Abstract
Decompensated hepatic failure occurred in a patient with a rare blood type. The patient had extreme hemodilution due to massive bleeding during liver transplantation. A shortage of matched and universal donor blood prompted us to transfuse albumin and fresh frozen plasma for intravascular volume resuscitation. The lowest hemoglobin was 0.6 g/dL, accompanied by ST depression and a serum lactate of 100 mg/dL. The accuracy of the measured value of 0.6 g/dL was confirmed. However, the patient recovered from this critical situation after transfusion, and he was eventually discharged from the hospital without significant sequelae. Maintaining normovolemia, administering pure oxygen, ensuring appropriate anesthetic depth, and maintaining minimal inotropic support were essential for this patient’s survival during massive bleeding.
Massive bleeding is a primary cause of intraoperative cardiac arrest. Affected patients often experience poor outcomes, including death.1 The tolerable limit of anemia, that is, the threshold hemoglobin value below which patients develop ischemic organ dysfunction associated with elevated serum lactate levels, is not known with certainty.
We report the case of a patient with a rare blood type who developed extreme hemodilution during liver transplantation. The lowest measured hemoglobin concentration was 0.6 g/dL, which was associated with an episode of ventricular tachycardia (VT), sustained ST segment depression on the electrocardiogram (ECG), and a precipitous increase in serum lactate level. Despite these serious conditions, the patient recovered and was discharged without significant sequelae.
The patient reviewed the Japanese translation of this manuscript and gave written permission for the authors to publish the report.
CASE DESCRIPTION
A 45-year-old Japanese man was admitted with decompensated hepatic failure resulting from primary biliary cirrhosis. He had previously been diagnosed with hepatocellular carcinoma and had been treated successfully with percutaneous radiofrequency ablation. However, because his state of decompensated hepatic failure had persisted, cadaveric split liver transplantation was indicated. The patient had a history of splenectomy and devascularization of the esophagus (the Hassab procedure). He also had a rare blood type, A-RhD(−), having a prevalence of 0.5% in Japan,2 and coagulopathy secondary to hepatic dysfunction. He did not have any cardiovascular disease. His height was 173 cm, and his weight was 91 kg.
Blood products (2800 mL of red cell concentrates–leukocytes reduced [RCC-LR], 6000 mL of fresh frozen plasma–leukocytes reduced [FFP-LR], and 250 mL of platelet concentrates) were available. General anesthesia was induced with thiopental and fentanyl. Vecuronium was administered to facilitate tracheal intubation. Mechanical ventilation was established, and general anesthesia was maintained with air, oxygen, sevoflurane, midazolam, and fentanyl. Central venous lines, a large-bore venous sheath, and a pulmonary arterial catheter were inserted. Dopamine and norepinephrine were administered by continuous infusion for inotropic support.
During separation of dense adhesions in the abdominal cavity, bleeding persisted at a rate of approximately 5000 mL/h. Seven hours after the initial incision was made, the patient’s hemoglobin concentration was 3.6 g/dL, after preoperatively prepared 2800 mL of RCC-LR blood product had been transfused, as well as large quantities of other blood derivatives, including FFP-LR and 5% albumin. We ordered additional units of A(−) and O(−) RCC-LRs.
Ten minutes after portal reperfusion, a transient ST elevation was noted in the inferior leads. Pure oxygen and nicorandil, a drug with coronary vasodilator properties attributable to nitrate and K+-ATP channel agonist activities, were started in an effort to improve the coronary oxygen supply. Monomorphic nonsustained VT developed after the first minute of ST segment elevation. The VT and ST segment elevation resolved immediately after lidocaine administration (Fig. 1). Arterial blood gas and laboratory values obtained just after ST segment resolution revealed acidemia (pH 7.157), normokalemia (4.2 mmol/L), a low ionized calcium level (0.64 mmol/L), and anemia (hemoglobin 8.0 g/dL). At that time, we had transfused 5600 mL of A(−) and O(−) RCC-LR in total. Acidemia and hypocalcemia were treated using boluses of sodium bicarbonate and calcium chloride. The next arterial laboratory values, measured 13 minutes after the VT, showed improved pH and ionized calcium, 7.275 and 0.91 mmol/L, respectively.
Figure 1.
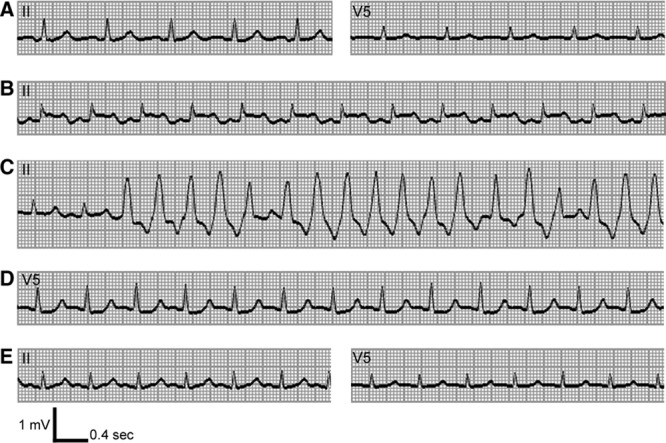
Traces from the monitored electrocardiogram of the patient, a 45-year-old Japanese man. Preincisional trace (A) (leads II and V5) showed sinus rhythm without ST-T change. The ST segment was elevated (B) 2 minutes after the portal reperfusion (lead II) and was followed by nonsustained ventricular tachycardia (C) (lead II). ST segment depression (D) occurred during extreme anemia (hemoglobin 0.6 g/dL) (lead V5). ST segment depression was resolved (E) concomitantly with red cell concentrates transfusion (hemoglobin 5.6 g/dL) (leads II and V5).
Because the ST segment elevation and VT had resolved, we proceeded to perform a microscopic hepatic arterial reconstruction to completely restore graft perfusion. After the hepatic arterial reconstruction, because of a shortage of A(−) and O(−) RCC-LRs, the procedure was temporarily interrupted until additional RCC-LRs arrived. During that waiting period, 5% albumin solution was transfused for intravascular volume resuscitation. This resulted in progressive extreme anemia, with a nadir hemoglobin level of 0.6 g/dL (Fig. 2, Table 1). This low hemoglobin level was associated with ST segment depression on the monitored ECG (Fig. 1D) and rapid increase in serum lactate to a maximum level of 100 mg/dL (Fig. 2).
Figure 2.
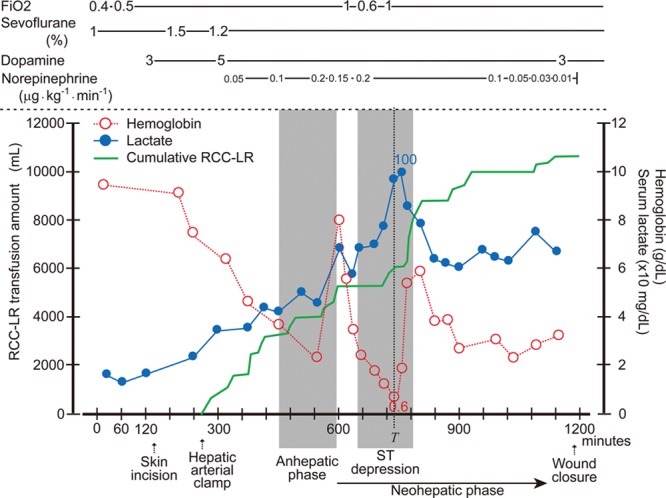
Overview of the patient’s anesthetic care. The serial changes in the serum lactate level, hemoglobin concentration, and cumulative amount of transfused red cell concentrates–leukocytes reduced (RCC-LR) are shown, along with the dosages of oxygen, sevoflurane, and inotropic drugs. T is the time when the hemoglobin concentration was the lowest. Fio2 = fraction of inspired oxygen.
Table 1.
Blood Count, Arterial Blood Gas, Electrolyte, and Metabolite Data Obtained During Nadir Hemoglobin Concentration (0.6 g/dL)

We confirmed that the patient had not previously received A(+) RCC transfusion, and A(+) RCC-LRs were ready to use in the operating room. However, we did not transfuse the A(+) RCC-LRs because the patient’s hemodynamics had been maintained, hemostasis had been achieved, and we were aware that additional A (−) and O (−) RCC-LR concentrates would arrive shortly.
The procedure resumed after A(−) and O(−)RCC-LRs arrived and were administered. The patient’s extreme anemia, ST depression, and excessive serum lactate concentration improved immediately after transfusion of an additional 2600 mL of RCC-LR (Figs. 1E and 2). The procedure was completed successfully, and the patient was transferred to the intensive care unit with a hemoglobin concentration of 3.1 g/dL, requiring only low-dose dopamine infusion for inotropic support.
Throughout the procedure, noninvasively measured systolic blood pressure was maintained >60 mm Hg, and central venous pressure was maintained at around 10 mm Hg. To protect the liver, we infused 0.01 μg/kg/min of prostaglandin E1 and 1 μg/kg/min of gabexate mesilate. Arterial pH was maintained between 7.2 and 7.5, except during the episode of VT, with administration of periodic boluses of sodium bicarbonate. Calcium chloride was administered to maintain the plasma ionized calcium level at normal values. Bladder temperature was maintained between 36°C and 38°C. Sevoflurane was administered at an end-tidal concentration of 1.2%, along with periodic boluses of midazolam and fentanyl for the maintenance of general anesthesia, up to 13 mg and 1.2 mg, respectively. Bispectral Index (Aspect Medical Systems, Norwood, MA) values were maintained in the 40s to 60s. The pulmonary arterial (mixed venous) oxyhemoglobin saturation was continuously monitored but not recorded.
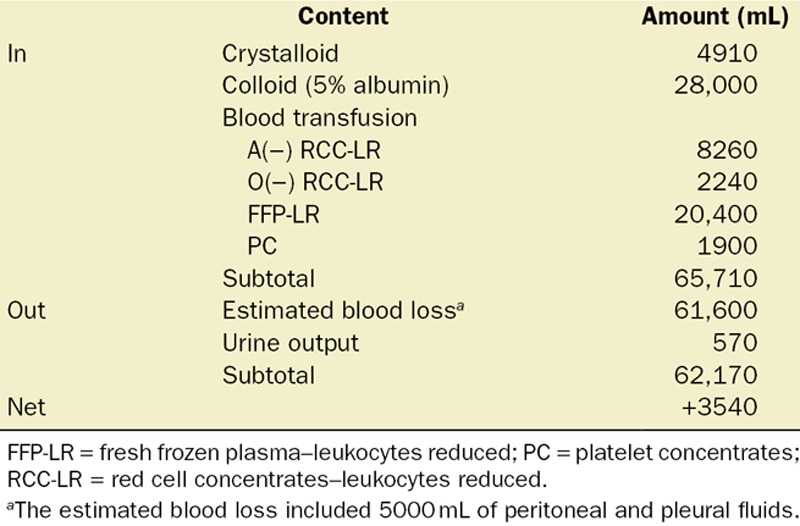
The total duration of general anesthesia was 20 hours and 18 minutes. The fluid balance of the case is summarized in Table 2. On postoperative day 7, the patient’s trachea was extubated uneventfully. He was discharged on postoperative day 57 without significant sequelae.
Table 2.
Intraoperative Fluid Balance
DISCUSSION
To the best of our knowledge, 0.6 g/dL is the lowest hemoglobin value ever observed in an anesthetized patient who survived. This extreme result led us to question the accuracy of the blood-counting instrument we used: a Celltac α Hematology Analyzer (MEK-6318; Nihon Kohden Corporation, Tokyo, Japan). For hemoglobin, this instrument has a measuring range of 0 to 29.9 g/dL and a reproducibility within 1.5% of the coefficient of variation.3 We tested the accuracy of the MEK-6318’s hemoglobin measurements at low concentrations using diluted whole blood with a hemoglobin concentration set to 0.64 g/dL. All 10 measurements produced the same result, 0.5 g/dL, confirming that the MEK-6318 is accurate to within 0.15 g/dL in measuring extremely low hemoglobin concentrations (approximately 0.6 g/dL). Therefore, we believe that the hemoglobin values obtained in the present case were accurate.
The patient’s extreme anemia is attributable to preoperative hepatic dysfunction, the large area of visceral adhesion to be separated, a long and highly invasive operation, and the scarcity of the RhD(−) phenotype in Asian countries. Because the prevalence of this phenotype is low in Japan (approximately 0.5%2 vs 15%4 in Western countries), blood products are not always readily available for RhD(−) patients with massive bleeding. We used 8260 mL A(−) and 2240 mL O(−) RCC-LRs and used up all the matched and universal donor RCC-LRs readily available in Japan at that time.
Because the patient’s hemodynamics were stable and hemostasis was nearly achieved, we did not transfuse A(+) RCC-LRs. In retrospect, we believe that we could have transfused A(+) RCC-LRs without much hesitancy because the patient had not undergone an A(+) RCC transfusion. From an ethical standpoint, if a patient has compromised hemodynamics due to anemia, ABO-incompatible RCCs should be considered as a lifesaving measure.
The physiological limit of dilutional anemia in clinical settings has been implied in many case reports. Dai et al.5 reported a nadir hemoglobin level of 0.7 g/dL without signs of hypoperfusion during surgery for axillary artery trauma in a 53-year-old man. Zollinger et al.6 reported a 1.1 g/dL hemoglobin level accompanied by ST depression during a spine operation in a patient with lumbar metastasis of renal cell carcinoma. In those cases, serum lactate levels were not measured.
We were able to record serum lactate levels, which increased more rapidly during profound anemia (hemoglobin <2 g/dL) in the neohepatic phase than before portal reperfusion. It has been reported that serum lactate levels normally decrease in the neohepatic phase of liver transplantation because the lactate clearance of the liver increases.7 Findings from animal studies suggest 2 major causes of hyperlactatemia in hemorrhagic shock: anaerobic glycolysis due to tissue hypoxia, and endogenous or exogenous catecholamine stimulation of β2 adrenoceptors with subsequent Na+, K+-ATPase activation, facilitating aerobic glycolysis in skeletal muscle.8–10 In the present case, we assume that facilitated anaerobic glucose metabolism contributed, at least in part, to the markedly elevated serum lactate levels. After RCC-LR transfusion, the serum lactate level decreased (Fig. 2).
The critical hemoglobin concentration (Hbcrit) is typically defined as the hemoglobin concentration at which the oxygen consumption of vital organs starts to decline, depending on the oxygen delivery during isovolemic hemodilution.11–16 Further hemodilution beyond Hbcrit results in tissue hypoxia; ST segment depression develops, and the serum lactate concentration increases.6,17,18
In humans, previous reports have emphasized the importance of maintaining normovolemia during extreme hemodilution for hemodynamic stabilization.5,6 During normovolemic hemodilution, hyperoxic ventilation with a fraction of inspired oxygen (Fio2) of 1.0 increases the amount of dissolved oxygen in the plasma, improving oxygen availability in the microcirculation.17 In pigs, rescue hyperoxic ventilation compared with room air ventilation during a period of Hbcrit resulted in maintenance of tissue oxygenation and reduced mortality.14,17 Also, prophylactic hyperoxic ventilation reduced the Hbcrit level in pigs.15 In dogs, increased anesthetic depth led to decreased tolerance to acute isovolemic anemia,19 suggesting that appropriate anesthetic depth should also be considered in normovolemic hemodilution.
In our patient, ST segment depression was seen for 80 minutes, while the patient’s hemoglobin was <2 g/dL (Fig. 1). The increasing serum lactate levels, as well as ST depression during this period, suggested that the patient’s Hbcrit with the new liver graft was approximately 2 g/dL. We estimated the patient’s oxygen supply and demand from the values in Table 1. The calculated arterial blood oxygen content (CaO2) was 2.41 mL per 100 mL of blood (1.34 × 0.6 × 1.0 + 535 × 0.003). Setting the hemoglobin concentration to 1.0 and 2.0 g/dL results in CaO2 values of 2.95 and 4.29 mL/dL, respectively. Given the increase in the patient’s cardiac output from 5 to 8 L/min due to the increase in the heart rate (from 70 to 110 bpm), systemic oxygen deliveries  are 192.72 (hemoglobin 0.6 g/dL), 235.60 (hemoglobin 1.0 g/dL), and 342.80 (hemoglobin 2.0 g/dL) mL/min.
are 192.72 (hemoglobin 0.6 g/dL), 235.60 (hemoglobin 1.0 g/dL), and 342.80 (hemoglobin 2.0 g/dL) mL/min.
Reportedly, systemic oxygen consumption 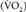 values during liver transplantation for a single patient with cirrhosis are 1.18 (preincisional), 0.62 (anhepatic phase), 3.49 (30 minutes after portal reperfusion), and 2.79 (60 minutes after portal reperfusion) mL/kg/min.20 The
values during liver transplantation for a single patient with cirrhosis are 1.18 (preincisional), 0.62 (anhepatic phase), 3.49 (30 minutes after portal reperfusion), and 2.79 (60 minutes after portal reperfusion) mL/kg/min.20 The 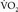 declines by 45% in the anhepatic phase and increases by 130% in the neohepatic phase compared with the preincisional value. In our patient, the increase of
declines by 45% in the anhepatic phase and increases by 130% in the neohepatic phase compared with the preincisional value. In our patient, the increase of 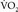 in the neohepatic phase partly explains why ST depression and precipitous lactate increase were seen only in the neohepatic phase. If the abovementioned values were simply applied to the present case, the
in the neohepatic phase partly explains why ST depression and precipitous lactate increase were seen only in the neohepatic phase. If the abovementioned values were simply applied to the present case, the  would be 56.42 (anhepatic phase), 317.59 (30 minutes after portal reperfusion), and 253.89 (60 minutes after portal reperfusion) mL/min. It is likely that the
would be 56.42 (anhepatic phase), 317.59 (30 minutes after portal reperfusion), and 253.89 (60 minutes after portal reperfusion) mL/min. It is likely that the 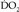 decreased below the
decreased below the 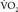 during the patient’s ST depression. The increased heart rate and cardiac output probably largely compensated for the decreased CaO2, thereby facilitating survival.
during the patient’s ST depression. The increased heart rate and cardiac output probably largely compensated for the decreased CaO2, thereby facilitating survival.
We maintained normovolemia by infusing 5% albumin solution and FFP-LR (because of the shortage of matched and universal donor RCC-LRs). Also, we maintained the Fio2 >0.5 and at 1.0 during the latter half of the period of anesthetic care. Furthermore, we monitored the anesthetic depth with a Bispectral Index system and avoided inappropriate fluctuations in anesthetic depth. Finally, minimal amounts of inotropes were administered to maintain perfusion pressure. We did not attempt to induce mild hypothermia due to concerns regarding causing platelet dysfunction, although its effectiveness in preventing the adverse effects of severe hemodilution has been discussed.21,22
In severe dilutional anemia, if matched and universal donor RCCs are unavailable, and the patient experiences hemodynamic collapse or has an uncontrollable lactate increase related to low oxygen supply, ABO-incompatible RCC transfusion should be considered. In massive bleeding with severe hemodilution, transfusing any available, even uncrossmatched, RCCs should always be considered to avoid patient death due to lack of red cells. An increase in serum lactate may be an indication for additional RCC transfusion or conversion to an abbreviated surgical procedure with plans for future definitive repair.
However, in our patient, despite extreme intraoperative hemodilution due to massive bleeding, administering 100% oxygen, maintaining normovolemia, ensuring an appropriate anesthetic depth, and minimizing inotropic support resulted in a good clinical outcome. These actions should be considered for other patients with massive bleeding if sufficient matched and universal donor RCCs are not available. Trends in serum lactate levels, as well as abnormal ECG findings, can serve as warnings of tissue hypoperfusion due to Hbcrit. Monitoring the mixed or central venous oxyhemoglobin saturation may also be helpful.
Previously, the lowest reported intraoperative hemoglobin level was 0.7 g/dL.5 In that trauma case, the patient had been previously healthy. In contrast, our patient had several preoperative comorbidities. The fact that our patient tolerated severe anemia supports the effectiveness of the strategies we used. Refining the best strategies for treating massive bleeding will require additional reports of similar cases with detailed monitoring and records.
Footnotes
Funding: None.
The authors declare no conflicts of interest.
REFERENCES
- 1.Irita K, Inada E, Yoshimura H, Warabi K, Tsuzaki K, Inaba S, Handa M, Uemura T, Kino S, Mashiko K, Yano T, Kamei Y, Kubo T. [Present status of preparatory measures for massive hemorrhage and emergency blood transfusion in regional hospitals with an accredited department of anesthesiology in 2006] (article in Japanese). Masui. 2009;58:109–23. [PubMed] [Google Scholar]
- 2.Akaishi S, Kudo T. Bood groups. In: Watanabe S, Kondo S, Matsunaga E, editors. In: Anthropological and Genetic Studies on the Japanese. Tokyo: University of Tokyo Press; 1975. pp. 77–107. [Google Scholar]
- 3.Nihon Kohden Corporation. MEK-6318J/K Celltac a Hematology Analyzer. Tokyo, Japan: Nihon Kohden Corporation; 2006. [Google Scholar]
- 4.Daniels G. Human Blood Groups. Oxford: Blackwell; 2002. [Google Scholar]
- 5.Dai J, Tu W, Yang Z, Lin R. Case report: intraoperative management of extreme hemodilution in a patient with a severed axillary artery. Anesth Analg. 2010;111:1204–6. doi: 10.1213/ANE.0b013e3181e668b8. [DOI] [PubMed] [Google Scholar]
- 6.Zollinger A, Hager P, Singer T, Friedl HP, Pasch T, Spahn DR. Extreme hemodilution due to massive blood loss in tumor surgery. Anesthesiology. 1997;87:985–7. doi: 10.1097/00000542-199710000-00036. [DOI] [PubMed] [Google Scholar]
- 7.Orii R, Sugawara Y, Hayashida M, Yamada Y, Kubota K, Takayama T, Harihara Y, Makuuchi M, Hanaoka K. Peri-operative blood lactate levels in recipients of living-related liver transplantation. Transplantation. 2000;69:2124–7. doi: 10.1097/00007890-200005270-00028. [DOI] [PubMed] [Google Scholar]
- 8.McCarter FD, James JH, Luchette FA, Wang L, Friend LA, King JK, Evans JM, George MA, Fischer JE. Adrenergic blockade reduces skeletal muscle glycolysis and Na(+), K(+)-ATPase activity during hemorrhage. J Surg Res. 2001;99:235–44. doi: 10.1006/jsre.2001.6175. [DOI] [PubMed] [Google Scholar]
- 9.Luchette FA, Jenkins WA, Friend LA, Su C, Fischer JE, James JH. Hypoxia is not the sole cause of lactate production during shock. J Trauma. 2002;52:415–9. doi: 10.1097/00005373-200203000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Levy B, Desebbe O, Montemont C, Gibot S. Increased aerobic glycolysis through beta2 stimulation is a common mechanism involved in lactate formation during shock states. Shock. 2008;30:417–21. doi: 10.1097/SHK.0b013e318167378f. [DOI] [PubMed] [Google Scholar]
- 11.van Woerkens EC, Trouwborst A, van Lanschot JJ. Profound hemodilution: what is the critical level of hemodilution at which oxygen delivery-dependent oxygen consumption starts in an anesthetized human? Anesth Analg. 1992;75:818–21. doi: 10.1213/00000539-199211000-00029. [DOI] [PubMed] [Google Scholar]
- 12.Fontana JL, Welborn L, Mongan PD, Sturm P, Martin G, Bünger R. Oxygen consumption and cardiovascular function in children during profound intraoperative normovolemic hemodilution. Anesth Analg. 1995;80:219–25. doi: 10.1097/00000539-199502000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Habler OP, Messmer KF. The physiology of oxygen transport. Transfus Sci. 1997;18:425–35. doi: 10.1016/S0955-3886(97)00041-6. [DOI] [PubMed] [Google Scholar]
- 14.Meier J, Kemming GI, Kisch-Wedel H, Wölkhammer S, Habler OP. Hyperoxic ventilation reduces 6-hour mortality at the critical hemoglobin concentration. Anesthesiology. 2004;100:70–6. doi: 10.1097/00000542-200401000-00014. [DOI] [PubMed] [Google Scholar]
- 15.Pape A, Meier J, Kertscho H, Steche M, Laout M, Schwerdel F, Wedel M, Zwissler B, Habler O. Hyperoxic ventilation increases the tolerance of acute normovolemic anemia in anesthetized pigs. Crit Care Med. 2006;34:1475–82. doi: 10.1097/01.CCM.0000215826.45839.36. [DOI] [PubMed] [Google Scholar]
- 16.Pape A, Stein P, Horn O, Habler O. Clinical evidence of blood transfusion effectiveness. Blood Transfus. 2009;7:250–8. doi: 10.2450/2008.0072-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kemming GI, Meisner FG, Kleen M, Meier JM, Tillmanns J, Hutter JW, Wojtczyk CJ, Packert KB, Bottino D, Habler OP. Hyperoxic ventilation at the critical haematocrit. Resuscitation. 2003;56:289–97. doi: 10.1016/s0300-9572(02)00408-2. [DOI] [PubMed] [Google Scholar]
- 18.Leung JM, Weiskopf RB, Feiner J, Hopf HW, Kelley S, Viele M, Lieberman J, Watson J, Noorani M, Pastor D, Yeap H, Ho R, Toy P. Electrocardiographic ST-segment changes during acute, severe isovolemic hemodilution in humans. Anesthesiology. 2000;93:1004–10. doi: 10.1097/00000542-200010000-00023. [DOI] [PubMed] [Google Scholar]
- 19.Van der Linden P, De Hert S, Mathieu N, Degroote F, Schmartz D, Zhang H, Vincent JL. Tolerance to acute isovolemic hemodilution. Effect of anesthetic depth. Anesthesiology. 2003;99:97–104. doi: 10.1097/00000542-200307000-00018. [DOI] [PubMed] [Google Scholar]
- 20.Irita K, Kawasaki T, Okamoto H, Matsukado T, Sakaguchi Y, Okabe H, Taniyama T, Takahashi S. The difference between the changes in systemic oxygen consumption during orthotopic liver transplantation and those during extracorporeal hepatic resection. Tohoku J Exp Med. 1996;180:217–23. doi: 10.1620/tjem.180.217. [DOI] [PubMed] [Google Scholar]
- 21.Koehntop DE, Belani KG. Acute severe hemodilution to a hemoglobin of 1.3 g/dl tolerated in the presence of mild hypothermia. Anesthesiology. 1999;90:1798–9. doi: 10.1097/00000542-199906000-00057. [DOI] [PubMed] [Google Scholar]
- 22.Perez-de-Sa V, Roscher R, Cunha-Goncalves D, Larsson A, Werner O. Mild hypothermia has minimal effects on the tolerance to severe progressive normovolemic anemia in swine. Anesthesiology. 2002;97:1189–97. doi: 10.1097/00000542-200211000-00024. [DOI] [PubMed] [Google Scholar]


