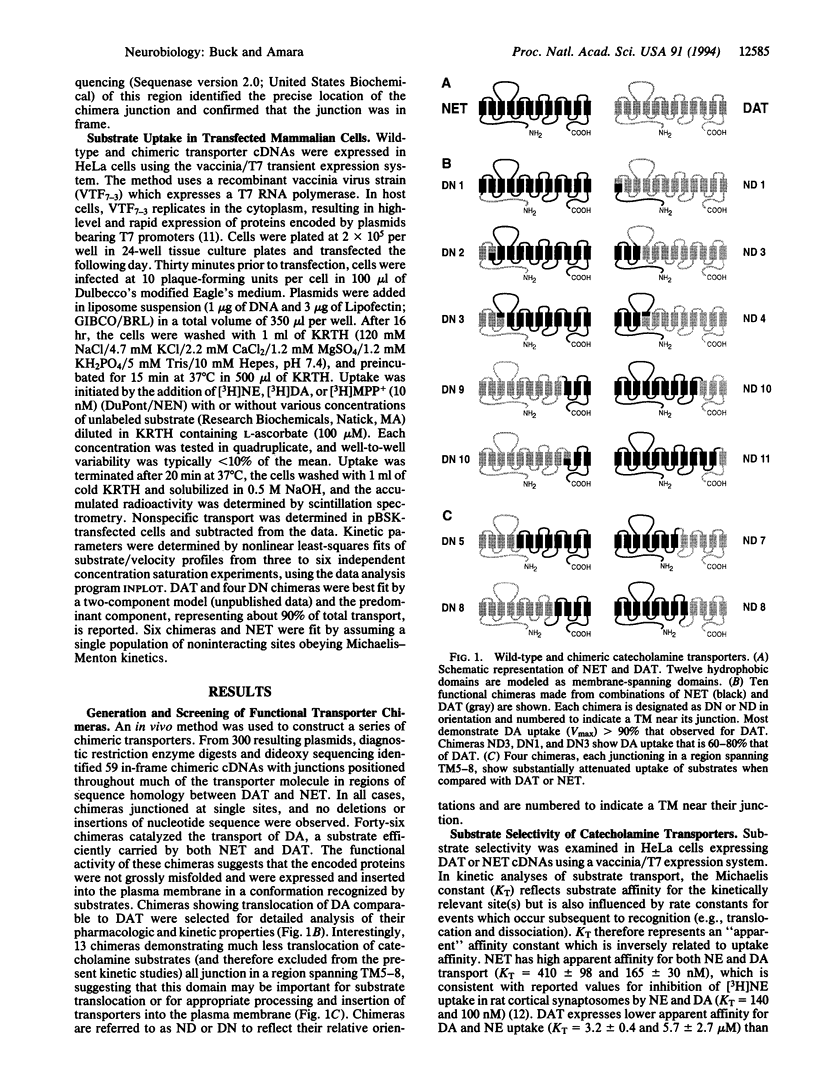
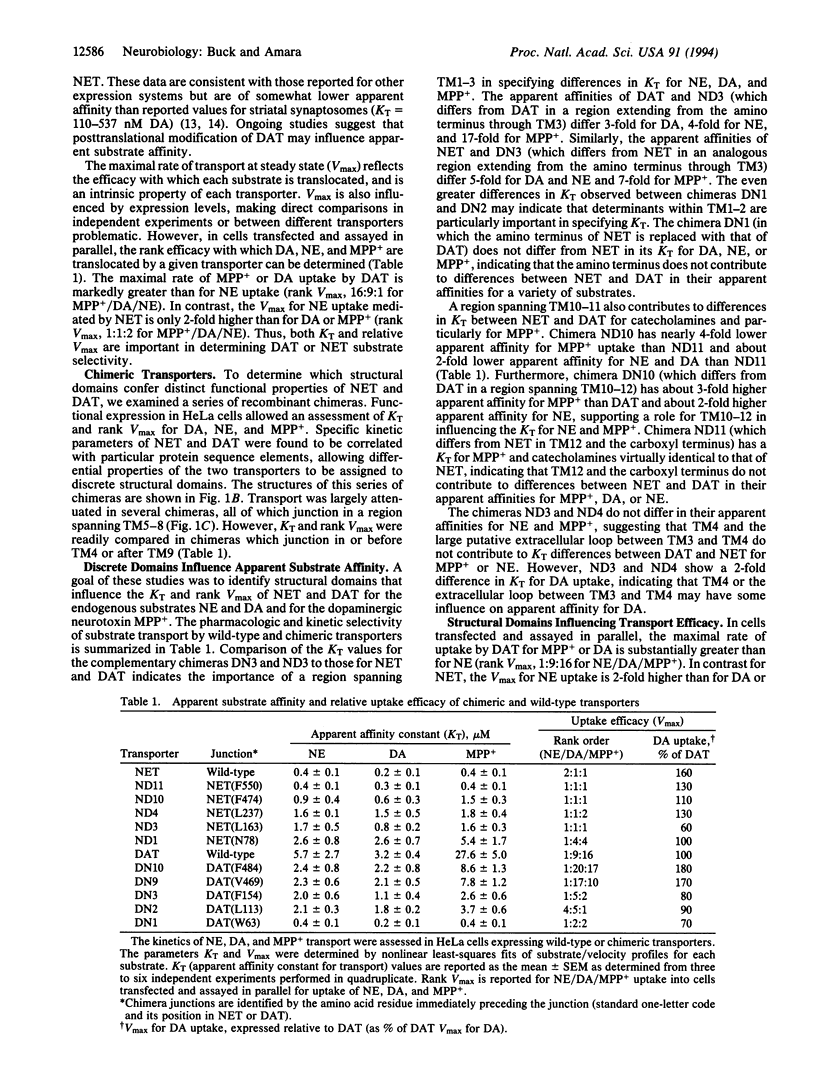
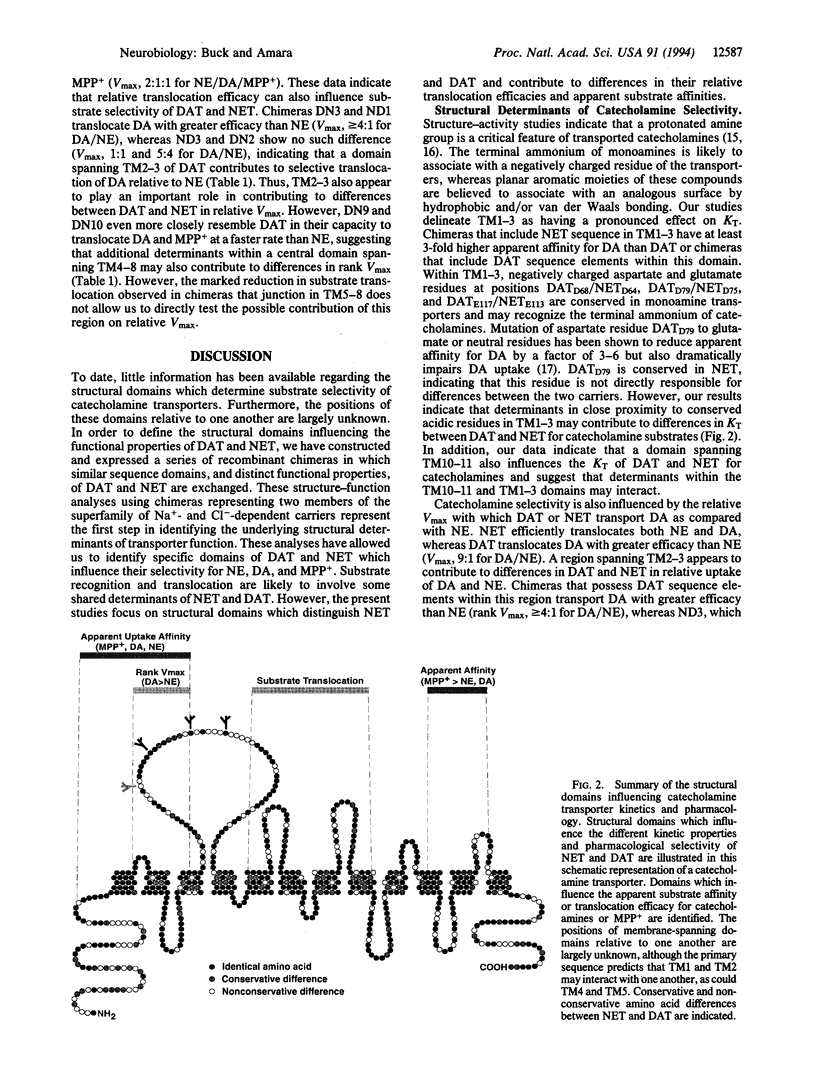
Abstract
The dopamine (DA) and norephinephrine (NE) transporters demonstrate important differences in their selectivity for catecholamines and the parkinsonism-inducing neurotoxin 1-methyl-4-phenylpyridinium (MPP+), yet their primary sequences and predicted topology are strikingly similar. To delineate discrete structural domains contributing to pharmacologic and kinetic differences between the DA and NE transporters, a series of recombinant chimeras was generated by a restriction site-independent method and expressed in mammalian cells. Functional analyses of the chimeras delineate two discrete regions spanning the first through the third transmembrane domains (TM1-3) and TM10-11 that contribute to differences in their apparent affinities for DA, NE, and MPP+. These studies also suggest that TM2-3 of the DA transporter have a role in selectively increasing the rate of DA uptake as compared with NE. TM4-8 of the DA transporter may influence the relative rate with which MPP+ is taken up into cells and could contribute to its selective toxicity in neurons expressing the DA transporter. These structure-function studies using chimeras of members of the superfamily of Na(+)- and Cl(-)-dependent transporters provide a framework for identifying the specific structural or regulatory determinants contributing to substrate recognition and translocation by the DA and NE transporters.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amara S. G., Kuhar M. J. Neurotransmitter transporters: recent progress. Annu Rev Neurosci. 1993;16:73–93. doi: 10.1146/annurev.ne.16.030193.000445. [DOI] [PubMed] [Google Scholar]
- Blakely R. D., Clark J. A., Rudnick G., Amara S. G. Vaccinia-T7 RNA polymerase expression system: evaluation for the expression cloning of plasma membrane transporters. Anal Biochem. 1991 May 1;194(2):302–308. doi: 10.1016/0003-2697(91)90233-j. [DOI] [PubMed] [Google Scholar]
- Boyson S. J. Parkinson's disease and the electron transport chain. Ann Neurol. 1991 Sep;30(3):330–331. doi: 10.1002/ana.410300303. [DOI] [PubMed] [Google Scholar]
- Fraser C. M., Chung F. Z., Wang C. D., Venter J. C. Site-directed mutagenesis of human beta-adrenergic receptors: substitution of aspartic acid-130 by asparagine produces a receptor with high-affinity agonist binding that is uncoupled from adenylate cyclase. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5478–5482. doi: 10.1073/pnas.85.15.5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giros B., Wang Y. M., Suter S., McLeskey S. B., Pifl C., Caron M. G. Delineation of discrete domains for substrate, cocaine, and tricyclic antidepressant interactions using chimeric dopamine-norepinephrine transporters. J Biol Chem. 1994 Jun 10;269(23):15985–15988. [PubMed] [Google Scholar]
- Giros B., el Mestikawy S., Bertrand L., Caron M. G. Cloning and functional characterization of a cocaine-sensitive dopamine transporter. FEBS Lett. 1991 Dec 16;295(1-3):149–154. doi: 10.1016/0014-5793(91)81406-x. [DOI] [PubMed] [Google Scholar]
- Giros B., el Mestikawy S., Godinot N., Zheng K., Han H., Yang-Feng T., Caron M. G. Cloning, pharmacological characterization, and chromosome assignment of the human dopamine transporter. Mol Pharmacol. 1992 Sep;42(3):383–390. [PubMed] [Google Scholar]
- Javitch J. A., Blaustein R. O., Snyder S. H. [3H]mazindol binding associated with neuronal dopamine and norepinephrine uptake sites. Mol Pharmacol. 1984 Jul;26(1):35–44. [PubMed] [Google Scholar]
- Kilty J. E., Lorang D., Amara S. G. Cloning and expression of a cocaine-sensitive rat dopamine transporter. Science. 1991 Oct 25;254(5031):578–579. doi: 10.1126/science.1948035. [DOI] [PubMed] [Google Scholar]
- Kitayama S., Shimada S., Xu H., Markham L., Donovan D. M., Uhl G. R. Dopamine transporter site-directed mutations differentially alter substrate transport and cocaine binding. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7782–7785. doi: 10.1073/pnas.89.16.7782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. M., Javitch J. A., Snyder S. H. Characterization of [3H]desipramine binding associated with neuronal norepinephrine uptake sites in rat brain membranes. J Neurosci. 1982 Oct;2(10):1515–1525. doi: 10.1523/JNEUROSCI.02-10-01515.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacholczyk T., Blakely R. D., Amara S. G. Expression cloning of a cocaine- and antidepressant-sensitive human noradrenaline transporter. Nature. 1991 Mar 28;350(6316):350–354. doi: 10.1038/350350a0. [DOI] [PubMed] [Google Scholar]
- Reith M. E., Meisler B. E., Sershen H., Lajtha A. Structural requirements for cocaine congeners to interact with dopamine and serotonin uptake sites in mouse brain and to induce stereotyped behavior. Biochem Pharmacol. 1986 Apr 1;35(7):1123–1129. doi: 10.1016/0006-2952(86)90148-6. [DOI] [PubMed] [Google Scholar]
- Shimada S., Kitayama S., Lin C. L., Patel A., Nanthakumar E., Gregor P., Kuhar M., Uhl G. Cloning and expression of a cocaine-sensitive dopamine transporter complementary DNA. Science. 1991 Oct 25;254(5031):576–578. doi: 10.1126/science.1948034. [DOI] [PubMed] [Google Scholar]
- Shoffner J. M., Watts R. L., Juncos J. L., Torroni A., Wallace D. C. Mitochondrial oxidative phosphorylation defects in Parkinson's disease. Ann Neurol. 1991 Sep;30(3):332–339. doi: 10.1002/ana.410300304. [DOI] [PubMed] [Google Scholar]
- Snyder S. H., D'Amato R. J. MPTP: a neurotoxin relevant to the pathophysiology of Parkinson's disease. The 1985 George C. Cotzias lecture. Neurology. 1986 Feb;36(2):250–258. doi: 10.1212/wnl.36.2.250. [DOI] [PubMed] [Google Scholar]
- Strader C. D., Sigal I. S., Candelore M. R., Rands E., Hill W. S., Dixon R. A. Conserved aspartic acid residues 79 and 113 of the beta-adrenergic receptor have different roles in receptor function. J Biol Chem. 1988 Jul 25;263(21):10267–10271. [PubMed] [Google Scholar]
- Usdin T. B., Mezey E., Chen C., Brownstein M. J., Hoffman B. J. Cloning of the cocaine-sensitive bovine dopamine transporter. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11168–11171. doi: 10.1073/pnas.88.24.11168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Paulis T., Kelder D., Ross S. B. On the topology of the norepinephrine transport carrier in rat hypothalamus. The site of action of tricyclic uptake inhibitors. Mol Pharmacol. 1978 Jul;14(4):596–606. [PubMed] [Google Scholar]