Abstract
Background: Polyunsaturated fatty acids (PUFAs) are associated with protection from obesity-related phenotypes in adults; however, the relation between reported intake of PUFAs with body-composition outcomes in children remains unknown.
Objective: Our objective was to examine how self-reported intakes of PUFAs, including total, n–6 (ω-6), and n–3 (ω-3) PUFAs and ratios of n–6 to n–3 PUFAs and PUFAs to saturated fatty acids (SFAs), are associated with measures of adiposity and lean mass (LM) in children. We hypothesized that higher self-reported intakes of PUFAs and the ratio of PUFAs to SFAs would be positively associated with LM and negatively associated with total adiposity.
Methods: Body composition and dietary intake were measured in a racially diverse sample of 311 children (39% European American, 34% African American, and 27% Hispanic American) aged 7–12 y. Body composition and abdominal fat distribution were measured by dual-energy X-ray absorptiometry and computed tomography scans, respectively. Self-reported dietary intakes (including total PUFAs, n–3 PUFAs, n–6 PUFAs, and SFAs) were assessed by using two 24-h recalls. Independent-sample t tests and multiple linear regression analyses were conducted.
Results: Total PUFA intake was positively associated with LM (P = 0.049) and negatively associated with percentage of body fat (%BF; P = 0.033) and intra-abdominal adipose tissue (IAAT; P = 0.022). A higher ratio of PUFAs to SFAs was associated with higher LM (P = 0.030) and lower %BF (P = 0.028) and IAAT (P = 0.048). Intakes of n–3 and n–6 PUFAs were positively associated with LM (P = 0.017 and P = 0.021, respectively), and the ratio of n–6 to n–3 PUFAs was negatively associated with IAAT (P = 0.014). All results were independent of biological, environmental, and genetic covariates.
Conclusions: Our results show that a higher self-reported intake of PUFAs and a higher ratio of PUFAs to SFAs are positively associated with LM and negatively associated with visceral adiposity and %BF in a healthy cohort of racially diverse children aged 7–12 y. This trial was registered at clinicaltrials.gov as NCT00726778.
Keywords: obesity, n–3 PUFA, n–6 PUFA, saturated fatty acid, fat mass, body composition, Hispanic, African American, non-Hispanic white
Introduction
Since 1980, the prevalence of obesity among youth has nearly tripled in the United States, with recent estimates suggesting that ∼12.5 million children aged 2–19 y are considered obese (1). Pediatric obesity, defined as sex- and age-specific BMI more than the 95% percentile in children aged 2–12 y (2), increases the probability of being obese in adulthood by 2-fold (3) and may ultimately increase the risk of developing type 2 diabetes (4), metabolic syndrome (5), and dyslipidemia (6). Because of this increased risk, it is important to characterize dietary factors beyond total energy intake that can improve pediatric body composition and prevent the onset of adult obesity-related comorbidities.
The FA composition of children’s diets may have an effect on their body composition (7–15). FAs are calorically dense and contribute substantially to daily energy intake. However, PUFAs do not appear to contribute to fat mass (FM)9 accumulation in the same way as other FAs (i.e., SFAs) (16–19). Moreover, all PUFAs do not appear to affect body composition in the same way, because there is evidence that n–6 and n–3 PUFAs have varying effects (7–16, 20). Total and n–3 PUFA intakes may be protective against excessive adiposity, but the contributions of n–6 PUFAs, the n–6 to n–3 PUFA ratio, and the PUFA to SFA ratio to FM, fat deposition, and lean mass (LM) remain poorly characterized.
Previous studies have been conducted primarily in white children of European descent (7–9, 13, 14); however, racial and ethnic background is an important genetic determinant of pediatric obesity (21, 22). Moreover, measurements of body composition beyond BMI that assess abdominal fat distribution and LM have not been widely used. Finally, objective measures of energy expenditure and physical activity are essential covariates when assessing the contributions of dietary components to body composition and obesity. In the current study, we used the previously described AMERICO Study data set composed of detailed genetic, dietary, behavioral, and metabolic data in >300 multiethnic children in the United States (21). With this robust data set, we were able to control for well-known determinants of obesity and better isolate the relations of dietary components. Therefore, the purpose of this study was to test the hypothesis that higher self-reported intakes of PUFAs (including total, n–6, n–3, and n–6:n–3) and the PUFA to SFA ratio would be negatively associated with measures of adiposity and positively associated with LM, independent of biological, environmental, and genetic covariates.
Methods
Subjects.
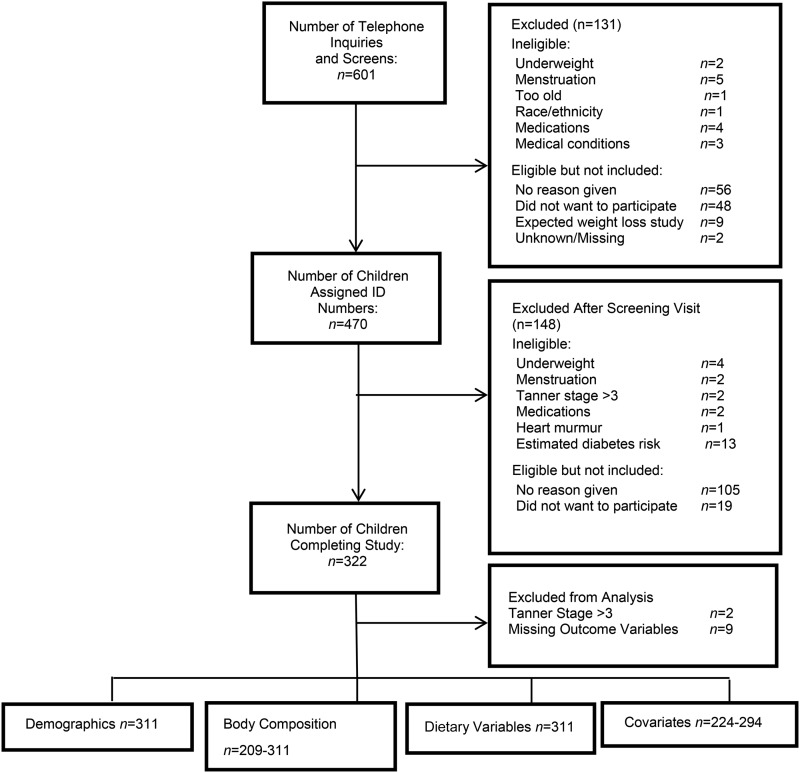
Participants were 311 children (47% female) aged 7–12 y, self-identified as African American (AA; n = 106), European American (EA; n = 121), or Hispanic American (HA; n = 84) from the Birmingham, Alabama, area. The majority of the children were of normal weight (67% normal weight, 23% overweight, and 10% obese). Children were recruited at schools, churches, and health fairs and through newspapers, magazines, and participant referrals. The children were peripubertal (pubertal stage ≤3 as assessed by a pediatrician according to the criteria of Marshall and Tanner) (23, 24) and had no medical diagnosis or medications contraindicated for study participation (i.e., medication known to affect metabolism, body composition). Before study participation, the children and their parents provided informed assent and consent, respectively, to the protocol, which was approved by the Institutional Review Board for human subjects at the University of Alabama at Birmingham. All measurements were performed at the Nutrition Obesity Research Center and the Department of Nutrition Sciences at the University of Alabama at Birmingham between 2005 and 2008. (See Figure 1 for information on how our sample size was determined.)
FIGURE 1.
Recruitment and final sample size flowchart.
Subjects participated in 2 visits. On the first visit, pubertal status, anthropometrics, questionnaire data, dietary recall, and body composition by DXA and/or computed tomography (CT) scanning were measured. Within 30 d, the children and parents returned for the second overnight visit, at which time blood for genetic admixture analysis was drawn, resting energy expenditure (REE) was assessed, and a second dietary recall was performed.
Anthropometric measurements.
Anthropometric measurements for all participants were obtained by the same registered dietitian. Participants were weighed (Scale-tronix 6702W; Scale-tronix, Carol Stream, IL) to the nearest 0.1 kg (wearing minimal clothing without shoes). Height was recorded to the nearest 0.1 cm without shoes by using a digital stadiometer (Heightronic 235; Measurement Concepts). Children’s BMI percentiles were calculated as indicated by the CDC guidelines (2).
Body composition and fat distribution.
Body composition [FM, LM, and percentage of body fat (%BF)] was measured by DXA with the use of a GE Lunar Prodigy densitometer (GE Lunar Radiation Corp.). Participants were scanned in light clothing while lying flat on their backs with arms at their sides. DXA scans were performed and analyzed with pediatric software enCORE 2002 version 6.10.029. DXA has been found to be highly reliable for body-composition assessment in children (25). Because of financial limitations, CT scanning was used to quantify the distribution of abdominal adipose tissue as total abdominal adipose tissue, intra-abdominal adipose tissue (IAAT), and subcutaneous abdominal adipose tissue (SAAT) in a subsample of children (n = 209). There were no significant differences in demographic variables between children who were assessed with CT and children who were not assessed by CT (data not shown). A HiLight/Advantage scanner (General Electric) was used to perform a single-slice (5-mm) scan of the abdomen at the level of the umbilicus. Scans were analyzed for cross-sectional area (cm2) of adipose tissue by using the density contour program with Hounsfield units for adipose tissue set at −190 to −30. CT has been shown to provide accurate measurements of body fat distribution in children (26).
Dietary recall.
Two 24-h dietary recalls (1 at each of the study visits) were administered and analyzed by a registered dietitian by using the “multiple pass” method, which provides a cup and bowl size to help estimate portion sizes (27). A parent/guardian was present for, and assisted with, each recall, and all 24-h dietary recalls were conducted on weekdays. A registered dietitian coded and entered the data into Nutrition Data System for Research, version 2006 (Nutrition Coordinating Center, University of Minnesota). Total energy intake (kcal/d), macronutrient composition (% of total energy), total PUFAs (g/d), SFAs (g/d), n–3 FAs (g/d), and n–6 FAs (g/d) were among the variables analyzed. Total energy intake was included in analyses as a covariate given that PUFA intake potentially increases with increasing energy intake and positive energy balance is known to have an effect on obesity-related variables (28–30). It is important to note that we are aware of the nontrivial errors associated with self-reported estimates of energy intake and we recognize that this limits the definitive conclusions that can be made from this report alone (31). Rather, we believe that this study provides a unique opportunity to explore our hypothesis of interest and to identify, on the basis of our results, potential future hypotheses directed toward the understanding of the physiologic differences of higher vs. lower reported PUFA and SFA consumption.
Physical activity assessment.
Given that physical activity is inversely associated with weight in children, we examined differences in physical activity between the groups (32). Children wore an MTI ActiGraph accelerometer (GT1M; ActiGraph Health Services) on their waist over the right hip for 7 d to objectively measure physical activity levels (removal was limited to times when the child was sleeping, bathing, or swimming). Epoch length was set at 1 min, and physical activity data were expressed as counts per minute (counts/min). As previously described, moderate and vigorous physical activity (MVPA) was defined as the daily counts per minute >1952 (33). The average minutes that children spent per day engaged in MVPA were summed and averaged.
REE.
The largest fraction of total daily energy expenditure is accounted for by REE (34), and alterations in energy expenditure are associated with FM and LM in children (35). Given the known influence of REE on children’s body weight, we examined differences in REE between groups. Children were provided with a standardized meal the night before their REE testing. REE was measured in a fasting state in the morning immediately after awakening during the overnight visit. A computerized, open-circuit, indirect calorimetry system with a ventilated canopy (Delta Trac II; Sensor Medics) was used. While lying supine on a bed, the subject’s head was enclosed in a clear plastic canopy. Subjects were instructed not to sleep and to remain quiet and still, breathing normally. One-minute-average intervals of oxygen uptake and carbon dioxide production were measured continuously for 30 min.
Socioeconomic status.
Socioeconomic status (SES) has been associated with body composition in children (36); therefore, SES was used as a covariate in all models related to child adiposity. SES was measured with the Hollingshead 4-factor index of social class (37), which combines educational attainment and occupational prestige for working parents in the child’s family. Scores range from 8 to 66, with higher scores indicating higher social status.
Pubertal status and sex.
Both pubertal stage and biological sex play a role in adiposity accrual and distribution (38, 39) and were included as covariates in the analysis. Tanner staging is an objective measure of pubertal development and a better predictor of body composition than age (38). Direct observation for the assessment of pubertal stage by the same pediatrician was used for differentiating among the 5 stages of maturity, and children at pubertal stage ≤3 were included in this study (23, 24). On the basis of the criteria of Marshall and Tanner, pubertal staging was assessed according to both breast and pubic hair development in girls and genitalia and pubic hair development in boys. One composite number was assigned for Tanner staging, representing the higher of the 2 values defined by breast/genitalia and pubic hair development (23, 24).
Genetic admixture.
Previous work in our group has demonstrated that ancestral genetic background is associated with FM and LM in children (21). African admixture (AFADM) and European admixture (EUADM) estimates were used to adjust for the genetic contribution to underlying population variability in body composition (21). The genetic admixture estimates were obtained from genotyping ∼142 ancestry informative markers across the human genome that provided information about European, African, and Amerindian parental ancestry for each participant. Genotyping for the measures of genetic admixture was performed at Prevention Genetics (www.preventiongenetics.org) by using the McSNP method and agarose gel electrophoresis, as previously described (21, 22). Individual admixture estimates were derived by using the maximum likelihood method, which estimates the proportion of genetic ancestry for an individual by using a range of proportions from 0 to 1 and identifies the most probable value of admixture on the basis of the observed genotypes (21, 22).
Statistical analysis.
For the demographic analyses, participants were stratified into either the high-PUFA group [% of total energy from PUFAs (%PUFA) ≥5.84%; HP] or the low-PUFA group (%PUFA <5.84%; LP) on the basis of the median %PUFA value (5.84%). Participants with missing data were not included in the analyses. Independent-sample t tests were used to detect differences in the variables of interest between the HP and LP groups. Multiple linear regression models were used to evaluate the relation of dietary FA intakes with body-composition variables. All residuals were tested for normality, and skewed variables were log-transformed for data analysis. Values of IAAT, SAAT, FM, total abdominal adipose tissue, and LM were successfully log-transformed for normality after visual inspection of residuals from the regression equations. Exploratory models were conducted to test for significant contributions of total energy intake, sex, age, pubertal status, height, race/ethnicity, AFADM, EUADM, REE, MVPA, and SES on each body-composition variable. Because of the exploratory nature of this analysis, P values were not adjusted for multiple comparisons. On the basis of the seminal work of Wells and Cole (40–42), we also explored the relation between PUFA variables of interest and the fat-free mass index [LM (kg)/height2(m2)] and the fat mass index [FM (kg)/height2(m2)] When taking into account the demographic characteristics of our diverse sample, assumptions of regression, distribution of residuals, and overall fit of the data, results with %BF, LM, and FM appear to be the most suitable for our study. Thus, we chose to report the unadjusted body-composition variables in the main article and report the height-adjusted variables in the Supplemental Table 1.
As previously described (21), the measured value for each genetic admixture component adds to 1; therefore, to avoid overspecification of the statistical models, only AFADM and EUADM were included as genetic covariates in the models. AFADM and EUADM were chosen because they have the greatest range of variability among the AA, HA, and EA participants in this sample. Data were analyzed by using SAS statistical software version 9.3 (SAS Institute). Significance was set at P < 0.05.
Results
Demographic characteristics.
Descriptive statistics for the total sample and comparisons between LP and HP groups are reported in Table 1. No significant differences were observed with regard to age, sex, pubertal stage, SES, EUADM, or AFADM between LP and HP groups. We observed a higher prevalence of AA children in the HP group relative to the LP group.
TABLE 1.
Child demographic characteristics, body composition, and dietary variables by LP and HP groups based on percentage of total energy derived from PUFAs1
| Total sample (n = 311) | LP group (n = 155) | HP group (n = 156) | |
| Demographic characteristics | |||
| Age, y | 9.6 ± 1.6 | 9.6 ± 1.6 | 9.6 ± 1.6 |
| Sex, % female | 47.0 | 43.9 | 51.0 |
| Tanner stage, % Tanner stage 3 | 13.1 | 9.7 | 16.7 |
| SES | 38.7 ± 14.5 | 39.9 ± 14.4 | 37.6 ± 14.5 |
| Race/ethnicity, % | |||
| European American | 39.0 | 41.9 | 36.1 |
| African American | 33.9 | 28.4 | 39.4* |
| Hispanic American | 27.1 | 29.7 | 24.5 |
| Genetic ancestry, % | |||
| European admixture | 52.3 ± 38.0 | 54.8 ± 37.5 | 49.8 ± 38.6 |
| African admixture | 29.8 ± 38.2 | 25.8 ± 3 6.4 | 33.8 ± 39.6 |
| Body composition | |||
| BMI-for-sex-and-age percentile | 66.1 ± 26.4 | 64.0 ± 28.4 | 68.7 ± 24.0 |
| Lean mass, kg | 25.6 ± 5.3 | 25.2 ± 5.0 | 26.0 ± 5.5 |
| Fat mass, kg | 8.9 ± 5.7 | 9.1 ± 6.1 | 8.8 ± 5.4 |
| Body fat, % | 23.5 ± 9.3 | 23.8 ± 9.5 | 23.2 ± 9.2 |
| IAAT,2 cm2 | 33.3 ± 22.4 | 36.0 ± 26.0 | 30.5 ± 17.6 |
| SAAT,2 cm2 | 93.0 ± 75.0 | 95.7 ± 75.2 | 90.2 ± 75.1 |
| TAAT,2 cm2 | 126 ± 94.2 | 132 ± 98.5 | 121 ± 89.5 |
| Daily intakes | |||
| Energy, kcal/d | 1887 ± 471 | 1860 ± 467 | 1915 ± 475 |
| Carbohydrate, % of energy | 51.2 ± 7.7 | 53.9 ± 7.3 | 48.5 ± 7.1† |
| Protein, % of energy | 15.0 ± 3.3 | 15.0 ± 3.4 | 15.0 ± 3.3 |
| Fat, % of energy | 35.0 ± 6.1 | 32.3 ± 5.7 | 37.6 ± 5.3† |
| SFAs, % of energy | 12.6 ± 2.7 | 12.6 ± 3.0 | 12.5 ± 2.3 |
| PUFAs, % of energy | 6.4 ± 2.6 | 4.7 ± 0.8 | 8.1 ± 2.6† |
| Total PUFAs, g/d | 13.5 ± 6.7 | 9.8 ± 3.2 | 17.2 ± 7.2† |
| n–3 FAs | 1.2 ± 0.6 | 0.9 ± 0.4 | 1.4 ± 0.7† |
| α-Linolenic acid | 1.1 ± 0.6 | 0.8 ± 0.3 | 1.4 ± 0.6† |
| EPA | 0.03 ± 0.1 | 0.01 ± 0.0 | 0.04 ± 0.1† |
| DHA | 0.06 ± 0.2 | 0.03 ± 0.1 | 0.08 ± 0.2** |
| EPA+DHA | 0.08 ± 0.2 | 0.05 ± 0.1 | 0.10 ± 0.3** |
| n–6 FAs | 12.2 ± 6.2 | 8.8 ± 2.9 | 15.6 ± 6.7† |
| Linoleic acid | 12.1 ± 6.2 | 7.9 ± 2.0 | 16.3 ± 6.1† |
| Arachidonic acid | 0.11 ± 0.1 | 0.09 ± 0.1 | 0.14 ± 0.1† |
| PUFA:SFA | 0.6 ± 0.3 | 0.4 ± 0.1 | 0.7 ± 0.3† |
| n-6:n–3 FAs | 11.0 ± 4.1 | 9.9 ± 2.8 | 12.2 ± 4.8† |
| Energy expenditure | |||
| Physical activity,3 MVPA min/d | 54.9 ± 29.1 | 52.7 ± 28.4 | 57.0 ± 29.9 |
| REE, kcal/d | 1192 ± 235 | 1207 ± 257 | 1178 ± 212 |
Values are means ± SDs unless otherwise indicated. Values may not add up to 100% due to rounding. *,**,†Different from LP: *P < 0.05, **P < 0.01, †P < 0.001. HP, high-PUFA intake; IAAT, intra-abdominal adipose tissue; LP, low-PUFA intake; MVPA, moderate and vigorous physical activity; REE, resting energy expenditure; SAAT, subcutaneous abdominal adipose tissue; SES, socioeconomic status; TAAT, total abdominal adipose tissue.
A subsample of children (n = 209) had measures of IAAT, SAAT, and TAAT assessed (n = 104 in LP group; n = 105 in HP group).
Average minutes spent in MVPA per day.
Body composition, dietary intakes, and energy expenditure.
Body-composition measures for the total sample and comparisons between the LP and HP groups are reported in Table 1. IAAT tended to be lower in the HP group than in the LP group (P = 0.08).
Dietary intake measures for the total sample and comparisons between LP and HP groups are reported in Table 1. The HP group reported consuming a significantly greater percentage of fat (P < 0.001), %PUFA (P < 0.001), and EPA+DHA (P < 0.001), and a lower percentage of carbohydrate (P < 0.001) relative to the LP group. The HP group also consumed more total PUFAs (P < 0.001), n–3 PUFAs (P < 0.001), α-linolenic acid (ALA, 18:3n−3; P < 0.001), EPA (P < 0.001), DHA (P = 0.008), and n–6 PUFAs (P < 0.001), linoleic acid (18:2n−6; P < 0.001), and arachidonic acid (20:4n−6; P < 0.001) and had higher PUFA to SFA (P < 0.001) and n–6 to n–3 (P = 0.002) ratios when compared with the LP group. No differences were detected between groups with regard to total energy intake, percentage of protein, percentage of SFAs, MVPA, or REE.
Acceptable macronutrient dietary recommendations (as a part of the Dietary Reference Intakes established by the Institute of Medicine) for n–6 PUFAs, ALA, and EPA+DHA have been established (43). The percentages of children consuming recommended amounts of n–6 PUFAs (5–10% of total energy), plant-derived n–3 PUFA ALA (0.6–1.2% of total energy), and marine-derived n–3 PUFA EPA+DHA (0.06–0.12% of total energy) were 57.7%, 24.8%, and 11.6%, respectively (data not shown). In the HP group, 87.7% met recommendations for n–6 PUFAs, 40.7% for ALA, and 16.1% for EPA+DHA. In the LP group, 27.7% met recommendations for n–6 PUFAs, 9.0% for ALA, and 7.1% for EPA+DHA (data not shown).
Relations between dietary fat and body composition.
Results for the multiple regression models assessing the contribution of dietary fat to variables of body composition and fat distribution while adjusting for pubertal stage, sex, SES, AFADM, EUADM, and total energy intake (with the exception of the %PUFA model) are shown in Table 2. Models for LM and FM were also adjusted for height. Higher LM was associated with higher intakes of %PUFA (P = 0.003), total PUFAs (P = 0.05), n–3 PUFAs (P = 0.02), n–6 PUFAs (P = 0.02), and PUFA to SFA ratio (P = 0.03). Lower %BF was associated with higher total PUFAs (P = 0.03) and PUFA to SFA ratio (P = 0.02). Lower IAAT was associated with higher %PUFA (P = 0.03), total PUFAs (P = 0.02), and PUFA to SFA (P = 0.05) and n–6 to n–3 (P = 0.01) ratios. On the basis of the seminal work of Wells and Cole (40–42), we also explored the relation between PUFA variables of interest and fat-free mass index and fat mass index (Supplemental Table 1). The relations between PUFA variables, fat-free mass index, and fat mass index remained in the same direction, although slightly attenuated (P < 0.08).
TABLE 2.
Multivariate linear regression analyses exploring the relation between dietary PUFA consumption and body-composition variables in children1
| Dependent variable | %PUFA | Total PUFA | n–3 FAs | n–6 FAs | PUFAs:SFAs | n–6:n–3 FAs |
| BMI% | ||||||
| β | 0.04 | 0.00 | 0.25 | 0.00 | 0.19 | −0.05 |
| P | 0.54 | 0.96 | 0.44 | 0.97 | 0.77 | 0.19 |
| Log lean mass | ||||||
| β | 0.006 | 0.002 | 0.023 | 0.002 | 0.047 | −0.001 |
| P | 0.003 | 0.049 | 0.017 | 0.021 | 0.030 | 0.371 |
| Log fat mass | ||||||
| β | −0.01 | −0.01 | −0.04 | −0.01 | −0.24 | −0.24 |
| P | 0.39 | 0.23 | 0.51 | 0.22 | 0.06 | 0.07 |
| Percentage body fat | ||||||
| β | −0.24 | −0.009 | −0.05 | −0.14 | −0.219 | −0.01 |
| P | 0.28 | 0.033 | 0.24 | 0.19 | 0.028 | 0.48 |
| Log IAAT | ||||||
| β | −0.814 | −0.014 | −0.01 | −0.01 | −0.229 | −0.015 |
| P | 0.031 | 0.022 | 0.79 | 0.11 | 0.048 | 0.014 |
| Log SAAT | ||||||
| β | −0.01 | −0.01 | −0.06 | −0.00 | −0.13 | 0.00 |
| P | 0.42 | 0.20 | 0.20 | 0.53 | 0.27 | 0.62 |
| Log TAAT | ||||||
| β | −0.01 | −0.00 | −0.03 | −0.00 | −0.13 | −0.00 |
| P | 0.23 | 0.52 | 0.39 | 0.34 | 0.20 | 0.47 |
Sample sizes are as follows: BMI%, n = 311; lean mass, n = 304; fat mass, n = 304; percentage body fat, n = 304; IAAT, n = 209; SAAT, n = 209; TAAT n = 209. All models adjusted for pubertal stage, sex, socioeconomic status, African genetic admixture, European genetic admixture, and total energy intake (with the exception of % of total energy from PUFAs). Models for log fat mass and log lean mass were also adjusted for height in the regression model. BMI%, BMI percentile for age and sex; IAAT, intra-abdominal adipose tissue; SAAT, subcutaneous abdominal adipose tissue; TAAT, total abdominal adipose tissue; %PUFA, percentage of total energy consumed from PUFAs.
Discussion
Our results demonstrate that reported higher intakes of PUFAs are associated with a lower-risk pattern of body composition in a healthy cohort of children 7–12 y of age, independent of biological, environmental, and genetic predictors of pediatric body composition. We found that all measures of PUFA intake (%PUFA, total PUFAs, n–3 PUFAs, n–6 PUFAs) and the PUFA to SFA ratio were positively related to LM. In addition, we found that higher total PUFA intake and a higher ratio of PUFAs to SFAs were associated with both lower %BF and IAAT and higher LM. A higher n–6 to n–3 ratio was related to lower IAAT and had a borderline association with lower FM. Taken together, our results support the hypothesis that the intake of PUFA is positively associated with LM and inversely associated with adiposity in children, independent of known genetic, biological, and environmental covariates.
To our knowledge this is the first study to observe a relation between PUFA intake and LM in children. Observational studies in children (8, 13, 14) and adults (20) have focused primarily on the relation of PUFAs and %BF or obesity status and have not reported on LM. Few human intervention studies have reported the effects of PUFA intake on muscle synthesis or LM and accounted for objective measures of physical activity. Smith et al. (44, 45) demonstrated that supplementation of n–3 PUFAs (4 g fish oil/d for 8 wk) augmented in vivo muscle protein synthesis rates in the presence of elevated blood insulin and amino acids in younger (25–45 y) (44) and older (≥65 y) adults (45). In a randomized controlled overfeeding trial in normal-weight adults (20–38 y), consumption of excess calories from n–6 PUFAs (sunflower oil) was associated with a nearly 3-fold increase in LM relative to those who consumed SFAs (palm oil) (16). Interestingly, the control supplement in both studies by Smith et al. was n–6 PUFAs (corn oil) and did not affect protein synthesis rates (44, 45). The proposed mechanism by which n–3 PUFAs may increase or protect LM is enhanced muscle mammalian target of rapamycin (mTOR) pathway signaling during an anabolic state (44), but this may be a different mechanism than muscle growth in a child. In sum, there is a small but promising amount of evidence to support the positive influence of PUFAs on LM.
Total PUFA intake and the ratio of PUFAs to SFAs were both inversely associated with %BF in children in our study, consistent with observations in other populations. Spanish children (5–18 y) in the highest quintile of PUFA intake had lower odds of being obese than those in the lowest quintile of intake (7), and Spanish youth (6–18 y) with the A allele of the FTO gene reporting a low PUFA to SFA ratio had a 2-fold increased risk of becoming obese (46). In Swedish and French youth (aged 10–16 y), higher blood concentrations of SFAs were observed in overweight and obese adolescents compared with nonobese controls (14, 15). In obese 9- to 11-y-old US children, self-reported %PUFA was not correlated with %BF in the fully adjusted model; however percentages of dietary fat, MUFAs, and SFAs remained positively related to %BF (8). Taken together, PUFA and SFA intake appears to have disparate associations with obesity, particularly in overweight children.
Beyond obesity, excessive abdominal fat (particularly in the visceral compartment) is associated with greater risk of obesity-related diseases (47, 48). In our study, lower IAAT (i.e., visceral fat) was associated with higher %PUFA, total PUFAs, and PUFA to SFA and n–6 to n–3 ratios. Total and subcutaneous abdominal fat measures were not associated with any dietary measures of PUFAs. Karlsson et al. (14) found that SAAT, but not IAAT, was correlated with changes in serum PUFAs and SFAs in adolescents, which differs from our findings. These disparities may be due to methodologic differences, such as assessing PUFA status in blood instead of diet, a higher percentage of obese youth in their study (40% vs. 10%), and studying a smaller, racially homogenous population in a case-control study design compared with our cross-sectional assessment of a larger multiethnic population. Consuming PUFAs (n–3 and n–6) in the place of SFAs has been shown to reduce abdominal fat in rats (18, 19), broiler chickens (n–6) (49), and adults (n–6 and n–3) (16, 50). The mechanism for the effect of PUFAs on FM may be mediated through beneficial fuel partitioning of PUFAs compared with SFAs (19, 51). In addition, PUFAs could be modulating adipogenesis through activating PPARs, which affect adipocyte growth, differentiation, and lipid oxidation (52, 53). We hypothesize that PUFAs may affect fuel storage in adipocytes, due to FA oxidation and/or a direct effect of less SFA on lipogenesis, or both. However, this is outside the scope of this study and we cannot speak to this hypothesis directly.
Although total dietary PUFAs were associated with lower adiposity in our study, the marine-derived n–3 PUFAs EPA+DHA were strongly related to lower adiposity in children in additional studies. Several studies in youth from France, Japan, Australia, and Sweden found inverse relations between BMI or adiposity and blood concentrations of EPA and/or DHA (12, 14, 15, 54). However, these studies differed methodologically from ours in that blood concentrations of n–3 PUFAs were measured. In addition, ALA provided most of the n–3 PUFAs in our study, whereas EPA+DHA intake was low. Overall, the few studies published in children have shown that n–3 PUFAs are correlated with normal body weight and more healthful levels of adiposity, but more research is needed to establish a causal relation.
An elevated ratio of n–6 to n–3 FAs in the Western diet, primarily due to increased dietary availability of n–6 PUFAs, has been implicated in causing greater FM accumulation and as a possible contributor to the increased prevalence of childhood obesity (11, 55). Our findings that higher n–6 PUFA intake was associated with higher LM and that a higher n–6 to n–3 FA ratio was related to lower IAAT and tended to be related to lower FM (P = 0.07) may seem paradoxical in light of this theory. However, the evidence supporting this theory derives primarily from mechanistic data on rodents (11). To our knowledge, clinical trials have not demonstrated that high n–6 PUFA intake or a higher n–6 to n–3 FA ratio contributes to excessive or dysfunctional adiposity in children (10, 16). Thus, the PUFA to SFA ratio may be a meaningful dietary ratio related to adiposity in children but more research is needed.
We acknowledge the limitations of a cross-sectional study design and do not ascribe causality to the relation observed between PUFA intake and body composition. The lack of biomarkers in our study, such as erythrocyte phospholipid FAs, to confirm the PUFA intake values from the 24-h dietary recalls is also a significant limitation. However, blood concentrations of PUFAs are fairly well correlated to PUFA dietary intake, depending on the tissue sample and dietary intake methods (56, 57), but differences in FA metabolism can cause changes in blood FA profiles unrelated to intake (58). An FFQ related to fish intake (an episodically consumed food in the United States) could have better captured n–3 PUFA intake estimates, which were quite low. However, an FFQ was not included in the study protocol. The study of overall dietary patterns, rather than a focus on FAs, was outside the scope of this study and thus we cannot speculate on how the inclusion of this may have affected our results.
The strengths of this study are the large cohort of multiethnic children with robust measures of factors that are known to affect energy balance. We used the most advanced imaging techniques to determine total adiposity and LM and abdominal fat distribution with DXA and CT scans, respectively. In addition, our analysis accounted for demographic covariates that have been shown to independently contribute to body composition, including SES, sex, and pubertal status (36). Given the established role of genetics in pediatric body composition (21), an additional strength of this study includes adjusting for ancestral genetic background. Finally, we accounted for energy expenditure, including objective physical activity, with robust measures of 7 d of accelerometry and objective measures of REE.
In summary, our results extend what is known about the relation between PUFA intake and body composition by demonstrating that higher reported dietary PUFA intakes are associated with lower %BF and visceral adiposity in a multiethnic cohort of children. Importantly, we also found that higher self-reported intakes of PUFAs (as measured by % of total energy from PUFAs, total PUFAs, n–3 PUFAs, n–6 PUFAs, and a high PUFA to SFA ratio) are associated with higher levels of LM, independent of genetic, environmental, and biological covariates. Further investigation is required to determine if dietary FA composition in children’s diets plays a causal role in maintaining or achieving a desirable body composition.
Acknowledgments
MC, DJL, and JRF designed the research (project conception, development of overall research plan, and study oversight); MC and JRF conducted the research (hands-on conduct of the experiments and data collection) and had primary responsibility for final content; and MC, DJL, KHJ, JEF, and JRF analyzed data or performed statistical analysis and wrote the manuscript. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: AA, African American; AFADM, African admixture; ALA, α-linolenic acid; CT, computed tomography; EA, European American; EUADM, European admixture; FM, fat mass; HA, Hispanic American; HP, high-PUFA group if % of total energy from PUFAs ≥ 5.84%; IAAT, intra-abdominal adipose tissue; LM, lean mass; LP, low-PUFA group if % of total energy from PUFAs <5.84%; mTOR, mammalian target of rapamycin; MVPA, moderate and vigorous physical activity; REE, resting energy expenditure; SAAT, subcutaneous abdominal adipose tissue; SES, socioeconomic status; %BF, percentage body fat; %PUFA, percentage of total energy consumed from PUFAs.
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA 2012;307:483–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, Wei R, Curtin LR, Roche AF, Johnson CL. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat 11 2002;246:1–190. [PubMed] [Google Scholar]
- 3.Serdula MK, Ivery D, Coates RJ, Freedman DS, Williamson DF, Byers T. Do obese children become obese adults? A review of the literature. Prev Med 1993;22:167–77. [DOI] [PubMed] [Google Scholar]
- 4.Pinhas-Hamiel O, Dolan LM, Daniels SR, Standiford D, Khoury PR, Zeitler P. Increased incidence of non-insulin-dependent diabetes mellitus among adolescents. J Pediatr 1996;128:608–15. [DOI] [PubMed] [Google Scholar]
- 5.Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW, Allen K, Lopes M, Savoye M, Morrison J, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med 2004;350:2362–74. [DOI] [PubMed] [Google Scholar]
- 6.Freedman DS, Dietz WH, Srinivasan SR, Berenson GS. The relation of overweight to cardiovascular risk factors among children and adolescents: the Bogalusa Heart Study. Pediatrics 1999;103:1175–82. [DOI] [PubMed] [Google Scholar]
- 7.Martín-Calvo N, Ochoa MC, Marti A, Martínez-González MÁ. Asociación entre los macronutrientes de la dieta y la obesidad en la infancia y adolescencia: un estudio de casos y controles. [The association between dietary macronutrients intake and obesity among children and adolescents; a case-control study.] Nutr Hosp 2013;28:1515–22 (in Spanish). [DOI] [PubMed] [Google Scholar]
- 8.Gazzaniga JM, Burns TL. Relationship between diet composition and body fatness, with adjustment for resting energy expenditure and physical activity, in preadolescent children. Am J Clin Nutr 1993;58:21–8. [DOI] [PubMed] [Google Scholar]
- 9.Damsgaard CT, Stark KD, Hjorth MF, Biltoft-Jensen A, Astrup A, Michaelsen KF, Lauritzen L. n-3 PUFA status in school children is associated with beneficial lipid profile, reduced physical activity and increased blood pressure in boys. Br J Nutr 2013;110:1304–12. [DOI] [PubMed] [Google Scholar]
- 10.Mac K, Shahkhalili Y, Aprikian O, Stan S. Dietary fat and fat types as early determinants of childhood obesity: a reappraisal. Int J Obes (Lond) 2006;30 Suppl 4:S50–7. [DOI] [PubMed] [Google Scholar]
- 11.Ailhaud G, Guesnet P. Fatty acid composition of fats is an early determinant of childhood obesity: a short review and an opinion. Obes Rev 2004;5:21–6. [DOI] [PubMed] [Google Scholar]
- 12.Saito E, Okada T, Abe Y, Kuromori Y, Miyashita M, Iwata F, Hara M, Ayusawa M, Mugishima H, Kitamura Y. Docosahexaenoic acid content in plasma phospholipids and desaturase indices in obese children. J Atheroscler Thromb 2011;18:345–50. [DOI] [PubMed] [Google Scholar]
- 13.Burrows T, Collins CE, Garg ML. Omega-3 index, obesity and insulin resistance in children. Int J Pediatr Obes 2011;6:e532–9. [DOI] [PubMed] [Google Scholar]
- 14.Karlsson M, Mårild S, Brandberg J, Lönn L, Friberg P, Strandvik B. Serum phospholipid fatty acids, adipose tissue, and metabolic markers in obese adolescents. Obesity (Silver Spring) 2006;14:1931–9. [DOI] [PubMed] [Google Scholar]
- 15.Klein-Platat C, Drai J, Oujaa M, Schlienger J-L, Simon C. Plasma fatty acid composition is associated with the metabolic syndrome and low-grade inflammation in overweight adolescents. Am J Clin Nutr 2005;82:1178–84. [DOI] [PubMed] [Google Scholar]
- 16.Rosqvist F, Iggman D, Kullberg J, Jonathan Cedernaes J, Johansson H-E, Larsson A, Johansson L, Ahlström H, Arner P, Dahlman I, et al. Overfeeding polyunsaturated and saturated fat causes distinct effects on liver and visceral fat accumulation in humans. Diabetes 2014;63:3356–68. [DOI] [PubMed] [Google Scholar]
- 17.Ruzickova J, Rossmeisl M, Prazak T, Flachs P, Sponarova J, Vecka M, Tvrzicka E, Bryhn M, Kopecky J. Omega-3 PUFA of marine origin limit diet-induced obesity in mice by reducing cellularity of adipose tissue. Lipids 2004;39:1177–85. [DOI] [PubMed] [Google Scholar]
- 18.Okuno M, Kajiwara K, Imai S, Kobayashi T, Honma N, Maki T, Suruga K, Goda T, Takase S, Muto Y, et al. Perilla oil prevents the excessive growth of visceral adipose tissue in rats by down-regulating adipocyte differentiation. J Nutr 1997;127:1752–7. [DOI] [PubMed] [Google Scholar]
- 19.Hill JO, Peters JC, Lin D, Yakubu F, Greene H, Swift L. Lipid accumulation and body fat distribution is influenced by type of dietary fat fed to rats. Int J Obes Relat Metab Disord 1993;17:223–36. [PubMed] [Google Scholar]
- 20.Micallef M, Munro I, Phang M, Garg M. Plasma n-3 polyunsaturated fatty acids are negatively associated with obesity. Br J Nutr 2009;102:1370–4. [DOI] [PubMed] [Google Scholar]
- 21.Cardel M, Higgins PB, Willig AL, Keita AD, Casazza K, Gower BA, Fernandez JR. African genetic admixture is associated with body composition and fat distribution in a cross-sectional study of children. Int J Obes (Lond) 2011;35:60–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fernández JR, Shriver MD. Using genetic admixture to study the biology of obesity traits and to map genes in admixed populations. Nutr Rev 2004;62:S69–74. [DOI] [PubMed] [Google Scholar]
- 23.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child 1969;44:291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child 1970;45:13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elberg J, McDuffie JR, Sebring NG, Salaita C, Keil M, Robotham D, Reynolds JC, Yanovski JA. Comparison of methods to assess change in children’s body composition. Am J Clin Nutr 2004;80:64–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fox K, Peters D, Armstrong N, Sharpe P, Bell M. Abdominal fat deposition in 11-year-old children. Int J Obes Relat Metab Disord 1993;17:11–6. [PubMed] [Google Scholar]
- 27.Johnson RK, Driscoll P, Goran MI. Comparison of multiple-pass 24-hour recall estimates of energy intake with total energy expenditure determined by the doubly labeled water method in young children. J Am Diet Assoc 1996;96:1140–4. [DOI] [PubMed] [Google Scholar]
- 28.Lemas DJ, Wiener HW, O’Brien DM, Hopkins S, Stanhope KL, Havel PJ, Allison DB, Fernandez JR, Tiwari HK, Boyer BB. Genetic polymorphisms in carnitine palmitoyltransferase 1A gene are associated with variation in body composition and fasting lipid traits in Yup’ik Eskimos. J Lipid Res 2012;53:175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vernarelli JA, Mitchell DC, Hartman TJ, Rolls BJ. Dietary energy density is associated with body weight status and vegetable intake in U.S. children. J Nutr 2011;141:2204–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cardel M, Willig AL, Dulin-Keita A, Casazza K, Cherrington A, Gunnarsdottir T, Johnson SL, Peters JC, Hill JO, Allison DB, et al. Home-schooled children are thinner, leaner, and report better diets relative to traditionally schooled children. Obesity (Silver Spring) 2014;22:497–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schoeller DA, Thomas D, Archer E, Heymsfield SB, Blair SN, Goran MI, Hill JO, Atkinson RL, Corkey BE, Foreyt J, et al. Self-report–based estimates of energy intake offer an inadequate basis for scientific conclusions. Am J Clin Nutr 2013;97:1413–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruiz JR, Rizzo NS, Hurtig-Wennlof A, Ortega FB, Warnberg J, Sjostrom M. Relations of total physical activity and intensity to fitness and fatness in children: the European Youth Heart Study. Am J Clin Nutr 2006;84:299–303. [DOI] [PubMed] [Google Scholar]
- 33.Willig AL, Hunter GR, Casazza K, Heimburger DC, Beasley TM, Fernandez JR. Body fat and racial genetic admixture are associated with aerobic fitness levels in a multiethnic pediatric population. Obesity (Silver Spring) 2011;19:2222–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horlick M, Thornton J, Wang J, Levine LS, Fedun B, Pierson RN Jr. Bone mineral in prepubertal children: gender and ethnicity. J Bone Miner Res 2000;15:1393–7. [DOI] [PubMed]
- 35.Marugán de Miguelsanz JM, Redondo del Río MP, Alonso-Franch M, Calvo Romero C., Torres Hinojal MdC. Increased resting energy expenditure by fat-free mass in children and teenagers with constitutional leanness. Nutr Hosp 2011;26:589–93. [DOI] [PubMed] [Google Scholar]
- 36.Cardel M, Willig AL, Dulin-Keita A, Casazza K, Mark Beasley T, Fernandez JR. Parental feeding practices and socioeconomic status are associated with child adiposity in a multi-ethnic sample of children. Appetite 2012;58:347–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hollingshead A. Four factor index of social status. New Haven (CT): Yale University Press; 1975.
- 38.Goulding A, Taylor RW, Gold E, Lewis-Barned NJ. Regional body fat distribution in relation to pubertal stage: a dual-energy X-ray absorptiometry study of New Zealand girls and young women. Am J Clin Nutr 1996;64:546–51. [DOI] [PubMed] [Google Scholar]
- 39.Demerath EW, Schubert CM, Maynard LM, Sun SS, Chumlea WC, Pickoff A, Czerwinski SA, Towne B, Siervogel RM. Do changes in body mass index percentile reflect changes in body composition in children? Data From the Fels Longitudinal Study. Pediatrics 2006;117:e487–95. [DOI] [PubMed] [Google Scholar]
- 40.Wells JC, Cole TJ.. Adjustment of fat-free mass and fat mass for height in children aged 8 y. Int J Obes Relat Metab Disord 2002;26:947–52. [DOI] [PubMed] [Google Scholar]
- 41.Wells JCK, Cole TJ. Adjustment of fat-free mass and fat mass for height in children aged 8 y. Int J Obes Relat Metab Disord 2002;26:947. [DOI] [PubMed] [Google Scholar]
- 42.Wells JCK, Cole TJ. Disentangling the size and adiposity components of obesity. Int J Obes (Lond) 2011;35:548–9. [DOI] [PubMed] [Google Scholar]
- 43.Institute of Medicine. Dietary Reference Intakes: the essential guide to nutrient requirements. Washington (DC): National Academies Press; 2006.
- 44.Smith GI, Atherton P, Reeds DN, Mohammed BS, Rankin D, Rennie MJ, Mittendorfer B. Omega-3 polyunsaturated fatty acids augment the muscle protein anabolic response to hyperaminoacidemia-hyperinsulinemia in healthy young and middle aged men and women. Clin Sci (Lond) 2011;121:267–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith GI, Atherton P, Reeds DN, Mohammed BS, Rankin D, Rennie MJ, Mittendorfer B. Dietary omega-3 fatty acid supplementation increases the rate of muscle protein synthesis in older adults: a randomized controlled trial. Am J Clin Nutr 2011;93:402–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moleres A, Ochoa MC, Rendo-Urteaga T, Martínez-González MA, Azcona San Julián MC, Martínez JA, Marti A. Dietary fatty acid distribution modifies obesity risk linked to the rs9939609 polymorphism of the fat mass and obesity-associated gene in a Spanish case-control study of children. Br J Nutr 2012;107:533–8. [DOI] [PubMed] [Google Scholar]
- 47.Chan JM, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Obesity, fat distribution, and weight gain as risk factors for clinical diabetes in men. Diabetes Care 1994;17:961–9. [DOI] [PubMed] [Google Scholar]
- 48.Larsson B, Svärdsudd K, Welin L, Wilhelmsen L, Björntorp P, Tibblin G. Abdominal adipose tissue distribution, obesity, and risk of cardiovascular disease and death: 13 year follow up of participants in the study of men born in 1913. Br Med J (Clin Res Ed) 1984;288(6428):1401–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sanz M, Lopez-Bote CJ, Menoyo D, Bautista JM. Abdominal fat deposition and fatty acid synthesis are lower and β-oxidation is higher in broiler chickens fed diets containing unsaturated rather than saturated fat. J Nutr 2000;130:3034–7. [DOI] [PubMed] [Google Scholar]
- 50.Summers LKM, Fielding BA, Bradshaw HA, Ilic V, Beysen C, Clark ML, Moore NR, Frayn KN. Substituting dietary saturated fat with polyunsaturated fat changes abdominal fat distribution and improves insulin sensitivity. Diabetologia 2002;45:369–77. [DOI] [PubMed] [Google Scholar]
- 51.Krishnan S, Cooper J. Effect of dietary fatty acid composition on substrate utilization and body weight maintenance in humans. Eur J Nutr 2014;53:691–710. [DOI] [PubMed] [Google Scholar]
- 52.Lombardo YB, Chicco AG. Effects of dietary polyunsaturated n-3 fatty acids on dyslipidemia and insulin resistance in rodents and humans: a review. J Nutr Biochem 2006;17:1–13. [DOI] [PubMed] [Google Scholar]
- 53.Luquet S, Lopez-Soriano J, Holst D, Gaudel C, Jehl-Pietri C, Fredenrich A, Grimaldi PA. Roles of peroxisome proliferator-activated receptor delta (PPARδ) in the control of fatty acid catabolism: a new target for the treatment of metabolic syndrome. Biochimie 2004;86:833–7. [DOI] [PubMed] [Google Scholar]
- 54.Harris WS, von Schacky C. The Omega-3 Index: a new risk factor for death from coronary heart disease? Prev Med 2004;39:212–20. [DOI] [PubMed] [Google Scholar]
- 55.Ailhaud G, Massiera F, Weill P, Legrand P, Alessandri J-M, Guesnet P. Temporal changes in dietary fats: role of n−6 polyunsaturated fatty acids in excessive adipose tissue development and relationship to obesity. Prog Lipid Res 2006;45:203–36. [DOI] [PubMed] [Google Scholar]
- 56.Hodson L, Skeaff CM, Fielding BA. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog Lipid Res 2008;47:348–80. [DOI] [PubMed] [Google Scholar]
- 57.Browning LM, Walker CG, Mander AP, West AL, Madden J, Gambell JM, Young S, Wang L, Jebb SA, Calder PC. Incorporation of eicosapentaenoic and docosahexaenoic acids into lipid pools when given as supplements providing doses equivalent to typical intakes of oily fish. Am J Clin Nutr 2012;96:748–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lattka E, Illig T, Heinrich J, Koletzko B. Do FADS genotypes enhance our knowledge about fatty acid related phenotypes? Clin Nutr 2010;29:277–87. [DOI] [PubMed] [Google Scholar]