Abstract
AIM: To determine the impact of partial reimbursement for antivirals on antiviral utilization and adherence for chronic hepatitis B patients.
METHODS: This was a retrospective cohort study. Two separate cohorts were enrolled, including 14163 and 16288 chronic hepatitis B outpatients, respectively. These patients were referred to Beijing You’an Hospital before and after the new partial reimbursement for antivirals, which was implemented on July 1, 2011. Demographic characteristics (including medical insurance status), routine biochemical, virological and serology laboratory test results, and antiviral agents’ prescription information were collected from an electronic database. Patients were also defined as new and existing patients according to treatment history. Antiviral utilization, medication possession ratio and persistence rate were calculated and compared among the patients with different characteristics. A questionnaire survey was conducted among 212 randomly sampled outpatients from the same hospital to confirm the validity of the electronic database. Propensity score matching was used to adjust the distribution of patient’s characteristics which may influence the antiviral utilization. χ2 test or ANOVA was adopted and multivariate logistic regression was used to determine the factors associated with antiviral utilization and good adherence.
RESULTS: A total of 13364 outpatients from each cohort were enrolled after the propensity score matching. The antiviral utilization rate for the insured patients increased from 57.4% to 75.9% (P < 0.0001) after the reimbursement, and the rate among those who paid out-of-pocket increased from 54.9% to 56.7% (P = 0.028). Approximately 71% of the patients had a medication possession ratio of more than 80% in each cohort before reimbursement. This increased to 79.2% and 73.1% for insured patients and those who paid out-of-pocket, respectively (P < 0.0001). Insured patients and those who paid out-of-pocket had the similar persistence rates before reimbursement. But after reimbursement, insured patients had higher persistence rates than those who paid out-of-pocket at 6 (86.5% vs 81.5%, P < 0.0001), 9 (79.7% vs 69.9%, P < 0.0001), 12 (73.4% vs 61.9%, P < 0.0001), and 15 mo (66.6% vs 53.1%, P < 0.0001). The reimbursement could significantly improve adherence for the insured patients than those who paid out-of-pocket even after adjusting other covariates, with an interaction odds ratio of 1.422 (95%CI: 1.220-1.657, P < 0.0001). The questionnaire survey supported the validity of the electronic database.
CONCLUSION: The reimbursement policy shows a positive impact on antiviral utilization as well as adherence for insured chronic hepatitis B patients.
Keywords: Antiviral therapy, Adherence, Chronic hepatitis B, Compliance, Reimbursement
Core tip: High adherence is the key to ensuring the effectiveness of antiviral therapy and adherence can be influenced by cost and affordability which can be strongly influenced by reimbursement scheme. This study uniquely analyzed the impact of medication reimbursement on hepatitis B antiviral usage as well as treatment adherence in Beijing, China, where chronic hepatitis B infection is endemic. The results showed a positive impact of partial reimbursement on antiviral utilization as well as adherence for insured chronic hepatitis B patients. The results of this study could address a more global overall question rather than something at the patient level.
INTRODUCTION
Chronic hepatitis B (CHB) is one of the most common chronic liver diseases worldwide, especially in China. Individuals with chronic hepatitis B virus (HBV) infections have a 15%-40% probability of developing compensated cirrhosis (CC), decompensated cirrhosis (DCC) or hepatocellular carcinoma (HCC)[1,2]. Therefore, the ultimate goal of therapy is to prevent these complications by suppressing HBV replication[3]. Over the past decades, there has been considerable improvement in the treatment of CHB, including two interferon (IFN)-α formulations and four nucleos(t)ide analogs (NAs) including lamivudine (LAM), adefovir (ADV), entecavir (ETV) and telbivudine (LdT), which have been approved in China[4].
Current guidelines highlight the optimal adherence of antiviral treatment to achieve the best results[3]. Medication adherence usually refers to whether patients take their medications as scheduled and continue the therapy. This has been studied in various chronic diseases, including CHB[5-10]. Several methods have been used to assess medication adherence, including measurement of drug levels in blood or urine, patient self-reporting, pill counts, electronic monitoring devices, and prescription record review[11]. Each method has its advantages and disadvantages. In recent years, pharmacy databases have been increasingly used to evaluate medication adherence. Although using the databases has a number of limitations including the inability to determine whether the patient actually consumed the dispensed medication, the relative efficiency in large populations in a “real-world” setting in a timely and efficient manner is highly advantageous if data are deemed complete and patients are unlikely to obtain the medications from other sources not captured by the database[12].
Several factors have been identified to influence the medication adherence, including age, education, marital status, social medical support; disease severity; therapy effectiveness; and cost and affordability which can be strongly influenced by reimbursement scheme[1,13]. In China, both generic and branded drugs are currently available for antivirals and annual cost differs greatly across modalities. The branded drugs cost more than the generics. Annual cost ranges from $600 to $900 for LAM, $600 to $1000 for ADV, $1500 to $2000 for ETV, and $1400 for the only branded LdT approved in China. The conventional IFN costs as much as $1500-$2500 annually, while the pegylated IFN-α costs $7000-$8000. In Beijing, no reimbursement for all anti-HBV agents had been implemented before July 1, 2011 and all expenses were covered by the patients. Patients bear out of pockets no matter whether they were with or without medical insurance, which may have led to a poor adherence of antiviral therapy. Currently, all antiviral agents including IFN and NAs are on the list of National Reimbursement Catalogs of Drugs for Basic Medical Insurance and partial reimbursement has been implemented since July 1, 2011 in Beijing[14]. Patients with medical insurance could receive a 75%-85% reimbursement of the cost between a deductible of $300 and a ceiling of $3300. While for those without medical insurance, they still need to bear out of pockets themselves. It was estimated that the disposable personal income in Beijing was $5353 in 2012. So, annual cost for CHB patients with antivirals was a great burden for patients and their families. Whether the partial reimbursement policy can increase the antiviral medication adherence by increasing the acceptance and affordability of anti-HBV therapy needs to be explored.
In this study, we used the outpatient electronic data from Beijing You’an Hospital, one of the two biggest infectious and liver disease hospitals in Beijing, China, to estimate the antiviral treatment pattern and treatment adherence in CHB outpatients and explore the impact of the new partial reimbursement policy on the antiviral treatment adherence.
MATERIALS AND METHODS
Study population
Two cohorts were employed in this study. Cohort 1 consisted of outpatients with CHB who had been referred to Beijing You’an Hospital between January 1, 2010 and December 30, 2010, and cohort 2 referred between July 1, 2011 and June 30, 2012. Follow-up ended on June 30, 2011 and December 31, 2012 for cohort 1 and cohort 2, respectively. The inclusion criteria were as follows: (1) Beijing residents; and (2) diagnosed according to the criteria by “Asian-Pacific Consensus Statement on management of CHB”[3]. Patients co-infected with hepatitis A, C, D or E virus, human immunodeficiency virus, cytomegalovirus, or who had been admitted to hospital due to other diseases or conditions, including pregnancy, glomerulonephritis, uremia, metabolic syndrome, tumor, and severe cardiovascular diseases were excluded. A total of 14163 outpatients in cohort 1 and 16228 outpatients in cohort 2 were enrolled.
All the patients in the two cohorts were categorized into new patients and existing patients. Patients who had not undergone antiviral treatment during an 18-mo period prior to the enrollment were defined as new patients; otherwise were defined as existing patients.
Data collection
Clinical information, antiviral utilization and on-study laboratory tests for each visit of each patient were retrieved from electronic medical records. Clinical information included patients’ ID number, gender, age, visit date, health insurance status, signs and symptoms of illness. Antiviral utilization included drug names, dosage and prescription date. Laboratory tests were retrieved to identify the disease severity, including routine biochemical tests [serum alanine aminotransferase (ALT)], serum HBV DNA and hepatitis B e antigen (HBeAg) status.
Outcome measurements
Antiviral agent utilization: Antiviral utilization rate was calculated as the number of patients who had received antiviral agents during their follow-up period divided by the total number of patients during the study period. The proportion of specific antiviral agent usage was also calculated as the number of patients who had received the specific antiviral agent during their follow-up period divided by the total number of patients with antiviral treatment by all the antiviral agents during the study period.
Adherence measurement: For patients with antiviral treatment, the medication possession ratio (MPR) was used to evaluate primary measurement of adherence, defined as the proportion of days within an observation period for which antivirals were supplied[15,16]. It was calculated for each patient as the total days prescribed during the treatment period divided by the days the antivirals should have been supplied (defined as the days between the first and the last antiviral agent prescription date during the study period for each patient). Also, the proportion of patients with an MPR of no less than 80% which was defined as good adherence was calculated at the same time.
The persistence rate was alternatively used as the secondary adherence measurement[15,16]. It was measured as percent of patients who were still receiving antiviral therapy at different study time points. Patients who had a gap greater than 28 d (a maximum of 28 d’ worth of pills was allowed to be prescribed during a 4 wk-period in Beijing) between the prescription month and the following month without resumed treatment were considered to be off therapy. Persistence rates for all antivirals and specific NAs were calculated. In addition, because each patient was followed for a different length of time, persistence rates at 3, 6, 9, 12, and 15 mo were calculated.
Questionnaire survey: Questionnaire surveys by face-to-face or telephone interview were conducted among 212 randomly sampled outpatients to confirm the validity of the information from the electronic database. Age, gender, education, income, insurance type, and antiviral treatment including drug names, dosage, amount, prescription date and the place where they took the agents before and after reimbursement were collected. For patients with medical insurance (PMI) and patients who paid out-of-pocket (PPO) before and after reimbursement, the proportion of patients who took antiviral agents outside You’an Hospital only was calculated, respectively, to infer the validity of influence of reimbursement on antiviral utilization rate.
Statistical analysis
A de-identified dataset was used to do analysis. The statistical methods of this study were reviewed by Tao Xu from Department of Epidemiology and Biostatistics, Peking Union Medical College.
Propensity score matching: Propensity score (PS) matching[17,18] was used to adjust the distribution of patient’s age, gender, medical insurance type and disease severity in the two cohorts, which may influence the antiviral utilization. The PS was calculated by logistic regression, where the dependent variable was cohort classification and the independent variables were above confounders. Disease severity status was defined as whether the patients had one of the following characteristics: (1) serum ALT over two times upper limit of normal; (2) HBV DNA ≥ 20000 IU/mL for HBeAg (+) patients; or (3) HBV DNA ≥ 2000 IU/mL for HBeAg (-) patients[11]. Cohort 2 was matched to cohort 1 within a range of 0.1 standard deviation of PS.
Patients were divided into four subgroups: PMI and PPO before and after reimbursement. Antiviral utilization, utilization of different antiviral agents, MPR, good adherence and persistence rate were calculated among the different groups. χ2 test or ANOVA was adopted to compare the difference in above indexes among the groups when appropriate. Multivariate logistic regression was used to determine the factors associated with antiviral utilization and good adherence. P-values < 0.05 were considered significant.
All analyses were performed with SAS software, version 9.2 (SAS Institute, Cary, NC).
RESULTS
Subject characteristics
A total of 14163 outpatients in cohort 1 and 16228 outpatients in cohort 2 were enrolled. The distribution of age, gender, health insurance type and HBV-related disease status was significantly different between the two cohorts (Table 1). More male (67.7% vs 64.7%, P < 0.001) and younger patients were involved in cohort 1. The proportion of PMI in cohort 1 was lower than that in cohort 2 (40.6% vs 52.4%, P < 0.001). After PS matching, a matched sample size of 13,364 outpatients for each cohort was acquired, with the same PS (0.4 ± 0.1) for each cohort. The distribution of the key confounders was similar between the two cohorts (Table 1).
Table 1.
Patient characteristics before and after propensity score matching
|
Before matching |
After matching |
|||
| Cohort 1 (n = 14163) | Cohort 2 (n = 16228) | Cohort 1 (n = 13364) | Cohort 2 (n = 13364) | |
| Age (mean ± SD)b | 38.2 ± 12.6 | 39.6 ± 12.6 | 38.7 ± 12.6 | 38.9 ± 12.4 |
| Male (%)b | 67.7 | 64.7 | 66.7 | 66.5 |
| Insurance type (%)b | ||||
| Medical insurance | 40.6 | 52.4 | 42.9 | 42.6 |
| Out-of-pocket | 58.2 | 47.0 | 56.3 | 56.6 |
| Others | 1.3 | 0.6 | 0.7 | 0.8 |
| Disease statusb | ||||
| Yes1 | 29.3% | 23.6% | 28.2% | 27.9% |
Having one of the following characteristics: (1) serum alanine aminotransferase (ALT) over two times upper limit of normal (ULN); (2) hepatitis B virus (HBV) DNA ≥ 20000 IU/mL for hepatitis B e antigen (HBeAg) (+) patients; or (3) HBV DNA ≥ 2000 IU/mL for HBeAg (-) patients. ANOVA was performed to compare age difference between cohort 1 vs cohort 2; χ2 test was conducted to compare male (%), insurance type (%) and disease status between cohort 1 vs cohort 2.
P < 0.01, cohort 1 vs cohort 2.
Further analysis of cohort 1 showed that PMI were older than PPO (42.9 ± 13.1 vs 35.6 ± 11.2, P < 0.0001) and had less male patients (65.7 vs 67.7, P = 0.0126). The proportion of PMI with severe disease status was also higher than that of PPO (29.9% vs 25.3%, P < 0.0001). A similar tendency was observed between PMI and PPO in cohort 2 (data not shown).
Antiviral agent utilization
Figure 1 shows the change of antiviral utilization among patients with different characteristics. Before the reimbursement, antiviral utilization and the rate of specific NA utilization was almost equal between PMI and PPO (Figure 1A). ADV was predominantly used, followed by LAM and ETV. The utilization of LdT was the lowest.
Figure 1.
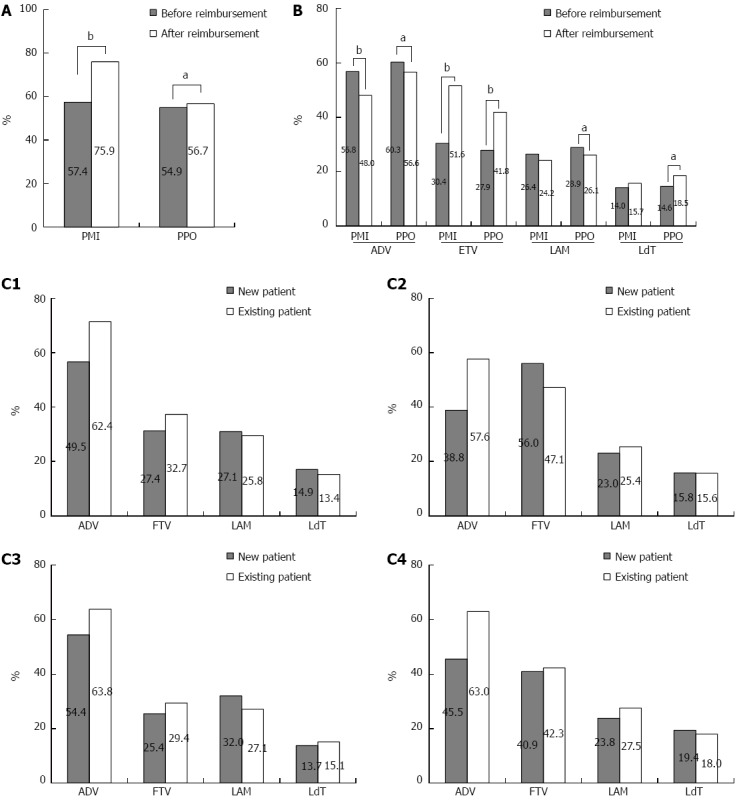
Antiviral agent utilization before and after reimbursement for patients with different characteristics. A: Antiviral agent utilization before and after reimbursement for patients with different insurance types; B: Utilization of different NAs for PMI vs PPO before and after reimbursement C1: Utilization of specific antiviral among new and existing PMI before reimbursement; C2: Utilization of specific antiviral among new and existing PMI after reimbursement; C3: Utilization of specific antiviral among new and existing PPO before reimbursement; C4: Utilization of specific antiviral among new and existing PPO after reimbursement. aP < 0.05, bP < 0.01, before reimbursement vs after reimbursement. PMI: Patients with medical insurance; PPO: Patients paid out-of-pocket.
After the reimbursement, a 19% increase of antiviral utilization was observed among PMI, and only a 2% increase was observed among PPO (Figure 1A). As the characteristics were differently distributed between PMI and PPO either before or after reimbursement, multiple logistic regression was adopted to adjust the above covariates. Our results showed that the reimbursement could significantly improve the antiviral utilization for PMI than PPO even after adjusting the covariates, with an interaction odds ratio (OR) of 2.194 (95%CI: 1.979-2.432, P < 0.0001) (data not shown).
For the specific NAs, a great increase rate of 21.2% for ETV utilization was observed among PMI (from 30.4% to 51.6%) and 13.9% (from 27.9% to 41.8%) among PPO. However, an 8.8% (from 56.8% to 48.0%) and 3.7% decrease of ADV utilization was observed among PMI and PPO, respectively. ETV was predominantly used among PMI while ADV was still preferred for PPO. The proportion of LdT or LAM had changed slightly after reimbursement (Figure 1B).
Further analysis showed that the utilization rate of ADV was significantly higher among existing patients than new patients either before or after the reimbursement. But for the PMI with ETV, a 10% higher rate was observed among new patients than the existing patients (56.0% vs 47.1%, P < 0.0001). For PPO with LAM, a 4.9% higher rate was observed among new patients than existing patients before the reimbursement while afterwards the rate was 3.7% lower (32.0% vs 27.1%, P = 0.0044; 23.8% vs 27.5%, P = 0.0250, respectively). No significant difference in LAM and LdT utilization was observed between new and existing patients for PMI (Figure 1C1-C4).
Adherence evaluation among patients with antiviral utilization
For the 6198 and 7721 patients with antivirals in cohort 1 and cohort 2, respectively, adherence was further evaluated, including MPR and the persistence rate. The mean follow-up period was 309.6 ± 155.5 and 375.2 ± 156.8 d for PMI before and after the reimbursement, and was 300.1 ± 155.2 and 305.5 ± 156.9 d for PPO, respectively.
MPR measurement: Before reimbursement, MPR for both PMI and PPO were more than 0.80 and no significant difference was observed (P = 0.8042). After reimbursement, a 5% increase was observed among PMI (83.4% ± 24.3% vs 88.5% ± 19.7%, P < 0.0001) and a less than 2% increase was observed among PPO (83.6% ± 24.2% vs 85.5% ± 23.0%, P = 0.0055) (Table 2).
Table 2.
Medication possession ratio and good adherence rate among patients with different insurance types before and after reimbursement
|
Adherence (%) |
Good adherence rate |
|||||||
| n | mean ± SD | New patients | Existing patients | Percent | New patients | Existing patients | ||
| Before | PMI | 2825 | 83.4 ± 24.3a | 82.3 ± 25.6d | 84.5 ± 22.9d | 70.6% | 69.2 | 71.8 |
| PPO | 3373 | 83.6 ± 24.2b | 83.6 ± 24.8 | 83.6 ± 23.7 | 70.2% | 70.5 | 69.9 | |
| After | PMI | 4102 | 88.5 ± 19.7ac | 87.1 ± 20.8e | 90.2 ± 18.1e | 79.2%g | 77.0h | 82.1h |
| PPO | 3619 | 85.2 ± 23.0bc | 84.0 ± 24.1f | 86.1 ± 22.1f | 73.1%g | 71.7 | 74.2 | |
P < 0.01 among PMI before reimbursement vs after reimbursement;
P < 0.01 among PPO before reimbursement vs after reimbursement;
P < 0.01 between PMI and PPO after reimbursement;
P < 0.05 between new and existing PMI before reimbursement;
P < 0.01 between new and existing PMI after reimbursement;
P < 0.01 between new and existing PPO after reimbursement;
P < 0.01 between PMI and PPO after reimbursement;
P < 0.01 between new and existing PMI after reimbursement. PMI: Patients with medical insurance; PPO: Patients paid out-of-pocket.
We also observed that PMI and PPO had a similar proportion of good adherence before reimbursement. However, after the reimbursement, the PMI had a higher proportion of patients with good adherence than PPO (79.2% vs 73.1%, P <0.0001) (Table 2).
No difference in the proportion of patients with good adherence was observed between existing and new patients before the reimbursement. However, a 5.1% higher proportion of existing PMI than new PMI (82.1% vs 77.0%, P < 0.0001) was subsequently observed after reimbursement (Table 2).
Persistence rate: Persistence rates declined within 15 mo, more rapidly during the first 6 mo and in new patients. PMI and PPO had the similar persistence rates before reimbursement. But after reimbursement, PMI had higher persistence rates than the PPO at 6 (86.5% vs 81.5%, P < 0.0001), 9 (79.7% vs 69.9%, P < 0.0001), 12 (73.4% vs 61.9%, P < 0.0001), and 15 mo (66.6% vs 53.1%, P < 0.0001) (Figure 2A). The similar tendency were observed in the new patients and existing patients, although the new patients had a lower persistence rate than the existing patients for each specific month (Figure 2B).
Figure 2.
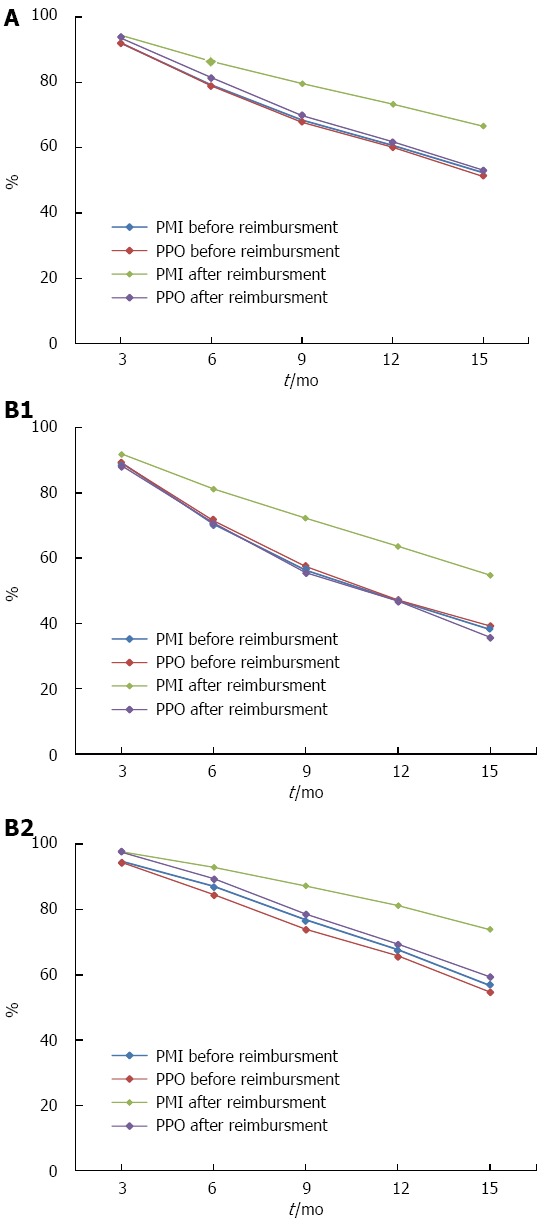
Persistence rate before and after reimbursement for patients with different characteristics. A: Persistence rate before and after reimbursement for patients with different insurance types; B1: Persistence rate for new patients with different insurance types; B2: Persistence rate for existing patients with different insurance types. PMI: Patients with medical insurance; PPO: Patients who paid out-of-pocket.
Factors associated with good adherence
Age, gender, insurance type, patient status (new vs existing patients), reimbursement implementation, disease severity, interactions between insurance type and policy implementation, as well as interactions between insurance type and patients characteristics were included in the logistic regression model to test the factors associated with good adherence. Results showed that compared to patients 18 years or younger, patients between the age of 18-45 and aged > 45 years had a lower probability to have good adherence, with an OR of 0.719 (95%CI: 0.559-0.926, P = 0.0105) and 0.667 (95%CI: 0.513-0.867, P = 0.0024). A significant interaction was observed between insurance type and patient status (OR = 0.820, 95%CI: 0.703-0.957; P = 0.0117) and patients’ insurance type and reimbursement implementation (OR = 1.422, 95%CI: 1.220-1.657; P < 0.0001). These interactions suggested that the reimbursement implementation can significantly improve more adherence for PMI than PPO, especially for existing patients (Table 3).
Table 3.
Factors associated with good adherence
| Parameter | β | OR (95%CI) |
| Age (yr) | ||
| 18-45 vs ≤ 18a | -0.3297 | 0.719 (0.559-0.926) |
| > 45 vs ≤ 18b | -0.4048 | 0.667 (0.513-0.867) |
| Gender (1 = male; 0 = female) | -0.0382 | 0.963 (0.886-1.046) |
| Insurance type (1 = medical insurance; 0 = paid out-of-pocket) | 0.1200 | 1.128 (0.987-1.288) |
| Disease status (1 =; 0 = reference;)b | 0.2516 | 1.286 (1.164-1.422) |
| Patient status (1 = new patients; 0 = existing patients) | -0.0341 | 0.966 (0.860-1.086) |
| Reimbursement implementation (1 = Yes; 0 = No)b | 0.1502 | 1.162 (1.047-1.290) |
| Insurance type patient statusa | -0.1980 | 0.820 (0.703-0.957) |
| Insurance type reimbursement implementationb | 0.3518 | 1.422 (1.220-1.657) |
P < 0.05;
P < 0.01, new vs existing patients. 1 = having one of the following characteristics: (1) serum alanine aminotransferase over two times upper limit of normal; (2) alanine aminotransferase DNA ≥ 20000 IU/mL for hepatitis B e antigen (HBeAg) (+) patients; or (3) HBV DNA ≥ 2000 IU/mL for HBeAg (-) patients.
Questionnaire
Questionnaire surveys were conducted in 212 outpatients, including 152 PMI and 60 PPO (Table 4). There was no difference in age, gender distribution, or household income per person per month between PMI and PPO. Before reimbursement, approximately 10% of patients had ever taken antivirals outside You’an Hospital regardless of medical insurance. After reimbursement, the proportion had decreased to 5.9% for PMI. We could still observe a higher antiviral utilization rate among PMI than PPO after reimbursement even adjusting the proportion of patients with different medical insurance status who took antiviral agents outside You’an Hospital only.
Table 4.
Characteristics of patients receiving questionnaire survey
| Insurance (n = 152) | Out-of-pocket (n = 60) | |
| Age (yr) | 38.8 ± 8.3 | 39.7 ± 11.6 |
| Male (%) | 99 (65.1) | 38 (64.4)1 |
| Income (Yuan) | ||
| Median (Q25-Q75) | 4000 (2500, 6300) | 3000 (1500, 5000) |
| Antivirals obtaining outside You’an Hospital only (%) | ||
| Before reimbursement | 9.8 | 10.8 |
| After reimbursement | 5.9 | 10.2 |
The number is 59.
DISCUSSION
Studies have shown that good adherence helps to maintain virologic response and prevent virologic resistance[19]. The reimbursement scheme may increase the adherence to antivirals by increasing their affordability. In this study, electronic data of 30391 outpatients from a university affiliated infection specialty hospital in Beijing, China, from 2010 to 2012 was used to determine the effect of partial reimbursement, which was firstly implemented for the treatment of CHB patients on July 1, 2011, on antiviral utilization and adherence. We found that partial reimbursement increased the antiviral utilization, although slightly for medical insured CHB patients.
Our study from electronic dataset showed that the antiviral utilization was almost the same (50%-60%) between PMI and PPO before reimbursement. After reimbursement, the rate increased to 75.9% for PMI while kept constant for PPO. Although questionnaire surveys found about 8.5% of PMI and 10% of PPO had taken medication outside You’an Hospital, which inferred that our results from electronic dataset might underestimate the antiviral utilization, the utilization was still much higher among PMI than PPO, indicating that reimbursement could improve antiviral utilization by reducing the economic burden. We also found that ADV was predominantly used for both PMI and PPO before reimbursement. But after reimbursement, ETV replaced ADV as the first choice NA for PMI. Monotherapy with ETV has been recommended as the first-line oral antiviral treatment for CHB by the Asian Pacific Association for the Study of the Liver, the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver[3,20,21]. This could explain that the reimbursement policy had mitigated the economic burden for PMI, thus treatment effectiveness was more of a concern than cost when choosing an antiviral agent. However, PPO may have chosen less costly and more common antivirals of ADV. A higher ADV utilization among existing patients was also observed, which may be explained by the fact that most of the patients who had received antiviral therapy for a long time before our enrollment might continue to select ADV.
MPR analysis showed that the mean MPR for both PMI and PPO was more than 80% before reimbursement, which had already reached an optimistic level, similar to the 80%-99% mean adherence rate in the other studies regarding oral antiviral adherence for CHB patients[22-24]. After the reimbursement the MPR had increased 5% for PMI and 2% for PPO. Even after adjusting other covariates, the reimbursement could still significantly improve adherence for PMI than PPO, with an interaction OR of 1.422 (1.220-1.657).
The persistence rate was used to assess whether a patient stays on therapy consistently. We found that persistence rates declined during the study period, especially more rapidly during the first 6 mo, indicating that patients tend to miss or stop the medication at the beginning of the treatment, probably due to side effects or lack of treatment effectiveness[12]. Similar to MPR, the persistence rate was observed higher among PMI. This was supported by a report by Liaw et al[1] that lack of adequate reimbursement was correlated with inadequate anti-HBV therapy according to treatment guidelines.
Our study also observed higher MPR and persistence rates and a more gradually decline of persistence rate for existing patients compared to new patients. Poorer adherence for new patients who have received antiviral therapy for a short duration might be due to inability to endure the side effects of antiviral agents, which was also suggested in other studies that a greater decline in adherence for chronic medications was often observed among new users[12,22].
Our study has several limitations. First, our patients might not represent the general CHB population in Beijing. However, the demographic characteristics in our study population showed that the proportion of male patients was 64%-67% and the mean age was 38-40 years, which are consistent with the gender and age distribution of CHB infection in other studies[25-29]. Second, the difference in patients’ characteristics which may result in different antiviral utilization may exist between the patients referred to You’an Hospital before and after reimbursement based on real-world electronic datasets. Although PS matching was adopted to try to balance the baseline characteristics, the bias generated by unmeasured confounding factors cannot be eliminated. Third, pharmacy database was used in our study to evaluate medication adherence which may not capture the exact amount of the agents used. But after the validation study by questionnaire surveys, we can still conclude that the reimbursement can improve the utilization for patients with medical insurance.
In conclusion, the utilization of antivirals and adherence for insured CHB patients had significantly increased after the new partial reimbursement implementation, especially for patients receiving ETV and ADV. Thus, the new policy had a positive impact on antiviral treatment pattern, thereby offering improved outcomes.
COMMENTS
Background
Chronic hepatitis B (CHB) is one of the most common chronic liver diseases worldwide, especially in China, leading to a high rate of incidence and mortality from development of cirrhosis and hepatocellular cancer (HCC). Effective antiviral treatment is the only way to prevent the development of cirrhosis and HCC after infection and the essential prerequisite is long-term adherence. There are many factors which influence the therapy adherence for CHB patients, and one of the most important factors is the cost and affordability for antiviral drugs, which can be strongly influenced by reimbursement scheme. In Beijing, no reimbursement for all antivirus agents for CHB patients had been implemented before July 1, 2011 and all expenses of drugs are borne by the patients, which may lead to a low compliance rate of antivirus therapy. However, all antivirus agents including IFN and NAs, have been in the list of National Reimbursement Catalogs of Drugs for Basic Medical Insurance and partial reimbursement has been implemented since July 1, 2011 in Beijing. Whether or not the compliance with NAs can be increased under the new partial reimbursement policy needs to be explored.
Research frontiers
Medication adherence has been studied in different chronic diseases and several methods have been used to assess medication adherence. In recent years, pharmacy databases have been increasingly used to evaluate medication adherence in large populations in a “real-world” setting and the relative efficiency in a timely and efficient manner is more advantageous than the other ways. Moreover, as the new reimbursement policy has just been implemented for a short period, this is the first study to evaluate its impact on antiviral therapy adherence in Beijing, China.
Innovations and breakthroughs
This study uniquely analyzed the impact of medication reimbursement on hepatitis B antiviral usage as well as treatment adherence in Beijing, China, where chronic hepatitis B infection is endemic. Two cohorts were contrasted, one before implementation of the new partial reimbursement policy, and the other after implementation. Propensity score matching was used to control the effects from confounding. Antiviral usage rates and adherence rates were compared between the two groups as well as for those with insurance and those paying out-of-pocket. Also, a questionnaire survey was conducted to infer the validity of reimbursement on antiviral usage. Results have shown that the antiviral utilization rate for the insured patients increased from 57.4% to 75.9% (P < 0.0001) after the reimbursement. The rate among those who paid out-of-pocket increased only from 54.9% to 56.7% (P = 0.028). Approximately 71% of the patients had an MPI of more than 80% in each cohort before reimbursement. This increased to 79.2% and 73.1% for insured patients and those who paid out-of-pocket, respectively (P < 0.0001). The reimbursement could significantly improve adherence for the insured patients than those who paid out-of-pocket even after adjusting other covariates, with an interaction odds ratio of 1.422 (95%CI: 1.220-1.657, P < 0.0001).
Applications
The results suggested a positive impact of partial reimbursement on antiviral utilization as well as adherence for insured chronic hepatitis B patients. The results of this study could address a more global overall question rather than something at the patient level.
Terminology
Reimbursement is an act of compensating someone for an expense. Often, a person is reimbursed for out-of-pocket expenses when the person incurs those expenses through government, employment or in an account of carrying out the duties for another party or member. Medication adherence usually refers to whether patients take their medications as scheduled and continue the therapy.
Peer-review
This study appears as a well-designed study that for the first time analyzed the impact of medication reimbursement on adherence to hepatitis B antiviral treatment in Beijing, China, where chronic hepatitis B infection is endemic. The study is interesting and results showed that partial reimbursement, implemented in 2011, improved adherence as well as influenced the choice of NAs selected by the patients which should improve the overall outcome. These types of studies are highly warranted not only in China but also from other parts of the world to design antiviral treatment for chronic hepatitis B.
Footnotes
Supported by Foundation of Ministry of Science and Technology of China, No. 2012ZX10004904 and No. 2013ZX10002002006002; Bristol-Myers Squibb Company, No. AI463-961; and Innovative Foundation of Beijing Union Medical College.
Institutional review board statement: The study protocol was approved by the Ethical Review Committee of Beijing You’an Hospital of Capital Medical University and Institute of Basic Medical Sciences Chinese Academy of Medical Sciences.
Informed consent statement: Given that the study poses no more than the minimal risk, and it would not be practicable to contact all the 30451 CHB patients in the two cohorts, a waiver of the informed consent was allowed by the Ethical Review Committee for the first part of study, involving secondary analysis of data of the two cohorts for a total of 30451 CHB patients. But for the validation study part, written or oral informed consent was obtained from the participants dependent on questionnaire survey by face-to-face interview or telephone interview. Deidentification was done to assure confidentiality of the study data for the two parts.
Conflict-of-interest statement: None declared.
Data sharing statement: Technical appendix and output files of the statistical analysis available from the corresponding author at liwang@ibms.pumc.edu.cn.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: March 14, 2015
First decision: April 13, 2015
Article in press: June 16, 2015
P- Reviewer: Akbar SMF, Labonte P, Stasi C S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Zhang DN
References
- 1.Liaw YF. Antiviral therapy of chronic hepatitis B: opportunities and challenges in Asia. J Hepatol. 2009;51:403–410. doi: 10.1016/j.jhep.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006;45:529–538. doi: 10.1016/j.jhep.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 3.Liaw YF, Kao JH. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2012 update. Hepatol Int. 2012;(6):531–561. doi: 10.1007/s12072-012-9365-4. [DOI] [PubMed] [Google Scholar]
- 4.Chinese Society of Hepatology and Chinese Society of Infectious Diseases, Chinese Medical Association. [The guideline of prevention and treatment for chronic hepatitis B (2010 version)] Zhonghua Liu Xing Bing Xue Zazhi. 2011;32:405–415. [PubMed] [Google Scholar]
- 5.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 6.Bramley TJ, Gerbino PP, Nightengale BS, Frech-Tamas F. Relationship of blood pressure control to adherence with antihypertensive monotherapy in 13 managed care organizations. J Manag Care Pharm. 2006;12:239–245. doi: 10.18553/jmcp.2006.12.3.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouchard MH, Dragomir A, Blais L, Bérard A, Pilon D, Perreault S. Impact of adherence to statins on coronary artery disease in primary prevention. Br J Clin Pharmacol. 2007;63:698–708. doi: 10.1111/j.1365-2125.2006.02828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paterson DL, Swindells S, Mohr J, Brester M, Vergis EN, Squier C, Wagener MM, Singh N. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2000;133:21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- 9.Kamezaki H, Kanda T, Wu S, Nakamoto S, Arai M, Maruyama H, Fujiwara K, Imazeki F, Yokosuka O. Emergence of entecavir-resistant mutations in nucleos(t)ide-naive Japanese patients infected with hepatitis B virus: virological breakthrough is also dependent on adherence to medication. Scand J Gastroenterol. 2011;46:1111–1117. doi: 10.3109/00365521.2011.584898. [DOI] [PubMed] [Google Scholar]
- 10.Ha NB, Ha NB, Garcia RT, Trinh HN, Chaung KT, Nguyen HA, Nguyen KK, Levitt BS, Nguyen MH. Medication nonadherence with long-term management of patients with hepatitis B e antigen-negative chronic hepatitis B. Dig Dis Sci. 2011;56:2423–2431. doi: 10.1007/s10620-011-1610-5. [DOI] [PubMed] [Google Scholar]
- 11.Vermeire E, Hearnshaw H, Van Royen P, Denekens J. Patient adherence to treatment: three decades of research. A comprehensive review. J Clin Pharm Ther. 2001;26:331–342. doi: 10.1046/j.1365-2710.2001.00363.x. [DOI] [PubMed] [Google Scholar]
- 12.Andrade SE, Kahler KH, Frech F, Chan KA. Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol Drug Saf. 2006;15:565–574; discussion 575-577. doi: 10.1002/pds.1230. [DOI] [PubMed] [Google Scholar]
- 13. Available from: https://openlibrary.org/works/OL11076328W/Threat_convergence_at_the_border.
- 14. Available from: http://govfile.beijing.gov.cn/Govfile/front/content/02005158_0.html.2005.
- 15.Lee M, Keeffe EB. Study of adherence comes to the treatment of chronic hepatitis B. J Hepatol. 2011;54:6–8. doi: 10.1016/j.jhep.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 16.Peterson AM, Nau DP, Cramer JA, Benner J, Gwadry-Sridhar F, Nichol M. A checklist for medication compliance and persistence studies using retrospective databases. Value Health. 2007;10:3–12. doi: 10.1111/j.1524-4733.2006.00139.x. [DOI] [PubMed] [Google Scholar]
- 17.Hemmila MR, Birkmeyer NJ, Arbabi S, Osborne NH, Wahl WL, Dimick JB. Introduction to propensity scores: A case study on the comparative effectiveness of laparoscopic vs open appendectomy. Arch Surg. 2010;145:939–945. doi: 10.1001/archsurg.2010.193. [DOI] [PubMed] [Google Scholar]
- 18.Newgard CD, Hedges JR, Arthur M, Mullins RJ. Advanced statistics: the propensity score--a method for estimating treatment effect in observational research. Acad Emerg Med. 2004;11:953–961. doi: 10.1197/j.aem.2004.02.530. [DOI] [PubMed] [Google Scholar]
- 19.Scaglione SJ, Lok AS. Effectiveness of hepatitis B treatment in clinical practice. Gastroenterology. 2012;142:1360–1368.e1. doi: 10.1053/j.gastro.2012.01.044. [DOI] [PubMed] [Google Scholar]
- 20.Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661–662. doi: 10.1002/hep.23190. [DOI] [PubMed] [Google Scholar]
- 21.European Association For The Study Of The Liver. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167–185. doi: 10.1016/j.jhep.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 22.Chotiyaputta W, Peterson C, Ditah FA, Goodwin D, Lok AS. Persistence and adherence to nucleos(t)ide analogue treatment for chronic hepatitis B. J Hepatol. 2011;54:12–18. doi: 10.1016/j.jhep.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 23.Chotiyaputta W, Hongthanakorn C, Oberhelman K, Fontana RJ, Licari T, Lok AS. Adherence to nucleos(t)ide analogues for chronic hepatitis B in clinical practice and correlation with virological breakthroughs. J Viral Hepat. 2012;19:205–212. doi: 10.1111/j.1365-2893.2011.01494.x. [DOI] [PubMed] [Google Scholar]
- 24.Berg T, Marcellin P, Zoulim F, Moller B, Trinh H, Chan S, Suarez E, Lavocat F, Snow-Lampart A, Frederick D, et al. Tenofovir is effective alone or with emtricitabine in adefovir-treated patients with chronic-hepatitis B virus infection. Gastroenterology. 2010;139:1207–1217. doi: 10.1053/j.gastro.2010.06.053. [DOI] [PubMed] [Google Scholar]
- 25.Fattovich G. Natural history and prognosis of hepatitis B. Semin Liver Dis. 2003;23:47–58. doi: 10.1055/s-2003-37590. [DOI] [PubMed] [Google Scholar]
- 26.Brunetto MR, Oliveri F, Coco B, Leandro G, Colombatto P, Gorin JM, Bonino F. Outcome of anti-HBe positive chronic hepatitis B in alpha-interferon treated and untreated patients: a long term cohort study. J Hepatol. 2002;36:263–270. doi: 10.1016/s0168-8278(01)00266-5. [DOI] [PubMed] [Google Scholar]
- 27.Fattovich G, Giustina G, Christensen E, Pantalena M, Zagni I, Realdi G, Schalm SW. Influence of hepatitis delta virus infection on morbidity and mortality in compensated cirrhosis type B. The European Concerted Action on Viral Hepatitis (Eurohep) Gut. 2000;46:420–426. doi: 10.1136/gut.46.3.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoon EL, Yim HJ, Lee HJ, Lee YS, Kim JH, Jung ES, Kim JH, Seo YS, Yeon JE, Lee HS, et al. Comparison of clevudine and entecavir for treatment-naive patients with chronic hepatitis B virus infection: two-year follow-up data. J Clin Gastroenterol. 2011;45:893–899. doi: 10.1097/MCG.0b013e31821f8bdf. [DOI] [PubMed] [Google Scholar]
- 29.Liaw YF, Sheen IS, Lee CM, Akarca US, Papatheodoridis GV, Suet-Hing Wong F, Chang TT, Horban A, Wang C, Kwan P, Buti M, Prieto M, Berg T, Kitrinos K, Peschell K, Mondou E, Frederick D, Rousseau F, Schiff ER. Tenofovir disoproxil fumarate (TDF), emtricitabine/TDF, and entecavir in patients with decompensated chronic hepatitis B liver disease. Hepatology. 2011;53:62–72. doi: 10.1002/hep.23952. [DOI] [PubMed] [Google Scholar]


