Abstract
Receptor tyrosine kinase-like orphan receptor 1 (ROR1) is an onco-embryonic antigen. Due to its expression on the cell surface of leukemia cells from patients with chronic lymphocytic leukemia (CLL), but not on normal B-cells or other post-partum tissues, ROR1 is an attractive candidate for targeted therapies. UC-961 is a first-in-class humanized monoclonal antibody (mAb) that binds the extracellular domain of ROR1. In this paper we outline some of the preclinical studies leading to an investigation new drug (IND) designation, enabling clinical studies in patients with CLL.
ROR1 is an onco-embryonic antigen not expressed by normal post-partum tissues
Receptor tyrosine kinase-like orphan receptor 1 (ROR1) is a type I surface protein expressed during embryogenesis, where it contributes polarized migration and organogenesis.1–3 It contains a transmembrane domain, a cytoplasmic tyrosine kinase-like domain, and extracellular ligand binding domains, including a cysteine-rich domain homologous to Frizzled receptors for various Wnt factors.2 The expression of ROR1 is developmentally regulated. Expression of ROR1 attenuates during fetal development becoming negligible at term. Normal post-partum tissues lack surface expression of the ROR1 protein, with the exception of hematogones.4
Gene expression studies identified distinctive expression of ROR1 in CLL cells, in contrast to normal B lymphocytes.5, 6 Analysis of autoantibodies produced by patients immunized with autologous leukemia cells transduced to express CD154 identified that these autoantibodies recognized ROR1 protein on the leukemia cell surface.7 Functional studies found that ROR1 could serve as a receptor for Wnt5a, which induces non-canonical Wnt signaling leading to enhanced leukemia-cell survival; also anti-ROR1 antibodies produced by some patents could neutralize the pro-survival effects of Wnt5a on leukemia cells in vitro.7 Downstream signaling from ROR1 apparently activates the PI3K / AKT / mTOR pathway,8, 9 and studies in other cancer cell lines suggest that ROR1 may be a pseudokinase that serves as a substrate for other signaling molecules, such as MET, also known as hepatocyte growth factor receptor (HGFR).10, 11
ROR1 targeted therapies and derivation of UC-961
Due to its tumor specific expression and potential functional significance, ROR1 has been of interest as a target for novel immunotherapies. An early report of anti-ROR1 mAbs screened by phage display for optimal binding found that these mAbs typically bound the N-terminal region of the extracellular immunoglobulin-like domain of ROR1, but had limited direct cytotoxicity for human CLL cells.12 One of these mAbs (designated 2A2) is currently under exploration as a part of antibody drug conjugates or chimeric antigen receptor (CAR) expressing T-cells.13–16
However, other groups have produced naked mAbs capable of directly inducing apoptosis of CLL cells.17 We screened hybridomas for production of mAbs mimicking the activity of anti-ROR1 autoantibodies that we observed in some patients vaccinated against autologous leukemia cells.7 We also examined the activity of various mAbs in vivo using a ROR1 transgenic mouse model of CLL.8 We identified one mAb, D10, of relatively low affinity that could inhibit activation of AKT and engraftment of ROR1+ leukemia cells in this model. Mapping the epitope bound by this mAb allowed us to generate mAbs of substantially higher affinity for ROR1 that retained this distinctive biologic activity. We humanized the variable regions of one such mAb (designated UC-961, or cirmtuzumab), which maintained high binding affinity (Kd = 2 nM) for the functional epitope of ROR1.
Pre-clinical specific and safety studies of UC-961
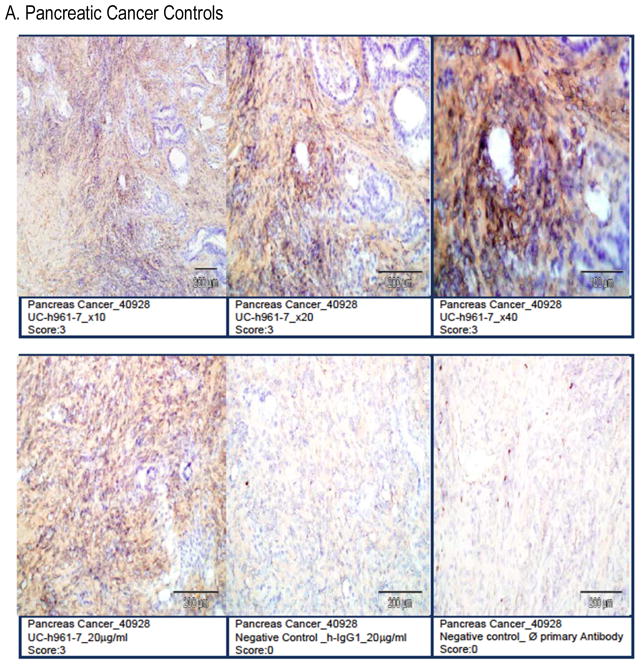
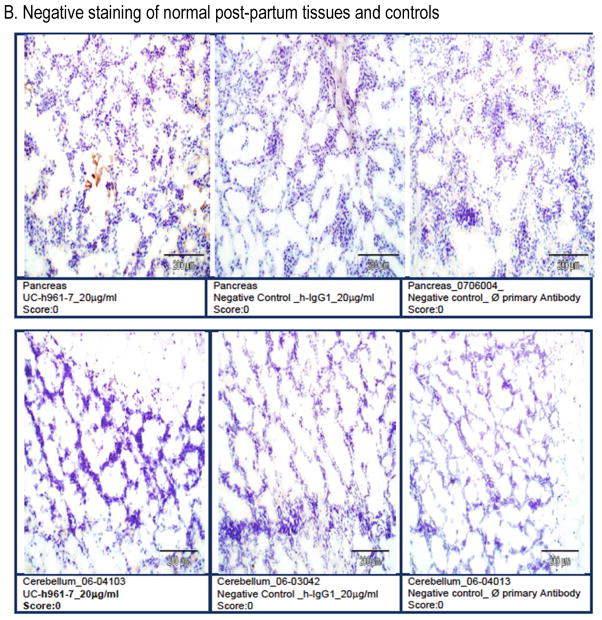
Studies with other anti-ROR1 mAbs have found potential expression of ROR1 on adipose tissue and pancreatic islet cells.15 Therefore, we performed a GLP-compliant human tissue cross-reactivity study with UC-961 prior to proceeding with clinical testing of this mAb. Samples from all human tissues from three separate donors were probed with UC-961 at the concentrations up to 5x the optimal staining concentration for ROR1+ cancer tissue.18 We did not observe any cross-reactivity with normal post-partum tissues, including the pancreas or adipose tissue (Figure 1).
Figure 1. UC-961 does not cross-react with normal adult human tissues.
UC-961 was applied to full tissue lists of human tissues from three separate donors, supplemented with a positive control specimen (pancreatic carcinoma). This was conducted in compliance with GLP regulations. A) The UC-961 staining resulted in and strong (Grade-3) positive-staining intensity on ROR1+ pancreatic tumor cells. B) There was no tissue cross-reactivity at the high concentration on any of the normal human tissues. Representative photomicrographs are shown.
We conducted rodent and primate studies to assess for off-target or non-ROR1 specific activity. Groups of Sprague-Dawley rats (15 of each gender) received UC-961 at doses of 40 to 400 mg/kg by IV administration weekly for 5 doses over 28 days. Clinical signs, body weight, clinical pathology, and safety pharmacology measurements were assessed during the study. Twenty animals (10 of each gender) in each dosing cohort were sacrificed three days after the final UC-961 injection, and the remaining animals were sacrificed on day 56. In all groups, UC-961 was well tolerated and no adverse effects were noted. At terminal sacrifice, gross pathologic exams were normal and no untoward pathology was observed.
We also performed studies in cynomolgus monkeys. UC-961 was administered once by IV injection at a dosage of 40 mg/kg. There were no changes in body weight, clinical chemistry values, or hematologic parameters, including absolute numbers of T-cells or B-cells. Pharmacokinetic evaluation of plasma samples indicated the elimination half-life of UC-961 was over 14 days.
Discussion
MAbs are effective agents in the therapy of patients with CLL or other cancers. Treatment of patients with mAbs has provided prolonged survival alone or in combination with chemotherapy.19, 20 To date, mAbs currently used in the treatment of patients with CLL are mostly directed against CD20, an antigen also found on normal B cells. As such, treatment with such mAbs can cause depletion of normal B cells and potentially enhance post-treatment hypogammaglobulinemia. Agents directed against CD19, CD37, CD52, or other antigens also are being evaluated.21 However, none of these antigens are unique to CLL cells, potentially limiting their therapeutic index by targeting non-cancer cells, resulting in immune suppression or other risks. Targeting ROR1 may be advantageous as it is a tumor-specific antigen that has functional importance to CLL cells.
UC-961 targets a distinctive epitope of ROR1 and has biologic activity against ROR1+ tumor cells. Preclinical studies did not detect toxicity. This may be due to the fact that UC-961 does not have detectable binding activity for normal post-partum tissues. However, human trials will be necessary to determine the safety and activity of this mAb in the therapy in patients with CLL. To this end, a phase 1 trial is currently enrolling (NCT0222688). This also will provide another opportunity to evaluate the functional impact of targeting ROR1 on CLL in vivo.
The implications of this work extend beyond CLL. ROR1 is broadly expressed in many types of cancer and not their normal tissue counterparts. (Cite reference 18 here, not 21) Although the prevalence of ROR1 expression in solid tumors does not appear to be as high as it is in CLL, its expression is found on cancers that are poorly differentiated and that have high relapse rates and high metastatic potential.9, 22, 23 More recent studies have identified ROR1 on the cancer-stem-cells in ovarian cancer.24 Moreover, treatment of immune-deficient mice engrafted with ovarian-cancer patient-derived xenografts (PDX) with UC-961 could induce senescence in the cancer-stem-cells, thereby inhibiting the growth of the ovarian-cancer PDX and its capacity to re-engraft other immune-deficient mice.24 As such, UC-961 may have broader applications in the treatment of patients with solid-tumor malignancies in addition to the treatment of patients with CLL.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Green J, Nusse R, van Amerongen R. The role of Ryk and Ror receptor tyrosine kinases in Wnt signal transduction. Cold Spring Harb Perspect Biol. 2014;6(2) doi: 10.1101/cshperspect.a009175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Masiakowski P, Carroll RD. A novel family of cell surface receptors with tyrosine kinase-like domain. J Biol Chem. 1992;267(36):26181–26190. [PubMed] [Google Scholar]
- 3.Yoda A, Oishi I, Minami Y. Expression and function of the Ror-family receptor tyrosine kinases during development: lessons from genetic analyses of nematodes, mice, and humans. J Recept Signal Transduct Res. 2003;23(1):1–15. doi: 10.1081/rrs-120018757. [DOI] [PubMed] [Google Scholar]
- 4.Broome HE, Rassenti LZ, Wang HY, Meyer LM, Kipps TJ. ROR1 is expressed on hematogones (non-neoplastic human B-lymphocyte precursors) and a minority of precursor-B acute lymphoblastic leukemia. Leuk Res. 2011;35(10):1390–1394. doi: 10.1016/j.leukres.2011.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klein U, Tu Y, Stolovitzky GA, Mattioli M, Cattoretti G, Husson H, et al. Gene expression profiling of B cell chronic lymphocytic leukemia reveals a homogeneous phenotype related to memory B cells. J Exp Med. 2001;194(11):1625–1638. doi: 10.1084/jem.194.11.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenwald A, Alizadeh AA, Widhopf G, Simon R, Davis RE, Yu X, et al. Relation of gene expression phenotype to immunoglobulin mutation genotype in B cell chronic lymphocytic leukemia. J Exp Med. 2001;194(11):1639–1647. doi: 10.1084/jem.194.11.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fukuda T, Chen L, Endo T, Tang L, Lu D, Castro JE, et al. Antisera induced by infusions of autologous Ad-CD154-leukemia B cells identify ROR1 as an oncofetal antigen and receptor for Wnt5a. Proc Natl Acad Sci U S A. 2008;105(8):3047–3052. doi: 10.1073/pnas.0712148105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Widhopf GF, II, Cui B, Ghia EM, Chen L, Messer K, Shen Z, et al. ROR1 can interact with TCL1 and enhance leukemogenesis in Emu-TCL1 transgenic mice. Proc Natl Acad Sci U S A. 2014;111(2):793–798. doi: 10.1073/pnas.1308374111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang S, Chen L, Cui B, Chuang HY, Yu J, Wang-Rodriguez J, et al. ROR1 is expressed in human breast cancer and associated with enhanced tumor-cell growth. PLoS One. 2012;7(3):e31127. doi: 10.1371/journal.pone.0031127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gentile A, Lazzari L, Benvenuti S, Trusolino L, Comoglio PM. Ror1 is a pseudokinase that is crucial for Met-driven tumorigenesis. Cancer Res. 2011;71(8):3132–3141. doi: 10.1158/0008-5472.CAN-10-2662. [DOI] [PubMed] [Google Scholar]
- 11.Gentile A, Lazzari L, Benvenuti S, Trusolino L, Comoglio PM. The ROR1 pseudokinase diversifies signaling outputs in MET-addicted cancer cells. Int J Cancer. 2014;135(10):2305–2316. doi: 10.1002/ijc.28879. [DOI] [PubMed] [Google Scholar]
- 12.Yang J, Baskar S, Kwong KY, Kennedy MG, Wiestner A, Rader C. Therapeutic potential and challenges of targeting receptor tyrosine kinase ROR1 with monoclonal antibodies in B-cell malignancies. PLoS One. 2011;6(6):e21018. doi: 10.1371/journal.pone.0021018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baskar S, Wiestner A, Wilson WH, Pastan I, Rader C. Targeting malignant B cells with an immunotoxin against ROR1. MAbs. 2012;4(3):349–361. doi: 10.4161/mabs.19870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berger C, Sommermeyer D, Hudecek M, Berger M, Balakrishnan A, Paszkiewicz PJ, et al. Safety of targeting ROR1 in primates with chimeric antigen receptor-modified T cells. Cancer Immunol Res. 2014 doi: 10.1158/2326-6066.CIR-14-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hudecek M, Schmitt TM, Baskar S, Lupo-Stanghellini MT, Nishida T, Yamamoto TN, et al. The B-cell tumor-associated antigen ROR1 can be targeted with T cells modified to express a ROR1-specific chimeric antigen receptor. Blood. 2010;116(22):4532–4541. doi: 10.1182/blood-2010-05-283309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mani R, Mao Y, Frissora FW, Chiang CL, Wang J, Zhao Y, et al. Tumor antigen ROR1 targeted drug delivery mediated selective leukemic but not normal B-cell cytotoxicity in chronic lymphocytic leukemia. Leukemia. 2014 doi: 10.1038/leu.2014.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daneshmanesh AH, Hojjat-Farsangi M, Khan AS, Jeddi-Tehrani M, Akhondi MM, Bayat AA, et al. Monoclonal antibodies against ROR1 induce apoptosis of chronic lymphocytic leukemia (CLL) cells. Leukemia. 2012;26(6):1348–1355. doi: 10.1038/leu.2011.362. [DOI] [PubMed] [Google Scholar]
- 18.Zhang S, Chen L, Wang-Rodriguez J, Zhang L, Cui B, Frankel W, et al. The onco-embryonic antigen ROR1 is expressed by a variety of human cancers. Am J Pathol. 2012;181(6):1903–1910. doi: 10.1016/j.ajpath.2012.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goede V, Fischer K, Busch R, Engelke A, Eichhorst B, Wendtner CM, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med. 2014;370(12):1101–1110. doi: 10.1056/NEJMoa1313984. [DOI] [PubMed] [Google Scholar]
- 20.Hallek M, Fischer K, Fingerle-Rowson G, Fink AM, Busch R, Mayer J, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376(9747):1164–1174. doi: 10.1016/S0140-6736(10)61381-5. [DOI] [PubMed] [Google Scholar]
- 21.Robak T. Current and emerging monoclonal antibody treatments for chronic lymphocytic leukemia: state of the art. Expert Rev Hematol. 2014;7(6):841–857. doi: 10.1586/17474086.2014.963048. [DOI] [PubMed] [Google Scholar]
- 22.Cui B, Zhang S, Chen L, Yu J, Widhopf GF, II, Fecteau JF, et al. Targeting ROR1 inhibits epithelial-mesenchymal transition and metastasis. Cancer Res. 2013;73(12):3649–3660. doi: 10.1158/0008-5472.CAN-12-3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang H, Qiu J, Ye C, Yang D, Gao L, Su Y, et al. ROR1 expression correlated with poor clinical outcome in human ovarian cancer. Sci Rep. 2014;4:5811. doi: 10.1038/srep05811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang S, Cui B, Lai H, Liu G, Ghia EM, Widhopf GF, II, et al. Ovarian cancer stem cells express ROR1, which can be targeted for anti-cancer-stem-cell therapy. Proc Natl Acad Sci U S A. 2014 doi: 10.1073/pnas.1419599111. [DOI] [PMC free article] [PubMed] [Google Scholar]