Abstract
A newly discovered B cell subset, Age Associated B Cells, expresses the transcription factor T-bet, has a unique surface phenotype, and progressively accumulates with age. Moreover, B cells with these general features are associated with viral infections and autoimmunity in both mice and humans. Here we review current understanding of the characteristics, origins, and functions of these cells. We also suggest that the protective versus pathogenic actions of these cells reflect appropriate versus aberrant engagement of regulatory mechanisms that control the antibody responses to nucleic acid containing antigens.
INTRODUCTION
Advancing age is accompanied by shifts in many qualitative and quantitative aspects of immune function. These changes, collectively termed immune senescence (1, 2), include blunted primary and memory immune responses, reduced vaccine efficacy, and increases in the prevalence of inflammatory and autoimmune pathologies (2–6). While the underlying mechanisms remain unclear, a growing literature documents contributions from age-associated changes at the systemic, molecular, and cellular levels. Systemically, serum and local concentrations of inflammatory cytokines are progressively elevated in both mice and humans, yielding an overall phenomenon described as “inflammaging” (7, 8). In addition, monoclonal gammopathies, as well as antibodies reactive with chromatin and dsDNA, frequently emerge with increasing age (9–12). Finally, with a few exceptions such as type 1 diabetes and juvenile rheumatoid arthritis, the frequency of autoimmune disease rises with age. These pathologies may reflect causal relationships with overall changes in the immune system, the cumulative impact of environmental insults, or combinations of these factors. Alternatively, some of these features may instead initiate in young individuals and stem from normal immune activity, but their pathogenic actions may only become manifest once the underlying effectors reach a minimum threshold with advancing age.
At a cellular level, the output of primary lymphoid organs wanes, reflecting a shift towards myeloid lineage preference in hematopoetic stem cell (HSC) specification (13, 14), reductions in key developmental gene expression (15–17), and altered microenvironmental and homeostatic feedback mechanisms (18, 19). Despite this dwindling lymphocyte production, total numbers of mature B and T cells remain relatively unchanged. Nevertheless, nearly all peripheral lymphoid pools exhibit altered dynamics, shifts in functional subset representation, and changes in clonal composition. Thus, the renewal rates of both T and B cell pools decline (15, 20, 21), in part explaining how overall numbers can be maintained in the absence of newly generated cells. Further, inordinate clonal expansions are observed in both T and B cell compartments. While some of these age-associated changes may result from immune dysregulation, others may simply reflect the cumulative influence of antigenic experiences and normal homeostatic processes. In accord with this notion, the ratio of T cells displaying naïve versus memory phenotype inverts with age. Similarly, a substantial shift in the composition of peripheral B cell pools accompanies advancing age, reflecting the gradual appearance of a novel B cell subset whose properties and origins are the focus of this review.
The emergence and characteristics of Age-associated B cells (ABCs)
Recently, our laboratories described a phenotypically and functionally unique B cell subset that accumulates with age that we have named Age Associated B cells (ABCs) (22, 23). These cells display a characteristic transcriptional profile, compete homeostatically with the naïve follicular (FO) and marginal zone (MZ) B cells, and bear hallmark features of antigen-experienced cells. ABCs are detected in the spleen, blood and bone marrow, but less frequently in the peritoneal cavity or lymph nodes. Detailed understanding of their locale relative to splenic follicles and MZs is lacking, but recently reported age-associated changes in the cells occupying MZs make these sites a potential candidate (24). Finally, ABCs are associated with appropriate humoral responses to certain classes of infectious and inflammatory stimuli, arise prematurely in autoimmune-prone mouse strains, and may be enriched for autoreactive antibody specificities (23, 25). The origins and roles of ABCs in normal immune responses, as well as in immune senescence and autoimmunity, remain areas of intense investigation.
While sharing many features, some heterogeneity exists among ABCs. Hao et al identified ABCs by the lack of both CD21 and CD23 expression (22). The frequency and the numbers of these B cells increased with the age, comprising as much as 30% of splenic B cells in 22 month old mice. Further phenotypic analysis of this CD23−/21− ABC population revealed that they differ from marginal zone (MZ), follicular (FO) or B1 B cells, and also showed that they express several markers shared with ‘exhausted’ memory B cells (26). Simultaneously, Rubstov et al (23) reported a population of CD11c+/CD11b+ B cells that appear in healthy aged female mice and in autoimmune-prone animals (23). These cells clearly overlapped with those reported by Hao et al, since they expressed low levels of CD21 and CD23 and elevated levels of CD5, Fas, CD138. However, in contrast to the more broadly defined cells described by Hao et al, the CD11c+/CD11b+ B cells described by Rubstov uniformly expressed high levels of the activation markers CD80, CD86 and MHCII. A comparison of surface markers among the ABCs defined by Hao et al. and Rubtsov et al. is shown in Table 1. Importantly, both groups found that ABCs accumulate with age and tend to arise earlier and more consistently in female mice. While this surface phenotype heterogeneity remains to be fully resolved, it likely reflects alternative routes of ABC generation.
Table 1.
A comparison of the expression of surface markers of mouse and human ABCs reported by Hao et al., Rubtsov et al. and exhausted human B cells.
| Hao at al. mice | Rubtsov et al. mice | Rubtsov et al. human | Exhausted B cells human | |
|---|---|---|---|---|
| CD19 | N/A | high | + | + |
| B220 | + | + | + | N/A |
| CD11c | +/− | + | + | + |
| CD11b | N/A | + | N/A | + |
| CD21 | neg | neg | neg | low |
| CD23 | neg | neg | neg | + |
| Fas | N/A | + | + | N/A |
| CD138 | N/A | int | N/A | low |
| CD5 | neg | int | + | N/A |
| CD80/86 | low | high | high | high |
| MHC II | low | high | N/A | N/A |
| T-bet | N/A | + | + | N/A |
| sIgM | + | +/− | neg | N/A |
| sIgD | low | +/− | neg | neg |
A key feature of ABCs is that they express and depend upon B-cell intrinsic expression of the transcription factor T-bet (25). Consistent with this notion, T-bet overexpression induces acquisition of the ABC phenotype (25), indicating it acts as a master regulator of ABC character. The exact mechanism whereby T-bet promotes and maintains the ABC phenotype remains unclear, but ongoing chromatin immunoprecipitation and deep sequencing studies will likely reveal both direct and indirect effects of T-bet on characteristic ABC gene expression patterns.
As might be anticipated from their unique T-bet driven transcriptional program, ABCs differ substantially from other B cell subsets in their activation requisites, functional capacities, and survival requirements. In contrast to FO or MZ B cells, ABCs survive but respond poorly to BCR engagement. However, they proliferate robustly to simulation with either TLR9 or TLR7 agonists, either alone or in combination with BCR ligation. Moreover, following TLR stimulation in vitro ABCs elaborate a unique spectrum of regulatory cytokines, with notably robust production of both IL10 and IFN-gamma. Recent in vivo studies have suggested that they are also an abundant source of TNF-alpha in vivo (27).
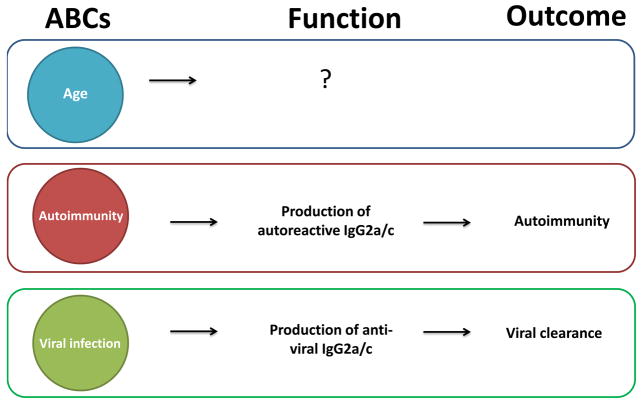
While most murine ABCs express IgM, they rapidly switch to IgG production after stimulation with TLR ligands (23, 25). Regardless of their source – autoimmunity, age or viral infection – ABCs are prone to IgG2a/c production (23, 25), consistent with the established role of T-bet in switching to this Ig heavy chain isotype (28–32). However, the specificity of the IgG produced by ABCs differs depending on their source; ABCs obtained from autoimmune or aged mice produce autoreactive IgG, whereas ABCs from virally infected mice produce predominantly anti-viral IgG (Figure 2) (23, 25). Together, these observations imply involvement of BCR signaling during the differentiation and recruitment of B cells into ABC subset, despite their apparently damped response to BCR ligation alone.
Figure 2. ABCs in age, autoimmunity and infection.
Function and the outcome of ABCs appearance in aged animals is still unknown. In autoimmune animals, ABCs produce high titers of autoantibodies (mostly of IgG2a/c isotype) upon stimulation, which may be the cause of autoimmunity. During the infection, ABCs produce anti-viral IgG (mostly IgG2a/c), which is required for the efficient viral clearance.
In addition to antibody secretion, ABCs can also serve as antigen presenters and, following activation they can produce regulatory cytokines capable of skewing the differentiation of other adaptive and innate cell subsets. For example, early studies showed that ABCs obtained from aged animals can present antigen and tend to induce Th17 polarization (22). More recent findings extend these ideas and also suggest that ABCs obtained from aged or autoimmune mice process and present antigen more efficiently that other B cells (33).
The accumulation of ABCs has profound effects on the dynamics and homeostasis of peripheral B cell pools. Interestingly, ABCs express the canonical BAFF receptors BR3 and TACI, but unlike FO and MZ B cells, they do not rely on BAFF for survival. Thus, as ABCs accumulate they engender reciprocal decreases in FO B cell numbers through competition for BAFF (22). Moreover, recent studies from the Riley laboratory suggest that ABCs negatively influence B lineage commitment or development of bone marrow progenitors, implying a causal role for ABCs in the decline of B cell lymphopoiesis with age (27). These observations may bear on reports that B lymphocyte ablation can rejuvenate B lymphopoiesis in aged individuals (34), in as much as ABCs do not reappear quickly during self-reconstitution.
It is tempting to speculate that the progressive dominance of ABCs at the expense of FO B cells impacts adaptive humoral responses, and a growing body of evidence suggests this may be the case. For example, adoptive transfer experiments have shown that multiple aspects of TFH differentiation – including those that depend upon B cell antigen presentation such as the upregulation of IL4 and IL21 production – are profoundly compromised in aged mice, regardless of T cell donor age (35, 36). Thus, the outcome of cognate presentation by ABCs may differ from other antigen presenting cells, failing to reinforce the TFH program or directing pre-TFH cells to alternative effector fates. In agreement with this idea, ABC presenters skew primed T cells to a TH17 fate in vitro (22).
ABC generation in health and disease
ABCs probably arise from activation-driven differentiation. Early work ruled out the possibility that ABCs represent the product of B cell genesis in the aged microenvironment, since they do not reappear after irradiation and autoreconstitution. Instead, multiple lines of evidence now suggest that they are a normal differentiative alternative taken by naïve B cells when responding to certain classes of exogenous and endogenous stimuli. Initial evidence that ABCs can arise from naïve B cells was suggested by experiments in which FO B cells from young donors were transferred to replete young or old congenic hosts. One month later, the recovered donor cells that had undergone extensive division had adopted an ABC phenotype, regardless of host age (22). Although these findings showed that ABC-like cells could be derived from quiescent pre-immune B cells, the activating stimuli were unclear and the paucity of recovered cells prevented detailed functional analyses.
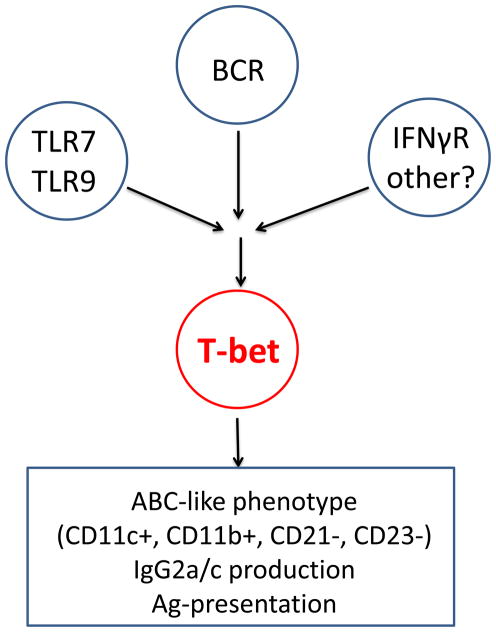
The early descriptions of ABCs also indicated that TLR7 and MyD88, but not IFNαR, were required for the accumulation of ABCs (23), consistent with their being derived from events driven by activating receptors. Subsequent in vitro analyses revealed that T-bet upregulation – the hallmark of ABC generation – was most effectively induced by concomitant receipt of BCR ligation, TLR7 stimulation, and IFN-gamma (25) (Figure 1).
Figure 1. Model for T-bet induction in B cells and its role in B cell fate.
Synergistic signaling via B cell antigen receptor (BCR), Toll-like receptor 7 (TLR7), and IFNγR in B cells leads to the induction of high levels of T-bet expression, which in turn drive the expression of an ABC phenotype and class switching to the production of IgG2a antibodies.
Taken together, these observations suggest ABCs originate under circumstances involving antigens that can not only engage the BCR, but that also contain ligands for endosomal nucleic acid sensors and induce a promoting cytokine milieu. If this is the case, then ABCs would be expected to arise both during normal adaptive responses to microbial pathogens, as well as during potentially autoreactive responses to self components, so long as this tripartite set of conditions is established. Indeed, several lines of evidence now indicate that ABCs arise and play key roles in both situations, providing clues about their emergence with age and connection with humoral autoimmunity.
ABCs in infection and immunity
B cells closely resembling ABCs arise during anti-viral immune responses (25). These T-bet+ CD11c+ B cells appear at the peak of the humoral immune response during infection with mouse gammaherpesvirus 68, mouse cytomegalovirus, lymphocyte choriomeningitis virus, and vaccinia. B cells with very similar phenotypic and functional characteristics have also been recently described in Ehrlichia muris infection (37). Importantly, ABCs derived during these responses secrete virus-specific IgG upon restimulation in vitro more efficiently than FO B cells from the same host, indicating recruitment of antigen-specific B cells into the ABC pool, rather than nonspecific enlargement of a bystander ABC pool. Further, ABC differentiation is a critical element of the successful immune response to viral infection. Mixed bone marrow chimeras in which the B cell compartment was T-bet deficient and unable to initiate ABC differentiation displayed dramatically reduced viral-specific IgG2a/c titers, less efficient viral clearance, and higher viral burden (Figure 2) (25). This is in agreement with prior studies indicating that IgG2a/c most effectively drives viral clearance due to its efficiency in ADCC and high affinity for activating Fc receptors (38–41).
These findings also strengthen the idea that ABCs arise via BCR-mediated activation in the context of TLR stimulation and appropriate cytokine milieus; BCR engagement will afford virus uptake and trafficking to endosomal nucleic acid sensors, while NK cells and T cells secrete abundant IFNγ in response to the virus and provide the appropriate cytokine microenvironment (Figure 1).
ABCs in autoimmunity
B cells phenotypically similar to ABCs also appear in young autoimmune-prone mice (42). Moreover, the appearance of ABCs is correlated with disease onset in several murine lupus models including MRLlpr, NZBxWF1, MER−/−, and BXSB.
The potential relevance of ABCs to human autoimmunity has been tested by screening human PBMC obtained from either healthy of autoimmune donors for the presence of a similar B cell subset. The results show that PBMCs from donors with some autoimmune diseases contained a high percentage of CD11c+/CD21− B cells. In addition, these human ABC-like cells, similar their murine counterparts, expressed low levels of CD23 and high levels of CD5 and CD86. However, unlike murine ABCs, the human ABC equivalents were isotype switched (Table 1) (23). Others have observed a similar B cell subset in the peripheral blood of autoimmune patients, but in these studies the cells were identified as CD19hi/CD21low (43–46). In concert with the more pronounced and reliable emergence of ABCs in female mice, these findings in toto may provide clues as to why some autoimmune diseases e more frequent in females.
B cells with similar phenotype have been described in HIV-viremic individuals (47) and where identified as FCRL4-expressing exhausted-like B cells. Moir et al. has reported FCRL-4 expressing B cells to have low levels of CD21 and high CD11c expression (refer to Table 1 for the comparison of exhausted B cells and ABCs). Since FCRL-4 expressing B cells (similar to ABCs) express CD11c and CXCR3, authors suggest that this B cell subset is similar to exhausted T cells (48) and can be driven by the persistent viral infection.
The exact combination of events that promote self-reactive ABCs in autoimmune prone individuals remains unclear. It is tempting to speculate that autoantigen specific B cells will engage and internalize autoantigens via their BCRs and, if these are chromatin or ribonuclear particles, will ligate endosomal TLRs. The third requisite for ABC generation – INFγ or other promoting cytokines - may be derived from TLR7 engagement in NK cells, or from bystander TH1 cells. It is noteworthy that TLR7 and IFNγR signaling are well-established factors in the etiology of humoral autoimmunity (49–55).
Support for this overall model comes from mixed bone marrow chimeras in which Mer−/− mice, which lack receptors for effective clearance of apoptotic debris, were reconstituted ABCs could be depleted by diphtheria toxin (23). Notably, ABC depletion reduced autoantibody titers in these animals (23). Also consistent with this idea, TLR7 deficiency in either MER−/− or Nba2 mice led to the absence of ABCs and significant reductions in autoantibody titers (Figure 2) (42). While these findings all suggest a role for ABCs in humoral autoimmunity, further work will be required to fully reveal underlying causal associations.
ABCs accumulate with age
While discovery of ABCs arose from studies in aged and autoimmune-prone mice, emerging findings suggest that this unique B cell subset reflects chronic or repeated exposures to stimuli that prompt a T-bet centered transcriptional program, and that these cells progressively accumulate throughout life, eventually displacing a substantial proportion of the preimmune B cell pool with advancing age. In this context, ABCs may represent a specialize memory B cell subset directed towards chonic or endogenous pathogenic microbes. They might also be the product of B cells that react with nucleic-acid containing autoantigens that, under normal circumstances are beneficial for “housekeeping” roles such as the clearance of apoptotic debris. However, under circumstances where inflammatory cytokines are persistently elevated – such as in advancing age – they might expand beyond normal homeostatic limits. These possibilities are not mutually exclusive, and are amenable to experimental interrogation.
However, it is not clear why the appearance of ABCs is gender-biased in aged animals. Sex hormones might contribute, but there is presently no evidence to support this idea. The X-linked Tlr7 gene might also be involved, since some regions on the lyonized chromosome can escape inactivation and yield to the overexpression of some X-linked genes (30). If Tlr7 is among these, at least in some cells, it might lead to consistently increased numbers of ABCs in females with age.
Conclusions
Current findings in toto suggest ABCs are antigen-experienced B cells that are characterized by a T-bet driven transcriptional program. Moreover, they play dichotomous roles in health and disease. ABCs are essential for effective immune responses against certain classes of infectious agents, likely reflecting the need for key effector functions mediated by IgG2a/c and inflammatory cytokines. Conversely, the sustained accumulation of ABCs can also have detrimental effects, including propensity for autoinflammatory and autoimmune pathologies. Based on the requisite for endosomal TLRs in ABC generation and activation, these seemingly paradoxical outcomes may reflect intricacies of the regulatory mechanisms that have evolved to control antibody response to nucleic acid containing antigens. Obviously, sensing pathogen-derived intracellular nucleic acids is critical to inducing immune effectors that eliminate or control such infections. We hypothesize that ABCs evolved, as a product of a specific set of B cell activating signals via BCR, TLR7, and IFNγR. Therefore we hypothesize that ABCs represent the stage of B cell activation, or a differentiated effector stage. We also suggest that ABCs are a transient stage of B cell activation and upon further TLR7 triggering they differentiate into antibody secreting plasma cells.
We also suggest that evolution selected for this pathway, components of which are so evident in virus infections, because it leads to the production of ABCs and effective anti-viral humoral immunity. Such a pathway is essential to health. However, the same mechanism can be triggered during autoimmune reactions in response to self-antigen and thus, in rare individuals causes damaging disease. Accordingly, interrogating the mechanisms that control ABC formation, activity and persistence may reveal targets for intervention in both microbial pathogenesis and autoinflammatory diseases.
Acknowledgments
Financial support
This work was supported by USPHS grants AI-18785, T32 AI074491 (K.R), and R01AG-16841, CDMRP PR130769 (MPC).
References
- 1.Ben-Yehuda A, Weksler ME. Immune senescence: mechanisms and clinical implications. Cancer investigation. 1992;10:525–531. doi: 10.3109/07357909209024815. [DOI] [PubMed] [Google Scholar]
- 2.Pawelec G. Immunosenescence and human longevity. Biogerontology. 2003;4:167–170. doi: 10.1023/a:1024185405946. [DOI] [PubMed] [Google Scholar]
- 3.Miller RA. The aging immune system: primer and prospectus. Science. 1996;273:70–74. doi: 10.1126/science.273.5271.70. [DOI] [PubMed] [Google Scholar]
- 4.Doria G, Frasca D. Genes, immunity, and senescence: looking for a link. Immunological reviews. 1997;160:159–170. doi: 10.1111/j.1600-065x.1997.tb01036.x. [DOI] [PubMed] [Google Scholar]
- 5.Franceschi C, Passeri M, De Benedictis G, Motta L. Immunosenescence. Aging. 1998;10:153–154. [PubMed] [Google Scholar]
- 6.Johnson SA, Cambier JC. Ageing, autoimmunity and arthritis: senescence of the B cell compartment - implications for humoral immunity. Arthritis research & therapy. 2004;6:131–139. doi: 10.1186/ar1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franceschi C, Bonafe M. Centenarians as a model for healthy aging. Biochemical Society transactions. 2003;31:457–461. doi: 10.1042/bst0310457. [DOI] [PubMed] [Google Scholar]
- 8.Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. The journals of gerontology Series A, Biological sciences and medical sciences. 2014;69(Suppl 1):S4–9. doi: 10.1093/gerona/glu057. [DOI] [PubMed] [Google Scholar]
- 9.Dolcetti R, Boiocchi M. Cellular and molecular bases of B-cell clonal expansions. Clin Exp Rheumatol. 1996;14(Suppl 14):S3–13. [PubMed] [Google Scholar]
- 10.LeMaoult J, Delassus S, Dyall R, Nikolic-Zugic J, Kourilsky P, Weksler ME. Clonal expansions of B lymphocytes in old mice. Journal of immunology. 1997;159:3866–3874. [PubMed] [Google Scholar]
- 11.LeMaoult J, Messaoudi I, Manavalan JS, Potvin H, Nikolich-Zugich D, Dyall R, Szabo P, Weksler ME, Nikolich-Zugich J. Age-related dysregulation in CD8 T cell homeostasis: kinetics of a diversity loss. Journal of immunology. 2000;165:2367–2373. doi: 10.4049/jimmunol.165.5.2367. [DOI] [PubMed] [Google Scholar]
- 12.Eaton-Bassiri AS, Mandik-Nayak L, Seo SJ, Madaio MP, Cancro MP, Erikson J. Alterations in splenic architecture and the localization of anti-double-stranded DNA B cells in aged mice. International immunology. 2000;12:915–926. doi: 10.1093/intimm/12.6.915. [DOI] [PubMed] [Google Scholar]
- 13.Zediak VP, Maillard I, Bhandoola A. Multiple prethymic defects underlie age-related loss of T progenitor competence. Blood. 2007;110:1161–1167. doi: 10.1182/blood-2007-01-071605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller JP, Allman D. The decline in B lymphopoiesis in aged mice reflects loss of very early B-lineage precursors. Journal of immunology. 2003;171:2326–2330. doi: 10.4049/jimmunol.171.5.2326. [DOI] [PubMed] [Google Scholar]
- 15.Labrie JE, 3rd, Sah AP, Allman DM, Cancro MP, Gerstein RM. Bone marrow microenvironmental changes underlie reduced RAG-mediated recombination and B cell generation in aged mice. The Journal of experimental medicine. 2004;200:411–423. doi: 10.1084/jem.20040845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sherwood EM, Blomberg BB, Xu W, Warner CA, Riley RL. Senescent BALB/c mice exhibit decreased expression of lambda5 surrogate light chains and reduced development within the pre-B cell compartment. Journal of immunology. 1998;161:4472–4475. [PubMed] [Google Scholar]
- 17.Frasca D, Van Der Put E, Riley RL, Blomberg BB. Age-related differences in the E2A-encoded transcription factor E47 in bone marrow-derived B cell precursors and in splenic B cells. Exp Gerontol. 2004;39:481–489. doi: 10.1016/j.exger.2003.09.027. [DOI] [PubMed] [Google Scholar]
- 18.Stephan RP, Lill-Elghanian DA, Witte PL. Development of B cells in aged mice: decline in the ability of pro-B cells to respond to IL-7 but not to other growth factors. Journal of immunology. 1997;158:1598–1609. [PubMed] [Google Scholar]
- 19.Stephan RP, V, Sanders M, Witte PL. Stage-specific alterations in murine B lymphopoiesis with age. International immunology. 1996;8:509–518. doi: 10.1093/intimm/8.4.509. [DOI] [PubMed] [Google Scholar]
- 20.Kline GH, Hayden TA, Klinman NR. B cell maintenance in aged mice reflects both increased B cell longevity and decreased B cell generation. Journal of immunology. 1999;162:3342–3349. [PubMed] [Google Scholar]
- 21.Tsukamoto H, Clise-Dwyer K, Huston GE, Duso DK, Buck AL, Johnson LL, Haynes L, Swain SL. Age-associated increase in lifespan of naive CD4 T cells contributes to T-cell homeostasis but facilitates development of functional defects. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:18333–18338. doi: 10.1073/pnas.0910139106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hao Y, O’Neill P, Naradikian MS, Scholz JL, Cancro MP. A B-cell subset uniquely responsive to innate stimuli accumulates in aged mice. Blood. 2011;118:1294–1304. doi: 10.1182/blood-2011-01-330530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rubtsov AV, Rubtsova K, Fischer A, Meehan RT, Gillis JZ, Kappler JW, Marrack P. Toll-like receptor 7 (TLR7)-driven accumulation of a novel CD11c B-cell population is important for the development of autoimmunity. Blood. 2011;118:1305–1315. doi: 10.1182/blood-2011-01-331462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Birjandi SZ, Ippolito JA, Ramadorai AK, Witte PL. Alterations in marginal zone macrophages and marginal zone B cells in old mice. Journal of immunology. 2011;186:3441–3451. doi: 10.4049/jimmunol.1001271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rubtsova K, Rubtsov AV, van Dyk LF, Kappler JW, Marrack P. T-box transcription factor T-bet, a key player in a unique type of B-cell activation essential for effective viral clearance. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:E3216–3224. doi: 10.1073/pnas.1312348110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ehrhardt GR, Hijikata A, Kitamura H, Ohara O, Wang JY, Cooper MD. Discriminating gene expression profiles of memory B cell subpopulations. The Journal of experimental medicine. 2008;205:1807–1817. doi: 10.1084/jem.20072682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ratliff M, Alter S, Frasca D, Blomberg BB, Riley RL. In senescence, age-associated B cells secrete TNFalpha and inhibit survival of B-cell precursors. Aging cell. 2013;12:303–311. doi: 10.1111/acel.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gerth AJ, Lin L, Peng SL. T-bet regulates T-independent IgG2a class switching. International immunology. 2003;15:937–944. doi: 10.1093/intimm/dxg093. [DOI] [PubMed] [Google Scholar]
- 29.Ehlers M, Fukuyama H, McGaha TL, Aderem A, Ravetch JV. TLR9/MyD88 signaling is required for class switching to pathogenic IgG2a and 2b autoantibodies in SLE. The Journal of experimental medicine. 2006;203:553–561. doi: 10.1084/jem.20052438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carrel L, Willard HF. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature. 2005;434:400–404. doi: 10.1038/nature03479. [DOI] [PubMed] [Google Scholar]
- 31.Wang NS, McHeyzer-Williams LJ, Okitsu SL, Burris TP, Reiner SL, McHeyzer-Williams MG. Divergent transcriptional programming of class-specific B cell memory by T-bet and RORalpha. Nature immunology. 2012;13:604–611. doi: 10.1038/ni.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peng SL, Szabo SJ, Glimcher LH. T-bet regulates IgG class switching and pathogenic autoantibody production. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:5545–5550. doi: 10.1073/pnas.082114899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rubtsov AV, Rubtsova K, Kappler JW, Jacobelli J, Friedman RS, Marrack P. CD11c-Expressing B Cells Are Located at the T Cell/B Cell Border in Spleen and Are Potent APCs. Journal of immunology. 2015 doi: 10.4049/jimmunol.1500055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keren Z, Naor S, Nussbaum S, Golan K, Itkin T, Sasaki Y, Schmidt-Supprian M, Lapidot T, Melamed D. B-cell depletion reactivates B lymphopoiesis in the BM and rejuvenates the B lineage in aging. Blood. 2011;117:3104–3112. doi: 10.1182/blood-2010-09-307983. [DOI] [PubMed] [Google Scholar]
- 35.Goenka R, Scholz JL, Naradikian MS, Cancro MP. Memory B cells form in aged mice despite impaired affinity maturation and germinal center kinetics. Exp Gerontol. 2014;54:109–115. doi: 10.1016/j.exger.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lefebvre JS, Maue AC, Eaton SM, Lanthier PA, Tighe M, Haynes L. The aged microenvironment contributes to the age-related functional defects of CD4 T cells in mice. Aging cell. 2012;11:732–740. doi: 10.1111/j.1474-9726.2012.00836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yates JL, Racine R, McBride KM, Winslow GM. T cell-dependent IgM memory B cells generated during bacterial infection are required for IgG responses to antigen challenge. Journal of immunology. 2013;191:1240–1249. doi: 10.4049/jimmunol.1300062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kipps TJ, Parham P, Punt J, Herzenberg LA. Importance of immunoglobulin isotype in human antibody-dependent, cell-mediated cytotoxicity directed by murine monoclonal antibodies. The Journal of experimental medicine. 1985;161:1–17. doi: 10.1084/jem.161.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coutelier JP, van der Logt JT, Heessen FW, Vink A, van Snick J. Virally induced modulation of murine IgG antibody subclasses. The Journal of experimental medicine. 1988;168:2373–2378. doi: 10.1084/jem.168.6.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nimmerjahn F, Bruhns P, Horiuchi K, Ravetch JV. FcgammaRIV: a novel FcR with distinct IgG subclass specificity. Immunity. 2005;23:41–51. doi: 10.1016/j.immuni.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 41.Markine-Goriaynoff D, Coutelier JP. Increased efficacy of the immunoglobulin G2a subclass in antibody-mediated protection against lactate dehydrogenase-elevating virus-induced polioencephalomyelitis revealed with switch mutants. Journal of virology. 2002;76:432–435. doi: 10.1128/JVI.76.1.432-435.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Larson JD, Thurman JM, Rubtsov AV, Claypool D, Marrack P, van Dyk LF, Torres RM, Pelanda R. Murine gammaherpesvirus 68 infection protects lupus-prone mice from the development of autoimmunity. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E1092–1100. doi: 10.1073/pnas.1203019109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rakhmanov M, Keller B, Gutenberger S, Foerster C, Hoenig M, Driessen G, van der Burg M, van Dongen JJ, Wiech E, Visentini M, Quinti I, Prasse A, Voelxen N, Salzer U, Goldacker S, Fisch P, Eibel H, Schwarz K, Peter HH, Warnatz K. Circulating CD21low B cells in common variable immunodeficiency resemble tissue homing, innate-like B cells. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:13451–13456. doi: 10.1073/pnas.0901984106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Isnardi I, Ng YS, Menard L, Meyers G, Saadoun D, Srdanovic I, Samuels J, Berman J, Buckner JH, Cunningham-Rundles C, Meffre E. Complement receptor 2/CD21− human naive B cells contain mostly autoreactive unresponsive clones. Blood. 2010;115:5026–5036. doi: 10.1182/blood-2009-09-243071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Warnatz K, Wehr C, Drager R, Schmidt S, Eibel H, Schlesier M, Peter HH. Expansion of CD19(hi)CD21(lo/neg) B cells in common variable immunodeficiency (CVID) patients with autoimmune cytopenia. Immunobiology. 2002;206:502–513. doi: 10.1078/0171-2985-00198. [DOI] [PubMed] [Google Scholar]
- 46.Wehr C, Eibel H, Masilamani M, Illges H, Schlesier M, Peter HH, Warnatz K. A new CD21low B cell population in the peripheral blood of patients with SLE. Clinical immunology. 2004;113:161–171. doi: 10.1016/j.clim.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 47.Moir S, Ho J, Malaspina A, Wang W, DiPoto AC, O’Shea MA, Roby G, Kottilil S, Arthos J, Proschan MA, Chun TW, Fauci AS. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. The Journal of experimental medicine. 2008;205:1797–1805. doi: 10.1084/jem.20072683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, Subramaniam S, Blattman JN, Barber DL, Ahmed R. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 49.Giltiay NV, Chappell CP, Sun X, Kolhatkar N, Teal TH, Wiedeman AE, Kim J, Tanaka L, Buechler MB, Hamerman JA, Imanishi-Kari T, Clark EA, Elkon KB. Overexpression of TLR7 promotes cell-intrinsic expansion and autoantibody production by transitional T1 B cells. The Journal of experimental medicine. 2013 doi: 10.1084/jem.20122798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Allman D, Pillai S. Peripheral B cell subsets. Current opinion in immunology. 2008;20:149–157. doi: 10.1016/j.coi.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leadbetter EA, I, Rifkin R, Hohlbaum AM, Beaudette BC, Shlomchik MJ, Marshak-Rothstein A. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416:603–607. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- 52.Jacob CO, van der Meide PH, McDevitt HO. In vivo treatment of (NZB X NZW)F1 lupus-like nephritis with monoclonal antibody to gamma interferon. The Journal of experimental medicine. 1987;166:798–803. doi: 10.1084/jem.166.3.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Balomenos D, Rumold R, Theofilopoulos AN. Interferon-gamma is required for lupus-like disease and lymphoaccumulation in MRL-lpr mice. The Journal of clinical investigation. 1998;101:364–371. doi: 10.1172/JCI750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ytterberg SR, Schnitzer TJ. Serum interferon levels in patients with systemic lupus erythematosus. Arthritis and rheumatism. 1982;25:401–406. doi: 10.1002/art.1780250407. [DOI] [PubMed] [Google Scholar]
- 55.Christensen SR, Shupe J, Nickerson K, Kashgarian M, Flavell RA, Shlomchik MJ. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity. 2006;25:417–428. doi: 10.1016/j.immuni.2006.07.013. [DOI] [PubMed] [Google Scholar]