Abstract
Purpose
To determine the frequency of biochemical cholestasis (direct bilirubin (DB) ≥ 2mg/dL) in children with short bowel syndrome and biopsy proven parenteral nutrition (PN) associated liver disease and to define predictive factors for the occurrence and degree of hepatic fibrosis.
Methods
Following IRB approval, a retrospective review was conducted of patients followed by two multidisciplinary intestinal rehabilitation programs between January 1st, 2000, and September 30th, 2008. Inclusion criteria were exposure to parenteral nutrition (>30 days) and having undergone a liver biopsy. Liver biopsies were graded from 0–3 based upon degree of fibrosis in the pathology report. The most recent DB within 10 days prior to biopsy was recorded.
Results
A total of 66 children underwent 83 liver biopsies. The most common diagnoses included necrotizing enterocolitis (NEC) (36.4%), gastroschisis (22.7%) and intestinal atresia (15.1%). Median age at biopsy was 6.1 months with a median duration of PN of 4.7 months. 70.3% of patients had a history of exposure to parenteral omega-3 lipid emulsion. 89% (74/83) of liver biopsies demonstrated some degree of fibrosis (fibrosis scale 1–3), while 9.6% (8/83) had evidence of cirrhosis. 83% of biopsies without fibrosis and 55% of biopsies with fibrosis were obtained in patients without evidence of biochemical cholestasis (P=0.20). 3 of the 8 patients with cirrhosis on liver biopsy (37%) had no evidence of biochemical cholestasis. Univariate analysis identified only gestational age at birth (GA) as significantly associated with the degree of liver fibrosis (P=0.03). A multivariate logistic regression model accounting for multiple biopsies in patients revealed that GA was a predictor of fibrosis only in patients with a diagnosis other than NEC (P <0.01).
Conclusions
In children with short bowel syndrome, biochemical cholestasis does not reflect the presence or degree of histologically confirmed parenteral nutrition-associated liver fibrosis. Careful follow-up, combined with further refinement of diagnostic and hepatoprotective strategies, may be warranted in this patient population.
Keywords: Short bowel syndrome, parenteral nutrition, liver fibrosis, biochemical cholestasis
Introduction
Since its introduction in 1968, parenteral nutrition (PN) has become a critical therapy in the treatment of infants with short bowel syndrome1. While therapeutic approaches to pediatric intestinal rehabilitation have since undergone several significant advances, parenteral nutrition associated liver disease (PNALD) remains a significant contributor to morbidity and mortality. In the infant population, up to 60% of patients on long term PN develop intestinal failure associated liver disease2, a condition linked with an increased mortality rate3,4. The current gold standard for the assessment of liver disease is the liver biopsy, an invasive procedure inherently restricted in its use.
Because of the invasive nature of percutaneous or direct liver biopsy, indirect non-invasive measures of liver function are often used to gauge the degree of hepatic dysfunction. While prothrombin time and plasma concentrations of bilirubin and albumin allow for frequent serial monitoring, they are not considered reliable markers of liver fibrosis or cirrhosis. What limited published data exists, in fact, seems to indicate that liver fibrosis resulting from PNALD persists despite normalization of previously elevated bilirubin levels5–8.
Early data on the use of parenteral omega-3 lipid emulsions indicate progress is being made in the ability to reverse PN associated cholestasis9. Similarly, the transition of PN patients to full enteral nutrition has analogous salutary effects10. While these hepatoprotective strategies can improve biochemical cholestasis, heightened attention to the resolution, or lack thereof, of established liver fibrosis or cirrhosis due to PN is warranted. The presence of compensated cirrhosis in these patients with resolved cholestasis must not be overlooked, given the potential long term clinical implications. We therefore sought to evaluate the correlation between liver fibrosis or cirrhosis and direct bilirubin levels in our cohort of PN dependent children with short bowel syndrome.
Methods
Following IRB approval, a retrospective chart review was performed at two multidisciplinary pediatric intestinal rehabilitation programs. Pediatric short bowel syndrome patients treated with PN for at least 30 days who had a liver biopsy between January 1st, 2000 and September 30th, 2008 were included in the study. All liver biopsies were obtained via a core needle biopsy technique. Throughout the period of study, all patients received Trophamine (B Braun Medical Inc., Irvine, CA, USA) 10% amino acid solution and lipid solution of either Intralipid (Fresenius Kabi AB, Uppsala, Sweden) or Omegaven (Fresenius Kabi AG, Bad Homburg vdh, Germany). Demographic data including age at biopsy, gestational age, gender, and primary diagnosis leading to SBS were collected. Clinical variables including length of treatment with PN, percentage of calories from enteral intake, and dose and type of parenteral fat (soy vs. fish oil) was recorded. Biochemical tests collected within 10 days prior to liver biopsy were recorded, including alanine aminotransferase (ALT), direct bilirubin, prothrombin time (PT), and international normalization ratio (INR). Co-temporal history of sepsis, as documented by a positive blood culture within 14 days before or after the date of liver biopsy, was recorded. In those patients without biochemical cholestasis (defined as a direct bilirubin level < 2mg/dL) at time of biopsy, historical lab results were checked for evidence of previous hyperbilirubinemia during the patient’s documented treatment with PN.
For the purposes of this study, an elevated PT was defined as >13 seconds. Prolonged INR was defined as >1.12 and an elevated ALT level was defined as >80 units/L. Cholestasis was defined as a direct bilirubin level ≥ 2 mg/dL. The degree of liver fibrosis on biopsy was based upon the extent of fibrosis recorded in the original pathologic report. A scale from 0 to 3 was used (0= no fibrosis, 1= fibrosis, 2= portal-portal bridging fibrosis, 3= cirrhosis). Biopsy evidence of cholestasis was recorded as absent, mild, moderate or severe, also based upon the original pathologic report.
Continuous data including age at biopsy, gestational age, time on PN, and intravenous lipid dose were expressed as medians, interquartile ranges and full ranges since they were skewed distributions as tested by the Wilk-Shapiro test. Nonparametric methods were used to compare associations between variables (χ2 for 2×4 tables, Spearman rho correlation, and Mann-Whitney U-test). Multivariate logistic regression analysis with backward selection was applied to determine variables predictive of fibrosis with age at biopsy, GA, diagnosis (NEC vs. other), and time on PN tested as covariates11. Two-way interactions were tested and a generalized estimating equations (GEE) strategy was used to handle the multiple biopsies from the same patient (i.e., correlated data). In addition, factors predictive of DB ≥2 mg/dL were evaluated, including the above variables as well as history of exposure to parenteral omega-3 lipid emulsions. The Wald test was used to assess significance. Statistical analysis was performed using the SPSS version 17.0 (SPSS Inc., Chicago, IL). Two-tailed P < 0.05 were considered statistically significant.
Results
A total of 66 children underwent 83 liver biopsies. The most common diagnoses included necrotizing enterocolitis (36%), gastroschisis (23%) and intestinal atresia (15%). The median age at time of biopsy was 6.1 months (IQR 3.9 – 13.8) while the median gestational age at birth was 32 weeks (range 23–41) (Table 1).
Table 1.
Patient demographic variables and liver biopsy distributions
| Number of patients | 66 |
|---|---|
| Males, n (%) | 47 (71) |
| Gestational age, weeks: median (range) | 32 (23–41) |
| Age at biopsy, months: | |
| median | 6.1 |
| IQR | 3.9–13.8 |
| range | 1–240 |
| Diagnosis: n (%) | |
| NEC | 24 (36) |
| Gastroschisis | 15 (23) |
| Intestinal atresia | 10 (15) |
| Hirschsprung’s disease | 4 (6) |
| Other* | 13 (20) |
| Duration of PN therapy, months: median (IQR) | 4.7 (3.0–8.4) |
| IV lipid dose, g/kg/day: median (range) | 1.0 (0.5–3.3) |
| Number of biopsies | 83 |
| Number of patients with multiple biopsies, n | |
| Two biopsies | 11 |
| Three biopsies | 3 |
IQR = interquartile range, NEC = necrotizing enterocolitis
Other includes intestinal volvulus, pseudo-obstruction, polyarteritis nodosa, meconium ileus, mesenteric cyst, duodenal atresia or web, and ileal perforation
The median duration of PN treatment at time of liver biopsy was 4.7 months (IQR 3.0 – 8.4). The average % of calories from enteral intake at time of biopsy was 27.3%, with 24 biopsies documented in patients receiving 100% parenteral nutrition. 70.3% of patients in our cohort had a history of exposure to parenteral omega-3 lipid emulsions. The median intravenous lipid dose for all patients at the time of biopsy was 1.0 g/kg/day (range 0.5–3.3) (Table 1).
Of the 66 patients, 14 had more than one biopsy recorded (11 had two and 3 had three) (Table 1). 89% (74/83) of liver biopsies demonstrated some degree of fibrosis (fibrosis scale ≥1) including 9.6% (8/83) with evidence of cirrhosis (fibrosis scale = 3) (Figure 1). 83% of biopsies without fibrosis and 55% of biopsies with fibrosis were obtained in patients without evidence of biochemical cholestasis. Three of the 8 patients with cirrhosis on liver biopsy (38%) had no evidence of biochemical cholestasis. Direct bilirubin ≥ 2mg/dL was not found to be an independent predictor of the degree of fibrosis (P= 0.19).
Figure 1.
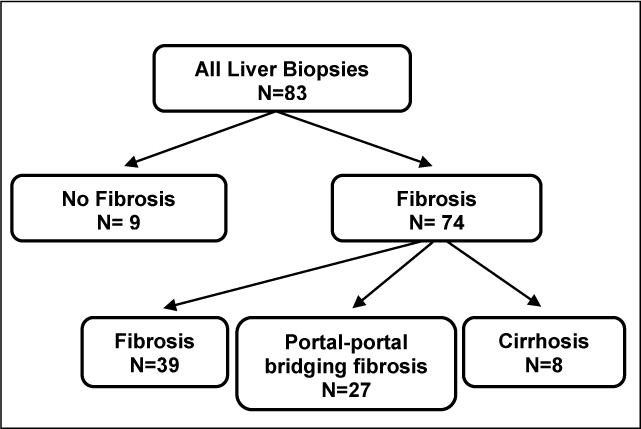
Breakdown of liver biopsy results according to degree of liver fibrosis. Score based upon the original pathologic report. Score 0=no fibrosis, score 1=fibrosis, score 2=portal-portal bridging fibrosis, score 3=cirrhosis.
Univariate analysis identified younger gestational age as associated with liver fibrosis (fibrosis score >0) (P=0.03). Accounting for the clustering effect of multiple biopsies within patients, multivariate logistic regression, using generalized estimating equations (GEE) with backward selection identified no significant predictors of the degree of fibrosis, although it did reveal that younger GA was predictive of a higher risk for occurrence of fibrosis (fibrosis score >0). However, this was evident only in patients without a diagnosis of NEC, as indicated by a significant interaction between GA and diagnosis (Wald test: P=0.007). GA was not predictive of fibrosis for patients with NEC since the occurrence of hepatic fibrosis in this population was very high, independent of a patient’s GA (Figure 2).
Figure 2.
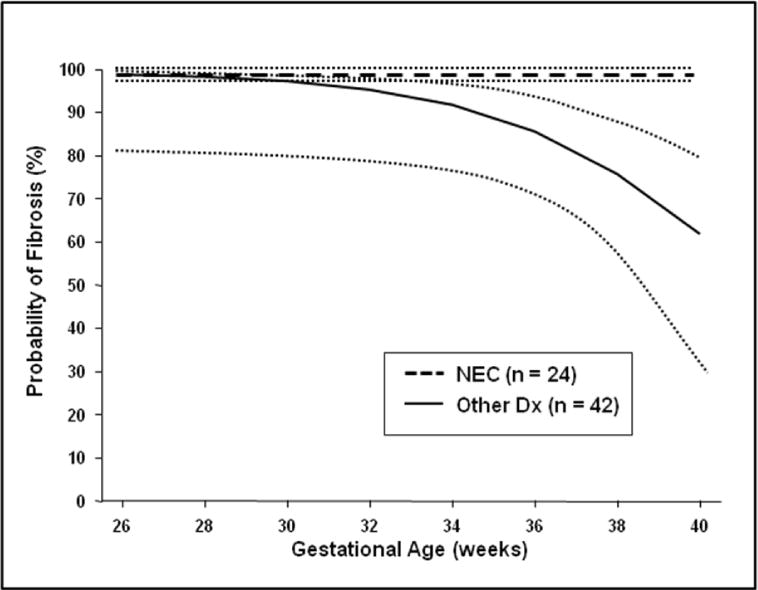
Exclusion of patients with a primary diagnosis of NEC identified 42 patients with a total of 54 liver biopsies. A multivariate logistic regression analysis of this subset of patients confirmed that lower GA was predictive of a higher probably of fibrosis (p = 0.036). In addition, this analysis identified elevated ALT as a second independent predictor of the presence of fibrosis (p = 0.029) in this subset of patients.
In the subgroup of patients with a history of exposure to parenteral omega-3 lipid emulsions, there was a significant negative correlation between time on PN and DB (Spearman rho = −0.67, P < .001). Median time on PN was significantly shorter in patients with biochemical cholestasis (DB ≥ 2 mg/dL) compared to those with DB <2 mg/dL (6.2 months vs. 3.1 months, respectively, P < .001, Mann-Whitney U-test). This implies that a longer time on PN is associated with a lower DB for patients exposed to parenteral omega-3 lipid emulsions.
Discussion
The first reported case of end stage liver disease following parenteral nutrition therapy in an infant was published in 197112. Despite thirty years of subsequent experience and research, the precise etiology of PNALD remains unknown. Various theories have been put forth to explain the observed injury to the liver following long term parenteral nutrition, including injury secondary to catheter related sepsis, direct toxic components of the PN, manganese toxicity, lipid emulsion toxicity, overfeeding of energy or one of the macronutrients, and injury subsequent to lack of enteral intake2. Despite the range of possible theories, however, no single hypothesis has been recognized as a definitive explanation.
Research into the histological evolution of PNALD has been more fruitful. It seems clear from pathologic review of liver specimens that a clear disease progression exists, and that this process is directly related to the length of PN treatment13,14. PNALD appears to begin with periportal inflammation and cholestasis, progressing from there to bile duct proliferation, fibrosis and cirrhosis. The majority of liver specimens with frank cirrhosis are observed in patients with over 12 weeks of exposure to PN13. The presence of fibrosis or cirrhosis is classically diagnosed on the basis of liver biopsy. Given the invasive nature of this procedure, however, physicians are typically not able to follow serial biopsies while tracking the course of liver disease progression or resolution. More commonly, clinicians rely upon indirect measures of liver function, including plasma direct bilirubin levels.
Recent data seem to demonstrate an improvement in serum direct bilirubin levels following treatment with parenteral omega-3 lipid emulsions9,15. Our results support these study conclusions, demonstrating that in patients with a history of exposure to parenteral omega-3 lipid emulsions, a longer course of treatment with PN is associated with a lower DB level.
While the resolution of PN-associated cholestasis is a significant clinical improvement, the limited published evidence seems to indicate that a corresponding resolution of established fibrosis, and certainly cirrhosis, may not take place. In 1981, Dahms et al reported that 6 infants with two serial biopsies demonstrated persistent liver fibrosis despite normalization of biochemical cholestasis6. Later, a retrospective review of 31 infants with PN associated cholestasis confirmed that marked histological damage in the form of liver fibrosis could remain despite resolution of PN associated cholestasis7. More recently, a case report from the transplant literature demonstrated that treatment of intestinal failure and associated PNALD with an isolated small bowel transplant resulted in successful normalization of hyperbilirubinemia but failed to improve the fibrosis demonstrated on follow up liver biopsy 11 months post transplant8.
Our data support these previous observations that reversal of PN-associated cholestasis can be observed despite persistent fibrosis or even cirrhosis. The vast majority, 89% (74/83), of liver biopsies in our cohort demonstrated some degree of fibrosis (fibrosis scale 1–3) (Figure 1). Over half (55%) of these cases were obtained from patients without evidence of biochemical cholestasis. Furthermore, while almost 10% (n=8) of all biopsies had evidence of cirrhosis, 38% of these (3 of the 8) were recorded in patients with a direct bilirubin less than 2 mg/dL.
The recognition of established liver damage in the form of persistent fibrosis or cirrhosis is of vital clinical importance. Patients carrying a diagnosis of compensated cirrhosis must undergo careful monitoring for signs of hepatic decompensation. Long term complications have been reported in the setting of this disease including, most significantly, the development of hepatocellular carcinoma (HCC). In patients who die from liver related complications of compensated cirrhosis, 54–70% of deaths are due to HCC16. While this mortality rate includes cirrhosis secondary to multiple etiologies, most commonly chronic hepatitis C infection, case reports in infants have underscored the potential for the development of this highly lethal disease in the setting of both PN associated cirrhosis and bridging fibrosis17,18. Additional long term complications of cirrhosis in children include both recurrent sepsis19 and the consequences of portal hypertension, such as the development of ascites and variceal bleeding20.
Due to the importance of recognizing this persistent liver damage in patients who have normal DB levels, we sought to identify patient factors predictive of liver fibrosis or cirrhosis. Univariate analysis identified younger gestational age as the only factor associated with the presence of liver fibrosis on biopsy (P=0.03). Further analyses, taking into account multiple biopsies in the same patient, revealed that younger gestational age was predictive of the presence of fibrosis specifically in individuals without a diagnosis of NEC. It is of note that this distinction was due to a very high occurrence of liver fibrosis in those affected by NEC (Figure 2). A plausible explanation is that severe NEC is associated with factors, such as frequent sub-clinical or clinical sepsis, and hypotension which may in turn increase the prevalence of liver fibrosis in these children. Finally, it is interesting to note that a subset analysis of those patients with a primary diagnosis other than NEC identified elevated ALT as a second independent variable predictive of the presence of fibrosis. ALT appears to remain elevated in children with PN associated liver disease after the resolution of biochemical cholestasis21. Our results would seem to corroborate the hypothesis of these authors that ALT elevation may reflect persistent hepatic injury.
Future advances in medical techniques may allow clinicians to better assess the degree of liver fibrosis or cirrhosis in those patients with normalized indirect measures of hepatic function. While imaging techniques such as ultrasound are currently being investigated as possible non-invasive instruments in the evaluation of this disease22, evidence is minimal and relatively inconsistent23. Novel techniques including hepatic elastography and quantitative breath tests for liver function such as the Methionine breath test offer promise, but remain in the early stages of investigation and development24,25. Liver biopsy continues to be the current gold standard in the diagnosis of cirrhosis.
Our data confirm the persistence of liver fibrosis and cirrhosis following resolution of biochemical cholestasis in children with a history of PNALD. Given the potential for malignant degeneration, as well as other complications, in patients with chronic, compensated fibrosis or cirrhosis, this data suggests the need for careful long term follow-up of this cohort. It also argues for trials employing preventative hepatoprotective strategies in pediatric patients at risk for intestinal failure.
DISCUSSION
Unidentified Speaker: Very nice presentation. You have taken away all of the metrics for determining when the hepatoprotective strategy needs to be employed. What are your suggestions as to – especially in the high-risk infant who is successfully recovering from necrotizing enterocolitis but whose gut function has not returned to normal, how, short of biopsy, do you propose that we manage or monitor this problem?
Brian A. Jones, M.D., Boston, MA: Our experience is that as soon as a patient is referred to our multidisciplinary center we initiate hepatoprotective parenteral nutrition strategies with reduced lipid emulsions or omega-3-based lipid emulsions, as well as including methods to reduce the incidence of sepsis, including ethanol locks on our central venous catheters.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dudrick SJ, Wilmore DW, Vars HM, et al. Long-term parenteral nutrition with growth, development and positive nitrogen balance. Surgery. 1968;64:132. [PubMed] [Google Scholar]
- 2.Kelly DA. Intestinal Failure-Associated Liver Diseases: What do we know today? Gastroenterology. 2006;130:S70–S77. doi: 10.1053/j.gastro.2005.10.066. [DOI] [PubMed] [Google Scholar]
- 3.Teitelbaum D, Drongowski R, Spivak D. Rapid development of hyperbilirubinemia in infants with short bowel syndrome as a correlate to mortality: possible indication for early small bowel transplantation. Transplant Proc. 1996;26:2699–700. [PubMed] [Google Scholar]
- 4.Quiros-Tejeira RE, Ament ME, Reyen L, et al. Long-term parenteral nutritional support and intestinal adaptation in children with short bowel syndrome: A 25-year experience. J Pediatr. 2004;145:157–163. doi: 10.1016/j.jpeds.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 5.Rodgers BM, Hollenbeck JI, Donnelly WH, et al. Intrahepatic cholestasis with parenteral alimentation. Am J Surg. 1976;131:149–155. doi: 10.1016/0002-9610(76)90088-x. [DOI] [PubMed] [Google Scholar]
- 6.Dahms BB, Halpin TC. Serial biopsies in parenteral nutrition-associated cholestasis of early infancy. Gastroenterology. 1981;81:136–144. [PubMed] [Google Scholar]
- 7.Moss RL, Das JB, Raffensperger JG. Total parenteral nutrition-associated cholestasis: clinical and histopathologic correlation. J Pediatr Surg. 1993;28(10):1270–1275. doi: 10.1016/s0022-3468(05)80311-2. [DOI] [PubMed] [Google Scholar]
- 8.Hasegawa T, Sasaki T, Kimura T, et al. Effects of isolated small bowel transplantation on liver dysfunction cause by intestinal failure and long-term total parenteral nutrition. Pediatr Transplant. 2002;6:235–239. doi: 10.1034/j.1399-3046.2002.01074.x. [DOI] [PubMed] [Google Scholar]
- 9.Gura KM, Duggan CP, Collier SB, et al. Reversal of parenteral nutrition-associated liver disease in two infants with short bowel syndrome using parenteral fish oil: implications for future management. Pediatrics. 2006;118:197–201. doi: 10.1542/peds.2005-2662. [DOI] [PubMed] [Google Scholar]
- 10.Javid PJ, Collier S, Richardson D, et al. The role of enteral nutrition in the reversal of parenteral nutrition-associated liver dysfunction in infants. J Pediatr Surg. 2005;40(6):1015–8. doi: 10.1016/j.jpedsurg.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 11.Katz MH. Multivariable Analysis: A Practical Guide for Clinicians. 2nd. New York: Cambridge University Press; 2006. pp. 158–183. [Google Scholar]
- 12.Peden VH, Witzleben CL, Skelton MA. Total parenteral nutrition. J Pediatr. 1971;78(1):180–183. doi: 10.1016/s0022-3476(71)80289-5. [DOI] [PubMed] [Google Scholar]
- 13.Zambrano E, El-Hennawy M, Ehrenkranz RA, et al. Total parenteral nutrition induced liver pathology: an autopsy series of 24 newborn cases. Pediatr Dev Pathol. 2004;7:425–432. doi: 10.1007/s10024-001-0154-7. [DOI] [PubMed] [Google Scholar]
- 14.Cohen C, Olsen MM. Pediatric total parenteral nutrition. Liver histopathology. Arch Pathol Lab Med. 1981;105:152–156. [PubMed] [Google Scholar]
- 15.Ekema G, Falchetti D, Boroni G, et al. Reversal of severe parenteral nutrition-associated liver disease in an infant with short bowel syndrome using parenteral fish oil (Omega-3 fatty acids) J Pediatr Surg. 2008;43:1191–1195. doi: 10.1016/j.jpedsurg.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 16.Fattovich G, Stroffolini T, Zagni I, et al. Hepatocellular carcinoma in cirrhosis; incidence and risk factors. Gastroenterology. 2004;127:S35–S50. doi: 10.1053/j.gastro.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 17.Patterson K, Kapur SP, Chandra RS. Hepatocellular carcinoma in a noncirrhotic infant after prolonged parenteral nutrition. J Pediatr. 1984;106(5):797–800. doi: 10.1016/s0022-3476(85)80360-7. [DOI] [PubMed] [Google Scholar]
- 18.Vileisis RA, Sorensen K, Gonzales-Crussi F, et al. Liver malignancy after parenteral nutrition. J Pediatr. 1982;100(1):88–90. doi: 10.1016/s0022-3476(82)80242-4. [DOI] [PubMed] [Google Scholar]
- 19.Kelly DA. Liver complications of pediatric parenteral nutrition – epidemiology. Nutrition. 1998;14(1):153–7. doi: 10.1016/s0899-9007(97)00232-3. [DOI] [PubMed] [Google Scholar]
- 20.Leonis MA, Balistreri WF. Evaluation and management of end-stage liver disease in children. Gastroenterology. 2008;134(6):1741–51. doi: 10.1053/j.gastro.2008.02.029. [DOI] [PubMed] [Google Scholar]
- 21.Yang CF, Lee M, Valim C, et al. Persistent alanine aminotransferase elevations in children with parenteral nutrition-associated liver disease. J Pediatr Surg. 2009;44(6):1084–7. doi: 10.1016/j.jpedsurg.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaiani S, Gramantieri L, Venturoli N, et al. What is the criterion for differentiating chronic hepatitis from compensated cirrhosis? A prospective study comparing ultrasonography and percutaneous liver biopsy. J Hepatol. 1997;27:979–985. doi: 10.1016/s0168-8278(97)80140-7. [DOI] [PubMed] [Google Scholar]
- 23.Ong TZ, Tan HJ. Ultrasonography is not reliable in diagnosing liver cirrhosis in clinical practice. Singapore Med J. 2003;44(6):293–295. [PubMed] [Google Scholar]
- 24.Duro D, Duggan C, Valim C, et al. Novel intravenous 13C-methionine breath test as a measure of liver function in children with short bowel syndrome. J Pediatr Surg. 2009;44(3):236–240. doi: 10.1016/j.jpedsurg.2008.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Friedrich-Rust M, Ong MF, Martens S, et al. Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology. 2007;134(4):1238–1239. doi: 10.1053/j.gastro.2008.01.034. [DOI] [PubMed] [Google Scholar]


