Abstract
A GO game can enhance mental health, but its effects on Alzheimer Disease (AD) remains unknown. To address the issue, 147 AD patients were randomly assigned into control (without GO-game intervention), Short-time GO-Game Intervention (SGGI, 1 h daily) and Long-time GO-game Intervention (LGGI, 2 h daily) groups. After 6-month follow-up, the game reduced the mean score of Montgomery-Asberg Depression Rating Scales (MADRS) of 4.72 (95% CI, 0.69 to 9.12) and Hospital Anxiety and Depression Scale (HADS) of 1.75 (95% CI, 0.17–3.68), and increased the mean score of Global Assessment of Functioning (GAF) of 4.95 (95% CI, −1.37–9.18) and RAND-36 of 4.61 (95% CI, −2.75–11.32) (P < 0.05 via controls). A GO-game intervention improved 9 of 11 items of KICA-dep (Kimberley Indigenous Cognitive Assessment of Depression). Meanwhile, serum levels of brain derived neurotrophic factor (BDNF) were higher in SGGI and LGGI groups (24.02 ± 7.16 and 28.88 ± 4.12 ng/ml respectively, P = 0.051) than those in controls (17.28 ± 7.75 ng/ml) (P < 0.001). The serum levels of BDNF showed a negative relation with MADRS and a positive relation with RAND-36 (P < 0.01). A GO-game intervention ameliorates AD manifestations by up-regulating BDNF levels.
Keywords: Alzheimer's disease, brain-derived neurotrophic factor, GO game, Montgomery-Asberg Depression Rating Scale, RAND-36
Introduction
Alzheimer's disease (AD) is one leading cause of dementia and affects more than 35 million people in the world (Gregori et al., 2015; Hamilton et al., 2015). AD is a kind of neurodegenerative disorder and mainly characterized by extracellular senile plaques and intracellular neurofibrillary tangles. The high incidence and financial global burden of AD manifestations greatly reduce the quality of elderly life. However, the etiology of AD is complex and medicine therapy is still the mainstay for AD patients. Cholinesterase inhibitor and memantine (1-amino-3,5-dimethyl-adamantane) are often used for the conventional therapy of AD patients (Ahn et al., 2014; Dysken et al., 2014; Evans et al., 2014). However, all the medicine has significant side effects. Cholinesterase inhibitors cause the side effects, such as bradycardia (Leikin et al., 2014), hypotension (van Beek et al., 2010), and low intraocular pressure (Sawada et al., 1971). Memantine causes common adverse effects, such as confusion, dizziness, drowsiness, headache, insomnia and hallucinations, and some less common adverse effects including vomiting, anxiety, hypertonia, cystitis and libido (Rossom et al., 2004; Jain et al., 2008). Furthermore, it can induce reversible neurological impairment in sclerosis (an inflammatory disease in the brain and spinal cord) patients, resulting in the halt of a clinical trial (Villoslada et al., 2009). High cost of the medicine and serious unwanted adverse effects, have limited its utilization. Therefore, it is highly demanded to explore low-cost non-pharmaceutical method for AD patients.
Brain exercises are widely known approaches to prevent dementia, increase neurogenesis and protect neurons (Gatz, 2005). Physicians often advise the elderly to engage in a mentally challenging activity to reduce dementia risk. The elderly spend time solving crossword puzzles, anagrams and figural logic puzzles, and may feel well if the activities are both challenging and successfully completed. More education has been found to be related to a lower incidence of AD. Typically, the risk of AD is 2–4 times higher in the persons with less education than those with more education (Qiu et al., 2001). On the other hand, the elderly participate in leisure activities, especially for mental stimulation, will also have a lower incidence of AD (Provencher et al., 2009). In addition, longitudinal studies have showed older adults have less dementia if they participate in intellectually challenging activity (Hultsch et al., 1999).
The GO game, a kind of Chinese chesses, has been practiced as a brain activity for more than 5000 years. Playing the game involves many aspects of cognition and improves mental health (Kim et al., 2014). A GO game involves the changes associated with many cognitive functions, including learning, abstract reasoning, and self-control, which facilitate cognitive behavioral therapy (Lee et al., 2010). However, the functional role of a GO game in AD patients remains elusive. Furthermore, brain derived neurotrophic factor (BDNF) is often deficient in AD patients or animal AD models (Voineskos et al., 2011; Lee et al., 2012; Boiocchi et al., 2013). BDNF protects cerebral cortical neurons against beta-amyloid 25–35 (the major AD toxic peptide) and inhibits beta-amyloid 25–35 aggregation in the brain (Xiao et al., 2010; Zhang et al., 2012). A GO game may ameliorate AD patients by affecting the levels of BDNF since BDNF plays a critical role in learning and memory functions (Fan et al., 2014). Thus, we explored the functions of a GO game in AD patients and measured BDNF levels in AD patients before and after the study.
Materials and methods
Participants
All the protocols were approved by the Institutional Ethics Committee from the Fourth Affiliated Hospital of China Medical University and conducted in accordance with the Declaration of Helsinki (Palacios, 2013). All subjects were Han Chinese from Shenyang city (China). An informed consent was obtained either from each subject or from his or her guardian. All AD patients were diagnosed with NINCDS-ADRDA (National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association) criteria according to a previous report (Tamaoka, 2011). All familial cases of AD were excluded in this study. The patients were excluded if they met any of the following criteria: (1) a history of suicidal behavior; (2) substance abuse; (3) verbal communication difficulty; (4) a family history of AD; (5) brain injury or other brain disorders; (6) disliking playing a GO game. From July 7th, 2014 to January 8th, 2015, a total of 147 AD patients were recruited at the Fourth Affiliated Hospital of China Medical University (Shenyang, China).
Study design
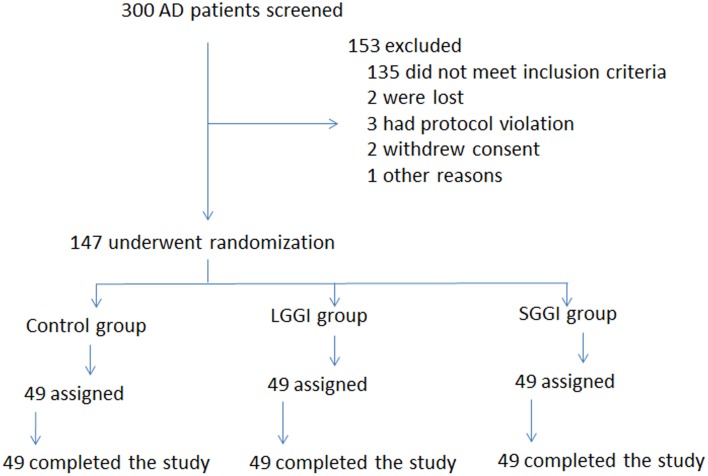
After scanning all AD patients, 147 patients were eligible to enter designated 6-month follow-up (Figure 1). A randomized and controlled trial was conducted using three groups: control (without GO-game intervention, n = 49), short-time GO-game intervention (SGGI, 1 h daily, n = 49) and long-time GO-game intervention (LGGI, 2 h daily, n = 49) groups. The three groups have the similar gender ratio, age, BMI (body mass index, the ratio of weight (kg) to height in meters squared), years of education, alcohol drinking, cigarette smoking, and diabetes. Each of them has a spouse and there is a good mutual relation. Among the AD patients, some subjects have complex disorders such as comorbid diabetes, depression, and overeating (Brownley et al., 2015).
Figure 1.
The flowchart of the study. LGGI, a long-time GO game intervention. SGGI, a short-time GO game intervention. Control group, without a GO game intervention.
To avoid the interference of the experiences for playing a GO game, none of them had ever played a GO game before the study. Before game training, all subjects from GO-game groups were invited to learn the rules for playing the game from websites, such as http://en.wikipedia.org/wiki/GO_(game). All participants were trained by a game player in the same club. After mastering the basic rules, each person from GO-game groups can finish the game as Figure 2 showed. In GO groups, two partners were combined randomly. If one patient could not find a partner, he or she watched the game played by other participants. Otherwise, he or she would play with one of GO-game staff.
Figure 2.
GO game profile.
Neuropsychological tests
According to Diagnostic and Statistical Manual of Mental Disorders (DSM), all the cases were diagnosed by licensed neurological physicians including neurological examination, blood tests, and neuroimaging data (computed tomography or magnetic resonance imaging). Clinical Dementia Rating (CDR) scale is often used to detect memory and executive functions (Inoue et al., 2012). Furthermore, CDR is used not only to detect memory impairment but also to quantify dementia severity. CDR is also used to assess domains of cognition (memory, orientation and problem solving) and the domains of functions (community affairs, home, and hobbies and personal care) (Cedarbaum et al., 2013). Furthermore, the Mini Mental State Examination (MMSE) has been widely used for testing memory problems. Clinicians often diagnose dementia and assess its progression and severity by MMSE scores.
Depression is a frequent condition for AD patients, so the depressive severity was examined based on the Montgomery and Asberg Depression Rating Scale (MADRS) (Alonzo et al., 2013). There are 10 items for MADRS scoring from 0 to 60. The severities of depression were regarded as severe, moderate and mild based on different cut-off scores (Birnbaum et al., 2009). Furthermore, all participants was evaluated with the Kimberley Indigenous Cognitive Assessment of Depression (KICA-Dep) (Salvatore et al., 2013). There are 11 items for KICA-Dep, each of which is assigned as from “never” to “sometimes,” “a lot” and “all the time” based on a frequency scale.
Some AD patients suffer from anxiety disorder (Ramakers et al., 2013; Mormont et al., 2014) and anxiety was measured based on Hospital Anxiety and Depression Scale (HADS) (Hinz et al., 2014). The scores of HADS are presented as from 0 to 21, and higher scores stand for more severe anxiety. General functioning was evaluated by a Global Assessment of Functioning (GAF) scale (Mello et al., 2007). Life quality was assessed using RAND-36 (Mattila et al., 2012), which counts for wellbeing and functioning in eight dimensions. Alexithymia is a psychological problem and was evaluated using the Toronto Alexithymia Scale–20 (TAS-20) (Melin et al., 2010).
ELISA analysis of BDNF
To determine serum levels of BDNF, 5 ml of blood samples were taken (8:00–11:00 a.m.) by venipuncture into a free-anticoagulant vacuum tube at before and after 6-month follow-up. The blood samples were centrifuged at 3000 g for 10 min, and serum was isolated and kept at −80°C until next step. The serum levels of BDNF were measured using a human BDNF ELISA kit (Adipo Bioscience, Santa Clara, CA, USA).
Statistical analyses
All statistical analyses were performed with a SPSS 20.0 package (IBM China Company Limited, Beijing, China). The AD patients were compared by t-tests between GO groups and the control group without a GO intervention. One-Way ANOVA was used to explore the effect of a GO game on the levels of BDNF in AD patients. Spearman's rank correlation coefficient was conducted to identify the correlation between the depressive severity (Montgomery-Asberg Depression Rating Scale or RAND-36) and BDNF levels. There were statistically significant differences if P < 0.05.
Results
The baseline characters of AD patients
All 147 AD patients had medical histories consistent with AD and took neurological examinations (Supplemental Table 1). Controls were matched well to AD patients by gender and age at onset (P > 0.05). A previous report has found that BMI is associated with the risk of AD (Besser et al., 2014). To avoid the effects of BMI on present results, all patients were selected to guarantee that there were no statistically significant differences among three groups (P > 0.05) (Supplemental Table 1). Diabetes is an increasing epidemic and affects millions of the elderly worldwide. The chronic disease also affects brain function and contributes to an AD risk (Butterfield et al., 2014). Similarly, high blood pressure and coronary heart disease are also contributing factors for the development of AD (Qiu et al., 2006; Lattanzi et al., 2014). Furthermore, cigarette smoking has been reported to be also associated with incipient AD (Chang et al., 2012). Alcohol drinking can impair brain function, and cause dementia and geriatric cognitive disorders (Wiscott et al., 2001). The patients with long-period education have better cognitive functions than the AD patients with short-period education (Pradier et al., 2014). Furthermore, the anterior temporal lobes play an important role in melody recognition, and that music can significantly affect AD patients (Johnson et al., 2011). To avoid these interfering factors, all the elements were carefully examined to exclude their effects on final results (Supplemental Table 1). In neuropsychological tests, all AD patients had MMSE scores less than 26 and the CDR scale of 0.5 or over 0.5 (Supplemental Table 1). The results indicated that all AD subjects had clinical dementia. Similarly, all AD patients had the similar psychiatric test values and the prevalence of KICA-dep items in AD patients among LGGI, SGGI, and control groups (P > 0.05) (Supplemental Table 1 and Table 1). Therefore, the baseline characters of patients were basically similar among three groups.
Table 1.
The comparison for Prevalence of KICA-Dep items among AD patients.
| KICA-Dep Items | Response | Control group | SGGI group | LGGI group | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline N(%) | 6 months N(%) | P-value | Baseline N(%) | 6 months N(%) | P-value | Baseline N(%) | 6 months N(%) | P-value | ||
| Felt down, sad, no good | Never | 5(10.2) | 5(11.3) | 0.587 | 5(10.2) | 10(20.8) | 0.001 | 5(10.2) | 12(25.0) | 0.001 |
| Sometimes | 30(61.2) | 26(59.1) | 0.182 | 29(59.2) | 30(62.5) | 0.183 | 28(57.1) | 31(64.6) | 0.043 | |
| A lot | 5(10.2) | 5(11.3) | 0.210 | 5(10.2) | 3(6.3) | 0.002 | 6(12.2) | 2(4.2) | 0.001 | |
| All the same | 9(18.4) | 8(18.2) | 0.859 | 10(20.4) | 5(10.4) | 0.001 | 10(20.4) | 2(4.2) | 0.001 | |
| Felt like doing things that you usually like doing | Never | 5(10.2) | 5(11.4) | 0.066 | 6(12.2) | 4(8.3) | 0.006 | 5(10.2) | 4(8.3) | 0.004 |
| Sometimes | 25(51.0) | 22(50.0) | 0.393 | 25(51.0) | 20(41.6) | 0.002 | 25(51.0) | 19(39.6) | 0.002 | |
| A lot | 6(12.2) | 5(11.4) | 0.299 | 6(12.2) | 7(14.6) | 0.015 | 6(12.2) | 8(16.7) | 0.005 | |
| All the same | 13(26.5) | 12(27.3) | 0.180 | 12(24.5) | 17(35.4) | 0.001 | 13(26.5) | 17(35.4) | 0.001 | |
| Had trouble going to sleep, staying asleep, or sleeping too much | Never | 5(10.2) | 5(11.4) | 0.804 | 5(10.2) | 18(37.5) | 0.001 | 5(10.2) | 20(41.7) | 0.001 |
| Sometimes | 30(61.2) | 26(59.1) | 0.185 | 29(59.2) | 20(41.7) | 0.001 | 30(61.2) | 18(37.5) | 0.001 | |
| A lot | 4(8.2) | 3(6.1) | 0.119 | 4(8.2) | 3(6.3) | 0.048 | 5(10.2) | 4(8.2) | 0.008 | |
| All the same | 10(20.4) | 6(22.7) | 0.083 | 11(22.4) | 7(14.6) | 0.001 | 9(18.4) | 6(12.5) | 0.001 | |
| Felt tired or slack, and had no energy | Never | 7(15) | 10(20.0) | 0.854 | 13(16.3) | 21(27.5) | 0.085 | 13(16.3) | 21(27.5) | 0.085 |
| Sometimes | 24(50) | 28(57.5) | 0.341 | 39(48.7) | 37(49.3) | 0.874 | 39(48.7) | 37(49.3) | 0.874 | |
| A lot | 12(25) | 8(17.5) | 0.246 | 19(23.8) | 14(18.8) | 0.440 | 19(23.8) | 14(18.8) | 0.440 | |
| All the same | 6(10) | 2(5.0) | 0.230 | 9(11.2) | 3(3.8) | 0.072 | 9(11.2) | 3(3.8) | 0.072 | |
| Eating too much or eating only a little bit. | Never | 5(10.2) | 4(9.1) | 0.197 | 5(10.2) | 15(31.3) | 0.001 | 5(10.2) | 16(33.3) | 0.001 |
| Sometimes | 4(8.2) | 4(9.1) | 0.133 | 5(10.2) | 25(52.1) | 0.001 | 4(8.2) | 25(52.1) | 0.001 | |
| A lot | 16(32.7) | 14(31.8) | 0.210 | 15(30.6) | 4(8.3) | 0.001 | 16(32.7) | 4(8.3) | 0.001 | |
| All the same | 24(49) | 22(50) | 0.512 | 24(49) | 4(8.3) | 0.001 | 24(49) | 3(6.3) | 0.001 | |
| Felt bad or shamed that you let yourself or your family down. | Never | 8(16.3) | 7(15.9) | 0.514 | 7(14.3) | 17(35.4) | 0.001 | 7(14.3) | 18(37.5) | 0.004 |
| Sometimes | 4(8.2) | 4(9.1) | 0.614 | 5(10.2) | 15(31.3) | 0.001 | 6(12.2) | 16(33.3) | 0.001 | |
| A lot | 17(34.7) | 15(34.1) | 0.693 | 17(34.7) | 8(16.7) | 0.001 | 16(32.7) | 7(14.6) | 0.001 | |
| All the same | 20(40.8) | 18(40.9) | 0.875 | 20(40.8) | 8(16.7) | 0.001 | 20(40.8) | 7(14.6) | 0.001 | |
| Had trouble paying attention or concentrating on things | Never | 6(12.2) | 5(11.4) | 0.449 | 7(14.3) | 16(33.3) | 0.001 | 7(14.3) | 17(35.4) | 0.001 |
| Sometimes | 10(20.4) | 9(20.5) | 0.526 | 11(22.4) | 21(43.8) | 0.001 | 11(22.4) | 22(45.8) | 0.001 | |
| A lot | 14(28.6) | 13(29.5) | 0.514 | 13(26.5) | 6(12.5) | 0.001 | 14(28.6) | 4(8.3) | 0.001 | |
| All the same | 19(38.8) | 17(38.6) | 0.948 | 18(36.7) | 5(10.4) | 0.001 | 17(34.7) | 5(10.4) | 0.001 | |
| Been told that you are speaking or moving too slowly or fast | Never | 5(10.2) | 4(9.1) | 0.194 | 5(10.2) | 15(31.3) | 0.001 | 5(10.2) | 15(31.3) | 0.001 |
| Sometimes | 6(12.2) | 5(11.4) | 0.445 | 6(12.2) | 18(37.5) | 0.001 | 6(12.2) | 18(37.5) | 0.001 | |
| A lot | 12(24.5) | 11(25) | 0.551 | 12(24.5) | 7(14.6) | 0.001 | 13(26.5) | 8(16.7) | 0.001 | |
| All the same | 26(53.1) | 24(54.5) | 0.303 | 26(53.1) | 8(16.7) | 0.001 | 25(51) | 7(14.6) | 0.001 | |
| Had thoughts that you would be better off dead | Never | 3(6.1) | 3(6.8) | 0.204 | 4(8.2) | 17(35.4) | 0.001 | 4(8.2) | 18(37.5) | 0.001 |
| Sometimes | 6(12.2) | 5(11.4) | 0.752 | 6(12.2) | 16(33.3) | 0.001 | 6(12.2) | 17(35.4) | 0.001 | |
| A lot | 12(24.5) | 11(25) | 0.822 | 11(22.4) | 9(18.8) | 0.001 | 11(22.4) | 7(14.6) | 0.001 | |
| All the same | 28(57.1) | 25(56.8) | 0.730 | 28(57.1) | 6(12.5) | 0.001 | 28(57.1) | 6(12.5) | 0.001 | |
| Thought of hurting yourself | Never | 4(8.2) | 4(9.1) | 0.105 | 4(8.2) | 14(29.2) | 0.001 | 4(8.2) | 15(31.3) | 0.001 |
| Sometimes | 4(8.2) | 4(9.1) | 0.138 | 4(8.2) | 19(39.6) | 0.001 | 4(8.2) | 19(39.6) | 0.001 | |
| A lot | 14(28.6) | 12(27.3) | 0.357 | 15(30.6) | 7(14.6) | 0.001 | 15(30.6) | 7(14.6) | 0.001 | |
| All the same | 27(55.1) | 24(54.5) | 0.548 | 26(53.1) | 8(16.7) | 0.001 | 26(53.1) | 7(14.6) | 0.001 | |
| Felt angry | Never | 2(4.1) | 2(4.5) | 0.627 | 2(4.1) | 18(37.5) | 0.001 | 3(6.1) | 17(35.4) | 0.001 |
| Sometimes | 3(6.1) | 3(6.8) | 0.322 | 3(6.1) | 17(35.4) | 0.001 | 2(4.1) | 18(37.5) | 0.001 | |
| A lot | 24(49) | 22(50) | 0.512 | 23(46.9) | 7(14.6) | 0.001 | 23(46.9) | 6(12.5) | 0.001 | |
| All the same | 20(40.8) | 17(38.6) | 0.142 | 21(42.9) | 6(12.5) | 0.001 | 21(42.9) | 7(14.6) | 0.001 | |
n = 49 in each group.
Statistical analyses for the outcomes at 6-month follow-up
Statistical analyses showed that a GO-game intervention significantly decreased the mean score of MADRS of 4.72 (95% CI, 0.69–9.12) compared with those in a control group after 6-month follow-up (P < 0.05) (Supplemental Table 2). In the similar way, a GO-game intervention also reduced mean score of HADS of 1.75 (95% CI, 0.17–3.68) when compared with those from controls (P < 0.05) (Supplemental Table 2). In contrast, a GO-game intervention significantly increased the mean score of GAF of 4.95 (95% CI, −1.37–9.18) and RAND-36 of 4.61 (95% CI, −2.75–11.32) when compared with a control group (P < 0.05) (Supplemental Table 2). For TAS-20, there was no statistically significant difference in three groups (P > 0.05) (Supplemental Table 2). The results may be caused by alexithymic personality traits, which are closely related with gender, advanced age, educational level and social intelligence (Koelkebeck et al., 2010).
A GO game improves the symptoms of AD
The analysis for KICA-Dep items showed that GO game relieved the symptoms of AD patients compared with those from a control group (Table 1). In the 11 items of KICA-Dep, a control group could not attenuate the symptoms of AD patients and there was no statistically significant difference for most items (P > 0.05) except of two ones (Table 1). Comparatively, a GO game ameliorated the symptoms of depression and there were statistically significant differences for most items (P < 0.05) except of two items (Table 1). After a 6-month GO-game intervention, the severity of AD was decreased significantly (P < 0.05) (Table 1).
The levels of BDNF are negatively related with stratification of CDR
When AD severity was stratified by CDR scales at onset (χ2 = 6.8, P = 0.03), significant differences of BDNF levels were observed in lower (CDR 0.5), middle (CDR 1), and higher CDR (CDR 2+). There were statistically significant differences for BDNF levels between CDR 0.5 and 2+ (P < 0.05). BDNF levels were negatively related with CDR scales, suggested that low levels of BDNF were related with AD risk since the BDNF levels were higher in lower CDR scores compared to those in higher CDR scores (χ2 = 214, P < 0.01) while CDR scores have been used to evaluate the severity of AD (Clark et al., 2014).
Association between an AD episode and the protein levels of BDNF
Elevated levels of BDNF were related with a decrease in MADRS scores and an increase in RAND-36 scales (P < 0.01). There was a strong negative relationship between the severity of AD and the levels of BDNF because the rho values were less than −0.5 based on the calculation of Spearman's rank correlation coefficient.
A GO game enhances the serum protein levels of BDNF in AD patients
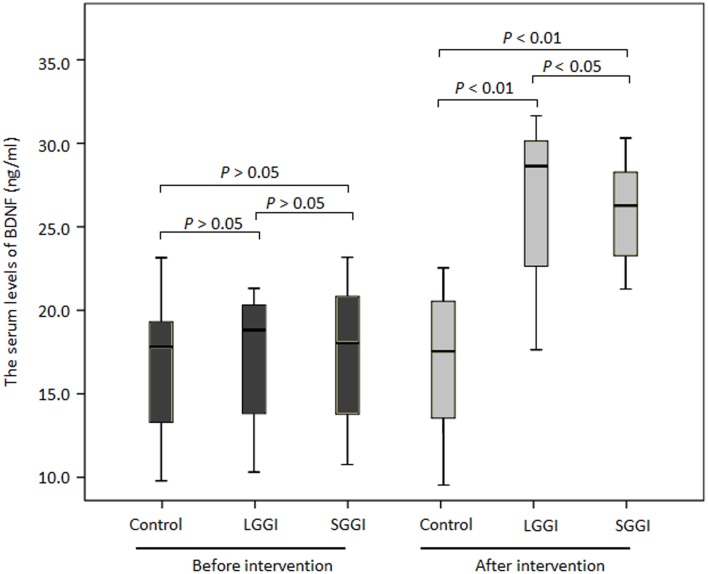
The study consisted of 147 AD patients before and after 6-month follow-up. The socio-demographic was list in Supplemental Table 1 and balanced based on age, gender, educational levels, life habits and medicine therapy. The results showed that BDNF levels were similar in three groups before GO game intervention. After 6-month intervention, serum BDNF levels were significantly higher in subjects receiving SGGI and LGGI (respectively, 24.02 ± 7.16 and 28.88 ± 4.12 ng/ml, t = 0.345, P = 0.051) when compared with controls (17.28 ± 7.75 ng/ml) (t = 3.423, P = 0.001) (Figure 3). All these results implied that a GO game promotes the production of BDNF.
Figure 3.
Serum BDNF levels in control, LGGI and SGGI groups before and after 6-month follow-up. Comparative analyses were carried out using ANOVA followed by a Duncan test. Values were expressed as mean ± SD. N = 49 cases in each group. The bars in the boxes were average expression and the boxes represented 95% of the samples. The error bars were above or below the boxes.
Discussion
A GO game is very popular in China, Japan, and Korean, and can be widely played. More importantly, a GO game has been proved to be very interesting for its rich strategies with simple rules and attracts many elderly adults. The game is more suitable to be developed to prevent the progression of AD. We investigated the effects of the game on AD and related molecular mechanisms. On the other hand, physical activities can certainly delay the loss of autonomy in AD patients and are also useful strategies for delaying the complications of AD (Rolland et al., 2000). During the recruitment, some AD patients like physical exercises but they dislike playing GO game. In the similar cases, some AD patients like playing the game but they dislike physical exercises. Only a few AD patients like playing a GO game and physical exercises. The sample size will be a problem for considering both physical exercises and a GO game. Furthermore, the effects of physical exercises on AD patients have been reported. Thus, we only consider the effects of a GO game on AD here. The patients will be excluded if they dislike playing the game. Importantly, a GO game showed effective anti-depression results with few side effects. Meanwhile, we interpreted the possible outcome by using GO-game-specific and computational methods with a particular motivation to detect therapeutically-relevant phenomena.
The strength of the paper is its technique in clinical practice. Furthermore, each person in GO groups could play the game well with basic rules. On the other hand, the combination of various techniques was utilized. Brain imaging and self-ratings, computational game analyses and psychiatric assessment was undertaken, resulting in complementary and myriad measures access to various underlying determinants of an intervention. Some brain imaging techniques, such as fMRI and PET, can provide distinct spatial information, but which cannot provide time and event-related correlations. Depression has been reported to be correlated with a hypo-activation of left brain activity and a hypo-inactivation of right brain activity (Richieri et al., 2012), which can be ameliorated with the time going after playing the game.
MADRS is one of the most effective tools for detecting the depression in AD patients and higher MADRS score suggests severe depression (Müller-Thomsen et al., 2005). We used the MADRS to measure the changes of depression severity. The results showed that a GO game significantly decreased mean score of MADRS of 4.72 (95% CI, 0.69–9.12) when compared with controls after 6-month follow-up (P < 0.05, Supplemental Table 2). HADS is also an important tool to detect the depression of AD (Pietrzak et al., 2015), so we also measured the HADS scores here. In the similar results, a GO game decreased the mean score of HADS of 1.75 (95% CI, 0.17–3.68) when compared with controls in the same period (P < 0.05, Supplemental Table 2). Furthermore, KICA-Dep has been reported to very useful for detecting the depressive symptoms in AD patients. The results indicated that a GO game improved 9 of 11 items of KICA-Dep compared with controls after 6-month follow-up (Table 1). On the other hand, GAF has been used to evaluate the overall improved quality of life in AD patients (Lu et al., 2006). Thus, we measured GAF and found that a GO game increased the mean score of GAF of 4.95 (95% CI, −1.37–9.18) and RAND-36 of 4.61 (95% CI, −2.75–11.32) when compared with those in a control group after 6-month follow-up (P < 0.05, Supplemental Table 2).
Although a GO game showed effective results for ameliorating the symptoms of AD, the molecular mechanism remains unknown. Previous work demonstrates that AD patients have reduced levels of BDNF in the brain and serum. Additionally, animal-based studies also showed protective effects of BDNF against Amyloid beta-protein (Abeta)-induced neurotoxicity (Psotta et al., 2015). Abeta1-42 exhibits neurotoxicity and induces neural death. Abeta1-40 can inhibit the fibril formation of Abeta1-42 (Zou et al., 2003). In contrast, Abeta1-16, Abeta25-35, and Abeta40-1 prevent neither the fibril formation of Abeta1-42 nor Abeta1-42-induced neural death. Here, we measured serum levels of BDNF in AD patients and found that a GO game increased serum BDNF levels in SGGI and LGGI groups (24.02 ± 7.16 and 28.88 ± 4.12 ng/ml respectively, t = 0.345, P = 0.051) compared those in controls (17.28 ± 7.75 ng/ml) (t = 3.423, P = 0.001) after 6-month follow-up. Furthermore, the serum levels of BDNF showed a negative relation with MADRS and a positive relation with RAND-36 scores (P < 0.01). A GO game ameliorates AD patients by up-regulating BDNF levels. Thus, A GO game is effective to prevent the development of AD.
Alexithymia is considered for its defects in regulating feelings (Goerlich et al., 2011) and typically co-occurs with depression (Gilbert et al., 2014). However, alexithymia is a complex brain disorder with a cluster of deficits in the recognition (Reker et al., 2010). Here we found that a GO game was not an effective way for the treatment of alexithymia compared with that from a control group (P > 0.05) (Table 1). In any way, a GO game can improve the life quality in other aspects (P < 0.05) (Supplemental Table 2).
In addition, evoking and dealing with emotions is usually related with a GO game, which fit the treatment of emotional disorders just like depression. Although all AD patients wanted to learn and play a GO game before the study, someone kept active exercises and a few kept passive exercises for the game with time going. We found that most winners were active for playing the game while a few losers were passive for playing the game. We would let the game trainers play with these passive losers and let them win on purpose. Most of them could become active again for playing the game finally. In order to gain an insight into the effectiveness of the game, we also aimed to develop novel analytical methods for clinical purposes, such as kinematics, electromyographic and electroencephalographic (EEG) data (Barlaam et al., 2011). The new concept is also needed to understand the mechanisms by studying symptoms, behaviors, or biomarkers, which are different to traditional classification for the mental disorders (Badcock and Hugdahl, 2014). Meanwhile, the study will provide valuable information for the therapy of depression.
Limitations
A GO game affects the progression of depression but the molecular mechanism remains unknown. Much evidence indicates that chronic stress and low levels of BNDF are the key causes of depression (Banerjee et al., 2014; Vásquez et al., 2014). However, the clinical relevance of a GO game intervention and the levels of BDNF remain unknown. Here, we firstly reported that a GO game ameliorated the depression by affecting the serum levels of BDNF. We found the relationships between SGGI or LGGI and levels of BDNF. The results showed that lower concentration of BDNF might be specific for the depressive state in AD patients. BDNF is a potential biomarker for adjuvant diagnosis and therapy of an AD episode. However, further studies are necessary to define a functional role of BDNF in preventing the development of depression.
Certainly, the present trial still has its limitations in a statistical sense. The sample size was sufficient to detect an effect in the primary outcome at 6-months, but not at longer period although we found the effect tended to persist. Second, the present trial was the GO game intervention. It remained unknown whether a long-term GO game was better than a short-term GO game. At least, there are no reports showing such indication yet. However, we still believe that a GO game intervention may be more suitable for a few populations only received medicine therapy.
Finally, a major problem with the design is that the control group did not receive any treatment. Therefore, it is not possible to know whether the changes reported are because the treatment groups received attention from and interacted with colleagues and staff or because of the game itself. A “placebo” procedure will be necessary to enable solid conclusions. Unfortunately, the “placebo” group is hard to be designed because no game can be matched GO game well according our experiences. Being interesting is very important to play a game. Gomoku, also called Five in a Row, can be played on a GO board. From May 6th, 2012 to August 12th, 2013, a total of 50 AD patients were recruited. All the participants did not have the experiences of playing Gomoku and showed interests for the game before the study. However, more than 50% patients lost their passions for the game after 2-month follow-up and the work was unavoidably stopped. Fortunately, less than 10% patients lost enthusiasm for GO game even after 6-month. Furthermore, these patients still could show enthusiasm again for GO game when they won in subsequent matches, which were designed on purpose by the GO game staff. We think that a GO game shows its beneficial functions on AD patients since the enthusiasm still can be maintained after 6-month follow-up.
Clinical and mechanistic implications
A GO game can improve the life quality of AD patients. None complained of playing a GO game and no routine neurological exam could identify any adverse effect. Playing a GO game should be developed a public activity since the game intervention can reduce the symptoms of AD. Its mechanistic implication may be that many strategies involve in the play and the number of possible games is vast (there are about 10120 possible results for playing the game), although the rules of game are very simple. Given the substantial changes in the game, the game attracts an ever-growing older population. Playing a simple and inexpensive game can improve the cognitive functions of AD patients. Millions of AD patients are looking for a technologically-oriented game to enhance their cognitive skills, which can prevent the development of AD.
In sum, a GO game is feasible and effective to improve life quality of AD patients by reducing their depression. A GO game intervention reduces AD severity by increasing the level of BDNF. Thus, a GO game should be developed as a new method for the therapy of AD.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are very grateful to all anonymous reviewers for their critical and strategic comments, which have significantly improved the quality of the paper.
Supplementary material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fnagi.2015.00163
References
- Ahn S. H., Choi N. K., Kim Y. J., Seong J. M., Shin J. Y., Jung S. Y., et al. (2014). Drug persistency of cholinesterase inhibitors for patients with dementia of Alzheimer type in Korea. Arch. Pharm. Res. 38, 1255–1262. 10.1007/s12272-014-0500-8 [DOI] [PubMed] [Google Scholar]
- Alonzo A., Chan G., Martin D., Mitchell P. B., Loo C. (2013). Transcranial direct current stimulation (tDCS) for depression: analysis of response using a three-factor structure of the Montgomery-Asberg depression rating scale. J. Affect. Disord. 150, 91–95. 10.1016/j.jad.2013.02.027 [DOI] [PubMed] [Google Scholar]
- Badcock J. C., Hugdahl K. (2014). A synthesis of evidence on inhibitory control and auditory hallucinations based on the Research Domain Criteria (RDoC) framework. Front. Hum. Neurosci. 8:180. 10.3389/fnhum.2014.00180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee R., Hazra S., Ghosh A. K., Mondal A. C. (2014). Chronic administration of bacopa monniera increases BDNF protein and mRNA expressions: a study in chronic unpredictable stress induced animal model of depression. Psychiatry Investig. 11, 297–306. 10.4306/pi.2014.11.3.297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlaam F., Descoins M., Bertrand O., Hasbroucq T., Vidal F., Assaiante C., et al. (2011). Time-Frequency and ERP Analyses of EEG to characterize anticipatory postural adjustments in a bimanual load-lifting task. Front. Hum. Neurosci. 5:163. 10.3389/fnhum.2011.00163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besser L. M., Gill D. P., Monsell S. E., Brenowitz W., Meranus D. H., Kukull W., et al. (2014). Body mass index, weight change, and clinical progression in mild cognitive impairment and Alzheimer disease. Alzheimer Dis. Assoc. Disord. 28, 36–43. 10.1097/WAD.0000000000000005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum H., Kessler R., Joish V., Kelley D., Ben-Hamadi R., Hsieh M., et al. (2009). Healthcare resource utilization and costs of mild, moderate, and severe depression in the workforce in the United States. Eur. Psychiatry 24, S614 10.1016/S0924-9338(09)70847-8 [DOI] [Google Scholar]
- Boiocchi C., Maggioli E., Zorzetto M., Sinforiani E., Cereda C., Ricevuti G., et al. (2013). Brain-derived neurotrophic factor gene variants and Alzheimer disease: an association study in an Alzheimer disease Italian population. Rejuvenation Res. 16, 57–66. 10.1089/rej.2012.1381 [DOI] [PubMed] [Google Scholar]
- Brownley K. A., Boettiger C. A., Young L., Cefalu W. T. (2015). Dietary chromium supplementation for targeted treatment of diabetes patients with comorbid depression and binge eating. Med. Hypotheses 85, 45–48. 10.1016/j.mehy.2015.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield D. A., Di Domenico F., Barone E. (2014). Elevated risk of type 2 diabetes for development of Alzheimer disease: a key role for oxidative stress in brain. Biochim. Biophys. Acta 1842, 1693–1706. 10.1016/j.bbadis.2014.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedarbaum J. M., Jaros M., Hernandez C., Coley N., Andrieu S., Grundman M., et al. (2013). Rationale for use of the Clinical Dementia rating sum of boxes as a primary outcome measure for Alzheimer's disease clinical trials. Alzheimer's Dement. 9, S45–S55. 10.1016/j.jalz.2011.11.002 [DOI] [PubMed] [Google Scholar]
- Chang C. C., Zhao Y., Lee C. W., Ganguli M. (2012). Smoking, death, and Alzheimer disease: a case of competing risks. Alzheimer Dis. Assoc. Disord. 26, 300–306. 10.1097/WAD.0b013e3182420b6e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D. G., Kapur P., Geldmacher D. S., Brockington J. C., Harrell L., DeRamus T. P., et al. (2014). Latent information in fluency lists predicts functional decline in persons at risk for Alzheimer disease. Cortex 55, 202–218. 10.1016/j.cortex.2013.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dysken M. W., Sano M., Asthana S., Vertrees J. E., Pallaki M., Llorente M., et al. (2014). Effect of vitamin E and memantine on functional decline in Alzheimer disease: the TEAM-AD VA cooperative randomized trial. JAMA 311, 33–44. 10.1001/jama.2013.282834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. A., Morris M. C., Rajan K. B. (2014). Vitamin E, memantine, and Alzheimer disease. JAMA 311, 29–30. 10.1001/jama.2013.282835 [DOI] [PubMed] [Google Scholar]
- Fan D., Li J., Zheng B., Hua L., Zuo Z. (2014). Enriched Environment Attenuates Surgery-Induced Impairment of Learning, Memory, and Neurogenesis Possibly by Preserving BDNF Expression. Mol. Neurobiol. [Epub ahead of print]. 10.1007/s12035-014-9013-1 [DOI] [PubMed] [Google Scholar]
- Gatz M. (2005). Educating the brain to avoid dementia: can mental exercise prevent Alzheimer disease? PLoS Med. 2:e7. 10.1371/journal.pmed.0020007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert P., McEwan K., Catarino F., Baião R., Palmeira L. (2014). Fears of happiness and compassion in relationship with depression, alexithymia, and attachment security in a depressed sample. Br. J. Clin. Psychol. 53, 228–244. 10.1111/bjc.12037 [DOI] [PubMed] [Google Scholar]
- Goerlich K. S., Witteman J., Aleman A., Martens S. (2011). Hearing feelings: affective categorization of music and speech in alexithymia, an ERP study. PLoS ONE 6:e19501. 10.1371/journal.pone.0019501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregori M., Masserini M., Mancini S. (2015). Nanomedicine for the treatment of Alzheimer's disease. Nanomedicine (Lond.) 10, 1203–1218. 10.2217/nnm.14.206 [DOI] [PubMed] [Google Scholar]
- Hamilton A., Zamponi G. W., Ferguson S. S. (2015). Glutamate receptors function as scaffolds for the regulation of beta-amyloid and cellular prion protein signaling complexes. Mol. Brain 8, 18. 10.1186/s13041-015-0107-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz A., Finck C., Gómez Y., Daig I., Glaesmer H., Singer S. (2014). Anxiety and depression in the general population in Colombia: reference values of the Hospital Anxiety and Depression Scale (HADS). Soc. Psychiatry Psychiatr. Epidemiol. 49, 41–49. 10.1007/s00127-013-0714-y [DOI] [PubMed] [Google Scholar]
- Hultsch D. F., Hertzog C., Small B. J., Dixon R. A. (1999). Use it or lose it: engaged lifestyle as a buffer of cognitive decline in aging? Psychol. Aging 14, 245–263. 10.1037/0882-7974.14.2.245 [DOI] [PubMed] [Google Scholar]
- Inoue K., Meguro K., Akanuma K., Meguro M., Yamaguchi S., Fukuda H. (2012). Impaired memory and executive function associated with decreased medial temporal and prefrontal blood flow in Clinical Dementia Rating 0.5 status: the Osaki−Tajiri project. Psychogeriatrics 12, 27–33. 10.1111/j.1479-8301.2011.00384.x [DOI] [PubMed] [Google Scholar]
- Jain S., Farooq S. J., Gottlob I. (2008). Resolution of superior oblique myokymia with memantine. J. AAPOS 12, 87–88. 10.1016/j.jaapos.2007.07.007 [DOI] [PubMed] [Google Scholar]
- Johnson J. K., Chang C. C., Brambati S. M., Migliaccio R., Gorno-Tempini M. L., Miller B. L., et al. (2011). Music recognition in frontotemporal lobar degeneration and Alzheimer disease. Cogn. Behav. Neurol. 24, 74–84. 10.1097/WNN.0b013e31821de326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. H., Han D. H., Lee Y. S., Kim B. N., Cheong J. H., Han S. H. (2014). Baduk (the Game of GO) Improved Cognitive Function and Brain Activity in Children with Attention Deficit Hyperactivity Disorder. Psychiatry Investig. 11, 143–151. 10.4306/pi.2014.11.2.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelkebeck K., Pedersen A., Suslow T., Kueppers K. A., Arolt V., Ohrmann P. (2010). Theory of Mind in first-episode schizophrenia patients: correlations with cognition and personality traits. Schizophr. Res. 119, 115–123. 10.1016/j.schres.2009.12.015 [DOI] [PubMed] [Google Scholar]
- Lattanzi S., Viticchi G., Falsetti L., Buratti L., Luzzi S., Provinciali L., et al. (2014). Visit-to-Visit Blood Pressure Variability in Alzheimer Disease. Alzheimer Dis. Assoc. Disord. 28, 347–351. 10.1097/WAD.0000000000000040 [DOI] [PubMed] [Google Scholar]
- Lee B., Park J. Y., Jung W. H., Kim H. S., Oh J. S., Choi C. H., et al. (2010). White matter neuroplastic changes in long-term trained players of the game of “Baduk” (GO): a voxel-based diffusion-tensor imaging study. Neuroimage 52, 9–19. 10.1016/j.neuroimage.2010.04.014 [DOI] [PubMed] [Google Scholar]
- Lee S. T., Chu K., Jung K. H., Kim J. H., Huh J. Y., Yoon H., et al. (2012). miR-206 regulates brain-derived neurotrophic factor in Alzheimer disease model. Ann. Neurol. 72, 269–277. 10.1002/ana.23588 [DOI] [PubMed] [Google Scholar]
- Leikin J. B., Braund V., DesLauris C. (2014). Cholinergic symptoms with low serum cholinesterase from therapeutic cholinesterase inhibitor toxicity. Am. J. Emerg. Med. 32, 815. e813–814. 10.1016/j.ajem.2013.12.048 [DOI] [PubMed] [Google Scholar]
- Lu P. H., Masterman D. A., Mulnard R., Cotman C., Miller B., Yaffe K., et al. (2006). Effects of testosterone on cognition and mood in male patients with mild Alzheimer disease and healthy elderly men. Arch. Neurol. 63, 177–185. 10.1001/archneur.63.2.nct50002 [DOI] [PubMed] [Google Scholar]
- Mattila K., Lahtela M., Hynynen M. (2012). Health-related quality of life following ambulatory surgery procedures: assessment by RAND-36. BMC Anesthesiol. 12:30. 10.1186/1471-2253-12-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melin E. O., Thulesius H. O., Persson B. A. (2010). Affect School for chronic benign pain patients showed improved alexithymia assessments with TAS-20. Biopsychosoc. Med. 4:5. 10.1186/1751-0759-4-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello A. F., Blay S. L., Kohn R. (2007). Global Assessment of Relational Functioning Scale (GARF): a validity study in patients with recurrent major depression in Brazil. Transcult. Psychiatry 44, 55–64. 10.1177/1363461507074969 [DOI] [PubMed] [Google Scholar]
- Mormont E., Jamart J., Jacques D. (2014). Symptoms of depression and anxiety after the disclosure of the diagnosis of Alzheimer disease. J. Geriatr. Psychiatry Neurol. 27, 231–236. 10.1177/0891988714532021 [DOI] [PubMed] [Google Scholar]
- Müller-Thomsen T., Arlt S., Mann U., Mass R., Ganzer S. (2005). Detecting depression in Alzheimer's disease: evaluation of four different scales. Arch. Clin. Neuropsychol. 20, 271–276. 10.1016/j.acn.2004.03.010 [DOI] [PubMed] [Google Scholar]
- Palacios R. (2013). Post-trial access and the new version of the Declaration of Helsinki. Colomb Med (Cali.) 44, 206–207. [PMC free article] [PubMed] [Google Scholar]
- Pietrzak R. H., Lim Y. Y., Neumeister A., Ames D., Ellis K. A., Harrington K., et al. (2015). Amyloid-beta, anxiety, and cognitive decline in preclinical Alzheimer disease: a multicenter, prospective cohort study. JAMA Psychiatry 72, 284–291. 10.1001/jamapsychiatry.2014.2476 [DOI] [PubMed] [Google Scholar]
- Pradier C., Sakarovitch C., Le Duff F., Layese R., Metelkina A., Anthony S., et al. (2014). The mini mental state examination at the time of Alzheimer's disease and related disorders diagnosis, according to age, education, gender and place of residence: a cross-sectional study among the French National Alzheimer database. PLoS ONE 9:e103630. 10.1371/journal.pone.0103630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencher V., Bier N., Audet T., Gagnon L. (2009). Long-term effect of a cognitive intervention on learning and participation in a significant leisure activity in early dementia of Alzheimer type: a case study. Psychol. Neuropsychiatr. Vieil. 7, 131–140. 10.1684/pnv.2009.0166 [DOI] [PubMed] [Google Scholar]
- Psotta L., Rockahr C., Gruss M., Kirches E., Braun K., Lessmann V., et al. (2015). Impact of an additional chronic BDNF reduction on learning performance in an Alzheimer mouse model. Front. Behav. Neurosci. 9:58. 10.3389/fnbeh.2015.00058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu C., Bäckman L., Winblad B., Aguero-Torres H., Fratiglioni L. (2001). The influence of education on clinically diagnosed dementia incidence and mortality data from the Kungsholmen Project. Arch. Neurol. 58, 2034–2039. 10.1001/archneur.58.12.2034 [DOI] [PubMed] [Google Scholar]
- Qiu C., Winblad B., Marengoni A., Klarin I., Fastbom J., Fratiglioni L. (2006). Heart failure and risk of dementia and Alzheimer disease: a population-based cohort study. Arch. Intern. Med. 166, 1003–1008. 10.1001/archinte.166.9.1003 [DOI] [PubMed] [Google Scholar]
- Ramakers I. H., Verhey F. R., Scheltens P., Hampel H., Soininen H., Aalten P., et al. (2013). Anxiety is related to Alzheimer cerebrospinal fluid markers in subjects with mild cognitive impairment. Psychol. Med. 43, 911–920. 10.1017/S0033291712001870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reker M., Ohrmann P., Rauch A. V., Kugel H., Bauer J., Dannlowski U., et al. (2010). Individual differences in alexithymia and brain response to masked emotion faces. Cortex 46, 658–667. 10.1016/j.cortex.2009.05.008 [DOI] [PubMed] [Google Scholar]
- Richieri R., Boyer L., Padovani R., Adida M., Colavolpe C., Mundler O., et al. (2012). Equivalent brain SPECT perfusion changes underlying therapeutic efficiency in pharmacoresistant depression using either high-frequency left or low-frequency right prefrontal rTMS. Prog. Neuropsychopharmacol. Biol. Psychiatry 39, 364–370. 10.1016/j.pnpbp.2012.07.012 [DOI] [PubMed] [Google Scholar]
- Rolland Y., Rival L., Pillard F., Lafont C., Rivére D., Albaréde J., et al. (2000). Feasibility [corrected] of regular physical exercise for patients with moderate to severe Alzheimer disease. J. Nutr. Health Aging 4, 109–113. [PubMed] [Google Scholar]
- Rossom R., Adityanjee Dysken, M. (2004). Efficacy and tolerability of memantine in the treatment of dementia. Am. J. Geriatr. Pharmacother. 2, 303–312. 10.1016/j.amjopharm.2004.12.006 [DOI] [PubMed] [Google Scholar]
- Salvatore P., Baldessarini R. J., Khalsa H. M., Amore M., Di Vittorio C., Ferraro G., et al. (2013). Predicting diagnostic change among patients diagnosed with first-episode DSM-IV-TR major depressive disorder with psychotic features. J. Clin. Psychiatry 74, 723–731. 10.4088/JCP.12m08328 [DOI] [PubMed] [Google Scholar]
- Sawada A., Hara K., Futa R., Ogata H. (1971). Pressure-lowering effect of a new cholinesterase inhibitor, Ubretid on glaucoma. Nihon Ganka Kiyo 22, 676–682. [PubMed] [Google Scholar]
- Tamaoka A. (2011). Alzheimer's disease: definition and National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association (NINCDS-ADRDA). Nippon. Rinsho 69(Suppl. 10), 240–245. [PubMed] [Google Scholar]
- van Beek A. H., Sijbesma J. C., Jansen R. W., Rikkert M. G., Claassen J. A. (2010). Cortical oxygen supply during postural hypotension is further decreased in Alzheimer's disease, but unrelated to cholinesterase-inhibitor use. J. Alzheimers. Dis. 21, 519–526. 10.3233/JAD-2010-100288 [DOI] [PubMed] [Google Scholar]
- Vásquez C. E., Riener R., Reynolds E., Britton G. B. (2014). NMDA receptor dysregulation in chronic state: a possible mechanism underlying depression with BDNF downregulation. Neurochem. Int. 79C, 88–97. 10.1016/j.neuint.2014.09.007 [DOI] [PubMed] [Google Scholar]
- Villoslada P., Arrondo G., Sepulcre J., Alegre M., Artieda J. (2009). Memantine induces reversible neurologic impairment in patients with MS. Neurology 72, 1630–1633. 10.1212/01.wnl.0000342388.73185.80 [DOI] [PubMed] [Google Scholar]
- Voineskos A. N., Lerch J. P., Felsky D., Shaikh S., Rajji T. K., Miranda D., et al. (2011). The brain-derived neurotrophic factor Val66Met polymorphism and prediction of neural risk for Alzheimer disease. Arch. Gen. Psychiatry 68, 198–206. 10.1001/archgenpsychiatry.2010.194 [DOI] [PubMed] [Google Scholar]
- Wiscott R., Kopera-Frye K., Seifert L. (2001). Possible consequences of social drinking in the early stages of Alzheimer disease. Geriatr Nurs. 22, 100–104; quiz 105. 10.1067/mgn.2001.115201 [DOI] [PubMed] [Google Scholar]
- Xiao Q., Wang C., Li J., Hou Q., Li J., Ma J., et al. (2010). Ginkgolide B protects hippocampal neurons from apoptosis induced by beta-amyloid 25-35 partly via up-regulation of brain-derived neurotrophic factor. Eur. J. Pharmacol. 647, 48–54. 10.1016/j.ejphar.2010.08.002 [DOI] [PubMed] [Google Scholar]
- Zhang R., Wang Z., Howson P. A., Xia Z., Zhou S., Wu E., et al. (2012). Smilagenin attenuates beta amyloid (25-35)-induced degeneration of neuronal cells via stimulating the gene expression of brain-derived neurotrophic factor. Neuroscience 210, 275–285. 10.1016/j.neuroscience.2012.03.017 [DOI] [PubMed] [Google Scholar]
- Zou K., Kim D., Kakio A., Byun K., Gong J. S., Kim J., et al. (2003). Amyloid beta-protein (Abeta)1-40 protects neurons from damage induced by Abeta1-42 in culture and in rat brain. J. Neurochem. 87, 609–619. 10.1046/j.1471-4159.2003.02018.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.