SECTION 1
A 65-year-old woman diagnosed with a right parietal-occipital glioblastoma after presenting with hemianopia was treated with 2 surgical resections including carmustine wafer placement, radiotherapy with concurrent chemotherapy with temozolomide, and adjuvant chemotherapy with temozolomide and bevacizumab, an anti-angiogenic agent targeting vascular endothelial growth factor (VEGF).
Seven months after the diagnosis, while receiving temozolomide and bevacizumab, the patient developed headaches, subtle gait ataxia, memory difficulties, lethargy, and back pain, progressive over 3 weeks. She eventually developed a generalized tonic-clonic seizure and was brought to an urgent care facility.
Questions for consideration:
What is the differential diagnosis?
Which examinations should be performed?
SECTION 2
In a patient with glioblastoma presenting with worsening neurologic symptoms, tumor progression with increased tumor size and edema, eventually resulting in a seizure, seems the most obvious diagnosis. However, the differential also includes other neurologic complications (table), some of which are not necessarily indicative of treatment failure, and that require specific management1:
Ischemic stroke can occur at increased incidence in patients with brain tumor as a result of the cancer-related prothrombotic state, in addition to radiotherapy-related vasculopathy in arteries included within the radiation port, and use of bevacizumab. As deep venous thrombosis and pulmonary embolism are common in these patients, paradoxical embolism may also occur.
Intratumoral bleeding may occur spontaneously, especially in glioblastomas and oligodendrogliomas, and in the setting of bevacizumab, anticoagulation, or thrombocytopenia.
Seizures may develop even in stable tumors, and can be difficult to recognize. This patient's slow deterioration could result from repeated nonconvulsive and subclinical seizures, which can mimic slow progression of disease, only identifiable through continuous EEG monitoring. Anticonvulsant intoxication is another possibility.
Hydrocephalus may result from direct mechanical blockade of CSF flow (noncommunicating hydrocephalus) or reduced CSF absorption due to radiotherapy or leptomeningeal spread (communicating hydrocephalus). Intracranial pressure is usually elevated, especially in patients with rapidly progressive symptoms, but in others normal-pressure hydrocephalus (NPH) may develop slowly. Symptoms include progressive gait difficulties, psychomotor slowness, and cognitive impairment; headaches, nausea, and vomiting may be absent. Carefully monitoring ventricle size throughout the disease course is important for the diagnosis.
Leptomeningeal tumor spread or metastasis: Tumor cells may gain access and thrive in the subarachnoid space, causing progressive neurologic deterioration even when the original tumor appears stable on the MRI. Symptoms may include worsening headache, back pain, ataxia, cognitive dysfunction, radiculopathy, cranial nerve palsies, and hydrocephalus. The diagnosis is confirmed by CSF cytology or typical MRI findings, including enhancement in cranial nerves, roots, and cerebellar folia, as well as cisterns and spinal cord nodules and deposits, sometimes with a sugar coating appearance.
Tumor pseudoprogression: Following radiotherapy, and usually within 2–3 months, some tumors may display radiographic worsening resulting from inflammation and necrosis in response to treatment, mimicking tumor progression. Recognizing pseudoprogression is important because these patients are not experiencing treatment failure and a change in treatment is not indicated.
Corticosteroid withdrawal: In some patients, it is difficult to taper off corticosteroids, as worsening of brain edema may develop in the setting of recent decreases in dose. Such patients promptly improve with re-institution of corticosteroids, and benefit from a slower taper.
Herpetic meningoencephalitis: Cranial radiotherapy and chemotherapy increase this infection risk. Signs of encephalitis may go unrecognized in the MRI, confounded by the presence of tumor-related abnormalities; the diagnosis requires a high degree of suspicion.
Metabolic causes such as systemic infection (especially during chemotherapy), hyperglycemia (secondary to corticosteroids), liver failure (chemotherapy-related hepatotoxicity or reactivation of viral hepatitis), and hypopituitarism (from radiotherapy) may all cause neurologic worsening in patients with a brain tumor.
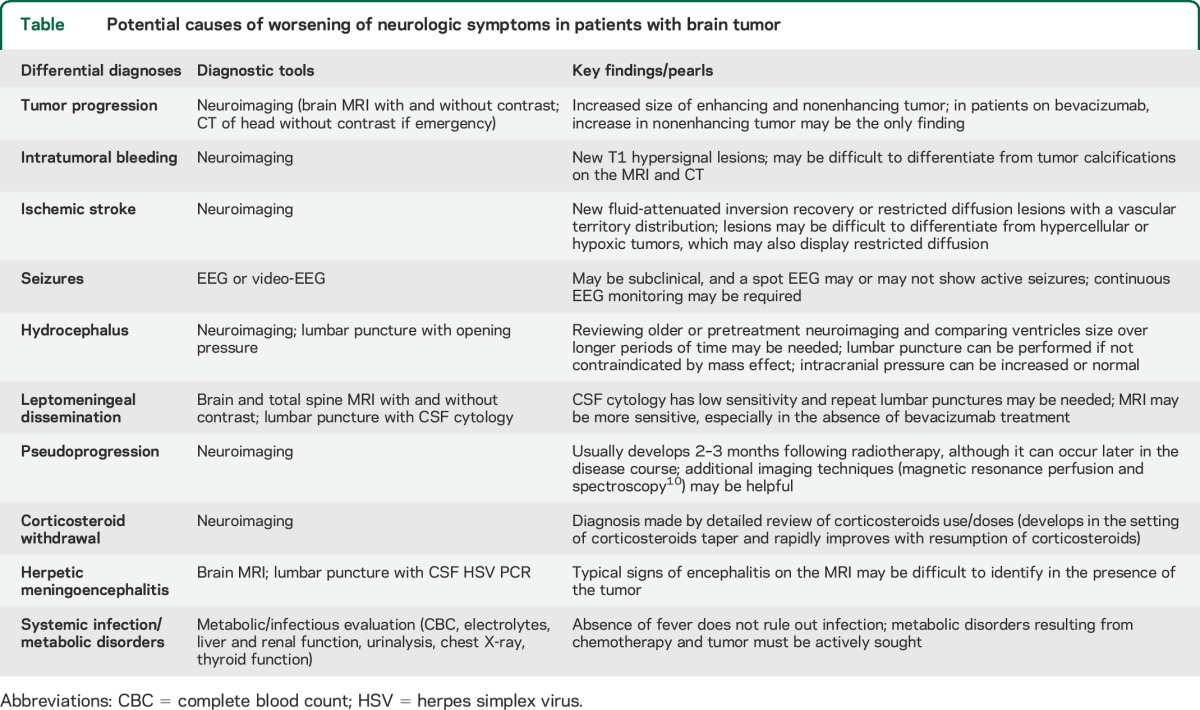
Table.
Potential causes of worsening of neurologic symptoms in patients with brain tumor
Our patient's MRI showed some ventricular dilation and faint contrast enhancement around the right lateral ventricle occipital horn (figure, A and D), without obvious tumor progression, bleeding, or stroke. A total spine MRI with gadolinium, performed to investigate leptomeningeal disease, was unremarkable. Platelets were normal and there was no mass effect contraindicating a lumbar puncture (LP), which was performed. CSF analysis showed protein of 125 mg/dL, negative cytology and herpes simplex PCR, and normal opening pressure. Video-EEG and routine urine and blood tests were unremarkable. The patient had been off corticosteroids for several months.
Figure. MRI and autopsy findings.
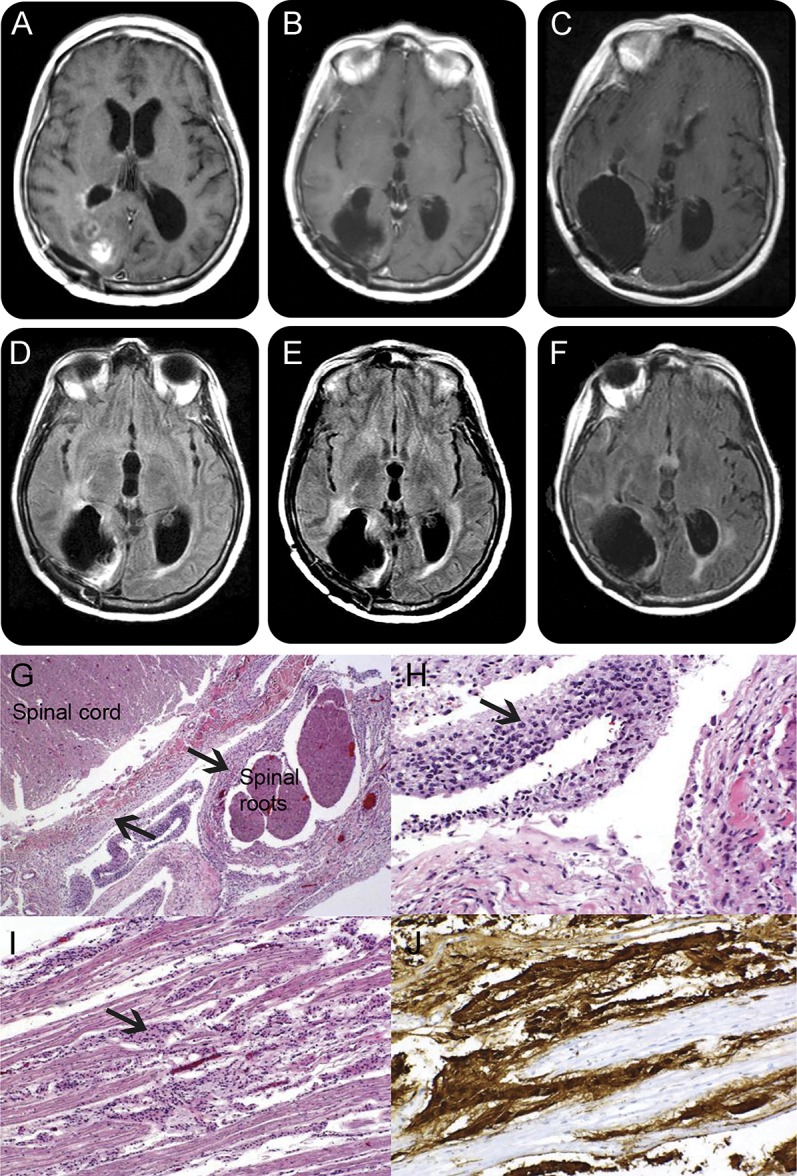
Brain MRI T1 postgadolinium images 7 months after the diagnosis show mild ventricular dilation and hyperintense signal corresponding to residual tumor (A). In spite of progressive clinical worsening, subsequent scans done 11 (B) and 12 months (C) after diagnosis show no significant progression of enhancing tumor. Corresponding fluid-attenuated inversion recovery sequences at 7 months (D), 11 months (E), and 12 months (F) after diagnosis confirm absence of progression of nonenhancing tumors, as occasionally seen in patients on bevacizumab. This dissociation between neurologic deterioration and absence of neurologic worsening poses a diagnostic dilemma. Autopsy shows extensive leptomeningeal spread of the tumor, with hematoxylin & eosin low power (G) and high power (H) demonstrating the coating of spinal cord and nerve roots by glioma (arrows). (I) Tumor cells coating the cauda equina; glial fibrillary acid protein immunohistochemistry further demonstrates the glial nature of the tumor, shown in brown (J). MRI of the spine (not shown) had normal results, reflecting bevacizumab masking effects.
Given the ventricular dilation, the patient received a ventriculoperitoneal shunt in an attempt to treat NPH, and was discharged. Questionable clinical improvement was seen over the next few weeks.
In the following 6 months, while still on bevacizumab, the patient developed progressive confusion and disorientation, worsening memory difficulties, urinary incontinence, and more severe gait ataxia. A repeat brain MRI showed no remarkable changes in contrast enhancement (figure, B and C) or fluid-attenuated inversion recovery (FLAIR) hyperintensities (figure, E and F). Her back pain worsened, and symptoms of lumbosacral radiculopathy developed, but a repeat spine MRI was unremarkable. CSF analysis showed protein 340 mg/dL, glucose 30 mg/dL, 2 leukocytes, and negative cytology. EEG and blood and urine tests were again noncontributory.
Questions for consideration:
What could be suspected despite normal CSF cytology and stable imaging studies?
What could be done to improve symptoms?
SECTION 3
An intriguing finding in this patient is the lack of radiographic worsening despite marked clinical deterioration. In patients on bevacizumab, tumor progression without increase in contrast enhancement is sometimes observed, in a process of tumor cell cooption of existing blood vessels that is independent of neoangiogenesis.2 In those patients, FLAIR hypersignal continues to increase, but our patient showed only minimal FLAIR changes, if any. Shunt malfunction could be a potential explanation, and neurosurgical consultation was obtained. Neurotoxicity from radiotherapy could be considered, but there was no significant leukoencephalopathy, and this type of complication typically occurs years later in the disease course, in the rare patients who achieve long-term survival.
Leptomeningeal spread remained the most likely diagnosis, given the back pain, radiculopathy, and increasing CSF protein, although cytology remained negative. A careful look at the brain MRI (figure, C) discloses some enhancement lining the ventricles, providing a diagnostic clue, but that finding is nonspecific and the spine MRI results were normal. Radiotherapy to the lumbosacral spine was considered for symptom relief, but not pursued given the absence of radiographic abnormalities or diagnostic confirmation.
The patient resumed bevacizumab and temozolomide but continued to decline. Several shunt revisions were performed with no improvement. Her neurologic symptoms rapidly deteriorated and she died a few weeks later.
On autopsy, macroscopic examination of the brain revealed infiltration of the septum pellucidum by a mucoid tumor tissue, with intraventricular and periventricular dissemination. Macroscopic examination of the spinal cord showed disseminated tumor coating of the leptomeninges and subarachnoid spaces, with nodularity and thickening of spinal roots and cauda equina. Microscopic findings are shown in the figure, G–J. The diagnosis of widespread leptomeningeal tumor was confirmed.
DISCUSSION
Our patient presented with symptoms suggestive of leptomeningeal metastasis (LM) during treatment with bevacizumab. Repeated CSF cytology and MRI failed to confirm LM, and there was a marked dissociation between symptoms and radiographic findings; the diagnosis could only be confirmed by autopsy.
LM is an increasingly frequent complication of cancer, and while therapeutic options are limited, establishing this diagnosis is important for prognosis and institution of palliative treatments such as focal radiotherapy.
Confirming the diagnosis of LM can be challenging. CSF cytology sensitivity is approximately only 50% at the first LP, increasing to up to 90% with 3 repeat LPs3,4; analysis of large volumes of CSF may also improve sensitivity. Cytology sensitivity is particularly lower in solid tumors, especially in glioblastoma.5 The identification of CSF circulating tumor cells through rare cell capture technology may facilitate the diagnosis,4 but this methodology is limited to epithelial tumors, and not helpful in gliomas. In solid tumors, the diagnosis of LM remains heavily dependent on the MRI, a more sensitive technique than cytology.6,7
This patient was receiving the VEGF inhibitor bevacizumab, known to confound interpretation of MRI contrast enhancement. Proangiogenic factors secreted by tumors lead to the formation of new capillaries, and these neovessels are fenestrated and lack blood–brain barrier (BBB) properties. This disrupted BBB provides the basis for imaging tumors on contrast-enhanced MRI. VEGF promotes vascular permeability in these vessels, and anti-VEGF therapies may thus decrease contrast enhancement, preventing accurate tumor visualization. This could explain why the exuberant leptomeningeal disease seen in the autopsy, with widespread coating of spinal cord and nerve roots, was not apparent on the patient's MRI despite obvious symptoms. This clinical and radiologic dissociation has also been reported in 2 patients receiving bevacizumab for lung cancer brain metastases, both presenting with LM and normal MRIs.8 In a retrospective study of bevacizumab to treat gliomas with LM, it was noted that in spite of radiographic improvements, some patients experienced worsening of symptoms, once again suggesting masking effects of this agent.9
It is important to fully evaluate patients with primary or metastatic brain tumors presenting with neurologic deterioration in order to recognize causes that require specific neurologic management. Leptomeningeal spread is a potential cause of unexplained neurologic deterioration in glioma patients treated with anti-angiogenic agents, and interpreting contrast-enhanced MRI in this setting can be challenging.
AUTHOR CONTRIBUTIONS
G.F.: drafting and revising the manuscript for content, including medical writing for content, study concept, analysis and interpretation of data. E.P.: drafting and revising the manuscript for content, including medical writing for content, study concept, analysis and interpretation of data. A.D.: revising the manuscript for content, including medical writing for content, analysis and interpretation of data. S.T.: revising the manuscript for content, including medical writing for content. M.R.: revising the manuscript for content, including medical writing for content, analysis and interpretation of data. A.O.: drafting and revising the manuscript for content, including medical writing for content, study concept, analysis and interpretation of data.
STUDY FUNDING
No targeted funding reported.
DISCLOSURE
G. Faivre, E. Pentsova, A. Demopoulos, S. Taillibert, and M. Rosenblum report no disclosures relevant to the manuscript. A. Omuro has participated in an advisory board for Roche. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Omuro A, DeAngelis LM. Glioblastoma and other malignant gliomas: a clinical review. JAMA 2013;310:1842–1850. [DOI] [PubMed] [Google Scholar]
- 2.Norden AD, Young GS, Setayesh K, et al. Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology 2008;70:779–787. [DOI] [PubMed] [Google Scholar]
- 3.Wasserstrom WR, Glass JP, Posner JB. Diagnosis and treatment of leptomeningeal metastases from solid tumors: experience with 90 patients. Cancer 1982;49:759–772. [DOI] [PubMed] [Google Scholar]
- 4.Nayak L, Fleisher M, Gonzalez-Espinoza R, et al. Rare cell capture technology for the diagnosis of leptomeningeal metastasis in solid tumors. Neurology 2013;80:1598–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lawton CD, Nagasawa DT, Yang I, Fessler RG, Smith ZA. Leptomeningeal spinal metastases from glioblastoma multiforme: treatment and management of an uncommon manifestation of disease. J Neurosurg Spine 2012;17:438–448. [DOI] [PubMed] [Google Scholar]
- 6.Straathof CS, de Bruin HG, Dippel DW, Vecht CJ. The diagnostic accuracy of magnetic resonance imaging and cerebrospinal fluid cytology in leptomeningeal metastasis. J Neurol 1999;246:810–814. [DOI] [PubMed] [Google Scholar]
- 7.Clarke JL, Perez HR, Jacks LM, Panageas KS, Deangelis LM. Leptomeningeal metastases in the MRI era. Neurology 2010;74:1449–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kleinschmidt-DeMasters BK, Damek DM. The imaging and neuropathological effects of bevacizumab (Avastin) in patients with leptomeningeal carcinomatosis. J Neurooncol 2010;96:375–384. [DOI] [PubMed] [Google Scholar]
- 9.Taillibert S, Guillevin R, Dehais C, Bellanger A, Omuro A. Bevacizumab and irinotecan (BI) for gliomatous leptomeningeal disease. J Clin Oncol 2010;28:e12526. [Google Scholar]
- 10.Sawlani V, Taylor R, Rowley K, Redfern R, Martin J, Poptani H. Magnetic resonance spectroscopy for differentiating pseudo-progression from true progression in GBM on concurrent chemoradiotherapy. Neuroradiol J 2012;25:575–586. [DOI] [PubMed] [Google Scholar]


