Abstract
Neurons in the medullary raphe are critical to opioid analgesia through descending projections to the dorsal horn. Work in anesthetized rats led to the postulate that nociceptive suppression results from tonic activation of nociceptive-inhibiting neurons and tonic inhibition of nociceptive-facilitating neurons. However, morphine does not cause tonic changes in raphe neuronal firing in unanesthetized rodents. Recent work suggests that a drop in activity of nociceptive-inhibiting neurons synchronizes nociceptive circuits and a burst of activity in nociceptive-facilitating neurons facilitates withdrawal magnitude. After morphine, the phasic responses of raphe cells are suppressed along with nociceptive withdrawals. The results suggest a new model of brainstem modulation of nociception in which the medullary raphe facilitates nociceptive reactions when noxious input occurs and may modulate other functions between injurious events.
Endogenous pain modulation operates through descending pathways from the brainstem that modulate nociceptive transmission within the spinal cord (reviewed in 1-3). Neurons within the raphe magnus and adjacent ventromedial reticular formation (collectively termed the rostral ventromedial medulla or RVM here) provide a major final common pathway for modulatory influences on the spinal cord arising from brainstem and forebrain areas. RVM neurons project to the superficial dorsal horn and intermediate gray and their activation modulates the activity and responses of nociceptive dorsal horn neurons. The pathway through RVM contributes significantly to systemic opioid analgesia and is necessary for supraspinal opioid analgesia [4]. This review focuses on an emerging reformulation of how RVM neurons participate in the modulation of nociceptive responsiveness.
In the early 1980s, Howard Fields and co-workers recorded the activity of RVM neurons in anesthetized rats during the heat-evoked tail flick [5]. The tail flick is a ballistic movement that removes the tail, after a few seconds, from a noxious stimulus and is thus analogous to the nociceptive flexor withdrawal reflex of the limbs. Fields and co-workers physiologically defined two classes of medullary neurons by their discharge during the heat-evoked tail flick. They hypothesized that these two neuronal classes were critical to both nociception and antinociception, the latter elicited either by opioid administration or midbrain stimulation (reviewed in 1). A large body of work from Fields’s and others’ laboratories led to the elaboration of a cellular model for RVM-mediated analgesia and eventually to a proposed mechanism for producing hyperalgesia as well [3, 6-7]. The circuit model proposed by Fields has been extraordinarily heuristic particularly in spurring advances in our understanding of the behavioral and pharmacological mechanisms involved in descending nociceptive modulation. Progress on understanding how physiological discharge patterns support RVM-mediated nociceptive modulation has been more measured and until recently, has been made primarily within the context of the anesthetized rodent. As highlighted in this review, physiological recordings from single units in RVM, particularly in unanesthetized rodents, challenge several specific components of the existing model and constrain future hypotheses.
The current model postulates that the tonic discharge of RVM cells modulates nociceptive state
The two RVM cell classes with putative nociceptive modulatory effects are termed on and off cells. The definition and name of on cells derive from this cell class’s consistent excitatory response to superficial noxious stimulation [5]. Similarly, off cells are defined by the pause in their discharge in response to superficial noxious stimulation. In the initial description, an analogy was made between off cells and the omnipause neurons of the paramedian pontine reticular formation, presciently highlighting the putative importance of the off cell pause in phasically gating movement through disinhibition [5]. Yet, as described below, it was the tonic responses of on and off cells to an analgesic dose of morphine in anesthetized rats that ultimately lent salience to the classification scheme and received the most attention [8-9].
Without question, the tonic effects of opioids on on and off cell discharge are dramatic in the anesthetized rat. On cells stop firing and off cells fire continuously after administration of an opioid at any dose and by any route that suppresses nociceptive withdrawals [see for example 10-13]. The robust and consistent finding that opioids change the tonic firing of on and off cells led to the suggestion that the tonic discharge of on and off cells mediates RVM modulation of nociception. In this formulation, continuous on cell firing produces a state of heightened sensitivity to noxious stimulation, termed hyperalgesia, and continuous off cell firing depresses the sensitivity to noxious stimulation, termed antinociception (Fig. 1A). Beyond the tonic responses of RVM cells to opioid administration, additional findings support the interpretation that tonic RVM discharge produce states of nociceptive responsiveness. First, withdrawal latency appears to be a function of the tonic discharge pattern at the time of stimulus application [14]. Withdrawals occur at a shorter latency, reflective of greater nociceptive sensitivity, when on cells are at peak firing rates and off cells relatively inactive at the time of noxious stimulation (Fig. 1B). Conversely, when noxious stimulation is applied while off cells are at peak firing rates and on cells inactive, withdrawals occur at a long latency or not at all. Second, the tonic discharge rate of on cells is elevated during the hyperalgesia associated with acute, naloxone-precipitated opioid withdrawal [15]. In sum, the strong association between tonic RVM cell activity and nociceptive sensitivity in anesthetized rats supports nociceptive-facilitating and -inhibiting roles for the tonic discharge of on and off cells, respectively.
Figure 1.
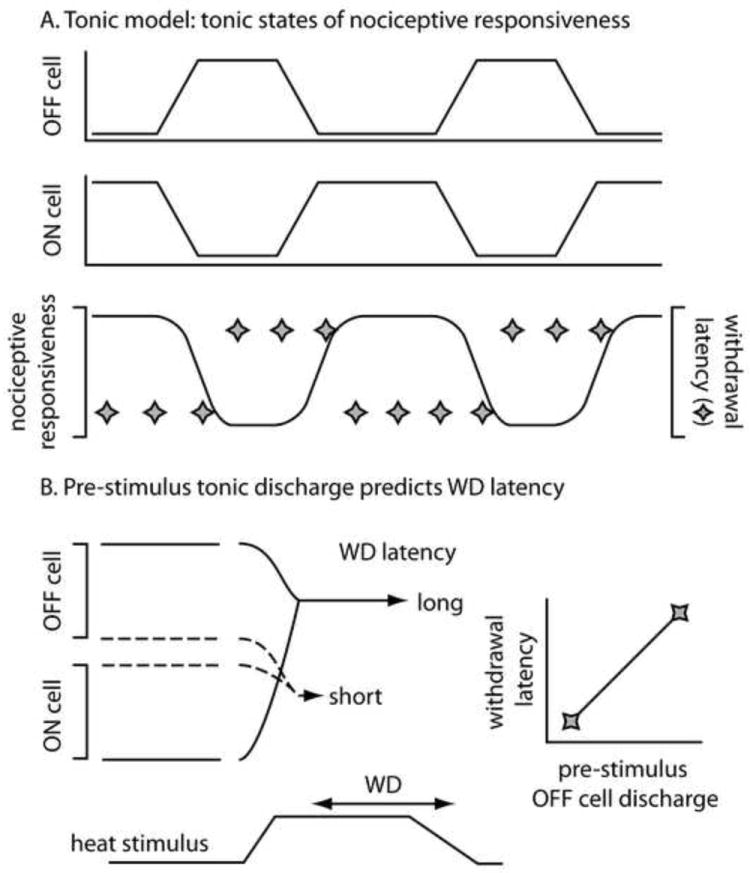
The tonic model of descending nociceptive modulation posits that different patterns of tonic activity in RM cells produce different states of nociceptive responsiveness. A: On cells tend to fire together as do off cells whereas on (middle trace) and off cells (top trace) show reciprocal patterns of tonic activity. According to the model, on cell activity combined with off cell inactivity produces an elevation in nociceptive responsiveness (bottom trace) that is marked by short withdrawal latencies (diamonds). In contrast, off cell activity combined with on cell inactivity depresses nociceptive responsiveness so that withdrawals occur at long latencies or not at all. B: According to the tonic model of RM nociceptive modulation, the pattern of tonic discharge in on and off cells (top two traces), just prior to noxious stimulation (marked heat stimulus), determines the ensuing withdrawal (WD) latency. If noxious stimulation is applied when off cells are active and on cells inactive (solid lines), antinociception, marked by a long withdrawal latency, results. In contrast, if noxious stimulation is applied when on cells are active and off cells inactive (dashed lines), hyperalgesia, marked by a short withdrawal latency, results. In this way, the withdrawal latency is expected to be directly proportional to the tonic level of off cell discharge prior to the stimulus (graph at right) and inversely proportional to pre-stimulus on cell discharge (not shown).
Because of the tonic response to opioids in anesthetized rats, a shorthand developed. A drug or manipulation that excites the tonic firing of off cells and inhibits the tonic firing of on cells is potentially antinociceptive whereas a drug or manipulation with the reverse effect is expected to produce hyperalgesia. There is indirect evidence in support of the tonic model of RVM nociceptive modulation. Since on but not off cells are directly sensitive to mu opioid receptor agonists (such as dermorphin) [12], dermorphin-conjugated saporin is thought to selectively kill RVM on cells. According to the shorthand then, injection of dermorphin-saporin into RVM should prevent the development of hyperalgesia. Indeed, dermorphin-saporin lesions of RVM prevent the development of several types of hyperalgesia, including the neuropathic variety following nerve injury [6, 16]. The interpretation of such studies is that without on cells, there can be no tonic on cell output and consequently RVM-mediated hyperalgesia cannot and does not occur. Tempering the weight ascribed to these findings is evidence that dermorphin-conjugated saporin decreases the number of both on and off cells in RVM rather than simply excising the on cell population as intended [17].
RVM on and off cells act through phasic rather than tonic modulation of nociception
The first attempt at determining whether opioids act similarly on RVM on and off cells in the absence of general anesthesia as in the presence of general anesthesia came when Jean-Louis Oliveras and colleagues recorded the activity of RVM cells in the awake rat before and after morphine [18]. In this study, only cells with on-like responses to somatosensory stimulation (hereafter referred to as simply on cells) were studied; no off-like cells were isolated (presumably because rats were not permitted to enter sleep, the behavioral state when off cells are primarily active; 19). In response to morphine, the tonic activity of on cells in the awake rat did not change. However, on cell responses to noxious stimulation were suppressed by morphine. These results, reported two decades ago, raised serious concerns about the tonic model of RVM nociceptive modulation introduced in the early 1980s and dominant to this day. Despite the clear inconsistency of the results from Martin et al [18] with the idea that tonic changes in on and off cell discharge produce antinociception, this work in awake rat has been largely ignored for the past 20 years. In fact, several recent reviews on RVM failed to cite this work while advancing the traditional, tonic model of RVM on and off cell function.
Recently, we confirmed and extended the results of Martin et al [18]. We recorded from both on and off cells in unanesthetized mice who cycled through wake and sleep states [20••]. Morphine suppressed the noxious stimulus-evoked responses of both cell classes while producing no change in background discharge rates. Thus, morphine suppressed the on cell burst and off cell pause elicited by noxious heat. The same depression of noxious stimulus-evoked responses coupled with no change in background discharge observed in unanesthetized rodents is also found in the anesthetized mouse [21]. Therefore, in both unanesthetized and anesthetized rodents, phasic responses of RVM on and off cells to noxious stimulation are suppressed by opioids. In contrast to their consistent effects on evoked responses, opioids alter the tonic discharge of on and off cells only in the anesthetized rat preparation.
The finding that opioids do not alter the tonic discharge of RVM cells in unanesthetized rodents shows that RVM modulates nociception on a phasic basis rather than producing tonic states of nociceptive responsiveness. In sum, RVM cells modulate nociceptive events on an on-demand basis, only in concert with incoming nociceptive input1.
A phasic decrease in off cell discharge times withdrawal onset
The studies reviewed above show that tonic off cell discharge is not necessary for antinociception. Striking evidence that tonic off cell activity is also insufficient for antinociception comes from the finding that background off cell discharge is greater before noxious stimulus trials that result in withdrawals than before those that do not produce withdrawals in the unanesthetized mouse [20••]. The observation of greater off cell activity before responding trials than before non-responding trials is frankly inconsistent with the tonic model described above which predicts that greater off cell activity should lead to decreased nociceptive responses (Fig. 1B). Furthermore, since off cells always pause in response to noxious stimulation that elicits a withdrawal, we can hypothesize that withdrawals are facilitated when off cells are both active prior to the noxious stimulus application and silenced by the noxious stimulus. According to this model, a decrease in off cell activity, rather than a lack of off cell activity, is key to off cells’ providing pronociceptive modulation (Fig. 2A). A sharp decrease in the discharge of an inhibitory input can result in post-inhibitory rebound excitation of a post-synaptic cell and is a common mechanism for synchronizing motor reactions [22-23]. Thus, rather than OFF cell silence simply serving a permissive role, it is likely that the decrease in OFF cell firing, the pause, provides a pronociceptive signal that synchronizes and therefore strengthens ensuing motor circuits in the spinal cord. This idea is both too new and sufficiently counter to the current model’s predictions that it has not been tested specifically. However, one study provides suggestive evidence in support. In the anesthetized rat, the magnitude of the off cell decrease in discharge elicited by noxious tail heat was greater in trials resulting in a withdrawal than in trials without a motor reaction [24].
Figure 2.
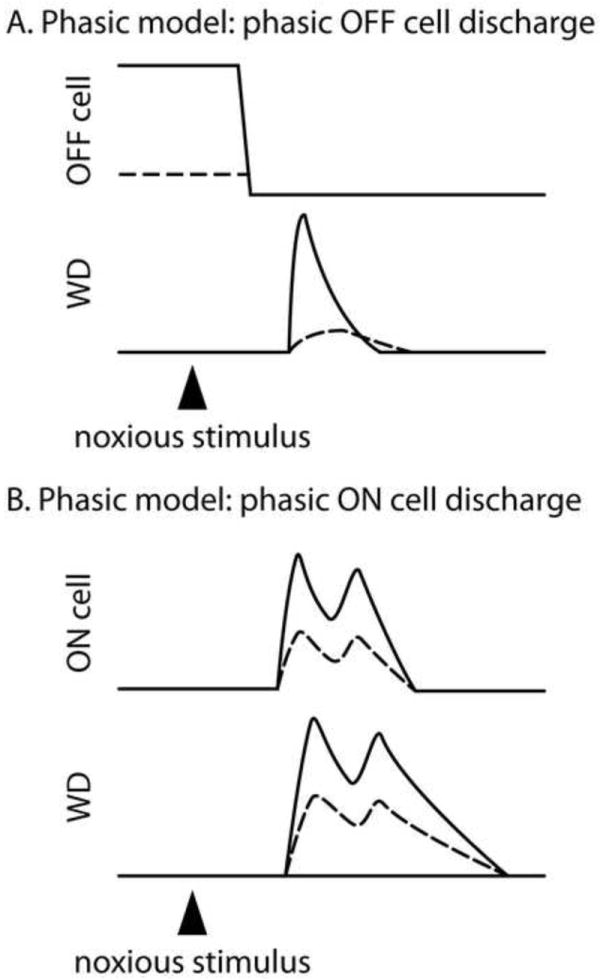
The phasic model proposes modulatory roles for the responses of on and off cells evoked by noxious stimulation. A: In the phasic model proposed here, the greater the magnitude of the decrease in off cell discharge (top trace) elicited by noxious stimulation, the greater the synchronization of the withdrawal, leading in turn to a larger withdrawal magnitude (bottom trace labeled WD). A large evoked decrease in off cell activity is associated with a rapidly rising, large magnitude withdrawal whereas a small evoked decrease in off cell activity is associated with a slowly rising, low magnitude withdrawal or no withdrawal at all (not shown). B: The on cell burst (top trace) evoked by noxious stimulation is proposed to sculpt the ensuing withdrawal, with larger bursts associated with larger magnitude withdrawals.
The on cell burst facilitates withdrawal magnitude and its absence is key to antinociception
Converging evidence suggests that the on cell burst in response to noxious stimulation positively sculpts motor withdrawals (Fig. 2B). In support of this idea, the envelope of the fully rectified electromyographic response closely resembles the envelope of the noxious stimulus-evoked on cell burst in both anesthetized and unanesthetized rodent [20••, 24]. Further, the peak magnitude of the EMG response correlates with the peak of the on cell burst [20••]. The attenuation of hyperalgesic withdrawals after dermorphin-saporin treatment of the RVM lends indirect support to the importance of on cell activation in facilitating nociceptive withdrawals (reviewed in 7). These findings suggest that on cells strongly facilitate motor withdrawals elicited by noxious stimulation.
It now appears that on cell facilitation of nociception is sufficiently effective that a reduction in on cell activity, even in the absence of off cell activation, can produce antinociception. For example, midbrain administration of the endocannabinoid palmitoylethanolamide (PEA) suppresses on cell discharge without activating off cells and causes a significant increase in the latency to the tail flick withdrawal; withdrawal magnitude was not measured in this study [25•]. Similarly, morphine administration to unanesthetized mice that resulted in antinociception suppressed on cell responses to noxious stimulation without producing tonic activation of off cells [20••]. In light of these results, it is particularly instructive to consider the sequelae resulting from microinjection of kynurenate into RVM. Kynurenate blocks the excitatory response of on cells to noxious stimulation and depresses on cell background activity but does not alter the background activity of off cells or the length of the off cell pause [26]. Notably kynurenate microinjection has no effect on tail flick latency. The finding that kynurenate has no effect on withdrawal onset is predicted by the absence of a change in off cell firing. The behavioral prediction stemming from kynurenate’s reduction in the on cell burst is a withdrawal of greatly reduced magnitude. Indeed, kynurenate microinjection greatly reduces movement magnitude in the stimulated limb and even more so in the other 3 limbs [27].
In conclusion, antinociception can result from the suppression of phasic rather than tonic on cell discharge. In support of this idea, morphine administration to unanesthetized rodents suppresses on cell bursts without either activating off cells or inhibiting on cells tonically [18, 20••]. The time course of PEA-induced antinociception matches that of the reduction in on cell burst and precedes the decrease in tonic on cell discharge [25•]. Thus, a reduction in the on cell burst is sufficient, by itself, to greatly reduce nociceptive responses.
The neurochemistry of on and off cell-mediated nociceptive modulation remains a challenge
The connectivity and neurotransmitters involved in the hypothesized off cell synchronization of dorsal horn circuits through disinhibition and on cell facilitation of those same circuits are unclear. Although serotonin is clearly an important player in spinal nociceptive modulation [3], neither on nor off cells contain serotonin [28-30]. Direct inhibition of dorsal horn neurons by off cells is certainly possible as most off cells contain GAD, the synthesizing enzyme for GABA [30]. Surprisingly, most on cells also contain GABA, a result which is harder to easily reconcile with the hypothesized facilitatory actions of on cells on dorsal horn neurons. Whereas net facilitation can result from disinhibition, the primary post-synaptic elements in the dorsal horn targeted by RVM cells are non-GABAergic dendrites, making on cell inhibition of an inhibitory interneuron unlikely [31•].
Implications of phasic rather than tonic RVM-mediated modulation
The data reviewed above suggest that RVM cells do not produce tonic states of nociceptive responsiveness but rather that they modulate nociceptive responsiveness if and when the need should arise. The restriction of RVM cells’ serving a nociceptive modulatory function to periods of injury means that RVM cells may perform other functions when no noxious stimulus is present. That on and off cells do contribute to functions beyond nociceptive modulation is supported by their discharge patterns. Even in the absence of intentional somatic stimulation and in the relatively constant conditions of light anesthesia, on and off cells discharge in bursts alternating with silent periods [32-33]. On and off cells in unanesthetized animals also discharge spontaneously in irregular patterns of bursting and silence [19, 34]. In fact without knowing the times of noxious stimulus application, discriminating between a stimulus-evoked change in discharge and a change in discharge that occurred “spontaneously” is impossible. Thus, inputs unrelated to nociception influence on and off cell discharge. Some of these non-nociceptive inputs are known; for example, blood pressure changes [35], sleep-wake state [19], active body movements [36-37], voiding [38], and eating [39] all influence RVM cell discharge. It is likely that additional non-nociceptive variables influence RVM cell discharge as well.
Despite their discharge being influenced by non-nociceptive inputs, RVM cells may have only one efferent function: nociceptive modulation. For example, the sleep-active discharge pattern exhibited by off cells [26] may act to modulate nociception in accordance with behavioral state rather than to modulate behavioral state per se. On the other hand, RVM cells may modulate non-nociceptive as well as nociceptive functions. For example, indirect evidence suggests that on cells facilitate the response to cold challenge by facilitating both tail vasoconstriction [40••] and thermogenesis from brown adipose tissue [41]. A major challenge for the future is a full understanding of how and if individual on and off cells influence nociceptive and non-nociceptive functions alike.
Highlights.
opioid analgesia depends on descending modulation from the medullary raphe
opioids produce analgesia without altering the tonic discharge of medullary neurons
normally, phasic neuronal responses of brainstem cells facilitate nociception
morphine produces analgesia by blocking the phasic responses of brainstem cells
brainstem cells may modulate non-nociceptive functions during pain-free periods
Acknowledgments
The author was supported by grants from National Institute of Drug Abuse (RO1 DA022978; R21 DA022429).
Footnotes
To a certain extent, this assertion suffers from the same uncertainty as does the question of the tree falling in the empty forest. In other words, asking whether a state of analgesia exists in the absence of noxious stimulation is akin to asking whether the aforementioned tree makes a sound when it falls.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
-
•
of special interest
-
••
of outstanding interest
- 1.Basbaum AI, Fields HL. Endogenous pain control mechanisms: review and hypothesis. Ann Neurol. 1978;4:451–62. doi: 10.1002/ana.410040511. [DOI] [PubMed] [Google Scholar]
- 2.Gebhart GF. Descending modulation of pain. Neurosci Biobehav Rev. 2004;27:729–37. doi: 10.1016/j.neubiorev.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 3.Ossipov MH, Dussor GO, Porreca F. Central modulation of pain. J Clin Invest. 2010;120:3779–87. doi: 10.1172/JCI43766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gilbert A-K, Franklin KBJ. The role of descending fibers from the rostral ventromedial medulla in opioid analgesia in rats. Eur J Pharmacol. 2002;449:75–84. doi: 10.1016/s0014-2999(02)01974-x. [DOI] [PubMed] [Google Scholar]
- 5.Fields HL, Bry J, Hentall I, Zorman G. The activity of neurons in the rostral medulla of the rat during withdrawal from noxious heat. J Neurosci. 1983;3:2545–2552. doi: 10.1523/JNEUROSCI.03-12-02545.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fields H. State-dependent opioid control of pain. Nat Rev Neurosci. 2004;5:565–75. doi: 10.1038/nrn1431. [DOI] [PubMed] [Google Scholar]
- 7.Vanegas H, Schaible HG. Descending control of persistent pain: inhibitory or facilitatory? Brain Res Rev. 2004;46:295–309. doi: 10.1016/j.brainresrev.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Barbaro N, Heinricher M, Fields H. Putative pain modulating neurons in the rostral ventral medulla: Reflex-related activity predicts effects of morphine. Brain Res. 1986;366:203–210. doi: 10.1016/0006-8993(86)91296-5. [DOI] [PubMed] [Google Scholar]
- 9.Fields HL, Vanegas H, Hentall ID, Zorman G. Evidence that disinhibition of brain stem neurones contributes to morphine analgesia. Nature. 1983;306:684–686. doi: 10.1038/306684a0. [DOI] [PubMed] [Google Scholar]
- 10.Fang F, Haws C, Drasner K, Williamson A, Fields H. Opioid peptides (DAGO-enkephalin, dynorphin A(1 13), BAM 22P) microinjected into the rat brainstem: comparison of their antinociceptive effect and their effect on neuronal firing in the rostral ventromedial medulla. Brain Res. 1989;501:116–128. doi: 10.1016/0006-8993(89)91033-0. [DOI] [PubMed] [Google Scholar]
- 11.Heinricher MM, Drasner K. Lumbar intrathecal morphine alters activity of putative nociceptive modulatory neurons in rostral ventromedial medulla. Brain Res. 1991;549:338–41. doi: 10.1016/0006-8993(91)90478-e. [DOI] [PubMed] [Google Scholar]
- 12.Heinricher M, Morgan M, Fields H. Direct and indirect actions of morphine on medullary neurons that modulate nociception. Neuroscience. 1992;48:533–543. doi: 10.1016/0306-4522(92)90400-v. [DOI] [PubMed] [Google Scholar]
- 13.Tortorici V, Aponte Y, Acevedo H, Nogueira L, Vanegas H. Tolerance to non-opioid analgesics in PAG involves unresponsiveness of medullary pain-modulating neurons in male rats. Eur J Neurosci. 2009;29:1188–1196. doi: 10.1111/j.1460-9568.2009.06678.x. [DOI] [PubMed] [Google Scholar]
- 14.Heinricher MM, Barbaro NM, Fields HL. Putative nociceptive modulating neurons in the rostral ventromedial medulla of the rat: firing of on- and off-cells is related to nociceptive responsiveness. Somatosens Mot Res. 1989;6:427–439. doi: 10.3109/08990228909144685. [DOI] [PubMed] [Google Scholar]
- 15.Bederson JB, Fields HL, Barbaro NM. Hyperalgesia during naloxone-precipitated withdrawal from morphine is associated with increased on-cell activity in the rostral ventromedial medulla. Somatosens Mot Res. 1990;7:185–203. doi: 10.3109/08990229009144706. [DOI] [PubMed] [Google Scholar]
- 16.Porreca F, Burgess SE, Gardell LR, Vanderah TW, Malan TP, Jr, Ossipov MH, Lappi DA, Lai J. Inhibition of neuropathic pain by selective ablation of brainstem medullary cells expressing the mu-opioid receptor. J Neurosci. 2001;21:5281–8. doi: 10.1523/JNEUROSCI.21-14-05281.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meng ID, Harasawa I, Lai J, Porreca F, Fields HL. Changes in rostral ventromedial medulla (RVM) neurons after the selective loss of mu-opioid receptor expressing cells. Neuroscience. 2002 Abstract 351.9. [Google Scholar]
- 18.Martin G, Montagne-Clavel J, Olivéras JL. Involvement of ventromedial medulla “multimodal, multireceptive” neurons in opiate spinal descending control system: a single-unit study of the effect of morphine in the awake, freely moving rat. J Neurosci. 1992;12:1511–1522. doi: 10.1523/JNEUROSCI.12-04-01511.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leung CG, Mason P. Physiological properties of raphe magnus neurons during sleep and waking. J Neurophysiol. 1999;81:584–595. doi: 10.1152/jn.1999.81.2.584. [DOI] [PubMed] [Google Scholar]
- 20••.Hellman KM, Mason P. Opioids disrupt pronociceptive modulation mediated by raphe magnus. J Neurosci. 2012 doi: 10.1523/JNEUROSCI.1551-12.2012. in press. In this electrophysiological study in unanesthetized mice, RVM cells were characterized by their responses to noxious stimulation and their activity recorded before and after an analgesic dose of morphine. The results suggest that both RVM on and off cells normally exert phasic pronociceptive effects which simply do not occur during opioid analgesia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hellman KM, Brink TS, Mason P. Activity of murine raphe magnus cells predicts tachypnea and on-going nociceptive responsiveness. J Neurophysiol. 2007;98:3121–3133. doi: 10.1152/jn.00904.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nusbaum MP, Beenhakker MP. A small-systems approach to motor pattern generation. Nature. 2002;417:343–350. doi: 10.1038/417343a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perkel DH, Mulloney B. Motor pattern production in reciprocally inhibitory neurons exhibiting postinhibitory rebound. Science. 1974;185:181–183. doi: 10.1126/science.185.4146.181. [DOI] [PubMed] [Google Scholar]
- 24.Jinks SL, Carstens E, Antognini JF. Isoflurane differentially modulates medullary on and off neurons while suppressing hind-limb motor withdrawals. Anesthesiology. 2004;100:1224–1234. doi: 10.1097/00000542-200405000-00026. [DOI] [PubMed] [Google Scholar]
- 25•.de Novellis V, Luongo L, Guida F, Cristino L, Palazzo E, Russo R, Marabese I, D’Agostino G, Calignano A, Rossi F, Di Marzo V, Maione S. Effects of intra-ventrolateral periaqueductal grey palmitoylethanolamide on thermoceptive threshold and rostral ventromedial medulla cell activity. Eur J Pharmacol. 2012;676:41–50. doi: 10.1016/j.ejphar.2011.11.034. This thorough electrophysiological study documents an example of decreased nociceptive responsiveness associated with a reduced on cell burst but not with an increase in the tonic discharge of off cells. [DOI] [PubMed] [Google Scholar]
- 26.Heinricher MM, McGaraughty S. Analysis of excitatory amino acid transmission within the rostral ventromedial medulla: implications for circuitry. Pain. 1998;75:247–55. doi: 10.1016/s0304-3959(97)00226-1. [DOI] [PubMed] [Google Scholar]
- 27.Jinks SL, Carstens EE, Antognini JF. Glutamate receptor blockade in the rostral ventromedial medulla reduces the force of multisegmental motor responses to supramaximal noxious stimuli. Neurosci Lett. 2007;426:175–80. doi: 10.1016/j.neulet.2007.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao K, Mason P. Serotonergic raphe magnus cells that respond to noxious tail heat are not ON or OFF cells. J Neurophysiol. 2000;84:1719–25. doi: 10.1152/jn.2000.84.4.1719. [DOI] [PubMed] [Google Scholar]
- 29.Potrebic SB, Fields HL, Mason P. Serotonin immunoreactivity is contained in one physiological cell class in the rat rostral ventromedial medulla. J Neurosci. 1994;14:1655–65. doi: 10.1523/JNEUROSCI.14-03-01655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Winkler CW, Hermes SM, Chavkin CI, Drake CT, Morrison SF, Aicher SA. Kappa opioid receptor (KOR) and GAD67 immunoreactivity are found in OFF and NEUTRAL cells in the rostral ventromedial medulla. J Neurophysiol. 2006;96:3465–73. doi: 10.1152/jn.00676.2006. [DOI] [PubMed] [Google Scholar]
- 31•.Aicher SA, Hermes SM, Whittier KL, Hegarty DM. Descending projections from the rostral ventromedial medulla (RVM) to trigeminal and spinal dorsal horns are morphologically and neurochemically distinct. J Chem Neuroanat. 2011 doi: 10.1016/j.jchemneu.2011.11.002. Epub ahead of print. This study provides critical data on the connection between RVM and the spinal dorsal horn, showing that GABAergic terminals from RVM neurons contact primarily non-GABAergic dendrites. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barbaro NM, Heinricher MM, Fields HL. Putative nociceptive modulatory neurons in the rostral ventromedial medulla of the rat display highly correlated firing patterns. Somatosens Mot Res. 1989;6:413–25. doi: 10.3109/08990228909144684. [DOI] [PubMed] [Google Scholar]
- 33.Leung CG, Mason P. Physiological survey of medullary raphe and magnocellular reticular neurons in the anesthetized rat. J Neurophysiol. 1998;80:1630–1646. doi: 10.1152/jn.1998.80.4.1630. [DOI] [PubMed] [Google Scholar]
- 34.Olivéras JL, Martin G, Montagne-Clavel J. Drastic changes of ventromedial medulla neuronal properties induced by barbiturate anesthesia. II. Modifications of the single-unit activity produced by Brevital, a short-acting barbiturate in the awake, freely moving rat. Brain Res. 1991;563:251–60. doi: 10.1016/0006-8993(91)91541-8. [DOI] [PubMed] [Google Scholar]
- 35.Leung CG, Mason P. Spectral analysis of arterial blood pressure and raphe magnus neuronal activity in anesthetized rats. Am J Physiol. 1996;271:R483–9. doi: 10.1152/ajpregu.1996.271.2.R483. [DOI] [PubMed] [Google Scholar]
- 36.Foo H, Mason P. Movement-related discharge of ventromedial medullary neurons. J Neurophysiol. 2005;93:873–83. doi: 10.1152/jn.00750.2004. [DOI] [PubMed] [Google Scholar]
- 37.Olivéras JL, Martin G, Montagne J, Vos B. Single unit activity at ventromedial medulla level in the awake, freely moving rat: effects of noxious heat and light tactile stimuli onto convergent neurons. Brain Res. 1990;506:19–30. doi: 10.1016/0006-8993(90)91194-l. [DOI] [PubMed] [Google Scholar]
- 38.Baez MA, Brink TS, Mason P. Roles for pain modulatory cells during micturition and continence. J Neurosci. 2005;25:384–394. doi: 10.1523/JNEUROSCI.3536-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Foo H, Mason P. Sensory suppression during feeding. Proc Natl Acad Sci USA. 2005;102:16865–16869. doi: 10.1073/pnas.0506226102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40••.Tanaka M, McKinley MJ, McAllen RM. Preoptic-raphé connections for thermoregulatory vasomotor control. J Neurosci. 2011;31:5078–88. doi: 10.1523/JNEUROSCI.6433-10.2011. This study demonstrates that preoptic neurons that respond to skin warming project to the medullary raphe and that bicuculline microinjection into the preoptic results in an increase in the activity of sympathetic nerves to the tail. Further, kynurenate microinjection into the raphe attenuates the tail vasoconstriction evoked by either skin cooling or preoptic activation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nason MW, Jr, Mason P. Medullary raphe neurons facilitate brown adipose tissue activation. J Neurosci. 2006;26:1190–8. doi: 10.1523/JNEUROSCI.4707-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]


