Abstract
Background
Endemic pemphigus foliaceus (EPF), is also known as “fogo selvagem” or “wild fire,” reflecting the intense burning sensation of the skin reported by patients with this disease. Based on this finding, we tested for neural autoreactivity in patients affected by a new variant of EPF (El Bagre-EPF).
Methods
We tested 20 El Bagre-EPF patients, 20 normal controls from the endemic area, and 20 age- and sex-matched normal controls from outside the endemic area. We tested for autoreactivity to several immunoglobulins and complement. Both human skin and bovine tail were used as antigens.
Results
We detected autoreactivity to neural structures, mechanoreceptors, nerves, perineural cell layers of the arachnoid envelope around the optic nerve, brain structures, and to neuromuscular spindles; these structures colocalized with several neural markers. The patient antibodies also colocalized with desmoplakins 1 and 2, with the armadillo repeat protein deleted in velo-cardio-facial syndrome and with p0071 antibodies. Autoreactivity was also found associated with neurovascular bundles innervating the skin, and immunoelectron microscopy using protein A gold against patient antibodies was positive against the nerve axons. Paucicellularity of the intraepidermal nerve endings and defragmentation of the neural plexus were seen in 70% of the cases and not in the controls from the endemic area (p<0.005). Neuropsychological and/or behavioral symptoms were detected in individuals from the endemic area, including sensorimotor axonal neuropathy.
Conclusions
Our findings may explain for the first time the “pose of pemphigus,” representing a dorsiflexural posture seen in EPF patients vis-a-vis the weakness of the extensor nerves, and furthermore, the autoreactivity to nerves in EPF could explain the “burning sensation” encountered in EPF disease.
Keywords: Autoantibodies, mechanoreceptor, pacinian corpuscle, nerves, endemic pemphigus foliaceus, epidermal nerve fiber density, myelin basic protein, glial fibrillary acidic protein, CD57, protein gene product 9.5, desmoplakins, optic nerve, arachnoid envelope antibodies
Introduction
We have identified a new variant of EPF in El Bagre, Colombia, S.A. (El Bagre-EPF) [1–8]. Our studies established that this focus exhibits alike features to those described in Senear–Usher syndrome, a disease with combined features of lupus erythematosus and pemphigus [1–8]. This new variant of El Bagre-EPF differs from classic endemic pemphigus foliaceus (EPF) in aspects such as the following: (1) no clinical involvement of children or persons under the age of 30 years occurs, contrary to fogo selvagem (FS) that primarily affects children and young adults, with the highest incidence at 10–30 years of age, and both sexes equally affected; (2) El Bagre-EPF affects predominantly 40- to 60-year-old men, as well as a few post-menopausal women, in contradistinction to EPF; and (3) the main autoantigens are plakins (desmoplakins, envoplakin, periplakin, and plakoglobin), in comparison to EPF, where most autoantigens are desmogleins1 [1–8]. Both EPF as well as in El Bagre-EPF patients have intercellular deposits of immunoglobulins and/or complement showing intercellular stain (ICS) between the keratynocytes, but contrary to El Bagre-EPF, EPF does not exhibit deposits of antibodies and complement at the basement membrane zone (BMZ) of the skin [1–12].
EPF is also known by the name “fogo selvagem” or “wild fire” because patients affected by this illness classically report a burning sensation on the skin [1–12]. Two main questions about EPF remain unknown over almost a century: what explains the burning sensation of the patients’ skin, which represents the hallmark symptom of this disease, and what explains the muscular weakness and the “pose of pemphigus” (a dorsiflexural position of the body), present in most chronically affected patients before the steroid era [1–12].
The population in El Bagre is exposed to tropical diseases, as well as to mercury and other pollutants as a result of gold mining and agricultural chores [3]. We reported the presence of mercury in skin biopsies from people living in the endemic area [3]. Given the burning sensation present in El Bagre-EPF and FS patients and noting that mercury is a neurotoxin, we searched for nerve alterations by hematoxylin and eosin (H&E), silver diamine ion (SDI), and modified Bielschowsky (MBS) staining. We performed direct and indirect immunofluorescence (DIF and IIF), immunohistochemistry (IHC), confocal microscopy (CFM), immunoblotting (IB), and immunoelectron microscopy (IEM) to study patient autoreactivity to several neural components.
Materials and Methods
We examined 20 El Bagre-EPF patients, 20 normal controls from the endemic area, and 20 normal controls from outside of the endemic area (all matched by age, sex, and work activity). In addition, we tested five patient samples of people suffering FS. El Bagre-EPF disease was characterized by epidemiological, immunological, histopathological, and clinical criteria reported elsewhere [1–3]. Simultaneously, we tested for neuropsychological, behavioral, and kinesiological alterations in the subjects of the study (all tested negative for Mycobacterium leprae). All cases displayed IgG4 intercellular staining between keratinocytes [1–3]. Sera of the cases were reactive to desmoglein 1 (Dsg1) by IB, by immunoprecipitation, and by enzyme-linked immunosorbent assay [1–3, 8]. In addition, in IB of the El Bagre-EPF, most sera reacted with plakins [including desmoplakins 1 and 2 (DPI and DPII), envoplakin, periplakin, plakoblobulin], to bullous pemphigoid (BP) antigens 1 and 2 and to other undetermined molecules [8]. For IIF, we used as substrates human chest skin, rat, mouse, and chipmunk nerve and brain tissue, and monkey esophagus (Oregon Primate Research Center, Portland, Oregon, USA). Selected samples for DIF and IIF were partially fixed in 3% paraformaldehyde [9, 10]. All samples were tested anonymously to comply with institutional review board requirements.
DIF and IIF
Slides were incubated with the sera at 1:20–1:40 dilutions in phosphate-buffered saline (PBS), rinsed, and then incubated with secondary antibodies [including Fluorescein isothiocyanate (FITC)-conjugated goat anti-human IgG, IgM, IgA, IgD, IgE, C1q, C3c, C3d, albumin, and fibrinogen (all from Dako, Carpinteria, CA, USA)]. We also utilized anti-human total IgG/IgM/IgA antiserum (Zymed®; Invitrogen, Carlsbad, CA, USA) and rhodamine-conjugated goat anti-human IgA/IgM/IgG anti-serum (Rockland Labs, Rockland, ME, USA). Neural marker colocalization was studied by utilizing anti-human S-100 antibody (Dako), mouse anti-human neuron specific enolase (NSE) (Dako), and Cy3 conjugated anti-glial fibrillary acidic protein (GFAP), (Sigma Aldrich, St. Louis, MO, USA). In addition, we used a multi-epitope cocktail containing anti-desmoplakin I and II (DPI and DPII), anti-armadillo repeat protein deleted in velo-cardio-facial syndrome (anti-ARVCF) and anti-p0071 antibodies (all from Progen). The secondary antibodies were conjugated with Texas Red and/or with Alexa 555 (both from Invitrogen). All sections were then examined with a Nikon Eclipse 50i microscope (Japan). All slides were counterstained with Dapi (Pierce, Rockford, IL, USA) or TOPRO®-3/DNA (Invitrogen).
IHC
Immunohistochemistry was used to study the colocalization of patient’s autoantibodies with nerves, employing a dual endogenous peroxidase blockage, with the addition of an Envision dual link (Dako). We tested for anti-human IgG, IgM, IgA, IgD, IgE, C1q, C3c, C3d, albumin, and fibrinogen (all from Dako). We also utilized anti-S-100 proteins, GFAP, NSE, and rabbit anti-human myelin basic protein (MBP), anti-neurofilament, CD57, and protein gene product 9.5 (PPG 9.5) (all from Dako).
SDI and Modified MBS
Samples were prepared as previously described [9], and all biopsies were taken from the chest area for accurate morphometric correlation [9–14]. Nerve density was determined by the average number of positive silver staining fibers in five low power fields under a light microscope [9, 10]. We quantified both the epidermal nerve fiber density (ENFD) and myelinated nerve endings per square millimeter [9–14].
Autoreactivity to Optic Nerve and Brain Structures
To define autoreactivty of the El Bagre-EPF patients, we assessed immunoreactivity utilizing optic nerve and brain from rat, mouse, and chipmunk sources, and we performed colocalization with DPI, DPII, ARVCF, and p0071 antibodies.
Colocalization of El Bagre-EPF Autoantibodies and Neural Markers Utilizing CFM
We performed CFM examinations using standard ×20 and ×40 objective lenses; each photo-frame included an area of approximately 440×330 μm. Images were obtained using EZ 1 image analysis software (Nikon, Japan).
Immunoblotting
IB testing for reactivity of IgM and IgG antibodies to bovine tail nerve was performed. The samples were extracted with 0.6 M Tris–HCI, pH 6.8, containing 2% sodium dodecyl sulfate (SDS), 5% ß-mercaptoethanol, and 1 mM EDTA, with 1 mM dithiothreitol and protease inhibitors. Extracts were run on a 10% SDS gel. The proteins were transferred to nitrocellulose membranes, and the immune reactions were carried out with patient and control sera. Chemiluminescence detection performed using an ECL kit including SuperSignal® West Pico Substrate ECL™ (Pierce, Rockford, IL, USA).
Neurological, Neuropsychological, Behavioral, and Kinesiological Testing
Clinical studies were performed to identify neurological alterations, including autonomic neuropathies [15–17]. Numbness, paresthesias, urinary incontinence, sensitivity to pressure, cold, heat and pain, bedside orthostatic hypotension, a Valsalva ratio, beat-to-beat blood pressure, pupillary responses, and cranial nerves II–XII were assessed.
Kinesiology Evaluation
We tested for kinesiological movement and skin shriveling in response to immersing the hand in hot or cold water and also utilized the Nottingham Health Profile (NHP) for the evaluation of muscular imbalance or derangement [11–17]. We used the Lysholm Knee Scale (LKS) to study limp support, impaired stair climbing, squatting, walking, running, jumping, atrophy of the thigh, pain, and swelling [16, 17]. All these parameters were rated as none, very mild, moderate, or severe.
ENFD, Nerve Morphometry, and Histomorphometric Analysis
SDI, MBS, and IHC stains were used on the skin biopsies to quantify the density of intraepidermal axons, as well as the ENFD [12–15].
Immunoelectron Microscopy
Postembedding immunogold labeling was performed on samples of El Bagre-EPF sera and controls. Peripheral rat nerve was used as an antigen; the tissue was dissected, fixed in 4% glutaraldehyde with 0.2% paraformaldehyde, and embedded in lowicryl® resin. The tissue was then sectioned at 70 nm thickness. The samples were blocked with a solution from Aurion™, Electron Microcopy Sciences (EMS, Hatfield, PA, USA). The grids were then washed with PBS-BSAC (Aurion™, EMS). The primary antibodies were incubated overnight at 4°C. The next day the grids were washed, and a secondary antibody solution, 10 nm gold-conjugated protein A PBS-BSAC (Aurion, EMS™) was applied. The samples were double-stained with uranyl acetate and lead citrate. The samples were observed under a Hitachi H7500 transmission electron microscope. Immunogold particles displaying any pattern of positivity were converted to TIF format.
Mercury Detection in Hair and Nails
We measured mercury in the hair in all of the cases and controls. After hair washing, 25 mg of hair and/or nails was cut and degreased with acetone. Hair and nail samples were packed separately in ash-free paper and analyzed separately. Samples were measured following methods described elsewhere [3]. Mercury was measured in an atomic absorption spectrophotometer (MAS 50). The World Health Organization defines a permissible level of mercury in hair of 7 ppm. We scale the results as negative (−) >7 ppm, weak positive >7–14 ppm (+), positive >14–28 ppm (++), very positive from 28>to 56 ppm (strong positive) (+++), and super strong (>56–144 ppm and upper) (++++).
Statistical Methods
ENFD and myelinated nerve endings per square millimeter were statistically analyzed using the nonparametric Spearman’s rank correlation, specifically with a p value of 0.05 and a single-tailed analysis. We determined that our data followed a normal distribution using the Kolmogorov–Smirnov test and used Student’s t test to evaluate differences in morphology.
Results
All El Bagre-EPF patients and the five FS patients have a skin burning sensation, and no control from the endemic or non endemic area has this symptom (p>0.005).
Nerve Paucicellularity (Free Ending and Thin Skin Myelinated Nerves) Was Found in Most El Bagre-EPF Patients
We detected reduced ENFD and decrease myelinated nerve fiber density in 70% (p>0.005) of the El Bagre-EPF patients and in three of five FS patients by the H&E, IHC, SDI, and MBS stains. We noted damage to subepidermal neural plexus areas in the El Bagre-EPF patients and in three of five FS patients, featuring fragmentation of both myelinated and non-myelinated fibers as well as reduction of the innervations of skin appendices. These findings were noted in only 6% of the control patients from the endemic area and in none normal controls from outside the endemic area. When utilizing antibodies to PPG9.5, CD57, neurofilament, GFAP, NSE, S-100, and MBP, fragmentation of the subepidermal nerve plexus fibers was appreciated, and specific loss of nerve fibers ascending vertically into the epidermis was observed (Figs. 1, 2, 3, 4, and 5).
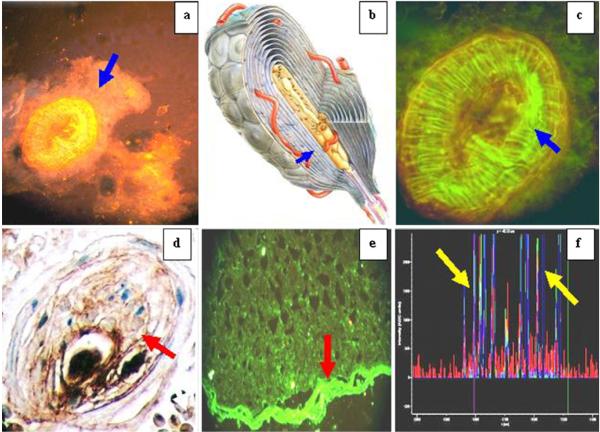
Fig. 1.
A series of IIF, DIF, IHC, and NDIC images demonstrating by multiple techniques the autoreactivity of the El Bagre-EPF patient sera against the PC and optic nerves
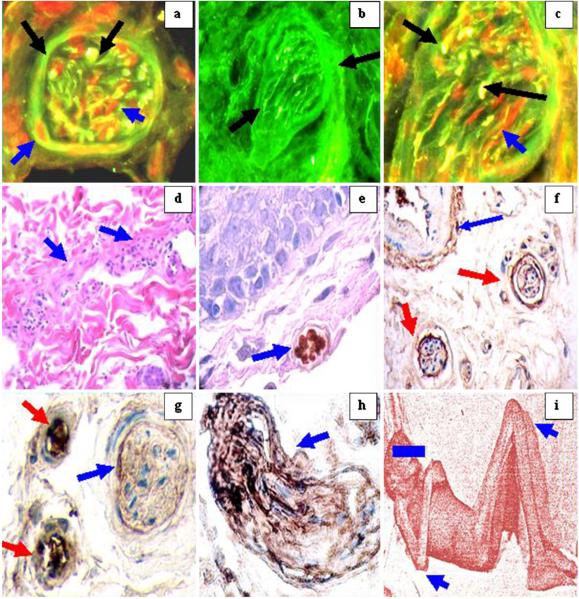
Fig. 2.
A series of IIF, DIF, and IHC images demonstrating the autoreactivity of the El Bagre-EPF patient sera against several peripheral nerves
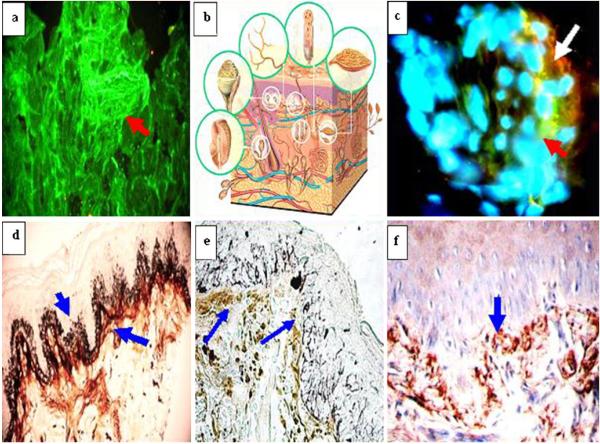
Fig. 3.
El Bagre-EPF patient sera recognize other mechanoreceptors, and their skin biopsies show nerve paucicellularity and defragmentation of small nerves. “Pose of pemphigus”
Fig. 4.
El Bagre-EPF patient sera recognize myelinated and non-myelinated nerves, the spindle cell apparatus, and some brain tissues by different techniques
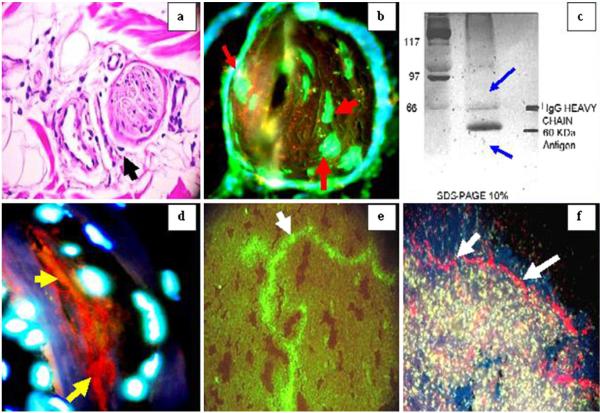
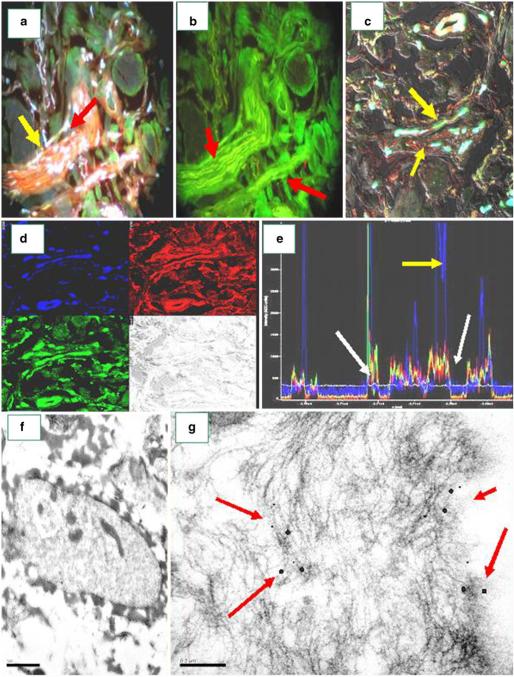
Fig. 5.
Immunoelectron microscopy reveals deposits of El Bagre-EPF patient autoantibodies within nerve axons, and CFM and IHC demonstrate colocalization with neural markers
Autoreactivity to Neural Structures Colocalizing with Neural Markers
We found autoreactivity to Pacinian corpuscles (PC), mechanoreceptors, nerves, neuromuscular spindles, and neurovascular packages in 70% of El Bagre-EPF and in three of five FS patients (p<0.005). No controls show this reactivity. We demonstrated colocalization of the autoreactivity with multiple neural markers, such CD57, neurofilament, PPG 9.5, CD57, neurofilament, PPG 9.5, GFAP, S-100, and MBP (Figs. 1, 2, 3, 4, and 5).
Autoreactivity to Optic Nerve and Brain Structures
Reactivity to perineural cell layers of the arachnoid envelope surrounding the optic nerve was noted in 12 of 20 El Bagre-EPF patients, versus no controls (p<0.005). This reactivity was not seen in the FS patients. The reactivity colocalized exactly with the antibodies to DPI, DPII, and ARVCF. Colocalization was also present in nearby vessels with p0071 by both IIF and CFM (p<0.005). In 12 of 20 El Bagre-EPF patients and in one of 20 controls from the endemic area (a brother of one El Bagre-EPF patients), autoreactivity was noted surrounding brain cisternae, colocalizing with the arachnoid envelope of the optic nerve (mainly, but some reactivity was seen inside the nerve) and with the brain gyral and the sulci surfaces (p<0.005). This reactivity was not seen in the FS patients, neither in control out of the endemic area (Figs. 1, 2, 3, 4, and 5).
Neuropsychological and Behavioral Performance
Seventy percent of the El Bagre-EPF and three of five FS patients displayed neurological alterations that were statistically significantly higher in comparison with the other controls (i.e., symptoms of depression, anxiety, insomnia, and restlessness; p<0,005). Intellectual sequelae (mainly alteration of executive function and constructional praxis) were not found to be different between patients and controls. Conversely, the kinesiological alterations and the burning sensation on the skin were present in 100% of the El Bagre-EPF and in three of five FS patients compared to none of the controls neither inside or outside the endemic area (p<0.005). Autonomic alterations were also higher in the El Bagre-EPF patients (p<0.005), including urinary incontinence, itching, burning sensations, esophageal dysmotility, and sphincter relaxation. Pupillary and other reflexes were normal, as well as testing for cranial nerves (II–XII), and non-statistical differences were found in comparison with the controls. Numbness, paresthesias, sensitivity to pressure, cold, heat and pain, bedside orthostatic hypotension, valsalva ratio, and beat-to-beat blood pressure show no differences either.
The NHP analysis showed higher depression among the El-Bagre-EPF patients (100%), in contrast to the controls from the endemic area (10%; p<0.005). Postural deformities in the flexor tonus (as well as joint ankylosis and muscular weakness) were present in 70% of the El Bagre-EPF and in three of five FS patients, which was statistically significant to controls (5%; p<0.0005).
Figure 1 shows a series of DIF, IIF, CFM, and IHC images, demonstrating by multiple techniques the autoreactivity of the El Bagre-EPF patient sera against PC and optic nerve envelope. Autoreactivity to PC in El Bagre-EPF patients is shown (×100 for Fig. 1a and ×400 for Fig. 1c, d). Figure 1a shows positive staining to PC using rhodamine-conjugated goat anti-human IgG/IgM/IgA antiserum (blue arrow, orange stain). Figure 1b is a cover picture of a PC, reproduced with permission from SKF 26, and GDAS (Ratingen, Germany). Figure 1c is the higher magnification of Fig. 1a (×400; blue arrow), but in this case using anti-human IgG/IgM/IgA, conjugated with FITC (reactivity in green; blue arrow). Nomarski differential interference contrast confocal microscopy (NDIC) demonstrate that these structures were PC (data not shown). In Fig. 1c, IHC stains on El Bagre-EPF biopsies confirms the reactivity to PC (blue arrows) with anti-human IgM and IgA (dark brown stain onion shape stain, red arrow). Figure 1d is the IIF of a representative picture of a rat optic nerve, showing reactivity in the perineural meningeal sheaths recognized by El Bagre-EPF FITC conjugated anti-human IgG FITCI conjugated antibodies (red arrow, green staining). In some cases, delicate intra-optic nerve reactivity was also seen (not shown). No controls were positive. Using CFM, we were able to show that El Bagre-EPF patients reactivity colocalized with anti-P0071 antibodies (data not shown). In Fig. 1e, CFM shows a colocalization with antibodies to DP I and II (red panel) with the El Bagre-EPF autoantibodies (green panel). The blue peak is Dapi nuclear counterstain. The combined pikes colocalizing are pointed by the yellow arrows.
El Bagre-EPF Patient Sera Reactive Against Some Peripheral Nerves
In Fig. 2 DIF, IIF, and IHC revealed autoreactivity against peripheral nerves. Figure 2a, c, d DIF reveals autoreactivity to peripheral nerve bundles, some directed against the epineururum, perineurum, and endoneurum. In Fig. 2a, the black arrows show positivity inside and in the periphery of the nerve with antibodies to IgG-FITCI conjugated from one El Bagre-EPF patient (yellowish-green stain); the red stain (blue arrows) is PP9.5 stain to show colocalization of El Bagre-EPF antibodies with neural components (Fig. 2b).El Bagre-EPF sera shows reactivity to peripheral nerves using FITC conjugated anti-human IgG/IgA/IgM polyclonal antibodies (black arrows) (green staining). Figure 2c is similar as Fig. 2a, but at ×100. Figure 2d shows H&E stain demonstrating a strong lymphohistiocytic infiltrate around one of the El Bagre-EPF dermal nerves and edema (blue arrows). Figure 2e shows IHC positive for anti-human S-100 in nerves (blue arrow). Figure 2g shows nerve positivity with anti-human IgA (blue arrow) and positivity to two vessel (red arrows). Figure 2h demonstrates strong staining of a nerve with anti-human IgM antibody (blue arrow; brown staining). Figure 2i shows the “pose of pemphigus” with dorsiflexion of extremities (blue arrows). By IIF, 11 of the 20 El Bagre-EPF sera showed nerve reactivity to the thin nerves, especially to their epineurium, perineurium, endoneurium, and to vessels around and inside the nerve bundles. El Bagre-EPF patient antibodies colocalized with neural markers demonstrated by DIF, IIF, and IHC; positivity with S-100, NSE, PPG 9.5, GFAP, CD57, and neurofilament supports the neural origin of these antigens. The autoantibodies in El Bagre-EPF were polyclonal; thus, nine of the 20 samples tested positive to thin nerves utilizing anti-human IgG/IgM/IgA antiserum. In contrast, two of the 20 normal controls from the endemic area showed autoreactivity to larger nerves with only IgM.
In Fig. 3, El Bagre-EPF patient sera recognize other mechanoreceptors, and their skin biopsies show nerve paucicellularity and defragmentation. Figure 3a shows positive staining of a Krause endbulb using FITC-conjugated anti-human IgM antibodies (red arrow) (green staining). Figure 3b is a diagram of mechanoreceptors of the skin. Figure 3c shows positivity with FITC-conjugated anti-human fibrinogen antiserum (red arrow, green staining), and with anti-human-ICAM-1/CD54 (orange staining, white arrow) in a Meissner corpuscle. The nuclei are counterstained with Dapi (blue). Figure 3d shows normal silver staining of the upper neural plexus of the skin of a normal control (dark brown netting stain; blue arrows). Figure 3e shows silver staining from one El Bagre-EPF skin biopsy, revealing defragmentation and loss of nerves (blue arrows). Figure 3f shows IHC of one El Bagre-EPF skin biopsy, utilizing anti-human C1q antibodies positive against the upper neurovascular bundle under the BMZ with anti-human C1q (blue arrow).
In Fig. 4. El Bagre-EPF patient sera recognize thin myelinated and non-myelinated nerves, the spindle cell apparatus, and brain tissue by different techniques. Figure 4a shows perineural inflammation and mild edema of a nerve in an H&E skin biopsy of an El Bagre-EPF patient (×100). Figure 4b and d is DIF showing positive colocalizations of El Bagre-EPF patient’s autoantibodies using FITC conjugated-anti-human IgG antibodies (in Fig. 4b, to mechanoreceptors; in Fig. 4d, to the neuromuscular spindle; we used anti-GFAP to demonstrate the neural nature of these structures). The Bagre-EPF antibodies are shown in green staining (red arrows). In Fig. 4d, the red-orange stain shows El Bagre-EPF atutoreactivity to the neural spindle (yellow arrows). Figure 4c shows IB using cow spinal cord extract. On the left are the molecular weight standards and on the right is a positive control. After SDS polyacrylamide gel electrophoresis separation of cow spinal cord and blotting of neural substrate antigens, El Bagre-EPF sera reactivity to several bands are shown, including 135, 97, 66 and 60 kDa(the last two, highlighted with blue arrows, were correlated using anti-human-IgG and IgM, respectively). We found that one third of the El Bagre-EPF sera were reactive to some of these four bands. No controls from outside or inside the endemic area were positive. Figure 4e illustrates positive FITC-conjugated IgG from an El Bagre-EPF patient, showing positive reactivity to the rat brain gyral and the sulci surface (green staining; white arrow). In Fig. 4f, similar pattern is seen with DPI-II (red staining; white arrows). We were able to demonstrate that El-Bagre-EPF patient autoantibodies colocalized in several areas of the brain and neurovascular structures with the commercial antibodies from Progen directed to DPI and DPII, to ARVCF, and with the P0071 antibodies (data no shown).
In Fig. 5, El Bagre-EPF patient autoantibodies colocalize with neural markers using IEM, CFM, and IHC. Figure 5a, c demonstrates colocalization of the neural components with El Bagre-EPF patient sera using FITC-conjugated human IgM and IgA antibodies (white staining; yellow arrows) and Texas red-conjugated GFAP (orange staining) (red arrows). In Fig. 5b, El Bagre-EPF autoantibodies colocalize using FITCI-conjugated human-IgM antibodies (yellow staining; red arrows). In Fig. 5d, e, CFM demonstrated that the El Bagre-EPF antibodies colocalized with neural markers. Figure 5d shows CFM image revealing anti-GFAP (red), anti-IgG (green), and Dapi (nuclei; blue) in their respective staining patterns, utilizing the EZ 1 Viewer software for image analysis. The light gray represents the overlapping of GFAP and IgG using NDIC. In Fig. 5e, we measured the staining of the overlap distances and found colocalization of the dyes in the same focal plane. The GFAP and the IgG peaks overlap (white arrows), in contrast to the Dapi (yellow arrow). Figure 5f, g shows IEM images. We observed 10 nm gold particles, representative of El Bagre-EPF autoantibodies deposited in the axons of the peripheral nerves at lower magnification (Fig. 5f, 60 kV) and at higher magnification (Fig. 5g, 150 kV) (red arrows, black particles).
Table I shows mercury levels in the patients and controls and documents neural alterations found in comparison with mercury levels at the time of the examination.
Table I.
Mercury levels in the patients and controls and neural alterations found in comparison with mercury levels at the time of the examination
| Subjects | Disease Course | Range of levels of mercury detected in nails and hair in parts per million (ppm) where (+ mild, ++ moderate and +++ severe) |
Presence of autoantibodies to neural structures, and isotypes of the antibodies |
Skin burning sensation |
Kinesiological and neurological alterations |
|---|---|---|---|---|---|
| El Bagre-EPF cases | Acute (<6 months after disease onset) |
40% (+++) 20% (++) 20% (+) 20% (5+) 0% (0) |
None | Positive in 100% cases |
Fine tremor 50% |
| El Bagre-EPF cases | Chronic cases(>3 years after disease onset) |
40% (+++) 20% (++) 20% (+) 20% (5+) 0% (0) |
IgM alone (40%) IgA alone (40%) IgA, IgM, fibrinogen, C3 and IgG (70%) |
Positive in 100% cases |
Depression (100%) |
| Controls living in the endemic area |
<6 months | 30% (+++) 20% (++) 10% (+) 20% (5+) 20% (0) |
None | Negative in 100% cases |
Fine tremor 50% |
| Controls living in the endemic area |
>3 years | 40% (+++) 20% (++) 20% (+) 20% (5+) 0% (0) |
IgM 1 control | Negative in 100% cases |
Depression (30%) |
| Controls from out of the endemic area |
100% (0) | None | Negative in 100% cases |
None |
Discussion
Patients affected by FS and El Bagre-EPF have burning sensation on the skin, combined with itching and occasional paresthesias statistically significant even comparing with the controls living in the same endemic area where mercury and other metals and metalloids prevail [4, 5, 7, 18]. Other neuromuscular symptoms reported before the steroid era for patients affected chronically by EPF included depression, mood disturbances, decalcifications, muscular atrophy (most of extensors muscle), contractual deformities, and ankylosis of the joints producing the “pose of pemphigus” (dosiflexion of extremities) [1–8]. Based on our studies, we can speculate that the “pose of pemphigus” is explained vis-a-vis for the weakness and/or direct damage of the extensor nerves for an unknown reason to us yet [1–12]. Our findings of polyclonal neural reactivity by colocalization of El Bagre-EPF autoantibodies with neurovascular markers could explain the skin burning and “be in fire sensation” symptoms and could provide valuable clues to the pathophysiological process. In addition, antibodies to the spindle cells of the neuromuscular apparatus may explain the clinical neuromuscular atrophy and the increased dorsiflexural tone in the “pose of pemphigus”. Furthermore, by IB, we detected reactivity of several molecules, including plakins and unknown molecules of 135, 97, 66, and 60 kDa in several sera from El Bagre-EPF patients. Of interest, p0071 is 135 kDa in molecular weight, and ARVCF approximates 97 kDa. IB revealed that IgM and IgG antibodies correlated with our DIF, IIF, and IHC testing.
We detected (1) autoantibodies to mechanoreceptors and mostly to thin nerves of the skin including some myelinated and some not. We also detected autoantibodies to the optic nerve, but mostly to the perineural meningeal sheaths [that are rich in desmoplakins (El Bagre-EPF antigens)]. In addition, we detected neural paucicellularity, decreases in the ENFD, and autoreactivity to parts of the brain that all colocalized with the patient’s autoantibodies and several neural markers. We demonstrated colocalization of the patient’s autoantibodies with DPI and DPII (known to be antigens for El Bagre-EPF) in the optic nerve and brain tissues. We recently described several ocular abnormalities and the presence of autoantibodies to the meibomian and other structures of the eyes in El Bagre-EPF patients [6]. Desmosomes are major intercellular junctions found in association with intermediate filaments in epithelial, cardiac, and arachnoidal tissue that surrounds the optic nerve [19]. DPI and DPII are part of the desmosomal plaque and seem to have a role for linking intermediate filaments in several tissues [19]. DPI and DPII are not restricted to stratified epithelia [19]. Interestingly, ARVCF is associated with E-, M-, and possibly N-cadherins [20]. Thus, we suggest that epitope spreading could occur in the disease process in El Bagre-EPF patients.
Within the skin, we observed defragmentation and alterations in the neural plexuses by several methods. Skin biopsy has been demonstrated to be of value in the diagnosis of clinical small fiber neuropathies; this technique is less invasive than nerve biopsy [12–15]. In autonomic neuropathies, diagnostic fibers are located in the dermis; thus, fibers innervating sweat glands and piloerector muscles can be assessed [12–15]. We recently described autoantibodies to sweat glands in patients affected by El Bagre-EPF [21]. Here, we demonstrate antibodies to multiple neurovascular areas. Thus, we have demonstrated physical and immunological evidence of autoreactivity to the peripheral and central nervous system using multiple techniques. However, the neural symptoms could result from a combination of sympathetic and parasympathetic nerves, both myelinated and non-myelinated damage, and therefore, larger and extended studies need to be pursue to answer these questions.
We speculate that damage to the nerves may occur because intraepidermal nerves exist in proximity to desmosomes; blisters, acantholysis, and separation would occur in these areas, potentially exposing neural antigens to autoreactivity. A similar process could occur at the neurovascular plexus below the basement membrane zone or within the dermal papillae. Additionally, mercury, other elements, and/or other diseases could induce epitope mimicry and/or alter the conformation of selected molecules, thus triggering the autoimmunity. p0071 is very homologous to the neural plakophilin-related armadillo repeat protein (NPRAP/δ catenin) and is located in several cell junctions [22]. p0071, DPI, and DPII are part of the complexus adherens meshwork, related to endothelial and lymphatic cells [23]. These cells are connected by the complexus adherens, with VE-cadherin joining with DPI and DPII, as well as several adherens junction plaque proteins, such α- and β-catenin, p120 catenin, and components of tight junctions, such claudin-5, JAM-A, and ZO-1 [23].
Most current literature and trends have clearly showed that most autoimmune skin blistering disease, such pemphigus and pemphigoids, are for the known desmogleins and BP18 and BP230. However, alterations of the nerves or other neural structures have been previously described in bullous diseases and loss over time, including a patient with paraneoplastic pemphigus (PNP) with a pseudotumor of the spinal nerve [24, 25]. Another case of PNP was associated with a myofibroblastic tumor [26]. Two Russian studies have shown alterations in adrenergic and cholinergic nerves of the skin in patients suffering chronic pemphigus [27, 28]. Changes in the Gasserian ganglion have been identified in cases of oral pemphigus [29]. Kaposi reported that many cases of fatal pemphigus showed anatomic changes of chronic myelitis in the spinal cord and/or in the sympathetic nerves [30]. Li et. al. reported that sera from a patient with BP with neurological changes recognized BP antigens by IB in the skin and brain [31]. In mice lacking the BPAG1 gene, neurons exhibited perturbations in their intermediate filaments and microtubules, leading to swellings and changes in the axons [31]. Our studies demonstrated autoantibodies by IEM using 10-nm gold particles in the axons of peripheral nerves; further IEM studies will be performed on the central neural system. Additional evidence of the importance of the neural system in pemphigus has been demonstrated by the detection of human alpha-acetylcholine and cholinergic receptors as pemphigus vulgaris antigens [32, 33].
Chronic exposure to mercury produces neural tissue alterations, as seen in acrodynia Minamata disease and in animal models exposed to mercury [34–36]. Symptoms typically include sensory impairment (vision, hearing, and speech), ataxia, disturbed sensation, and a lack of coordination. The type and degree of symptoms exhibited depend upon the individual toxin, the dose, and the method and duration of exposure [34–36]. These alterations differ from the severe burning sensation seen in El Bagre-EPF patients. Organic mercury affects primarily sensory peripheral nerves, with swelling and degeneration of Schwann cells and changes in both myelin sheaths and axons [32–35] Also differing, the skin biopsies from El Bagre-EPF showed several inflammatory cells as reported before and showed here [5, 8]. Most of autoantibodies produced by chronic mercury exposure alone in human and animals have been reported to be antinucleolar, differing to our findings.
In El Bagre-EPF patients, we have documented autoanti-bodies in the axons by IEM. Since autoantibodies to neural structures following exposure to mercury, metalloids or organophosphates are primarily directed against MBP and GFAP and of IgG, IgA, and IgM subclasses, a mercury association cannot be excluded in El Bagre-EPF etiology [34–36]. However, mercury exposure alone is not sufficient to develop pemphigus. In Minamata disease, no patients have been described with EPF [34–36]. In addition, in the control group from the endemic area, the predominance of any autoreactivity was seen using IgM and present only in some individuals; in contrast, most of the neurological alterations and the skin burning sensations presented exclusively in El Bagre-EPF patients. Finally, the autoanti-bodies in the patients were expressed against perineurium, epineurum, endoneurium, and blood vessels Both the NHP and the LKS clinical evaluation scale results correlate with our findings [16, 17]. We note that most of our affected nerves innervated extensor muscles; the significance of this finding is beyond the extent of our current study.
A paraformaldehyde prefixation of skin biopsies improved our assessment of neural tissues; we suggest larger studies to assess technique efficacy. Furthermore, the El Bagre-EPF patient focus exists in a rural area, with minimal medical resources. Ideal testing would address specific antigens with neural microarrays, quantitative sensory nerve testing (for large and small fibers), quantitative sudomotor axonal reflex testing, single photon and positron emission tomography, and electromyography.
Plakins are the major El Bagre-EPF antigens [1–12]. Homozygous mice with a desmoplakin gene ablation and subsequent rescue utilizing extra-embyonic ectoderm have shown that desmoplakin is important in embryonic development, affecting heart, neuroepithelium, skin, and vasculature integrity [37, 38]. Finally Balo and Foldavari [38] reported in 1948 that in the Gasserian ganglia and in other spinal ganglia, a chronic inflammatory process with perivascular infiltration, vacuolar degeneration of ganglionic cells, and proliferation of amphicytes [38] occurs. In addition, there is the presence of some granulomas, which were found at the juncture of posterior roots and spinal ganglia. These and other authors speculated that the disturbance of fluid circulation is caused by the granulomas consisting of arachnoidal cells. Our findings of autoreactivity against the arachnoid envelope antibodies may explain these findings. In summary, we suggest that neural autoreactivity may contribute to burning skin sensations, paresthesias, and the “pose of pemphigus” encountered in patients with EPF.
Acknowledgement
We thank Jonathan S. Jones, HT, and Lynn K. Nabers, HT, HTL for their expertise and excellent technical assistance at Georgia Dermatopathology Associates.
Funding sources Georgia Dermatopathology Associates, Atlanta, GA, USA (MSH, AMAV). The El Bagre-EPF samples were collected through previous grants from the University of Antioquia, the Embassy of Japan in Colombia, the Mineros de Antioquia SA, DSSA, the Hospital Nuestra Senora del Carmen, all in Medellin, Colombia, South America (AMAV). Confocal studies were performed with funds from the Department of Ophthalmology, Emory University Medical Center, Atlanta, GA, USA (HG) (NIH NEI EY06360).
Abbreviations
- EPF
Endemic pemphigus foliaceus
- El
Bagre-EPF endemic pemphigus foliaceus in El Bagre, Colombia
- FS
fogo selvagem
- ICS
intercellular stain between the keratynocytes
- BMZ
basement membrane zone
- DIF and IIF
direct and indirect immunofluorescence
- ENFD
epidermal nerve fiber density
- SDI
silver diamine ion stain
- BP
bullous pemphigoid
- MBS
modified Bielschowsky stain
- NHP
Nottingham Health Profile
- LKS
Lysholm Knee Scale
- NDIC
Normarski differential interference contrast confocal microscopy
- IHC
immunohistochemistry
- CFM
confocal microscopy
- IB
immunoblotting
- IEM
immunoelectron microscopy
- ARVCF
armadillo repeat protein deleted in velo-cardio-facial syndrome
References
- 1.Abrèu-Velez AM, Beutner EH, Montoya F, Bollag WB, Hashimoto T. Analyses of autoantigens in a new form of endemic pemphigus foliaceus in Colombia. J Am Acad Dermatol. 2003;49:609–14. doi: 10.1067/s0190-9622(03)00852-1. [DOI] [PubMed] [Google Scholar]
- 2.Abréu-Vélez AM, Hashimoto T, Bollag WB. A unique form of endemic pemphigus in Northern Colombia. J Am Acad Dermatol. 2003;4:599–608. doi: 10.1067/s0190-9622(03)00851-x. [DOI] [PubMed] [Google Scholar]
- 3.Abréu-Vélez AM, Warfvinge G, Leon-Herrera W, et al. Detection of mercury and other undetermined materials in skin biopsies of endemic pemphigus foliaceus. Am J Dermatopathol. 2003;25:384–91. doi: 10.1097/00000372-200310000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Hisamatsu Y, Abreu Velez AM, Amagai M, Ogawa MM, Kanzaki T, Hashimoto T. Comparative study of autoantigen profile between Colombian and Brazilian types of endemic pemphigus foliaceus by various biochemical and molecular biological techniques. J Dermatol Sci. 2003;32:33–41. doi: 10.1016/s0923-1811(03)00034-3. [DOI] [PubMed] [Google Scholar]
- 5.Howard MS, Yepes MM, Maldonado-Estrada JG, et al. Broad histopathologic patterns of non-glabrous skin and glabrous skin from patients with a new variant of endemic pemphigus foliaceus—part 1. J Cutan Pathol. 2010;37:222–30. doi: 10.1111/j.1600-0560.2009.01315.x. [DOI] [PubMed] [Google Scholar]
- 6.Abreu-Velez AM, Howard MS, Hashimoto T, Grossniklaus HE. Human eyelid meibomian glands and tarsal muscle are recognized by autoantibodies from patients affected by a new variant of endemic pemphigus foliaceus in El-Bagre, Colombia, South America. J Am Acad Dermatol. 2010;62:437–47. doi: 10.1016/j.jaad.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abreu Velez AM, Howard MS, Hashimoto T. Palm tissue displaying a polyclonal autoimmune response in patients affected by a new variant of endemic pemphigus foliaceus in Colombia, South America. Eur J Dermatol. 2010;20:74–81. doi: 10.1684/ejd.2010.0834. [DOI] [PubMed] [Google Scholar]
- 8.Castro RM, Proença NG. Similarities and differences between Brazilian wild fire and pemphigus foliaceus Cazenave. Hautarzt. 1982;11:574–7. [PubMed] [Google Scholar]
- 9.Diaz LA, Sampaio SA, Rivitti EA, et al. Endemic pemphigus foliaceus (fogo selvagem). I. Clinical features and immunopathology. J Am Acad Dermatol. 1989;4:657–69. doi: 10.1016/s0190-9622(89)70079-7. [DOI] [PubMed] [Google Scholar]
- 10.Vieira JP, Fonzari M, Goldman A. Some recent studies in Brazilian pemphigus. Am J Trop Med Hyg. 1954;3:868–77. doi: 10.4269/ajtmh.1954.3.868. [DOI] [PubMed] [Google Scholar]
- 11.Wendell G, Zander E. A critical evaluation of methods used to demonstrate tissue neural elements, illustrated by reference to the cornea. J Anat (London) 1950;84:168–94. [PMC free article] [PubMed] [Google Scholar]
- 12.Kennedy WR, Wendelschafer-Crabb G. The innervation of human epidermis. J Neurol Sci. 1993;115:184–90. doi: 10.1016/0022-510x(93)90223-l. [DOI] [PubMed] [Google Scholar]
- 13.Griffin JW, McArthur JC. Small fiber sensory neuropathies: clinical course and neuropathology of idiopathic cases. Ann Neurol. 1998;144:47–59. doi: 10.1002/ana.410440111. [DOI] [PubMed] [Google Scholar]
- 14.Singer W, Spies JM, McArthur J, et al. Prospective evaluation of somatic and autonomic small fibers in selected autonomic neuropathies. Neurology. 2004;4:612–8. doi: 10.1212/01.wnl.0000110313.39239.82. [DOI] [PubMed] [Google Scholar]
- 15.Williams GN, Taylor DC, Gangel TJ, Uhorchak JM, Arciero RA. Comparison of the single assessment numeric evaluation method and the Lysholm score. Clin Orthop Relat Res. 2000;373:184–92. doi: 10.1097/00003086-200004000-00022. [DOI] [PubMed] [Google Scholar]
- 16.Ebrahim S, Barer D, Nouri F. Use of the Nottingham Health Profile with patients after a stroke. J Epidemiol Community Health. 1986;40:166–9. doi: 10.1136/jech.40.2.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.da Justa Pinheiro CH, de Soussa Filho WM, Gongalvez Moura Pinhero D, de Olivera Brasil AC. Consideraous sobre rehabilitacao fisica e fisioterpia nas penfigo foliaceo endemico. Rev Bras Provoăcao Saude. 2007;20:124–32. [Google Scholar]
- 18.Angst BD, Nilles LA, Green KJ. Desmoplakin II expression is not restricted to stratified epithelia. J Cell Sci. 1990;97:247–57. doi: 10.1242/jcs.97.2.247. [DOI] [PubMed] [Google Scholar]
- 19.Kaufmann U, Zuppinger C, Waibler Z, Rudiger M, Urbich C, Martin B, et al. The armadillo repeat region targets ARVCF to cadherin-based cellular junctions. J Cell Sci. 2000;113:4121–35. doi: 10.1242/jcs.113.22.4121. [DOI] [PubMed] [Google Scholar]
- 20.Abreu-Velez AM, Howard MS, Hashimoto K, Hashimoto T. Autoantibodies to sweat glands detected by different methods in serum and in tissue from patients affected by a new variant of endemic pemphigus foliaceus. Arch Dermatol Res. 2009;301:711–8. doi: 10.1007/s00403-009-0972-4. [DOI] [PubMed] [Google Scholar]
- 21.Deguchi M, Iizuka T, Hata Y, Nishimura W, Hirao K, Yao I, et al. 2000;22(275):29875–80. doi: 10.1074/jbc.M005384200. [DOI] [PubMed] [Google Scholar]
- 22.Moll R, Sievers E, Hämmerling B, Schmidt A, Barth M, Kuhn C, et al. Endothelial and virgultar cell formations in the mammalian lymph node sinus: endothelial differentiation morphotypes characterized by a special kind of junction (complexus adherens) Cell Tissue Res. 2009;335:109–41. doi: 10.1007/s00441-008-0700-y. [DOI] [PubMed] [Google Scholar]
- 23.Eto K, Yasutake A, Miyamoto K, Tokunaga H, Otsuka Y. Chronic effects of methylmercury in rats. II Pathological aspects. Tohoku J Exp Med. 1997;182:197–205. doi: 10.1620/tjem.182.197. [DOI] [PubMed] [Google Scholar]
- 24.Lee SH, Sung JK. Inflammatory pseudotumor of the spinal nerve complicated by paraneoplastic pemphigus. Case illustration. J Neurosurg Spine. 2006;6:514. doi: 10.3171/spi.2006.4.6.514. [DOI] [PubMed] [Google Scholar]
- 25.Lee DH, Lee SH, Sung JK. Inflammatory myofibroblastic tumor on intercostal nerve presenting as paraneoplastic pemphigus with fatal pulmonary involvement. J Korean Med Sci. 2007;4:735–9. doi: 10.3346/jkms.2007.22.4.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tseraidis GS, Bavykina EA. Changes in adrenergic and cholinergic nerves of the skin in chronic pemphigus. Vestn Dermatol Venerol. 1977;10:17–20. [PubMed] [Google Scholar]
- 27.Torsuev NA, Kimbarovskaia EM, Romanenko VN, et al. Neuromorphology of the skin in patients with pemphigus vulgaris. Vestn Dermatol Venerol. 1977;10:13–27. [PubMed] [Google Scholar]
- 28.Foldvarif F, Balo J. Changes of the Gasserian ganglion in cases of oral pemphigus. Ann Dermatol Syphiligr. 1954;5:507–20. [PubMed] [Google Scholar]
- 29.Kaposi M. Vesicular eruptions: pemphigus. William Wood and Company; New York: 1985. Pathology and treatment of diseases of the skin for practitioners and students; pp. 390–7. special edition. Lecture XXIX. Copyright to the Classics of Medicine Library Division of Gryphon Editions. [Google Scholar]
- 30.Li L, Chen J, Wang B, Yao Y, Zuo Y. Sera from patients with bullous pemphigoid (BP) associated with neurological diseases; recognized BP antigen in the skin and brain. Br J Dermatol. 2009;6:1343–5. doi: 10.1111/j.1365-2133.2009.09122.x. [DOI] [PubMed] [Google Scholar]
- 31.Nguyen VT, Ndoye A, Grando SA. Novel human alpha9 acetylcholine receptor regulating keratinocyte adhesion is targeted by pemphigus vulgaris autoimmunity. Am J Pathol. 2000;4:1377–91. doi: 10.1016/s0002-9440(10)64651-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vu TN, Lee TX, Ndoye A, Shultz LD, Pittelkow MR, Dahl MV, et al. The pathophysiological significance of nondesmoglein targets of pemphigus autoimmunity. Development of antibodies against keratinocyte cholinergic receptors in patients with pemphigus vulgaris and pemphigus foliaceus. Arch Dermatol. 1998;134:971–80. doi: 10.1001/archderm.134.8.971. [DOI] [PubMed] [Google Scholar]
- 33.Miyakawa T, Deshimaru M, Sumiyoshi S, Teraoka A, Tatetsu S. Experimental organic mercury poisoning; pathological changes in peripheral nerves. Acta Neuropathol. 1970;15:45–55. doi: 10.1007/BF00690688. [DOI] [PubMed] [Google Scholar]
- 34.Fabriziomaria G. Occupational exposure to chemicals and sensory organs: a neglected research field. Neurotoxicology. 2003;24:675–91. doi: 10.1016/S0161-813X(03)00038-X. [DOI] [PubMed] [Google Scholar]
- 35.Anniko M, Sarkady L. The effects of mercurial poisoning on the vestibular system. Acta Otolaryngol. 1978;2:96–104. doi: 10.3109/00016487809121428. [DOI] [PubMed] [Google Scholar]
- 36.Lechler T, Fuchs E. Desmoplakin: an unexpected regulator of microtubule organization in the epidermis. J Cell Biol. 2007;176:147–54. doi: 10.1083/jcb.200609109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gallicano GI, Bauer C, Fuchs E. Rescuing desmoplakin function in extra-embryonic ectoderm reveals the importance of this protein in embryonic heart, neuroepithelium, skin and vasculature. Development. 2001;128:929–41. doi: 10.1242/dev.128.6.929. [DOI] [PubMed] [Google Scholar]
- 38.Baló J, Földvári F. Pemphigus and the nervous system. Int J Dermatol. 1972;11:223–30. doi: 10.1111/j.1365-4362.1972.tb01755.x. [DOI] [PubMed] [Google Scholar]