Abstract
The ethnomedicinal plant Curatella americana L. (Dilleniaceae) is a common shrub in the Brazilian cerrado, in which crude extract showed antifungal activity in a preliminary study. In this work, the antifungal and cytotoxic properties of the crude extract, fractions, and isolated compounds from C. americana were evaluated against the standard yeast strains Candida albicans, C. tropicalis, and C. parapsilosis, clinical isolates, and fluconazole-resistant strains. The combinatory effects between subfractions and isolated compounds and effects on cell morphology, virulence factors, and exogenous ergosterol were also evaluated. The MIC obtained against the Candida species including fluconazole-resistant strain ranged from 15.3 to 31.3 µg/mL for crude extract, 3.9 to 15.6 µg/mL for ethyl acetate fraction, and 7.8 to 31.3 µg/mL for subfractions. The isolated compounds identified as 4′-O-methyl-catechin, epicatechin-3-O-gallate, and 4′-O-methyl-catechin-3-O-gallate showed lower antifungal activity than the crude extract and fractions (MIC ranging from 31.3 to 125.0 µg/mL). The addition of exogenous ergosterol to yeast culture did not interfere in the antifungal activity of the extract and its fractions. Synergistic antifungal activity was observed between subfractions and isolated compounds. The effects on virulence factors and the different mechanisms of action compared to fluconazole and nystatin suggest that this ethnomedicinal plant may be an effective alternative treatment for candidiasis.
1. Introduction
In the Brazilian cerrado there is a large and still unknown biodiversity [1]. Studies of various plant species with therapeutic use have been carried out, and many compounds with biological activity were obtained from medicinal plants of the Brazilian cerrado [2–4]. Considering the antimicrobial activity, several studies have been conducted [5] to investigate the potential use of herbal drugs in the treatment or control of infectious diseases searching for drugs with fewer side effects, lower costs, and broad-spectrum. This approach is also relevant in case of diseases caused by opportunistic and drug-resistant microorganisms, mainly in immunocompromised patients [6].
The species Curatella americana L. (Dilleniaceae) is a very common shrub in the Brazilian cerrado, and it is also characteristic of the Neotropical savanna, occurring from southern Mexico to Bolivia [7]. The plant stem bark, popularly known as “Lixeira” and “Sambaíba,” is used in folk medicine due to its anti-inflammatory and antiulcer effects in the treatment of wounds and other skin diseases [8–10].
Fungal infections of the oral cavity are caused by a group of saprophytic fungi that includes eight species of the genus Candida. Candida albicans is the most common species that resides in the oral cavity in humans, accounting for 70–80% of oral isolates. Candida parapsilosis, C. tropicalis, and C. glabrata comprise approximately 10% of oral isolates [11]. Candida albicans is also the main cause of vaginal candidiasis, a common mucosal infection that occurs with the highest prevalence in women aged 20–30 years [12]. Because of the resistance of yeast pathogens to pharmacotherapeutics, candidiasis therapy frequently fails. The resistance of Candida species to azole antifungals is the most prevalent type of resistance to antifungals [13]. Researchers have shown that 3.6% of C. albicans vaginal isolates were found to be resistant to fluconazole, and 16.2% were resistant to itraconazole [14, 15]. In addition to resistance to antifungals, fungal infections caused by opportunistic pathogens have become more frequent, partially as a result of prolonged antibiotic therapy and the increasing number of immunocompromised patients [16]. Therefore, developing therapeutic strategies for the treatment of candidiasis in immunocompromised patients is particularly important, with the goal of developing drugs with better effectiveness, reduced side effects, and lower cost. The price of antifungal agents is an important factor when considering that the prevalence of opportunistic infections also depends on economic factors, involving health, hygiene, and nutritional status. We performed a survey of plants that are commonly used in traditional medicine, and extracts from the stem bark of C. americana had very promising antifungal activity [17]. Thus, considering obtaining a possible alternative for the treatment of oral and vaginal candidiasis, the present study evaluated the antifungal properties of extracts, fractions, and isolated compounds from the bark of the plant species Curatella americana (L.).
2. Materials and Methods
2.1. Plant Material
The stem bark from Curatella americana L. was collected at the Tocantins cerrado (altitude 267 m; S 10°11.884′; HO 48°17.714′) under authorization number 14043-1 from the Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis (IBAMA). Identification occurred at the Tocantins Herbarium, Universidade Federal de Tocantins, by Dr. Solange F. Lolis. Voucher specimens were deposited in the same herbarium (registration HTO2234).
2.2. Preparation of Crude Extract and Fractionation
The stem bark was dried under shade in the open air to reduce deterioration of the plant drug material. It was then comminuted and dried at room temperature in the dark. After drying, the plant material was powdered (Tigre ASN5), and the crude extract (CE; 10% w/v) was obtained by turboextraction according to Toledo et al. [17] using acetone/water (7 : 3, v/v) as the extractor liquid. The lyophilized CE was then dissolved in acetone/water (2 : 8, v/v) and partitioned with ethyl acetate to obtain a water fraction (F1) and an ethyl acetate fraction (F2). The F2 fraction (2 g) was chromatographed on a Sephadex LH-20 column (h = 750 mm, Ø = 55 mm) using 50% ethanol (4 L), absolute ethanol (1 L), and acetone/water (7 : 3, v/v; 2 L) as the eluent system. Nineteen fractions were obtained, in which the most active (F2#5 and F2#6) were subfractionated by high-speed countercurrent chromatography (HSCCC). For identification, the isolated compounds were acetylated, and their nuclear magnetic resonance spectra were determined on a Varian instrument (Mercury 300BB, 300 MHz for 1H, 75 MHz for 13C) using TMS as an internal standard. The spectra were analyzed and compared with data from the literature [18–21].
2.3. Evaluation of Antifungal Properties
The standard yeast strains Candida albicans (ATCC 10231) and C. parapsilosis (ATCC 22019; supplied by Fundação Oswaldo Cruz, Rio de Janeiro, Brazil) were maintained at 4°C on Sabouraud dextrose agar plates and subcultured at 37°C in Sabouraud dextrose broth before each experiment to ensure viability and purity. The yeast strains evaluated in the present study also included three isolates from vaginal fluid (C. albicans, C. parapsilosis, and C. tropicalis) obtained from women with no previous history of immunodeficiency, four strains that were resistant to fluconazole (C. albicans 32res, 32Bres, 48res, and 103res) and one susceptible strain (C. albicans 48sen). All of these isolates are part of the yeast culture collection at our laboratory [22]. The MICs of the crude extracts were determined against the yeast using broth microdilution techniques (CLSI, 2008). Nystatin (Sigma) and fluconazole (kindly donated by Pfizer) were used as a control. The assays were done in triplicate in three independent experiments.
2.4. Cytotoxicity Assay
A suspension of Vero cells (ATCC CCL-81) was counted in a Neubauer hemocytometer and diluted in Dulbecco's Modified Eagle Medium (DMEM) that contained 10% fetal bovine serum (FBS) and 50 μg/mL gentamycin to obtain a suspension of 2.0 × 105 cells/mL. One hundred microliters of cell suspension were placed in each well of 96-well plate and incubated at 37°C with 5% CO2 for 24 h or until formation of confluent cell monolayers. The pure compound was initially dissolved in dimethyl sulfoxide (DMSO; final concentration, 1%) and then in DMEM to obtain the initial solution that was subsequently diluted in DMEM and added to each cell monolayer. The negative control consisted of the addition of only the medium and antibiotics. The 96-well plates were incubated for 72 h in a humidified chamber at 37°C with 5% CO2. Cell viability was determined by the sulforhodamine B colorimetric method [23]. The results were obtained using an enzyme-linked immunosorbent assay (ELISA) reader (Bio-Tek Power Wave XS Microplate Fluorescence Reader) at an optical density (OD) of 530 nm. Cytotoxicity is expressed as a percentage of the OD of the control. The assays were done in triplicate in three independent experiments.
2.5. Hemolysis Assay
Human erythrocytes from a healthy donor (type A+) were collected and washed with saline solution that contained 1% glucose, buffered to pH 7.25 (SSG). To determine hemolytic activity, a 3% erythrocyte suspension was incubated for 2 h with the crude extracts in 96-well microplates at 37°C (tested concentrations: 15.6, 31.3, 62.5, 125.0, 250.0, 500.0, and 1000 μg/mL). Hemolysis was determined by reading the absorbance of the supernatant from each well using a spectrophotometer at 550 nm. The positive control (100% hemolysis) and negative control (0% hemolysis) were also evaluated by incubating the erythrocytes with 1% Triton X-100 in SSG and SSG alone, respectively [24, 25]. The assays were done in triplicate in three independent experiments.
2.6. Evaluation of Synergistic Effect: Checkerboard Test
Checkerboard tests were performed using the CLSI [26] broth microdilution reference procedure with a final inoculum of 0.5–2.5 × 103 CFU/mL and RPMI 1640 medium buffered with 0.165 M MOPS. The final concentrations were 1.6–100 μg/mL for fluconazole, 0.8–50.0 μg/mL for nystatin, and 0.5–500 μg/mL for the CE, fractions, and subfractions. The plates were incubated at 37°C for 48 h, and the endpoints were defined as the lowest concentration of antifungal agent that resulted in the total inhibition of visual growth. The fractional inhibitory concentration (FIC) index is defined as the sum of the MIC of each drug in combination, divided by the MIC of the drug used alone. An FIC index <0.5 is considered synergism. An FIC index >4 is considered antagonism. An FIC index >0.5 but <4 is considered indifferent [27]. The assays were done in triplicate in three independent experiments.
2.7. Effect of Exogenous Ergosterol on the MIC
To determine whether the effects of the CE, fractions, and subfractions on yeast are attributable to their interaction with ergosterol, a competition assay using ergosterol during the MIC determination assay was performed. The MIC was determined according to the CLSI [26] guidelines described above in the presence and absence of several concentrations (50, 100, and 200 μg/mL each) of ergosterol (Sigma). Amphotericin B was used as a control drug. The MIC was read at 24 h according to the growth of the control fungus. The assays were done in triplicate in three independent experiments.
2.8. Effect on Virulence Factors
Effect on C. albicans Budding. Different concentrations of CE and subfractions diluted in RPMI 1640 medium were tested to assess the effect on C. albicans budding. Cells (0.5–2.5 × 103 CFU/mL) were incubated at 37°C for 48 h and observed using a negative-staining technique with 7% aqueous nigrosin. Three hundred cells were counted in each smear by light microscopy, and the percentage of budding cells was calculated [28].
Effect on Germ-Tube Formation. A yeast suspension of 1.0 × 106 CFU/mL was treated (MIC, sub-MIC, and 2x MIC) with the CE and subfraction diluted in sterile fetal bovine serum (FBS) and observed by phase-contrast microscopy (Zeiss Axiovert 25) after 2-3 h incubation at 37°C. Nystatin was used as the standard antifungal drug. For quantification, the cells were considered germinated if they had a germ tube at least twice the length of the cell [29].
Evaluation of Adherence Inhibition on a Glass Surface. The yeasts were treated with different concentrations (MIC, sub-MIC, and 2x MIC) of CE, subfractions, and nystatin for 24 h at 37°C, washed with 10 mM phosphate-buffered saline (PBS) at pH 7.2, and counted in a Neubauer chamber to obtain 1.0 × 106 CFU/mL. A 500 μL aliquot of the yeast suspension was added on cover slips alone. The interaction between the yeast and the abiotic surface was performed for 1 h at 37°C. The cover slips were then washed in 10 mM PBS, pH 7.2. In the analysis of yeast adherence to the abiotic surface, 20–100 fields were counted with a 100x objective [22].
Evaluation of Effect of the Extracts on the Ability to Adhere to Buccal Epithelial Cells (BECs). Samples of C. albicans were grown in medium with glucose for 24 h at 37°C. They were then washed twice with PBS (pH 7.2) and resuspended in PBS, adjusting the concentration to 1.0 × 107 cells/mL. We collected BECs from a healthy volunteer using sterile swab, which were shaken in PBS and washed twice with PBS to remove possible microorganisms. The cells were resuspended in PBS, adjusting the concentration to 1.0 × 105 cells/mL. The yeasts were treated with the drugs (MIC, sub-MIC, and 2x MIC) for 1 h at 37°C under agitation. A 500 μL volume of the epithelial cell suspension (1.0 × 105 cells/mL) was added to 500 μL of treated and untreated yeast (1.0 × 107 yeast/mL) and incubated for 30 min at 37°C. The suspension was then filtered through a polycarbonate membrane (12 μm, 47 mmØ, Millipore, Billerica, MA, USA), which was washed with PBS to remove the nonadhered yeast. The cells were then fixed with methanol, stained with crystal violet solution, and observed under an optical microscope. The number of treated yeast that adhered to 100 epithelial cells was counted and compared with untreated yeast [30].
Effect on Cell Surface Hydrophobicity (CSH). Yeasts that were treated with the CE and subfraction (MIC and sub-MIC) were washed with 50 mM PBS (pH 7.4) and resuspended in the same buffer. Turbidity was determined in a spectrophotometer at 660 nm. The cell suspensions were then mixed with xylene 2.5 : 1 (v/v; Merck) and shaken for 2 min. The tube was left for 20 min at room temperature to obtain phase separation. The turbidity of the aqueous phase was read at 660 nm. The hydrophobicity index (HI) was calculated as HI = (A control − A test) × 100/A control, where A control indicates the OD of the strains before xylene treatment and A test indicates the OD of the strains after xylene treatment [31]. The assays were done in triplicate in three independent experiments.
2.9. Scanning Electron Microscopy
C. albicans treated with the CE and subfractions were fixed with 2.5% glutaraldehyde for 2 h at room temperature. After fixation, small drops of the sample were placed on a specimen support with poly-L-lysine. Subsequently, the samples were dehydrated in graded ethanol, critical-point-dried in CO2, coated with gold, and observed in a Shimadzu SS-550 scanning electron microscope.
2.10. Evaluation of Oxygen Consumption
To evaluate oxygen uptake [32], stationary-phase cells (approximately 1.5 × 108 cells/mL) were grown with and without the test drugs in RPMI 1640 broth at 37°C overnight, harvested, washed with 0.025 M PBS (pH 7.2), and resuspended in 0.025 M phosphate buffer (pH 7.2) at a cell density of 5.0 × 108/mL. Oxygen uptake measurements were made at 30°C using a Clark-type oxygen electrode. Oxygen uptake rates were calculated as nmoles of oxygen consumed/60 s/108 cells, and the results are expressed as a percentage relative to controls (untreated yeast). The assays were done in triplicate in three independent experiments.
3. Results and Discussion
The phytochemical analysis of the bark of C. americana L. has demonstrated the presence of compounds as flavonoids, terpenes, phenolics, saponins, and steroids that have also been isolated in other species of the Dilleniaceae family [33]. Among the phenolic compounds, tannins have been found in considerable amounts. Researchers identified oligomeric and polymeric proanthocyanidins by high-performance liquid chromatography and discussed the possibility that they are responsible for its gastroprotective effect [34]. In the present work, from 1 kg of the dried and powdered bark of C. americana and 10 L of acetone/water (7 : 3, v/v), we obtained 148.79 g of dried extract (CE; 14.88% yield). From the liquid-liquid partition from the CE (120 g), we obtained 72.03 g of the aqueous phase (60.03% yield) and 34.17 g of the ethyl acetate phase (F2; 28.48% yield). From the ethyl acetate fraction F2 (20.00 g), we obtained 19 subfractions by column chromatography (Sephadex LH-20), of which the highest yields and best antifungal activity were obtained for F2#5 (3.21 g), F2#6 (2.47 g), and F2#7 (1.68 g). Using HSCCC, we obtained 4′-O-methyl-catechin (F2#5.4.1) and F2#5.2.2 (unidentified substance) from F2#5 and epicatechin-3-O-gallate (F2#6.2.1) and 4′-O-methyl-catechin-3-O-gallate (F2#6.1.2) from F2#6.
The antifungal activity of the crude extract, fractions, and subfractions obtained by broth microdilution techniques is shown in Table 1. The F2 fractionation by column chromatography yielded 19 subfractions monitored by TLC (Thin Layer Chromatography). Of these, the subfractions F2#5 and F2#6 had the lowest MIC against C. albicans (31.3 and 15.6 μg/mL, resp.). The isolated compounds had greater MICs than the subfractions, fractions, and extracts, ranging from 31.3 to 125.0 μg/mL. In the tests that evaluated fungicidal activity, we observed the growth of yeast cells after drug removal, demonstrating that the CE and its fractions had fungistatic activity.
Table 1.
Minimum inhibitory concentration (MIC) of crude extract (CE), fractions, subfractions, and isolated compounds from C. americana.
| Samples | MIC (µg/mL) | ||
|---|---|---|---|
| C. albicans | C. tropicalis | C. parapsilosis | |
| CE | 15.6 | 31.3 | 31.3 |
| Aqueous fraction (F1) | 500.0 | 500.0 | 500.0 |
| Ethyl acetate fraction (F2) | 3.9 | 15.6 | 7.8 |
| F2#5 subfraction | 31.3 | 31.3 | 15.6 |
| F2#6 subfraction | 15.6 | 31.3 | 7.8 |
| 4′-O-Methyl-catechin (F2#5.4.1) | 62.5 | 125.0 | 125.0 |
| Epicatechin-3-O-gallate (F2#6.2.1) | 62.5 | 125.0 | 125.0 |
| 4′-O-Methyl-catechin-3-O-gallate | 31.3 | 62.5 | 62.5 |
| Nystatin | 1.6 | 3.1 | 1.6 |
In order to verify whether the subfractions could have combinatorial effect among themselves, checkerboard test was performed, and the F2#5 and F2#6 subfractions exhibited synergic activity (FIC 0.28) as shown in Figure 1. Because the subfractions exhibited less activity than F2, the compounds in F2 should act together in a synergistic way for effective antifungal activity. These various substances that act synergistically are known as “phytocomplexes,” in which different substances that do not necessarily have the same mechanism of action, when together, have higher therapeutic efficacy than each isolated substance.
Figure 1.
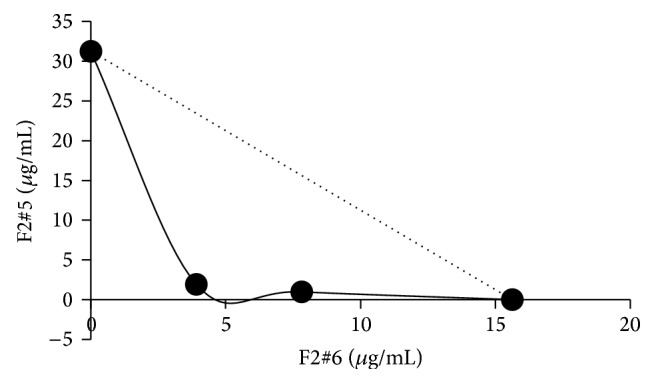
Isobologram showing synergic interaction between F2#5 and F2#6 against Candida albicans. FIC = 0.28. The dotted lines indicate a theoretical additive activity. The measured lines yielded a highly concave curve, a characteristic of strong drug synergism (fractional inhibitory concentration index ≤0.5).
Synergistic effects between CE or each subfraction and fluconazole or nystatin were also evaluated. The CE, F2#5, and F2#6 resulted in a FIC ≤ 0.5 (data not shown), which means, according to Odds [27], that the CE, ethyl acetate fraction, and its subfractions increased the antifungal activity of fluconazole and nystatin (i.e., a synergistic effect).
The CE and its fractions were effective against yeasts that were sensitive or resistant to fluconazole and also to clinical isolates (Table 2). The MICs remained close to those found for the antifungal activity against standard yeast, between 7.8 and 31.3 μg/mL. Fluconazole had a MIC between 3.1 and 12.5 μg/mL against clinical yeast isolates; however, against fluconazole-resistant strains the MIC was >50 μg/mL. These results suggest that derivatives of the plant species C. americana exert their effects through mechanisms of action that are different from fluconazole.
Table 2.
Minimum inhibitory concentration (MIC) of extracts, fractions, and subfractions against strains of Candida spp. fluconazole-sensitive and fluconazole-resistant and from clinical isolates.
| Yeasts | MIC (µg/mL) | ||||
|---|---|---|---|---|---|
| CE | F2 | F2#5 | F2#6 | Fluconazole | |
| Candida albicans (LC352) | 7.8 | 7.8 | 15.6 | 7.8 | 12.5 |
| Candida tropicalis (LC299) | 7.8 | 7.8 | 7.8 | 7.8 | 6.3 |
| Candida parapsilosis (LC144) | 7.8 | 7.8 | 7.8 | 7.8 | 3.1 |
| Candida albicans (32res) | 15.6 | 15.6 | 31.3 | 15.6 | 100.0 |
| Candida albicans (32Bres) | 31.3 | 31.3 | 15.6 | 15.6 | >100.0 |
| Candida albicans (48res) | 15.6 | 15.6 | 15.6 | 15.6 | 50.0 |
| Candida albicans (103res) | 15.6 | 15.6 | 31.3 | 15.6 | 100.0 |
| Candida albicans (48sen) | 15.6 | 15.6 | 31.3 | 15.6 | 50.0 |
In vitro cytotoxicity was evaluated in a Vero cell culture and hemolysis assay (Table 3). The cytotoxic concentration (CC50) of the CE in Vero cells was 250 μg/mL, showing greater toxicity than their fractions. The F2#5 subfraction had the lowest toxicity against Vero cells (CC50 = 714 μg/mL). The evaluation of hemolytic ability in human erythrocytes showed that the extracts and their fractions had low toxicity. Even when considering a concentration of 125 μg/mL for the test drugs, hemolytic activity was observed only with F2 (15.2%), which was lower than amphotericin B (65.8%). When considering the concentration near the MIC (15.6 μg/mL), the highest percentage of hemolysis for F2 was only 5.8%. The presence of tannins and saponins has already been described for Curatella species. The tannin was characterized as having a high ability to interact with proteins [35], and saponins can interact with the cell membrane, causing cell disruption [10]. Thus, in-depth evaluations are necessary to ensure the safety of the herbal preparation.
Table 3.
Cytotoxicity and hemolysis assay of crude extract and their fractions.
| Samples | CC50 (µg/mL) | Hemolytic activity (%) | |
|---|---|---|---|
| 15.6 μg/mL | 125 μg/mL | ||
| CE | 250 ± 12.0 | 1.4 | 8.5 |
| F2 | 650 ± 7.0 | 5.8 | 15.2 |
| F2#5 | 714 ± 20.5 | 1.1 | 2.4 |
| F2#6 | 357 ± 3.5 | 0.4 | 2.0 |
| Amphotericin B | — | 3.3 | 65.8 |
In evaluating the effect on ergosterol, the CE and F2 subfractions maintained their antifungal effects, even in the presence of different concentrations of ergosterol in the growth medium (Figure 2), suggesting that its mode of action is not the same as amphotericin B. Amphotericin B is a polyene that acts on the induction of membrane permeability to potassium, sodium, hydrogen, and nanoelectrolytes in the phospholipid bilayer and forms a complex with fungal ergosterol located in the membrane, resulting in the leakage of intracellular components and subsequent cell death [36]. Because of substrate competition, the increasing concentration of ergosterol in the medium is inversely proportional to its antifungal activity, meaning that the ergosterol concentration is essential for maintenance of the efficacy of amphotericin B. These results show that the extracts, fractions, and subfractions do not depend on ergosterol for their antifungal action, reinforcing the notion that they have a fungistatic effect.
Figure 2.
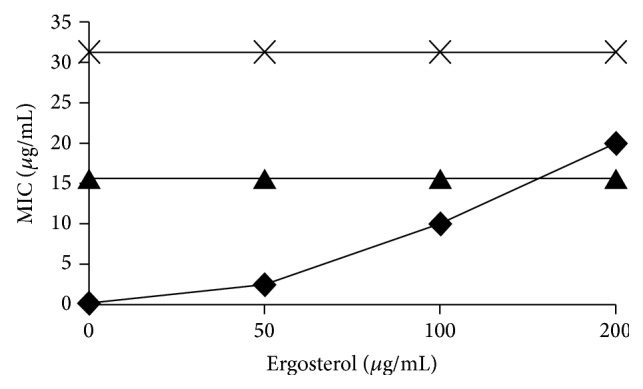
Effect of exogenous ergosterol (0–200 µg/mL) on the MIC of amphotericin B (◆), CE and subfraction F2#6 (▲), and subfraction F2#5 (×) against C. albicans.
To better understand the action of phytocomplexes on yeast, the effects of the CE, fractions, and subfractions on virulence factors of C. albicans were evaluated. The virulence of C. albicans includes recognition of the host and the binding of yeast to their cells and host cell proteins, and this linkage can be facilitated by the transition between the yeast cell and filamentous growth (morphogenesis) compared with isotropic growth from a single yeast cell. Therefore, the presence of filamentous cells or budding is associated with virulence and pathogenicity [37]. In the test of budding inhibition, we found that the CE and F2 had an inhibitory effect that was significantly different from the control group with regard to the average number of cells with budding (Figure 3). Even at the highest concentration, equivalent to 2x MIC, the CE, fractions, and subfractions showed no effect on germ-tube formation, whereas 6.3 μg/mL nystatin visibly inhibited it.
Figure 3.
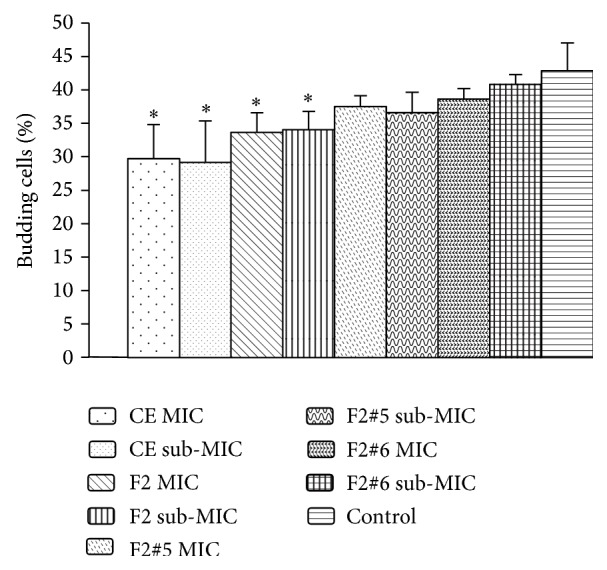
Effect on budding of C. albicans. Only the crude extract and the ethyl acetate fraction showed effect on budding. ∗Statistically significant difference (p < 0.05).
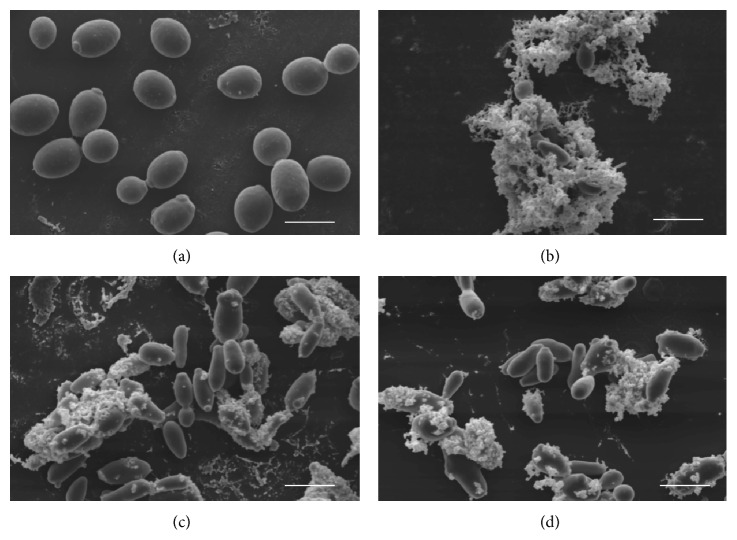
The analysis of the ability of the CE, ethyl acetate fraction, and subfractions at their respective sub-MICs to inhibit adherence on an abiotic surface, with nystatin as a comparison, showed that the number of adhered cells decreased by 98-99%, demonstrating a clear effect on the adherence of yeast to glass slides. All of the samples were able to inhibit the adherence of C. albicans in BECs (Figure 4), which was significantly different from the control group. The F2#6 subfraction had the greatest effect, in which the number of yeast cells adhered to BECs decreased approximately 57%, a higher proportion than for the CE at 2x MIC. This demonstrates a reduction of adhesion ability in yeast treated with the drugs derived from plant species C. americana, which is an important factor for decreasing the virulence of this yeast. These results are consistent with the theory that tannins bind with proteins in the cell wall of yeast [38, 39]. Scanning electron microscopy suggested that this decrease in adherence may be attributable to the changes observed in treated cells, which form an assemblage between yeast and the deposition of a granular consistency outside the cell walls of fungi (Figure 5). These same ultrastructural characteristics of proanthocyanidins of the plant species Stryphnodendron adstringens (Leguminosae) and their tannins can induce modifications of plasmalemma permeability and changes in cytoplasm content, in which the cell wall showed a loss of integrity and low electrodensity [22]. These formations indicate an interaction between the polyphenols present in the CE and its fractions and proteins in the cell wall and could be related to the decreased adhesion ability of C. albicans.
Figure 4.
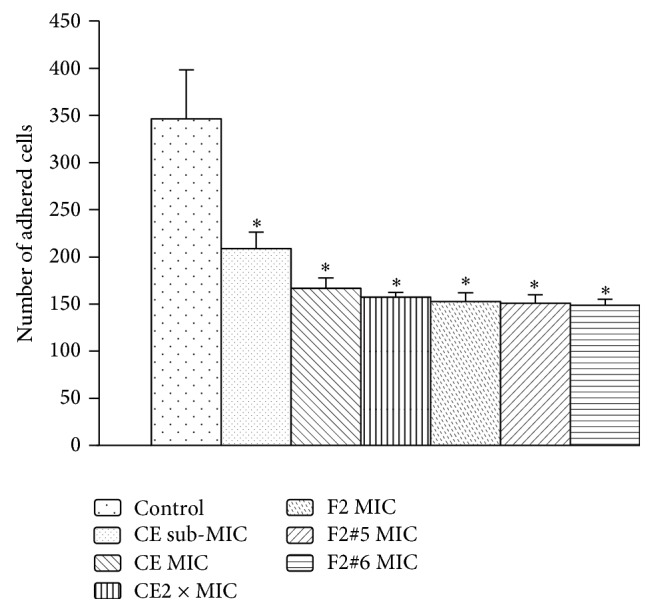
Evaluation of the ability of C. albicans to adhere to buccal epithelial cells (BEC). ∗Statistically significant differences (p < 0.05).
Figure 5.
Scanning electron microscopy (Bar: 5 μm; Mag.: 3000x). The morphological alterations observed in treated cells were yeast agglutination and granular matter deposited on the cell walls of the fungi. (a) Control; (b) CE; (c) F2; (d) F2#5 and F2#6 had similar characteristics.
Additionally, the hydrophobicity assay revealed that the extract of C. americana and its fractions at their respective MICs significantly decreased cell surface hydrophobicity (Table 4). Hydrophobic yeast cells bind profusely in many tissues, whereas a decrease in hydrophobicity reduces the adhesion of yeast [40]. The CE at 4x MIC (62.5 μg/mL), F2 at MIC and 4x MIC (3.2 and 12.8 μg/mL, resp.), F2#5 at MIC and 4x MIC (31.3 and 125.0 μg/mL, resp.), and F2#6 at sub-MIC, MIC, and 4x MIC (7.8, 15.6, and 62.5 μg/mL, resp.) were significantly different from controls (Table 4). The exposure of C. albicans to these compounds for 48 h interfered with CSH. The F2#6 subfraction decreased the HI from 95.1% to 84.5% at MIC and 36.2% at 4x MIC (62.5 μg/mL).
Table 4.
Effect on cell surface hydrophobicity (CSH) with hydrophobicity index (HI), Candida albicans (HIControl = 95.1% ± 0.8).
| Samples | HI (%) | ||
|---|---|---|---|
| Sub-MIC | MIC | 4x MIC | |
| CE | 94.2 ± 0.7 | 93.6 ± 0.6 | 47.2 ± 0.4∗ |
| F2 | 96.4 ± 0.8 | 91.8 ± 0.7∗ | 47.4 ± 0.6∗ |
| F2#5 | 93.7 ± 1.1 | 90.1 ± 1.0∗ | 39.9 ± 0.6∗ |
| F2#6 | 86.7 ± 2.2∗ | 84.5 ± 3.1∗ | 36.2 ± 1.1∗ |
∗Statistically significant difference (p < 0.05).
The influence on oxygen consumption by yeast was confirmed for extracts and fractions tested (Figure 6). Oxygen is essential for cell division and the maintenance of cell viability. According to Thati et al. [32] and Geraghty and Kavanagh [41] reduced cellular respiration causes a reduction of the synthesis of ergosterol, a steroid that is essential for maintaining membrane integrity and cell division. The F2#5 and F2#6 subfractions caused a greater reduction of oxygen consumption by yeast (33.6% and 31.3% decreases, resp.). The CE and F2 caused 45.4% and 35.2% decreases, respectively. The level of oxygen may limit metabolic regulators, and low oxygen consumption can then reduce the pathogenicity of yeast [42]. This decrease in oxygen consumption may be linked to the mechanism of action [38], in which tannins act directly on the metabolism of the microorganism by inhibiting oxidative phosphorylation. Herpetomonas samuelpessoai treated with extracts rich in tannins obtained from Stryphnodendron adstringens Mart. presented evident mitochondrial swelling and a reduction of activity of the enzyme succinate cytochrome c reductase, interfering with the energy metabolism of the cell [43]. Transmission electron microscopy indicated that the cell wall of C. albicans treated with tannins of the same plant species exhibited a loss of integrity and low electrodensity, inducing modifications of plasmalemma permeability and changes in cytoplasm content, reflected by a difference in the electrodensity of treated cells compared with controls [30].
Figure 6.
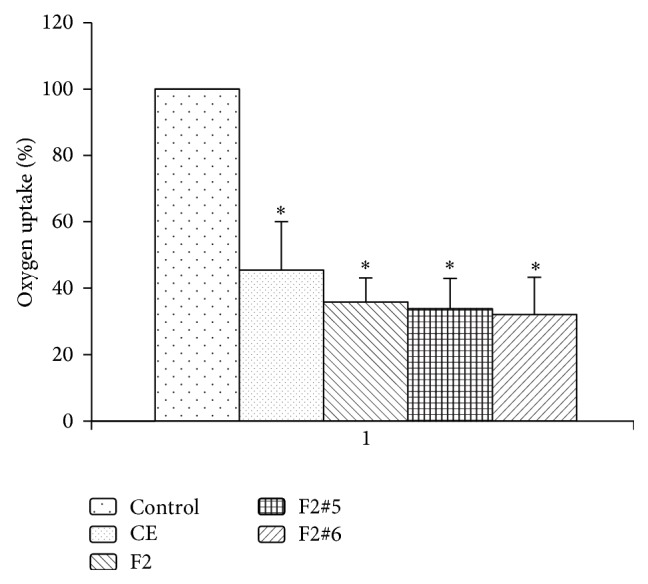
Evaluation of oxygen consumption of C. albicans treated with crude extract, fractions, and subfractions. All samples tested showed effective activity, decreasing oxygen consumption by the yeast C. albicans. ∗Statistically significant difference (p < 0.05).
The present study confirmed the antifungal activity of the ethnomedicinal species Curatella americana L. against Candida species. Although home preparation has presented a pharmacological effect, it can produce potential toxic effects, indicating that the preparation and use of medicinal plants should be performed under professional supervision. From the active subfractions (F2#5 and F2#6), we were able to isolate the substances 4′-O-methyl-catechin (F2#5.4.1), epicatechin-3-O-gallate (F2#6.2.1), and 4′-O-methyl-catechin-3-O-gallate (F2#6.1.2); however, they showed relatively low antifungal activity. This suggests a synergistic action between different substances in the plant drug. If rigorous quality control is performed, then the CE or its fractions could be safe and effective herbal drugs with low production costs. Another important property of the plant species under study is that being able to maintain the activity of the CE and its fractions against fluconazole-sensitive and fluconazole-resistant strains may open new perspectives for alternative treatments.
Acknowledgments
The authors are very grateful to Dr. Solange Lolis for botanic identification, the support of CNPq, PRONEX, CAPES, FINEP, COMCAP, and Fundação Araucária and Programa de Pós-graduação em Ciências Farmacêuticas da Universidade Estadual de Maringá (PCF-UEM).
Abbreviations
- F1:
Aqueous fraction
- ATCC:
American type culture collection
- BEC:
Buccal epithelial cells
- CC50:
50% cytotoxic concentration
- CE:
Crude extract
- CFU:
Colony-forming units
- CPE:
Cytopathic effect
- CSH:
Cell surface hydrophobicity
- EC50:
50% effective concentration
- F2:
Ethyl-acetate fraction
- F2#:
Subfraction of F2
- FBS:
Fetal bovine serum
- FIC:
Fractional inhibitory concentration
- HSCCC:
High-speed countercurrent chromatography
- HTO:
Tocantins herbarium
- MIC:
Minimal inhibitory concentration
- OD:
Optical density
- SI:
Selectivity index
- SSG:
Saline solution containing 1% glucose and buffered to pH 7.25
- subMIC:
Half the MIC
- TCID50:
50% tissue culture infective dose.
Conflict of Interests
The authors have declared that no conflict of interests exists.
References
- 1.Neto G. G., Morais R. G. Recursos medicinais de espécies do cerrado de Mato Grosso: um estudo bibliográfico. Acta Botanica Brasilica. 2003;17(4):561–584. [Google Scholar]
- 2.Espindola L. S., Vasconcelos J. R. E., Jr., De Mesquita M. L., et al. Trypanocidal activity of a new diterpene from Casearia sylvestris var. lingua. Planta Medica. 2004;70(11):1093–1095. doi: 10.1055/s-2004-832655. [DOI] [PubMed] [Google Scholar]
- 3.De Mesquita M. L., Grellier P., Blond A., et al. New ether diglycosides from Matayba guianensis with antiplasmodial activity. Bioorganic and Medicinal Chemistry. 2005;13(14):4499–4506. doi: 10.1016/j.bmc.2005.04.043. [DOI] [PubMed] [Google Scholar]
- 4.Rodrigues A. M. S., de Paula J. E., Roblot F., Fournet A., Espíndola L. S. Larvicidal activity of Cybistax antisyphilitica against Aedes aegypti larvae. Fitoterapia. 2005;76(7-8):755–757. doi: 10.1016/j.fitote.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 5.Saleem M., Nazir M., Ali M. S., et al. Antimicrobial natural products: an update on future antibiotic drug candidates. Natural Product Reports. 2010;27(2):238–254. doi: 10.1039/b916096e. [DOI] [PubMed] [Google Scholar]
- 6.Maregesi S. M., Pieters L., Ngassapa O. D., et al. Screening of some Tanzanian medicinal plants from Bunda district for antibacterial, antifungal and antiviral activities. Journal of Ethnopharmacology. 2008;119(1):58–66. doi: 10.1016/j.jep.2008.05.033. [DOI] [PubMed] [Google Scholar]
- 7.Kubitzki K. Dilleniaceae. In: Smith N., editor. Flouring Plants of the Neotropics. Princenton, NJ, USA: Princeton University Press; 2004. pp. 128–130. [Google Scholar]
- 8.Guerrero M. F., Puebla P., Carrón R., Martín M. L., Arteaga L., Román L. S. Assessment of the antihypertensive and vasodilator effects of ethanolic extracts of some Colombian medicinal plants. Journal of Ethnopharmacology. 2002;80(1):37–42. doi: 10.1016/S0378-8741(01)00420-2. [DOI] [PubMed] [Google Scholar]
- 9.De Souza C. D., Felfili J. M. Uso de plantas medicinais na região de Alto Paraíso de Goiás, GO, Brasil. Acta Botanica Brasilica. 2006;20(1):135–142. doi: 10.1590/s0102-33062006000100013. [DOI] [Google Scholar]
- 10.Costa E. S., Hiruma-Lima C. A., Lima E. O., et al. Antimicrobial activity of some medicinal plants of the Cerrado, Brazil. Phytotherapy Research. 2008;22(5):705–707. doi: 10.1002/ptr.2397. [DOI] [PubMed] [Google Scholar]
- 11.Vazquez J. A., Sobel J. D. Mucosal candidiasis. Infectious Disease Clinics of North America. 2002;16(4):793–820. doi: 10.1016/S0891-5520(02)00042-9. [DOI] [PubMed] [Google Scholar]
- 12.Sojakova M., Liptajova D., Simonicova M., Borovsky M., Subik J. Vulvovaginal candidiasis and susceptibility of pathogens to antifungal agents. Ceská Gynekologie. 2003;68:24–29. [PubMed] [Google Scholar]
- 13.Cross E. W., Park S., Perlin D. S. Cross-resistance of clinical isolates of Candida albicans and Candida glabrata to over-the-counter azoles used in the treatment of vaginitis. Microbial Drug Resistance. 2000;6(2):155–161. doi: 10.1089/107662900419474. [DOI] [PubMed] [Google Scholar]
- 14.Sobel J. D., Zervos M., Reed B. D., et al. Fluconazole susceptibility of vaginal isolates obtained from women with complicated Candida vaginitis: clinical implications. Antimicrobial Agents and Chemotherapy. 2003;47(1):34–38. doi: 10.1128/aac.47.1.34-38.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richter S. S., Galask R. P., Messer S. A., Hollis R. J., Diekema D. J., Pfaller M. A. Antifungal susceptibilities of Candida species causing vulvovaginitis and epidemiology of recurrent cases. Journal of Clinical Microbiology. 2005;43(5):2155–2162. doi: 10.1128/jcm.43.5.2155-2162.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castón-Osorio J. J., Rivero A., Torre-Cisneros J. Epidemiology of invasive fungal infection. International Journal of Antimicrobial Agents. 2008;32(2):S103–S109. doi: 10.1016/s0924-8579(08)70009-8. [DOI] [PubMed] [Google Scholar]
- 17.Toledo C. E. M., Britta E. A., Ceole L. F., et al. Antimicrobial and cytotoxic activities of medicinal plants of the Brazilian cerrado, using Brazilian cachaça as extractor liquid. Journal of Ethnopharmacology. 2011;133(2):420–425. doi: 10.1016/j.jep.2010.10.021. [DOI] [PubMed] [Google Scholar]
- 18.Davis A. L., Cai Y., Davies A. P., Lewis J. R. 1H and 13C NMR assignments of some green tea polyphenols. Magnetic Resonance in Chemistry. 1996;34(11):887–890. doi: 10.1002/(SICI)1097-458X(199611)34:11&lt;887::AID-OMR995>3.0.CO;2-U. [DOI] [Google Scholar]
- 19.Toledo C. E. M. Estudos anatômico, químico e biológico de cascas e extratos obtidos de barbatimão [Stryphnodendron adstringens (Mart.) Coville, Leguminosae] [Master Degree in Pharmaceutical Sciences] São Paulo, Brazil: UNESP; 2002. [Google Scholar]
- 20.Khallouki F., Haubner R., Hull W. E., et al. Isolation, purification and identification of ellagic acid derivatives, catechins, and procyanidins from the root bark of Anisophyllea dichostyla R. Br. Food and Chemical Toxicology. 2007;45(3):472–485. doi: 10.1016/j.fct.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 21.Toledo C. E. M. Propriedade antimicrobiana de espécies vegetais do cerrado brasileiro utilizadas na etnomedicina [Doctoral thesis in Pharmaceutical Sciences] Maringá, Brazil: State University of Maringá; 2011. [Google Scholar]
- 22.Ishida K., Palazzo de Mello J. C., Garcia Cortez D. A., Dias Filho B. P., Ueda-Nakamura T., Nakamura C. V. Influence of tannins from Stryphnodendron adstringens on growth and virulence factors of Candida albicans . Journal of Antimicrobial Chemotherapy. 2006;58(5):942–949. doi: 10.1093/jac/dkl377. [DOI] [PubMed] [Google Scholar]
- 23.Skehan P., Storeng R., Scudiero D., et al. New colorimetric cytotoxicity assay for anticancer-drug screening. Journal of the National Cancer Institute. 1990;82(13):1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- 24.Conceição K., Konno K., Richardson M., et al. Isolation and biochemical characterization of peptides presenting antimicrobial activity from the skin of Phyllomedusa hypochondrialis . Peptides. 2006;27(12):3092–3099. doi: 10.1016/j.peptides.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 25.Tempone A. G., Pimenta D. C., Lebrun I., et al. Antileishmanial and antitrypanosomal activity of bufadienolides isolated from the toad Rhinella jimi parotoid macrogland secretion. Toxicon. 2008;52(1):13–21. doi: 10.1016/j.toxicon.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 26.Clinical and Laboratory Standards Institute (CLSI) Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts: Approved Standard M27-A3. Wayne, Pa, USA: NCCLS; 2008. [Google Scholar]
- 27.Odds F. C. Synergy, antagonism, and what the chequerboard puts between them. Journal of Antimicrobial Chemotherapy. 2003;52(1):p. 1. doi: 10.1093/jac/dkg301. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura C. V., Ishida K., Faccin L. C., et al. In vitro activity of essential oil from Ocimum gratissimum L. against four Candida species. Research in Microbiology. 2004;155(7):579–586. doi: 10.1016/j.resmic.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 29.Mincoff P. C., Garcia Cortez D. A., Ueda-Nakamura T., Nakamura C. V., Dias Filho B. P. Isolation and characterization of a 30 kD antifungal protein from seeds of Sorghum bicolor. Research in Microbiology. 2006;157(4):326–332. doi: 10.1016/j.resmic.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 30.Biasoli M. S., Tosello M. E., Magaró H. M. Adherence of Candida strains isolated from the human gastrointestinal tract. Mycoses. 2002;45(11-12):465–469. doi: 10.1046/j.1439-0507.2002.00793.x. [DOI] [PubMed] [Google Scholar]
- 31.Teixeira L. A., Figueiredo A. M. S., Ferreira B. T., et al. Sialic acid content and surface hydrophobicity of group B streptococci. Epidemiology and Infection. 1993;110(1):87–94. doi: 10.1017/s0950268800050718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thati B., Noble A., Rowan R., et al. Mechanism of action of coumarin and silver(I)-coumarin complexes against the pathogenic yeast Candida albicans . Toxicology in Vitro. 2007;21(5):801–808. doi: 10.1016/j.tiv.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 33.El-Azizi M. M., Ateya A. M., Svoboda G. H., Schiff P. L., Jr., Slatkin D. J., Knapp J. E. Chemical constituents of Curatella americana (Dilleniaceae) Journal of Pharmaceutical Sciences. 1980;69(3):360–361. doi: 10.1002/jps.2600690333. [DOI] [PubMed] [Google Scholar]
- 34.Hiruma-Lima C. A., Rodrigues C. M., Kushima H., et al. The anti-ulcerogenic effects of Curatella americana L. Journal of Ethnopharmacology. 2009;121(3):425–432. doi: 10.1016/j.jep.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 35.Haslam E. Vegetable tannins—lessons of a phytochemical lifetime. Phytochemistry. 2007;68(22–24):2713–2721. doi: 10.1016/j.phytochem.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 36.Mcginnis M. R., Rinaldi M. G. Antifungal drugs: mechanisms of action, drug resistence, susceptibility testing, and assays of activity in biologic fluids. In: Lorian V., editor. Antibiotics in Laboratory Medicine. 5th. Lippincott Williams & Wilkins; 2005. pp. 176–329. [Google Scholar]
- 37.Calderone R. A., Fonzi W. A. Virulence factors of Candida albicans . Trends in Microbiology. 2001;9(7):327–335. doi: 10.1016/s0966-842x(01)02094-7. [DOI] [PubMed] [Google Scholar]
- 38.Scalbert A. Antimicrobial properties of tannins. Phytochemistry. 1991;30(12):3875–3883. doi: 10.1016/0031-9422(91)83426-l. [DOI] [Google Scholar]
- 39.Mello J. C. P., Santos S. C. Taninos. In: Simões C. M. O., Schenkel E. P., Gosmann G., Mello J. C. P., Mentz L. A., Petrovick P. R., editors. Farmacognosia da planta ao medicamento. 6th. Florianópolis, Brazil: Universidade UFRGS, Porto Alegre, Brazil; Editora da UFSC; 2007. pp. 615–656. [Google Scholar]
- 40.Hazen K. C., Brawner D. L., Riesselman M. H., Jutila M. A., Cutler J. E. Differential adherence of hydrophobic and hydrophilic Candida albicans yeast cells to mouse tissues. Infection and Immunity. 1991;59(3):907–912. doi: 10.1128/iai.59.3.907-912.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Geraghty P., Kavanagh K. Disruption of mitochondrial function in Candida albicans leads to reduced cellular ergosterol levels and elevated growth in the presence of amphotericin B. Archives of Microbiology. 2003;179(4):295–300. doi: 10.1007/s00203-003-0530-y. [DOI] [PubMed] [Google Scholar]
- 42.Setiadi E. R., Doedt T., Cottier F., Noffz C., Ernst J. F. Transcriptional response of Candida albicans to hypoxia: linkage of oxygen sensing and Efg1p-regulatory networks. Journal of Molecular Biology. 2006;361(3):399–411. doi: 10.1016/j.jmb.2006.06.040. [DOI] [PubMed] [Google Scholar]
- 43.Holetz F. B., Ueda-Nakamura T., Dias Filho B. P., et al. Biological effects of extracts obtained from Stryphnodendron adstringens on Herpetomonas samuelpessoai . Memorias do Instituto Oswaldo Cruz. 2005;100(4):397–401. doi: 10.1590/s0074-02762005000400010. [DOI] [PubMed] [Google Scholar]