Abstract
This is a dose-response (DR) meta-analysis to evaluate the association of coffee consumption on endometrial cancer (EC) risk. A total 1,534,039 participants from 13 published articles were added in this meta-analysis. The RR of total coffee consumption and EC were 0.80 (95% CI: 0.74–0.86). A stronger association between coffee intake and EC incidence was found in patients who were never treated with hormones, 0.60 (95% CI: 0.50–0.72), and subjects with a BMI ≥25 kg/m2, 0.57 (95% CI: 0.46–0.71). The overall RRs for caffeinated and decaffeinated coffee were 0.66 (95% CI: 0.52–0.84) and 0.77 (95% CI: 0.63–0.94), respectively. A linear DR relationship was seen in coffee, caffeinated coffee, decaffeinated coffee and caffeine intake. The EC risk decreased by 5% for every 1 cup per day of coffee intake, 7% for every 1 cup per day of caffeinated coffee intake, 4% for every 1 cup per day of decaffeinated intake of coffee, and 4% for every 100 mg of caffeine intake per day. In conclusion, coffee and intake of caffeine might significantly reduce the incidence of EC, and these effects may be modified by BMI and history of hormone therapy.
EC is a common type of gynaecologic cancer. The overall cases reported each year is third in rank among the number of new cases and one of the major (4th Rank) cause of deaths in the female around the world1. In the developed countries, the incidence is more than two fold than in developing countries2. In United States (US), it is anticipated that approximately 54870 women will be diagnosed with EC in 20153. Coffee is commonly consumed among all the beverages globally. More than 2.5 billion cups of coffee are consumed worldwide each day4. It contains various phytochemicals having potential antioxidant and anti-mutagenic properties5 and minor health effects may lead to impact the public health in large. Recently, the connection between coffee consumption and associated risk of cancer has gained great interest6,7,8. As per EC report from the World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR), coffee is a protective factor against EC9.
As per our current knowledge, only four published meta-analyses were focused on the involvement of coffee for and EC risk10,11,12,13. In a meta-analysis, 200913, it was found an inverse association between high coffee consumption and EC. In another meta-analysis10 of four cohort studies, it has been reported RR of 0.74 between high vs. low coffee intake group. Recently, an article11 that combined a cohort study and meta-analysis was published, and a weak relation between coffee consumption and EC was found. However, these four meta-analyses did not further explore the relationship between different caffeine dosages or coffee types, and graph of DR relationship. Moreover, recent meta-analysis11 did not include four large cohort studies, i.e. approximately 2000 cases and 369624 participants were ignored.
Therefore, we designed this meta-analysis with the following objectives: (1) to investigate the epidemiological evidence on the relationship between total coffee intake and EC risk. It also quantifies the association between different caffeine dosages and coffee types and risk of EC; (2) to calculate the DR graph of these associations; and (3) to evaluate the statistical power of the associations and the quality of evidence.
Methods and Materials
This meta-analysis was designed as per the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines14.
Date source and search strategy
The PubMed, Embase and the Cochrane Library databases were searched from inception to May 1, 2015 for studies describing the association between coffee consumption and incidence of EC. The key words used during searching were : (“endometrial cancer” OR “endometrium cancer” OR “endometrial carcinoma” OR “endometrium carcinoma” OR “endometrial neoplasms” OR “endometrium neoplasms” OR “corpus uteri cancer” OR “corpus uteri neoplasm” OR “corpus uteri carcinoma” OR “uterus tumor” OR “uterine tumor”) AND (“Coffee” OR “coffee” OR “coffee consumption” OR “coffee intake” OR “coffee drinking” OR “Caffeine” OR “caffeine consumption”). In addition, the references from applicable research papers and review papers were screened to identify studies. In case of duplicated data, the most complete study was only considered for this Meta analysis.
Study selection and data extraction
The selection of titles and abstracts of the studies were done as the first step for scrutinization. The second selection was done based on a complete review of the paper. The studies meeting the following conditions were only included in this study:
(1) Study design was a prospective cohort study;
(2) Exposure of interest was coffee intake;
(3) Outcome of interest was EC;
(4) RR or HR and the corresponding CI of the EC for the maximum versus the minimum level were provided.
The RRs or HRs had to be adjusted for potential confounders. Two independent investigators (QZ and ML-L) selected the studies. If the studies did not not meet the all the above criteria, were excluded from this analysis. From the selected studies the information isolated were: first author’s last name; publication year; country of origin; study period; cohort name; duration of follow-up; age at baseline; coffee intake category; number of cases and cohort size of each category;relative risks (with their 95% CI) for each category of coffee consumption, and covariates adjusted for in the multivariable analysis. Two investigators (QZ and HL) independently retrieved the data. This disagreement in the independent analysis was resolved by mutual agreement.
Quality assessment of studies and evidence
The Newcastle-Ottawa Scale15 (NOS) were used to assess the quality of the included studies by two reviewers (QZ and ML). This scale ranged from 0 (poor) to 9 stars (excellent) and awards four stars for selection of study participants, two stars for comparability of studies, and three stars for the adequate ascertainment of outcomes. Scores of 0–3, 4–6, and 7–9 are regarded as low, moderate, and high quality, respectively16. The quality of evidence was evaluated using the GRADE system17,18 (GRADE profiler 3.6.1) by two reviewers (QZ and JG-Z). There are several reasons for downgrade of data which are; inconsistency, indirectness, imprecision or publication bias, and three reasons to upgrade the evidence: large effect size, dose-response relationship and plausible confounders that would not decrease the apparent effect size19. The categories for the different evidences are: High; Moderate; Low and very low.
Statistical analysis
Because incidence of EC is low, HR is mathematically similar to RR20. The results were reported as RR. We quantified the associations between total coffee caffeine dosage, caffeinated coffee, decaffeinated coffee intake and risk of EC comparing the highest versus the lowest (the referent) categories. For the dose-response meta-analysis, The dosage value assigned to each coffee intake category is the median provided by the original research.For the studies that did not report a median, we used the midpoint for closed categories. In case of open ended highest category, the midpoint of the category was set at 1.5 times the lower boundary and in case of open ended lowest category, the lower boundary was set to zero21. The least squares estimation was done by Greenland and Longneck method22,23 to account for the correlation with the log RR estimates across the exposures. The potential DR relationship was estimated in two stages24; firstly, restricted cubic spline model with 4 knots at percentiles 5%, 35%, 65% and 75% of the distribution of exposure consumption was estimated, and the 3 regression coefficients (4 knots minus 1) were calculated; Secondly, the variance or covariance matrix within each study was combined. Non-linearity test was made by testing the null hypothesis that both the coefficients of the second and third spline are all equal to zero.If the p value more than 0.05 was considered as linear and less than 0.05 was considered as nonlinear. Heterogeneity among studies was assessed using the χ2 test and was defined as a P value less than 0.10. Quantification of heterogeneity was assessed by the I2statistic25. An I2 above 50% indicated high heterogeneity. A random effect was implemented26. Publication bias was judged with the Begg rank correlation test27 and Egger’s regression test28. The results were considered to show publication bias when p < 0.10. If publication bias existed, we used a trim and fill method29 to evaluate the number of missing studies. A stratified analysis was performed for study, location, BMI, smoking status, hormone therapy, menopausal status, and interval of follow-up, total of participants, number of cases and results were adjusted whether for BMI or hormone therapy. A power calculation was performed using the method described by Cafri et al.30. Highest versus lowest meta-analysis and subgroup analysis were performed in Review Manager Version 5.3 (The Nordic Cochrane Center, Copenhagen, Denmark). The sensitivity analyses were estimated in Stata version 12.0 (StataCorp, College Station, Texas, USA). The trim and fill method was conducted in R version 3.2.0 (R Foundation for Statistical Computing, Vienna, Austria). A signifance level is said when the p was less than 0.05, unless otherwise specified.
Results
Literature search
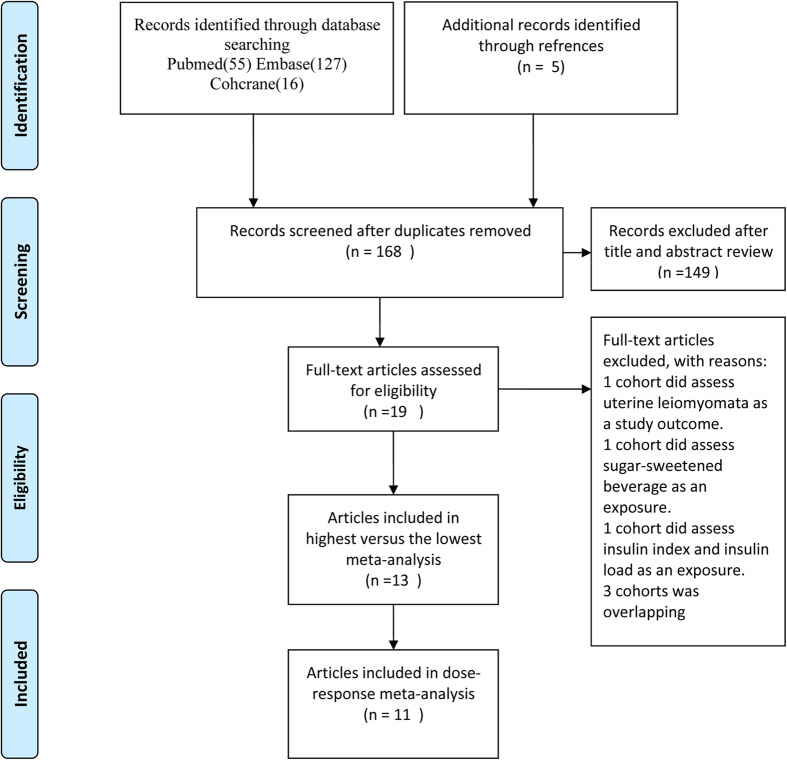
The Fig. 1 shows the flow chart for literature retrieval and selection. In first search a total 198 published articles from PubMed, Embase and Cochrane Library prior to May 1, 2015 were identified. 5 associated papers were also identified by the manual literature search. All the identified articles were screened and, after screening only 19 papers were selected. From these 19 papers, 6 studies were excluded due to following reasons:
Figure 1. Flowchart of literature search and study selection.
(1) One study31 assessed uterine leiomyomata as a study outcome;
(2) Two studies32,33, did not assess coffee intake;
(3) Three34,35,36 were overlapping cohorts.
Finally 13 articles11,37,38,39,40,41,42,43,44,45,46,47,48 were selected and these papers were included this meta-analysis. Among these articles, one study provided information both on type I and type II EC. Thus; our main meta-analysis were done in 14 comparisons obtained from 13 studies.
In the DR meta-analysis, we excluded papers of Jacobsen et al.43 and Merritt et al.48 because in the first paper only two levels of coffee intake categorised and in the second paper coffee intakes were calculated at each center in grams/day to account for differences in cup sizes by region. Finally, the DR meta-analysis was done in 12 comparisons from 11 studies only11,37,38,39,40,41,42,44,45,46,47.
Study characteristics
The Table 1 shows the characteristics of all the selected studies. All 13 selected studies were prospective cohort with subjects without EC at baseline. A total 1,534,039 participants were included in this study. A total of 10,100 cases of EC were documented during the follow-up period of 11–20 years. From the total studies eight studies11,37,39,41,43,46,47,48 were conducted in Europe, three38,42,44,45 in the USA and one in Asia (Japan40). The maximum numbers (560,356) of participants were from UK Million Women Study11 and minimum numbers (2891) were from Norwegian cohorts43. The participant’s age at baseline ranged from 30 to 79 years. Only three studies38,45,46 included postmenopausal women, and other studies11,37,39,40,41,42,43,44,47,48 included both premenopausal and postmenopausal women. For the assessment of dietary coffee intake, the Nurses’ Health Study (NHS42) used seven food frequency questionnaires (FFQs), whereas the remaining studies used one11,37,38,39,43,46,48 or two41,45,47 FFQs. Four papers38,42,44,45 (five comparisons) provided information on caffeinated coffee intake, and three articles37,38,42 (four comparisons) measured the total caffeine intake per day and the risk of EC. All studies provided adjusted risk estimates, the RRs were adjusted for age (12 studies), BMI (11 studies), smoking (12 studies) and use of exogenous hormones (9 studies). NOS scores ranged from six to nine. The most common reason for deductions was an insufficient adjustment for the potential confounders39,41,43,46,47 (Supplementary Appendix 1).
Table 1. Characteristics of prospectivestudies included that assessed the association between coffee intake and EC.
| Reference | Country | Cohort Name | Age at baseline | Follow up | Cases/Cohort Size | NOS | Highest vs. lowest (Coffee intake) | RR (95% CI) | Adjustments |
|---|---|---|---|---|---|---|---|---|---|
| Jacobsen,1986 | Norway | Norwegian cohorts | NA | 11.5 | 11/2891 | 7 | ≥7 cups/day vs. ≤2 cups/day | 0.52(0.06–4.44) | age, residence |
| Stensvold,1994 | Norway | Norwegian prospective study | 35–54 | 10.1 | 84/21238 | 6 | ≥7 cups/day vs. ≤2 cups/day | 0.8(0.34–1.90) | age, cigarettes per day, county of residence |
| Shimazu,2008 | Japan | Japan Public Health Center-based Prospective Study | 40–69 | 15 | 117/53724 | 9 | ≥3 cups/day vs. ≤2 cups/week | 0.38(0.16–0.91) | age, study center, BMI, menopausal status, age at menopause, use of exogenous hormones, smoking status, green vegetable consumption, beef consumption, pork consumption, green tea consumption |
| Friberg,2009 | Sweden | Swedish Mammography Cohort | 40–76 | 17.6 | 677/60634 | 6 | ≥4 cups/day VS.≤1 cups/day | 0.75(0.58–0.97) | age in months, BMI and smoking |
| Nilsson,2010 | Sweden | Vasterbotten Intervention Project (VIP) | 50 | 15 | 108/30639 | 7 | ≥4 cups/day vs. <1 cups/day | 0.88(0.44–1.78) | age, BMI, smoking, education, and recreational physical activity |
| Giri,2011 | USA | Women’s Health Initiative (WHI) Observational Study | 50–79 | 7.5 | 427/45696 | 8 | ≥4 cups/day vs. 0 or <1 cup/day | 0.86(0.63–1.18) | age, ethnicity, unopposed oestrogen use, progestin + oestrogen use, smoking and BMI |
| Je,2011 | USA | Nurses’Health Study (NHS) | 30–55 | 26 | 672/67470 | 7 | ≥4 cups/day vs. <1cup/day | 0.75(0.57–0.97) | age, BMI, age at menopause, age at menarche, parity and age at last birth, parity, duration of oral contraceptive use, postmenopausal hormone use, pack-years of smoking, alcohol intake and total energy intake |
| Gunter,2012 | USA | The NIH-AARP Diet and Health Study | 50–71 | 9.3 | 1486/226732 | 7 | >3cups/day vs. 0 cups/day | 0.64(0.51–0.80) | age, smoking, BMI, age at menarche, age at first child’s birth, parity, age at menopause, HT use, oral contraceptive use, diabetes and physical activity |
| Uccella1,2013 | USA | Iowa Women’s Health Study (IWHS) | 55–69 | 20 | 471/23356(Type I) 71/23356(Type II) | 8 | ≥4 cups/day vs. never or ≤1 cups/month | 0.71(0.51–0.99) (Type I) 0.84(0.33–2.12) (Type II) | age, diabetes, duration of HT use, hypertension, age at menarche, age at menopause, BMI, waist-to-hip ratio, smoking status, pack years of smoking, total energy and alcohol use. |
| Gavrilyuk,2014 | Norway | Norwegian Women and Cancer study(NOWAC) | 30–70 | 18 | 462/ 97926 | 8 | ≥ 8 cups/day vs. ≤ 1 cups/day | 0.52 (0.34–0.79) | parity, smoking status, BMI, duration of OC and HRT use |
| Weiderpass,2014 | Sweden | Swedish Women’s Lifestyle and Health | 30–49 | 10.9 | 144/ 42270 | 9 | >3cups/day vs. <2 cups/day | 0.64(0.39–1.06) | age, education, duration of hormonal contraceptive use, parity, duration of breastfeeding, smoking status and number of cigarettes/day, menopausal status, BMI, and diabetes mellitus |
| Merritt,2015 | Europe | European Prospective Investigation into Cancer and Nutrition study (EPIC) | 25–70 | 11 | 1303/301107 | 9 | 750.0 g/day vs.8.6 g/day | 0.81(0.68–0.97) | Age, BMI, total energy intake, smoking status, age at menarche, oral contraceptive use, menopausal status, postmenopausal hormone use, parity, the age of recruitment and the study center. |
| Owenyang,2015 | United Kingdom | UK Million Women Study | NA | 9.3 | 4067/560356 | 8 | ≥5cups/day vs.1-2cups/day | 0.92(0.82–1.03) | age, region, socioeconomic status, height, age at menarche, parity, duration of oral contraceptive use, age and status of menopause at study baseline, duration of HT for menopause, BMI, smoking, alcohol consumption, strenuous exercise, and other non-alcoholic fluid intake. |
NOS: Newcastle-Ottawa Scale, USA: the United States of America, UK: United Kingdom, NA: not available, BMI: Body Mass Index, HT: Hormone therapy.
Highest versus lowest meta-analysis
Fourteen comparisons from thirteen11,37,38,39,40,41,42,43,44,45,46,47,48 studies had a link between coffee ingestion and EC risk. The summary of RR for the highest and lowest categories was 0.80 (95% CI: 0.74–0.86) (Fig. 2). Heterogeneity among studies was not statistically significant (P = 0.13, I2 = 31%). Egger regression tests (P = 0.03) were used to showed publication bias, but not by Begg correlation test (P = 0.66). The estimate was found to be 0.86 (0.80–0.91) after adjusting the six missing studies (Fig. 3). Four comparisons from three studies37,40,46 provided the caffeine dose per day and EC risk. The summary RR of the highest versus the lowest categories was 0.77 (95% CI: 0.65–0.92).Heterogeneity among studies was not statistically significant (P = 0.87, I2 = 0%) and both Egger regression test(P = 0.61) and Begg correlation test (P = 0.75) did not detected publication bias. Five comparisons of the four studies38,42,44,45 reported a link between different types of coffee and EC risk. The summary RR for caffeinated coffee was 0.66 (95% CI: 0.52–0.84) along with evidence of heterogeneity (P = 0.07, I2 = 53%). As such Begg correlation test (P = 0.23) or the Egger regression test (P = 0.47) showed no evidence of substantial publication bias. For the decaffeinated coffee, the summary RR was 0.77 (95% CI: 0.63–0.94). No heterogeneity (P = 0.76, I2 = 0%) and publication bias among studies indicated by the Begg correlation test (P = 1.00) and the Egger regression test (P = 0.88). Tests for subgroup differences showed no significant differences between caffeinated and decaffeinated coffee (P = 0.35) (Table 2).
Figure 2. Forest plot of total coffee intake and relative risk of EC.
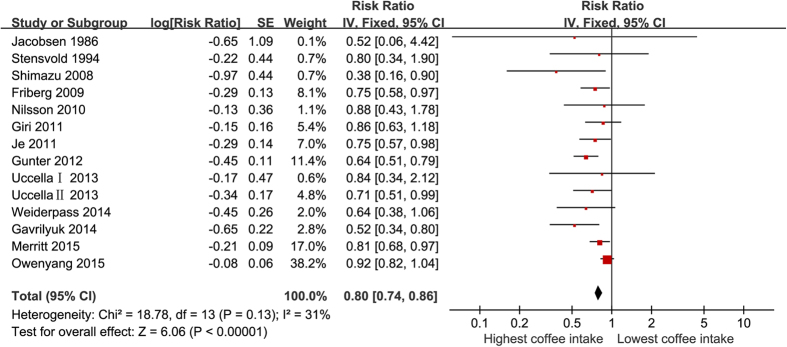
Figure 3. Filled funnel plot of total coffee intake and relative risk of EC.
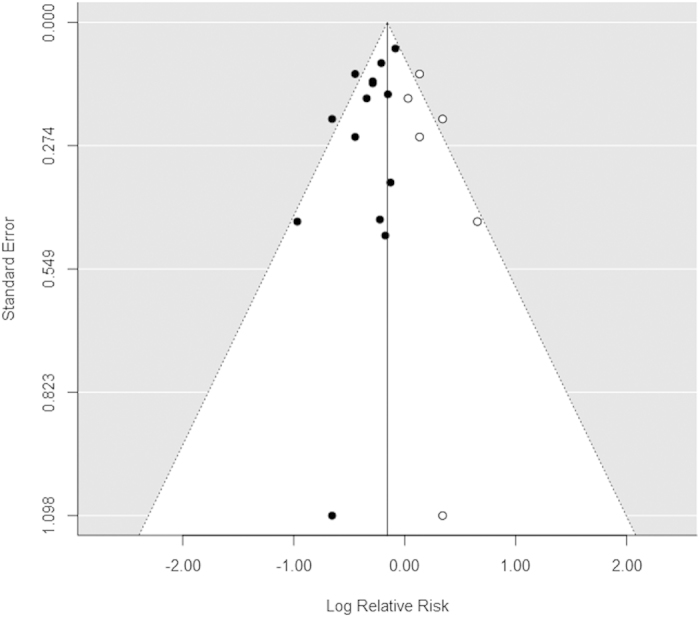
Table 2. Meta-analysis of intake of coffee, caffeine, different types of coffee and risk of EC (highest vs lowest coffee intake).
| Variables | Number of |
Test of association |
Test of heterogeneity |
Publication bias |
P* (value) | |||
|---|---|---|---|---|---|---|---|---|
| comparisons | Pooled RR(95% CI) | P value | I2 (%) | P value | Begg’s test | Egger’s test | ||
| Total coffee | 14 | 0.80(0.74–0.86) | 0.00 | 31 | 0.13 | 0.66 | 0.03 | |
| Caffeine intake | 4 | 0.77(0.65–0.92) | 0.00 | 0 | 0.87 | 0.75 | 0.61 | |
| Type of coffee | 0.35 | |||||||
| Caffeinated coffee | 5 | 0.66(0.52–0.85) | 0.00 | 53 | 0.07 | 0.23 | 0.47 | |
| Decaffeinated coffee | 5 | 0.77(0.63–0.94) | 0.01 | 0 | 0.76 | 1.00 | 0.88 | |
Subgroup and sensitivity analysis
The subgroup analyses are shown in the Table 3. The observed inverse association was more pronounced in the group of BMI more than 25 kg/m2 (RR = 0.57, 95% CI: 0.46–0.71) and in those without hormone therapy (RR = 0.60, 95% CI: 0.50–0.72). However, no association was observed in the group of BMI less than 25 kg/m2 (RR = 0.99, 95% CI: 0.86–1.15) or among hormone therapy participants (RR = 0.85, 95% CI: 0.65–1.11). Sensitivity analyses results are in the range from 0.73 (95% CI: 0.67–0.81) to 0.82 (95% CI: 0.76–0.89) for total coffee intake.
Table 3. Subgroup analysis to investigate differences between studies included in the meta-analysis (highest vs. lowest coffee intake).
| Variables | Number of comparisons |
Test of association |
Test of heterogeneity |
Publication bias |
P*(value) | |||
|---|---|---|---|---|---|---|---|---|
| Pooled RR(95% CI) | P value | I2 (%) | P value | Begg’s P value | Egger’s P value | |||
| Study location | 0.14 | |||||||
| Europe | 8 | 0.80(0.71–0.91) | 0.00 | 26 | 0.22 | 0.90 | 0.09 | |
| United States | 5 | 0.72(0.63–0.82) | 0.00 | 0 | 0.62 | 1.00 | 0.86 | |
| Asia | 1 | 0.38(0.16–0.90) | 0.03 | NA | NA | NA | NA | |
| Body Mass Index | ||||||||
| <25 kg/m2 | 7 | 0.99(0.86–1.15) | 0.94 | 0 | 0.58 | 0.07 | 0.03 | 0.00 |
| ≥25 kg/m2 | 4 | 0.57(0.46–0.71) | 0.00 | 0 | 0.48 | 1.00 | 0.84 | |
| Smoking status | 0.68 | |||||||
| Never | 7 | 0.66(0.55–0.79) | 0.00 | 0 | 0.67 | 0.13 | 0.00 | |
| Ever | 6 | 0.70(0.56–0.88) | 0.00 | 37 | 0.16 | 0.71 | 0.99 | |
| Hormone therapy | 0.04 | |||||||
| Never | 4 | 0.60(0.50–0.72) | 0.00 | 0 | 0.72 | 1.00 | 0.44 | |
| Ever | 3 | 0.85(0.65–1.11) | 0.24 | 0 | 0.92 | 1.00 | 0.80 | |
| Menopausal status | 0.30 | |||||||
| postmenopausal only | 4 | 0.72(0.60–0.88) | 0.00 | 14 | 0.001 | 0.75 | 0.78 | |
| both | 10 | 0.81(0.75–0.88) | 0.00 | 37 | 0.12 | 0.72 | 0.06 | |
| Length of follow-up | 0.77 | |||||||
| ≤15 years | 9 | 0.82(0.75–0.89) | 0.00 | 52 | 0.03 | 0.47 | 0.11 | |
| >15 years | 5 | 0.73(0.63–0.85) | 0.00 | 0 | 0.98 | 0.81 | 0.80 | |
| Number of participants | 0.57 | |||||||
| <50 000 | 7 | 0.77(0.64–0.93) | 0.01 | 0 | 0.96 | 0.44 | 0.12 | |
| >50 000 | 7 | 0.72(0.60–0.85) | 0.00 | 72 | 0.00 | 0.23 | 0.00 | |
| Number of cases | 0.55 | |||||||
| <200 | 6 | 0.67(0.49–0.92) | 0.01 | 0 | 0.74 | 1.00 | 0.87 | |
| >200 | 8 | 0.75(0.65–0.86) | 0.00 | 64 | 0.00 | 0.26 | 0.02 | |
| Adjust for BMI | 0.95 | |||||||
| Yes | 12 | 0.74(0.65–0.84) | 0.00 | 52 | 0.02 | 0.73 | 0.04 | |
| NO | 2 | 0.76(0.34–1.68) | 0.49 | 0 | 0.72 | 1.00 | 0.72 | |
| Adjust for hormone therapy | 0.74 | |||||||
| Yes | 10 | 0.73(0.63–0.84) | 0.00 | 60 | 0.00 | 0.37 | 0.02 | |
| NO | 4 | 0.76(0.61–0.96) | 0.02 | 0 | 0.96 | 0.75 | 0.99 | |
P* was utilized to assess the subgroup differences.
DR analysis
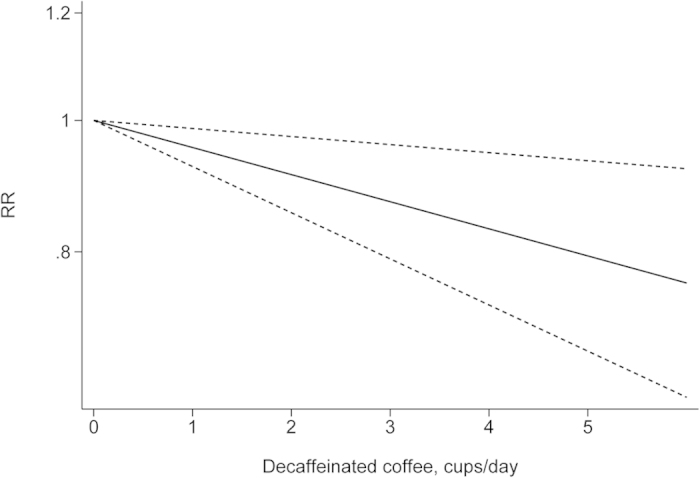
The departure from nonlinearity was not significant (P = 0.90), and an increase of 1 cup of coffee per day was associated with a 5% (RR = 0.95, 95% CI: 0.93–0.97) lower risk of EC (Fig. 4). Four comparisons from three studies37,38,42 included DR analysis of caffeine intake per day and risk of EC. The test for nonlinearity (P = 0.89) supported a linear model and indicated that an increased intake of 100 mg caffeine per day was associated with a 4% (RR = 0.96, 95% CI: 0.93–0.98) lower risk of EC (Fig. 5). Five comparisons from four studies42,44,45,46 included the DR analysis of different types of coffee and risk of EC. For caffeinated coffee, the test for nonlinearity was not significant (P = 0.82), and the results showed that increased intake of 1 cup of caffeinated coffee per day was associated with a 7% (RR = 0.93, 95% CI: 0.89–0.97) lower risk of EC (Fig. 6). For decaffeinated coffee, the test for nonlinearity was not significant (P = 0.50), and the results showed that increased intake of 1 cup of decaffeinated coffee per day was associated with a 4% (RR = 0.96, 95% CI: 0.92–0.99) lower risk of EC (Fig. 7).
Figure 4. Dose-response analysis of total coffee intake and relative risk of EC.
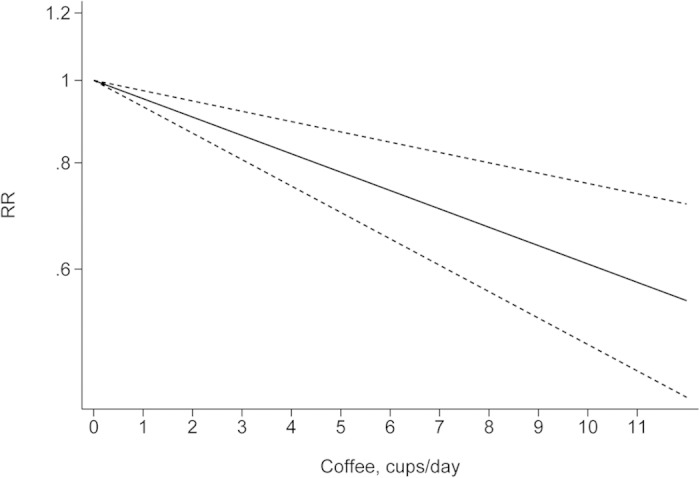
Figure 5. Dose-response analysis of caffeine intake per day and risk of EC.
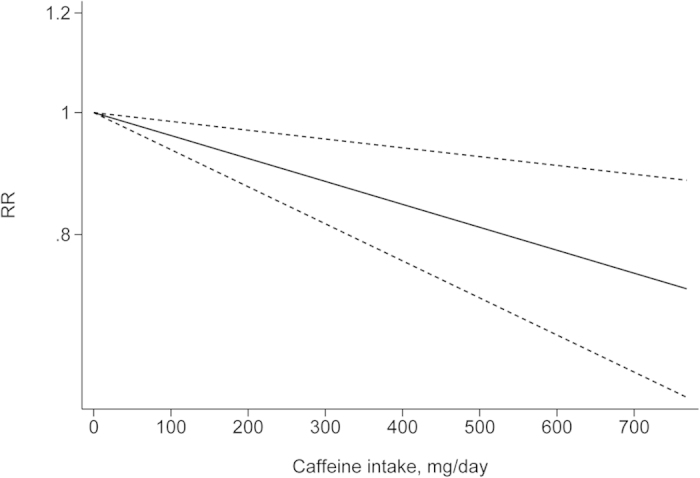
Figure 6. Dose-response analysis of caffeinated coffee intake and risk of EC.
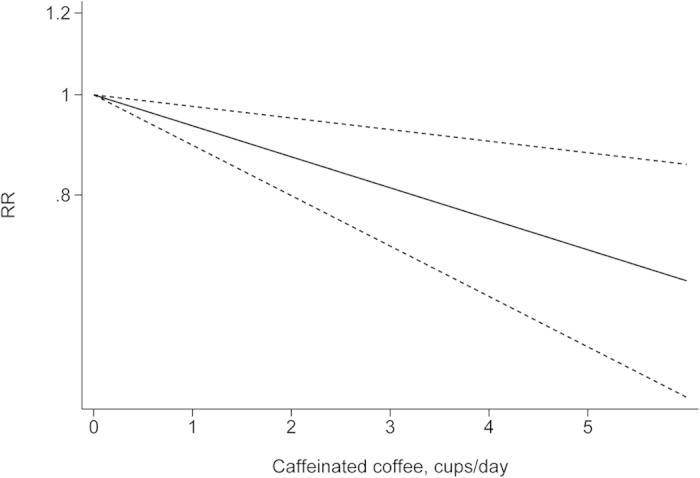
Figure 7. Dose-response analysis of decaffeinated coffee intake and risk of EC.
Power analysis and quality of evidence
Power calculations were performed post hoc as per the method described by Cafri et al.30. The power to detect an RR of 0.80 for the total coffee intake was 100%, 84.98% for an RR of 0.77 for caffeine intake per day, 92.09% for an RR of 0.66 for caffeinated coffee intake and 72.47% for an RR of 0.77 for decaffeinated coffee intake (Supplementary Appendix 2). RR of total coffee intake and EC was the critical outcome. The others were important outcomes. The evidence of quality for caffeine dosage, decaffeinated coffee intake and risk of EC were moderate. The evidence of quality for total coffee intake, caffeinated coffee intake and risk of EC were low (Table 4).
Table 4. Assessment of quality using the GRADE system.
| Outcome | Quality assessment |
Quality | Importance | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | Other considerations | |||
| Total coffee intake and EC incidence | 11 | cohort study | no serious risk of bias | no serious inconsistency | no serious indirectness | no serious imprecision | detected1 | dose response gradient2 | low | critical |
| Caffeine dosage and EC incidence | 3 | cohort study | no serious risk of bias | no serious inconsistency | no serious indirectness | no serious imprecision | undetected | dose response gradient3 | moderate | important |
| Caffeinated coffee intake and EC incidence | 4 | cohort study | no serious risk of bias | serious inconsistency4 | no serious indirectness | no serious imprecision | undetected | dose response gradient5 | low | important |
| Decaffeinated coffee intake and EC incidence | 4 | cohort study | no serious risk of bias | no serious inconsistency | no serious indirectness | no serious imprecision | undetected | dose response gradient6 | moderate | important |
1We conducted Egger’s test to detect the publication bias; the p value was 0.03.
2We conducted dose-response analysis of total coffee consumption and EC, and the result showed that increased intake of1 cup of coffee per day was associated with 5% lower risk of EC.
3We conducted dose-response analysis of caffeine dosage and EC, and the result showed that increased intake of 100 mg caffeine per day was associated with a 4% lower risk of EC.
4Moderate heterogeneity was detected (P = 0.08, I2 = 52%).
5We conducted dose-response analysis of caffeinated coffee consumption and EC, and the result showed that increased intake of 1 cup of coffee per day was associated with a 7% lower risk of EC.
6We conducted dose-response analysis of decaffeinated coffee consumption and EC, and the result showed that increased intake of 1 cup of coffee per day was associated with a 4% lower risk of EC.
Discussion
The possible relationship between the intake of coffee and EC risk was first explored in a Norwegian prospective study in 198643; many cohort studies have attempted to confirm this hypothesis. However, the role of coffee intake in EC is controversial. Six studies40,42,44,46,47,48 have found a decreased risk of EC with high coffee intake, but five studies11,37,39,41,45 failed to find a significant association. Additionally, in an Iowa Women’s Health Study (IWHS)38, a significant inverse association was found only with Type I EC. The intake of total coffee, caffeine, and caffeinated and decaffeinated coffee was inversely associated with EC in a highest versus lowest meta-analyses. The intake of caffeine results in the percentage decrease in risk of EC, which was shown by DR analyses. This study indicated a 5% lower risk of EC by increase the intake of total coffee having each 1 cup/day, a 4% lower risk of EC with each 100 mg/day by an increase in the intake of total caffeine, a 7% lower risk of EC with 1 cup/day by an increase in the intake of caffeinated coffee and a 4% lower risk of EC with each 1 cup/day as we increases the intake of decaffeinated coffee. Our results are consistent with the EC 2013 report of the continuous update project WCRF/AICR9. A DR meta-analysis showed a 7% and 8% decreased risk of EC with an increased intake of one cup of total coffee and decaffeinated coffee per day, respectively.
The prospective cohort study design was included in almost all the studies and little heterogeneity was found in our meta-analysis. Except one study in Japan40, various studies conducted in Western countries where most of the population shared genetic background, lifestyle, dietary pattern49; and the EC incidences in most studies were comparable. Mostly the results of our subgroup analyses were quite similar and robust. There were no differences in study location, smoking status, menopausal status, follow-up length, number of participants and number of cases, but the associations was modified by BMI and history of hormone therapy. The inverse association was more among participants having a BMI greater than 25 kg/m2 (RR = 0.57, 95% CI: 0.46–0.71) and among participants never received hormone therapy (RR = 0.66, 95% CI: 0.550.79). However, whether the study adjusted for BMI or hormone therapy did not affect the results in our study. The statistically non-significant subgroup differences occurred because of the small number of studies, which did not adjust for BMI (n = 2) or hormone therapy (n = 4) such that there was insufficient statistical power.
Several mechanisms were explained for the association of the coffee with EC. Caffeine and methylxanthine in coffee may increase amount of sex-hormone-binding globulin, thus reducing the concentrations of sex-steroids and leading to down regulation of endometrial hyper proliferation50. These compounds include phenol compounds, chlorogenic acid, which produces catechins, caffeic, ferulic and coumaric acids that are partly lost during roasting51, melanoidins that are mainly produced during roasting and diterpenes that may have anticarcinogenic effects8,52. In addition, it is also reported that coffee may be an insulin sensitizer53. Coffee and caffeine intake were inversely related with intensities of circulating C-peptide, this association was much higher in overweight and obese women54.
The current study has several advantages, compared to the previous published meta-analyses10,11,12,13. A DR analysis was also conducted to see the possible associations so the results are more reliable. In addition, the association between different caffeine dosages and types of coffee was examined. Furthermore, the statistical power was calculated to assess the probability of correctly rejecting the null hypothesis. In this meta-analysis, the power for the RR value of coffee intake and risk of EC incidence was 100%.
There are several limitations of this study as listed below:
(1) Most of the studies from America and Europe and only one study conducted in Japan. So more studies need to be included from other part of world much as Asia and South Africa.
(2) Coffee intake data were collected using food frequency questionnaires (FFQs). Findings from large population-based studies in Sweden48,55,56 (EPIC cohort) and the US44 (NIH-AARP cohort) have shown that coffee intake tends to be stable over a very long period of time.
(3) Most studies did not distinguish between types of EC. Only one study38 reported a statistically non-significant protective effect between coffee intake and type II EC. However, recent studies from the University of Southern California have shown that two EC types share many common aetiologic factors. The aetiology of type II tumours may not be completely oestrogen independent, as previously believed57.
(4) The possibility of residual confounding due to other risk factors cannot be excluded, although most investigators had adjusted BMI, hormone therapy, and smoking status.
(5) Publication bias was detected in the total coffee intake and EC risk. However, we added six studies with null results trend in the funnel plot, the overall effect size still showed a protective effect.
(6) The GRADE quality of the evidence was moderate or low, which lowers confidence in any subsequent recommendations.
This study meets several of the Hill criteria for causation58,59: Temporality; Strength; Consistency; Biological gradient; Plausibility.
In summary, coffee, regardless of coffee type and caffeine dosage, is a potential protective factor against EC in a dose-dependent fashion; the results corroborate the findings of the conclusion of the EC 2013 Report by WCRF/AICR. Further, the association of coffee intake and EC risk may be adapted by BMI and history of hormone therapy.
Additional Information
How to cite this article: Zhou, Q. et al. Coffee consumption and risk of endometrial cancer: a dose-response meta-analysis of prospective cohort studies. Sci. Rep. 5, 13410; doi: 10.1038/srep13410 (2015).
Supplementary Material
Acknowledgments
This study was not supported by any of the funding agencies or any private funder.
Footnotes
Author Contributions Q.Z. and Z.J.G. conceived of and designed the experiments. Q.Z., Z.J.G., M.L., H.L. and M.L. performed the experiments.Q.Z. and Z.J.G. analyzed the data. H.L., M.L., Q.Z. and Z.J.G. wrote the paper.
References
- Jemal A. et al. Global cancer statistics. CA-Cancer J Clin 61, 69–90, 10.3322/caac.20107 (2011). [DOI] [PubMed] [Google Scholar]
- Olesen T. B. et al. Prevalence of Human Papillomavirus in endometrial cancer: a systematic review and meta-analysis. Gynecol Oncol 134, 206–215, 10.1016/j.ygyno.2014.02.040 (2014). [DOI] [PubMed] [Google Scholar]
- Siegel R. L., Miller K. D. & Jemal A. Cancer statistics, 2015. CA-Cancer J Clin 65, 5–29, 10.3322/caac.21254 (2015). [DOI] [PubMed] [Google Scholar]
- Weisse A. B. Coffee: grounds for concern? Proceedings (Baylor University. Medical Center) 28, 122–123 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn S. K., Blomhoff R. & Paur I. Coffee and cancer risk, epidemiological evidence, and molecular mechanisms. Mol Nutr Food Res 58, 915–930, 10.1002/mnfr.201300526 (2014). [DOI] [PubMed] [Google Scholar]
- Je Y. & Giovannucci E. Coffee consumption and total mortality: a meta-analysis of twenty prospective cohort studies. Br J Nutr 111, 1162–1173, 10.1017/s0007114513003814 (2014). [DOI] [PubMed] [Google Scholar]
- Crippa A., Discacciati A., Larsson S. C., Wolk A. & Orsini N. Coffee consumption and mortality from all causes, cardiovascular disease, and cancer: a dose-response meta-analysis. Am J Epidemiol 180, 763–775, 10.1093/aje/kwu194 (2014). [DOI] [PubMed] [Google Scholar]
- Malerba S. et al. A meta-analysis of prospective studies of coffee consumption and mortality for all causes, cancers and cardiovascular diseases. Eur J Epidemiol 28, 527–539, 10.1007/s10654-013-9834-7 (2013). [DOI] [PubMed] [Google Scholar]
- Research, W. C. R. F. A. I. f. C. Continuous Update Project Report: Endometrial Cancer 2013 Report. (2013).
- Yu X., Bao Z., Zou J. & Dong J. Coffee consumption and risk of cancers: a meta-analysis of cohort studies. BMC Cancer 11, 96, 10.1186/1471-2407-11-96 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W. et al. Tea and coffee and risk of endometrial cancer: cohort study and meta-analysis. Am J Clin Nutr 101, 570–578, 10.3945/ajcn.113.081836 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Je Y. & Giovannucci E. Coffee consumption and risk of endometrial cancer: findings from a large up-to-date meta-analysis. Int J cancer. 131, 1700–1710, 10.1002/ijc.27408 (2012). [DOI] [PubMed] [Google Scholar]
- Bravi F. et al. Coffee drinking and endometrial cancer risk: a meta-analysis of observational studies. Am J Obstet Gynecol 200, 130–135, 10.1016/j.ajog.2008.10.032 (2009). [DOI] [PubMed] [Google Scholar]
- Stroup D. F. et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA 283, 2008–2012 (2000). [DOI] [PubMed] [Google Scholar]
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25, 603–605, 10.1007/s10654-010-9491-z (2010). [DOI] [PubMed] [Google Scholar]
- Fang X. et al. Dietary intake of heme iron and risk of cardiovascular disease: A dose-response meta-analysis of prospective cohort studies. Nutr Metab Cardiovasc Dis, 10.1016/j.numecd.2014.09.002 (2014). [DOI] [PubMed] [Google Scholar]
- Zhou J. G. et al. Treatment on advanced NSCLC: platinum-based chemotherapy plus erlotinib or platinum-based chemotherapy alone? A systematic review and meta-analysis of randomised controlled trials. Med Oncol 32, 471, 10.1007/s12032-014-0471-0 (2015). [DOI] [PubMed] [Google Scholar]
- Atkins D. et al. Grading quality of evidence and strength of recommendations. BMJ 328, 1490, 10.1136/bmj.328.7454.1490 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J. et al. Intramedullary nail versus dynamic compression plate fixation in treating humeral shaft fractures: grading the evidence through a meta-analysis. PloS One 8, e82075, 10.1371/journal.pone.0082075 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol rev 9, 1–30 (1987). [DOI] [PubMed] [Google Scholar]
- Wu W. et al. Coffee consumption and bladder cancer: a meta-analysis of observational studies. Sci Rep 5, 9051, 10.1038/srep09051 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenland S. & Longnecker M. P. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol 135, 1301–1309 (1992). [DOI] [PubMed] [Google Scholar]
- Orsini N., Bellocco R. & Greenland S. Generalized least squares for trend estimation of summarized dose-response data. Stata J 6, 40 (2006). [Google Scholar]
- Orsini N., Li R., Wolk A., Khudyakov P. & Spiegelman D. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol 175, 66–73, 10.1093/aje/kwr265 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J. & Thompson S. G. Quantifying heterogeneity in a meta-analysis. Stat Med 21, 1539–1558 (2002). [DOI] [PubMed] [Google Scholar]
- DerSimonian R. & Laird N. Meta-analysis in clinical trials. Control Clin Trials 7, 177–188 (1986). [DOI] [PubMed] [Google Scholar]
- Begg C. B. & Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 50, 1088–1101 (1994). [PubMed] [Google Scholar]
- Egger M., Davey Smith G., Schneider M. & Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval S. & Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 56, 455–463 (2000). [DOI] [PubMed] [Google Scholar]
- Cafri G., Kromrey J. D. & Brannick M. T. A SAS macro for statistical power calculations in meta-analysis. Behav Res Methods 41, 35–46, 10.3758/BRM.41.1.35 (2009). [DOI] [PubMed] [Google Scholar]
- Wise L. A. et al. Risk of uterine leiomyomata in relation to tobacco, alcohol and caffeine consumption in the Black Women’s Health Study. Hum Reprod 19, 1746–1754, 10.1093/humrep/deh309 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue-Choi M., Robien K., Mariani A., Cerhan J. R. & Anderson K. E. Sugar-sweetened beverage intake and the risk of type I and type II endometrial cancer among postmenopausal women. Cancer Epidemiol Biomarkers Pre 22, 2384–2394 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott J. et al. Dietary insulin index and insulin load in relation to endometrial cancer risk in the Nurses’ Health Study. Cancer Epidemiol Biomarkers Pre 23, 1512–1520 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilyuk O., Braaten T., Skeie G. & Lund E. Coffee consumption and endometrial cancer risk among postmenopausal women in the norwegian women and cancer (NOWAC) study. Int J Gynecol Cancer 21, S509 (2011). [Google Scholar]
- Giri A., Reeves K., Luisi N., Balasubramanian R. & Sturgeon S. Coffee, tea consumption and endometrial cancer risk: A prospective cohort study. Am J Epidemiol 173, S233 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uccella S. et al. Coffee consumption and the risk of endometrial cancer in postmenopausal women. Gynecol Oncol 116, S75–S76 (2010). [Google Scholar]
- Weiderpass E. et al. Endometrial cancer in relation to coffee, tea, and caffeine consumption: a prospective cohort study among middle-aged women in sweden. Nut Cancer 66, 1132–1143, 10.1080/01635581.2014.948214 (2014). [DOI] [PubMed] [Google Scholar]
- Uccella S. et al. Intake of coffee, caffeine and other methylxanthines and risk of Type I vs Type II endometrial cancer. Brit J Cancer 109, 1908–1913, 10.1038/bjc.2013.540 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stensvold I. & Jacobsen B. K. Coffee and cancer: a prospective study of 43,000 Norwegian men and women. Cancer Cause Control 5, 401–408 (1994). [DOI] [PubMed] [Google Scholar]
- Shimazu T. et al. Coffee consumption and risk of endometrial cancer: a prospective study in Japan. Int J Cancer 123, 2406–2410, 10.1002/ijc.23760 (2008). [DOI] [PubMed] [Google Scholar]
- Nilsson L. M., Johansson I., Lenner P., Lindahl B. & Van Guelpen B. Consumption of filtered and boiled coffee and the risk of incident cancer: A prospective cohort study. Cancer Cause Control 21, 1533–1544 (2010). [DOI] [PubMed] [Google Scholar]
- Je Y., Hankinson S. E., Tworoger S. S., DeVivo I. & Giovannucci E. A prospective cohort study of coffee consumption and risk of endometrial cancer over a 26-year follow-up. Cancer Epidemiol Biomarkers Pre 20, 2487–2495 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen B. K., Bjelke E., Kvale G. & Heuch I. Coffee drinking, mortality, and cancer incidence: results from a Norwegian prospective study. J Natl Cancer Inst 76, 823–831 (1986). [PubMed] [Google Scholar]
- Gunter M. J. et al. A prospective investigation of coffee drinking and endometrial cancer incidence. Int J Cancer 131, E530–536, 10.1002/ijc.26482 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri A. et al. Caffeinated coffee, decaffeinated coffee and endometrial cancer risk: a prospective cohort study among US postmenopausal women. Nutrients 3, 937–950, 10.3390/nu3110937 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilyuk O. et al. High coffee consumption and different brewing methods in relation to postmenopausal endometrial cancer risk in the Norwegian women and cancer study: a population-based prospective study. BMC Womens Health 14, 48, 10.1186/1472-6874-14-48 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friberg E., Orsini N., Mantzoros C. S. & Wolk A. Coffee drinking and risk of endometrial cancer - A population-based cohort study. Int J Cancer 125, 2413–2417 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt M. A. et al. Investigation of dietary factors and endometrial cancer risk using a nutrient-wide association study approach in the EPIC and Nurses’ Health Study (NHS) and NHSII. Cancer Epidemiol Biomarkers Pre 24, 466–471, 10.1158/1055-9965.EPI-14-0970 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., He M. & Zhang X. Combined intravitreal anti-VEGF and photodynamic therapy versus photodynamic monotherapy for polypoidal choroidal vasculopathy: a systematic review and meta-analysis of comparative studies. PloS one 9, e110667, 10.1371/journal.pone.0110667 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrini R. L. & Barrett-Connor E. Caffeine intake and endogenous sex steroid levels in postmenopausal women. The Rancho Bernardo Study. Am J Epidemiol 144, 642–644 (1996). [DOI] [PubMed] [Google Scholar]
- Garattini S. Caffeine, coffee, and health. Vol. 5 (Raven Press: New York, , 1993). [Google Scholar]
- Cavin C. et al. Cafestol and kahweol, two coffee specific diterpenes with anticarcinogenic activity. Food Chem Toxicol 40, 1155–1163 (2002). [DOI] [PubMed] [Google Scholar]
- Loopstra-Masters R. C., Liese A. D., Haffner S. M., Wagenknecht L. E. & Hanley A. J. Associations between the intake of caffeinated and decaffeinated coffee and measures of insulin sensitivity and beta cell function. Diabetologia 54, 320–328, 10.1007/s00125-010-1957-8 (2011). [DOI] [PubMed] [Google Scholar]
- Wu T., Willett W. C., Hankinson S. E. & Giovannucci E. Caffeinated coffee, decaffeinated coffee, and caffeine in relation to plasma C-peptide levels, a marker of insulin secretion, in U.S. women. Diabetes Care 28, 1390–1396 (2005). [DOI] [PubMed] [Google Scholar]
- Oh J. K. et al. Prospective study of breast cancer in relation to coffee, tea and caffeine in Sweden. Int J Cancer, 10.1002/ijc.29569 (2015). [DOI] [PubMed] [Google Scholar]
- Nilsson L. M. et al. Consumption of filtered and boiled coffee and the risk of first acute myocardial infarction: a nested case/referent study. Nutr Metab Cardiovasc Dis 20, 527–535, 10.1016/j.numecd.2009.05.004 (2010). [DOI] [PubMed] [Google Scholar]
- Setiawan V. W. et al. Type I and II endometrial cancers: have they different risk factors? J Clin Oncol 31, 2607–2618, 10.1200/jco.2012.48.2596 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J. Y., Zhang Y. H. & Qin L. Q. Erectile dysfunction and risk of cardiovascular disease: meta-analysis of prospective cohort studies. J Am Coll Cardiol 58, 1378–1385, 10.1016/j.jacc.2011.06.024 (2011). [DOI] [PubMed] [Google Scholar]
- Hill A. B. The environment and disease: association or causation? Proceedings of the Royal Society of Medicine 58, 295 (1965). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.