 2004; Hidalgo et al., 2005; Iruretagoyena et al., 2005). Andrographolide down regulated COX-2 and iNOS gene expression by inhibiting NF-κB and signal transducer and activator of transcription-3 (STAT3) expression via suppressing the expression of suppressor of cytokine signaling 1 and 3 (Lee et al., 2011). Zhang et al. (2014) conducted a study to determine the effect of andrographolide on insulinoma tumor growth. Andrographolide was found to inhibit the progression of insulinoma tumor by targeting the TLR4/NF-κB signaling pathway (Zhang et al., 2014).
In spite of this vast knowledge, researchers have recently conducted numerous trials to expand its fields of application. But most of the trials have been conducted by using the crude extract. A double blind study was conducted on 101 patients with moderately active Crohn’s disease to assess the efficacy and safety of aqueous ethanol extract of A. paniculata. At week 8 of treatment, a significant decrease in the mean C-reactive protein level was observed in extract treated group as compared with the placebo group (Sandborn et al., 2010). A phase II study in patients with mildly to moderately active ulcerative colitis demonstrated that the efficacy of the aqueous ethanol extract of A. paniculata was similar to that of slow release mesalazine (Tang et al., 2011). In contrast to the patients with mildly to moderately active ulcerative colitis receiving placebo, those treated with A. paniculata extract were more probable to attain clinical response (Sandborn et al., 2013). In a double blind against placebo controlled clinical trial, the standardized patented A. paniculata extract’s (Paractin®) effect was evaluated in chronic rheumatoid arthritis patients. A significant reduction in total grade of tender joints, number of tender joints, total grade of swollen joint, number of swollen joints was observed. Serological parameters and rheumatoid factor (C4 and IgA) of disease were found to be reduced (Burgos et al., 2009). Currently, five different studies related to the various actions of andrographolide could be found on http://www.clinicaltrials.gov/. Two trials are registered on http://www.clinicaltrials.gov/ that will use pure andrographolide. A double blind, interventional, randomized, and placebo controlled phase II trial will determine the safety, acceptability, and effectiveness of andrographolide in active rheumatoid arthritis-affected patients and effects on the immunological functions that effect chronic inflammation will also be determined. A study will be conducted to determine the efficacy of andrographolide in retarding the progression of brain atrophy in patients with progressive forms of multiple sclerosis. Besides, the efficacy and safety of andrographolide will also be evaluated for the treatment of colorectal cancer.
Genistein
Genistein, chemically 4,5,7-trihydroxyisoflavone, is a naturally occurring phytoestrogen, present in soy and soy-derived items. Genistein is a renowned tyrosine kinase inhibitor. In vitro, genistein inhibited IL-1b/IFNγ-induced COX-2 and iNOS expression and in addition to producing PGE2 and NO in human islets that may prevent pathogenesis of diabetes and improve insulin resistance (Corbett et al., 1996). Like other topoisomerase inhibitors, genistein induced apoptosis (Mccabe and Orrenius, 1993). Genistein strongly inhibited angiogenesis, exerted inhibiting effect on proliferating cells (Fotsis et al., 1995). Genistein regulates vascular function and protects from atherosclerosis (Si and Liu, 2007). The administration of genistein in human brain microvascular endothelial cells and HUVECs significantly inhibited the expression of TNF-α-induced cell adhesion molecule CD106 and CD62E, and monocyte adhesion (Lee and Lee, 2008). Genistein also reduced the interaction between endothelial cells and monocyte via activation of PPARs that reduced monocyte adhesion in culture cells and animals (Kim et al., 2004; Chacko et al., 2007). The LPS-stimulated secretion of MCP-1 from macrophages was inhibited by genistein that resulted in decreased migration of monocyte in vitro (Nagarajan et al., 2008). Genistein inhibited LPS-induced expression of nitro tyrosine protein and iNOS in vascular tissue that inhibits vascular alterations and hypotension in vivo (Bermejo et al., 2003). Genistein exerted potential effect on chronic colitis, neurodegenerative diseases, rheumatoid arthritis, metabolic disorders, and diabetes by modulating inflammatory response. For example, genistein inhibited production of pro-inflammatory molecules called as markers of cartilage catabolism (HC gp-39, IL-1b, and NO) in LPS-induced human chondrocytes (Hooshmand et al., 2007). Genistein modulated Th1-predominant immune response by increasing IL-4 production and suppressing the secretion of IFN-γ in a collagen-induced rheumatoid arthritis rat (Wang et al., 2008). NAFLD is an obesity-linked fatty liver illness sourced by pro-inflammatory cytokines and TNF-α and results in increase fatty acid uptake and the dysfunction of hepatocytes. Genistein reduced the high fat diet-induced steatohepatitis by improving liver function and decreasing plasma TNF-α level in rat (Yalniz et al., 2007). Moreover, genistein reduced LPS-stimulated dopamine uptake loss in rat mesencephalic neuron glial cells by decreasing production of TNF-α and NO and microglia activation (Wang et al., 2005) this might protect the pathogenesis of Parkinson’s disease caused by dopaminergic neuron injury. The increase of astrocytes at Ab deposition sites is the initial neuropathological fluctuation that starts inflammation in Alzheimer’s disease. The production of Ab-stimulated inflammatory mediators IL-1β, TNF-α, COX-2, and iNOS in astrocytes was decreased by genistein treatment, probably by PPARs’ activation (Valles et al., 2010). Likewise, oral administration of genistein decreased TNBS-stimulated chronic colitis by inhibition of COX-2 mRNA and protein expression together with the colonic myeloperoxidase (MPO) activity in rat that applies useful anti-inflammatory effects in the treatment of IBD (Seibel et al., 2009).
Genistein has undergone clinical trials for a variety of diseases like type 2 diabetes mellitus, menopause, osteopenia, and various types of cancer. The most noticeable diseases for which trails had been conducted were cancer. Genistein is undergoing clinical trials for chemopreventive and chemotherapeutic purposes in various cancers. Currently 11 open clinical trials have been in recruiting or planned according to http://www.clinicaltrials.gov. The main field of interest is cancer (colon cancer; rectal cancer; colorectal cancer, prostate cancer, bladder cancer, adenocarcinoma, pancreatic adenocarcinoma) followed by osteoporosis, hypercholesterolemia, Alzheimer’s disease and menopause.
Conclusions and Future Prospects
Natural products and folklore medicines are the main contributors of the leads in the design and development of therapeutic agents. Several plant derived compounds have been identified over the years for their immunomodulatory characteristics. Numerous illnesses can be alternatively treated by immunomodulation using medicinal plants, instead of chemotherapy. The discovery and isolation of more specific immunomodulatory agents from plant origin possesses potential to counteract the side effects and high cost of synthetic compounds. This review highlights the significance of medicinal plants as producers of immunomodulatory molecules of very varied chemistries with possible uses in animal and human health. The challenges encountered by the application of plant-derived immunomodulators need to be addressed. Though, the path from traditional medicines to western pharmaceutical practices is not always easy. The inconsistency of responses of phytomedical practices can be clarified in terms of the typically strong reliance of plant secondary metabolite profiles on environmental signals that can disturb reproducibility of results with extracts. This can be decreased if the principles of standardization of extracts and enriched fractions are thoroughly applied.
In most of studies conducted to determine the effect on immune system, no adequate microbial contamination control protocols were applied. Researchers report that the microbial endotoxin can change the parameters of immune system. Therefore, appropriate precautions must be taken to counter the microbial contamination. An additional task is the classification of plant-derived immunomodulatory agents in a specific class or among classes as per inherent risk. This classification of novel plant derived immunomodulatory agents by level of risk can be endeavored from collective knowledge of meta-analyses of clinical trials, national registries and physicians. Another significant constraint with natural products is inadequate quantities, needed for development and clinical use. So, the development of novel isolation techniques to improve the amount for pharmaceutical applications needs more attention of researchers. A major clinical restraint of these substances is the low bioavailability. Nanotechnology and other delivery strategies are being applied to enhance their efficacy when administered to humans. Lastly, there is a deficiency of standard checking and quality control procedures to assure the efficacy and quality of the plant derived medicinal products for prospective pharmaceutical applications. A few plant derived compounds, including polysaccharides, are extremely diversified in terms of molecular weight and structure; therefore, it is challenging to produce the similar quality in every batch. Accumulative requirement for these plant-derived products would motivate improvement to overwhelming these obstacles for reaching market.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Abbey E. L., Rankin J. W. (2011). Effect of quercetin supplementation on repeated-sprint performance, xanthine oxidase activity, and inflammation. Int. J. Sport Nutr. Exerc. Metab. 21 91–96. [DOI] [PubMed] [Google Scholar]
- Ammon H. P. (2006). Boswellic acids in chronic inflammatory diseases. Planta Med. 72 1100–1116. 10.1055/s-2006-947227 [DOI] [PubMed] [Google Scholar]
- Anand P., Kunnumakkara A. B., Newman R. A., Aggarwal B. B. (2007). Bioavailability of curcumin: problems and promises. Mol. Pharm. 4 807–818. 10.1021/mp700113r [DOI] [PubMed] [Google Scholar]
- Andújar I., Recio M. C., Bacelli T., Giner R. M., Rios J. L. (2010). Shikonin reduces oedema induced by phorbol ester by interfering with IκBα degradation thus inhibiting translocation of NF-κB to the nucleus. Br. J. Pharmacol. 160 376–388. 10.1111/j.1476-5381.2010.00696.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auffenberg C., Rosenthal L. J., Dresner N. (2013). Levamisole: a common cocaine adulterant with life-threatening side effects. Psychosomatics 54 590–593. 10.1016/j.psym.2013.02.012 [DOI] [PubMed] [Google Scholar]
- Bae Y., Lee S., Kim S.-H. (2011). Chrysin suppresses mast cell-mediated allergic inflammation: involvement of calcium, caspase-1 and nuclear factor-κB. Toxicol. Appl. Pharmacol. 254 56–64. 10.1016/j.taap.2011.04.008 [DOI] [PubMed] [Google Scholar]
- Bakker G. C., Van Erk M. J., Pellis L., Wopereis S., Rubingh C. M., Cnubben N. H., et al. (2010). An antiinflammatory dietary mix modulates inflammation and oxidative and metabolic stress in overweight men: a nutrigenomics approach. Am. J. Clin. Nutr. 91 1044–1059. 10.3945/ajcn.2009.28822 [DOI] [PubMed] [Google Scholar]
- Bandele O. J., Osheroff N. (2008). (-)-Epigallocatechin gallate, a major constituent of green tea, poisons human type II topoisomerases. Chem. Res. Toxicol. 21 936–943. 10.1021/tx700434v [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartelds G. M., Krieckaert C. L., Nurmohamed M. T., Van Schouwenburg P. A., Lems W. F., Twisk J. W., et al. (2011). Development of antidrug antibodies against adalimumab and association with disease activity and treatment failure during long-term follow-up. JAMA 305 1460–1468. 10.1001/jama.2011.406 [DOI] [PubMed] [Google Scholar]
- Basu A., Du M., Sanchez K., Leyva M. J., Betts N. M., Blevins S., et al. (2011). Green tea minimally affects biomarkers of inflammation in obese subjects with metabolic syndrome. Nutrition 27 206–213. 10.1016/j.nut.2010.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudouin V., Crusiaux A., Haddad E., Schandene L., Goldman M., Loirat C., et al. (2003). Anaphylactic shock caused by immunoglobulin E sensitization after retreatment with the chimeric anti-interleukin-2 receptor monoclonal antibody basiliximab. Transplantation 76 459–463. 10.1097/01.TP.0000073809.65502.8F [DOI] [PubMed] [Google Scholar]
- Bauer J., Koeberle A., Dehm F., Pollastro F., Appendino G., Northoff H., et al. (2011). Arzanol, a prenylated heterodimeric phloroglucinyl pyrone, inhibits eicosanoid biosynthesis and exhibits anti-inflammatory efficacy in vivo. Biochem. Pharmacol. 81 259–268. 10.1016/j.bcp.2010.09.025 [DOI] [PubMed] [Google Scholar]
- Baxter D. (2007). Active and passive immunity, vaccine types, excipients and licensing. Occup. Med. 57 552–556. 10.1093/occmed/kqm110 [DOI] [PubMed] [Google Scholar]
- Berger J. R., Houff S. A., Major E. O. (2009). Monoclonal antibodies and progressive multifocal leukoencephalopathy. MAbs 1 583–589. 10.4161/mabs.1.6.9884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermejo A., Zarzuelo A., Duarte J. (2003). In vivo vascular effects of genistein on a rat model of septic shock induced by lipopolysaccharide. J. Cardiovasc. Pharmacol. 42 329–338. 10.1097/00005344-200309000-00003 [DOI] [PubMed] [Google Scholar]
- Bhattacharyya B., Panda D., Gupta S., Banerjee M. (2008). Anti-mitotic activity of colchicine and the structural basis for its interaction with tubulin. Med. Res. Rev. 28 155–183. 10.1002/med.20097 [DOI] [PubMed] [Google Scholar]
- Bhaumik S., Jyothi M. D., Khar A. (2000). Differential modulation of nitric oxide production by curcumin in host macrophages and NK cells. FEBS Lett. 483 78–82. 10.1016/S0014-5793(00)02089-5 [DOI] [PubMed] [Google Scholar]
- Bi X. L., Yang J. Y., Dong Y. X., Wang J. M., Cui Y. H., Ikeshima T., et al. (2005). Resveratrol inhibits nitric oxide and TNF-α production by lipopolysaccharide-activated microglia. Int. Immunopharmacol. 5 185–193. 10.1016/j.intimp.2004.08.008 [DOI] [PubMed] [Google Scholar]
- Boots A. W., Drent M., De Boer V. C., Bast A., Haenen G. R. (2011). Quercetin reduces markers of oxidative stress and inflammation in sarcoidosis. Clin. Nutr. 30 506–512. 10.1016/j.clnu.2011.01.010 [DOI] [PubMed] [Google Scholar]
- Boots A. W., Haenen G. R., Bast A. (2008). Health effects of quercetin: from antioxidant to nutraceutical. Eur. J. Pharmacol. 585 325–337. 10.1016/j.ejphar.2008.03.008 [DOI] [PubMed] [Google Scholar]
- Borisy G. G., Taylor E. W. (1967). The mechanism of action of colchicine. colchicine binding to sea urchin eggs and the mitotic apparatus. J. Cell Biol. 34 535–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinker A. M., Ma J., Lipsky P. E., Raskin I. (2007). Medicinal chemistry and pharmacology of genus Tripterygium (Celastraceae). Phytochemistry 68 732–766. 10.1016/j.phytochem.2006.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgos R. A., Hancke J. L., Bertoglio J. C., Aguirre V., Arriagada S., Calvo M., et al. (2009). Efficacy of an Andrographis paniculata composition for the relief of rheumatoid arthritis symptoms: a prospective randomized placebo-controlled trial. Clin. Rheumatol. 28 931–946. 10.1007/s10067-009-1180-5 [DOI] [PubMed] [Google Scholar]
- Cameron M., Chrubasik S. (2013). Topical herbal therapies for treating osteoarthritis. Cochrane Database Syst. Rev. 5 CD010538. 10.1002/14651858.CD010538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campoli-Richards D., Sorkin E., Heel R. (1986). Inosine Pranobex. Drugs 32 383–424. 10.2165/00003495-198632050-00001 [DOI] [PubMed] [Google Scholar]
- Caterina M. J., Schumacher M. A., Tominaga M., Rosen T. A., Levine J. D., Julius D. (1997). The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389 816–824. 10.1038/39807 [DOI] [PubMed] [Google Scholar]
- Chacko B. K., Chandler R. T., D’alessandro T. L., Mundhekar A., Khoo N. K., Botting N., et al. (2007). Anti-inflammatory effects of isoflavones are dependent on flow and human endothelial cell PPAR gamma. J. Nutr. 137 351–356. [DOI] [PubMed] [Google Scholar]
- Chandrashekar N., Selvamani A., Subramanian R., Pandi A., Thiruvengadam D. (2012). Baicalein inhibits pulmonary carcinogenesis-associated inflammation and interferes with COX-2, MMP-2 and MMP-9 expressions in-vivo. Toxicol. Appl. Pharmacol. 261 10–21. 10.1016/j.taap.2012.02.004 [DOI] [PubMed] [Google Scholar]
- Chen C. Y., Peng W. H., Tsai K. D., Hsu S. L. (2007). Luteolin suppresses inflammation-associated gene expression by blocking NF-κB and AP-1 activation pathway in mouse alveolar macrophages. Life Sci. 81 1602–1614. 10.1016/j.lfs.2007.09.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D., Bi A., Dong X., Jiang Y., Rui B., Liu J., et al. (2014). Luteolin exhibits anti-inflammatory effects by blocking the activity of heat shock protein 90 in macrophages. Biochem. Biophys. Res. Commun. 443 326–332. 10.1016/j.bbrc.2013.11.122 [DOI] [PubMed] [Google Scholar]
- Chen D. P., Wong C. K., Leung P. C., Fung K. P., Lau C. B., Lau C. P., et al. (2011). Anti-inflammatory activities of Chinese herbal medicine sinomenine and Liang Miao San on tumor necrosis factor-alpha-activated human fibroblast-like synoviocytes in rheumatoid arthritis. J. Ethnopharmacol. 137 457–468. 10.1016/j.jep.2011.05.048 [DOI] [PubMed] [Google Scholar]
- Chen Y.-C., Yang L. L., Lee T. J. F. (2000). Oroxylin A inhibition of lipopolysaccharide-induced iNOS and COX-2 gene expression via suppression of nuclear factor-κB activation. Biochem. Pharmacol. 59 1445–1457. 10.1016/S0006-2952(00)00255-0 [DOI] [PubMed] [Google Scholar]
- Chiou W. F., Chen C. F., Lin J. J. (2000). Mechanisms of suppression of inducible nitric oxide synthase (iNOS) expression in RAW 264.7 cells by andrographolide. Br. J. Pharmacol. 129 1553–1560. 10.1038/sj.bjp.0703191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S. J., Tai B., Cuong N., Kim Y. H., Jang H. D. (2012). Antioxidative and anti-inflammatory effect of quercetin and its glycosides isolated from mampat (Cratoxylum formosum). Food Sci. Biotechnol. 21 587–595. 10.1007/s10068-012-0075-4 [DOI] [Google Scholar]
- Chung J., Choi R., Seo E.-K., Nam J.-W., Dong M.-S., Shin E., et al. (2012). Anti-inflammatory effects of (Z)-ligustilide through suppression of mitogen-activated protein kinases and nuclear factor-κB activation pathways. Arch. Pharm. Res. 35 723–732. 10.1007/s12272-012-0417-z [DOI] [PubMed] [Google Scholar]
- Chung J. Y., Park J. O., Phyu H., Dong Z., Yang C. S. (2001). Mechanisms of inhibition of the Ras-MAP kinase signaling pathway in 30.7b Ras 12 cells by tea polyphenols (-)-epigallocatechin-3-gallate and theaflavin-3,3’-digallate. FASEB J. 15 2022–2024. 10.1096/fj.01-0031fje [DOI] [PubMed] [Google Scholar]
- Corbett J. A., Kwon G., Marino M. H., Rodi C. P., Sullivan P. M., Turk J., et al. (1996). Tyrosine kinase inhibitors prevent cytokine-induced expression of iNOS and COX-2 by human islets. Am. J. Physiol. 270 C1581–C1587. [DOI] [PubMed] [Google Scholar]
- Daikonya A., Katsuki S., Kitanaka S. (2004). Antiallergic agents from natural sources 9. Inhibition of nitric oxide production by novel chalcone derivatives from Mallotus philippinensis (Euphorbiaceae). Chem. Pharm. Bull. 52 1326–1329. 10.1248/cpb.52.1326 [DOI] [PubMed] [Google Scholar]
- Deftereos S., Giannopoulos G., Kossyvakis C., Efremidis M., Panagopoulou V., Kaoukis A., et al. (2012). Colchicine for prevention of early atrial fibrillation recurrence after pulmonary vein isolation: a randomized controlled study. J. Am. Coll. Cardiol. 60 1790–1796. 10.1016/j.jacc.2012.07.031 [DOI] [PubMed] [Google Scholar]
- De Silva V., El-Metwally A., Ernst E., Lewith G., Macfarlane G. J. (2011). Evidence for the efficacy of complementary and alternative medicines in the management of osteoarthritis: a systematic review. Rheumatology 50 911–920. 10.1093/rheumatology/keq379 [DOI] [PubMed] [Google Scholar]
- Dhandapani K. M., Mahesh V. B., Brann D. W. (2007). Curcumin suppresses growth and chemoresistance of human glioblastoma cells via AP-1 and NFκB transcription factors. J. Neurochem. 102 522–538. 10.1111/j.1471-4159.2007.04633.x [DOI] [PubMed] [Google Scholar]
- Donnelly L. E., Newton R., Kennedy G. E., Fenwick P. S., Leung R. H., Ito K., et al. (2004). Anti-inflammatory effects of resveratrol in lung epithelial cells: molecular mechanisms. Am. J. Physiol. Lung. Cell. Mol. Physiol. 287 4 10.1152/ajplung.00110.2004 [DOI] [PubMed] [Google Scholar]
- Fan G. W., Zhang Y., Jiang X., Zhu Y., Wang B., Su L., et al. (2013). Anti-inflammatory activity of baicalein in LPS-stimulated RAW264.7 macrophages via estrogen receptor and Nf-κB-dependent pathways. Inflammation 36 1584–1591. 10.1007/s10753-013-9703-2 [DOI] [PubMed] [Google Scholar]
- Fotsis T., Pepper M., Adlercreutz H., Hase T., Montesano R., Schweigerer L. (1995). Genistein, a dietary ingested isoflavonoid, inhibits cell proliferation and in vitro angiogenesis. J. Nutr. 125 790S–797S. [DOI] [PubMed] [Google Scholar]
- Fürst R., Zündorf I. (2014). Plant-derived anti-inflammatory compounds: hopes and disappointments regarding the translation of preclinical knowledge into clinical progress. Mediat. Inflamm. 2014:146832 10.1155/2014/146832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X., Kuo J., Jiang H., Deeb D., Liu Y., Divine G., et al. (2004). Immunomodulatory activity of curcumin: suppression of lymphocyte proliferation, development of cell-mediated cytotoxicity, and cytokine production in vitro. Biochem. Pharmacol. 68 51–61. 10.1016/j.bcp.2004.03.015 [DOI] [PubMed] [Google Scholar]
- Gao X., Xu Y. X., Janakiraman N., Chapman R. A., Gautam S. C. (2001). Immunomodulatory activity of resveratrol: suppression of lymphocyte proliferation, development of cell-mediated cytotoxicity, and cytokine production. Biochem. Pharmacol. 62 1299–1308. 10.1016/S0006-2952(01)00775-4 [DOI] [PubMed] [Google Scholar]
- Gao Y., Jiang W., Dong C., Li C., Fu X., Min L., et al. (2012). Anti-inflammatory effects of sophocarpine in LPS-induced RAW 264.7 cells via NF-κB and MAPKs signaling pathways. Toxicol. In Vitro 26 1–6. [DOI] [PubMed] [Google Scholar]
- Gilman’s G. (2008). Manual of Pharmacology and Therapeutics. San Diego LaJolla, CA: Professor of Pharmacology & Medicine University of California. [Google Scholar]
- Golan D. E. (2008). Principles of Pharmacology. The Pathophysiologic Basic of Drug Therapy, 2nd Edn Pennsylvania, PA: Lippincott Williams & Wilkins, 795–809. [Google Scholar]
- Guo Q., Zhao L., You Q., Yang Y., Gu H., Song G., et al. (2007). Anti-hepatitis B virus activity of wogonin in vitro and in vivo. Antiviral Res. 74 16–24. 10.1016/j.antiviral.2007.01.002 [DOI] [PubMed] [Google Scholar]
- Haanpaa M., Treede R. D. (2012). Capsaicin for neuropathic pain: linking traditional medicine and molecular biology. Eur. Neurol. 68 264–275. 10.1159/000339944 [DOI] [PubMed] [Google Scholar]
- Hamalainen M., Nieminen R., Vuorela P., Heinonen M., Moilanen E. (2007). Anti-inflammatory effects of flavonoids: genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-κB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-κB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediators Inflamm. 2007:45673 10.1155/2007/45673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansel T. T., Kropshofer H., Singer T., Mitchell J. A., George A. J. (2010). The safety and side effects of monoclonal antibodies. Nat. Rev. Drug Discov. 9 325–338. 10.1038/nrd3003 [DOI] [PubMed] [Google Scholar]
- Hara Y., Fujino M., Adachi K., Li X. K. (2006). The reduction of hypoxia-induced and reoxygenation-induced apoptosis in rat islets by epigallocatechin gallate. Transplant. Proc. 38 2722–2725. 10.1016/j.transproceed.2006.08.010 [DOI] [PubMed] [Google Scholar]
- Hartog A., Smit H. F., Van Der Kraan P. M., Hoijer M. A., Garssen J. (2009). In vitro and in vivo modulation of cartilage degradation by a standardized Centella asiatica fraction. Exp. Biol. Med. 234 617–623. 10.3181/0810-RM-298 [DOI] [PubMed] [Google Scholar]
- Heinz S. A., Henson D. A., Nieman D. C., Austin M. D., Jin F. (2010). A 12-week supplementation with quercetin does not affect natural killer cell activity, granulocyte oxidative burst activity or granulocyte phagocytosis in female human subjects. Br. J. Nutr. 104 849–857. 10.1017/S000711451000156X [DOI] [PubMed] [Google Scholar]
- Hidalgo M. A., Romero A., Figueroa J., Cortes P., Concha I. I., Hancke J. L., et al. (2005). Andrographolide interferes with binding of nuclear factor-kappaB to DNA in HL-60-derived neutrophilic cells. Br. J. Pharmacol. 144 680–686. 10.1038/sj.bjp.0706105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden N. S., Squires P. E., Kaur M., Bland R., Jones C. E., Newton R. (2008). Phorbol ester-stimulated NF-κB-dependent transcription: roles for isoforms of novel protein kinase C. Cell. Signal. 20 1338–1348. 10.1016/j.cellsig.2008.03.001 [DOI] [PubMed] [Google Scholar]
- Holmes-McNary M., Baldwin A. S., Jr. (2000). Chemopreventive properties of trans-resveratrol are associated with inhibition of activation of the IκB kinase. Cancer Res. 60 3477–3483. [PubMed] [Google Scholar]
- Hong M. H., Kim M. H., Chang H. J., Kim N. H., Shin B. A., Ahn B. W., et al. (2007). (-)-Epigallocatechin-3-gallate inhibits monocyte chemotactic protein-1 expression in endothelial cells via blocking NF-κB signaling. Life Sci. 80 1957–1965. 10.1016/j.lfs.2007.02.024 [DOI] [PubMed] [Google Scholar]
- Hooshmand S., Soung Do Y., Lucas E. A., Madihally S. V., Levenson C. W., Arjmandi B. H. (2007). Genistein reduces the production of proinflammatory molecules in human chondrocytes. J. Nutr. Biochem. 18 609–614. 10.1016/j.jnutbio.2006.11.006 [DOI] [PubMed] [Google Scholar]
- Hougee S., Sanders A., Faber J., Graus Y. M. F., Van Den Berg W. B., Garssen J., et al. (2005). Decreased pro-inflammatory cytokine production by LPS-stimulated PBMC upon in vitro incubation with the flavonoids apigenin, luteolin or chrysin, due to selective elimination of monocytes/macrophages. Biochem. Pharmacol. 69 241–248. 10.1016/j.bcp.2004.10.002 [DOI] [PubMed] [Google Scholar]
- Hu J.-Z., Huang J. H., Xiao Z. M., Li J. H., Li X. M., Lu H. B. (2013). Tetramethylpyrazine accelerates the function recovery of traumatic spinal cord in rat model by attenuating inflammation. J. Neurol. Sci. 324 94–99. 10.1016/j.jns.2012.10.009 [DOI] [PubMed] [Google Scholar]
- Imazio M., Bobbio M., Cecchi E., Demarie D., Demichelis B., Pomari F., et al. (2005a). Colchicine in addition to conventional therapy for acute pericarditis: results of the colchicine for acute PEricarditis (COPE) trial. Circulation 112 2012–2016. 10.1161/CIRCULATIONAHA.105.542738 [DOI] [PubMed] [Google Scholar]
- Imazio M., Bobbio M., Cecchi E., Demarie D., Pomari F., Moratti M., et al. (2005b). Colchicine as first-choice therapy for recurrent pericarditis: results of the CORE (Colchicine for REcurrent pericarditis) trial. Arch. Intern. Med. 165 1987–1991. 10.1001/archinte.165.17.1987 [DOI] [PubMed] [Google Scholar]
- Imazio M., Brucato A., Cemin R., Ferrua S., Belli R., Maestroni S., et al. (2011). Colchicine for recurrent pericarditis (CORP): a randomized trial. Ann. Intern. Med. 155 409–414. 10.7326/0003-4819-155-7-201110040-00359 [DOI] [PubMed] [Google Scholar]
- Imazio M., Brucato A., Cemin R., Ferrua S., Maggiolini S., Beqaraj F., et al. (2013). A randomized trial of colchicine for acute pericarditis. N. Engl. J. Med. 369 1522–1528. 10.1056/NEJMoa1208536 [DOI] [PubMed] [Google Scholar]
- Imazio M., Trinchero R., Brucato A., Rovere M. E., Gandino A., Cemin R., et al. (2010). Colchicine for the prevention of the post-pericardiotomy syndrome (COPPS): a multicentre, randomized, double-blind, placebo-controlled trial. Eur. Heart J. 31 2749–2754. 10.1093/eurheartj/ehq319 [DOI] [PubMed] [Google Scholar]
- Iruretagoyena M. I., Tobar J. A., Gonzalez P. A., Sepulveda S. E., Figueroa C. A., Burgos R. A., et al. (2005). Andrographolide interferes with T cell activation and reduces experimental autoimmune encephalomyelitis in the mouse. J. Pharmacol. Exp. Ther. 312 366–372. 10.1124/jpet.104.072512 [DOI] [PubMed] [Google Scholar]
- Issuree P. D., Pushparaj P. N., Pervaiz S., Melendez A. J. (2009). Resveratrol attenuates C5a-induced inflammatory responses in vitro and in vivo by inhibiting phospholipase D and sphingosine kinase activities. FASEB J. 23 2412–2424. 10.1096/fj.09-130542 [DOI] [PubMed] [Google Scholar]
- Ito T., Warnken S. P., May W. S. (1999). Protein synthesis inhibition by flavonoids: roles of eukaryotic initiation factor 2alpha kinases. Biochem. Biophys. Res. Commun. 265 589–594. 10.1006/bbrc.1999.1727 [DOI] [PubMed] [Google Scholar]
- Jain S. (2008). Handbook of Pharmacology. London: Pars Publication. [Google Scholar]
- Joe B., Rao U. J., Lokesh B. R. (1997). Presence of an acidic glycoprotein in the serum of arthritic rats: modulation by capsaicin and curcumin. Mol. Cell. Biochem. 169 125–134. 10.1023/a:1006877928703 [DOI] [PubMed] [Google Scholar]
- Jurenka J. S. (2009). Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Altern. Med. Rev. 14 141–153. [PubMed] [Google Scholar]
- Kang H.-K., Ecklund D., Liu M., Datta S. K. (2009). Apigenin, a non-mutagenic dietary flavonoid, suppresses lupus by inhibiting autoantigen presentation for expansion of autoreactive Th1 and Th17 cells. Arthritis Res. Ther. 11 R59. 10.1186/ar2682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J., Zhang Y., Cao X., Fan J., Li G., Wang Q., et al. (2012). Lycorine inhibits lipopolysaccharide-induced iNOS and COX-2 up-regulation in RAW264.7 cells through suppressing P38 and STATs activation and increases the survival rate of mice after LPS challenge. Int. Immunopharmacol. 12 249–256. 10.1016/j.intimp.2011.11.018 [DOI] [PubMed] [Google Scholar]
- Kang S. R., Park K. I., Park H. S., Lee D. H., Kim J. A., Nagappan A., et al. (2011). Anti-inflammatory effect of flavonoids isolated from Korea Citrus aurantium L. on lipopolysaccharide-induced mouse macrophage RAW 264.7 cells by blocking of nuclear factor-kappa B (NF-κB) and mitogen-activated protein kinase (MAPK) signalling pathways. Food Chem. 129 1721–1728. 10.1016/j.foodchem.2011.06.039 [DOI] [Google Scholar]
- Kannaiyan R., Shanmugam M. K., Sethi G. (2011). Molecular targets of celastrol derived from Thunder of God Vine: potential role in the treatment of inflammatory disorders and cancer. Cancer Lett. 303 9–20. 10.1016/j.canlet.2010.10.025 [DOI] [PubMed] [Google Scholar]
- Katzung G. B., Trevor J. A. (2009). Basic and Clinical Pharmacology. New York, NY: Lange Medical Books/McGraw Hill, 2004. [Google Scholar]
- Keane J., Gershon S., Wise R. P., Mirabile-Levens E., Kasznica J., Schwieterman W. D., et al. (2001). Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N. Engl. J. Med. 345 1098–1104. 10.1056/NEJMoa011110 [DOI] [PubMed] [Google Scholar]
- Khajuria A., Gupta A., Garai S., Wakhloo B. P. (2007). Immunomodulatory effects of two sapogenins 1 and 2 isolated from Luffa cylindrica in Balb/C mice. Bioorg. Med. Chem. Lett. 17 1608–1612. 10.1016/j.bmcl.2006.12.091 [DOI] [PubMed] [Google Scholar]
- Kim C. S., Kawada T., Kim B. S., Han I. S., Choe S. Y., Kurata T., et al. (2003). Capsaicin exhibits anti-inflammatory property by inhibiting IkB-a degradation in LPS-stimulated peritoneal macrophages. Cell. Signal. 15 299–306. 10.1016/S0898-6568(02)00086-4 [DOI] [PubMed] [Google Scholar]
- Kim G. Y., Kim K. H., Lee S. H., Yoon M. S., Lee H. J., Moon D. O., et al. (2005). Curcumin inhibits immunostimulatory function of dendritic cells: MAPKs and translocation of NF-κB as potential targets. J. Immunol. 174 8116–8124. 10.4049/jimmunol.174.12.8116 [DOI] [PubMed] [Google Scholar]
- Kim S. Y., Moon K. A., Jo H. Y., Jeong S., Seon S. H., Jung E., et al. (2012). Anti-inflammatory effects of apocynin, an inhibitor of NADPH oxidase, in airway inflammation. Immunol. Cell Biol. 90 441–448. 10.1038/icb.2011.60 [DOI] [PubMed] [Google Scholar]
- Kim S., Shin H. J., Kim S. Y., Kim J. H., Lee Y. S., Kim D. H., et al. (2004). Genistein enhances expression of genes involved in fatty acid catabolism through activation of PPARalpha. Mol. Cell. Endocrinol. 220 51–58. 10.1016/j.mce.2004.03.011 [DOI] [PubMed] [Google Scholar]
- Kleemann R., Verschuren L., Morrison M., Zadelaar S., Van Erk M. J., Wielinga P. Y., et al. (2011). Anti-inflammatory, anti-proliferative and anti-atherosclerotic effects of quercetin in human in vitro and in vivo models. Atherosclerosis 218 44–52. 10.1016/j.atherosclerosis.2011.04.023 [DOI] [PubMed] [Google Scholar]
- Kobuchi H., Roy S., Sen C. K., Nguyen H. G., Packer L. (1999). Quercetin inhibits inducible ICAM-1 expression in human endothelial cells through the JNK pathway. Am. J. Physiol. 277 C403–C411. [DOI] [PubMed] [Google Scholar]
- Koeberle A., Pollastro F., Northoff H., Werz O. (2009). Myrtucommulone, a natural acylphloroglucinol, inhibits microsomal prostaglandin E(2) synthase-1. Br. J. Pharmacol. 156 952–961. 10.1111/j.1476-5381.2009.00070.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong L., Luo C., Li X., Zhou Y., He H. (2013). The anti-inflammatory effect of kaempferol on early atherosclerosis in high cholesterol fed rabbits. Lipids Health Dis. 12 112–115. 10.1186/1476-511X-12-115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalski J., Samojedny A., Paul M., Pietsz G., Wilczok T. (2005). Effect of apigenin, kaempferol and resveratrol on the expression of interleukin-1beta and tumor necrosis factor-alpha genes in J774.2 macrophages. Pharmacol. Rep. 57 390–394. [PubMed] [Google Scholar]
- Kumar A., Dhawan S., Hardegen N. J., Aggarwal B. B. (1998). Curcumin (Diferuloylmethane) inhibition of tumor necrosis factor (TNF)-mediated adhesion of monocytes to endothelial cells by suppression of cell surface expression of adhesion molecules and of nuclear factor-kappa B activation. Biochem. Pharmacol. 55 775–783. 10.1016/S0006-2952(97)00557-1 [DOI] [PubMed] [Google Scholar]
- Kumar R. A., Sridevi K., Kumar N. V., Nanduri S., Rajagopal S. (2004). Anticancer and immunostimulatory compounds from Andrographis paniculata. J. Ethnopharmacol. 92 291–295. 10.1016/j.jep.2004.03.004 [DOI] [PubMed] [Google Scholar]
- Kumar S., Ahuja V., Sankar M. J., Kumar A., Moss A. C. (2012). Curcumin for maintenance of remission in ulcerative colitis. Cochrane Database Syst. Rev. 10 CD008424 10.1002/14651858.CD008424.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H. N., Cho H. J., Lim D. Y., Kang Y.-H., Lee K. W., Park J. H. Y. (2013a). Mechanisms by which licochalcone e exhibits potent anti-inflammatory properties: studies with phorbol ester-treated mouse skin and lipopolysaccharide-stimulated murine macrophages. Int. J. Mol. Sci. 14 10926–10943. 10.3390/ijms140610926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I. T., Lin C. C., Lee C. Y., Hsieh P. W., Yang C. M. (2013b). Protective effects of (-)-epigallocatechin-3-gallate against TNF-α-induced lung inflammation via ROS-dependent ICAM-1 inhibition. J. Nutr. Biochem. 24 124–136. 10.1016/j.jnutbio.2012.03.009 [DOI] [PubMed] [Google Scholar]
- Lee K. C., Chang H. H., Chung Y. H., Lee T. Y. (2011). Andrographolide acts as an anti-inflammatory agent in LPS-stimulated RAW264.7 macrophages by inhibiting STAT3-mediated suppression of the NF-κB pathway. J. Ethnopharmacol. 135 678–684. 10.1016/j.jep.2011.03.068 [DOI] [PubMed] [Google Scholar]
- Lee W., Ku S.-K., Bae J.-S. (2015). Anti-inflammatory effects of Baicalin, Baicalein, and Wogonin in vitro and in vivo. Inflammation 38 110–125. 10.1007/s10753-014-0013-0 [DOI] [PubMed] [Google Scholar]
- Lee W. J., Shim J. Y., Zhu B. T. (2005). Mechanisms for the inhibition of DNA methyltransferases by tea catechins and bioflavonoids. Mol. Pharmacol. 68 1018–1030. 10.1124/mol.104.008367 [DOI] [PubMed] [Google Scholar]
- Lee Y. W., Lee W. H. (2008). Protective effects of genistein on proinflammatory pathways in human brain microvascular endothelial cells. J. Nutr. Biochem. 19 819–825. 10.1016/j.jnutbio.2007.10.006 [DOI] [PubMed] [Google Scholar]
- Li H., Gong X., Zhang L., Zhang Z., Luo F., Zhou Q., et al. (2009). Madecassoside attenuates inflammatory response on collagen-induced arthritis in DBA/1 mice. Phytomedicine 16 538–546. 10.1016/j.phymed.2008.11.002 [DOI] [PubMed] [Google Scholar]
- Li P., Yin Y. L., Li D., Kim S. W., Wu G. (2007). Amino acids and immune function. Br. J. Nutr. 98 237–252. 10.1017/S000711450769936X [DOI] [PubMed] [Google Scholar]
- Lin W. C., Lin J. Y. (2011). Berberine down-regulates the Th1/Th2 cytokine gene expression ratio in mouse primary splenocytes in the absence or presence of lipopolysaccharide in a preventive manner. Int. Immunopharmacol. 11 1984–1990. 10.1016/j.intimp.2011.08.008 [DOI] [PubMed] [Google Scholar]
- Liu H.-T., Du Y.-G., He J.-L., Chen W.-J., Li W.-M., Yang Z., et al. (2010). Tetramethylpyrazine inhibits production of nitric oxide and inducible nitric oxide synthase in lipopolysaccharide-induced N9 microglial cells through blockade of MAPK and PI3K/Akt signaling pathways, and suppression of intracellular reactive oxygen species. J. Ethnopharmacol. 129 335–343. 10.1016/j.jep.2010.03.037 [DOI] [PubMed] [Google Scholar]
- Liu L., Li Y. (2014). The unexpected side effects and safety of therapeutic monoclonal antibodies. Drugs Today 50 33–50. 10.1358/dot.2014.50.1.2076506 [DOI] [PubMed] [Google Scholar]
- Liu X., Mei Z., Qian J., Zeng Y., Wang M. (2013). Puerarin partly counteracts the inflammatory response after cerebral ischemia/reperfusion via activating the cholinergic anti-inflammatory pathway. Neural Regen. Res. 8 3203 10.3969/j.issn.1673-5374.2013.34.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X. H., Pan L. L., Yang H. B., Gong Q. H., Zhu Y. Z. (2012). Leonurine attenuates lipopolysaccharide-induced inflammatory responses in human endothelial cells: involvement of reactive oxygen species and NF-κB pathways. Eur. J. Pharmacol 680 108–114. 10.1016/j.ejphar.2012.01.012 [DOI] [PubMed] [Google Scholar]
- Lucas C. D., Dorward D. A., Sharma S., Rennie J., Felton J. M., Alessandri A. L., et al. (2015). Wogonin induces eosinophil apoptosis and attenuates allergic airway inflammation. Am. J. Respir. Crit. Care Med. 191 626–636. 10.1164/rccm.201408-1565OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiti K., Gantait A., Mukherjee K., Saha B., Mukherjee P. K. (2006). Therapeutic potentials of andrographolide from Andrographis paniculata: a review. J. Nat. Remed. 6 1–13. [Google Scholar]
- Manna S. K., Mukhopadhyay A., Aggarwal B. B. (2000). Resveratrol suppresses TNF-induced activation of nuclear transcription factors NF-κB, activator protein-1, and apoptosis: potential role of reactive oxygen intermediates and lipid peroxidation. J. Immunol. 164 6509–6519. 10.4049/jimmunol.164.12.6509 [DOI] [PubMed] [Google Scholar]
- Mccabe M. J., Jr., Orrenius S. (1993). Genistein induces apoptosis in immature human thymocytes by inhibiting topoisomerase-II. Biochem. Biophys. Res. Commun. 194 944–950. 10.1006/bbrc.1993.1912 [DOI] [PubMed] [Google Scholar]
- Middleton E., Jr., Kandaswami C., Theoharides T. C. (2000). The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 52 673–751. [PubMed] [Google Scholar]
- Moradali M. F., Mostafavi H., Ghods S., Hedjaroude G. A. (2007). Immunomodulating and anticancer agents in the realm of macromycetes fungi (macrofungi). Int. Immunopharmacol. 7 701–724. 10.1016/j.intimp.2007.01.008 [DOI] [PubMed] [Google Scholar]
- Muraoka K., Shimizu K., Sun X., Tani T., Izumi R., Miwa K., et al. (2002). Flavonoids exert diverse inhibitory effects on the activation of NF-κB. Transplant. Proc. 34 1335–1340. 10.1016/S0041-1345(02)02795-1 [DOI] [PubMed] [Google Scholar]
- Mythili M., Nair S., Gunasekaran S. (2004). Effect of cyclophosphamide pretreatment on hematological indices of Indian Bonnet monkeys. Indian J. Pharmacol. 36 175. [Google Scholar]
- Nagarajan S., Burris R. L., Stewart B. W., Wilkerson J. E., Badger T. M. (2008). Dietary soy protein isolate ameliorates atherosclerotic lesions in apolipoprotein E-deficient mice potentially by inhibiting monocyte chemoattractant protein-1 expression. J. Nutr. 138 332–337. [DOI] [PubMed] [Google Scholar]
- Nicholas C., Batra S., Vargo M. A., Voss O. H., Gavrilin M. A., Wewers M. D., et al. (2007). Apigenin blocks lipopolysaccharide-induced lethality in vivo and proinflammatory cytokines expression by inactivating NF-κB through the suppression of p65 phosphorylation. J. Immunol. 179 7121–7127. 10.4049/jimmunol.179.10.7121 [DOI] [PubMed] [Google Scholar]
- Niu X. F., Zhou P., Li W. F., Xu H. B. (2011). Effects of chelerythrine, a specific inhibitor of cyclooxygenase-2, on acute inflammation in mice. Fitoterapia 82 620–625. 10.1016/j.fitote.2011.01.020 [DOI] [PubMed] [Google Scholar]
- Nuki G. (2008). Colchicine: its mechanism of action and efficacy in crystal-induced inflammation. Curr. Rheumatol. Rep. 10 218–227. 10.1007/s11926-008-0036-3 [DOI] [PubMed] [Google Scholar]
- Oberlies N. H., Kroll D. J. (2004). Camptothecin and taxol: historic achievements in natural products research. J. Nat. Prod. 67 129–135. 10.1021/np030498t [DOI] [PubMed] [Google Scholar]
- Oh S. R., Kinjo J., Shii Y., Ikeda T., Nohara T., Ahn K. S., et al. (2000). Effects of triterpenoids from Pueraria lobata on immunohemolysis: beta-D-glucuronic acid plays an active role in anticomplementary activity in vitro. Planta Med. 66 506–510. 10.1055/s-2000-8614 [DOI] [PubMed] [Google Scholar]
- Oh Y. C., Kang O. H., Kim S. B., Mun S. H., Park C. B., Kim Y. G., et al. (2012). Anti-inflammatory effect of sinomenine by inhibition of pro-inflammatory mediators in PMA plus A23187-stimulated HMC-1 Cells. Eur. Rev. Med. Pharmacol. Sci. 16 1184–1191. [PubMed] [Google Scholar]
- O’Neill J., Brock C., Olesen A. E., Andresen T., Nilsson M., Dickenson A. H. (2012). Unravelling the mystery of capsaicin: a tool to understand and treat pain. Pharmacol. Rev. 64 939–971. 10.1124/pr.112.006163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H. H., Lee S., Son H. Y., Park S. B., Kim M. S., Choi E. J., et al. (2008). Flavonoids inhibit histamine release and expression of proinflammatory cytokines in mast cells. Arch. Pharm. Res. 31 1303–1311. 10.1007/s12272-001-2110-5 [DOI] [PubMed] [Google Scholar]
- Park H. J., Jeong S. K., Kim S. R., Bae S. K., Kim W. S., Jin S. D., et al. (2009). Resveratrol inhibits Porphyromonas gingivalis lipopolysaccharide-induced endothelial adhesion molecule expression by suppressing NF-κB activation. Arch. Pharm. Res. 32 583–591. 10.1007/s12272-009-1415-7 [DOI] [PubMed] [Google Scholar]
- Park H. J., Shin D. H., Chung W. J., Leem K., Yoon S. H., Hong M. S., et al. (2006). Epigallocatechin gallate reduces hypoxia-induced apoptosis in human hepatoma cells. Life Sci. 78 2826–2832. 10.1016/j.lfs.2005.11.001 [DOI] [PubMed] [Google Scholar]
- Park J. S., Choi M. A., Kim B. S., Han I. S., Kurata T., Yu R. (2000). Capsaicin protects against ethanol-induced oxidative injury in the gastric mucosa of rats. Life Sci. 67 3087–3093. 10.1016/S0024-3205(00)00890-0 [DOI] [PubMed] [Google Scholar]
- Parkin J., Cohen B. (2001). An overview of the immune system. Lancet 357 1777–1789. 10.1016/S0140-6736(00)04904-7 [DOI] [PubMed] [Google Scholar]
- Parnham M. J., Nijkamp F. P. (2005). Principles of Immunopharmacology. Basel: Birkhauser Verlag. [Google Scholar]
- Pfeuffer M., Auinger A., Bley U., Kraus-Stojanowic I., Laue C., Winkler P., et al. (2013). Effect of quercetin on traits of the metabolic syndrome, endothelial function and inflammation in men with different APOE isoforms. Nutr. Metab. Cardiovasc. Dis. 23 403–409. 10.1016/j.numecd.2011.08.010 [DOI] [PubMed] [Google Scholar]
- Puri A., Saxena R., Saxena R. P., Saxena K. C., Srivastava V., Tandon J. S. (1994). Immunostimulant activity of Nyctanthes arbor-tristis L. J. Ethnopharmacol. 42 31–37. 10.1016/0378-8741(94)90020-5 [DOI] [PubMed] [Google Scholar]
- Qin L. H., Kong L., Shi G. J., Wang Z. T., Ge B. X. (2006). Andrographolide inhibits the production of TNF-alpha and interleukin-12 in lipopolysaccharide-stimulated macrophages: role of mitogen-activated protein kinases. Biol. Pharm. Bull. 29 220–224. 10.1248/bpb.29.220 [DOI] [PubMed] [Google Scholar]
- Rajagopal S., Kumar R. A., Deevi D. S., Satyanarayana C., Rajagopalan R. (2003). Andrographolide, a potential cancer therapeutic agent isolated from Andrographis paniculata. J. Exp. Ther. Oncol. 3 147–158. 10.1046/j.1359-4117.2003.01090.x [DOI] [PubMed] [Google Scholar]
- Ramgolam V., Ang S. G., Lai Y. H., Loh C. S., Yap H. K. (2000). Traditional Chinese medicines as immunosuppressive agents. Ann. Acad. Med. Singapore 29 11–16. [PubMed] [Google Scholar]
- Rang H. P., Dale M. M. (2007). Rang and Dale Pharmacology. Edinburgh: Churchill Livingestone. [Google Scholar]
- Richard A. H., Pamela C. C. (2009). Lippincott’s Illustrated Reviews: Pharmacology, 4th Edn New Delhi: Wolters Kluwer. [Google Scholar]
- Ruiz P. A., Braune A., Holzlwimmer G., Quintanilla-Fend L., Haller D. (2007). Quercetin inhibits TNF-induced NF-κB transcription factor recruitment to proinflammatory gene promoters in murine intestinal epithelial cells. J. Nutr. 137 1208–1215. [DOI] [PubMed] [Google Scholar]
- Sadava D., Whitlock E., Kane S. E. (2007). The green tea polyphenol, epigallocatechin-3-gallate inhibits telomerase and induces apoptosis in drug-resistant lung cancer cells. Biochem. Biophys. Res. Commun. 360 233–237. 10.1016/j.bbrc.2007.06.030 [DOI] [PubMed] [Google Scholar]
- Sandborn W., Targan S., Byers V., Tang T. (2010). Randomized, double-blind, placebo controlled trial of Andrographis paniculata extract (HMPL-004) in patients with moderately active Crohn’s disease. Am. J. Gastroenterol. 105(Suppl. 1), s429–s430. [Google Scholar]
- Sandborn W. J., Targan S. R., Byers V. S., Rutty D. A., Mu H., Zhang X., et al. (2013). Andrographis paniculata extract (HMPL-004) for active ulcerative colitis. Am. J. Gastroenterol. 108 90–98. 10.1038/ajg.2012.340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seibel J., Molzberger A. F., Hertrampf T., Laudenbach-Leschowski U., Diel P. (2009). Oral treatment with genistein reduces the expression of molecular and biochemical markers of inflammation in a rat model of chronic TNBS-induced colitis. Eur. J. Nutr. 48 213–220. 10.1007/s00394-009-0004-3 [DOI] [PubMed] [Google Scholar]
- Sengupta P. R. (2009). Medical Pharmacology. New Delhi: CBS Publisher. [Google Scholar]
- Sgro C. (1995). Side-effects of a monoclonal antibody, muromonab CD3/orthoclone OKT3: bibliographic review. Toxicology 105 23–29. 10.1016/0300-483X(95)03123-W [DOI] [PubMed] [Google Scholar]
- Shaik Y. B., Castellani M. L., Perrella A., Conti F., Salini V., Tete S., et al. (2006). Role of quercetin (a natural herbal compound) in allergy and inflammation. J. Biol. Regul. Homeost. Agents 20 47–52. [PubMed] [Google Scholar]
- Shen Y. C., Chen C. F., Chiou W. F. (2002). Andrographolide prevents oxygen radical production by human neutrophils: possible mechanism(s) involved in its anti-inflammatory effect. Br. J. Pharmacol. 135 399–406. 10.1038/sj.bjp.0704493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin E. K., Kwon H. S., Kim Y. H., Shin H. K., Kim J. K. (2009). Chrysin, a natural flavone, improves murine inflammatory bowel diseases. Biochem. Biophys. Res. Commun. 381 502–507. 10.1016/j.bbrc.2009.02.071 [DOI] [PubMed] [Google Scholar]
- Si H., Liu D. (2007). Phytochemical genistein in the regulation of vascular function: new insights. Curr. Med. Chem 14 2581–2589. 10.2174/092986707782023325 [DOI] [PubMed] [Google Scholar]
- Singh B. N., Shankar S., Srivastava R. K. (2011). Green tea catechin, epigallocatechin-3-gallate (EGCG): mechanisms, perspectives and clinical applications. Biochem. Pharmacol. 82 1807–1821. 10.1016/j.bcp.2011.07.093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S., Aggarwal B. B. (1995). Activation of transcription factor NF-κB is suppressed by curcumin (diferuloylmethane) [corrected]. J. Biol. Chem. 270 24995–25000. 10.1074/jbc.270.42.24995 [DOI] [PubMed] [Google Scholar]
- Singhal K. C. (2007). Essentials of Pharmacotherapeutics. New Delhi: CBS Publishers. [Google Scholar]
- Son D. J., Akiba S., Hong J. T., Yun Y. P., Hwang S. Y., Park Y. H., et al. (2014). Piperine inhibits the activities of platelet cytosolic phospholipase A2 and thromboxane A2 synthase without affecting cyclooxygenase-1 activity: different mechanisms of action are involved in the inhibition of platelet aggregation and macrophage inflammatory response. Nutrients 6 3336–3352. 10.3390/nu6083336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J. Y., Han S. K., Son E. H., Pyo S. N., Yun Y. S., Yi S. Y. (2002). Induction of secretory and tumoricidal activities in peritoneal macrophages by ginsan. Int. Immunopharmacol. 2 857–865. 10.1016/S1567-5769(01)00211-9 [DOI] [PubMed] [Google Scholar]
- Song X., Wang T., Zhang Z., Jiang H., Wang W., Cao Y., et al. (2015). Leonurine exerts anti-inflammatory effect by regulating inflammatory signaling pathways and cytokines in lps-induced mouse mastitis. Inflammation 38 79–88. 10.1007/s10753-014-0009-9 [DOI] [PubMed] [Google Scholar]
- Song Y., Qu R., Zhu S., Zhang R., Ma S. (2012). Rhynchophylline attenuates LPS-induced pro-inflammatory responses through down-regulation of MAPK/NF-κB signaling pathways in primary microglia. Phytother. Res. 26 1528–1533. 10.1002/ptr.4614 [DOI] [PubMed] [Google Scholar]
- Spellberg B., Edwards J. E., Jr. (2001). Type 1/Type 2 immunity in infectious diseases. Clin. Infect. Dis 32 76–102. 10.1086/317537 [DOI] [PubMed] [Google Scholar]
- Stanton R. A., Gernert K. M., Nettles J. H., Aneja R. (2011). Drugs that target dynamic microtubules: a new molecular perspective. Med. Res. Rev. 31 443–481. 10.1002/med.20242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanska J., Pawliczak R. (2008). Apocynin: molecular aptitudes. Mediators Inflamm. 2008:106507 10.1155/2008/106507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strayer D. R., Carter W. A. (2012). Recombinant and natural human interferons: analysis of the incidence and clinical impact of neutralizing antibodies. J. Interferon Cytokine Res. 32 95–102. 10.1089/jir.2011.0069 [DOI] [PubMed] [Google Scholar]
- Surh Y. J., Chun K. S., Cha H. H., Han S. S., Keum Y. S., Park K. K., et al. (2001). Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: down-regulation of COX-2 and iNOS through suppression of NF-κB activation. Mutat. Res. 481 243–268. 10.1016/S0027-5107(01)00183-X [DOI] [PubMed] [Google Scholar]
- Tang T., Targan S. R., Li Z. S., Xu C., Byers V. S., Sandborn W. J. (2011). Randomised clinical trial: herbal extract HMPL-004 in active ulcerative colitis - a double-blind comparison with sustained release mesalazine. Aliment. Pharmacol. Ther. 33 194–202. 10.1111/j.1365-2036.2010.04515.x [DOI] [PubMed] [Google Scholar]
- Tome-Carneiro J., Larrosa M., Yanez-Gascon M. J., Davalos A., Gil-Zamorano J., Gonzalvez M., et al. (2013). One-year supplementation with a grape extract containing resveratrol modulates inflammatory-related microRNAs and cytokines expression in peripheral blood mononuclear cells of type 2 diabetes and hypertensive patients with coronary artery disease. Pharmacol. Res. 72 69–82. 10.1016/j.phrs.2013.03.011 [DOI] [PubMed] [Google Scholar]
- Tortora G. J., Derrickson B. P. (2008). Principles of Anatomy and Physiology, 12th Edn Hoboken, NJ: John Wiley & Sons Inc. [Google Scholar]
- Tsai H. R., Yang L. M., Tsai W. J., Chiou W. F. (2004). Andrographolide acts through inhibition of ERK1/2 and Akt phosphorylation to suppress chemotactic migration. Eur. J. Pharmacol 498 45–52. 10.1016/j.ejphar.2004.07.077 [DOI] [PubMed] [Google Scholar]
- Vaibhav K., Shrivastava P., Javed H., Khan A., Ahmed M. E., Tabassum R., et al. (2012). Piperine suppresses cerebral ischemia–reperfusion-induced inflammation through the repression of COX-2, NOS-2, and NF-κB in middle cerebral artery occlusion rat model. Mol. Cell. Biochem. 367 73–84. 10.1007/s11010-012-1321-z [DOI] [PubMed] [Google Scholar]
- Vaillancourt F., Silva P., Shi Q., Fahmi H., Fernandes J. C., Benderdour M. (2011). Elucidation of molecular mechanisms underlying the protective effects of thymoquinone against rheumatoid arthritis. J. Cell. Biochem. 112 107–117. 10.1002/jcb.22884 [DOI] [PubMed] [Google Scholar]
- Valles S. L., Dolz-Gaiton P., Gambini J., Borras C., Lloret A., Pallardo F. V., et al. (2010). Estradiol or genistein prevent Alzheimer’s disease-associated inflammation correlating with an increase PPAR gamma expression in cultured astrocytes. Brain Res. 2 138–144. 10.1016/j.brainres.2009.11.044 [DOI] [PubMed] [Google Scholar]
- Vang O., Ahmad N., Baile C. A., Baur J. A., Brown K., Csiszar A., et al. (2011). What is new for an old molecule? Systematic review and recommendations on the use of resveratrol. PLoS ONE 6:e19881 10.1371/journal.pone.0019881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesely M. D., Kershaw M. H., Schreiber R. D., Smyth M. J. (2011). Natural innate and adaptive immunity to cancer. Annu. Rev. Immunol. 29 235–271. 10.1146/annurev-immunol-031210-101324 [DOI] [PubMed] [Google Scholar]
- Wang J., Zhang Q., Jin S., He D., Zhao S., Liu S. (2008). Genistein modulate immune responses in collagen-induced rheumatoid arthritis model. Maturitas 59 405–412. 10.1016/j.maturitas.2008.04.003 [DOI] [PubMed] [Google Scholar]
- Wang T., Liu B., Zhang W., Wilson B., Hong J. S. (2004). Andrographolide reduces inflammation-mediated dopaminergic neurodegeneration in mesencephalic neuron-glia cultures by inhibiting microglial activation. J. Pharmacol. Exp. Ther. 308 975–983. 10.1124/jpet.103.059683 [DOI] [PubMed] [Google Scholar]
- Wang X., Chen S., Ma G., Ye M., Lu G. (2005). Genistein protects dopaminergic neurons by inhibiting microglial activation. Neuroreport 16 267–270. 10.1097/00001756-200502280-00013 [DOI] [PubMed] [Google Scholar]
- Wang Z., Lee Y., Eun J. S., Bae E. J. (2014). Inhibition of adipocyte inflammation and macrophage chemotaxis by butein. Eur. J. Pharmacol. 738 40–48. 10.1016/j.ejphar.2014.05.031 [DOI] [PubMed] [Google Scholar]
- Wu C. J., Wang Y. H., Lin C. J., Chen H. H., Chen Y. J. (2011). Tetrandrine down-regulates ERK/NF-κB signaling and inhibits activation of mesangial cells. Toxicol In Vitro 25 1834–1840. 10.1016/j.tiv.2011.09.024 [DOI] [PubMed] [Google Scholar]
- Wung B. S., Hsu M. C., Wu C. C., Hsieh C. W. (2005). Resveratrol suppresses IL-6-induced ICAM-1 gene expression in endothelial cells: effects on the inhibition of STAT3 phosphorylation. Life Sci. 78 389–397. 10.1016/j.lfs.2005.04.052 [DOI] [PubMed] [Google Scholar]
- Xia Y. F., Ye B. Q., Li Y. D., Wang J. G., He X. J., Lin X., et al. (2004). Andrographolide attenuates inflammation by inhibition of NF-κB activation through covalent modification of reduced cysteine 62 of p50. J. Immunol. 173 4207–4217. 10.4049/jimmunol.173.6.4207 [DOI] [PubMed] [Google Scholar]
- Xiang M. X., Xu Z., Su H. W., Hu J., Yan Y. J. (2011). Emodin-8-O-beta-D-glucoside from Polygonum amplexicaule D. Don var. sinense Forb. promotes proliferation and differentiation of osteoblastic MC3T3-E1 cells. Molecules 16 728–737. 10.3390/molecules16010728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y.-K., Liao S.-G., Na Z., Hu H.-B., Li Y., Luo H.-R. (2012). Gelsemium alkaloids, immunosuppressive agents from Gelsemium elegans. Fitoterapia 83 1120–1124. 10.1016/j.fitote.2012.04.023 [DOI] [PubMed] [Google Scholar]
- Xu Y. X., Pindolia K. R., Janakiraman N., Chapman R. A., Gautam S. C. (1997). Curcumin inhibits IL1 alpha and TNF-alpha induction of AP-1 and NF-kB DNA-binding activity in bone marrow stromal cells. Hematopathol. Mol. Hematol. 11 49–62. [PubMed] [Google Scholar]
- Yalniz M., Bahcecioglu I. H., Kuzu N., Poyrazoglu O. K., Bulmus O., Celebi S., et al. (2007). Preventive role of genistein in an experimental non-alcoholic steatohepatitis model. J. Gastroenterol. Hepatol. 22 2009–2014. 10.1111/j.1440-1746.2006.04681 [DOI] [PubMed] [Google Scholar]
- Yamakuchi M., Bao C., Ferlito M., Lowenstein C. J. (2008). Epigallocatechin gallate inhibits endothelial exocytosis. Biol. Chem. 389 935–941. 10.1515/BC.2008.095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C. S., Wang H., Li G. X., Yang Z., Guan F., Jin H. (2011a). Cancer prevention by tea: evidence from laboratory studies. Pharmacol. Res. 64 113–122. 10.1016/j.phrs.2011.03.001 [DOI] [PubMed] [Google Scholar]
- Yang H., Landis-Piwowar K., Chan T. H., Dou Q. P. (2011b). Green tea polyphenols as proteasome inhibitors: implication in chemoprevention. Curr. Cancer Drug Targets 11 296–306. 10.2174/156800911794519743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye M., Wang Q., Zhang W., Li Z., Wang Y., Hu R. (2014). Oroxylin A exerts anti-inflammatory activity on lipopolysaccharide-induced mouse macrophage via Nrf2/ARE activation. Biochem. Cell Biol. 92 337–348. 10.1139/bcb-2014-0030 [DOI] [PubMed] [Google Scholar]
- Yoo H., Ku S.-K., Baek Y.-D., Bae J.-S. (2014). Anti-inflammatory effects of rutin on HMGB1-induced inflammatory responses in vitro and in vivo. Inflam. Res. 63 197–206. 10.1007/s00011-013-0689-x [DOI] [PubMed] [Google Scholar]
- Yoon J. Y., Kwon H. H., Min S. U., Thiboutot D. M., Suh D. H. (2013). Epigallocatechin-3-gallate improves acne in humans by modulating intracellular molecular targets and inhibiting P. acnes. J. Invest. Dermatol. 133 429–440. 10.1038/jid.2012.292 [DOI] [PubMed] [Google Scholar]
- Youn J., Lee J. S., Na H. K., Kundu J. K., Surh Y. J. (2009). Resveratrol and piceatannol inhibit iNOS expression and NF-κB activation in dextran sulfate sodium-induced mouse colitis. Nutr. Cancer 61 847–854. 10.1080/01635580903285072 [DOI] [PubMed] [Google Scholar]
- Yu H. N., Ma X. L., Yang J. G., Shi C. C., Shen S. R., He G. Q. (2007). Comparison of effects of epigallocatechin-3-gallate on hypoxia injury to human umbilical vein, RF/6A, and ECV304 cells induced by Na(2)S(2)O(4). Endothelium 14 227–231. 10.1080/10623320701547299 [DOI] [PubMed] [Google Scholar]
- Yun K. J., Shin J. S., Choi J. H., Back N. I., Chung H. G., Lee K. T. (2009). Quaternary alkaloid, pseudocoptisine isolated from tubers of Corydalis turtschaninovi inhibits LPS-induced nitric oxide, PGE2, and pro-inflammatory cytokines production via the down-regulation of NF-κB in RAW 264.7 murine macrophage cells. Int. Immunopharmacol. 9 1323–1331. 10.1016/j.intimp.2009.08.001 [DOI] [PubMed] [Google Scholar]
- Zhang B., Liu Z. Y., Li Y. Y., Luo Y., Liu M. L., Dong H. Y., et al. (2011). Antiinflammatory effects of matrine in LPS-induced acute lung injury in mice. Eur. J. Pharm. Sci. 44 573–579. 10.1016/j.ejps.2011.09.020 [DOI] [PubMed] [Google Scholar]
- Zhang Q. Q., Ding Y., Lei Y., Qi C. L., He X. D., Lan T., et al. (2014). Andrographolide suppress tumor growth by inhibiting TLR4/NF-κB signaling activation in insulinoma. Int. J. Biol. Sci. 10 404–414. 10.7150/ijbs.7723 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zhang Y., Wang S., Li Y., Xiao Z., Hu Z., Zhang J. (2008). Sophocarpine and matrine inhibit the production of TNF-α and IL-6 in murine macrophages and prevent cachexia-related symptoms induced by colon26 adenocarcinoma in mice. Int. Immunopharmacol. 8 1767–1772. 10.1016/j.intimp.2008.08.008 [DOI] [PubMed] [Google Scholar]
- Zhao F., Nozawa H., Daikonnya A., Kondo K., Kitanaka S. (2003). Inhibitors of nitric oxide production from hops (Humulus lupulus L.). Biol. Pharm. Bull. 26 61–65. 10.1248/bpb.26.61 [DOI] [PubMed] [Google Scholar]
- Ziyan L., Yongmei Z., Nan Z., Ning T., Baolin L. (2007). Evaluation of the anti-inflammatory activity of luteolin in experimental animal models. Planta Med. 73 221–226. 10.1055/s-2007-967122 [DOI] [PubMed] [Google Scholar]