Abstract
Background: Caloric restriction alone has been shown to improve insulin action and fasting glucose metabolism; however, the mechanism by which this occurs remains uncertain.
Objective: We sought to quantify the effect of caloric restriction on β cell function and glucose metabolism in people with type 2 diabetes.
Methods: Nine subjects (2 men, 7 women) with type 2 diabetes [BMI (in kg/m2): 40.6 ± 1.4; age: 58 ± 3 y; glycated hemoglobin: 6.9% ± 0.2%] were studied using a triple-tracer mixed meal after withdrawal of oral diabetes therapy. The oral minimal model was used to measure β cell function. Caloric restriction limited subjects to a pureed diet (<900 kcal/d) for the 12 wk of study. The studies were repeated after 6 and 12 wk of caloric restriction.
Results: Fasting glucose concentrations decreased significantly from baseline after 6 wk of caloric restriction with no further reduction after a further 6 wk of caloric restriction (9.8 ± 1.3, 5.9 ± 0.2, and 6.2 ± 0.3 mmol/L at baseline and after 6 and 12 wk of caloric restriction, respectively; P = 0.01) because of decreased fasting endogenous glucose production (EGP: 20.4 ± 1.1, 16.2 ± 0.8, and 17.4 ± 1.1 μmol · kg−1 · min−1 at baseline and after 6 and 12 wk of caloric restriction, respectively; P = 0.03). These changes were accompanied by an improvement in β cell function measured by the disposition index (189 ± 51, 436 ± 68, and 449 ± 67 10−14 dL · kg−1 · min−2 · pmol−1 at baseline and after 6 and 12 wk of caloric restriction, respectively; P = 0.01).
Conclusions: Six weeks of caloric restriction lowers fasting glucose and EGP with accompanying improvements in β cell function in people with type 2 diabetes. An additional 6 wk of caloric restriction maintained the improvement in glucose metabolism. This trial was registered at clinicaltrials.gov as NCT01094054.
Keywords: caloric restriction, endogenous glucose production, bariatric surgery, gastric emptying, insulin secretion, insulin action, disposition index
Introduction
Bariatric surgery ameliorates type 2 diabetes to a degree that depends in part on the nature of the procedure (1) and in part on underlying subject factors such as duration and severity of diabetes preceding surgery (2). Nevertheless, initial metabolic responses to intervention are quite dramatic and occur independently of weight loss (3). Because caloric restriction per se is shown to have salutary effects on glucose metabolism (4, 5), it is likely that this contributes substantially to the early amelioration of glucose metabolism after bariatric surgery. However, the response to a meal challenge after caloric restriction remains ill defined.
Glucose uptake during hyperinsulinemic, euglycemic conditions is reported to be increased after 7 d of caloric restriction (∼800 kcal/d), despite minimal weight loss (5). More recently, a very-low-calorie diet (500 kcal/d) and Roux-en-Y gastric bypass (RYGB)9 resulted in a comparable improvement in insulin action (Si) and β cell function measured by an intravenous glucose tolerance test in patients with type 2 diabetes (6). In contrast, an 800-kcal/d diet produced less improvement in β cell function than a matched cohort of subjects after RYGB with equivalent weight reduction, implying an additive effect of RYGB (7). The lack of concordance between studies may in part be due to the use of differing, qualitative measures of Si and secretion (8). More importantly, the effects of caloric restriction on fasting and postprandial glucose metabolism remain indeterminate (9).
To address these questions, we examined the effect of caloric restriction on glucose metabolism by using a labeled mixed meal (10) to simultaneously measure β cell function, Si, and fasting and postprandial glucose metabolism (11). People with type 2 diabetes were studied before and after 6 and 12 wk of caloric restriction.
Methods
Subjects.
After approval from the Mayo Institutional Review Board, we recruited 9 subjects (2 men, 7 women) with type 2 diabetes [BMI (in kg/m2): 40.6 ± 1.4; age: 58 ± 3 y; glycated hemoglobin: 6.9% ± 0.2%] who gave written, informed consent to participate in the study. All subjects had no active microvascular or macrovascular complications of diabetes, were weight stable, and did not actively exercise. They were instructed to follow a weight maintenance diet (∼55% carbohydrate, 30% fat, and 15% protein) in the run-up to the baseline study. All glucose-lowering medication was withdrawn for the 3 wk before the study, and this withdrawal was subsequently maintained for the duration of the study.
Subjects met with the study dietician on a weekly basis. They were instructed to consume a diet of 740 kcal daily by using meals derived from the Nutritional Guidelines after Bariatric Surgery (Supplemental Table 1). After 4 wk, subjects increased caloric consumption to 875 kcal daily. Compliance was monitored by weekly meetings with the dietician using an electronic record of food intake. Subjects also met with a behavioral psychologist every other week during the study period. The psychologist used motivational enhancement and behavior change techniques to promote compliance with the dietary guidelines. Body composition was measured with DXA (DPX scanner; Lunar) before each meal study.
Experimental design.
Subjects were studied on 3 occasions: at baseline and then after 6 and 12 wk of caloric restriction. The study was registered at clinicaltrials.gov as NCT01094054. Subjects were admitted to the clinical research unit at 1700 on the evening before all meal studies. They consumed a standardized low-calorie meal (10 kcal/kg body weight: 40% carbohydrate, 30% fat, and 30% protein) and then fasted overnight. At 0630 the next morning (−180 min), an 18-gauge needle was inserted into a forearm vein to allow infusions to be performed. An 18-gauge cannula was inserted retrogradely into a vein on the dorsum of the contralateral hand. This hand was placed in a heated acrylic plastic box maintained at 55°C to allow sampling of arterialized venous blood. A primed (12 mg/kg) continuous (0.12 mg · kg−1 · min−1) infusion of [6,6-2H2] glucose was initiated. At time 0 (0930), subjects consumed a meal (220 kcal, 56% carbohydrate, 25% fat, 19% protein) consisting of 1 scrambled egg, 55 g Canadian bacon, 100 mL water, and gelatin that contained 35 g glucose labeled with [1-13C] glucose (4% enrichment). To enable measurement of solid-phase gastric emptying, the egg was labeled with 0.1 mCi 111In-DTPA. An infusion of [6-3H] glucose was started at time 0. The infusion rates for the tracers were varied to minimize changes in specific activity as previously described (12). At the end of the meal subjects drank 30 mL water. The meal was consumed in the sitting position. For 1 subject intravenous access was lost during the third meal study (after 12 wk of caloric restriction), and data from that study were not used.
Analytical techniques.
Plasma samples were placed on ice, centrifuged at 4°C, separated, and stored at −20°C until assayed. Glucose concentrations were measured with a glucose oxidase method (Yellow Springs Instruments). Plasma insulin was measured with a chemiluminescence assay (Access Assay; Beckman). Plasma glucagon and C-peptide were measured by radioimmunoassay (Linco Research). Plasma [6,6-2H2] glucose and [1-13C] glucose enrichments were measured with gas chromatographic mass spectrometry (Thermoquest) to simultaneously monitor the C-1 and C-2 and C-3 to C-6 fragments, as described by Beylot et al. (13). In addition, [6-3H] glucose-specific activity was measured by liquid scintillation counting after deproteinization and passage over anion and cation exchange columns (12).
Gastric emptying was measured by anterior and posterior gamma camera images obtained in the supine position immediately after meal ingestion, and then every 15 min for the first 2 h, then every 30 min for the next 2 h (total 4 h after the meal). A region of interest analysis with counts corrected for radionuclide decay and tissue attenuation was used as previously described (14).
Calculations.
The systemic Meal Rate of Appearance (Meal Ra), endogenous glucose production (EGP), and glucose disappearance (Rd) were calculated with Steele’s model (15). Meal Ra was calculated by multiplying rate of appearance of [1-13C] glucose (obtained from the infusion rate of [6-3H] glucose and the clamped plasma ratio of [6-3H] glucose and [1-13C] glucose) by the meal enrichment. EGP was calculated from the infusion rate of [6,6-2H2] glucose and the ratio of [6,6-2H2] glucose to endogenous glucose production. Rd was calculated by subtracting the change in glucose mass from the overall rate of glucose appearance (i.e., Meal Ra + EGP). Values from −30 to 0 min were averaged and considered as basal. Incremental, integrated excursions were calculated with the trapezoidal rule.
Net Si was measured with the oral minimal model (16). β Cell responsivity (ϕ) (11) was estimated with the oral C-peptide minimal model (17), incorporating age-associated changes in C-peptide kinetics (18). Disposition indexes (DIs) were subsequently calculated as ϕ × Si.
Statistical analysis.
Data in the text are presented as (observed) means ± SEMs. Differences between the baseline and 6- and 12-wk studies were assessed with a repeated-measures ANOVA and assumed the absence of a Gaussian distribution (Friedman test). Subsequently, in the presence of significant between time point differences, a post hoc Student’s paired t test was used to examine differences between the 3 study days (baseline vs. 6 wk, baseline vs. 12 wk, and 6 wk vs. 12 wk). A Dunn test was used if values were not normally distributed. The statistical analysis was undertaken in Primer 5 (GraphPad Software). P < 0.05 was considered statistically significant.
Results
Volunteer characteristics.
Subject characteristics are summarized in Table 1.
TABLE 1.
Anthropometric characteristics of subjects in the study at the time of their screening visit and then after 6 and 12 wk of caloric restriction1
| Characteristic | Screening2 | 6 wk | 12 wk |
| Sex, M/F | 2/7 | — | — |
| Age, y | 58 ± 3 | — | — |
| Glycated hemoglobin, % | 6.9 ± 0.23 | NM | NM |
| Fasting glucose, mmol/L | 7.0 ± 0.33 | 5.9 ± 0.2 | 6.2 ± 0.3 |
| Weight, kg | 114 ± 6 | 105 ± 5 | 101 ± 6 |
| BMI, kg/m2 | 40.6 ± 1.4 | 37.3 ± 1.6 | 35.8 ± 1.8 |
| Lean body mass, kg | 56 ± 3 | 53 ± 2 | 53 ± 3 |
| Weight lost, kg | NA | 8.9 ± 1.0 | 14.8 ± 1.4 |
| Weight lost, % | NA | −8 ± 1 | −14 ± 1 |
Values are means ± SEMs, n = 9 except at 12 wk, n = 8. NA, not applicable; NM, not measured.
Medical therapy for type 2 diabetes included metformin (n = 6) and glimepiride (n = 2). One participant received no medication. Therapy was withdrawn 3 wk before baseline.
On treatment at the time of screening.
Plasma glucose, insulin, C-peptide, and glucagon concentrations before and after 6 and 12 wk of caloric restriction.
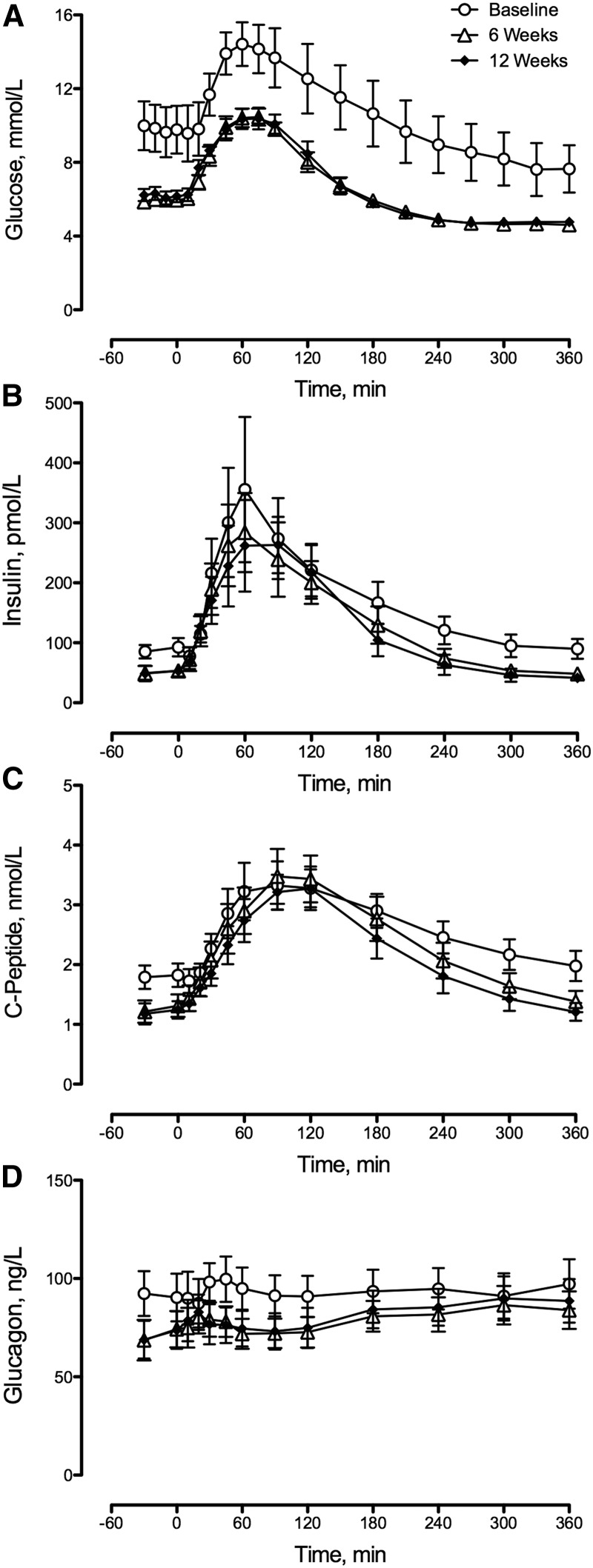
Fasting glucose decreased after 6 wk of caloric restriction (Figure 1A). No further decrease in fasting glucose was observed after 6 additional weeks of caloric restriction (Table 2). Peak postprandial glucose concentrations also decreased after 6 wk with no further decrease observed at 12 wk (Table 2). Of note, caloric restriction did not alter area above basal postprandial glucose concentrations (Table 2).
FIGURE 1.
Plasma glucose (A), insulin (B), C-peptide (C), and glucagon (D) concentrations during meal studies at baseline and after 6 and 12 wk of caloric restriction for patients with type 2 diabetes. The changes in fasting concentrations of glucose, insulin, C-peptide, and glucagon and peak postprandial glucose concentrations after 6 and 12 wk of caloric restriction are detailed in Table 2. Values are means ± SEMs, n = 9 except at 12 wk, n = 8.
TABLE 2.
Fasting and postprandial glucose and hormone concentrations during the baseline meal study and after 6 and 12 wk of caloric restriction for patients with type 2 diabetes1
| Concentrations | Baseline study | 6 wk study | 12 wk study | P2 | Post hoc comparisons |
| Fasting glucose, mmol/L | 9.8 ± 1.3 | 5.9 ± 0.2 | 6.2 ± 0.3 | <1 × 10−4 | BL > (6 wk = 12 wk) |
| Peak postprandial glucose, mmol/L | 14.9 ± 1.4 | 11.0 ± 0.4 | 10.9 ± 0.5 | <1 × 10−4 | BL > (6 wk = 12 wk) |
| AAB glucose, mmol/L × 6 h | 197 ± 101 | 186 ± 68 | 160 ± 38 | 0.28 | NA |
| Fasting insulin, pmol/L | 89 ± 13 | 51 ± 11 | 51 ± 11 | <1 × 10−4 | BL > (6wk = 12 wk) |
| Peak postprandial insulin, pmol/L | 369 ± 120 | 325 ± 66 | 310 ± 74 | 0.97 | NA |
| AAB insulin, nmol/L × 6 h | 29.5 ± 8.9 | 29.5 ± 6.2 | 23.8 ± 5.4 | 0.69 | NA |
| Fasting C-peptide, nmol/L | 1.8 ± 0.2 | 1.3 ± 0.2 | 1.2 ± 0.2 | <1 × 10−4 | BL > (6 wk = 12 wk) |
| Peak postprandial C-peptide, nmol/L | 3.5 ± 0.5 | 3.7 ± 0.5 | 3.5 ± 0.3 | 0.40 | NA |
| AAB C-peptide, nmol/L × 6 h | 296 ± 20 | 402 ± 48 | 339 ± 32 | 0.19 | NA |
| Fasting glucagon, ng/L | 91 ± 12 | 71 ± 10 | 71 ± 9 | 0.02 | BL > (6 wk = 12 wk) |
| Peak postprandial glucagon, ng/L | 107 ± 12 | 95 ± 9 | 96 ± 10 | 0.19 | NA |
| AAB glucagon, μg/L × 6 h | 0.7 ± 1.0 | 2.9 ± 0.9 | 3.8 ± 0.6 | 0.006 | BL < (6 wk = 12 wk) |
Values are means ± SEMs, n = 9 except at 12 wk, n = 8. AAB, area above basal; BL, baseline; NA, not applicable.
Determined by repeated-measures ANOVA.
Fasting insulin concentrations decreased after 6 wk of caloric restriction, with no further decrease thereafter (Figure 1B, Table 2). Caloric restriction did not alter peak or area above basal postprandial insulin concentrations.
Fasting C-peptide concentrations decreased after caloric restriction with no difference between 6- and 12-wk values (Figure 1C, Table 2). Neither peak nor area above basal postprandial C-peptide concentrations was altered by 6 or 12 wk of caloric restriction (Table 2).
Fasting glucagon concentrations were decreased by caloric restriction with no difference between 6- and 12-wk values (Figure 1D, Table 2). Peak postprandial glucagon concentrations were unchanged by caloric restriction (Table 2). However, the postprandial area above basal glucagon concentrations was increased by caloric restriction (Table 2), with no difference between 6- and 12-wk values. However, area under the curve for postprandial glucagon concentrations was unchanged by caloric restriction.
EGP, Meal Ra, and Rd before and after 6 and 12 wk of caloric restriction.
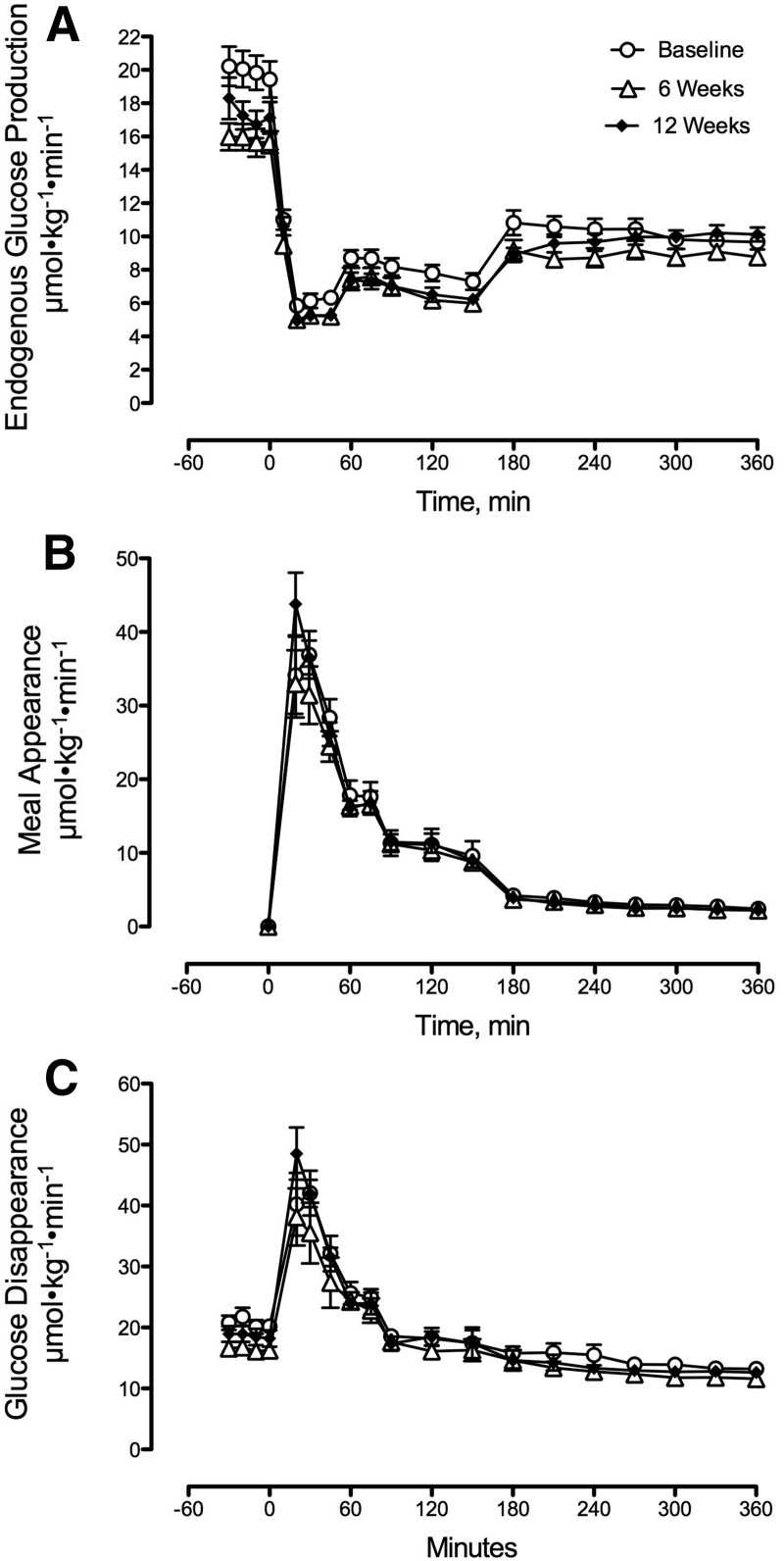
Six weeks of caloric restriction decreased fasting EGP with no difference between 6- and 12-wk values (Figure 2A, Table 3). Nadir postprandial EGP was unchanged by caloric restriction (Table 3).
FIGURE 2.
Rates of endogenous glucose production (A), meal appearance (B), and glucose disappearance (C) during meal studies at baseline and after 6 or 12 wk of caloric restriction for patients with type 2 diabetes. The changes in fasting endogenous glucose production after 6 and 12 wk of caloric restriction are detailed in Table 3. Values are means ± SEMs, n = 9 except at 12 wk, n = 8.
TABLE 3.
Fasting and postprandial variables of glucose metabolism during the baseline meal study and after 6 and 12 wk of caloric restriction for patients with type 2 diabetes1
| Variables | Baseline study | 6 wk study | 12 wk study | P2 | Post hoc comparisons |
| Fasting EGP, μmol · kg−1 · min−1 | 20.4 ± 1.2 | 16.2 ± 0.8 | 17.4 ± 1.1 | 0.003 | BL > (6 wk = 12 wk) |
| Nadir EGP, μmol · kg−1 · min−1 | 5.9 ± 0.4 | 4.8 ± 0.2 | 5.1 ± 0.3 | 0.07 | NA |
| Peak Meal Ra, μmol · kg−1 · min−1 | 40.1 ± 3.0 | 32.1 ± 3.4 | 38.1 ± 3.7 | 0.33 | NA |
| AAB Meal Ra, mmol/kg × 6 h | 3.2 ± 0.3 | 3.0 ± 0.1 | 3.0 ± 0.3 | 0.82 | NA |
| Fasting Rd, μmol · kg−1 · min−1 | 20.7 ± 1.1 | 16.4 ± 0.8 | 18.6 ± 1.3 | <1 × 10−4 | BL > (6 wk = 12 wk) |
| Peak Rd, μmol · kg−1 · min−1 | 45.0 ± 3.3 | 38.7 ± 2.9 | 44.9 ± 2.6 | 0.33 | NA |
| AAB Rd, mmol/kg × 6 h | 6.4 ± 0.2 | 6.2 ± 0.4 | 6.1 ± 0.3 | 0.15 | NA |
Values are means ± SEMs, n = 9 except at 12 wk, n = 8. AAB, area above basal; BL, baseline; EGP, endogenous glucose production; NA, not applicable; Meal Ra, rate of meal appearance; Rd, rate of disappearance.
Determined by repeated-measures ANOVA.
Caloric restriction did not alter either peak or area above basal postprandial Meal Ra (Figure 2B, Table 3).
Fasting Rd was decreased by caloric restriction with no difference between 6- and 12-wk values (Figure 2C, Table 3). Peak and integrated rates of postprandial glucose disposal were not altered by caloric restriction (Figure 2C, Table 3).
Gastric emptying before and after 6 and 12 wk of caloric restriction.
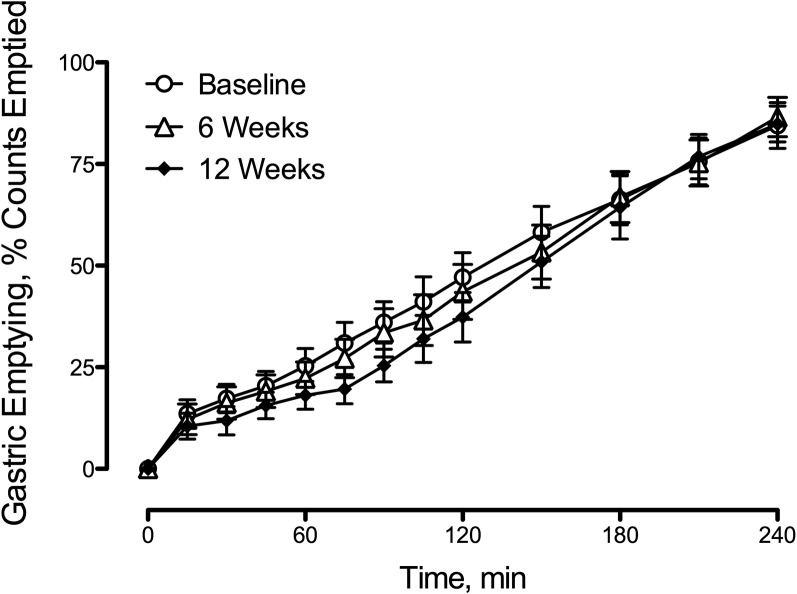
Caloric restriction did not alter the rate of gastric emptying of radiolabeled solids (Figure 3).
FIGURE 3.
Gastric emptying after meal ingestion at baseline and after 6 and 12 wk of caloric restriction for patients with type 2 diabetes. The rate of gastric emptying was unchanged by caloric restriction. Values are means ± SEMs, n = 9 except at 12 wk, n = 8.
Si, ϕ, and DI before and after 6 and 12 wk of caloric restriction.
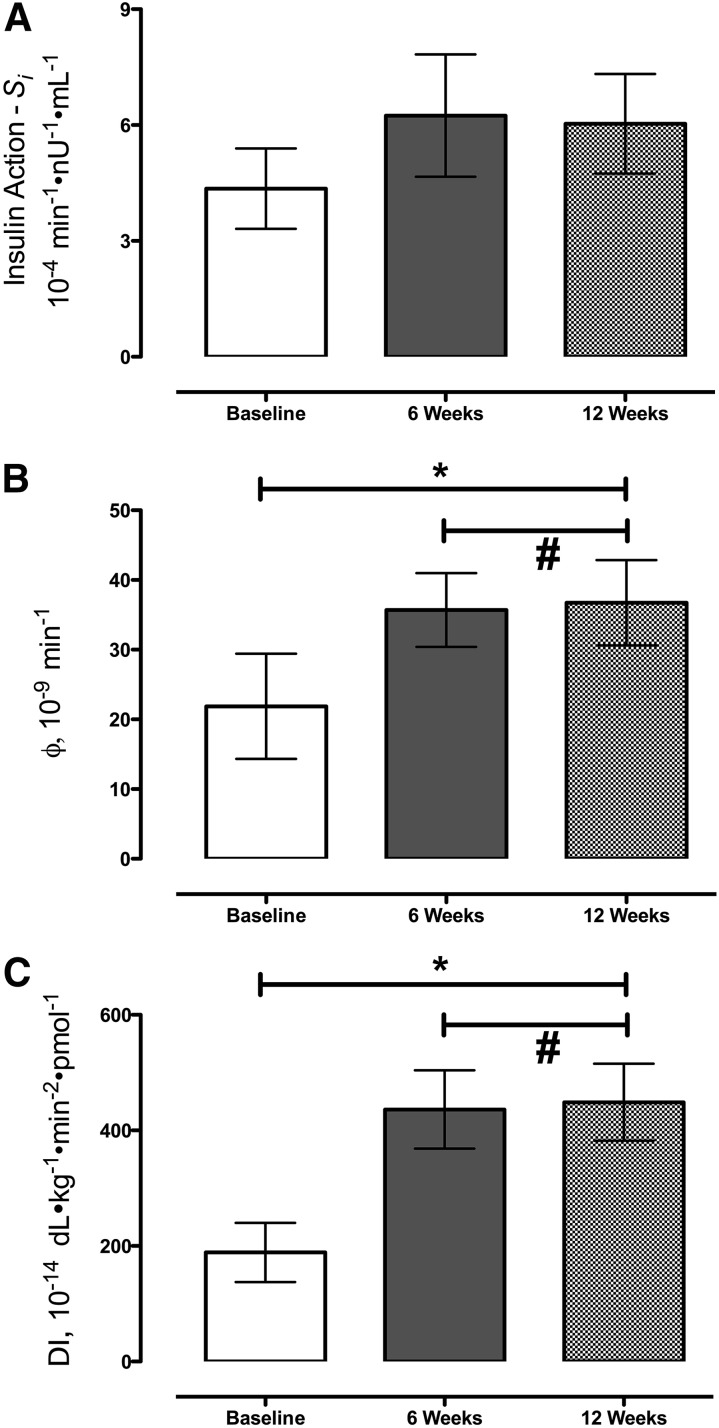
Si did not change significantly after caloric restriction (4.4 ± 1.0 vs. 6.2 ± 1.6 vs. 6.0 ± 1.3 10−4 min−1 · nU−1 · mL−1 at baseline and 6 and 12 wk, respectively; P = 0.19) (Figure 4A).
FIGURE 4.
Si (A), ϕ (B), and DI (C) during meal studies at baseline and after 6 and 12 wk of caloric restriction for patients with type 2 diabetes. Si was unchanged by caloric restriction. Values are means ± SEMs, n = 9 except at 12 wk, n = 8.*Different from baseline, P < 0.05 (ANOVA). #P > 0.05 (post hoc t test). DI, disposition index; Si, insulin action; ϕ, β cell responsivity.
However, caloric restriction increased ϕ (25 ± 8 vs. 36 ± 5 vs. 37 ± 6 10−9 min at baseline and 6 and 12 wk, respectively; P < 0.01; Figure 4B), primarily because of an increase in the static component of ϕ (data not shown) with no difference between the 6- and 12-wk values.
When the ϕ was considered in light of the prevailing degree of Si by calculating the DI, ϕ increased after caloric restriction (189 ± 51 vs. 436 ± 68 vs. 449 ± 67 10−14 dL · kg−1 · min−2 · pmol−1 at baseline and 6 and 12 wk, respectively; P < 0.01; Figure 4C), with no difference between the 6 and 12 wk values. The 95% CIs are shown in Supplemental Figure 1.
Discussion
Six weeks of caloric restriction in people with type 2 diabetes lowered fasting and postprandial glucose concentrations to values that approached those typically observed in people who do not have diabetes (19, 20). The decrease in fasting glucose was attributable to a decrease in fasting EGP. However, caloric restriction did not alter either postprandial Meal Ra or stimulation of postprandial Meal Ra, accounting for the lack of change in postprandial glycemic excursion as measured by the area above basal. The improvement in glycemic control was accompanied by an increase in insulin secretion and a trend toward improved postprandial Si. Despite ongoing caloric restriction and additional weight loss, no further improvement was found in insulin secretion or Si and fasting EGP and on the pattern of postprandial glucose metabolism. Adherence to the caloric restriction is confirmed by the average 8% and 14% weight loss at 6 and 12 wk, respectively.
The decrease in fasting glucose and EGP observed in the present study are consistent with prior studies in which people with type 2 diabetes lost ∼15 kg after consuming a very-low-calorie diet (330 kcal/d) over 40 d. As in the present study, the effects on fasting glucose were rapid with most of the effect seen within the first 10 d of caloric restriction (4). Similarly, despite minimal weight loss, subjects fed 800 kcal/d for 1 wk had a decrease in fasting glucose and EGP equal in magnitude to about one-half of the change observed after weight loss of ∼12 kg with greater caloric restriction (400 kcal/d) for 8 wk (5). In the present experiment, the decrease in fasting glucose was accompanied by an improvement in insulin secretion with a trend toward improvement in Si. The improvement in insulin secretion was primarily because of an increase in the static response to glucose which is believed to represent the provision of new insulin to the releasable pool (17). Previously, β cell function measured with a hyperglycemic clamp was shown to improve after caloric restriction of 400 kcal/d for 7 d (21) or caloric restriction of ∼1100 kcal/d (22).
Improvements in β cell secretion are typically accompanied by decreased α-cell secretion (12). In addition, ingestion of a mixed meal that contained fat and protein is typically accompanied by an increase in postprandial glucagon concentrations (23). During the present experiment, postprandial peak glucagon concentrations were unchanged from baseline by caloric restriction. The increase in incremental glucagon concentrations we observed after caloric restriction is explained not by increased peak postprandial glucagon concentrations but by the greater change from (the lower) fasting concentrations observed after caloric restriction. The effect of caloric restriction on glucagon was previously examined by Kelley et al. (5) who reported a decrease in fasting glucagon concentrations. Vetter et al. (9) reported a similar decrease in fasting glucagon after caloric restriction similar to that undertaken in this experiment. However, peak and incremental postprandial glucagon concentrations were also decreased by caloric restriction. Whether that reflects differences in the composition of the meal ingested and rate of its emptying (liquid vs. solid meal) remains to be ascertained (24).
The lack of improvement in Si contrasts with reports that used a greater degree (400–500 kcal/d) of caloric restriction (5, 6, 21) but is in concordance with other studies that used less (∼1100 kcal/d) caloric restriction (22). Notably, however, both fasting glucose and insulin decreased after caloric restriction and were accompanied by a decrease in fasting EGP, suggesting an improvement in hepatic insulin sensitivity. However, the systemic Meal Ra that represented the net balance of gastric emptying, intestinal absorption, and hepatic extraction of absorbed glucose was unchanged. Given that gastric emptying was unchanged by caloric restriction, this would suggest that hepatic extraction of ingested glucose is also unaltered.
As in the present experiment, Isbell et al. (8) noted a significant effect of caloric restriction alone on glucose metabolism, an effect that did not differ from that of RYGB. Two other studies demonstrated similar (7) or equivalent (6) effects of caloric restriction on glucose metabolism compared with bariatric surgery. Taken together these data strongly suggest that caloric restriction can account for many of the early effects of bariatric surgery (9, 25).
The study had certain limitations. The sample size of 9 subjects certainly allowed detection of the large changes in fasting glucose metabolism observed after 6 wk of caloric restriction (Supplemental Table 2). However, the study may have been underpowered to detect small(-er) changes in other variables or differences between the 6- and 12-wk studies. We have provided a power estimate in Supplemental Table 1. Nevertheless, our observations are congruent with those observed by Vetter et al. (9) in 10 subjects. We measured net Si rather than the individual contributions of insulin-induced suppression of glucose production and insulin-induced stimulation of glucose uptake. Although it is possible that caloric restriction resulted in offsetting effects on hepatic and extrahepatic Si, this seems to be an unlikely explanation for the lack of a significant change in Si after caloric restriction. We only studied the effect of restricting caloric intake to ∼800 kcal/d. We chose this degree of caloric restriction because it mimics that which patients undergoing bariatric surgery consume in the first postoperative weeks.
In conclusion, 6 wk of caloric restriction lowered EGP and fasting glucose. Six weeks of caloric restriction also increased insulin secretion and tended to improve postprandial Si. However, caloric restriction did not alter the pattern and degree of postprandial suppression of EGP, the postprandial stimulation of glucose uptake, or the systemic Meal Ra. Although 6 additional weeks of caloric restriction resulted in further weight loss, it had no additional effects on fasting glucose concentrations, EGP, insulin secretion, or Si. Because caloric restriction per se lowers fasting EGP and fasting glucose and increases insulin secretion, these effects need to be considered in experiments that attempt to determine the mechanisms by which other interventions that also cause caloric restriction (e.g., bariatric surgery) ameliorate type 2 diabetes. These data suggest that caloric restriction or other therapeutic approaches that lower EGP will result in substantial improvement in glycemic control in people with type 2 diabetes.
Acknowledgments
M Sathananthan and M Shah compiled the data and ran the studies; KLE provided dietary advice and monitored the food intake at each visit while KBG provided the motivational enhancement and behavior change techniques to promote compliance with the dietary guidelines; FP, FM, and CDM undertook the mathematical modeling of insulin secretion and action; LPF compiled the data and prepared the results for publication; CC supervised the mathematical modeling of insulin secretion and action and reviewed/edited the manuscript; RAR contributed to the discussion and reviewed/edited the manuscript; MC supervised the gastric emptying studies and reviewed/edited the manuscript; and AV designed and supervised the experiment and wrote the manuscript. AV is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: DI, disposition index; EGP, endogenous glucose production; Meal Ra, Meal Rate of Appearance; Rd, glucose disappearance; RYGB, Roux-en-Y gastric bypass; Si, insulin action; ϕ, β cell responsivity.
References
- 1.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, Schoelles K. Bariatric surgery: a systematic review and meta-analysis. JAMA 2004;292:1724–37. [DOI] [PubMed] [Google Scholar]
- 2.Still CD, Wood GC, Benotti P, Petrick AT, Gabrielsen J, Strodel WE, Ibele A, Seiler J, Irving BA, Celaya MP, et al. . Preoperative prediction of type 2 diabetes remission after Roux-en-Y gastric bypass surgery: a retrospective cohort study. Lancet 2014;2:38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wickremesekera K, Miller G, Naotunne TD, Knowles G, Stubbs RS. Loss of insulin resistance after Roux-en-Y gastric bypass surgery: a time course study. Obes Surg 2005;15:474–81. [DOI] [PubMed] [Google Scholar]
- 4.Henry RR, Scheaffer L, Olefsky JM. Glycemic effects of intensive caloric restriction and isocaloric refeeding in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 1985;61:917–25. [DOI] [PubMed] [Google Scholar]
- 5.Kelley DE, Wing R, Buonocore C, Sturis J, Polonsky K, Fitzsimmons M. Relative effects of calorie restriction and weight loss in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 1993;77:1287–93. [DOI] [PubMed] [Google Scholar]
- 6.Jackness C, Karmally W, Febres G, Conwell IM, Ahmed L, Bessler M, McMahon DJ, Korner J. Very low-calorie diet mimics the early beneficial effect of Roux-en-Y gastric bypass on insulin sensitivity and beta-cell function in type 2 diabetic patients. Diabetes 2013;62:3027–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plum L, Ahmed L, Febres G, Bessler M, Inabnet W, Kunreuther E, McMahon DJ, Korner J. Comparison of glucostatic parameters after hypocaloric diet or bariatric surgery and equivalent weight loss. Obesity (Silver Spring) 2011;19:2149–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Isbell JM, Tamboli RA, Hansen EN, Saliba J, Dunn JP, Phillips SE, Marks-Shulman PA, Abumrad NN. The importance of caloric restriction in the early improvements in insulin sensitivity after Roux-en-Y gastric bypass surgery. Diabetes Care 2010;33:1438–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vetter ML, Wadden TA, Teff KL, Khan Z, Carvajal R, Ritter S, Moore RH, Chittams JL, Iagnocco A, Murayama K, et al. . GLP-1 plays a limited role in improved glycemia shortly after Roux-en-Y gastric bypass: a comparison to intensive lifestyle modification. Diabetes 2015;64:434–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vella A, Rizza RA. Application of isotopic techniques using constant specific activity or enrichment to the study of carbohydrate metabolism. Diabetes 2009;58:2168–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cobelli C, Toffolo GM, Dalla Man C, Campioni M, Denti P, Caumo A, Butler P, Rizza R. Assessment of beta-cell function in humans, simultaneously with insulin sensitivity and hepatic extraction, from intravenous and oral glucose tests. Am J Physiol Endocrinol Metab 2007;293:E1–15. [DOI] [PubMed] [Google Scholar]
- 12.Dalla Man C, Bock G, Giesler PD, Serra DB, Ligueros Saylan M, Foley JE, Camilleri M, Toffolo G, Cobelli C, Rizza RA, et al. . Dipeptidyl peptidase-4 inhibition by vildagliptin and the effect on insulin secretion and action in response to meal ingestion in type 2 diabetes. Diabetes Care 2009;32:14–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beylot M, Previs SF, David F, Brunengraber H. Determination of the 13C-labeling pattern of glucose by gas chromatography-mass spectrometry. Anal Biochem 1993;212:526–31. [DOI] [PubMed] [Google Scholar]
- 14.Cremonini F, Mullan BP, Camilleri M, Burton DD, Rank MR. Performance characteristics of scintigraphic transit measurements for studies of experimental therapies. Aliment Pharmacol Ther 2002;16:1781–90. [DOI] [PubMed] [Google Scholar]
- 15.Steele R, Bjerknes C, Rathgeb I, Altszuler N. Glucose uptake and production during the oral glucose tolerance test. Diabetes 1968;17:415–21. [DOI] [PubMed] [Google Scholar]
- 16.Dalla Man C, Caumo A, Basu R, Rizza R, Toffolo G, Cobelli C. Minimal model estimation of glucose absorption and insulin sensitivity from oral test: validation with a tracer method. Am J Physiol Endocrinol Metab 2004;287:E637–43. [DOI] [PubMed] [Google Scholar]
- 17.Breda E, Cavaghan MK, Toffolo G, Polonsky KS, Cobelli C. Oral glucose tolerance test minimal model indexes of beta-cell function and insulin sensitivity. Diabetes 2001;50:150–8. [DOI] [PubMed] [Google Scholar]
- 18.Van Cauter E, Mestrez F, Sturis J, Polonsky KS. Estimation of insulin secretion rates from C-peptide levels. Comparison of individual and standard kinetic parameters for C-peptide clearance. Diabetes 1992;41:368–77. [DOI] [PubMed] [Google Scholar]
- 19.Bock G, Dalla Man C, Campioni M, Chittilapilly E, Basu R, Toffolo G, Cobelli C, Rizza R. Pathogenesis of pre-diabetes: mechanisms of fasting and postprandial hyperglycemia in people with impaired fasting glucose and/or impaired glucose tolerance. Diabetes 2006;55:3536–49. [DOI] [PubMed] [Google Scholar]
- 20.Bock G, Dalla Man C, Micheletto F, Basu R, Giesler PD, Laugen J, Deacon CF, Holst JJ, Toffolo G, Cobelli C, et al. . The effect of DPP-4 inhibition with sitagliptin on incretin secretion and on fasting and postprandial glucose turnover in subjects with impaired fasting glucose. Clin Endocrinol (Oxf) 2010;73:189–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malandrucco I, Pasqualetti P, Giordani I, Manfellotto D, De Marco F, Alegiani F, Sidoti AM, Picconi F, Di Flaviani A, Frajese G, et al. . Very-low-calorie diet: a quick therapeutic tool to improve cell function in morbidly obese patients with type 2 diabetes. Am J Clin Nutr 2012;95:609–13. [DOI] [PubMed] [Google Scholar]
- 22.Markovic TP, Jenkins AB, Campbell LV, Furler SM, Kraegen EW, Chisholm DJ. The determinants of glycemic responses to diet restriction and weight loss in obesity and NIDDM. Diabetes Care 1998;21:687–94. [DOI] [PubMed] [Google Scholar]
- 23.Butler PC, Rizza RA. Contribution to postprandial hyperglycemia and effect on initial splanchnic glucose clearance of hepatic glucose cycling in glucose-intolerant or NIDDM patients. Diabetes 1991;40:73–81. [PubMed] [Google Scholar]
- 24. Camilleri M. Clinical practice. Diabetic gastroparesis. N Engl J Med 2007;356:820–9. [DOI] [PubMed]
- 25.Vella A. Does caloric restriction alone explain the effects of Roux-en-Y gastric bypass on glucose metabolism? Not by a long limb. Diabetes 2013;62:3017–8. [DOI] [PMC free article] [PubMed] [Google Scholar]