Abstract
Background: Low chronic vitamin B-6 status can occur in a subset of women who use oral contraceptives (OCs) with uncertain metabolic consequences. An insufficiency of cellular pyridoxal 5′-phosphate (PLP), which is the coenzyme form of vitamin B-6, may impair many metabolic processes including one-carbon and tryptophan metabolism.
Objective: We investigated the effects of vitamin B-6 supplementation on the in vivo kinetics of one-carbon metabolism and the concentration of one-carbon and tryptophan metabolites in vitamin B-6–deficient OC users.
Design: A primed, constant infusion of [13C5]methionine, [3-13C]serine, and [2H3]leucine was performed on 10 OC users (20–40 y old; plasma PLP concentrations <30 nmol/L) before and after 28 d of supplementation with 10 mg pyridoxine hydrochloric acid/d. In vivo fluxes of total homocysteine remethylation, the remethylation of homocysteine from serine, and rates of homocysteine and cystathionine production were assessed. Targeted metabolite profiling was performed, and data were analyzed by using orthogonal partial least-squares–discriminant analysis and paired t tests adjusted for multiple testing.
Results: Pyridoxine supplementation increased the mean ± SD plasma PLP concentration from 25.8 ± 3.6 to 143 ± 58 nmol/L (P < 0.001) and decreased the leucine concentration from 103 ± 17 to 90 ± 20 nmol/L (P = 0.007) and glycine concentration from 317 ± 63 to 267 ± 58 nmol/L (P = 0.03). Supplementation did not affect in vivo rates of homocysteine remethylation or the appearance of homocysteine and cystathionine. A multivariate analysis showed a clear overall effect on metabolite profiles resulting from supplementation. Leucine, glycine, choline, cysteine, glutathione, trimethylamine N-oxide, and the ratios glycine:serine, 3-hydroxykynurenine:kynurenine, 3-hydroxykynurenine:3-hydroxyanthranilic acid, and 3-hydroxykynurenine:anthranilic acid were significant discriminating variables.
Conclusions: Consistent with previous vitamin B-6–restriction studies, fluxes of one-carbon metabolic processes exhibited little or no change after supplementation in low–vitamin B-6 subjects. In contrast, changes in the metabolic profiles after supplementation indicated perturbations in metabolism, suggesting functional vitamin B-6 deficiency. This study was registered at clinicaltrials.gov as NCT01128244.
Keywords: metabolomics, one-carbon metabolism, oral contraceptives, tryptophan catabolism, vitamin B-6
INTRODUCTION
Vitamin B-6 plays important roles in metabolism, and thus, normal vitamin B-6 status is important for health. Approximately 11% of the US population has vitamin B-6 deficiency as reflected by a plasma pyridoxal 5′-phosphate (PLP)9 concentration <20 nmol/L (1). The prevalence of vitamin B-6 deficiency increases with age and is higher in non-Hispanic individuals, higher in women than in men, higher in women of reproductive age, and higher in women who use oral contraceptives (OCs) than in nonusers (1, 2). Plasma PLP is lower in chronic inflammatory conditions (3–6). Epidemiologic studies have shown associations of vitamin B-6 deficiency with cardiovascular disease, stroke, and venous thrombosis (7–10); however, the underlying mechanisms are still unknown.
PLP is the coenzyme of vitamin B-6 involved in metabolic processes including amino acid, carbohydrate, and lipid metabolism; the synthesis of neurotransmitters; and one-carbon metabolism. PLP is also a cofactor in the tryptophan catabolic pathway that yields the de novo synthesis of niacin and the formation of several kynurenines (Kyns) that have various physiologic properties (11–14).
Studies in animals and humans and mathematical modeling have shown effects of vitamin B-6 deficiency on several aspects of metabolism that are consistent with the multiple metabolic functions of PLP (15–25). Our previous studies showed that low vitamin B-6 status with a plasma PLP concentration <30 nmol/L, which is consistent with marginal deficiency (26), also induces metabolic effects including alterations in concentrations of certain constituents of one-carbon and tryptophan pathways (17, 18, 20, 27).
Controlled dietary protocols and an in vivo kinetic evaluation by using stable isotopic tracers have shown little or no changes in the flux of one-carbon and transsulfuration pathways during short-term (28-d) vitamin B-6 insufficiency (∼0.35 mg dietary vitamin B-6/d) that yielded plasma PLP in the deficient or marginal range. With this approach, Davis et al. (23) showed no effect of vitamin B-6 restriction on the fluxes of total homocysteine remethylation or the remethylation of homocysteine from serine-derived one-carbon units (23). Lamers et al. (19) showed no effects of the restriction on remethylation, transmethylation, and transsulfuration fluxes in the methionine cycle and little effect on glycine kinetics (18). The effects of chronic vitamin B-6 deficiency or marginal status and vitamin B-6 supplementation have not been fully evaluated.
In the current study of women who were using OCs, we investigated the metabolic consequences of chronic vitamin B-6 insufficiency and effects of supplementation with pyridoxine for 28 d on one-carbon metabolism and the tryptophan catabolic pathway. This study involved the assessment of in vivo kinetics of one-carbon metabolism by using a previously established multitracer protocol (23) along with a targeted metabolite profile analysis. Results from this study provide information to extend our understanding of the impact of chronic vitamin B-6 deficiency or marginal status and the metabolic effects of vitamin B-6 supplementation.
METHODS
Materials
l-[13C5]methionine, l-[3-13C]serine, and l-[5,5,5-2H3]leucine were purchased from Cambridge Isotope Laboratories. These compounds were prepared in saline, filter sterilized, and analyzed to ensure sterility and the lack of pyrogenicity. Concentrations of labeled amino acids in these infusion solutions were 2.33 mg [3-13C]serine/mL, 0.583 mg[13C5]methionine/mL, and 0.583 mg [2H3]leucine/mL, which were verified by using gas chromatography–mass spectrometry.
Human participants
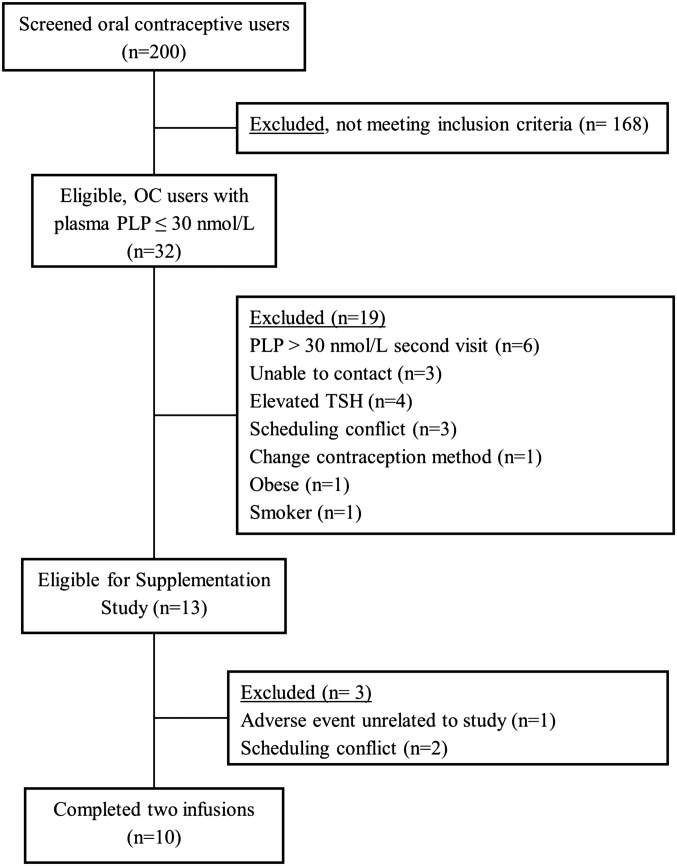
We screened a total of 200 women (20–40 y of age) who used common combined or single formulations of OCs that contained 20–30 μg ethinyl estradiol and progesterone/d between August 2010 and May 2014 in Gainesville, Florida (Figure 1). Inclusion criteria were as follows: low vitamin B-6 status (PLP concentration <30 nmol/L), OC usage >6 mo; no history of gastrointestinal surgery, abnormal kidney or thyroid function, or any other chronic disease; no smoking or chronic drug use or alcoholism; BMI (in kg/m2) <32; no vitamin, amino acid, or protein supplementation; nonpregnant; and normal plasma concentrations of folate (>7 nmol/L), vitamin B-12 (>200 pmol/L), and total homocysteine (<12 μmol/L). Subjects (n = 32) with a plasma PLP concentration <30 nmol/L in the initial plasma PLP evaluation were selected for additional screening. We initially calculated a power of 0.88 to detect a 20% change in the fractional synthesis rate of homocysteine (FSRHcy) because of supplementation with n = 15. Thirteen women remained eligible after the clinical chemistry screen, whereas the remainder of women could not participate in the protocol because of an inability to contact, scheduling conflicts, or no longer meeting the inclusion criteria (Figure 1). During the study, one participant was withdrawn because of an unrelated adverse event, and 2 subjects were withdrawn because of scheduling conflicts (Figure 1). Results are reported with n = 10. Because the protocol was conducted with fewer participants than intended, the actual power on the basis of n = 10 calculated using the results obtained, yielded 0.83 power to detect a 17% change in the FSRHcy, at α = 0.05.
FIGURE 1.
Consolidated Standards of Reporting Trials diagram of study participants. OC, oral contraceptive; PLP, pyridoxal 5′-phosphate; TSH, thyrotropin (thyroid-stimulating hormone).
All participants signed an informed consent form. All procedures were reviewed and approved by the University of Florida Institutional Review Board and the University of Florida Clinical Research Center (CRC) Scientific Advisory Committee. This study was registered at clinicaltrials.gov as NCT01128244.
Study design and samples
Two 9-h primed, constant infusions of labeled amino acids [13C5]methionine, [3-13C]serine and [2H3]leucine were performed for each participant; one infusion done was before, and one infusion was done after, 28 d of daily supplementation with custom-formulated 10 mg US Pharmacopoeia pyridoxine-HCl capsules. Before each infusion, participants received a 2-d controlled diet with a constant protein intake prepared by the Bionutrition Unit of the CRC with the diet formulation done by the research dietitian with Minnesota Nutrition Data Systems software version 2005/2007 (Nutrition Coordinating Center, University of Minnesota). Subjects consumed morning meals in the CRC and took a sack or bag of lunch, dinner, and snacks home in these 2-d periods. During the 28 d of the supplementation period, participants were asked to consume the daily pyridoxine supplement provided and continue with their usual diets. Weekly visits to the CRC were scheduled to obtain blood samples for the analysis of plasma PLP to monitor supplementation compliance. Food-frequency questionnaires (Block 2005 FFQ; NutritionQuest) were administered before and after the supplementation period to collect information regarding the usual diets of participants.
Infusion protocol
The primed, constant-infusion protocol was based on that reported previously (19, 23). Briefly, after an overnight fast, subjects were admitted to the CRC, and a catheter was inserted into the antecubital vein of each arm (one catheter was used for the infusion solution, and the other catheter was used for blood collection). Blood samples were taken in preprandial and postprandial states for the measurement of metabolite concentrations. Each participant received a nutritive formula immediately after fasting samples were taken and then hourly for the duration of the infusion. The hourly nutritive formula provided an intake of energy with a balanced composition of amino acids on the basis of requirements of 0.8 g protein/kg per day (30 kcal/kg per day) and maintained a fed state during the infusion. Infusions started with a priming dose of ∼20 mL over 5 min to deliver 9.48 μmol [3-13C]serine/kg, 1.63 μmol [13C5]methionine/kg, and 1.87 μmol [2H3]leucine/kg. After the priming dose, the 9-h constant infusion of ∼20 mL/h delivered 9.48 μmol [3-13C]serine/kg per hour, ∼1.63 μmol [13C5]methionine/kg per hour, and ∼1.87 μmol [2H3]leucine/kg per hour. Specific infusion rates and priming doses were calculated according to each participant’s body weight. Blood samples were taken at 0, 0.5, 1, 1.5, 2, 2.5, 3, 4, 5, 6, 7.5, and 9 h of the infusion. Samples were placed immediately in ice and were centrifuged within 15 min at 1500 × g for 10 min at 4°C. Plasma samples were stored at −80°C. Blood samples taken at time zero were used to determine the natural isotopic abundance of the amino acids.
Analytic methods
Quantitative analysis of metabolite concentrations
Plasma PLP and plasma aminothiols (including total homocysteine, total cysteine, total glutathione, and total cysteinylglycine) were determined by using reverse-phase HPLC methods with fluorescence detection (28, 29). Serum folate and vitamin B-12 were determined by using an Elecsys chemiluminesence system (Roche Diagnostics). Plasma concentrations of the amino acids methionine, serine, leucine, cystathionine, and glycine were measured by using isotope-dilution gas chromatography–mass spectrometry with the following labeled amino acids as internal standards: [13C5]methionine, [13C3]serine, [2H3]leucine, [13C4]cystathionine, and [13C2]glycine as previously described (30).
The following one-carbon and tryptophan catabolic pathway metabolites were measured by using liquid chromatography–tandem mass spectrometry (31) at Bevital: betaine, choline, dimethylglycine, creatine, creatinine, arginine, asymmetric dimethylarginine, symmetric dimethylarginine, homoarginine, trimethyllysine, histidine, trimethyl N-oxide, riboflavin; Kyn, 3-hydroxykynurenine (HK), 3-hydroxyanthranilic acid (HAA), kynurenic acid (KA), anthranilic acid (AA), xanthurenic acid (XA), quinolinic acid, nicotinamide, nicotinic acid, and N1-methylnicotinamide. Selected biomarkers of inflammation included neopterin measured by using liquid chromatography–tandem mass spectrometry (31) and plasma C-reactive protein measured by using immunoturbidimetry (Cobas 8000; Roche Diagnostics).
The following ratios were calculated: betaine:choline and dimethylglycine:betaine, which reflects reactions of production and the use of betaine; asymmetric dimethylarginine:arginine as a potential marker of endothelial dysfunction (20, 32); creatine:cystathionine, which was previously shown to be sensitive to vitamin B-6 restriction (20); glycine:serine, which provides information on the reaction catalyzed by serine hydroxymethyltransferase; the PAr index, which is the ratio of 4-pyridoxic acid (PA) divided by the sum of PLP plus pyridoxal (PL), i.e.,
 |
which is a marker of vitamin B-6 catabolism during inflammation (33); kynurenine:tryptophan, which is marker of inflammation; and the ratios HK:KA, HK:HAA, HK:XA, HK:AA, and HK:Kyn, which reflect PLP-dependent reactions in the tryptophan catabolic pathway.
Quantitative analysis of amino acid isotopic enrichment
Plasma amino acids were extracted and converted to n-propyl ester heptafluorobutyramide derivatives as previously described (19, 23). Isotopic enrichment was determined by using an Equity-5 fused silica capillary column (Supelco) followed by chemical ionization–mass spectrometry operated in the negative mode as previously described (19, 23). The relative abundance of specific ions was determined by selected-ion monitoring at the following m/z: methionine (367–372), serine (519–520), leucine (349–352), homocysteine (549–553), and cystathionine (677–682). Isotopic enrichments were expressed as molar ratios of labeled:nonlabeled isotopomers after correction for the natural abundance of stable isotopes (19, 23). Calculations of kinetic variables were done as previously reported (23). To correct for overestimation of the intracellular enrichment of serine, the [3-13C]serine enrichment was multiplied by a factor of 0.4 as previously reported (23, 34). The enrichment of intracellular [13C5]methionine was estimated by using [13C4]homocysteine enrichment (23, 35).
The simultaneous infusion of [13C5]methionine and [3-13C]serine allows for the determination fluxes of total remethylation of homocysteine, the remethylation of homocysteine by using one-carbon units from serine (vitamin B-6–dependent remethylation) (23), and the transsulfuration pathway. For total homocysteine remethylation, the flux of the methionine carbon skeleton and the methyl group of methionine were assessed. The flux of the methionine carbon skeleton includes its appearance from protein breakdown, dietary intake, and the infused methionine, whereas the flux of the methyl group involves its appearance from protein breakdown, dietary intake, the infused methionine, and the remethylation of homocysteine (34).
Statistical analysis
Kinetic analysis
Data were logarithmically transformed before the analysis to meet Gaussian assumption. Differences in the in vivo kinetics and selected amino acids in the preprandial state between before and after vitamin B-6 supplementation were investigated by using the paired t test. All data are presented as means ± SDs. Statistical significance was determined at the 0.05 level. Kinetic calculations were performed with the SigmaPlot 11.0 software package (Systat Software).
Targeted quantitative metabolite profiling
An orthogonal partial least-squares–discriminant analysis (OPLS-DA) was performed by using the pooled data for one-carbon metabolites, kinetic variables, tryptophan metabolites, and biomarkers of inflammation separate for preprandial and postprandial states with the SIMCA version 13 program (MKS Umetrics) (36). To account for the paired-data structure (i.e., measurements before and after supplementation), data were processed according to the method of Westerhuis et al. (37). The mean between observations (before and after supplementation) was calculated, and each value was divided by the mean. These adjusted data were evaluated by using an OPLS-DA to determine the effects of vitamin B-6 supplementation. The SIMCA algorithm interpolates any missing data points but does not influence the model (36). Overall differences in metabolite profiles according to vitamin B-6 status (i.e., before and after vitamin B-6 supplementation) were visualized by using score plots. S-plots and jack-knifed CIs shown in the loading column plots were used together to identify the variables responsible for differences between classes (36, 38). Variables that were shown to be significant in the loading columns at 99% CI and that showed a high correlation [p(corr)[1] less than −0.6 and >0.6] and high covariance (p[1]) in the S-plots, were considered the strongest contributors to the differences between treatments in the OPLS-DA analysis (38). A 99% CI was chosen in the analysis to increase the stringency in the identification of biomarkers. A validation of the models was determined by R2(cum) and Q2(cum). R2(cum) indicates good class separation, whereas Q2(cum) indicates the good predictability of the model. Values for Q2 > 0.5 were considered to be good models (36).
In addition, a paired t test that was adjusted for multiple testing by using the Sidak method (39) was performed separately for preprandial and postprandial states and for 3 classes of metabolites (one-carbon, tryptophan, and inflammatory markers) to examine the effects of supplementation on each variable. If missing data occurred in any metabolite analyses, the participant was not included in the paired t test. The test was performed on log-transformed data with SAS 9.4 software (SAS Institute Inc.) with the overall statistical significance determined at the 0.05 level.
RESULTS
Participant characteristics
Demographic and baseline characteristics of the 10 OC users who completed the 2 infusions are shown in Table 1. The mean age of subjects was 24.4 ± 5.5 y, and the mean BMI was 24.3 ± 3.50. Participants had adequate folate and vitamin B-12 status at baseline but exhibited low vitamin B-6 status (PLP concentration <30 nmol/L), consistent with deficient or marginal status (26, 40). The initial PLP concentration for 9 subjects was in the marginal deficiency range (20–30 nmol/L), whereas only one subjects had a plasma PLP concentration <20 nmol/L. Vitamin B-6 supplementation for 28 d increased the mean plasma PLP concentration from 25.8 ± 3.6 to 143 ± 57.6 nmol/L (P < 0.001) (Table 2). There were no significant changes in folate, vitamin B-12, and total homocysteine concentrations after supplementation (Table 2).
TABLE 1.
Demographic information and baseline characteristics of oral contraceptive users who completed the study intervention
| Characteristic | Oral contraceptive user (n = 10) |
| Race-ethnicity distribution, n | |
| African American | 2 |
| Asian | 0 |
| Caucasian | 5 |
| Hispanic | 3 |
| Age, y | 24.4 ± 5.51 |
| BMI, kg/m2 | 24.3 ± 3.5 |
| Plasma PLP,2 nmol/L | 25.8 ± 3.6 |
| Serum folate, nmol/L | 30.8 ± 7.8 |
| Serum vitamin B-12, pmol/L | 319 ± 123 |
| Plasma total homocysteine, μmol/L | 6.46 ± 1.9 |
Mean ± SD (all such values).
PLP, pyridoxal 5′-phosphate.
TABLE 2.
Preprandial plasma concentrations of PLP and amino acids before and after 28 d of vitamin B-6 supplementation with 10 mg pyridoxine/d in OC users1
| Baseline | Supplemented | P | |
| B vitamins | |||
| PLP, nmol/L | 25.8 ± 3.6 | 143 ± 58* | <0.001 |
| Folate,2 nmol/L | 30.8 ± 7.8 | 25.6 ± 10 | 0.29 |
| Vitamin B-12, pmol/L | 319 ± 123 | 295 ± 99.3 | 0.47 |
| Amino acids | |||
| Total homocysteine, μmol/L | 6.46 ± 1.9 | 6.06 ± 1.6 | 0.13 |
| Cystathionine, μmol/L | 0.14 ± 0.06 | 0.13 ± 0.06 | 0.45 |
| Total cysteine, μmol/L | 211 ± 33 | 210 ± 27 | 0.84 |
| Total glutathione, μmol/L | 4.68 ± 0.80 | 4.86 ± 1.2 | 0.98 |
| Cysteinylglycine, μmol/L | 26.5 ± 3.6 | 24.2 ± 2.8 | 0.12 |
| Methionine, μmol/L | 27.4 ± 2.4 | 27.3 ± 5.2 | 0.18 |
| Serine, μmol/L | 106 ± 18 | 110 ± 24 | 0.30 |
| Leucine, μmol/L | 103 ± 17 | 90.3 ± 20* | 0.007 |
| Glycine, μmol/L | 317 ± 63 | 267 ± 58* | 0.03 |
| Glycine:serine ratio | 3.07 ± 0.79 | 2.48 ± 0.57* | 0.01 |
All values are means ± SDs (n = 10). Data were analyzed by using the paired t test. *Significantly different from baseline, P < 0.05. OC, oral contraceptive; PLP, pyridoxal 5′-phosphate.
Folate after supplementation (n = 8). Two participants had folate concentrations >45.3 nmol/L and were excluded for the mean calculation.
Data from the food-frequency questionnaires showed that individual vitamin B-6 intake ranged from 0.6 to 2.2 mg/d (mean ± SD: 1.27 ± 0.49 mg/d) in the month before the first infusion and from 0.8 to 2.0 mg/d (mean ± SD: 1.13 ± 0.33 mg/d) during the 28-d supplementation period. These values do not include vitamin B-6 supplements given during the intervention. One-half of OC users reported vitamin B-6 intakes <1.3 mg/d, and the other one-half of OC users reported intakes higher than the Recommended Dietary Allowance for vitamin B-6 of 1.3 mg/d (40).
Kinetic analysis and preprandial amino acid concentrations
The primed, constant infusion protocol yielded enrichment curves for the infused amino acids [13C]serine, [13C5]methionine, and [2H3]leucine and for the metabolic products [13C4]methionine, [13C1]methionine, [13C4]homocysteine, and [13C4]cystathionine (Supplemental Figures 1 and 2). The enrichment plateau for the [13C]serine, [13C5]methionine, and [2H3]leucine was reached at ∼0.5 h. There were no effects of vitamin B-6 supplementation on the mean whole-body flux of leucine (Table 3), which suggested minimal effects of supplementation on the rate of protein turnover and leucine kinetics. The mean whole-body flux of serine did not change as a function of supplementation (Table 3). In addition, vitamin B-6 administration did not alter the mean whole fluxes of the methionine carbon skeleton (QC) and methyl group (QM), or the fluxes for serine and methionine adjusted for leucine flux.
TABLE 3.
In vivo kinetics at baseline and after 28 d of vitamin B-6 supplementation with 10 mg pyridoxine/d in OC users1
| Baseline | Supplemented2 | P | |
| Total remethylation, μmol/kg per hour | 6.07 ± 1.1 | 5.63 ± 1.1 | 0.20 |
| Vitamin B-6–dependent remethylation, μmol/kg per hour | 6.60 ± 1.9 | 6.92 ± 2.2 | 0.62 |
| Remethylation from serine, % | 109 ± 31 | 126 ± 45 | 0.25 |
| QSer for remethylation, % | 1.99 ± 0.58 | 1.96 ± 0.48 | 0.88 |
| QLeu, μmol/kg per hour | 120 ± 18 | 129 ± 21 | 0.21 |
| QSer, μmol/kg per hour | 339 ± 78 | 349 ± 48 | 0.44 |
| QSer/QLeu, μmol/kg per hour | 2.80 ± 0.32 | 2.75 ± 0.43 | 0.70 |
| QM, μmol/kg per hour | 25.7 ± 4.7 | 24.8 ± 3.5 | 0.82 |
| QC, μmol/kg per hour | 19.7 ± 3.7 | 20.6 ± 5.5 | 0.71 |
| QM/QLeu, μmol/kg per hour | 0.21 ± 0.01 | 0.21 ± 0.05 | 0.56 |
| FSRHcy, fraction/h | 0.19 ± 0.03 | 0.17 ± 0.03 | 0.06 |
| FSRCsn, fraction/h | 0.26 ± 0.05 | 0.26 ± 0.04 | 0.87 |
All values are means ± SDs (n = 10). Data were analyzed by using the paired t test. Csn, cystathionine; FSR, fractional synthesis rate; Hcy, homocysteine; Q, flux; QC, flux of carbon skeleton of methionine; QM, flux of methyl group of methionine.
Not significantly different from baseline, P > 0.05.
No differences were shown between before and after vitamin B-6 supplementation for total homocysteine remethylation, the remethylation from serine one-carbon units, the percentage of remethylation from serine, and the percentage of serine flux used for total remethylation (Table 3). In addition, the FSRHcy and the fractional synthesis rate of cystathionine were not significantly affected by supplementation (Table 3), although note that the mean FSRHcy value showed an apparent 10.5% reduction after supplementation, which exhibited a trend toward significance (P = 0.06). Because the study had sufficient power to detect a 17% change, this result must be interpreted with caution.
Vitamin B-6 supplementation did not change concentrations of preprandial homocysteine, cystathionine, cysteine, glutathione, and the glutathione degradation product cysteinylglycine (Table 2). There were also no changes in plasma concentrations of serine or methionine after supplementation. However, decrements were measured in plasma leucine concentrations from 103 ± 16.7 to 90.3 ± 20.3 nmol/L (P = 0.007) and glycine concentrations from 317 ± 62.5 to 267 ± 58.3 nmol/L (P = 0.03) after supplementation. The ratio of glycine:serine was also lower after supplementation (P = 0.01).
Targeted metabolite-profile analysis
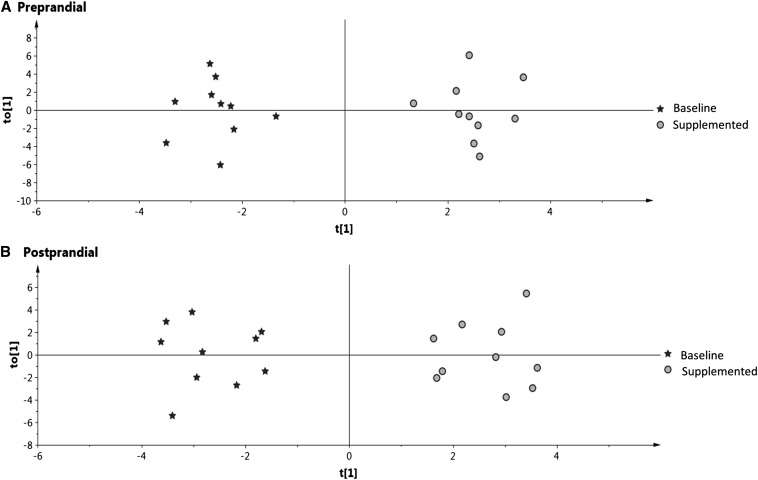
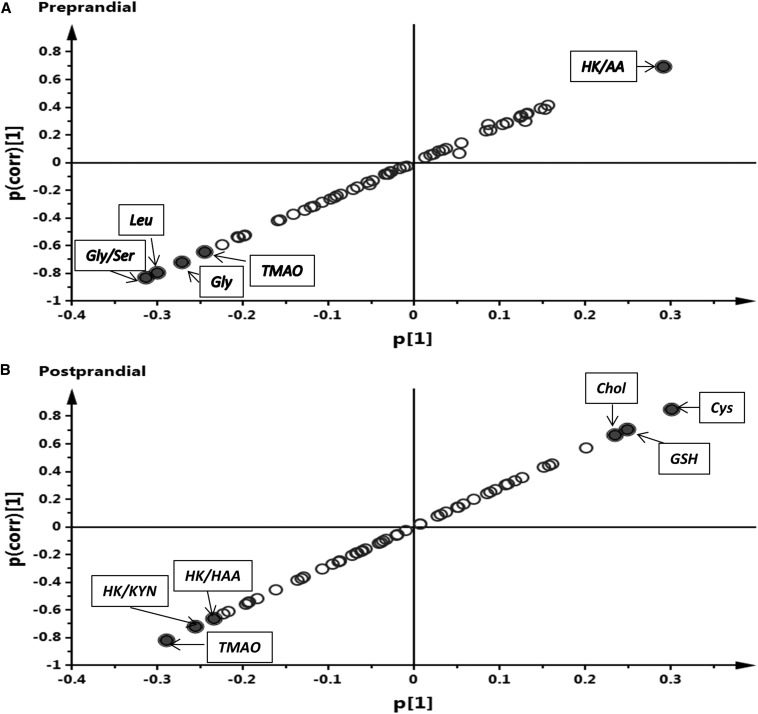
We used an OPLS-DA, which is a supervised method that has classes identified (i.e., before and after supplementation). The visualization of results was done by using score plots (Figure 2), S-plots (Figure 3), and loading column plots from the OPLS-DA analysis (Supplemental Figure 3). Score plots showed a clear grouping according to vitamin B-6 status for preprandial (Figure 2A) and postprandial (Figure 2B) states, which showed the overall effect of supplementation on the aggregate metabolite profiles. The overall good fit and predictability of the OPLS-DA models were indicated for the preprandial state [R2(cum) = 0.962, Q2(cum) = 0.905] and the postprandial state [R2(cum) = 0.928, Q2(cum) = 0.847). For the preprandial state, the significant discriminating metabolites were leucine, glycine, and trimethylamine N-oxide (TMAO) and the ratios glycine:serine and HK:AA (Figure 3A). For the postprandial state, the significant discrimination metabolites were cysteine, glutathione, choline, and TMAO and the ratios HK:Kyn and HK:HAA (Figure 3B).
FIGURE 2.
Score plots from a multilevel orthogonal partial least-squares–discriminant analysis for the overall pooled data of one-carbon metabolites and kinetic variables, tryptophan metabolites, and biomarkers of inflammation analyzed in plasma samples at baseline and after 28 d of vitamin B-6 supplementation in OC users. (A) Preprandial state. (B) Postprandial state. Each data point represents a function of the entire metabolite profile of each participant. The cumulative R2 and Q2 for the preprandial state were 0.962 and 0.905, respectively. The cumulative R2 and Q2 for the postprandial state were 0.928 and 0.847, respectively. t[1], principal component 1; to[1], orthogonal score vector 1.
FIGURE 3.
S-plots showing the relative contribution of variables for the separation between groups. (A) Preprandial state. (B) Postprandial state. The p(corr)[1] axis represents the correlation of the variables with the model classes, whereas the p[1] axis represents the magnitude of variables of the discriminating component of the orthogonal partial least-squares–discriminant analysis. Variables that showed a high p(corr)[1] (less than −0.6 and >0.6) and high p[1] were considered to be the variables that most strongly contributed to differences between groups, which are potential biomarkers (closed circles with labels). Negative p[1] values indicate variables that declined after pyridoxine supplementation, whereas positive p[1] values designate variables that increased because of supplementation. AA, anthranilic acid; Chol, choline; GSH, total glutathione; HAA, 3-hydroxyanthranilic acid; HK, 3-hydroxykynurenine; KYN, kynurenine; TMAO, trimethylamine N-oxide.
Results from the paired t test adjusted for multiple testing in the preprandial state showed no differences between before and after supplementation for the metabolites and ratios (Supplemental Tables 1–4). However, in the postprandial state cysteine was higher after supplementation (179 ± 16.3 compared with 195 ± 24.0 μmol/L after the supplementation; adjusted P = 0.04) (Supplemental Table 1).
Circulating concentrations of the inflammatory biomarkers neopterin and C-reactive protein and the kynurenine:tryptophan ratio or PAr index did not change after vitamin B-6 supplementation in either preprandial or postprandial states (Supplemental Table 4).
DISCUSSION
We investigated the effects of pyridoxine supplementation on one-carbon metabolism and the tryptophan catabolic pathway in vitamin B-6–deficient OC users. Our results showed little or no effect of supplementation on the in vivo fluxes of one-carbon metabolism and the transsulfuration reactions examined. The modest nonsignificant effect of supplementation on the FSRHcy (P = 0.06) was not associated with an alteration in the plasma homocysteine concentration or cystathionine kinetics. Despite the small sample size, differences in metabolite profiles before and after supplementation were observed. In addition, we showed that the use of a multivariate profile analysis was more powerful than are collective individual comparisons, which showed that this approach can be very useful in relatively small metabolic studies.
We showed that 13% of OC users had plasma PLP in the marginal deficiency range (20–30 nmol/L), and 3% of OC users had deficiency indicated by a plasma PLP concentration <20 nmol/L (26, 40). These percentages were much lower than those previously reported in studies that compared OC users with nonusers in the same age group (2, 41). Morris et al. (2) reported that 78% of OC users had a plasma PLP concentration <20 nmol/L compared with only 25% of nonusers, and Lussana et al. (41) reported that 44% of OC users had a plasma PLP concentration <21.7 nmol/L compared with 25% of nonusers. The low percentage of women who had low PLP in the current study could have been due to a higher overall dietary vitamin B-6 intake or to the intake of the growing number of products that contain added pyridoxine, including fortified foods and fortified beverages, compared with levels recorded approximately one decade earlier (2, 41). In addition, an overestimation of the number of vitamin B-6–deficient OC users in the study of Morris et al. (2) could have be due to the analytic method used. The National Center for Health Statistics reported that the enzymatic assay used in the study of Morris et al. (2) exhibited a systematic bias that yielded almost twice the number of low PLP results than did a validated HPLC method (42). Nevertheless, the occurrence of low vitamin B-6 status, even in a smaller subset of OC users, has the potential for negative health effects that must be evaluated.
The modest effects of pyridoxine supplementation on the in vivo kinetics examined in this group of OC users were consistent with previous findings regarding the effects of short-term dietary vitamin B-6 restriction (18, 19, 23). Together, the results showed the ability of the body to maintain fluxes of these critical processes during the reduced availability of PLP. Note that this protocol yielded a slightly higher percentage of homocysteine remethylation from serine relative to that for total remethylation. This result may have been due to the recycling of methionine in the methionine salvage pathway, also known as the 5′-methylthioadenosine cycle, which can recycle the infused [13C5]methionine via S-adenosylmethionine (43) to yield singly labeled [13C]methionine. A pilot study (JF Gregory; unpublished data, 2005) in which just [13C5]methionine was infused in the absence of [13C]serine showed the appearance of singly labeled methionine to a much-lesser extent than that observed when [13C]serine was concurrently infused. Another possible explanation for this observation could have been a minor error in the assumed correction factor of 0.4 that was used to predict intracellular serine enrichment in this calculation. Despite this apparent minor overestimation, these results strongly suggest that serine is the major donor of one-carbon groups used in homocysteine methylation, which was consistent with earlier findings (23, 30).
To our knowledge, no effects of estrogen on homocysteine remethylation, transmethylation fluxes, and plasma homocysteine were previously reported (44), but inconsistent effects of OCs on homocysteine concentration have been reported (27, 41, 45, 46). In this study, homocysteine concentrations were within the normal range (<12 μmol/L) in all subjects and was unchanged by supplementation.
The elevation of plasma cystathionine is a sensitive functional biomarker of vitamin B-6 insufficiency in animals (21, 47) and humans (18, 19, 48) because of the greater sensitivity of cystathionine γ-lyase than of cystathionine β-synthase to PLP insufficiency (21, 22). An elevation in cystathionine has occurred in vitamin B-6–restriction studies despite no change in the overall transsulfuration flux (19) or cysteine flux (48). In the current study, the baseline plasma cystathionine concentration was within the typical normal range seen in our studies. Moreover, cystathionine was not changed after supplementation, which suggested that the extent of vitamin B-6 insufficiency in our OC users was not sufficient to alter the activity of cystathionine γ-lyase.
Although the plasma cystathionine concentration and lack of response to supplementation suggested no functional effect of the low vitamin B-6 status by these criteria, plasma glycine and leucine concentrations and the glycine:serine ratio were significantly decreased by pyridoxine supplementation. Increased glycine has been reported in liver (49, 50), muscle, and plasma of vitamin B-6–deficient rats (50, 51), in the plasma of vitamin B-6–deficient humans (16, 18, 20, 23), and in vitamin B-6–restricted cultured cells (52), concurrent with modeling predictions (24). Major processes for the metabolism of glycine include PLP-dependent reactions catalyzed by serine hydroxymethyltransferase isoforms and by glycine decarboxylase in the glycine cleavage system (53). An elevation in glycine can be explained primarily by the reduced activity of glycine decarboxylase activity during vitamin B-6 deficiency (24). Short-term vitamin B-6 restriction has little effect on plasma leucine (16, 20). However, the chronic low vitamin B-6 status in this study likely reduced branched-chain aminotransferase activity that was restored by supplementation.
TMAO was identified as a discriminating metabolite that responded to supplementation. TMAO is produced from the oxidation of trimethylamine in the liver, which is not a PLP-dependent process. Increased TMAO has been associated with cardiovascular disease risk (54). Higher TMAO has been reported in female compared with male mice, suggesting hormonal effects on its metabolism (55). Increased TMAO also has been reported after vitamin B-6 restriction (17). The mechanism of increased TMAO and the role of cellular PLP requires additional investigation. Choline, which is a precursor of TMAO, was a discriminating biomarker with an increase after supplementation in the postprandial state. These findings suggest that the response of TMAO may be an indirect effect.
Our OPLS-DA analysis also showed that the tryptophan catabolic pathway was perturbed at the level of vitamin B-6 insufficiency of our participants. These results are in agreement with previous studies in animals (56), humans (6, 20, 25, 57), and mathematical modeling (58). Increased plasma HK and decreased KA have been reported after vitamin B-6 restriction (20). Increased plasma HK has also been reported in patients with systemic inflammation who had plasma PLP concentrations <20 nmol/L (25) and has been associated with inflammatory biomarkers (57). Unlike HK, the ratios HK:HAA, HK:KA, and HK:XA showed a strong inverse association with PLP but little or no association with inflammation, and thus, they have been proposed as better functional biomarkers of vitamin B-6 status (57). We recently showed the significant nonlinear negative association between PLP and the ratios HK:HAA and HK:KA in OC users (27). In the current study, the ratios HK:HAA, HK:Kyn, and HK:AA were identified as discriminating biomarkers that responded to supplementation. The higher response of HK:AA in this study is in contrast to the lower sensitivity of HK:Kyn than of other ratios reported recently (57). A mathematical simulation supported these findings (58).
Although the main objective of this study was to determine the effect of long-term vitamin B-6 deficiency associated with OC use, we tried to clarify whether the apparently low status shown in this group of women was due to low dietary vitamin B-6 intake or potentially the result of OC use. On the basis of the dietary evaluation, the cause of the deficiency remained inconclusive.
In conclusion, the fluxes of one-carbon metabolic processes examined in this study exhibit little or no change because of supplementation. However, changes in metabolite concentrations including glycine and leucine and the response of the ratios glycine:serine, HK:HAA, HK:Kyn, and HK:AA after supplementation indicate that functional metabolic consequences occur from this level of vitamin B-6 insufficiency. Vitamin B-6 supplementation would be appropriate for such individuals who chronically use OCs.
Acknowledgments
The authors’ responsibilities were as follows—LR-A, BC, MR, ØM, and PMU: conducted the research; JFG and PWS: designed the research; LR-A, Y-YC, and JFG: analyzed data; LR-A and JFG: wrote the manuscript; and all authors: read and approved the final manuscript. None of the authors reported a conflict of interest related to the study.
Footnotes
Abbreviations used: AA, anthranilic acid; CRC, Clinical Research Center; FSRHcy, fractional synthesis rate of homocysteine; HAA, 3-hydroxyanthranilic acid; HK, 3-hydroxykynurenine; KA, kynurenic acid; Kyn, kynurenine; OC, oral contraceptive; OPLS-DA, orthogonal partial least-squares–discriminant analysis; PLP, pyridoxal 5′-phosphate; TMAO, trimethylamine N-oxide; XA, xanthurenic acid.
REFERENCES
- 1.U.S. Center for Disease Control and Prevention. Second national report on biochemical indicators of diet and nutrition in the U.S. population. Atlanta (GA): Department of Health and Human Services; 2012. [Google Scholar]
- 2.Morris MS, Picciano MF, Jacques PF, Selhub J. Plasma pyridoxal 5′-phosphate in the US population: the National Health and Nutrition Examination Survey, 2003-2004. Am J Clin Nutr 2008;87:1446–54. [DOI] [PubMed] [Google Scholar]
- 3.Chiang EP, Bagley PJ, Selhub J, Nadeau M, Roubenoff R. Abnormal vitamin B(6) status is associated with severity of symptoms in patients with rheumatoid arthritis. Am J Med 2003;114:283–7. [DOI] [PubMed] [Google Scholar]
- 4.Saibeni S, Cattaneo M, Vecchi M, Zighetti M, Lecchi A, Lombardi R, Meucci G, Spina L, de Franchis R. Low vitamin B(6) plasma levels, a risk factor for thrombosis, in inflammatory bowel disease: role of inflammation and correlation with acute phase reactants. Am J Gastroenterol 2003;98:112–7. [DOI] [PubMed] [Google Scholar]
- 5.Chiang EP, Smith DE, Selhub J, Dallal G, Wang YC, Roubenoff R. Inflammation causes tissue-specific depletion of vitamin B6. Arthritis Res Ther 2005;7:R1254–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ulvik A, Midttun Ø, Pedersen ER, Nygård O, Ueland PM. Association of plasma B-6 vitamers with systemic markers of inflammation before and after pyridoxine treatment in patients with stable angina pectoris. Am J Clin Nutr 2012;95:1072–8. [DOI] [PubMed] [Google Scholar]
- 7.Friso S, Girelli D, Martinelli N, Olivieri O, Lotto V, Bozzini C, Pizzolo F, Faccini G, Beltrame F, Corrocher R. Low plasma vitamin B-6 concentrations and modulation of coronary artery disease risk. Am J Clin Nutr 2004;79:992–8. [DOI] [PubMed] [Google Scholar]
- 8.Lin PT, Cheng CH, Liaw YP, Lee BJ, Lee TW, Huang YC. Low pyridoxal 5′-phosphate is associated with increased risk of coronary artery disease. Nutrition 2006;22:1146–51. [DOI] [PubMed] [Google Scholar]
- 9.Robinson K, Arheart K, Refsum H, Brattstrom L, Boers G, Ueland P, Rubba P, Palma-Reis R, Meleady R, Daly L, et al. Low circulating folate and vitamin B6 concentrations: risk factors for stroke, peripheral vascular disease, and coronary artery disease. European COMAC Group. Circulation 1998;97:437–43. [DOI] [PubMed] [Google Scholar]
- 10.Kelly PJ, Shih VE, Kistler JP, Barron M, Lee H, Mandell R, Furie KL. Low vitamin B6 but not homocyst(e)ine is associated with increased risk of stroke and transient ischemic attack in the era of folic acid grain fortification. Stroke 2003;34:e51–4. [DOI] [PubMed] [Google Scholar]
- 11.Frumento G, Rotondo R, Tonetti M, Damonte G, Benatti U, Ferrara GB. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J Exp Med 2002;196:459–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morita T, Saito K, Takemura M, Maekawa N, Fujigaki S, Fujii H, Wada H, Takeuchi S, Noma A, Seishima M. 3-Hydroxyanthranilic acid, an L-tryptophan metabolite, induces apoptosis in monocyte-derived cells stimulated by interferon-gamma. Ann Clin Biochem 2001;38:242–51. [DOI] [PubMed]
- 13.Schwarcz R, Bruno JP, Muchowski PJ, Wu HQ. Kynurenines in the mammalian brain: when physiology meets pathology. Nat Rev Neurosci 2012;13:465–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pedersen ER, Tuseth N, Eussen SJ, Ueland PM, Strand E, Svingen GF, Midttun Ø, Meyer K, Mellgren G, Ulvik A, et al. Associations of plasma kynurenines with risk of acute myocardial infarction in patients with stable angina pectoris. Arterioscler Thromb Vasc Biol 2015;35:455–62. [DOI] [PubMed] [Google Scholar]
- 15.Park YK, Linkswiler H. Effect of vitamin B6 depletion in adult man on the excretion of cystathionine and other methionine metabolites. J Nutr 1970;100:110–6. [DOI] [PubMed] [Google Scholar]
- 16.Park YK, Linkswiler H. Effect of vitamin B6 depletion in adult man on the plasma concentration and the urinary excretion of free amino acids. J Nutr 1971;101:185–91. [DOI] [PubMed] [Google Scholar]
- 17.Gregory JF, Park Y, Lamers Y, Bandyopadhyay N, Chi Y-Y, Lee K, Kim S, da Silva V, Hove N, Ranka S, et al. Metabolomic analysis reveals extended metabolic consequences of marginal vitamin B-6 deficiency in healthy human subjects. PLoS One 2013;8:e63544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lamers Y, Williamson J, Ralat M, Quinlivan EP, Gilbert LR, Keeling C, Stevens RD, Newgard CB, Ueland PM, Meyer K, et al. Moderate dietary vitamin B-6 restriction raises plasma glycine and cystathionine concentrations while minimally affecting the rates of glycine turnover and glycine cleavage in healthy men and women. J Nutr 2009;139:452–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lamers Y, Coats B, Ralat M, Quinlivan EP, Stacpoole PW, Gregory JF. Moderate vitamin B-6 restriction does not alter postprandial methionine cycle rates of remethylation, transmethylation, and total transsulfuration but increases the fractional synthesis rate of cystathionine in healthy young men and women. J Nutr 2011;141:835–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.da Silva VR, Rios-Avila L, Lamers Y, Ralat M, Middtun Ø, Quinlivan EP, Garrett T, Coats B, Shankar M, Percival S, et al. Metabolite profile analysis reveals functional effects of 28-day vitamin B-6 restriction on one-carbon metabolism and tryptophan catabolic pathways in healthy men and women. J Nutr 2013;143:1719–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lima CP, Davis SR, Mackey AD, Scheer JB, Williamson J, Gregory JFI. Vitamin B-6 deficiency suppresses the hepatic transsulfuration pathway but increases glutathione concentration in rats fed AIN-76A or AIN-93G diets. J Nutr 2006;136:2141–7. [DOI] [PubMed] [Google Scholar]
- 22.Finkelstein JD, Chalmers FT. Pyridoxine effects on cystathionine synthase in rat liver. J Nutr 1970;100:467–9. [DOI] [PubMed] [Google Scholar]
- 23.Davis SR, Scheer JB, Quinlivan EP, Coats BS, Stacpoole PW, Gregory JF. Dietary vitamin B-6 restriction does not alter rates of homocysteine remethylation or synthesis in healthy young women and men. Am J Clin Nutr 2005;81:648–55. [DOI] [PubMed] [Google Scholar]
- 24.Nijhout HF, Gregory JF, Fitzpatrick C, Cho E, Lamers KY, Ulrich CM, Reed MC. A mathematical model gives insights into the effects of vitamin B-6 deficiency on 1-carbon and glutathione metabolism. J Nutr 2009;139:784–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Midttun O, Ulvik A, Ringdal Pedersen E, Ebbing M, Bleiei O, Schartum-Hansen H, Nilsen RM, Nygard O, Ueland PM. Low plasma vitamin B-6 status affects metabolism through the kynurenine pathway in cardiovascular patients with systemic inflammation. J Nutr 2011;141:611–7. [DOI] [PubMed] [Google Scholar]
- 26.Leklem JE. Vitamin B-6: a status report. J Nutr 1990;120(Suppl 11):1503–7. [DOI] [PubMed] [Google Scholar]
- 27.Rios-Avila L, Coats B, Chi YY, Middtun O, Ueland PM, Stacpoole PW, Gregory JF. Metabolite profile analysis reveals association of vitamin B-6 with metabolites related to one-carbon metabolism and tryptophan catabolism but not with biomarkers of inflammation in oral contraceptive users and reveals the effects of oral contraceptives on these processes. J Nutr 2015;145:87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ubbink JB, Serfontein WJ, de Villiers LS. Stability of pyridoxal-5-phosphate semicarbazone: applications in plasma vitamin B6 analysis and population surveys of vitamin B6 nutritional status. J Chromatogr 1985;342:277–84. [DOI] [PubMed] [Google Scholar]
- 29.Pfeiffer CM, Huff DL, Gunter EW. Rapid and accurate HPLC assay for plasma total homocysteine and cysteine in a clinical laboratory setting. Clin Chem 1999;45:290–2. [PubMed] [Google Scholar]
- 30.Davis SR, Stacpoole PW, Williamson J, Kick L, Quinlivan E, Coats B, Shane B, Bailey L, Gregory JF. Tracer-derived total and folate-dependent homocysteine remethylation and synthesis rates in humans indicate that serine is the main one-carbon donor. Am J Physiol Endocrinol Metab 2004;286:E272–9. [DOI] [PubMed] [Google Scholar]
- 31.Midttun Ø, Hustad S, Ueland PM. Quantitative profiling of biomarkers related to B-vitamin status, tryptophan metabolism and inflammation in human plasma by liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom 2009;23:1371–9. [DOI] [PubMed] [Google Scholar]
- 32.Böger RH, Bode-Böger SM, Szuba A, Tsao PS, Chan JR, Tangphao O, Blaschke TF, Cooke JP. Asymmetric dimethylarginine (ADMA): a novel risk factor for endothelial dysfunction: its role in hypercholesterolemia. Circulation 1998;98:1842–7. [DOI] [PubMed] [Google Scholar]
- 33.Ulvik A, Midttun O, Pedersen ER, Eussen SJ, Nygard O, Ueland PM. Evidence for increased catabolism of vitamin B-6 during systemic inflammation. Am J Clin Nutr 2014;100:250–5. [DOI] [PubMed] [Google Scholar]
- 34.Storch KJ, Wagner DA, Burke JF, Young VR. Quantitative study in vivo of methionine cycle in humans using [methyl-2H3]- and [1-13C]methionine. Am J Physiol 1988;255:E322–31. [DOI] [PubMed] [Google Scholar]
- 35.MacCoss MJ, Fukagawa NK, Matthews DE. Measurement of intracellular sulfur amino acid metabolism in humans. Am J Physiol Endocrinol Metab 2001;280:E947–55. [DOI] [PubMed] [Google Scholar]
- 36.Eriksson LBT, Byrne T, Johansson E, Trygg J, Vikstrom C. Multi-and megavariate data analysis. Basic principles and applications. 3rd revised ed. Malmo (Sweden) Sweden: MKS Umetrics AB; 2013. [Google Scholar]
- 37.Westerhuis JA, van Velzen EJ, Hoefsloot HC, Smilde AK. Multivariate paired data analysis: multilevel PLSDA versus OPLSDA. Metabolomics 2010;6:119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wiklund S, Johansson E, Sjostrom L, Mellerowicz E, Edlund U, Shockcor J, Gottfires J, Moritz T, Trygg J. Visualization of GC/TOF-MS-based metabolomics data for identification of biochemically interesting compounds using OPLS class models. Anal Chem 2008;80:115–22. [DOI] [PubMed] [Google Scholar]
- 39.Timm NH. Applied multivariate analysis. New York: Springer-Verlag New York Inc.; 2002. [Google Scholar]
- 40.Institute of Medicine. Dietary reference intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. Washington (DC): The National Academies Press; 1998. [PubMed] [Google Scholar]
- 41.Lussana F, Zighetti ML, Bucciarelli P, Cugno M, Cattaneo M. Blood levels of homocysteine, folate, vitamin B6 and B12 in women using oral contraceptives compared to non-users. Thromb Res 2003;112:37–41. [DOI] [PubMed] [Google Scholar]
- 42.National Center for Health Statistics [Internet]. Center for Disease Control and Prevention, National Health and Nutrition Examination Survey (NHANES). Data documentation, codebook, and frequencies. Laboratory component. Vitamin B6, Survey years: 2005-2006 [Internet]. 2010 Sep. Available from: http://www.cdc.gov/nchs/nhanes/2005-2006/VIT_B6_D.htm.
- 43.Albers E. Metabolic characteristics and importance of the universal methionine salvage pathway recycling methionine from 5′-methylthioadenosine. IUBMB Life 2009;61:1132–42. [DOI] [PubMed] [Google Scholar]
- 44.Smolders RG, de Meer K, Kenemans P, Jakobs C, Kulik W, van der Mooren MJ. Oral estradiol decreases plasma homocysteine, vitamin B6, and albumin in postmenopausal women but does not change the whole-body homocysteine remethylation and transmethylation flux. J Clin Endocrinol Metab 2005;90:2218–24. [DOI] [PubMed] [Google Scholar]
- 45.Norouzi V, Seifi M, Fallah S, Korani M, Samadikuchaksaraei A. Effect of oral contraceptive therapy on homocysteine and C-reactive protein levels in women: an observational study. Anadolu Kardiyol Derg 2011;11:698–702. [DOI] [PubMed] [Google Scholar]
- 46.Cauci S, Di Santolo M, Culhane JF, Stel G, Gonano F, Guaschino S. Effects of third-generation oral contraceptives on high-sensitivity C-reactive protein and homocysteine in young women. Obstet Gynecol 2008;111:857–64. [DOI] [PubMed] [Google Scholar]
- 47.Sally S, David S, Li-Ping W, Robert A. Elevations of serum cystathionine and total homocysteine in pyridoxine-, folate-, and cobalamin-deficient rats. Nutr Biochem 1997;8:279–89. [Google Scholar]
- 48.Davis SR, Quinlivan EP, Stacpoole PW, Gregory JF. Plasma glutathione and cystathionine concentrations are elevated but cysteine flux is unchanged by dietary vitamin B-6 restriction in young men and women. J Nutr 2006;136:373–8. [DOI] [PubMed] [Google Scholar]
- 49.Scheer JB, Mackey AD, Gregory JF 3rd. Activities of hepatic cytosolic and mitochondrial forms of serine hydroxymethyltransferase and hepatic glycine concentration are affected by vitamin B-6 intake in rats. J Nutr 2005;135:233–8. [DOI] [PubMed] [Google Scholar]
- 50.Swendseid ME, Villalobos J, Friedrich B. Free amino acids in plasma and tissues of rats fed a vitamin B6-deficient diet. J Nutr 1964;82:206–8. [DOI] [PubMed] [Google Scholar]
- 51.Runyan TJ, Gershoff SN. Glycine metabolism in vitamin B6-deficient and deoxypyridoxine-treated rats. J Nutr 1969;98:113–8. [DOI] [PubMed] [Google Scholar]
- 52.da Silva VR, Ralat MA, Quinlivan EP, Deratt BN, Garrett TJ, Chi Y-Y, Nijhout F, Reed MC, Gregory JF. Targeted metabolomics and mathematical modeling demonstrate that vitamin B-6 restriction alters one-carbon metabolism in cultured HepG2 cells. Am J Physiol Endocrinol Metab 2014;307:E93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kikuchi G. The glycine cleavage system: composition, reaction mechanism, and physiological significance. Mol Cell Biochem 1973;1:169–87. [DOI] [PubMed] [Google Scholar]
- 54.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011;472:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bennett BJ, de Aguiar Vallim TQ, Wang Z, Shih DM, Meng Y, Gregory J, Allayee H, Lee R, Graham M, Crooke R, et al. Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab 2013;17:49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yeh JK, Brown RR. Effects of vitamin B-6 deficiency and tryptophan loading on urinary excretion of tryptophan metabolites in mammals. J Nutr 1977;107:261–71. [DOI] [PubMed] [Google Scholar]
- 57.Ulvik A, Theofylaktopoulou D, Midttun O, Nygard O, Eussen SJ, Ueland PM. Substrate product ratios of enzymes in the kynurenine pathway measured in plasma as indicators of functional vitamin B-6 status. Am J Clin Nutr 2013;98:934–40. [DOI] [PubMed] [Google Scholar]
- 58.Rios-Avila L, Nijhout HF, Reed MC, Sitren HS, Gregory JF 3rd. A mathematical model of tryptophan metabolism via the kynurenine pathway provides insights into the effects of vitamin B-6 deficiency, tryptophan loading, and induction of tryptophan 2,3-dioxygenase on tryptophan metabolites. J Nutr 2013;143:1509–19. [DOI] [PMC free article] [PubMed] [Google Scholar]