Abstract
The plastid genome in higher plants contains >50 genes for rRNAs, tRNAs and proteins for transcriptional and translational functions, besides the genes encoding photosynthetic proteins. Considering the totipotency of most higher plant cells and the differentiation capacity of plastids, it can be inferred that at least the genes for genetic functions must be constitutively expressed in all plant organs, including non-photosynthetic roots, to maintain a basal level of transcriptional and translational activities. To test this hypothesis, transcription, RNA accumulation and polysome formation were analyzed in root amyloplasts, and in plastids from hypocotyls and cotyledons of dark-grown spinach seedlings. The results for 10 representative genes show that they are constitutively transcribed at relative rates which are similar in root amyloplasts and leaf chloroplasts. The differential accumulation of their mRNAs in roots and other non-photosynthetic plant organs is controlled at the post-transcriptional level by a developmental program. Although mRNAs for photosynthetic proteins are detectable in root amyloplasts, some of them are specifically depleted from polysomes relative to mRNAs for ribosomal proteins. This translational discrimination does not result from modifications in splicing or 5'- and 3' -end processing of mRNAs for photosynthetic proteins, since processing is identical in root amyloplasts and leaf chloroplasts. The results support the model of constitutive transcription of the plastid genome, and indicate that the expression of most plastid genes in spinach plants is controlled primarily by post-transcriptional and translational mechanisms.
Keywords: plastid, transcription, translation, gene expression, chloroplasts
Full text
PDF




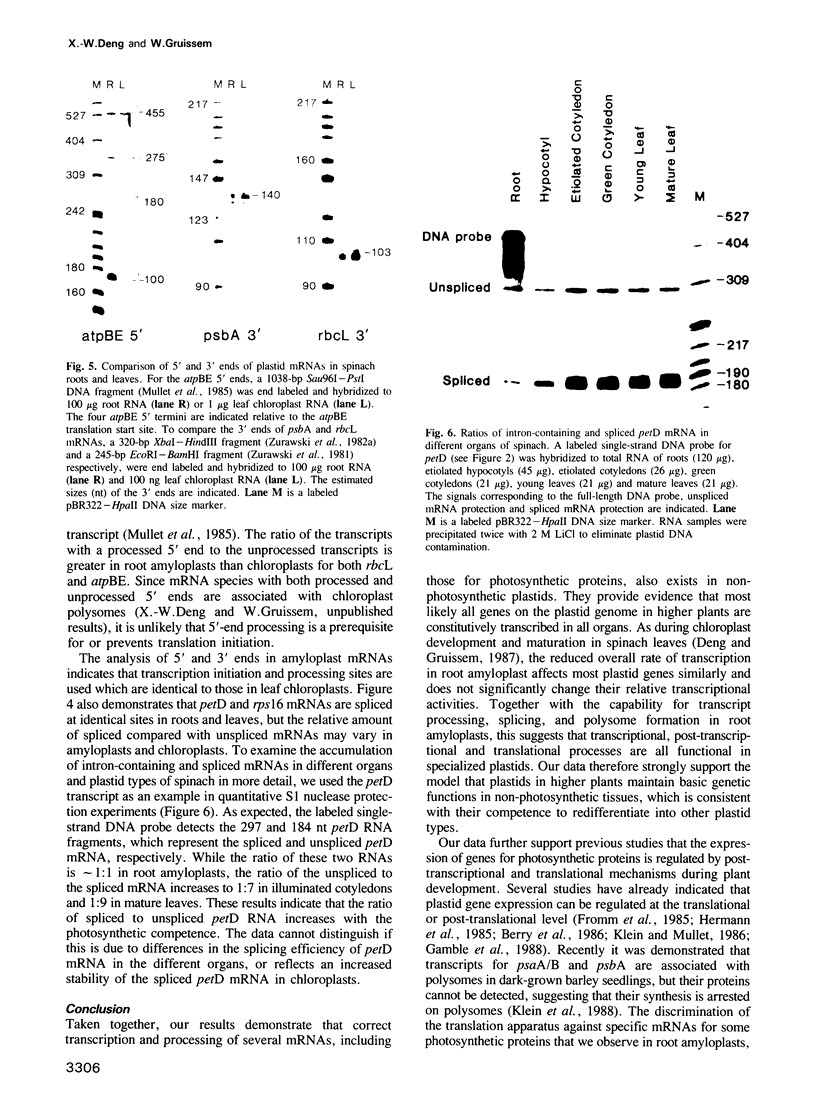


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berry J. O., Nikolau B. J., Carr J. P., Klessig D. F. Translational regulation of light-induced ribulose 1,5-bisphosphate carboxylase gene expression in amaranth. Mol Cell Biol. 1986 Jul;6(7):2347–2353. doi: 10.1128/mcb.6.7.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X. W., Gruissem W. Control of plastid gene expression during development: the limited role of transcriptional regulation. Cell. 1987 May 8;49(3):379–387. doi: 10.1016/0092-8674(87)90290-x. [DOI] [PubMed] [Google Scholar]
- Deng X. W., Stern D. B., Tonkyn J. C., Gruissem W. Plastid run-on transcription. Application to determine the transcriptional regulation of spinach plastid genes. J Biol Chem. 1987 Jul 15;262(20):9641–9648. [PubMed] [Google Scholar]
- Fromm H., Devic M., Fluhr R., Edelman M. Control of psbA gene expression: in mature Spirodela chloroplasts light regulation of 32-kd protein synthesis is independent of transcript level. EMBO J. 1985 Feb;4(2):291–295. doi: 10.1002/j.1460-2075.1985.tb03628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble P. E., Sexton T. B., Mullet J. E. Light-dependent changes in psbD and psbC transcripts of barley chloroplasts: accumulation of two transcripts maintains psbD and psbC translation capability in mature chloroplasts. EMBO J. 1988 May;7(5):1289–1297. doi: 10.1002/j.1460-2075.1988.tb02943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruissem W., Zurawski G. Analysis of promoter regions for the spinach chloroplast rbcL, atpB and psbA genes. EMBO J. 1985 Dec 16;4(13A):3375–3383. doi: 10.1002/j.1460-2075.1985.tb04093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A. O., Larkins B. A. Influence of Ionic Strength, pH, and Chelation of Divalent Metals on Isolation of Polyribosomes from Tobacco Leaves. Plant Physiol. 1976 Jan;57(1):5–10. doi: 10.1104/pp.57.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A. O., Larkins B. A. Influence of Ionic Strength, pH, and Chelation of Divalent Metals on Isolation of Polyribosomes from Tobacco Leaves. Plant Physiol. 1976 Jan;57(1):5–10. doi: 10.1104/pp.57.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R. R., Mason H. S., Mullet J. E. Light-regulated translation of chloroplast proteins. I. Transcripts of psaA-psaB, psbA, and rbcL are associated with polysomes in dark-grown and illuminated barley seedlings. J Cell Biol. 1988 Feb;106(2):289–301. doi: 10.1083/jcb.106.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubayashi T., Wakasugi T., Shinozaki K., Yamaguchi-Shinozaki K., Zaita N., Hidaka T., Meng B. Y., Ohto C., Tanaka M., Kato A. Six chloroplast genes (ndhA-F) homologous to human mitochondrial genes encoding components of the respiratory chain NADH dehydrogenase are actively expressed: determination of the splice sites in ndhA and ndhB pre-mRNAs. Mol Gen Genet. 1987 Dec;210(3):385–393. doi: 10.1007/BF00327187. [DOI] [PubMed] [Google Scholar]
- Mullet J. E., Klein R. R. Transcription and RNA stability are important determinants of higher plant chloroplast RNA levels. EMBO J. 1987 Jun;6(6):1571–1579. doi: 10.1002/j.1460-2075.1987.tb02402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NORTON J. P., BOLL W. G. Callus and shoot formation from tomato roots in vitro. Science. 1954 Feb 12;119(3085):220–221. doi: 10.1126/science.119.3085.220. [DOI] [PubMed] [Google Scholar]
- Ophir I., Ben-Shaul Y. Separation and Ultrastructure of Proplastids from Dark-grown Euglena Cells. Plant Physiol. 1973 Jun;51(6):1109–1116. doi: 10.1104/pp.51.6.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piechulla B., Glick R. E., Bahl H., Melis A., Gruissem W. Changes in Photosynthetic Capacity and Photosynthetic Protein Pattern during Tomato Fruit Ripening. Plant Physiol. 1987 Jul;84(3):911–917. doi: 10.1104/pp.84.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso S. W. The ultrastructure of chromoplast development in red tomatoes. J Ultrastruct Res. 1968 Nov;25(3):307–322. doi: 10.1016/s0022-5320(68)80076-0. [DOI] [PubMed] [Google Scholar]
- Shinozaki K., Ohme M., Tanaka M., Wakasugi T., Hayashida N., Matsubayashi T., Zaita N., Chunwongse J., Obokata J., Yamaguchi-Shinozaki K. The complete nucleotide sequence of the tobacco chloroplast genome: its gene organization and expression. EMBO J. 1986 Sep;5(9):2043–2049. doi: 10.1002/j.1460-2075.1986.tb04464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern D. B., Gruissem W. Control of plastid gene expression: 3' inverted repeats act as mRNA processing and stabilizing elements, but do not terminate transcription. Cell. 1987 Dec 24;51(6):1145–1157. doi: 10.1016/0092-8674(87)90600-3. [DOI] [PubMed] [Google Scholar]
- Zurawski G., Bohnert H. J., Whitfeld P. R., Bottomley W. Nucleotide sequence of the gene for the M(r) 32,000 thylakoid membrane protein from Spinacia oleracea and Nicotiana debneyi predicts a totally conserved primary translation product of M(r) 38,950. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7699–7703. doi: 10.1073/pnas.79.24.7699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawski G., Bottomley W., Whitfeld P. R. Junctions of the large single copy region and the inverted repeats in Spinacia oleracea and Nicotiana debneyi chloroplast DNA: sequence of the genes for tRNAHis and the ribosomal proteins S19 and L2. Nucleic Acids Res. 1984 Aug 24;12(16):6547–6558. doi: 10.1093/nar/12.16.6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawski G., Bottomley W., Whitfeld P. R. Structures of the genes for the beta and epsilon subunits of spinach chloroplast ATPase indicate a dicistronic mRNA and an overlapping translation stop/start signal. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6260–6264. doi: 10.1073/pnas.79.20.6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawski G., Perrot B., Bottomley W., Whitfeld P. R. The structure of the gene for the large subunit of ribulose 1,5-bisphosphate carboxylase from spinach chloroplast DNA. Nucleic Acids Res. 1981 Jul 24;9(14):3251–3270. doi: 10.1093/nar/9.14.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]









