Abstract
Objective
Impairments in cognitive emotion regulation (CER) have been linked to functional neural abnormalities and the pathogenesis of major depressive disorder (MDD). Few functional magnetic resonance imaging (fMRI) studies have investigated the neural underpinnings of CER in samples with depression. As CER develops in childhood, understanding dysfunctional CER-related alterations in brain function during this period could advance knowledge of the developmental psychopathology of MDD.
Method
This study tested whether neural activity in brain regions known to support cognitive reappraisal differed between healthy 7- to 15-year-old children and same-age peers with a history of MDD (MDD-ever). A total of 64 children participated in this event-related fMRI study, which used a developmentally appropriate and validated fMRI reappraisal task. Children were instructed to passively view sad or neutral images and to decrease negative emotions using cognitive reappraisal.
Results
MDD-ever and healthy children showed similar patterns of cortical activation during reappraisal, but with a significant difference found in 1 key CER region, the left inferior frontal gyrus (IFG). In addition, individual differences in CER were associated with left IFG activity during reappraisal.
Conclusion
Alterations in the neurocircuitry of reappraisal are evident in children with a depression history compared to healthy controls. The finding that MDD-ever children showed reappraisal-related neural responses in many regions similar to healthy controls has clinical implications. Findings suggest that identification of alterations in reappraisal in children with remitted depression, for whom much, although not all, of the neural circuitry remains intact, may be an important window of opportunity for intervention.
Keywords: childhood depression, emotion regulation, cognitive reappraisal, fMRI, MDD
Dysfunctional cognitive emotion regulation (CER) strategies have been linked to functional neural abnormalities as well as risk, course, and outcomes associated with major depressive disorder (MDD) in adults.1,2 One CER strategy that has received the bulk of empirical focus is cognitive reappraisal.3,4 By reinterpreting (i.e., reappraising) the affective meaning of emotion-eliciting situations, one may regulate and modify one’s emotional responses to a distressing event. Neuroimaging studies examining patterns of neural activation during the use of reappraisal in healthy adults and those with depression are consistent with decades of cognitive-behavioral research on mechanisms (i.e., cognitive vulnerabilities for depression) and treatment (e.g., cognitive-behavioral therapy [CBT]) that implicate impaired CER processes in the etiology and course of MDD.5 Related to these deficits, individuals at risk for MDD are more likely to process everyday life events as being negative, resulting in more experiences of negative mood, emotion, and affect.6 When these cognitive vulnerabilities are paired with an inability to use adaptive CER strategies such as reappraisal, risk for MDD increases.7 There are known associations between the infrequent and/or inefficient use of cognitive reappraisal tactics in relation to the development and course of MDD.8 Nonetheless, functional magnetic resonance imaging (fMRI) studies investigating the neural underpinnings of reappraisal in children at risk for, currently diagnosed with, or in a remitted phase of depression are absent from the translational developmental neuroscience literature.
Developmental Course of Emotion Regulation in the Context of Developing MDD
Developmentally informed research has established that depressotypic emotion regulation (ER) strategies are evident early in children’s development.9 Although ER strategies continue to evolve across the lifespan, strong correlations exist between cognitive-based ER strategies that develop and are practiced in late childhood and behavioral patterns of CER strategy used in later life.10–17 That is, extant findings indicate that late childhood is a critical developmental period for acquiring adaptive CER strategies to self-regulate affective arousal.18
From a developmental affective neuroscience perspective, cortical and especially the prefrontal cortex (PFC) brain regions that support the development of complex thought processes necessary for CER undergo significant changes in function as well as structure starting in early adolescence. In addition to normative neurodevelopment that occurs in adolescence, this is also the age at which individuals are most likely to be diagnosed with MDD. The dynamic interplay between pubertal changes, cognitive transitions, heightened social awareness, and maturing brain regions that support the use of CER may contribute aberrant trajectories of adolescent emotion development, which in turn may increase the risk of MDD episodes. Given that adolescence is a period of high risk for MDD, understanding dysfunction in CER processes and related alterations in brain function before and during this developmental period could have important implications for advancing our knowledge of risk and occurrence of MDD in adolescents. Using a previously validated approach,19 the current study tested whether neural response in brain regions known to support the reappraisal of negative stimuli in adults differed between typically developing children aged 7 to 15 years and those with 1 or more past episodes of MDD.
Neural Circuitry of Cognitive Reappraisal in Healthy Adults
fMRI research conducted in healthy adults has informed our understanding of the source brain regions that provide the neural underpinning for implementing reappraisals as well as the target neural systems that are acted upon during reappraisal. Although fMRI studies of reappraisal have varied along a number of experimentally significant dimensions (distancing versus reappraising, stimuli valence, and regulatory direction; increase versus decrease emotion response), when results are taken as a whole, findings indicate that the implementation of reappraisal to modulate emotion responsivity is supported by many of the same frontoparietal and cognitive control regions that regulate memory, attention, and numerous other thought processes. The most commonly observed regions involved in implementing reappraisals (i.e., source regions) include but are not limited to the following: dorsolateral (dlPFC) and the inferior parietal cortex (when combined this is also known as the frontoparietal network; in the context of reappraisal, this network is thought to direct attention to reappraisal-relevant stimulus features, to hold in mind appraisal goals, and to manipulate information during the construction of new appraisals20; dorsal anterior cingulate cortex (dACC) and posterior dorsomedial prefrontal cortex (dmPFC) regions, which, in the context of reappraisal, are thought to support the monitoring and tracking of the effectiveness of reappraisals21; and the ventrolateral PFC (vlPFC), which is thought to support goal-appropriate selection of a new reappraisal of the initial stimuli.22 The left vlPFC is thought to be of particular importance in reappraisal paradigms that focus on reinterpretation tactics. The left vlPFC may be used to deliberately select semantic elements needed to construct a new stimulus-appropriate reappraisal and is involved in inner-speech processes.
These cortical regions and networks provide the neural substrates for using cognitive reappraisal effectively. Supporting evidence for the importance of these cortical brain regions has been found in several studies that indicate that increased neural activation in cortical brain regions while engaged in reappraisal correlates with effective modulation of self-reported emotional experience as well as other effectively modulated measures such as behavioral and physiological correlates of emotion responsivity. Given that reappraisal is associated with modulation of emotion experience, it should also modulate in subcortical/limbic regions involved in the generation of emotion. Although results have been mixed, likely due to variation in methodological approach, the consensus remains that reappraisal modulates activity in subcortical structures associated with emotion generation.23,24 Specifically, findings from healthy adults have shown that using reappraisal to down-regulate negative emotions after presentation of negative stimuli is associated with decreased neural activation in the amygdala,25 ventral striatum, and, to a lesser extent, the ventromedial prefrontal cortex (vmPFC). These findings in healthy adults suggest that individuals who have difficulties with reappraisal, such as adults with depression, should show reduced activity in cortical regions, especially the vlPFC. Based on observations in healthy adults, it is also expected that adults with depression will show increased activation in emotion generation regions such as the amygdala, which may result from reduced modulation by cortical regions.
Reappraisal in Healthy Children
To date, few fMRI studies have examined neural activation during reappraisal in children and adolescent samples.19,26–29 Results indicate that childhood development is associated with both linear and quadratic (inverted U-shaped) increases in activity in dorsal and lateral PFC regions. For instance, researchers have found a linear association between participants’ age (i.e., 10–23) and reappraisal ability at both the neural and behavioral levels.27 Specifically, activation in a portion of the left inferior frontal gyrus (IFG: a.k.a. vlPFC) demonstrated a positive linear association with age. Increasing age was also associated with increased ratings of reappraisal successes during the fMRI task, which were both related to increased activation of the left IFG during reappraisal. The IFG may be especially relevant, given recent findings demonstrating that this region becomes more effective at supporting reappraisal with age. Although the strength of neural activation may differ between children and adults, findings suggest that children will show activation patterns in cortical and subcortical regions similar to those seen in healthy adults.
Although developmental affective neuroscience research specific to CER processes is in its infancy, 2 highlights have emerged from the existing literature. First, neurodevelopment within brain regions that support one’s ability to regulate emotion are highly similar to what has been observed in studies of “cold” cognitive tasks. Second, improved capacities and efficacy of using CER strategies such as reappraisal are supported by structural and functional maturation of prefrontal regions known to support reappraisal in healthy adults.
Cognitive Reappraisal in Adults and Adolescents With Depression
Dysfunctional ER is a core tenet of MDD and is thought to contribute to the chronic experience of negative affect and low mood commonly seen in individuals with depression. The few studies that have examined the neural mechanisms of reappraisal in adults with depression have varied in the use of reappraisal tactics, goals, and emotional valence. However, several findings consistently emerge when comparing healthy adults to those with depression. First, healthy adults and those with depression rarely differ significantly from each other on self-report measures of reappraisal success during fMRI reappraisal tasks. That is, regardless of diagnostic group status, healthy adults and those with depression report being equally successful at using reappraisal during fMRI tasks. Second, MDD influences regulation of subcortical responses to affective stimuli; however, the results are mixed. Some studies have found that participants with MDD show enhanced amygdala response during the reappraisal of negative affect, whereas others have found that adults with depression fail to sustain reappraisal-related modulation of the amygdala.30,31 The former suggests that participants with depression fail to recruit cortical regions responsible for modulating subcortical emotion generation regions. The latter suggests that individuals with MDD may initially be able to modulate emotion generation regions but cannot sustain this modulation, putting them at risk for returning to negative mood states. Blunted reappraisal effects on amygdala activation in both remitted32 and unmedicated samples with depression have also been reported.33 In a study of untreated adults with depression and healthy controls, both groups recruited regions of the dlPFC and vlPFC during CER, but only in controls was this associated with reduced activity in the amygdala, suggesting impairments in participants with depression.34,35
Third, healthy adults compared to those with depression recruit a broader area of the PFC during reappraisal. Thus, it has been suggested that adults with MDD exhibited less efficiency during reappraisal.2,33 In the only study examining reappraisal in healthy adolescents compared to those with depression, the latter demonstrated reduced activation in the vlPFC compared to that in healthy adolescents.28 Fourth, several studies have found that connectivity between the PFC and the amygdala or ventral striatum in adults with depression either is diminished or exhibits an opposite pattern of what is commonly observed in healthy controls.2,30 The only functional connectivity MRI (fcMRI) study that has compared adolescents with depression to healthy controls during reappraisal found that adolescents with depression showed less connectivity between the amygdala and both the insula and medial PFC compared to those in healthy controls while passively viewing negative stimuli.28 Taken together, all of these findings suggest that the effects of MDD on neural activation and connectivity during reappraisal are not yet well understood in adults, and even less is known about late childhood and adolescence, the period when CER skills are rapidly developing and the incidence of MDD is rising.
Present Study
The aim of the current study was to further investigate differences in neural activation during reappraisal in healthy controls compared to a group of school-age children previously diagnosed with MDD. Children with an MDD history are at increased risk for additional MDD episodes and thus may help shed light on neural alterations associated with dysfunctional CER during development. As the fMRI results in adults with depression are based on widely varying methodologies, and as there is only 1 fMRI study of reappraisal in adolescents with depression, we focused on regions most commonly associated with reappraisal in healthy adults based on a recent meta-analysis.4 These same regions of interest (ROIs) have also been identified as supporting reappraisal of sadness in a sample of 19 healthy children.5 Focusing on ROIs established from a meta-analysis has the benefit of targeting stable and representative regions. To date, fMRI studies of reappraisal in children and adults have emphasized the down-regulation of highly arousing negative emotions such as disgust and fear. The current study addresses a novel and important gap in the reappraisal literature by examining sad stimuli. Although sad stimuli may be lower in arousal compared to disgust or fear, they were the focus of the current study because of their prevailing role in MDD.
We hypothesized that children with previous episodes of depression would show significantly less activation in the brain regions thought to support reappraisal (e.g., dlPFC, vlPFC, and dACC) compared to healthy controls when instructed to reappraise sad stimuli. Furthermore, given the extensive literature on at-risk adolescents and those with depression that has demonstrated increased amygdala activation in response to negative emotion, we hypothesized that children with an MDD history would show increased neural activity in emotion-generative brain regions (e.g., amygdala and striatum) when processing sad stimuli, particularly in the reappraisal condition, when they should be downregulating this activity. We also expected that children’s neural activation in brain regions supporting reappraisal (dlPFC, dACC, inferior parietal regions) would show positive associations with parent report of children’s emotion regulation capacities. Activation in the amygdala during active reappraisal was expected to be negatively associated with children’s emotion regulation scores. We also hypothesized that higher MDD severity scores in children would correlate with decreased activation in cortical reappraisal regions and increased activation in the amygdala.
METHOD
Participants
A sample of 64 children (34 female) aged 8 to 15 years (mean, 11 years 6 months) participated in the current study after providing consent according to the guidelines of the Washington University School of Medicine Institutional Review Board. These children were recruited from a larger ongoing study of preschool-onset depression, but the current study was a different and separate fMRI study. Given the goals of the current study, we focused our analyses on healthy children and same-age peers with 1 or more prior diagnoses of MDD during the course of the study (i.e., MDD-ever group), resulting in a total sample of 55 children (28 MDD-ever). Thus, 9 children with a psychiatric disorder diagnosis other than MDD were excluded from these analyses. Table 1 summarizes demographic and clinical data for healthy and MDD-ever diagnostic groups.
TABLE 1.
Demographic, Clinical, and Scanning Characteristics by Diagnostic Group
| Controls n = 27 |
MDD-Ever N = 28 |
Statistic | p | |
|---|---|---|---|---|
| Child Demographic Factors | ||||
| Gender; % female (n) | 52% (14) | 57% (16) | χ2(1) = .16 | .69 |
| Age at scan, mean (SD) | 10.8 (2.00) | 11.4 (1.60) | t(53) = −1.19 | .24 |
| IQ, mean (SD) | 109 (13.5) | 103 (16.8) | t(52) = 1.56 | .13 |
| Pubertal status at scan; % prepubertal (n) | 41% (11) | 25% (7) | χ2(1) = 1.55 | .21 |
| Ethnicity; % white (n) | 63% (17) | 46% (13) | χ2(1) = 1.52 | .22 |
| Handedness; % right handed (n) | 82% (22) | 79% (22) | χ2(1) = .07 | .79 |
| Psychotropic use ever; % yes (n) | 3.7% (1) | 39% (11) | χ2(1) = 10.21 | .001 |
| Antidepressant use ever | 0 | 6% (3) | ||
| Antidepressant use at time of scan | 0 | 4% (2) | ||
| Family Demographic Factors | ||||
| Gross income at scan, % <$60K (n) | 48% (13) | 64% (18) | χ2(1) = 1.50 | .23 |
| Education, % ≥4-year degree (n) | 63% (17) | 25% (7) | χ2(1) = 8.05 | .005 |
| Clinical Variables | ||||
| MDD diagnosis at time of scan | 0 | 0 | ||
| MDD diagnosis within 6 mo of scan | 0 | 4% (2) | ||
| MDD diagnosis within 2 y of scan | 0 | 25% (14) | ||
| ERC emotion regulation | 29.06 (2.33) | 26.48 (3.31) | t(53) = 3.31 | .002 |
| MDD core symptoms at closest annual wave | 1.5 (1.45) | 2.70 (1.68) | t(53) = −2.74 | .008 |
| Average mood rating | ||||
| View neutral | 3.32 (0.55) | 3.43 (0.34) | t(52) = −0.98 | .33 |
| View sad | 2.40 (0.74) | 2.32 (0.55) | t(51) = 0.45 | .65 |
| Reappraise sad | 2.64 (0.75) | 2.41 (0.70) | t(51) = 1.16 | .25 |
Note: ERC = Emotion Regulation Checklist; MDD = major depressive disorder.
DSM-IV Psychiatric Diagnoses
For assessments before age 8 years, an age-appropriate, semi-structured, parent-report diagnostic interview was used to assess children’s psychiatric symptoms, namely, the Preschool-Age Psychiatric Assessment (PAPA). After age 8 years, the Childhood and Adolescent Psychiatric Assessment (CAPA) was used. This includes child report and caregiver report of psychiatric symptoms (which are combined) to inform diagnostic classification. Detailed descriptions of the parent study can be found in prior publications.6–8
Depression Severity Score
Depression severity scores were obtained at the children’s annual wave conducted in closest proximity to their scan for the current study. These scores comprised the total number of core DSM-IV MDD symptoms (0−9 possible) endorsed by caregiver and/or child on the MDD module of the PAPA or CAPA.
Emotion Regulation Score
Parent report of their children’s capacities to self-regulate emotion was assessed using the 8-item ER subscale of the Emotion Regulation Checklist.9 This is a well-validated subscale (α = 0.77 in our sample) of children’s emotion self-regulation.
Procedure
A pre-scan training procedure was used to ensure that children understood how to use reappraisal in response to negative stimuli. Details are provided in supplemental materials (see Supplemental 1, available online, and Belden et al.19 for description). Using a developmentally appropriate, validated fMRI reappraisal task, children were instructed to either passively view negative or neutral images, or to decrease their experience of negative emotions in response to viewing sad images, by using cognitive reappraisal strategies. Similar to other CER studies,28,29,40–42, at the start of each trial, a photograph (i.e., neutral or sad) was presented for a 4-second interval (Figure 1). Next, an instruction, “VIEW,” appeared to indicate nonregulation trials or “MAKE-POSITIVE,” to indicate regulation trials (the instruction and photograph remained on the screen together for another 4 seconds). Then, the photograph disappeared, but the instruction remained on screen for an additional 4 seconds. Following each picture, children were prompted to answer the question, “How do you feel?”. Children had 4 seconds to rate their negative affect on a scale from 1 to 4. Responses were made on a 4-button box. After the affect-rating period, the word “RELAX” appeared on the screen for 4 to 8 seconds (for example, see Figure 1). The combinations of neutral and sad photographs with nonregulate versus regulate instructions resulted in 3 conditions: view neutral (nonemotional), view sad (sadness without reappraisal), and reappraise sad (reappraise while viewing sad photo).
FIGURE 1.

Example of a single trial for the reappraise sad condition.
Stimuli were taken from the International Affective Picture Series (IAPS)43,44 and were supplemented from an in-house set of images selected to be appropriate for viewing by children (e.g., photographs of other children crying). IAPS stimuli have been rated for valence (1–9; extremely negative to extremely positive) and arousal (1–9; no arousal to extreme arousal). The IAPS images that we used had valence scores of less than 4 and arousal scores of greater than 4. We used 20 total neutral and 40 total sad pictures during the fMRI task. Each run presented 12 trials divided equally among view neutral, sad, and reappraise. Trial orders were pseudo-randomized, and stimuli used for the VIEW versus MAKE-POSITIVE conditions were counterbalanced so that stimuli were not confounded with condition. The CER task included 5 runs of 12 trials each (60 trials total, 20 in each condition). Each trial lasted 16 seconds (followed by a 4- to 8-second jitter), and each run lasted approximately 4 minutes and 40 seconds.
fMRI Methods
We acquired both T1 and blood oxygen level–dependent (BOLD) images in the same session (see Supplement 1, available online, for more details). The functional images were collected with a 12-channel head coil in runs using an asymmetric spin-echo echo-planar sequence sensitive to BOLD contrast (T2) (TR = 2000 ms, TE = 27 ms, FOV = 256 mm, flip = 90°). During each functional run, sets of 32 contiguous axial images with isotropic voxels (3 mm3) were acquired parallel to the anterior–posterior commissure plane.
Data Processing
Magnetic resonance (MR) data were reconstructed into images and aligned to correct for head motion. All MR data (structural and functional) were registered to a common space atlas optimized for children’s heads (Talairach space) using a 12-parameter linear (affine) transformation.45,46 Frames with excessive movement were identified and removed following a procedure suggested by Power et al.,47 as those for which the sum of the displacement across all 6 rigid body movement correction parameters exceeded 0.5 mm (more detail provided in Pagliaccio et al.48).
Data Analysis
Similarity of children with a history of 1 or more MDD episodes and healthy children on demographic and clinical/behavioral variables was examined using t tests and χ2 and bivariate correlational analyses (Table 1). Repeated-measures analysis of variance (ANOVA) was used to test for diagnostic group differences in children’s emotional state ratings during the fMRI task (view neutral versus view sad, and reappraise sad versus view sad). Furthermore, to test for potential covariates of noninterest, we examined whether children’s gender and/or their age at time of scan differentiated and/or associated with their neural activation when comparing their response to the reappraise versus view sad conditions using ROIs. If gender and/or age were found to have a significant effect on neural activation, they were included as a covariate in all analyses.
fMRI Data Analysis
For each participant, we computed a general linear model (GLM). We did not assume a hemodynamic response shape because of concerns about potential developmental differences in the shape or timing of this response. Instead, we used a finite impulse response approach where 16 time points (taken at 2-second repetition times [TRs]) were estimated. This included 8 time points to cover the 16 seconds of the trial (plus an additional 16 seconds for the evolution of the hemodynamic response. The results of these fixed effects analyses for each individual participant were entered into second-level analyses, treating participants as a random factor. Frames 5 to 8 (10–18 seconds into each trial) were used for the current analyses, reflecting BOLD responses hypothesized to be related to picture viewing and reappraisal of sad emotion when taking into account the lag in the hemodynamic response.
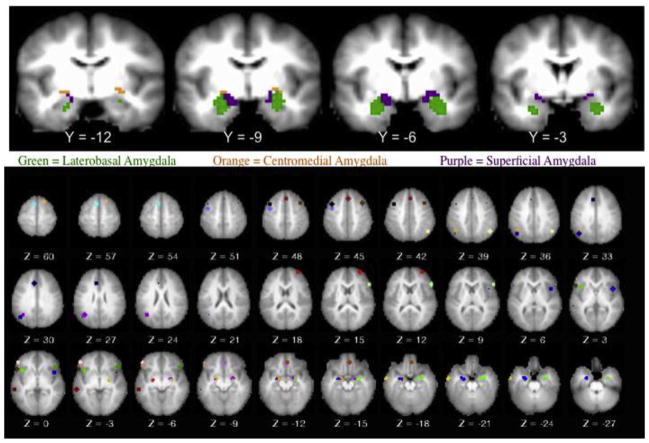
We used an ROI analysis to enhance power. We used ROIs for 3 subdivisions of the amygdala, namely, centromedial, laterobasal, and superficial, for right and left hemispheres (Figure 2). These amygdala ROIs were based on prior findings from Roy et al., who provided our ROI mask.49 For the cognitive control regions, we focused on the regions identified in a recent meta-analysis that included studies focused on cognitive down-regulation of negative emotion (albeit primarily fear stimuli) similar to the current study.36 In the Diekhof et al. meta-analysis, 23 regions showed greater activation during the reappraisal of negative stimuli consistently across all studies. We used these coordinates to create spherical ROIs that were 12 mm in diameter (Figure 2). A 12-mm diameter was chosen so as to be similar in size to that used in prior published studies. Larger ROIs risk including activation from multiple regions but are also associated with higher signal-to-noise ratio (SNR). Smaller ROIs are more focal but can suffer from lower SNR.
FIGURE 2.
Amygdala subdivisions and Diekhof region of interest.
Analysis Plan
Hypothesis 1a
Children previously diagnosed with MDD compared to controls will show greater neural response in the amygdala when viewing sad compared to neutral stimuli and when reappraising compared to passively viewing sad photographs.
Approach/Analysis
A targeted ROI analysis was performed using repeated-measures ANOVAs within each group separately. Amygdala subdivisions showing a significant main effect of condition or a condition × time interaction effect within the control group and/or group with prior depression were then tested using diagnostic group status as the between-subjects factor using false discovery rate (FDR) p < .05 correction.
Hypothesis 1b
Children previously diagnosed with MDD compared to controls will show significantly less activation in prefrontal regions commonly activated when implementing reappraisal.
Approach/Analysis
A targeted ROI analysis was performed using repeated-measures ANOVA within each group separately. Within-group analyses were conducted using 23 ROIs previously identified as being consistently activated during active reappraisal and were tested as the criterion variables. Brain regions that showed a significant condition and/or condition × time effect after using FDR correction were further tested with diagnostic group as a between-subjects factor.
Hypothesis 2a
Children’s higher MDD severity scores will be associated with increased neural activation in the amygdala (6 ROI) and decreased activation in prefrontal control regions (23 Diekhof ROI) during the reappraise versus view sad conditions.
Hypothesis 2b
Children’s higher ER scores will be associated with decreased neural response in the amygdala and increased activation in prefrontal control regions during the reappraise versus passive viewing conditions. Significant correlations are expected to remain significant when children’s current MDD severity at time of scan is included as a covariate in follow-up analyses.
Approach/Analysis
A targeted ROI approach was used for analyses examining the associations between MDD severity and ER scores in relation to neural activation during reappraisal minus view sad contrast. For each amygdala and Diekhof ROI, we averaged the percent change in BOLD signal across frames 5 to 8 separately for the reappraise and view sad conditions. Then we created difference/contrast scores for each ROI by subtracting children’s view sad activity from reappraise sad activity. We then conducted bivariate partial correlations in the total sample using the amygdala ROIs in 1 set of analyses and Diekhof ROIs in the second set of analyses. Regions showing a significant correlation after FDR p < .05 corrections were then tested as outcome variables in a 2-step hierarchical regression analysis in which the independent variables (IVs) were MDD severity in step 1 and ER scores in step 2. To reduce the number of comparisons, we examined only those Diekhof regions that showed a significant main effect of condition or a significant interaction effect of condition and time after using FDR corrections of p < .05 within the healthy and/or within the MDD-ever groups in approach 1 above.
RESULTS
Demographic and Clinical Characteristics
There was no significant effect of diagnostic group in relation to children’s gender, age, IQ, pubertal status, handedness, ethnicity, or family income (Table 1). Children in the MDD-ever group had caregivers who completed significantly fewer years of education than caregivers of the control group. As expected, children in the MDD-ever group had significantly higher MDD severity scores at their assessment closest to time of scan and had significantly lower ER scores compared to healthy peers (Table 1).
Self-Report of Negative Affect During fMRI
Results indicated that children reported significantly more positive affect after viewing neutral compared to sad photographs (F1,51 = 109.34, p < .00001). At a trend level, children also reported more positive affect after reappraise sad versus view sad trials (F1,51 = 3.63, p = .06; see Figure S1, available online). There were no significant interaction effects between diagnostic group and condition on children’s emotion state ratings during the view neutral versus view sad (F1,51 = .87, p = .35) or reappraise sad versus view sad condition (F1,51 = .70, p = .41).
Potential Covariates
There was no significant effect (all p > .12) of children’s gender on activation in the amygdala or Diekhof ROI when testing the view sad minus view neutral contrast and the reappraise minus view sad contrast. Results from the bivariate correlations using an FDR of p < .05 indicated that age was not significantly correlated with neural response within the amygdala or Diekhof ROI during the 2 contrasts (both p > .11). Given that age and gender did not have a significant effect on neural activation in the amygdala or Diekhof ROI, they were not included as covariates in the following analyses.
Passive Viewing of Sad Versus Neutral Photographs
Amygdala
Separate within-group analyses indicated no significant main effect of condition or condition × time interaction effect on amygdala activation (all p >.05 after FDR correction).
Diekhof Regions
Within-group analyses indicated that healthy children showed no main effect of condition on activation in the 23 Diekhof ROIs. Healthy children showed significant condition × time interaction effects on bilateral activation in the middle frontal gyrus (Table 2). Left and right middle frontal gyrus (MFG) showed greater magnitude over time for sad compared to neutral stimuli (see Figure S2, available online). Children with a history of MDD did not exhibit any significant effects of condition or condition × time within the 23 ROIs.
TABLE 2.
Significant Within-Group Differences for View Sad vs. View Neutral Using Diekhof Regions of Interest (ROIs)
| Regions | x | Y | z | Healthy Always
|
MDD-Ever
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Condition
|
Condition × Time
|
Condition
|
Condition × Time
|
||||||||
| z | p | z | p | z | p | z | p | ||||
| R MFG | 37 | 16 | 46 | 2.76 | .08 | 2.99 | .02 | 0.00 | .97 | 0.00 | .88 |
| L MFG | −41 | 13 | 44 | 1.16 | .64 | 3.25 | .02 | 0.99 | .97 | 0.00 | .88 |
Note: p Values are false discovery rate corrected at p < .05 using all 23 Diekhof ROIs. L = left; MDD = major depressive disorder; MFG = middle frontal gyrus; r = right.
Reappraise Sad Versus Passive View Sad: Within-Group Analyses
Amygdala
There were no significant condition or condition × time interaction effects on amygdala ROI for the within-group analyses.
Diekhof Regions
As shown in Table 3, after using an FDR of p < .05, children in the healthy group showed significant main effects of condition in 17 of the 23 Diekhof regions. All of these regions demonstrated greater activation during the reappraise compared to view sad conditions (Figure S3, available online). One region in the vlPFC located on the left IFG showed a significant interaction between condition and time (Figure S4, available online).
TABLE 3.
Group Comparisons in Diekhof Regions of Interest (ROIs) During Reappraise Sad vs. View Sad Trials: False Discovery Rate (FDR) Corrected Results p <.05
| Regions | x | y | z | Healthy Always
|
MDD-Ever
|
Group Comparison
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Condition
|
Condition × Time
|
Condition
|
Condition × Time
|
Condition × Diagnosis
|
Condition × Time × Diagnosis
|
||||||||||
| z | p | z | p | z | p | z | p | z | p | Z | p | ||||
| L dorsomedial PFC/ACC | −7 | 11 | 58 | 3.85 | .0005 | 2.69 | .046 | 3.66 | .005 | 5.28 | .0000 | 0 | .53 | 0 | .73 |
| R dorsomedial PFC/ACC | 1 | 26 | 46 | 3.29 | .0023 | 1.57 | .253 | 1.94 | .144 | 2.79 | .0164 | ||||
| L middle frontal gyrus/ | −41 | 13 | 44 | 3.12 | .0023 | 1.78 | .253 | 1.57 | .265 | 1.59 | .1807 | ||||
| L middle frontal gyrus/ | −41 | 0 | 47 | 3.88 | .0005 | 2.63 | .046 | 2.32 | .066 | 4.15 | .0002 | 1.52 | .21 | 1.82 | .21 |
| R middle frontal gyrus/ | 37 | 16 | 46 | 1.28 | .2300 | 1.33 | .296 | 1.12 | .352 | .92 | .3738 | ||||
| L inferior frontal gyrus/anterior insula | −48 | 26 | −6 | 4.35 | .0001 | 2.77 | .046 | 0 | .899 | 1.86 | .8400 | 2.83 | .03 | 0.92 | .61 |
| L inferior frontal gyrus/anterior insula | −52 | 18 | 1 | 4.15 | .0002 | 3.24 | .023 | 1.66 | .207 | 1.96 | .0836 | 2.01 | .11 | 0 | .73 |
| L inferior frontal gyrus/anterior insula | −50 | 37 | −2 | 3.22 | .0023 | 1.38 | .296 | 0 | .617 | 1.02 | .3833 | ||||
| R inferior frontal gyrus | 48 | 25 | −4 | 2.46 | .0164 | 1.00 | .460 | 0 | .930 | 2.35 | .0256 | ||||
| L intraparietal cortex | −45 | −66 | 32 | 2.25 | .0271 | 1.33 | .296 | 2.92 | .017 | 3.45 | .0023 | ||||
| L intraparietal cortex | −41 | −57 | 35 | 2.28 | .0271 | 0.99 | .460 | 1.58 | .230 | 1.96 | .0958 | ||||
| L intraparietal cortex | −37 | −61 | 27 | 2.45 | .0164 | 1.65 | .253 | 3.05 | .017 | 4.22 | .0002 | 0 | .71 | 1.11 | .61 |
| R intraparietal cortex | 47 | −60 | 40 | 1.91 | .0639 | .00 | .666 | 0.00 | .899 | 0.00 | .4600 | ||||
| L inferior temporal sulcus | −57 | −37 | −3 | 4.48 | .0001 | 1.67 | .253 | 1.74 | .204 | 0 | .4600 | 1.98 | .11 | 0 | .73 |
| L anterior insula/frontal operculum | −36 | 16 | −1 | 3.25 | .0023 | .00 | .528 | 2.81 | .018 | 2.33 | .0256 | ||||
| R anterior insula/frontal operculum | 44 | 9 | 4 | 2.8 | .0105 | 1.44 | .293 | 1.15 | .352 | 4.01 | .0003 | 0 | .54 | 2.23 | .14 |
| L VMPFC | 6 | 35 | −16 | .00 | .8155 | .00 | .940 | 1.20 | .337 | 1.76 | .1238 | ||||
| R VMPFC | 1 | 33 | −12 | .00 | .8400 | .00 | .562 | 0.00 | .899 | 1.07 | .3788 | ||||
| L middle temporal gyrus | −61 | −6 | −20 | 2.46 | .0199 | .00 | .940 | 0.98 | .409 | 0 | .7527 | ||||
| R frontomarginal sulcus | 32 | 53 | 14 | 1.25 | .2415 | .00 | .789 | 1.21 | .337 | 0.99 | .3874 | ||||
| R inferior frontal gyrus | 57 | 21 | 11 | 2.61 | .0164 | 1.61 | .253 | 2.42 | .038 | 1.31 | .2913 | ||||
| L ACC | −8 | 23 | 30 | 3.45 | .0019 | 1.76 | .253 | 2.91 | .017 | 2.83 | .0153 | ||||
| R superior frontal gyrus | 16 | 18 | 59 | 1.07 | .3067 | 0 | .702 | 1.51 | .249 | 2.07 | .0690 | ||||
Note: Boldface values meet FDR p < .001 threshold for further group comparison analyses. ACC = anterior cingulate cortex; L = left; MDD = major depressive disorder; PFC = prefrontal cortex; R = right; VMPFC = ventromedial prefrontal cortex.
Using an FDR of p < .05, children in the MDD-ever group showed a significant main effect of condition in 6 of the 23 Diekhof ROIs (Table 3 and Figure S5, available online). Furthermore, 9 of the 23 Diekhof ROIs showed a significant condition × time interaction effect (see Figure S6, available online, for example time courses). All of these regions demonstrated greater activation during the reappraise compared to view sad.
Next we tested whether any of the regions showing within-group effects of condition or condition × time differed between groups. To reduce the risk of making a type 1 error, we conducted between-group analyses only on regions that were significant after FDR corrections at a very stringent threshold of p < .001. That is, to be included in the group comparison analyses, the region had to show a significant within-group main effect of condition or a condition × time interaction effect with an FDR significance threshold of p < .001. Based on this criterion, we tested for diagnosis × condition and diagnosis × condition × time interaction effects in 7 of the 23 Diekhof regions (Table 3, boldface data). Specifically, we tested for group differences in the left dACC, left MFG, left IFG (2 areas), left intra-pariatal cortex, left inferior temporal sulcus, and right anterior insula.
As shown in Table 3 and Figure S7, available online, only a single region remained significant after conducting FDR corrections at p < .05. That is, healthy children compared to MDD-ever children showed significantly greater activation in the left IFG (−48, 26, −6) regions when instructed to reappraise versus view sad photographs. It is important to note that this result remained significant (p <.01) after controlling for children’s MDD severity at the time of scan. Results indicated no significant condition × diagnosis × time interaction effects within the 7 ROIs tested.
Dimensional ROI Analyses Examining Depression Severity and ER Scores
Amygdala
Children’s MDD severity scores were significantly negatively correlated with the difference between reappraise and view sad in the right superficial amygdala (r = −0.38, p = .01, FDR corrected). That is, MDD severity was associated with greater activation in the view sad compared to reappraise sad condition. There were no significant relationships between activity in any of the amygdala regions and ER scores.
Diekhof ROIs
There were no significant relationships between activity in the 23 Diekhof ROI and MDD severity scores. After applying an FDR of p < .05 to the correlations between ER scores and the 23 ROIs, 6 regions remained significant (p = .02–.04), as follows: right MFG, left and right IFG, left inferior temporal sulcus, and left and right anterior insula. Next we conducted follow-up hierarchical regressions to test whether ER scores were associated with the 6 ROIs for the reappraise versus view sad contrast when covarying for children’s MDD severity around the time of scan. In 5 of 6 models, children’s ER scores accounted for an additional and significant portion of the variance above and beyond MDD severity in children’s neural activation during the reappraise minus view sad contrast. The left anterior insula was the only region that did not show a significant effect of ER scores on neural response for the reappraise versus view sad contrast when covarying for MDD severity.
DISCUSSION
The goal of the current study was to test for group differences in neural activation during a cognitive reappraisal task in children previously diagnosed with MDD compared to healthy controls. Four key findings were obtained. First, amygdala activation did not differ significantly within or between groups in relation to regulating negative affect during reappraisal. Second, when trained and explicitly instructed to do so, MDD-ever and healthy children showed extensive similarities in patterns of neural activation within cortical brain regions identified in a meta-analysis of healthy adults. Third, MDD-ever and healthy children exhibited significantly different patterns of neural activation within left IFG for the reappraise versus view sad conditions. Fourth, dimensional analyses indicated that higher MDD severity scores were associated with decreased right superficial amygdala activation during the reappraise minus view negative contrast. Furthermore, better ER abilities were significantly associated with increased activation in the left IFG when covarying for children’s MDD severity at time of scan.
With regard to between-group differences, children with a history of MDD compared to healthy peers showed significantly less activation in 2 regions of the left IFG while reappraising versus passively viewing sad photographs. Importantly, reappraisal-related neural activation within IFG has been widely implicated in healthy ER and related to self-reports of successful ER in healthy controls.23,41 A recent meta-analysis indicated that increased activation in the IFG during the reappraisal of negative stimuli is the norm in adult reappraisal.25 In healthy samples, the IFG is thought to support, and has been implicated in, selecting goal-appropriate responses and inhibiting goal-inappropriate responses.50 The IFG has also been implicated in the deliberate selection of new stimulus-appropriate reappraisals.51 Our findings suggest that the IFG may be a key region in which neural activation patterns differentiate healthy children from those with a history of MDD. Dimensional analyses provided further support for the importance of left IFG activation during reappraisal. That is, increased left IFG activation during the reappraise minus view contrast was significantly associated with parent reports of their children’s more successful use of ER. Taken together, results suggest that patterns of significantly decreased neural activation in the left IFG may function as a trait marker of prior MDD episodes as well as a marker and possible mechanism of poor CER.
Although amygdala activation during reappraise sad versus view sad did not differ within or between groups, correlational analyses revealed that higher MDD severity was associated with greater activation in the right superficial amygdala subdivision during the view sad condition. The significant correlation between children’s MDD severity and increased superficial amygdala activation to sad stimuli suggests that group differences may be more apparent in children with more acute depression. In fact, the only study examining reappraisal in healthy children and those with acute depression found that healthy children were better able to modulate amygdala activation compared to peers with depression.3 However, consistent with our results, the authors clarified that the group with depression showing greater neural activation to the view negative condition drove their finding, rather than the result of reduced amygdala activation associated with reappraisal in healthy controls. As such, our finding is consistent with the notion that children with depression as a group are more emotionally reactive to negative stimuli, although it is also possible that such reactivity may be further accentuated by not actively engaging in an effective CER strategy. It is also possible that the use of sad rather than fear stimuli influenced the lack of between- or within-group effects on amygdala response. The sad stimuli used (e.g., funeral scenes, hospital scenes, and suffering children) likely did not induce the type of emotional activation similar to fear or disgust elicited by stimuli used in a number of reappraisal studies in which amygdala findings have been generated.29,36
We found evidence for diagnostic group differences during reappraisal in several brain regions previously identified in adults. Equally important was the finding of a very similar pattern of activation in a number of regions in children with a history of MDD and healthy controls. The finding that children with an MDD history showed reappraisal-related neural responses that were in many ways more similar than different from those of healthy controls has important clinical implications. These findings suggest that early identification of alterations in reappraisal processes in children with remitted depression, for whom much of the neural circuitry remains intact, may be an important window of opportunity for intervention. Study findings suggest that interventions should focus on methods of enhancing reappraisal in children with depression. Furthermore, if study findings are replicated, they would suggest that functions of the IFG such as inhibiting goal-inappropriate responses and enhancing goal-appropriate responses with regard to mood regulation could also be a direct target in treatment development.
Limitations of the findings presented include that we collected data from both boys and girls but were under-powered to test for gender effects. Given the differing patterns of brain maturation and emotion development in boys and girls, future studies that examine gender effects on reappraisal-related brain activity are needed to inform the increased rates of MDD in adolescent girls. Second, the current study focused on the down-regulation of a single emotion (sadness) when using a single CER strategy (reappraisal). An additional limitation was that the current sample included both right- and left-handed participants, which could have affected power in the between-group analyses. It is also a concern that a few participants were taking psychotropic drugs at the time of scan. However, when the 2 participants taking antidepressants were removed from analyses, the results were essentially identical. Future studies that include additional emotions, compare different CER strategies, and include conditions of up-regulation (i.e., increasing negative emotions) may elucidate key differences in the neural processes related to CER in healthy children and those with depression. Furthermore, MDD has been theorized to be a result of maladaptive functional interactions among a highly integrated network of limbic×cortical regions responsible for maintaining emotional homeostasis in response to negative affect or events. Thus, future research aimed at elucidating the connectivity between emotion-regulating versus emotion-regulated brain regions in the context of reappraisal and how these connections may differ in individuals with depression versus healthy controls during childhood is imperative for advancing knowledge about mechanisms of impairment in childhood depression.
This study suggests that children previously diagnosed with MDD can reappraise negative emotion and show patterns of neural activation in several brain regions that are highly similar to healthy same-age peers. However, reappraisal-related group differences were found in the left IFG, with MDD-ever children showing diminished neural responses in this region during reappraisal compared to healthy controls. Furthermore, poorer ER scores were associated with decreased activation in the left IFG during reappraisal. In sum, diminished reappraisal-related neural response, especially in the left IFG, may be a trait-like biomarker of remitted childhood MDD as well as neuromarker of poorer ability to effectively regulate negative emotions. Future studies that inform the function of the IFG and its connections to other emotion regions may be critical to the design of novel intervention strategies in childhood depression.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (5K01MH090515 to A.C.B., 2R01MH064769 to J.L.L., and R01MH090786 to Co-Principal Investigators J.L.L. and D.M.B); and the McDonnell Center for Systems Neuroscience (N.R.G. to A.C.B). Dr. Murphy’s work on this project was supported by grant 1T32MH100019-01.
Footnotes
Disclosure: Dr. Luby has received research support from the National Institute of Mental Health and has received royalties from Guilford Press. Dr. Barch has received research support from the National Institute of Mental Health and Brain and Behavior Research Foundation (formerly NARSAD). She has served as a consultant to Pfizer, Amgen, Roche, and Takeda. Drs. Belden, Pagliaccio, and Murphy report no biomedical financial interests or potential conflicts of interest.
Contributor Information
Dr. Andy C. Belden, Washington University in St. Louis.
Dr. David Pagliaccio, Washington University in St. Louis. Program in Neuroscience, Washington University in St. Louis.
Dr. Eric R. Murphy, Washington University in St. Louis.
Dr. Joan L. Luby, Washington University in St. Louis.
Dr. Deanna M. Barch, Washington University in St. Louis. Program in Neuroscience, Washington University in St. Louis.
References
- 1.Drevets WC. Neuroimaging and neuropathological studies of depression: implications for the cognitive-emotional features of mood disorders. Curr Opin Neurobiol. 2001;11:240–249. doi: 10.1016/s0959-4388(00)00203-8. [DOI] [PubMed] [Google Scholar]
- 2.Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J Neurosci. 2007;27:8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ochsner KN, Silvers JA, Buhle JT. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann N Y Acad Sci. 2012;1251:E1–E24. doi: 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buhle JT, Silvers JA, Wager TD, et al. Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cereb Cortex. 2014;24:2981–2990. doi: 10.1093/cercor/bht154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Disner S, Beevers C, Haigh E, Beck A. Neural mechanisms of the cognitive model of depression. Nature Rev Neurosci. 2011;12:467–477. doi: 10.1038/nrn3027. [DOI] [PubMed] [Google Scholar]
- 6.Beck A. The evolution of the cognitive model of depression and its neurobiological correlates. Am J Psychiatry. 2008;165:969–977. doi: 10.1176/appi.ajp.2008.08050721. [DOI] [PubMed] [Google Scholar]
- 7.Gross JJ, Munoz RF. Emotion regulation and mental health. Clin Psychol. 1995;2:151–164. [Google Scholar]
- 8.Green MJ, Malhi GS. Neural mechanisms of the cognitive control of emotion. Acta Neuropsychiatrica. 2006;18:144–153. doi: 10.1111/j.1601-5215.2006.00149.x. [DOI] [PubMed] [Google Scholar]
- 9.Feng X, Keenan K, Hipwell AE, et al. Longitudinal associations between emotion regulation and depression in preadolescent girls: moderation by the caregiving environment. Dev Psychol. 2009;45:798–808. doi: 10.1037/a0014617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garnefski N, Doets T. Perceived social support and dysfunctioning in ‘clinical’ and ‘normal’ adolescents. J Adol. 2000;23:753–762. doi: 10.1006/jado.2000.0357. [DOI] [PubMed] [Google Scholar]
- 11.Garnefski N, Legerstee J, Kraaij VV, Van Den Kommer T, Teerds J. Cognitive coping strategies and symptoms of depression and anxiety: a comparison between adolescents and adults. J Adolesc. 2002;25:603–611. doi: 10.1006/jado.2002.0507. [DOI] [PubMed] [Google Scholar]
- 12.Garnefski N, Boon S, Kraaij V. Relationships between cognitive strategies of adolescents and depressive symptomatology across different types of life event. J Youth Adolesc. 2003;32:401–408. [Google Scholar]
- 13.Garnefski N, Kraaij V, van Etten M. Specificity of relations between adolescents’ cognitive emotion regulation strategies and internalizing and externalizing psychopathology. J Adolesc. 2005;28:619–631. doi: 10.1016/j.adolescence.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 14.Garnefski N, Kraaij V. Relationships between cognitive emotion regulation strategies and depressive symptoms: a comparative study of five specific samples. Personal Indiv Diff. 2006;40:1659–1669. [Google Scholar]
- 15.Garnefski N, Rieffe C, Jellesma F, Meerum Terwogt M, Kraaij V. Cognitive emotion regulation strategies and emotional problems in 9–11-year-old children: the development of an instrument. Eur Child Adolesc Psychiatry. 2007;16:1–9. doi: 10.1007/s00787-006-0562-3. [DOI] [PubMed] [Google Scholar]
- 16.Kraaij V, Pruymboom E, Garnefski N. Cognitive coping and depressive symptoms in the elderly: a longitudinal study. Aging Mental Health. 2002;6:275–281. doi: 10.1080/13607860220142387. [DOI] [PubMed] [Google Scholar]
- 17.Kraaij V, Garnefski N, de Wilde EJ, et al. Negative life events and depressive symptoms in late adolescence: bonding and cognitive coping as vulnerability factors? J Youth Adolesc. 2003;32:185–193. [Google Scholar]
- 18.Silvers JA, McRae K, Gabrieli JDE, Gross JJ, Remy KA, Ochsner KN. Age-related differences in emotional reactivity, regulation, and rejection sensitivity in adolescence. Emotion. 2012;12:1235–1247. doi: 10.1037/a0028297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Belden AC, Luby JL, Pagliaccio D, Barch DM. Neural activation associated with the cognitive emotion regulation of sadness in healthy children. Dev Cogn Neurosci. 2014;9:136–147. doi: 10.1016/j.dcn.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Otto B, Misra S, Prasad A, McRae K. Functional overlap of top-down emotion regulation and generation: an fMRI study identifying common neural substrates between cognitive reappraisal and cognitively generated emotions. Cogn Affect Behav Neurosci. 2014;14:923–938. doi: 10.3758/s13415-013-0240-0. [DOI] [PubMed] [Google Scholar]
- 21.Kanske P, Heissler J, Schönfelder S, Bongers A, Wessa M. How to regulate emotion? Neural networks for reappraisal and distraction. Cereb Cortex. 2011;21:1379–1388. doi: 10.1093/cercor/bhq216. [DOI] [PubMed] [Google Scholar]
- 22.Dörfel D, Lamke JP, Hummel F, Wagner U, Erk S, Walter H. Common and differential neural networks of emotion regulation by detachment, reinterpretation, distraction, and expressive suppression: a comparative fMRI investigation. Neuroimage. 2014;101:298–309. doi: 10.1016/j.neuroimage.2014.06.051. [DOI] [PubMed] [Google Scholar]
- 23.Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 24.Ochsner KN, Gross JJ. Cognitive emotion regulation: insights from social cognitive and affective neuroscience. Curr Direct Psychol Sci. 2008;17:153–158. doi: 10.1111/j.1467-8721.2008.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buhle JT, Silvers JA, Wager TD, et al. Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cereb Cortex. 2014;24:2981–2990. doi: 10.1093/cercor/bht154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lévesque J, Joanette Y, Mensour B, et al. Neural basis of emotional self-regulation in childhood. Neuroscience. 2004;129:361–369. doi: 10.1016/j.neuroscience.2004.07.032. [DOI] [PubMed] [Google Scholar]
- 27.McRae K, Gross JJ, Weber J, et al. The development of emotion regulation: an fMRI study of cognitive reappraisal in children, adolescents and young adults. Soc Cogn Affect Neurosci. 2012;7:11–22. doi: 10.1093/scan/nsr093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perlman G, Simmons A, Wu J, et al. Amygdala response and functional connectivity during emotion regulation: a study of 14 depressed adolescents. J Affect Disord. 2012;139:75–84. doi: 10.1016/j.jad.2012.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pitskel NB, Bolling DZ, Kaiser MD, Crowley MJ, Pelphrey KA. How grossed out are you? The neural bases of emotion regulation from childhood to adolescence. Dev Cogn Neurosci. 2011;1:324–337. doi: 10.1016/j.dcn.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Erk S, Mikschl A, Stier S, et al. Acute and sustained effects of cognitive emotion regulation in major depression. J Neurosci. 2010;30:15726–15734. doi: 10.1523/JNEUROSCI.1856-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beauregard M, Levesque J, Bourgouin P. Neural correlates of conscious self-regulation of emotion. J Neurosci. 2001;21:RC165. doi: 10.1523/JNEUROSCI.21-18-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kanske P, Heissler J, Schonfelder S, Wessa M. Neural correlates of emotion regulation deficits in remitted depression: the influence of regulation strategy, habitual regulation use, and emotional valence. Neuroimage. 2012;61:686–693. doi: 10.1016/j.neuroimage.2012.03.089. [DOI] [PubMed] [Google Scholar]
- 33.Beauregard M, Paquette V, Lévesque J. Dysfunction in the neural circuitry of emotional self-regulation in major depressive disorder. Neuroreport. 2006;17:843–846. doi: 10.1097/01.wnr.0000220132.32091.9f. [DOI] [PubMed] [Google Scholar]
- 34.Greening SG, Lee TH, Mather M. A dual process for the cognitive control of emotional significance: implications for emotion regulation and disorders of emotion. Front Hum Neurosci. 2014;8:253. doi: 10.3389/fnhum.2014.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sheline YI, Barch DM, Price JL, et al. The default mode network and self-referential processes in depression. Proc Natl Acad Sci. 2009;106:1942–1947. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diekhof E, Geier K, Falkai P, Gruber O. Fear is only as deep as the mind allows: a coordinate-based meta-analysis of neuroimaging studies on the regulation of negative affect. Neuroimage. 2011;58:275–285. doi: 10.1016/j.neuroimage.2011.05.073. [DOI] [PubMed] [Google Scholar]
- 37.Barch DM, Gaffrey MS, Botteron KN, Belden AC, Luby JL. Functional brain activation to emotionally valenced faces in school-aged children with a history of preschool-onset major depression. Biol Psychiatry. 2012;72:1035–1042. doi: 10.1016/j.biopsych.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luby JL, Barch DM, Belden A, et al. Maternal support in early childhood predicts larger hippocampal volumes at school age. Proc Natl Acad Sci USA. 2012;109:2854–2859. doi: 10.1073/pnas.1118003109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luby JL, Gaffrey MS, Tillman R, April LM, Belden AC. Trajectories of preschool disorders to full DSM depression at school age and early adolescence: continuity of preschool depression. Am J Psychiatry. 2014;171:768–776. doi: 10.1176/appi.ajp.2014.13091198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ochsner KN, Ray RD, Cooper JC, et al. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 42.Perlman S, Pelphrey K. Developing connections for affective regulation: age-related changes in emotional brain connectivity. J Exp Child Psychol. 2011;108:607–620. doi: 10.1016/j.jecp.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): Affective Ratings of Pictures and Instruction Manual. Bethesda, MD: NIMH Center for the Study of Emotion & Attention; 2005. [Google Scholar]
- 44.Lang P, Bradley MM. The International Affective Picture System (IAPS) in the study of emotion and attention. In: Coan JA, Allen JJB, editors. Handbook of Emotion Elicitation and Assessment. New York: Oxford University Press; 2007. pp. 29–46. [Google Scholar]
- 45.Kang HC, Burgund ED, Lugar HM, Petersen SE, Schlaggar BL. Comparison of functional activation foci in children and adults using a common stereotactic space. Neuroimage. 2003;19:16–28. doi: 10.1016/s1053-8119(03)00038-7. [DOI] [PubMed] [Google Scholar]
- 46.Burgund ED, Kang HC, Kelly JE, et al. The feasibility of a common stereotactic space for children and adults in fMRI studies of development. Neuroimage. 2002;17:184–200. doi: 10.1006/nimg.2002.1174. [DOI] [PubMed] [Google Scholar]
- 47.Siegel JS, Power JD, Dubis JW, et al. Statistical improvements in functional magnetic resonance imaging analyses produced by censoring high-motion data points. Hum Brain Mapp. 2014;35:1981–1996. doi: 10.1002/hbm.22307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pagliaccio D, Luby J, Gaffrey M, et al. Functional brain activation to emotional and nonemotional faces in healthy children: evidence for developmentally undifferentiated amygdala function during the school-age period. Cogn Affect Behav Neurosci. 2013;13:771–789. doi: 10.3758/s13415-013-0167-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roy AK, Shehzad Z, Margulies DS, et al. Functional connectivity of the human amygdala using resting state fMRI. Neuroimage. 2009;45:614–626. doi: 10.1016/j.neuroimage.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thompson-Schill SL, Bedny M, Goldberg RF. The frontal lobes and the regulation of mental activity. Curr Opin Neurobiol. 2005;15:219–224. doi: 10.1016/j.conb.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 51.Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45:2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.