Abstract
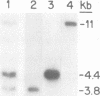
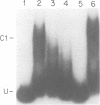
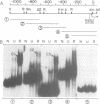
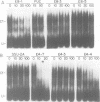
To understand how fruit development is controlled, we have begun experiments to identify DNA sequences and proteins that regulate gene expression during tomato (Lycopersicon esculentum) fruit ripening. We have focused on the E8 gene because its transcription is responsive to ethylene hormone and is activated at the onset of fruit ripening. We report here that sequences required for ethylene-responsive and developmentally regulated E8 gene expression in transgenic tomato plants are contained on a 4.4 kb restriction fragment which includes sequences 2 kb 5' and 0.5 kb 3' to the gene. In addition, we have identified a DNA-binding factor that specifically interacts with DNA sequences that flank the E8 gene. This DNA-binding activity is low in unripe fruit and increases during fruit ripening. This factor also binds to the 5'-flanking region of another ethylene-responsive gene which is coordinately expressed during tomato fruit ripening. These data suggest that the DNA binding-factor may be involved in the regulation of gene expression during fruit ripening.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumann G., Raschke E., Bevan M., Schöffl F. Functional analysis of sequences required for transcriptional activation of a soybean heat shock gene in transgenic tobacco plants. EMBO J. 1987 May;6(5):1161–1166. doi: 10.1002/j.1460-2075.1987.tb02349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. W. A catalogue of splice junction and putative branch point sequences from plant introns. Nucleic Acids Res. 1986 Dec 22;14(24):9549–9559. doi: 10.1093/nar/14.24.9549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Block M., Herrera-Estrella L., Van Montagu M., Schell J., Zambryski P. Expression of foreign genes in regenerated plants and in their progeny. EMBO J. 1984 Aug;3(8):1681–1689. doi: 10.1002/j.1460-2075.1984.tb02032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellapenna D., Alexander D. C., Bennett A. B. Molecular cloning of tomato fruit polygalacturonase: Analysis of polygalacturonase mRNA levels during ripening. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6420–6424. doi: 10.1073/pnas.83.17.6420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favaloro J., Treisman R., Kamen R. Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 gel mapping. Methods Enzymol. 1980;65(1):718–749. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- Fischer R. L., Goldberg R. B. Structure and flanking regions of soybean seed protein genes. Cell. 1982 Jun;29(2):651–660. doi: 10.1016/0092-8674(82)90181-7. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Holdsworth M. J., Bird C. R., Ray J., Schuch W., Grierson D. Structure and expression of an ethylene-related mRNA from tomato. Nucleic Acids Res. 1987 Jan 26;15(2):731–739. doi: 10.1093/nar/15.2.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaulen Hildegard, Schell Jeff, Kreuzaler Fritz. Light-induced expression of the chimeric chalcone synthase-NPTII gene in tobacco cells. EMBO J. 1986 Jan;5(1):1–8. doi: 10.1002/j.1460-2075.1986.tb04169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln J. E., Cordes S., Read E., Fischer R. L. Regulation of gene expression by ethylene during Lycopersicon esculentum (tomato) fruit development. Proc Natl Acad Sci U S A. 1987 May;84(9):2793–2797. doi: 10.1073/pnas.84.9.2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln J. E., Fischer R. L. Diverse mechanisms for the regulation of ethylene-inducible gene expression. Mol Gen Genet. 1988 Apr;212(1):71–75. doi: 10.1007/BF00322446. [DOI] [PubMed] [Google Scholar]
- Miskimins W. K., Roberts M. P., McClelland A., Ruddle F. H. Use of a protein-blotting procedure and a specific DNA probe to identify nuclear proteins that recognize the promoter region of the transferrin receptor gene. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6741–6744. doi: 10.1073/pnas.82.20.6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson J., VAN Montagu M., Herrera-Estrella L. Photosynthesis-associated gene families: differences in response to tissue-specific and environmental factors. Science. 1986 Jul 4;233(4759):34–38. doi: 10.1126/science.233.4759.34. [DOI] [PubMed] [Google Scholar]
- Singh H., Sen R., Baltimore D., Sharp P. A. A nuclear factor that binds to a conserved sequence motif in transcriptional control elements of immunoglobulin genes. Nature. 1986 Jan 9;319(6049):154–158. doi: 10.1038/319154a0. [DOI] [PubMed] [Google Scholar]
- Stougaard J., Sandal N. N., Grøn A., Kühle A., Marcker K. A. 5' Analysis of the soybean leghaemoglobin lbc(3) gene: regulatory elements required for promoter activity and organ specificity. EMBO J. 1987 Dec 1;6(12):3565–3569. doi: 10.1002/j.1460-2075.1987.tb02686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Haute E., Joos H., Maes M., Warren G., Van Montagu M., Schell J. Intergeneric transfer and exchange recombination of restriction fragments cloned in pBR322: a novel strategy for the reversed genetics of the Ti plasmids of Agrobacterium tumefaciens. EMBO J. 1983;2(3):411–417. doi: 10.1002/j.1460-2075.1983.tb01438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Walling L., Drews G. N., Goldberg R. B. Transcriptional and post-transcriptional regulation of soybean seed protein mRNA levels. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2123–2127. doi: 10.1073/pnas.83.7.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambryski P., Joos H., Genetello C., Leemans J., Montagu M. V., Schell J. Ti plasmid vector for the introduction of DNA into plant cells without alteration of their normal regeneration capacity. EMBO J. 1983;2(12):2143–2150. doi: 10.1002/j.1460-2075.1983.tb01715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]