Abstract
Background: Postmenopausal estrogen depletion is a major contributing factor to bone loss. Soy isoflavones have variable effects on the prevention of postmenopausal bone loss, which is possibly related to the specific isoflavone content or the variable equol-producing capacity of individuals.
Objective: We aimed to determine the effects of the content of isoflavones in a soy supplement and the equol-producing ability of the individual on postmenopausal bone calcium retention.
Design: The study was a blinded, randomized, crossover intervention trial in 24 postmenopausal women who were prescreened for their ability to convert daidzein to equol. Women were equilibrated with 41Ca before the intervention. Interventions were 5 soy isoflavone oral supplements (2 doses of a genistein-rich soy supplement and 3 doses of mixed isoflavones in various proportions) and a bisphosphonate (risedronate). Each intervention was given sequentially for 50 d followed by a 50-d washout period. The percentage of bone calcium retention was determined from the change in urinary 41Ca:calcium.
Results: Interventions that ranged from 52 to 220 mg total isoflavones/d increased bone calcium retention between 3.4% and 7.6% (P < 0.05), which was a moderate effect compared with that of risedronate at 15.3% (95% CI: 7.1%, 22.7%; P = 0.0014). The most-effective soy intervention delivered 105.23 mg total isoflavones/d as genistein, daidzein, and glycitein in their natural ratios and increased bone calcium retention by 7.6% (95% CI: 4.9%, 10.2%; P < 0.0001). Genistein, at 52.85 mg/d, increased bone calcium retention by 3.4% (95% CI: 0.5%, 6.2%; P = 0.029); but there was no benefit at higher amounts (113.52 mg/d). There was no difference (P = 0.5) in bone calcium retention between equol producers and nonproducers.
Conclusion: Soy isoflavones, although not as potent as risedronate, are effective bone-preserving agents in postmenopausal women regardless of their equol-producing status, and mixed isoflavones in their natural ratios are more effective than enriched genistein. This trial was registered at clinicaltrials.gov as NCT00244907.
Keywords: bone resorption, postmenopausal women, soy isoflavones, equol, genistein, 41Ca
INTRODUCTION
Postmenopausal estrogen depletion causes increased net bone resorption and is a major contributing factor of osteoporosis, which can lead to a decreased quality of life and premature death (1). Several antiresorptive treatments are available, including hormone-replacement therapy (HRT)9 and bisphosphonates. However, HRT has been linked to increased risk of coronary heart disease, pulmonary embolism, and stroke (2), and bisphosphonate use can lead to osteonecrosis of the jaw (3) and atypical fracture. These adverse events, along with an increasing trend in Americans to use complementary and alternative medicine (4), have prompted many patients to seek complementary and alternative treatments that are safe and effective.
Dietary alternatives to Food and Drug Administration–approved drugs have focused on phytoestrogens, especially isoflavones, which are a biologically active, nonnutritive subcategory of flavonoids. Isoflavones are mainly synthesized in leguminous plants, such as soybeans, with genistein, daidzein, and glycitein comprising the 3 most-abundant isoflavones in soy foods. The efficacy of whole soy foods and isoflavones in preventing postmenopausal bone loss has not been clearly defined (5–11), possibly because of the varying ratio and content of isoflavones in dietary products or the variation in metabolites produced by the gut microbiota of the subject.
Purified genistein, which is one of 2 major soy isoflavones, was as effective as HRT for preserving bone in mineral density in a 1-y trial in postmenopausal women (12), which suggested that genistein supplements may be superior to naturally occurring mixed isoflavones. However, little is known about the dose-response in bone of individual isoflavones or the possible synergistic or antagonistic effects of daidzein, which is the other major soy isoflavone (13). Moreover, the metabolites of isoflavones may have more of an effect than the parent compound. Setchell et al. (14) proposed that those subjects who have gut microflora that convert daidzein to equol have a greater response to isoflavones than do those who do not. Equol is selective for estrogen receptor (ER)-β (15), which is the ER associated with a bone response.
We sought to determine the effect of the equol-producing capability on bone calcium retention in postmenopausal women who were given various isoflavone mixtures by using a sensitive screening technique with the rare isotope 41Ca. The long half-life (105 y) of 41Ca and the sensitivity of urine measurements by using accelerator mass spectrometry allow bone to be labeled with this tracer and the effects of a series of interventions to be compared in a crossover design. We measured other potential modulators of bone turnover including calcium absorption, serum estrogen, and soy metabolites and mineral homeostasis as covariates. We hypothesized that the capability to convert daidzein to equol would enhance the suppression of bone resorption. In addition, we hypothesized that genistein would suppress bone resorption in a dose-dependent manner whether given in a relatively pure form or as part of a mixed-isoflavone supplement.
METHODS
Study participants
Study participants were 24 healthy postmenopausal women from the Lafayette, Indiana, area who did not have a history of gut, bone, liver, or kidney disease, hormonal disorders, or cancer and had blood chemistry panels that were within normal ranges. Subjects were >4 y postmenopausal and did not have any allergies to soy. Study staff enrolled participants on a rolling basis between 2006 and 2009, and the trial ended in June 2010. All women gave written, informed consent, and the study was approved by the Purdue University and Indiana University Purdue University institutional review boards.
Exclusionary criteria were currently taking osteoporosis or HRT drugs; nonprescription drugs, corticosteroids, or thiazide diuretics; and thyroid-replacement therapy and antibiotics. Dual-energy X-ray absorptiometry (General Electric Lunar DPX IQ) was performed to measure total-body bone mineral density (BMD), and anthropometric measurements were recorded. Subjects were tested for their ability to produce equol from the urinary excretion of the daidzein metabolite after the consumption of a high-daidzein soy food. Subjects consumed a soy food bar (Revival Products), which contained 160 mg isoflavones (64 mg daidzein)/d for 3 consecutive days and collected their morning urine voids on the fourth day. Equol producers were considered those subjects with urine equol >10 μM to avoid misclassification for those who consumed cow milk with low amounts of equol produced by cows that consumed a diet rich in daidzein. Subjects were screened until ≥8 equol producers were enrolled for testing the effect of equol production, and 24 total subjects were enrolled for multiple soy comparisons.
Study design
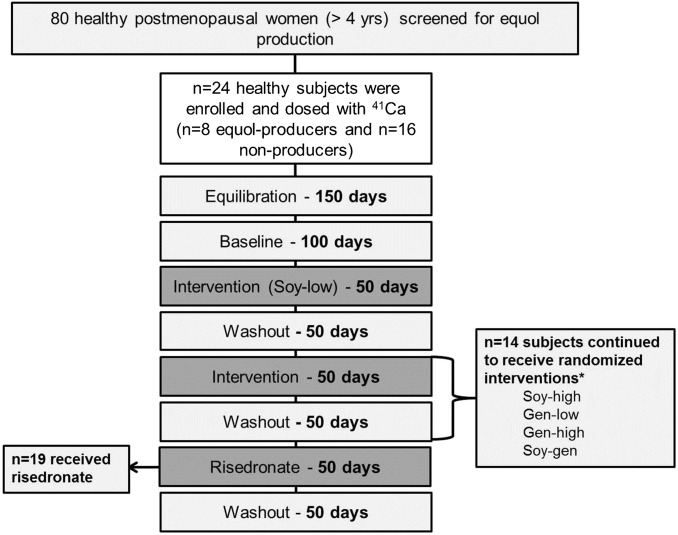
The study was a blinded (to participants and staff), randomized, crossover intervention trial in which subjects received 5 soy-isoflavone–extract interventions and or bisphosphonate in the form of risedronate as a control (Figure 1). Subjects were intravenously dosed with 50 nCi 41Ca (Argonne National Laboratories) in sterile saline. Two participants had been dosed with 100 nCi or 1 μCi 41Ca from a previous study. An equilibration period of ≥150 d facilitated the clearance of 41Ca from soft tissues so that urinary 41Ca reflected the steady state of 41Ca resorbed from bone and 41Ca re-entering bone (16). Subjects were instructed to collect a 24-h urine sample once every 10 d. Baseline urine collections were made over the next 100 d followed by treatment and washout periods of 50 d each. Urine samples were processed and measured by using accelerator mass spectrometry to determine the ratio of 41Ca to total calcium as described previously (17, 18).
FIGURE 1.
Study design. Twenty-four healthy postmenopausal women were enrolled in the study with 8 equol producers and 16 nonproducers. Subjects were dosed with 41Ca, and baseline values were collected after a 150-d equilibration period. All subjects received Soy-low as the first intervention. After the intervention with Soy-low, subjects had the option to continue directly with risedronate therapy or to receive 4 randomized soy interventions followed by risedronate. *One subject dropped out during the randomized soy interventions and had low compliance with taking the supplements; therefore, this subject was excluded in all analyses. Gen-high, high-dose genistein; Gen-low, low-dose genistein; Soy-gen, soy isoflavones enriched in genistein; Soy-high, high-dose soy; Soy-low, low-dose soy.
Interventions consisted of 5 different types of soy-isoflavone tablets of similar physical appearance (Archer Daniels Midland Co.) and varying isoflavone compositions and total isoflavone contents (Table 1). Subjects were instructed to consume 5 tablets throughout the day with meals. Isoflavone interventions were selected on the basis of the following criteria: to test for a dose effect of nearly purified genistein [low-dose genistein (Gen-low) vs. high-dose genistein (Gen-high)] and a natural mix of isoflavones [low-dose soy (Soy-low) vs. high-dose soy (Soy-high)] and to test compositional effects at a lower dose (Gen-low vs. Soy-low) and a higher dose [Gen-high vs. soy enriched with genistein (Soy-gen) and Soy-high]. Gen-low delivered 52.85 mg total isoflavones/d in 5 tablets as 83.4% genistein, 15.6% daidzein, and 1.0% glycitein compared with Gen-high, which delivered double the amount of total isoflavones (113.52 mg/d) in a similar proportion (82.6% genistein, 16.1% daidzein, and 1.4% glycitein). Similarly, the mixed-isoflavone–containing supplement (Soy-high), delivered double the amount of total isoflavones as the Soy-low supplement (219.67 mg/d in 5 tablets compared with 105.23 mg/d) in a similar proportion of ∼43.5% genistein, 42.3% daidzein, and 14.1% glycitein. To compare the effect of the composition at similar doses of genistein (∼45 mg genistein/d), we compared Gen-low (44.06 mg genistein/d, 8.25 mg daidzein/d, and 0.54 mg glycitein/d) to Soy-high (46.20 mg genistein/d, 44.27 mg daidzein/d, and 14.76 mg glycitein/d). Gen-high, Soy-gen, and Soy-high were compared for compositional effects because they contained similar amounts of genistein at 93.75, 91.02, and 95.66 mg genistein/d, respectively, but differed in their daidzein and glycitein contents (18.22, 54.53, and 92.99 mg daidzein/d, respectively, and 1.55, 15.52, and 31.02 mg glycitein/d, respectively.
TABLE 1.
Tablet isoflavone composition1
| Isoflavone content, mg/d (% of total isoflavones) |
||||
| Intervention | Genistein equivalent | Daidzein equivalent | Glycitein equivalent | Total |
| Gen-low | 44.06 (83.4) | 8.25 (15.6) | 0.54 (1.0) | 52.85 |
| Gen-high | 93.75 (82.6) | 18.22 (16.1) | 1.55 (1.4) | 113.52 |
| Soy-low | 46.20 (43.9) | 44.27 (42.1) | 14.76 (14.0) | 105.23 |
| Soy-high | 95.66 (43.5) | 92.99 (42.3) | 31.02 (14.1) | 219.67 |
| Soy-gen | 91.02 (56.5) | 54.53 (33.9) | 15.52 (9.6) | 161.07 |
Totals reflect isoflavones as aglycone equivalents in 5 tablets as analyzed. Gen-high, high-dose genistein; Gen-low, low-dose genistein; Soy-gen, soy isoflavones enriched in genistein; Soy-high, high-dose soy; Soy-low, low-dose soy.
The first intervention given to each subject was the commercially available mixed soy-isoflavone supplement [Soy-low (Novasoy)] to compare the response of equol producers (n = 8) with nonproducers (n = 16) because this was a primary aim of the study (Figure 1). Subsequently, equol producers and nonproducers were randomly assigned separately. The order of the interventions, except for the positive control, was assigned by using an allocation sequence, which was determined by the statistician, which represented unique product sequences that accounted for the varied number of interventions for each subject. The risedronate control was given last to prevent confounding from residual effects on bone. Fourteen subjects (6 equol producers and 8 nonproducers) elected to continue the study by receiving the other 4 soy isoflavone products (Gen-low, Gen-high, Soy-high, and Soy-gen), which were tested in a randomized order. The order was determined by the statistician by using a random-allocation sequence.
Soy interventions were mostly in the glycoside form, but amounts are reported in the aglycone form (standard units without sugar moiety). Tablets were analyzed by Archer Daniels Midland Co. by using reversed-phase HPLC and liquid chromatography–mass spectrometry (19), which were modified from the Association of Official Analytic Chemists method for isoflavone analysis. The analysis was validated in the laboratory of William Helferich, University of Illinois.
During the positive control period, subjects took 5 mg risedronate/d (Actonel; Procter & Gamble) daily with water while upright and did not consume food or supplements for 30 min after the drug was administered. The risedronate intervention was administered last because of its long half-life in bone (17), whereas the half-life of isoflavones is 7.77 h (20). Compliance was assessed by measuring serum isoflavone and by counting returned pills.
Subjects ingested 500 mg Ca/d as Viactiv chews (McNeil Nutritionals LLC) or Chewable Calcium for Women (Walgreen Co.) and 600 IU vitamin D/d [200 IU from a calcium chew and 400 IU from Geritol Complete; GlaxoSmithKline) beginning at baseline. Subjects completed 4-d diet records, which included 3 weekdays and 1 weekend day for each washout and intervention period beginning with the baseline period and continuing throughout the study. The Nutrition Data System for Research Version 2007 (University of Minnesota) program was used to calculate nutrient intakes. Adverse events were monitored by an interview and documented during study visits.
Biochemical analysis
Fasting blood and urine were obtained at the end of each baseline, intervention, and washout period to analyze for biochemical markers of bone turnover hormones and serum isoflavones. Urinary type I cross-linked N-telopeptides, serum bone-specific alkaline phosphatase, serum osteocalcin, and urinary deoxypyridinoline cross-links were analyzed by using ELISA kits (Quidel Corp.). Serum parathyroid hormone (PTH) and 25-hydroxyvitamin D were analyzed by using radioimmunoassays (DiaSorin). Serum phosphorus, alkaline phosphatase, calcium, and creatinine and urinary phosphorus, calcium, and creatinine were measured by using a COBAS MIRA chemistry spectrophotometric analyzer (Roche Diagnostics). Baseline values of serum follicle-stimulating hormone (FSH), estrone, and estradiol were also measured by using ELISAs (Alpha Diagnostic International Inc.), and estrone sulfate was measured by using a radioimmunoassay (Diagnostic Systems Laboratories Inc.). Serum genistein, daidzein, equol, dihydrodaidzein, glycitein, biochanin A, formononetin, coumestrol, enterodiol, enterolactone, O-desmethylangolensin (O-DMA), and 6′-OH-O-desmethylangolensin were measured by using reversed-phase HPLC–electrospray ionization–mass spectrometry by using a 4000 triple-quadrupole mass spectrometer (AB Sciexa) as described previously (21, 22).
Calcium absorption
A fractional calcium absorption test was performed at the end of the baseline period and at each intervention period by using 15.2 mg 44Ca that was administered as a simple oral tracer. Subjects ingested the stable isotope as CaCO3 midway through the consumption of a calcium-containing meal (250 mg Ca) with the soy treatment tablets. Subjects participated in a 5-h blood draw to determine the effect of soy isoflavones on calcium absorption. The 44Ca isotope was detected in the blood by using inductively coupled plasma and mass spectrometry, and fractional calcium absorption was calculated by using the equation
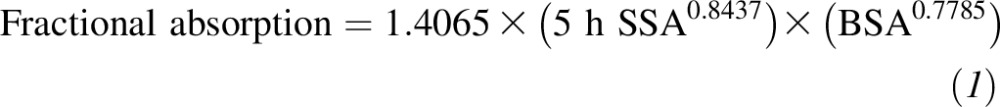 |
as previously described (23, 24), where SSA denotes the serum specific activity (fraction of dose per gram of calcium) and BSA denotes body surface area and
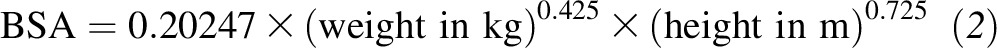 |
Statistical analysis
Sample sizes ranged from 14 to 24. For a sample size of 14, we had 80% power to detect an 8% improvement in bone calcium retention. For a sample size of 24, we had 80% power to detect a 6% improvement in bone calcium retention. The 41Ca:calcium ratio was transformed by using the ln. For each subject, a simple linear regression model was fit through all baseline and washout values to create a regression line, and the predicted 41Ca:calcium value for each 24-h urine collection during the intervention was subtracted from the observed value. Means of these differences were computed for each individual and averaged across individuals to provide estimates of treatment effects. Bootstrap resampling was used to calculate 95% CIs. To examine the effect of equol status, the 5 soy treatments were analyzed in a repeated-measures mixed model that included equol status, treatment, and the interaction. Dose and compositional effects were determined by using t tests with α = 0.05 with a Bonferroni correction (α = 0.007 after correcting for multiple comparisons). Means and 95% CIs were transformed back to the original scale and were reported as percentages of change in bone calcium retention. To examine possible covariates, correlations between covariates and a composite of treatment effects were used. The composite was obtained by first computing z scores for each intervention (by subtracting the mean and dividing by the SD in the log scale) and averaging these values across interventions for each subject.
Means ± SDs were calculated for baseline characteristics. Serum isoflavones and biochemistry data were transformed by using the ln to correct for a skewed distribution. To compare the effects of the interventions and equol-producing status, data were analyzed by using a mixed model with the Tukey-Kramer post hoc test for differences between intervention means. The model included terms to account for subject effects that were due to the crossover design, time, subject-by-time interaction, equol-producing ability, and intervention and a term to account for the interaction between equol-producing ability and intervention. All isoflavone and biochemistry data were transformed back to the original scale and reported as means with 95% CIs. Fractional calcium-absorption values were analyzed by using a mixed model with contrasts testing differences between baseline and risedronate, risedronate and soy, and soy interventions compared with baseline. Results were reported as means with 95% CIs. SAS software (version 9.2; SAS Institute) was used for all analyses.
RESULTS
Baseline characteristics
In comparison with NHANES anthropometric (25) and dual-energy X-ray absorptiometry body-composition (26) reference data for non-Hispanic white women ≥60 y of age, our participants (Table 2) were of similar height and weight but had higher bone density. Eighteen of the women had had a natural menopause, whereas 6 women had undergone A surgical menopause. Food records were consistent in nutrient compositions and mineral intakes across all interventions and washout periods (Table 2). Only one subject was under the estimated average requirement of 1000 mg Ca/d for this age group (411 mg/d through dietary intake plus 500 mg/d through supplementation). The equol-producing status had no effect on baseline serum estradiol, estrone, and FSH; however, equol producers had higher baseline serum estrone sulfate than that of nonproducers (1.1 ± 0.5 vs. 0.8 ± 0.3 ng/mL, respectively; P = 0.04).
TABLE 2.
Baseline characteristics and average nutrient intakes
| Characteristic | Mean ± SD |
| Age at time of dosing, y | 59 ± 9 |
| Height, m | 1.65 ± 0.06 |
| Weight, kg | 74.5 ± 15.5 |
| BMI, kg/m2 | 27.4 ± 5.9 |
| Time after menopause, y | 14 ± 8 |
| Total body bone mineral density, g/cm3 | 1.08 ± 0.10 |
| Total body bone mineral content, g | 2297.3 ± 386.6 |
| Dietary calcium,1 mg | 961 ± 368 |
| Dietary phosphorus, mg | 1238 ± 304 |
| Dietary magnesium, mg | 289 ± 87 |
| Serum follicle-stimulating hormone, U/L | 92.5 ± 25.0 |
| Serum estrone, pg/mL | 27.2 ± 8.3 |
| Serum estrone sulfate,2 ng/mL | 0.9 ± 0.4 |
| Serum estradiol, pg/mL | 12.1 ± 4.2 |
With the exclusion of calcium intake from supplements. Subjects were supplemented with 500 mg Ca/d and 600 IU vitamin D/d.
Equol producers had higher baseline serum estrone sulfate than did nonproducers (1.1 ± 0.5 compared with 0.8 ± 0.3 ng/mL, respectively; P = 0.04).
Of 6 subjects who did not complete the risedronate intervention, one subject experienced a headache, another subject experienced heartburn, and 4 subjects chose not to participate in the risedronate phase at the commencement of the study for fear of side effects and the time commitment. One woman with a strong family history of cardiovascular disease had a nonfatal myocardial infarction during the last recovery period, and her data were included in the analysis. One participant who dropped out during the third soy-intervention period was excluded from the 24 subjects included in the analyses for being noncompliant with taking pills (Figure 1). Twenty-one of the 24 participants were >90% compliant, and all subjects were >80% compliant with taking soy supplements and risedronate on the basis of counts of returned pills. Serum genistein amounts were substantially different from those at baseline in all interventions (Table 3).
TABLE 3.
Serum isoflavones and biochemistries for all interventions and baseline1
| Intervention |
|||||||
| Variable | Baseline (n = 24) | Risedronate (n = 19) | Gen-low (n = 14) | Gen-high (n = 14) | Soy-low (n = 24) | Soy-gen (n = 14) | Soy-high (n = 14) |
| Serum Alk Phos, U/L | 0.45 (0.07, 3.17)a | 0.19 (0.02, 1.57)a | 17 (2, 141)b | 49 (6, 401)b | 40 (6, 281)b | 140 (17, 1148)b | 154 (19, 1257)b |
| Serum BAP, ng/mL | 18.4 (16.1, 20.9)a,b | 17.5 (15.4, 20.0)b | 17.7 (15.4, 20.3)a,b | 18.2 (15.9, 20.9)a,b | 18.9 (16.6, 21.5)a | 18.5 (16.1, 21.2)a,b | 17.6 (15.4, 20.2)a,b |
| Serum osteocalcin, ng/mL | 9.5 (8.3, 10.9)a,b | 9.4 (8.1, 10.8)a,b | 9.5 (8.1, 11.2)a,b | 11.0 (9.3, 12.9)a | 9.5 (8.3, 10.9)a,b | 10.5 (8.9, 12.3)a,b | 9.2 (7.8, 10.8)b |
| Fasting urine NTxs, BCE/mmol creatinine2 | 48.3 (36.8, 63.5)a | 30.7 (23.2, 40.7)b | 44.0 (32.4, 59.9)a | 52.6 (38.7, 71.5)a | 50.3 (38.4, 65.9)a | 42.5 (31.3, 57.8)a | 44.0 (32.1, 60.1)a |
| Fasting urine DPDs, nmol/mmol creatinine3 | 9.3 (8.1, 10.5)a | 7.7 (6.5, 8.9)b | 8.2 (6.9, 9.5)a,b | 8.3 (7.0, 9.6)a,b | 9.2 (8.0, 10.3)a | 8.1 (6.8, 9.4)a,b | 7.9 (6.6, 9.2)a,b |
| Serum genistein, nM | 5.7 (3.1, 10.4)a | 5.4 (2.7, 10.7)a | 140 (63, 309)b | 762 (345, 1684)c | 257 (139, 474)b,c | 596 (270, 1317)b,c | 408 (185, 902)b,c |
| Serum daidzein, nM | 0.3 (0.1, 0.8)c | 1.2 (0.4, 3.1)c | 121 (38, 384)b | 504 (158, 1608)a,b | 883 (363, 2147)a,b | 1258 (395, 4008)a,b | 1466 (460, 4673)a |
| Serum glycitein, nM | 1.5 (0.7, 3.2)b | 1.1 (0.5, 2.4)b | 1.7 (0.6, 4.3)b | 1.5 (0.6, 3.8)b | 21.4 (10.2, 45.1)a | 20.2 (7.7, 52.9)a | 56.7 (21.7, 148.2)a |
| Serum equol nonproducers, nM | 0.53 (0.20, 1.35)a | 1.21 (0.43, 3.39)a | 1.75 (0.48, 6.42)a | 1.75 (0.48, 6.42)a | 1.76 (0.69, 4.53)a | 1.89 (0.51, 6.92)a | 0.61 (0.17, 2.25)a |
| Serum equol producers,3 nM | 0.46 (0.07, 3.17)a | 0.19 (0.02, 1.57)a | 17 (2, 141)b | 49 (6, 401)b | 40 (6, 281)b | 140 (17, 1148)b | 154 (19, 1257)b |
All values are means; 95% CIs in parentheses. Means within a row that have no superscript letter in common are significantly different from each other as determined by using Tukey-Kramer post hoc tests for multiple comparisons, P < 0.05. Alk Phos, alkaline phosphatase; BAP, bone-specific alkaline phosphatase; BCE, bone collagen equivalents; DPD, deoxypyridinoline cross-link; Gen-high, high-dose genistein; Gen-low, low-dose genistein; NTx, type I cross-linked N-telopeptide; Soy-gen, soy isoflavones enriched in genistein; Soy-high, high-dose soy; Soy-low, low-dose soy.
For serum equol producers, n = 8. For serum equol nonproducers, n = 16. For fasting urine phosphorus, calcium:creatinine, NTxs, and DPDs, n = 22 and n = 23 during baseline and the Soy-low intervention, respectively.
For fasting urine NTxs and DPDs, n = 13 during the Soy-high intervention.
Bone calcium retention
Although not a direct indication of bone strength, an increase in bone calcium retention is indicative of a positive change in bone balance. In lieu of measuring BMD and bone strength, interventions are directly comparable because changes in bone calcium retention directly correspond to changes in bone balance.
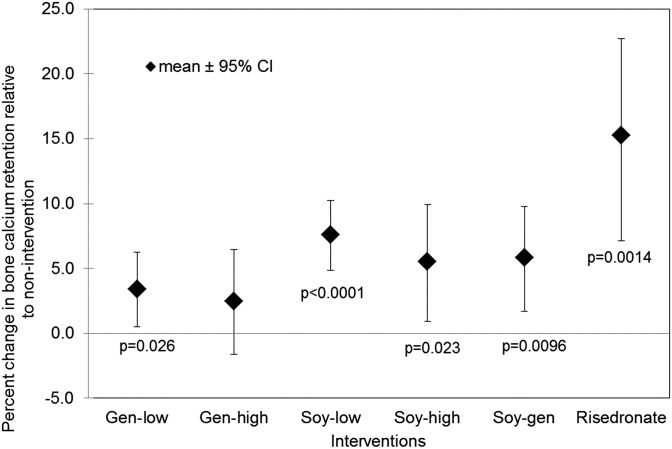
The risedronate intervention compared with nonintervention resulted in an increase in bone calcium retention of 15.3% (P = 0.0014) (Figure 2). Of soy interventions, Soy-low had the greatest effect with a 7.6% increase in bone calcium retention (P < 0.0001). All isoflavone interventions except for Gen-high significantly increased bone calcium retention with Gen-low, Soy-high, and Soy-gen, which resulted in increased bone calcium retention by 3.4% (P = 0.0263), 5.5% (P = 0.0232), and 5.8% (P = 0.0096), respectively. To examine the effect of equol status, the 5 soy treatments were analyzed in a mixed model that included equol status, treatment, and the interaction. The main effect of equol status and the interaction with soy treatment were NS (P = 0.52 and P = 0.35, respectively).
FIGURE 2.
Mean ± 95% CI effects of soy and risedronate interventions on bone calcium retention in postmenopausal women. A 95% CI that does not include zero indicates a significant change from baseline at α = 0.05 by using a linear regression model. Interventions with Gen-low (n = 14), Soy-low (n = 24), Soy-high (n = 14), Soy-gen (n = 14), and risedronate (n = 19) resulted in significant increases in bone calcium retention compared with baseline. the intervention with Gen-high (n = 14) did not result in a change in bone calcium retention compared with baseline. t tests were performed to determine differences between interventions with a Bonferroni-corrected α = 0.007 used to adjust for 7 multiple comparisons. The intervention with Soy-low resulted in a significantly greater bone calcium retention (P = 0.001) than the intervention with Gen-low. Gen-high, high-dose genistein; Gen-low, low-dose genistein; Soy-gen, soy isoflavones enriched in genistein; Soy-high, high-dose soy; Soy-low, low-dose soy.
There was no difference in bone calcium retention between Gen-low and Gen-high or between Soy-low and Soy-high. At the lower dose of genistein, the addition of daidzein and glycitein appeared to enhance the antiresorptive effect because Soy-low was more effective than Gen-low was (P = 0.001). However, no beneficial effect of mixed isoflavones was seen with higher doses of genistein (Gen-high vs. Soy-high). Daidzein did not exhibit antagonistic or agonistic behavior at this amount of genistein (91–96 mg/d) for comparisons of Gen-high vs. Soy-gen, Soy-gen vs. Soy-high, and Gen-high vs. Soy-high.
The following variables were examined as possible covariates: FSH, estrone, estrone sulfate, estradiol, serum calcium, serum phosphorus, the urinary potassium-to-creatinine ratio, the urinary calcium-to-creatinine ratio, and calcium. None of these variables were significant.
Calcium absorption
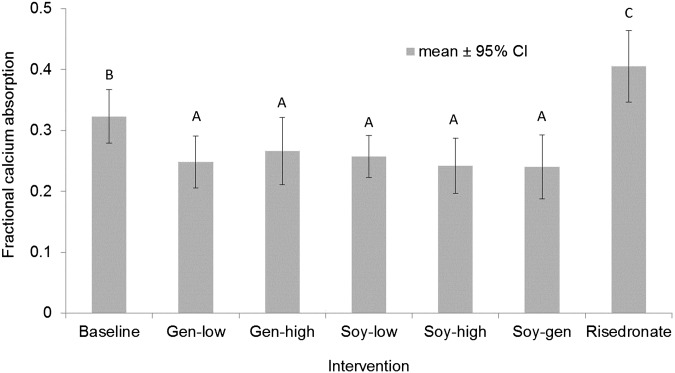
Fractional calcium absorption in soy interventions ranged from 0.240 to 0.266 (Figure 3) and was significantly lower than during baseline (0.323; P = 0.0026) and risedronate (0.405; P < 0.0001) periods. These differences remained when all soy treatments were pooled or when subjects who only completed all soy treatments were tested. There was no difference in fractional calcium absorption between equol producers and nonproducers in any of soy interventions (P = 0.3).
FIGURE 3.
Mean ± 95% CI effects of soy and risedronate interventions on fractional calcium absorption in postmenopausal women. Significant differences were determined by contrasts after 2-factor ANOVA. Fractional calcium absorption for pooled soy interventions (A; n = 77) was significantly lower than at baseline (B; n = 24; P = 0.0026) and with the risedronate intervention (C; n = 19; P = 0.0017). Fractional calcium absorption at baseline was significantly lower than with the risedronate intervention (P < 0.0001). Gen-high, high-dose genistein; Gen-low, low-dose genistein; Soy-gen, soy isoflavones enriched in genistein; Soy-high, high-dose soy; Soy-low, low-dose soy.
Biochemistries
Serum alkaline phosphatase and bone-specific alkaline phosphatase were not different in isoflavone-intervention periods (Table 3). Serum osteocalcin was higher during the intervention with Gen-high (11.0 ng/mL) than with Soy-high (9.2 ng/mL). Type I cross-linked N-telopeptides and deoxypyridinoline cross-links were decreased with the risedronate intervention (30.7 bone collagen equivalents/mmol creatinine and 7.7 nmol/mmol creatinine, respectively) compared with at baseline (48.3 bone collagen equivalents/mmol creatinine and 9.3 nmol/mmol creatinine, respectively) but were unaffected by the isoflavone interventions. There were no significant differences in serum 25-hydroxyvitamin D and serum calcium. Soy-gen had a higher urinary phosphorus:creatinine ratio(0.44; 95% CI: 0.36, 0.53) than during baseline (0.27; 95% CI: 0.24, 0.32), the risedronate intervention (0.30; 95% CI: 0.25, 0.35), and Soy-low (0.30; 95% CI: 0.26, 0.35) (Supplemental Table 1). The urinary phosphorus:creatinine ratio for Gen-low (0.36; 95% CI: 0.30, 0.43), Gen-high (0.37; 95% CI: 0.31, 0.44), and Soy-high (0.37; 95% CI: 0.31, 0.45) did not differ significantly from the control or risedronate intervention. Urinary calcium:creatinine was not significantly different because of the intervention with soy than at baseline; risedronate lowered urinary calcium:creatinine excretion more than the isoflavone interventions did [0.04 (95% CI: 0.03, 0.05) for risedronate compared with 0.08 to 0.10 for soy] but not compared with at baseline. Risedronate lowered serum phosphorus (3.3 mg/dL; 95% CI: 3.2, 3.5 mg/dL) compared with baseline values (3.6 mg/dL; 95% CI: 3.5, 3.8 mg/dL), but soy interventions had no effect. Serum PTH did not vary from that at baseline during isoflavone and risedronate interventions; however, serum PTH (pg/mL) was higher during the risedronate intervention (40.6; 95% CI: 34.3, 48.0) than during Gen-low (28.9; 95% CI: 24.0, 34.7) and Soy-gen (30.2; 95% CI: 25.1, 36.3) but not than during Gen-high (34.0; 95% CI: 28.2, 40.9), Soy-low (36.6; 95% CI: 31.2, 42.9), and Soy-high (33.1; 95% CI: 27.5, 39.8) (Supplemental Table 1). None of the biochemistries were different as a result of equol-producing status.
Serum isoflavones
Serum genistein, daidzein, dihydrodaidzein, and O-DMA were higher with the soy isoflavone intervention than at baseline (Supplemental Table 1, Table 3); there was a dose-dependent effect on serum genistein. The estimate of the difference between the average of high-genistein treatments (Gen-high, Soy-gen, and Soy-high) and low-genistein treatments (Gen-low and Soy-low) was 581 (95% CI: 332, 830; P = 0.0005). Serum daidzein was higher during Soy-high than during Gen-low (1466 compared with 121 nM, respectively; P < 0.05). Serum glycitein was higher with mixed-isoflavone–containing supplements Soy-low (21.4 nM), Soy-high (56.7 nM), and Soy-gen (20.2 nM) that at baseline (1.5 nM) and in Gen-low (1.7 nM), and Gen-high (1.5 nM). Serum dihydrodaidzein was higher in the high-daidzein–containing interventions Soy-low (156 nM; 95% CI: 70, 348 nM), Soy-high (190 nM; 95% CI: 69, 522 nM), and Soy-gen (202 nM; 95% CI: 74, 555 nM) than in Gen-low (15 nM; 95% CI: 5, 41 nM) (Supplemental Table 1). Serum O-DMA was significantly greater in Gen-high (99 nM; 95% CI: 28, 352 nM), Soy-low (88 nM; 95% CI: 32, 241 nM), Soy-high (83 nM; 95% CI: 24, 296 nM), and Soy-gen (178 nM; 95% CI: 50, 632 nM) than in Gen-low (4.3 nM; 1.2, 1.7 nM). Serum equol was not affected by soy interventions in individuals classified as equol nonproducers. Equol producers had serum equol that was higher during soy interventions than at baseline, but there were no differences in serum equol between the different soy interventions (Table 3). There were no differences between interventions for biochanin A, coumestrol, and enterolactone. Serum 6′-OH-O-desmethylangolensin was significantly greater from the intervention with Gen-high (1.3 nM; 95% CI: 0.7, 2.5 nM) than from the intervention with risedronate (0.4 nM; 95% CI: 0.2, 0.6 nM). Serum enterodiol was greater with Soy-high (2.34 nM; 95% CI: 0.65, 8.38 nM) than with all other interventions except for Soy-low (0.69 nM; 95% CI: 0.24, 1.99 nM). Serum formononetin was higher at baseline (37.0 nM; 95% CI: 19.5, 70.2 nM) and with Soy-low (25.9 nM; 95% CI: 13.7, 49.2 nM) and Soy-gen (21.1 nM; 95% CI: 9.3, 47.9 nM) than with Soy-high (8.3 nM; 95% CI: 3.6, 18.7 nM).
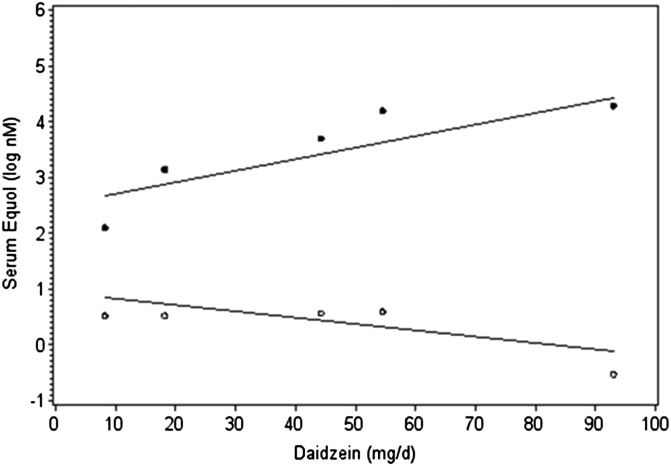
To determine predictors of serum equol, the 5 soy treatments were analyzed in a mixed model that included the ability to produce equol, treatment, and interaction. The interaction between the treatment and equol-producing capacity, both of which were expressed as categorical variables, was NS (P = 0.068). However, when treatments were expressed as the amount of daidzein in the diet (Table 1), there was a significant interaction (P = 0.0036). For equol producers, there was an increase of 0.0236 nM in serum equol for each milligram of daidzein in the diet (P = 0.009), and the slope for nonproducers was NS (P = 0.13) (Figure 4).
FIGURE 4.
Five soy treatments were analyzed in a repeated-measures mixed model that included equol status, dietary intake of daidzein, and the interaction to examine the effect of equol status on serum equol. Equol producers are indicated by filled circles, and nonproducers are indicated by open circles. There was a significant (P = 0.0036) interaction; in equol producers, there was an increase of 0.0236 nM serum equol for each milligram of daidzein in the diet (P = 0.009), whereas the slope for nonproducers was NS (P = 0.13).
DISCUSSION
In this randomized-order, crossover intervention trial in postmenopausal women, we showed that the ability to convert daidzein to its bioactive metabolite equol did not affect the antiresorption response of bone to soy consumption. We also determined that a moderate dose (105.23 mg total isoflavones/d) of naturally occurring mixed isoflavones was the most-effective soy intervention at increasing bone calcium retention in postmenopausal women. This naturally occurring isoflavone mixture was more effective than genistein-rich supplements were despite differences in total isoflavone contents; however, bisphosphonate, which is an osteoporosis pharmaceutical, was still more effective at increasing bone calcium retention.
Despite the enhanced bioavailability of equol (27) and the estrogenic effect (14) than with other isoflavones, we did not find differences between equol producers and nonproducers in the various measures of bone response, bone calcium retention, calcium absorption, or biochemical markers of bone turnover. To our knowledge, we are the first to a priori screen individuals for their equol-producing ability to compare differences in bone responses to a soy intervention. Our initial screening method was validated by an increase in serum equol during soy interventions than at baseline, and producers were distinguished from those who could not produce equol. Furthermore, serum equol increased with the daidzein content of interventions in equol-producers only. Serum genistein, glycitein, and daidzein were significantly correlated with each other, suggesting that the bioavailability of all isoflavones may be related to a variation in gut microbiota and that there are responders and nonresponders. Tablet genistein, daidzein, glycitein, and total isoflavone amounts generally predicted serum isoflavone amounts.
A retrospective analysis of literature and equol-intervention studies have given support for the hypothesis that the ability to produce equol would be favorable to bone. Equol-producing postmenopausal Japanese women conserved significantly greater BMD than did nonproducers in the sub-whole body and total hip after supplementation with 75 mg isoflavones/d for 24 wk (28). The supplement used in the study by Wu et al. (28) was given in the proportion of 12.5% genistein, 55% daidzein, and 32.5% glycitein, which was comparatively higher in daidzein and glycitein and lower in genistein than tested in the current study (105.23 mg/d as 43.9% genistein, 42.1% daidzein, and 14.0% glycitein). The equol-producing ability was a significant effect when dietary isoflavones were directionally lower in genistein, but the effect was suppressed when genistein was present in greater amounts, which may have indicated that genistein is more competitive than equol is for binding on ERs. Equol and genistein were shown to have a comparable binding affinity for ER-α and ER- β, but equol exhibited a more-estrogenic effect through the transcription with ER-α (29). The only other, although minor, source of dietary equol is milk from dairy cows fed a diet rich in isoflavones (30). The dietary intake of equol was significantly associated with bone density in postmenopausal women, but once the dietary intake of calcium was added to the model, this effect became nonsignificant (31). It has been previously reported that ∼30–50% of humans are equol producers (14, 32, 33). In our study, we screened 80 women, only 10 of whom were equol producers (<13% of those screened).
We hypothesized that genistein, in a relatively pure form or as part of a mixed-isoflavone supplement, would suppress bone resorption in a dose-dependent manner. Serum genistein amounts best predicted the respective dose suppression of bone resorption in various sources of isoflavones from soy cotyledon, soy germ, red clover, and kudzu in postmenopausal women (17). However, we did not observe a dose-dependent effect; the intervention with a moderate amount of isoflavones (105.23 mg/d) in their naturally occurring proportion was most effective. The lack of a dose response in serum genistein might have been be attributed to the short half-life of genistein [t1/2 =7.77 h (20)] and the difference in timing between the ingestion of the supplement and the fasting blood draw (20). In our study, lower amounts of genistein (Gen-low: 44.06 mg genistein/d) were more effective than higher amounts (Gen-high: 93.75 mg genistein/d) were at reducing bone resorption . This result was consistent with a biphasic response to soy isoflavones observed in an ovariectomized rat model (34) where a moderate dose had the maximal effect on bone. In addition, treatment with 54 mg genistein/d in the aglycone form, which was a similar dose to that in our Gen-low intervention, for 1 y in osteopenic postmenopausal women was shown to increase the BMD of the lumbar spine and femoral neck (35) and to protect bone as well as HRT (12) in early postmenopausal women.
Mixed-isoflavone rather than genistein-rich interventions had stronger antiresorptive effects on bone. Similarly, a mixture of naturally occurring soy isoflavones compared with purified genistein maintained trabecular separation, bone volume, and bone mass to the level of sham in ovariectomized mice (36) and was the most bioavailable supplement tested (37). In a 2-y intervention trial, a daily intake of 76 mg isoflavones from soymilk was effective at preventing bone loss at the lumbar spine in postmenopausal women compared with intake if isoflavone-poor soymilk, which showed the efficacy of a moderate dose of mixed isoflavones over several bone-remodeling cycles (9). In addition, 120 mg isoflavone supplementation/d protected whole-body BMD but not spine or hip BMD at 1 and 2 y in postmenopausal women (11). Of randomized controlled trials of soy isoflavones, the trial of Levis et al. (6) administered the same naturally occurring mixture of isoflavones as in our study; however, a significant bone-protective effect was not shown (6). Although aglycone equivalents were not reported, we estimate that the dose we showed to be effective was at a concentration that was 20% higher than the highest dose used in the trial of Levis et al. (6). Our findings confirmed the results of our previous study that mixed isoflavones of sufficient dose have antiresorptive effects (17).
Postmenopausal women taking soy interventions had lower fractional calcium absorption their baseline or risedronate periods, possibly related to the inhibitory effect of phytate on calcium absorption (38); although the soy tablets contained low amounts of phytate (<3 mg/d). Nonetheless, the improvement in bone calcium retention indicated an improved bone balance (16) with soy consumption despite the reduced fractional calcium absorption. There was no difference in the dietary consumption of calcium during interventions, and the average consumed intake of 961 mg Ca/d in addition to 500 mg supplemental calcium/d exceeded the recommended 1200 mg Ca/d intake for this age group.
The long half-life (105 y) of 41Ca and the sensitivity of urine measurements by using accelerator mass spectrometry allowed bone to be labeled with this tracer and the effects of a series of interventions to be compared in a crossover design. It can take from 6 mo to several years to capture the effects of a single intervention on traditional bone-strength measurements such as BMD; thus, the ability to perform multiple interventions of a shorter duration on a single subject is advantageous and efficient. The signal from 41Ca is less variable than from other biochemical markers of bone turnover, which allowed for greater power to detect intervention differences in a fewer number of subjects. A disadvantage of a shorter screening approach compared with longer trials is the inability to measure the effect of interventions on bone strength or BMD.
In conclusion, the equol-producing ability did not enhance the bone-protective effect of soy isoflavones in postmenopausal women. A moderate dose (but higher than that used in randomized controlled trials of BMD) of mixed isoflavones in their natural proportion was the most-effective isoflavone supplement at improving bone calcium retention compared with the effects of supplements that were more rich in genistein and higher in the total isoflavone content. We have shown that soy isoflavones, although not as potent as risedronate, are an effective antiresorptive therapy for postmenopausal women at a sufficient dose. Compared with bisphosphonates and HRT, the use of soy isoflavones presents minimal to negligible risk to postmenopausal women (39, 40) and can be used long term for some protection against postmenopausal bone loss.
Acknowledgments
The authors’ responsibilities were as follows—JWP: conducted the research; BRM, MP, GPM, LM, and CMW: designed the research plan; GSJ: performed the isotope analysis; SB and MP: coordinated analyses of isoflavones and biochemical measures, respectively; JWP, GPM, and LM: performed the statistical analysis; JWP and CMW: wrote the manuscript; JWP: had primary responsibility for final content of the manuscript; and all authors: read and approved the final manuscript. CMW is on the scientific advisory board of Pharmavite. SB has a US patent on the use of conjugated isoflavones and the prevention of osteoporosis. JWP, BRM, GPM, LM, GSJ, and MP reported no conflicts of interest related to the study.
Footnotes
Abbreviations used: BMD, bone mineral density; BSA, body surface area; ER, estrogen receptor; FSH, follicle-stimulating hormone; Gen-high, high-dose genistein; Gen-low, low-dose genistein; HRT, hormone-replacement therapy; O-DMA, O-desmethylangolensin; PTH, parathyroid hormone; Soy-gen, soy isoflavones enriched in genistein; Soy-high, high-dose soy; Soy-low, low-dose soy.
REFERENCES
- 1.Leboime A, Confavreux CB, Mehsen N, Paccou J, David C, Roux C. Osteoporosis and mortality. Joint Bone Spine 2010;77:S107–12. [DOI] [PubMed] [Google Scholar]
- 2.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the women’s health initiative randomized controlled trial. JAMA 2002;288:321–33. [DOI] [PubMed] [Google Scholar]
- 3.Arrain Y, Masud T. Recent recommendations on bisphosphonate-associated osteonecrosis of the jaw. Dent Update 2008;35:238–40, 242. [DOI] [PubMed] [Google Scholar]
- 4.Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report 2008;12:1–23. [PubMed] [Google Scholar]
- 5.Kreijkamp-Kaspers S, Kok L, Grobbee DE, de Haan EHF, Aleman A, Lampe JW, van der Schouw YT. Effect of soy protein containing isoflavones on cognitive function, bone mineral density, and plasma lipids in postmenopausal women: a randomized controlled trial. JAMA 2004;292:65–74. [DOI] [PubMed] [Google Scholar]
- 6.Levis S, Strickman-Stein N, Ganjei-Azar P, Xu P, Doerge DR, Krischer J. Soy isoflavones in the prevention of menopausal bone loss and menopausal symptoms. Arch Intern Med 2011;171:1363–9. [DOI] [PubMed] [Google Scholar]
- 7.Brink E, Coxam V, Robins S, Wahala K, Cassidy A, Branca F. Long-term consumption of isoflavone-enriched foods does not affect bone mineral density, bone metabolism, or hormonal status in early postmenopausal women: a randomized, double-blind, placebo controlled study. Am J Clin Nutr 2008;87:761–70. [DOI] [PubMed] [Google Scholar]
- 8.Alekel DL, St Germain A, Peterson CT, Hanson KB, Stewart JW, Toda T. Isoflavone-rich soy protein isolate attenuates bone loss in the lumbar spine of perimenopausal women. Am J Clin Nutr 2000;72:844–52. [DOI] [PubMed] [Google Scholar]
- 9.Lydeking-Olsen E, Beck-Jensen JE, Setchell KDR, Holm-Jensen T. Soymilk or progesterone for prevention of bone loss. Eur J Nutr 2004;43:246–57. [DOI] [PubMed] [Google Scholar]
- 10.Potter SM, Baum JA, Teng H, Stillman RJ, Shay NF, Erdman JW. Soy protein and isoflavones: their effects on blood lipids and bone density in postmenopausal women. Am J Clin Nutr 1998;68:1375S–9S. [DOI] [PubMed] [Google Scholar]
- 11.Wong WW, Lewis RD, Steinberg FM, Murray MJ, Cramer MA, Amato P, Young RL, Barnes S, Ellis KJ, Shypailo RJ, et al. Soy isoflavone supplementation and bone mineral density in menopausal women: a 2-y multicenter clinical trial. Am J Clin Nutr 2009;90:1433–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morabito N, Crisafulli A, Vergara C, Gaudio A, Lasco A, Frisina N, D’Anna R, Corrado F, Pizzoleo MA, Cinotta M, et al. Effects of genistein and hormone-replacement therapy on bone loss in early postmenopausal women: a randomized double-blind placebo-controlled study. J Bone Miner Res 2002;17:1904–12. [DOI] [PubMed] [Google Scholar]
- 13.Weaver CM, Cheong JMK. Soy isoflavones and bone health: the relationship is still unclear. J Nutr 2005;135:1243–7. [DOI] [PubMed] [Google Scholar]
- 14.Setchell KDR, Brown NM, Lydeking-Olsen E. The clinical importance of the metabolite equol–a clue to the effectiveness of soy and its isoflavones. J Nutr 2002;132:3577–84. [DOI] [PubMed] [Google Scholar]
- 15.Setchell KDR, Clerici C, Lephart ED, Cole SJ, Heenan C, Castellani D, Wolfe BE, Nechemias-Zimmer L, Brown NM, Lund TD, et al. S-equol, a potent ligand for estrogen receptor beta, is the exclusive enantiomeric form of the soy isoflavone metabolite produced by human intestinal bacterial flora. Am J Clin Nutr 2005;81:1072–9. [DOI] [PubMed] [Google Scholar]
- 16.Lee WH, Wastney ME, Jackson GS, Martin BR, Weaver CM. Interpretation of 41Ca data using compartmental modeling in postmenopausal women. Anal Bioanal Chem 2011;399:1613–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weaver CM, Martin BR, Jackson GS, McCabe GP, Nolan JR, McCabe LD, Barnes S, Reinwald S, Boris ME, Peacock M. Antiresorptive effects of phytoestrogen supplements compared with estradiol or risedronate in postmenopausal women using 41Ca methodology. J Clin Endocrinol Metab 2009;94:3798–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheong JMK, Martin BR, Jackson GS, Elmore D, McCabe GP, Nolan JR, Barnes S, Peacock M, Weaver CM. Soy isoflavones do not affect bone resorption in postmenopausal women: a dose-response study using a novel approach with 41Ca. J Clin Endocrinol Metab 2007;92:577–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griffith AP, Collison MW. Improved methods for the extraction and analysis of isoflavones from soy-containing foods and nutritional supplements by reversed-phase high-performance liquid chromatography and liquid chromatography-mass spectrometry. J Chromatogr A 2001;913:397–413. [DOI] [PubMed] [Google Scholar]
- 20.Setchell KDR, Faughnan MS, Avades T, Zimmer-Nechemias L, Brown NM, Wolfe BE, Brashear WT, Desai P, Oldfield MF, Botting NP, et al. Comparing the pharmacokinetics of daidzein and genistein with the use of 13-C-labeled tracers in premenopausal women. Am J Clin Nutr 2003;77:411–9. [DOI] [PubMed] [Google Scholar]
- 21.Prasain JK, Arabshahi A, Moore DR, Greendale G, Wyss JM, Barnes S. Simultaneous determination of eleven phytoestrogens in human serum using a two minute liquid chromatography/tandem mass spectrometry method. J Chromatogr B Analyt Technol Biomed Life Sci 2010;878:994–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coward L, Kirk M, Albin N, Barnes S. Analysis of plasma isoflavones by reversed-phase hplc-multiple reaction ion monitoring-mass spectrometry. Clin Chim Acta 1996;247:121–42. [DOI] [PubMed] [Google Scholar]
- 23.Lee WH, McCabe GP, Martin BR, Weaver CM. Simple isotopic method using oral stable or radioactive tracers for estimating fractional calcium absorption in adult women. Osteoporos Int 2011;22:1829–34. [DOI] [PubMed] [Google Scholar]
- 24.Weaver CM, Rothwell AP, Wood KV. Measuring calcium absorption and utilization in humans. Curr Opin Clin Nutr Metab Care 2006;9:568–74. [DOI] [PubMed] [Google Scholar]
- 25.McDowell MA, Fryar CD, Ogden CL, Flegal KM. Anthropometric reference data for children and adults, United States, 2003-2006. Natl Health Stat Report 2008;10:1–48. [PubMed] [Google Scholar]
- 26.Kelly TL, Wilson KE, Heymsfield SB. Dual energy X-ray absorptiometry body composition reference values from NHANES. PLoS One 2009;4:e7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Setchell KDR, Zhao X, Jha P, Heubi J, Brown N. The pharmacokinetic behavior of the soy isoflavone metabolite S-(-)equol and its diastereroisomer R-(+)equol in healthy adults determined by using stable-isotope-labeled tracers. Am J Clin Nutr 2009;90:1029–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu J, Oka J, Higuchi M, Tabata I, Toda T, Fujioka M, Fuku N, Teramoto T, Okuhira T, Ueno T, et al. Cooperative effects of isoflavones and exercise on bone and lipid metabolism in postmenopausal Japanese women: a randomized placebo-controlled trial. Metabolism 2006;55:423–33. [DOI] [PubMed] [Google Scholar]
- 29.Morito K, Hirose T, Kinjo J, Hirakawa T, Okawa M, Nohara T, Ogawa S, Inoue S, Muramatsu M, Masamune Y. Interaction of phytoestrogens with estrogen receptors alpha and beta. Biol Pharm Bull 2001;24:351–6. [DOI] [PubMed] [Google Scholar]
- 30.Mustonen EA, Tuori M, Saastamoinen I, Taponen J, Wähälä K, Saloniemi H, Vanhatalo A. Equol in milk of dairy cows is derived from forage legumes such as red clover. Br J Nutr 2009;102:1552–6. [DOI] [PubMed] [Google Scholar]
- 31.Kuhnle GGC, Ward HA, Vogiatzoglou A, Luben RN, Mulligan A, Wareham NJ, Forouhi NG, Khaw KT. Association between dietary phytoestrogens and bone density in men and postmenopausal women. Br J Nutr 2011;106:1063–9. [DOI] [PubMed] [Google Scholar]
- 32.Rowland IR, Wiseman H, Sanders TA, Adlercreutz H, Bowey EA. Interindividual variation in metabolism of soy isoflavones and lignans: influence of habitual diet on equol production by the gut microflora. Nutr Cancer 2000;36:27–32. [DOI] [PubMed] [Google Scholar]
- 33.Lampe JW, Karr SC, Hutchins AM, Slavin JL. Urinary equol excretion with a soy challenge: influence of habitual diet. Proc Soc Exp Biol Med 1998;217:335–9. [DOI] [PubMed] [Google Scholar]
- 34.Anderson JJB, Ambrose WW, Garner SC. Biphasic effects of genistein on bone tissue in the ovariectomized, lactating rat model. Proc Soc Exp Biol Med 1998;217:345–50. [DOI] [PubMed] [Google Scholar]
- 35.Marini H, Minutoli L, Polito F, Bitto A, Altavilla D, Atteritano M, Gaudio A, Mazzaferro S, Frisina A, Frisina N, et al. Effects of the phytoestrogen genistein on bone metabolism in osteopenic postmenopausal women: a randomized trial. Ann Intern Med 2007;146:839–47. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y, Li Q, Wan HY, Helferich WG, Wong MS. Genistein and a soy extract differentially affect three-dimensional bone parameters and bone-specific gene expression in ovariectomized mice. J Nutr 2009;139:2230–6. [DOI] [PubMed] [Google Scholar]
- 37.Sepehr E, Cooke G, Robertson P, Gilani GS. Bioavailability of soy isoflavones in rats part 1: application of accurate methodology for studying the effects of gender and source of isoflavones. Mol Nutr Food Res 2007;51:799–812. [DOI] [PubMed] [Google Scholar]
- 38.Heaney RP, Weaver CM, Fitzsimmons ML. Soybean phytate content: effect on calcium absorption. Am J Clin Nutr 1991;53:745–7. [DOI] [PubMed] [Google Scholar]
- 39.Steinberg FM, Murray MJ, Lewis RD, Cramer MA, Amato P, Young RL, Barnes S, Konzelmann KL, Fischer JG, Ellis KJ, et al. Clinical outcomes of a 2-y soy isoflavone supplementation in menopausal women. Am J Clin Nutr 2011;93:356–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hooper L, Madhavan G, Tice JA, Leinster SJ, Cassidy A. Effects of isoflavones on breast density in pre- and postmenopausal women: a systematic review and meta-analysis of randomized controlled trials. Hum Reprod Update 2010;16:745–60. [DOI] [PMC free article] [PubMed] [Google Scholar]