Abstract
Purpose/Objectives
To describe the development and feasibility of a protocol for nonpsychiatric subspecialty research staff members to screen research participants who endorse suicidal ideations or behaviors during data collection.
Design
Descriptive protocol development.
Setting
The Children’s Hospital of Philadelphia and the University of Pennsylvania.
Sample
186 mother caregivers and 134 adolescent or young adult survivors of childhood brain tumors, with the protocol implemented for 5 caregivers and 11 survivors.
Methods
During telephone- and home-based interviews, the interviewer assessed the participant using the Columbia-Suicide Severity Rating Scale (C-SSRS).
Main Research Variables
Expressed suicidal ideation or behavior.
Findings
Implementation of the C-SSRS by nonpsychiatric subspecialty staff members was feasible and valid. Interviewers’ conclusions based on this instrument matched those of the mental health professional who followed up with participants. Process notes contained themes about the participants, including anger and sadness in survivors and the physical and emotional demands of the survivor in caregivers. Progress notes for the interviewer included a reiteration of events, whether the assessment was successful, and whether the recommendation of the interviewer was in agreement with that of the mental health professional.
Conclusions
The protocol based on the C-SSRS was useful and feasible for nonpsychiatric subspecialty staff members to use in the collection of data from survivors of childhood brain tumors and their caregivers.
Implications for Nursing
Survivors of childhood brain tumors and their caregivers may experience psychosocial distress. Nurses, as research assistants or in other roles, can use tools such as the C-SSRS to assist in front-line assessments.
Keywords: suicidal ideation, suicide, brain neoplasms, survivors
Suicidal ideation and behavior assessments have been studied in many different cancer populations at diagnosis and during therapy (Anguiano, Mayer, Piven, & Rosenstein, 2012; Bjorkenstam, Edberg, Ayoubi, & Rosen, 2005; Filiberti & Ripamonti, 2002; Hem, Loge, Haldorsen, & Ekeberg, 2004). However, they have been studied less frequently in pediatric populations and long-term survivors of cancer (Recklitis et al., 2010), including survivors of childhood brain tumors (Brinkman et al., 2013). Compared to survivors of other childhood cancers, survivors of childhood brain tumors have an increased risk of suicidal ideation (Recklitis et al., 2010). Comparable rates (12%) of suicidal ideation were found in a separate sample of adult survivors of childhood brain tumors (Brinkman et al., 2013). These rates are higher than the 12-month suicidal ideation prevalence in adults (4%) in the general population of the United States (Crosby, Han, Ortega, Parks, & Gfroerer, 2011).
Several submissions to the U.S. Food and Drug Administration (FDA) demonstrated significant variation in the methods used to report suicidal thoughts and behaviors during traditional interviews by clinicians supervising antidepressant clinical trials. These findings challenge the validity of the clinical interview as the gold standard for the assessment of suicidal ideation in the clinical trial setting. The presence of variations in reporting methods supported the need to improve the evaluation of suicidal thoughts to detect small fluctuations that may be related to treatment efforts (Posner et al., 2011; Zimmerman, Chelminski, & Posternak, 2004, 2005). Suicidal ideation was reported in clinical trials of pharmaceuticals that were not being tested for the treatment of psychiatric disorders. To prevent this untoward event, effective assessment was merited (FDA, 2012).
The specific assessment of suicidal ideation and behavior during the course of research was not required until the 2000s, when the FDA began the requirement in antidepressant drug trials involving adolescents. Because of the increasing survival rates of once-fatal childhood health conditions, particularly those that may involve neurocognitive changes like brain tumors (Howlader et al., 2013), investigators need appropriate, feasible assessments that will meet the ethical requirements of grant agencies and institutional review boards (IRBs). In addition, initial assessments that can be used by research staff members without formal psychiatric assessment training are needed.
This research project, protocol development, and report was approached with a family focus; families are dynamic, and the chronic, health-related concerns of one member can affect all members. The purpose of this study is to discuss the incorporation of suicidal ideation and behavior assessments in research on adolescent and young adult (AYA) survivors of childhood brain tumors and their mother caregivers. This includes the development of a protocol to be followed in the event that a participant expresses suicidal ideations or behaviors and a discussion of how this protocol was adapted for in-home data collection. How this relates to human participant issues in research also is discussed.
Background
Children who survive brain tumors often experience decades-long sequelae. As more children survive this disease and its treatment (the five-year survival rate is about 75% [Howlader et al., 2013]), pediatric, family, and adult practitioners will encounter more survivors of childhood brain tumors and their caregivers. This population may be at risk for developing post- traumatic stress symptoms (Bruce, Gumley, Isham, Fearon, & Phipps, 2011; Kazak et al., 2004), which can include suicidal ideation and behavior (Krysinska & Lester, 2010). The term suicidality clusters the suicidal events of ideation, self-injurious behavior, suicide attempts, and (completed) suicide. Meyer et al. (2010) suggested abandoning the term suicidality and creating operational definitions for the component parts. Although Meyer et al. (2010) did not present definitions, they did suggest measurement strategies, including continuous measurement for suicidal ideation and “time to event” measurement for suicidal behaviors.
The most recent statistics from the Centers for Disease Control and Prevention (CDC) regarding self-inflicted injuries in the United States state that 494,169 nonfatal injuries (one every minute) occurred in 2013, and 40,600 self-inflicted injuries resulting in death (one every 13 minutes) occurred in 2012 (CDC, 2014). Although it is ranked as the 10th-leading cause of death in any given lifespan, suicide is the second-leading cause of death in people aged 15–34 years, accounting for more deaths than those caused by malignant neoplasms (CDC, 2014).
Using data from the Childhood Cancer Survivor Study, Recklitis et al. (2010) identified several risk factors for suicidal ideation following childhood cancer, including diseases of the central nervous system, lower levels of education, a lower household income, a lack of recent employment, an unmarried status, an early age at diagnosis, and a poor health rating. Treatment, including brain irradiation and surgery, also increases a patient’s risk of experiencing psychological distress (Zeltzer et al., 2009). AYA survivors of childhood brain tumors often are exposed to these risk factors and report dysfunction in multiple domains as compared to survivors of other childhood cancers (Ellenberg et al., 2009; Maunsell, Pogany, Barrera, Shaw, & Speechley, 2006; Ness et al., 2010; Speechley, Barrera, Shaw, Morrison, & Maunsell, 2006). They also have a lower likelihood of living independently (Kunin-Batson et al., 2011). Bruce et al. (2011) found that 35% of short-term (0.5–7 years after treatment) survivors of childhood brain tumors experienced post-traumatic stress symptoms. Given the essential nature of continued cancer research, attending to these psychological concerns in research samples is important.
Psychological Distress in Research
Psychological distress can arise during the course of any study, particularly when the participating population meets multiple risk factors, as do survivors of childhood brain tumors. Research involving psychological risk is not inappropriate or unethical (Labott & Johnson, 2004), and, generally, research on sensitive topics is well tolerated by most participants (Draucker, Martsolf, & Poole, 2009). When a member of the research team identifies participant distress, a systematized process should be initiated by the research team to identify and treat these participants.
The FDA began investigating reports of treatment-emergent suicidal ideation and behavior during trials of antidepressant drugs in children and adolescents in 2003. This investigation caused significant controversies and affected the use of some classes of antidepressants in children and adolescents, limiting their use in the United States (Adegbite-Adeniyi, Gron, Rowles, Demeter, & Findling, 2012; Chen & Toh, 2011). The FDA’s review revealed significant heterogeneity in the clinical evaluation of suicidal thoughts and behaviors by experienced clinicians (FDA, 2012). The FDA’s response was to commission researchers from Columbia University to oversee the classification of adverse events that might represent suicidality, resulting in the Columbia Classification Algorithm of Suicide Assessment (C-CASA) (Posner, Oquendo, Gould, Stanley, & Davies, 2007). Subsequently, the FDA required that all participants in trials with central nervous system (CNS)-acting drugs be evaluated with a scale that maps to C-CASA. Elements within these evaluations should include completed suicide, suicide attempt (with intent assessment), preparatory acts, suicidal ideation, self-injurious behavior (intent unknown), not enough information (fatal and nonfatal), nonsuicidal self-injury, and other injuries such as accidents (Meyer et al., 2010). The Columbia-Suicide Severity Rating Scale (C-SSRS) meets these criteria (Posner et al., 2011) and, despite the lack of research on its cross-cultural validity, the FDA considers this the instrument to which all new instruments should be compared (Meyer et al., 2010).
Although trials for CNS-acting drugs now are under a blanket regulation regarding assessments for suicidality, a potential gap exists in other drug and nondrug trials. In nondrug trials, individual IRBs determine the significance of risk and the need for measures to assess for and react to suicidal ideation and behavior. In the current study, the principal investigator included suicidality as a potential risk, and the IRB approved the distribution of tailored information based on geography and the specific needs of participants. This resource was provided to study participants identified by investigators as those who could benefit from such resources. This plan proved insufficient, however, when multiple participants endorsed (or acknowledged) suicidal ideation or behavior in their responses to the Brief Symptom Inventory (BSI), which warranted additional assessment. In response, the authors developed a systematic approach to addressing potential suicidality in study participants. Therefore, the purpose of this article is to discuss the feasibility of the C-SSRS as an assessment of suicidal ideation and behavior by nonpsychiatric subspecialty research personnel in the context of a descriptive research study. In addition, the development and implementation of a protocol in reaction to participant endorsement of suicidal ideation or behavior will be discussed, explaining the construction of such a protocol in a descriptive study with telephone- and home-based components.
Methods
The primary study in which the C-SSRS was used is well described elsewhere (Deatrick et al., 2014; Lucas, Barakat, Jones, Ulrich, & Deatrick, 2014). In brief, it described the caregiving competence of and demand placed on mother caregivers, and the quality of life of their children, who are AYA survivors of childhood brain tumors. Data were collected in two phases. First, research staff members conducted telephone-based, structured-instrument interviews with mothers (n = 186) and survivors (n = 134). The interviews lasted 45–90 minutes and followed a structured script. The caregivers provided demographic data about the survivors, including those survivors who did not participate (n = 52). Then, in-home, qualitative, semistructured interviews were conducted by two of the authors with a subsample of participants (45 caregivers and 41 survivors). The interviews occurred simultaneously but separately. Interviews lasted 20–90 minutes and consisted of open-ended discussions regarding the participants’ experiences during treatment and survivorship.
Setting and Sample
After the study was approved by the IRB, mother caregivers were approached regarding their interest in participation. If the caregiver enrolled, participation was offered to the survivor. The mother caregivers determined whether the survivors were able to participate, which could be precluded because of neurocognitive or functional issues (e.g., hearing). Survivors had to be at least five years postdiagnosis, at least two years post-treatment cessation, aged 14–40 years old, and residing in the same household as the mother-caregiver. Excluded from participation were dyads including caregivers younger than age 21 years, survivors who were married or living in a partnered relationship, survivors diagnosed with a genetic cause of brain tumor (e.g., neurofibromatosis), and intellectual disability or developmental delay prior to brain tumor diagnosis. Caregivers provided consent for themselves and survivors younger than age 18 years. Survivors provided consent for themselves if they were older than age 18 years. When survivors did not wish to answer questions about themselves, they provided consent or assented to their caregivers providing this information. The consent document disclosed the risk that the project could be stressful for participants. Study participants were referred to a clinical psychologist or a psychiatric mental health clinical nurse specialist if necessary.
Instruments
Participants were considered to have endorsed potential suicidality either when making general comments regarding suicide when interviewed or by answering the survey item, “thoughts of ending your life” within the past seven days with anything other than “not at all” on the BSI (Derogatis & Melisaratos, 1983). The BSI is a self-reported, normed measure of psychopathology available for use in people aged 13 years or older. The Global Severity Index subscale measures overall psychological distress and has adequate test-retest reliability (0.8–0.9), high internal consistency for caregivers (0.97), and construct validity demonstrated by the Minnesota Multiphasic Personality Inventory (Barakat et al., 1997; Barakat, Kazak, Gallagher, Meeske, & Stuber, 2000; Best, Streisand, Catania, & Kazak, 2001; Kazak, Stuber, Barakat, & Meeske, 1996; Santacroce, 2002). Study staff members informally interviewed participants regarding comments or responses related to suicidal ideations (or the risk of them) and recorded the discussion in a consultation document (see Appendix A). The document described the reason for the consultation, a summary of the discussion with the participant, and a detailed account of any actions taken, including additional descriptions of the response of a follow-up mental health professional. These consultation documents were used to replace missing data from interviews that were conducted before the implementation of the C-SSRS.
After the original study guidelines related to potential suicidal ideation and behavior proved insufficient, the study team met internally and contacted the IRB with a revised protocol. The C-SSRS (Posner et al., 2009) was adopted because of its ability to assist nonpsychiatric subspecialty research staff members in consistently assessing suicidal ideations and behavior. The C-SSRS is a brief instrument that distinguishes the domains of suicidal ideation and behavior. It measures the severity of the ideation, the intensity of the ideation, types of behaviors, and potential lethality. It has several scale versions for use in clinical trials or in practices (see www.cssrs.columbia.edu for more information). The C-SSRS has convergent and divergent validity with related scales and is highly sensitive and specific in assessing suicidal behavior; the “worst-point lifetime suicidal ideation” is predictive of suicide attempts (Posner et al., 2011). C-SSRS administration training was provided for research staff members via a webinar with the instrument developers, and it was reinforced internally through vignettes and role-playing activities.
Data Analysis
Participant demographics were analyzed using descriptive statistics. Process notes were coded by hand and analyzed for themes using conventional content analysis (Hsieh & Shannon, 2005).
Results
A primary outcome of this report was the development of the suicidality protocol. The protocol was successfully used in telephone and in-home data collection. Research staff members found the protocol feasible, and its implementation reflected important human participant issues in research. The protocol was deemed feasible and effective within the context of this study by nonpsychiatric subspecialty research staff members. Feasibility was determined when the protocol was completed and the results and guidances generated by the research staff using the C-SSRS matched those from the follow-up evaluation by the mental health professional. All study participants, from survivors with developmental delays to caregivers with university-level educations, were open to and cooperative with the protocol.
Sample
Sixteen participants (11 survivors and 5 caregivers, or 5% of the sample) from the final study sample of 186 caregivers and 134 survivors endorsed suicidal ideation. All endorsements occurred during telephone interviews. The AYA survivors of childhood brain tumors who expressed these endorsements were younger than other survivors in the cohort at the time of their endorsements. Endorsees were otherwise similar to the full sample with the majority of diagnoses being low-grade gliomas (50% and 51%, respectively) and primitive neuroectodermal tumors (31% and 27%, respectively) located in the posterior fossa (63% and 51%, respectively). See Table 1 for a comparison of demographics between survivor or caregiver endorsees and the full sample.
Table 1.
Endorsee and General Sample Characteristics
| Endorseesa (N = 16)
|
General Sample (N = 186)
|
|||||
|---|---|---|---|---|---|---|
| Characteristic | X̄ | SD | Range | X̄ | SD | Range |
| Caregiver age (years) | 49.4 | 3.05 | 45–53 | 50.48 | 6.29 | 30–69 |
| Survivor age (years) | 18.18 | 5.69 | 14–34 | 20.52 | 5.28 | 14–39 |
| Time since diagnosis (years) | 11.94 | 5.53 | 6–26 | 13.12 | 6.26 | 5–32 |
| Characteristic | n | % | n | % |
|---|---|---|---|---|
| Survivor gender (male; endorsees only) | 5 | 46 | 105 | 57 |
| Caregiver partnered | 11 | 69 | 146 | 79 |
| Caregiver employed full-time | 7 | 44 | 88 | 47 |
| Caregiver education | ||||
| Some high school | – | – | 3 | 2 |
| High school graduate | 5 | 31 | 53 | 28 |
| Some college | 4 | 25 | 41 | 22 |
| College graduate or higher | 7 | 44 | 89 | 48 |
| Survivor education or work | ||||
| In school | 12 | 75 | 117 | 63 |
| Workingb | 4 | 25 | 78 | 42 |
| Currently not in school or working | 3 | 19 | 33 | 18 |
| Tumor type | ||||
| Low-grade glioma | 8 | 50 | 94 | 51 |
| Primitive neuroectodermal tumor | 5 | 31 | 51 | 27 |
| Craniopharyngioma | – | – | 14 | 8 |
| High-grade glioma | – | – | 7 | 4 |
| Ependymoma | – | – | 5 | 3 |
| Germ cell | 1 | 6 | 5 | 3 |
| Choroid plexus | 1 | 6 | 4 | 2 |
| Other | 1 | 6 | 6 | 3 |
| Tumor location | ||||
| Posterior fossa | 10 | 63 | 94 | 51 |
| Cortical | 2 | 13 | 33 | 18 |
| Sellar | – | – | 29 | 16 |
| Pineal | 1 | 6 | 7 | 4 |
| Thalamic | 2 | 13 | 6 | 3 |
| Ventricle | 1 | 6 | 6 | 3 |
| Brain stem | – | – | 5 | 3 |
| Optic nerve | – | – | 4 | 2 |
| Other | – | – | 2 | 1 |
Includes 5 caregivers and 11 survivors
Three survivor endorsees and 42 survivors from the general sample worked while in school.
Note. The characteristics for endorsees include summary information for all 16 caregivers and survivors unless otherwise indicated.
Note. Because of rounding, percentages may not total 100.
Protocol Description
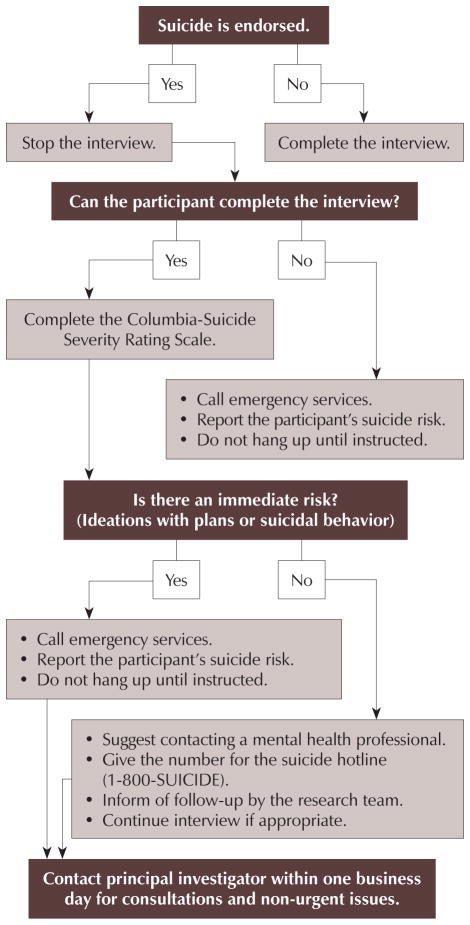
During telephone-based quantitative interviews, the protocol (see Figure 1) was initiated when a participant mentioned suicidal ideation or behavior or responded to question nine on the BSI, “thoughts of ending your life,” with any answer other than “not at all.” The revised protocol included a brief script that expressed the interviewer’s concern for the participant’s well-being and explained the need to ask additional questions (from the C-SSRS) to assess their safety. The interview paused, and the interviewer assessed whether the participant was able to continue the conversation. If the participant continued, the C-SSRS was completed. If the interview ended (e.g., the participant hung up the phone, further screening was refused), emergency services were contacted in case the participant intended to attempt suicide. If the C-SSRS did not suggest an immediate risk (e.g., current suicidal ideation with plans or suicidal behaviors), the interviewer recommended a consultation with a mental health professional, provided a suicide hotline phone number, and informed the participant that a member of the study team would follow up within 24 hours. The quantitative interview continued if the participant agreed that he or she was able to complete the interview. If the C-SSRS suggested an immediate risk (e.g., current suicidal ideation with plan or suicidal behaviors), emergency services were contacted to interrupt a potential suicide attempt. In either event, a licensed mental health professional called participants immediately (imminent risk) or within 24 hours (not an immediate risk) to clinically assess the degree of risk and offer follow-up as needed.
Figure 1.
Protocol for Participant Endorsement of Thoughts of Ending Life
The protocol was modified before the in-home, qualitative interviews to incorporate off-site safety precautions and to maximize safety for the research participants and research staff members. Concerns about participant safety during the first (telephone-based) phase initiated an evaluation of safety in general during the second (in-home) phase. These modifications were based on home healthcare (Fazzone, Barloon, McConnell, & Chitty, 2000; Sylvester & Reisener, 2002) and research safety literature (Paterson, Gregory, & Thorne, 1999). If any discussion of suicidal ideations or behaviors occurred, the protocol was initiated. Data collection was performed in pairs whenever possible to ensure staff member safety. When this was not possible, a member of the research team remained available to follow up with the interviewer by phone. This member called emergency services if the staff member conducting the home interview did not contact (or could not be contacted by) the on-call staff member 90 minutes postinterview. The full protocol is available in Appendix B.
Telephone implementation
Endorsements of suicidal ideation only occurred during the first phase when participants were asked, from the BSI, about “thoughts of ending your life” in the past seven days. One care-giver denied (score = 0) current suicidal ideation but disclosed that she experienced suicidal ideation nine years earlier when her daughter was diagnosed. Six participants (three survivors and three caregivers) endorsed suicidal ideation before the C-SSRS was added to the protocol. For those participants, data consistent with C-SSRS language are missing, but interviewer consultation documents included direct participant quotes. The number and intensity of participants’ endorsements of suicidal ideation are shown in Table 2. The mental health professional who provided next-day follow-up care agreed with the results of the C-SSRS and the decisions made by the interviewer based on them.
Table 2.
Endorsement of Suicidal Ideation: C-SSRS and Interviewer Documentation
| Survivor (n = 11)
|
Caregiver (n = 5)
|
|
|---|---|---|
| Characteristic | n | n |
| Survivors age at diagnosis (years) | ||
| 1–5 | 4 | – |
| 6–10 | 5 | – |
| 11–15 | 2 | – |
| Greater than 15 | – | – |
| Caregiver age at diagnosis (years) | ||
| 21–25 | – | 1 |
| 26–30 | – | – |
| 31–35 | – | – |
| 36–40 | – | 3 |
| 41–45 | – | 1 |
| Years since diagnosis | ||
| 1–5 | – | – |
| 6–10 | 4 | 2 |
| 11–15 | 5 | 2 |
| Greater than 15 | 2 | 1 |
| Thought of ending life | ||
| A little bit | 5 | 3a |
| Moderately | 1 | 1 |
| Quite a bit | 3 | – |
| Extremely often | 2 | 1 |
| C-SSRS ideation severity | ||
| None | 1 | 1 |
| Wish to be dead | 4 | 3 |
| Nonspecific, active suicidal thoughts | 4 | 1 |
| Active suicidal ideation with any methods (no plan) without intent to act | 2 | – |
| Active suicidal ideation with some intent to act, without specific plan | – | – |
| Active suicidal ideation with specific plan and intent | – | – |
| Psychotherapy use | ||
| None | 4 | 1 |
| Previous | 2 | 4 |
| Current | 5 | – |
One caregiver disclosed previous thoughts of suicide when child was first diagnosed.
C-SSRS—Columbia-Suicide Severity Rating Scale
Process notes contained themes about participants, including anger and sadness in survivors and the physical and emotional demands of the survivor in caregivers. Process notes for the interviewer included a reiteration of events, whether the assessment was successful, and whether the recommendation was in agreement with that of the mental health professional. When asked about suicidal ideation, survivors often mentioned anger (“When I get frustrated and angry, those are the only times I think about ending my life”) or sadness (“When I am super depressed, stressed out, and overwhelmed”), but almost all verbalized that they would not act on their ideations. Caregivers said they felt overwhelmed or “trapped” by current life situations and financial burdens. Interviewer notes included quotes from participants, lengthy descriptions of encounters with participants, and summaries of recommendations from the follow-up mental health professional.
In-home use
The protocol (see Appendix C) was feasible and excellent for planning and executing visits. When preparing for the visits, any previous suicidal ideation or behavior disclosed during the telephone interviews was noted. One survivor endorsed suicidal ideation during the telephone interview but did not disclose any similar ideations during the in-home interview. The in-home safety protocol was initiated once to contact an interviewer, who was alone with a participant, because the interviewer delayed contacting the on-call staff member during a long interview. The on-call staff member also was contacted twice when the home of a participant could not be located.
Discussion
AYA survivors of childhood brain tumors are vulnerable because of the neurodevelopmental insults of tumors and their treatment. These insults have a decades-long impact on individuals and their family members, which may be reflected in research reports of suicidal ideations or behavior. The iterative development of the C-SSRS protocol and the safety protocol for in-home data collection demonstrates responsiveness to the needs of this population. It was feasible to train study staff members to use the C-SSRS to generate an immediate response to participants endorsing suicidal ideations or behaviors. Research participants also were responsive to and engaged in the process.
The C-SSRS is widely used in clinical practice and pharmacologic effectiveness trials (Giddens, Sheehan, & Sheehan, 2014). This study shows that its use also is feasible in descriptive, sociobehavioral research. The tool offers a systematic assessment for quickly and thoroughly determining whether a participant is at an immediate risk for self-harm and identifying whether psychological distress was caused by the research study. Of note, Giddens et al. (2014) suggested some concerns about its use as a gold standard. However, when used with this study’s protocol, nonpsychiatric subspecialty research staff members were able to appropriately refer participants for additional help or immediate follow-up with a mental health professional as needed.
This research project and report was approached with a family focus; families are dynamic, and chronic health issues in one member can affect all members. Negative life events are strongly associated with suicidal ideation and behavior (Liu & Miller, 2014). Suicidal ideation is a common issue during adolescence, with about 20% of this age group having thoughts of self-harm (Kann et al., 2014). Although suicide attempts (8%) and attempts requiring treatment (3%) occur at significantly lower rates nationally, the late effects of treatment and disease in the AYA survivor population presumptively increase risk. Access to protective activities involving peer support, successful development, and accomplishment in school, sports, or other ego-enhancing exercises may be limited for survivors. In addition, a close relationship exists between bullying and suicide (National Center for Injury Prevention and Control, 2014). Therefore, passive or active ideations, such as the ones seen in this study, merit a more comprehensive evaluation to discern the risk of this population separately from their peers. At minimum, ongoing suicidal ideation suggests that the participant is struggling psychosocially and might benefit from additional resources or more formal support.
The 5% prevalence of suicidal ideation and behavior in the current article’s sample is higher than the national estimate of 4% (Crosby et al., 2011). Individuals with physical illnesses often have comorbid psychiatric disorders, and the confounding effects of the two health states exacerbate suicide risk (Qin, Hawton, Mortensen, & Webb, 2014). Suicidal ideations and behaviors arise from a number of internalized and externalized psychiatric disorders present in adolescence, including major depressive disorder, obsessive compulsive disorder, conduct disorder, substance use disorders, and impulsive acts. In mothers who are caregivers, the additional stress of care may increase the risk of suicidal thoughts and behaviors related to major depression.
Knowledge Translation.
Nonpsychiatric subspecialty research staff members can be trained to screen research participants who endorse suicidal ideations or behaviors during data collection.
Data collection in potentially vulnerable populations feasibility can include a suicide risk safety protocol to evaluate expressed ideations and behaviors and provide assistance as needed.
The Columbia-Suicide Severity Rating Scale is an easily integrated tool that can assist nonpsychiatric subspecialty research staff members in evaluating survivors of childhood brain tumors and their mother caregivers when suicidal ideation is endorsed.
An important component of this study’s methodology was reactivity to the needs of the research population. In this case, their needs reflected the ongoing psychosocial distress caused by the diagnosis of a brain tumor and its treatment. The existence of suicidal ideation and behavior was informative for the overall purpose of the study, and responsiveness to the participants was considered part of the ethical duty of researchers.
Implications for Nursing
The C-SSRS is useful in sociobehavioral research studies to assess for suicidal ideation and behavior in AYA survivors of childhood brain tumors and their family members. Nonpsychiatric subspecialty staff members can feasibly include the C-SSRS in such research. However, an enhanced evaluation of survivors should be included because of their unique risks. The presence of an available mental health professional to take referrals during psychiatric emergencies also is recommended while the study is in progress.
The C-SSRS may potentially be useful in other areas of research as an assessment tool for suicidal ideation and behavior. It also may be valuable in clinical (non-research) settings when a nonpsychiatric subspecialty staff member is concerned about a patient’s endorsement of suicidal ideation or behavior.
Conclusion
The novel additions of this study to the literature include the incorporation of the C-SSRS in a nondrug research study and the development of a protocol that is reactive to the needs of a research population. A careful approach must be taken when designing and implementing research plans with AYA survivors of childhood brain tumors and their families. Just as informed consent is an ongoing process, the researchers’ reaction to and flexibility about the ongoing research process should be dynamic. Researchers’ reactions to incidental findings in the sociobehavioral research process show responsiveness to the population under examination.
Acknowledgments
This research was funded by grants from the United States National Institutes of Health via the National Institute of Nursing Research (T32NR007100, F31NR013091 [MSL]; R01NR009651 [JAD]), the American Cancer Society (122552-DSCN-10-089 [MSL]), and the Oncology Nursing Society Foundation (Neuro-Oncology Nursing Grant [JAD]).
Appendix A. Example of a Consultation Form

Appendix B. Brain Tumor Survivors In-Home Interviewer Safety Protocol
Study Protocols.
The health and safety of all participants during an in-home research interview is a top concern for research staff, and a protocol must be in place for the protection of participants and staff members. The role of a research interviewer differs from that of a healthcare or mental health professional, and a researcher cannot adequately function as an emergency responder if an acute risk of self-harm is disclosed during an in-home interview. To accompany in-home interviews by research staff members for an at-risk population, the authors devised a protocol for participant safety in the event of risk for self-harm in participants. Communication between the interviewer and an off-site staff member is crucial to determine pre- and postinterview safety and the status of the interviewer after a set time period if contact is not made. Should a participant reference suicidal ideation, the interviewer will question whether the participant can continue and, if so, assess the participant using the Columbia-Suicide Severity Rating Scale. The interviewer will note if the participant has a plan, its lethality, and accessibility. Appropriate interventions will be made according to the participant’s response and the interviewer’s assessment of the situation.
Check the date, time, and location arranged for the in-home visit. Call the participant’s home prior to leaving for the interview (or night before, if early morning) to confirm.
Arrive at location.
Call contact to confirm arrival, address, and participant’s phone number.
-
Enter participant’s home and assess for cellular phone service availability.
If cell service is available, call or SMS contact to state this. If cell service unavailable, confirm with the participant or family that it is OK to receive a call to the home phone. Send an SMS to contact with information to receive check-in after (predetermined time: 60–90 minutes, depending on interview type).
-
If participant references suicidal ideation, stop the interview and ask the participant if he or she can continue the interview.
If the answer is “no,” determine the extent of immediate danger.
If it is safe, contact emergency services and principal investigator (PI), and stay with the participant until assistance arrives. If it is unsafe, leave the premises immediately and call emergency services.
If the answer is “yes,” ask questions from the Columbia-Suicide Severity Rating Scale (particularly regarding plan, lethality, and accessibility).
-
If there is an acute risk of suicide, inform the participant that you must call emergency services. If there is no acute risk (i.e., past thoughts),
Suggest seeing a mental health professional.
Provide the suicide hotline phone number.
Inform the participant that a member of the research team will contact them the next business day.
Continue the interview if the participant is comfortable.
Contact the PI within one business day regarding the incident.
After completing the interview and leaving participant’s home, call contact to state as much.
Appendix C. Suicide Risk Assessment Protocol
When to Consult the Research Team
Consult the research team any time a survivor or caregiver mentions suicidal thoughts or plans even if you are not sure of whether he or she will act on them.
Issues that require immediate consultation with supervisors include current suicidality and imminent risk. For example, the participant has thought about ending his or her own life, has a plan, and has the means to carry out that plan.
Issues that require consultation the next business day include recent suicidality. For example, the participant mentions that he or she had thought about ending his or her own life in the past but has not felt that way in months or years.
When to Use the Columbia-Suicide Severity Rating Scale
When participants endorse more than one item on Form 11, Item 9 (Brief Symptom Inventory), “Thoughts of ending your life,” or mention suicidal intention at any point during the interview,
Stop interview proceedings and state, “Whenever our research participants mention that they may have thoughts of ending their lives, we always want to check in with them to make sure that they are OK. I’m just going to ask you a few questions about how you’re feeling.”
Continue with the Columbia-Suicide Severity Rating Scale.
Lower-Risk Situations
For participants who have never attempted suicide and deny having the intention, plan, or means to carry out a suicide attempt,
Suggest that the participant contact a mental health professional so that someone can help with his or her concerns.
Provide the participant with the suicide hotline number (1-800-SUICIDE) and encourage him or her to call if they have suicidal thoughts.
Inform the participant that a member of the research team will contact him or her on the next business day to check in and see if there is anything else that can be done to assist them.
Continue with the interview if the participant is comfortable with doing so.
High-Risk Situations
For those who have ever attempted suicide or admit to having the intention, plan, or means to carry out a suicide attempt,
Thank the participant for being open and honest.
Express concern for his or her safety and let him or her know that emergency services have to be notified.
If someone else is around, ask that person to call emergency services and keep the participant on the line until the police are dispatched. If you are alone, hang up and call emergency services.
Inform the emergency services operator that you were conducting a telephone interview for a research study and that you have a participant who is at risk of suicide. Stay on the line to provide the requested information, and do not hang up until instructed to do so.
If the participant hangs up at any point after they have disclosed their suicidal ideation, contact emergency services immediately and seek immediate consultation with supervisors.
Important Points.
You do not have to be certain that danger is present to consult with the principal investigator. This will help remove any doubts as to the best course of action.
Use the Consultation Documentation form to thoroughly document the survivor’s or caregiver’s statements to you, your observations, and any actions taken. Do this as soon as possible to record the most accurate information.
Do not feel bad if a participant becomes upset with you for notifying the research team or the police. Remember that you actions can help save someone’s life!
For more information about the Columbia-Suicide Severity Rating Scale, see www.cssrs.columbia.edu.
Contact the principal investigator (PI) within the same day for immediate consultation issues and within one business day for nonurgent consultation issues. If the PI is unavailable, call the first back-up. If you cannot reach the PI or first back-up, call the second back-up. Document everything on the Consultation Documentation form as soon as possible. The PI will review the case and, if necessary, seek consultation from mental health experts. Necessary contact information includes
PI contact information
First back-up contact information
Second back-up contact information
Mental health expert 1
Mental health expert 2.
References
- Adegbite-Adeniyi C, Gron B, Rowles BM, Demeter CA, Findling RL. An update on antidepressant use and suicidality in pediatric depression. Expert Opinion on Pharmacotherapy. 2012;13:2119–2130. doi: 10.1517/14656566.2012.726613. [DOI] [PubMed] [Google Scholar]
- Anguiano L, Mayer DK, Piven ML, Rosenstein D. A literature review of suicide in cancer patients. Cancer Nursing. 2012;35:E14–E26. doi: 10.1097/NCC.0b013e31822fc76c. [DOI] [PubMed] [Google Scholar]
- Barakat LP, Kazak AE, Gallagher PR, Meeske K, Stuber M. Posttraumatic stress symptoms and stressful life events predict the long-term adjustment of survivors of childhood cancer and their mothers. Journal of Clinical Psychology in Medical Settings. 2000;7:189–196. doi: 10.1023/A:1009516928956. [DOI] [Google Scholar]
- Barakat LP, Kazak AE, Meadows AT, Casey R, Meeske K, Stuber ML. Families surviving childhood cancer: A comparison of posttraumatic stress symptoms with families of healthy children. Journal of Pediatric Psychology. 1997;22:843–859. doi: 10.1093/jpepsy/22.6.843. [DOI] [PubMed] [Google Scholar]
- Best M, Streisand R, Catania L, Kazak AE. Parental distress during pediatric leukemia and posttraumatic stress symptoms after treatment ends. Journal of Pediatric Psychology. 2001;26:299–307. doi: 10.1093/jpepsy/26.5.299. [DOI] [PubMed] [Google Scholar]
- Bjorkenstam C, Edberg A, Ayoubi S, Rosen M. Are cancer patients at higher suicide risk than the general population? Scandinavian Journal of Public Health. 2005;33:208–214. doi: 10.1080/14034940410019226. [DOI] [PubMed] [Google Scholar]
- Brinkman TM, Liptak CC, Delaney BL, Chordas CA, Muriel AC, Manley PE. Suicide ideation in pediatric and adult survivors of childhood brain tumors. Journal of Neuro-Oncology. 2013;113:425–432. doi: 10.1007/s11060-013-1130-6. [DOI] [PubMed] [Google Scholar]
- Bruce M, Gumley D, Isham L, Fearon P, Phipps K. Post-traumatic stress symptoms in childhood brain tumour survivors and their parents. Child: Care, Health and Development. 2011;37:244–251. doi: 10.1111/j.1365-2214.2010.01164.x. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Injury prevention and control: Data and statistics (WISQARS™) 2014 Retrieved from http://www.cdc.gov/injury/wisqars/index.html.
- Chen SY, Toh S. National trends in prescribing antidepressants before and after an FDA advisory on suicidality risk in youths. Psychiatric Services. 2011;62:727–733. doi: 10.1176/ps.62.7.pss6207_0727. [DOI] [PubMed] [Google Scholar]
- Crosby AE, Han B, Ortega LA, Parks SE, Gfroerer J. Suicidal thoughts and behaviors among adults aged ≥ 18 years—United States, 2008–2009. Morbidity and Mortality Weekly Report. 2011;60(SS13):1–22. [PubMed] [Google Scholar]
- Deatrick JA, Hobbie W, Ogle S, Fisher MJ, Barakat L, Hardie T, Ginsberg JP. Competence in caregivers of adolescent and young adult childhood brain tumor survivors. Health Psychology. 2014;33:1103–1112. doi: 10.1037/a0033756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derogatis LR, Melisaratos N. The Brief Symptom Inventory: An introductory report. Psychological Medicine. 1983;13:595–605. [PubMed] [Google Scholar]
- Draucker CB, Martsolf DS, Poole C. Developing distress protocols for research on sensitive topics. Archives of Psychiatric Nursing. 2009;23:343–350. doi: 10.1016/j.apnu.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Ellenberg L, Liu Q, Gioia G, Yasui Y, Packer RJ, Mertens A, Zeltzer LK. Neurocognitive status in long-term survivors of childhood CNS malignancies: A report from the Childhood Cancer Survivor Study. Neuropsychology. 2009;23:705–717. doi: 10.1037/a0016674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazzone PA, Barloon LF, McConnell SJ, Chitty JA. Personal safety, violence, and home health. Public Health Nursing. 2000;17(1):43–52. doi: 10.1046/j.1525-1446.2000.00043.x. [DOI] [PubMed] [Google Scholar]
- Filiberti A, Ripamonti C. Suicide and suicidal thoughts in cancer patients. Tumori. 2002;88:193–199. doi: 10.1177/030089160208800303. [DOI] [PubMed] [Google Scholar]
- Giddens JM, Sheehan KH, Sheehan DV. The Columbia-Suicide Severity Rating Scale (C-SSRS): Has the “gold standard” become a liability? Innovations in Clinical Neuroscience. 2014;11:66–80. [PMC free article] [PubMed] [Google Scholar]
- Hem E, Loge JH, Haldorsen T, Ekeberg O. Suicide risk in cancer patients from 1960 to 1999. Journal of Clinical Oncology. 2004;22:4209–4216. doi: 10.1200/JCO.2004.02.052. [DOI] [PubMed] [Google Scholar]
- Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, Cronin KA. SEER Cancer Statistics Review, 1975–2011. Bethesda, MD: National Institutes of Health; 2013. [Google Scholar]
- Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qualitative Health Research. 2005;15:1277–1288. doi: 10.1177/1049732305276687. [DOI] [PubMed] [Google Scholar]
- Kann L, Kinchen S, Shanklin SL, Flint KH, Kawkins J, Harris WA. Youth risk behavior surveillance—United States, 2013. Morbidity and Mortality Weekly Report. 2014;63(SS4):1–168. [PubMed] [Google Scholar]
- Kazak AE, Alderfer M, Rourke MT, Simms S, Streisand R, Grossman JR. Posttraumatic stress disorder (PTSD) and post-traumatic stress symptoms (PTSS) in families of adolescent childhood cancer survivors. Journal of Pediatric Psychology. 2004;29:211–219. doi: 10.1093/jpepsy/jsh022. [DOI] [PubMed] [Google Scholar]
- Kazak AE, Stuber ML, Barakat LP, Meeske K. Assessing posttraumatic stress related to medical illness and treatment: The impact of traumatic stressors interview schedule (ITSIS) Families, Systems, and Health. 1996;14:365–380. doi: 10.1037/h0089795. [DOI] [Google Scholar]
- Krysinska K, Lester D. Post-traumatic stress disorder and suicide risk. Archives of Suicide Research. 2010;14(1):1–23. doi: 10.1080/13811110903478997. [DOI] [PubMed] [Google Scholar]
- Kunin-Batson A, Kadan-Lottick N, Zhu L, Cox C, Bordes-Edgar V, Srivastava DK, Krull KR. Predictors of independent living status in adult survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Pediatric Blood and Cancer. 2011;57:1197–1203. doi: 10.1002/pbc.22982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labott SM, Johnson TP. Psychological and social risks of behavioral research. IRB: Ethics and Research. 2004;26(3):11–15. [PubMed] [Google Scholar]
- Liu RT, Miller I. Life events and suicidal ideation and behavior: A systematic review. Clinical Psychology Review. 2014;34:181–192. doi: 10.1016/j.cpr.2014.01.006. [DOI] [PubMed] [Google Scholar]
- Lucas MS, Barakat LP, Jones NL, Ulrich CM, Deatrick JA. Expectations for function and independence by childhood brain tumor survivors and their mothers. Narrative Inquiry in Bio-ethics. 2014;4:233–251. doi: 10.1353/nib.2014.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maunsell E, Pogany L, Barrera M, Shaw AK, Speechley KN. Quality of life among long-term adolescent and adult survivors of childhood cancer. Journal of Clinical Oncology. 2006;24:2527–2535. doi: 10.1200/JCO.2005.03.9297. [DOI] [PubMed] [Google Scholar]
- Meyer RE, Salzman C, Youngstrom EA, Clayton PJ, Goodwin FK, Mann JJ, Sheehan DV. Suicidality and risk of suicide—Definition, drug safety concerns, and a necessary target for drug development: A consensus statement. Journal of Clinical Psychiatry. 2010;71(8):E1–E21. doi: 10.4088/JCP.10cs06070blu. [DOI] [PubMed] [Google Scholar]
- National Center for Injury Prevention and Control. The relationship between bullying and suicide: What we know and what it means for schools. 2014 Retrieved from http://www.cdc.gov/violenceprevention/pdf/bullying-suicide-translation-final-a.pdf.
- Ness KK, Morris EB, Nolan VG, Howell CR, Gilchrist LS, Stovall M, Neglia JP. Physical performance limitations among adult survivors of childhood brain tumors. Cancer. 2010;116:3034–3044. doi: 10.1002/cncr.25051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson BL, Gregory D, Thorne S. A protocol for researcher safety. Qualitative Health Research. 1999;9:259–269. doi: 10.1177/104973299129121820. [DOI] [PubMed] [Google Scholar]
- Posner K, Brent D, Lucas C, Gould M, Stanley B, Brown G, Mann J. Columbia-Suicide Severity Rating Scale (C-SSRS) New York, NY: New York State Psychiatric Institute; 2009. [Google Scholar]
- Posner K, Brown GK, Stanley B, Brent DA, Yershova KV, Oquendo MA, Mann JJ. The Columbia-Suicide Severity Rating Scale: Initial validity and internal consistency findings from three multisite studies with adolescents and adults. American Journal of Psychiatry. 2011;168:1266–1277. doi: 10.1176/appi.ajp.2011.10111704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner K, Oquendo MA, Gould M, Stanley B, Davies M. Columbia Classification Algorithm of Suicide Assessment (C-CASA): Classification of suicidal events in the FDA’s pediatric suicidal risk analysis of antidepressants. American Journal of Psychiatry. 2007;164:1035–1043. doi: 10.1176/appi.ajp.164.7.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin P, Hawton K, Mortensen PB, Webb R. Combined effects of physical illness and comorbid psychiatric disorder on risk of suicide in a national population study. British Journal of Psychiatry. 2014;204:430–435. doi: 10.1192/bjp.bp.113.128785. [DOI] [PubMed] [Google Scholar]
- Recklitis CJ, Diller LR, Li X, Najita J, Robison LL, Zeltzer L. Suicide ideation in adult survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Journal of Clinical Oncology. 2010;28:655–661. doi: 10.1200/JCO.2009.22.8635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santacroce S. Uncertainty, anxiety, and symptoms of posttraumatic stress in parents of children recently diagnosed with cancer. Journal of Pediatric Oncology Nursing. 2002;19:104–111. doi: 10.1177/104345420201900305. [DOI] [PubMed] [Google Scholar]
- Speechley KN, Barrera M, Shaw AK, Morrison HI, Maunsell E. Health-related quality of life among child and adolescent survivors of childhood cancer. Journal of Clinical Oncology. 2006;24:2536–2543. doi: 10.1200/JCO.2005.03.9628. [DOI] [PubMed] [Google Scholar]
- Sylvester B, Reisener L. Scared to go to work: A home care performance improvement initiative. Journal of Nursing Care Quality. 2002;17:71–82. doi: 10.1097/00001786-200210000-00009. [DOI] [PubMed] [Google Scholar]
- U.S. Food and Drug Administration. Guidance for industry: Suicidal ideation and behavior: Prospective assessment of occurrence in clinical trials. Silver Spring, MD: U.S. Department of Health and Human Services; 2012. [Google Scholar]
- Zeltzer LK, Recklitis C, Buchbinder D, Zebrack B, Casillas J, Tsao JC, Krull K. Psychological status in childhood cancer survivors: A report from the Childhood Cancer Survivor Study. Journal of Clinical Oncology. 2009;27:2396–2404. doi: 10.1200/JCO.2008.21.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman M, Chelminski I, Posternak MA. Exclusion criteria used in antidepressant efficacy trials: Consistency across studies and representativeness of samples included. Journal of Nervous and Mental Disease. 2004;192(2):87–94. doi: 10.1097/01.nmd.0000110279.23893.82. [DOI] [PubMed] [Google Scholar]
- Zimmerman M, Chelminski I, Posternak MA. Generalizability of antidepressant efficacy trials: Differences between depressed psychiatric outpatients who would or would not qualify for an efficacy trial. American Journal of Psychiatry. 2005;162:1370–1372. doi: 10.1176/appi.ajp.162.7.1370. [DOI] [PubMed] [Google Scholar]