Abstract
Objective
B cells are likely to contribute to the pathogenesis of systemic lupus erythematosus (SLE), and rituximab induces depletion of B cells. The Exploratory Phase II/III SLE Evaluation of Rituximab (EXPLORER) trial tested the efficacy and safety of rituximab versus placebo in patients with moderately-to-severely active extrarenal SLE.
Methods
Patients entered with ≥1 British Isles Lupus Assessment Group (BILAG) A score or ≥2 BILAG B scores despite background immunosuppressant therapy, which was continued during the trial. Prednisone was added and subsequently tapered. Patients were randomized at a ratio of 2:1 to receive rituximab (1,000 mg) or placebo on days 1, 15, 168, and 182.
Results
In the intent-to-treat analysis of 257 patients, background treatment was evenly distributed among azathioprine, mycophenolate mofetil, and methotrexate. Fifty-three percent of the patients had ≥1 BILAG A score at entry, and 57% of the patients were categorized as being steroid dependent. No differences were observed between placebo and rituximab in the primary and secondary efficacy end points, including the BILAG-defined response, in terms of both area under the curve and landmark analyses. A beneficial effect of rituximab on the primary end point was observed in the African American and Hispanic subgroups. Safety and tolerability were similar in patients receiving placebo and those receiving rituximab.
Conclusion
The EXPLORER trial enrolled patients with moderately-to-severely active SLE and used aggressive background treatment and sensitive cutoffs for nonresponse. No differences were noted between placebo and rituximab in the primary and secondary end points. Further evaluation of patient subsets, biomarkers, and exploratory outcome models may improve the design of future SLE clinical trials.
Systemic lupus erythematosus (SLE) is a heterogeneous autoimmune disease that can cause severe organ damage (1,2). SLE predominantly affects women, and the prevalence is highest among African Americans, African Caribbeans, Hispanics, and Asians (3,4). Comprehensive care is required to prevent serious sequelae (1,5,6); however, no new medication for SLE has been approved by the US Food and Drug Administration (FDA) for the past 50 years. Current therapies, including use of corticosteroids, antimalarial agents, and immunosuppressive drugs, are largely empiric. These interventions are not always effective and may contribute to organ damage (7,8).
B cells have critical roles in the pathogenesis of SLE, including cytokine production, presentation of self antigen, T cell activation, and autoantibody production (9–12). Loss of B cell tolerance may be a pivotal event in the pathogenesis of SLE (9–11,13), providing a strong rationale for the study of targeted treatments that modify the effects of B cells on immunity.
Rituximab, a chimeric monoclonal antibody that selectively targets CD20-positive B cells while sparing stem cells and plasma cells (14–16), is approved for the treatment of non-Hodgkin's lymphoma and rheumatoid arthritis (RA) (17–19). The results of several small uncontrolled trials have suggested that rituximab might have potential efficacy and be steroid-sparing in SLE (20–32).
The Exploratory Phase II/III SLE Evaluation of Rituximab (EXPLORER) trial, a placebo-controlled, double-blind, multicenter study of rituximab in patients with moderately-to-severely active extrarenal SLE, was undertaken to assess the efficacy and safety of rituximab over 52 weeks. (See Appendix A for the principal EXPLORER study centers and investigators.)
PATIENTS AND METHODS
Institutional review board approval was obtained at each trial site. The study was conducted in accordance with FDA Good Clinical Practice guidelines and the Health Insurance Portability and Accountability Act of 1996. Patients provided written informed consent prior to participation.
Patients
Inclusion criteria included age 16–75 years; a history of meeting 4 American College of Rheumatology (ACR) criteria for SLE (33), including a positive test for antinuclear antibodies; active disease at screening, defined as ≥1 organ system with a British Isles Lupus Assessment (BILAG) A score (severe disease activity) or ≥2 organ systems with a BILAG B score (moderate disease activity) (34,35); and stable use of 1 immunosuppressive drug at entry, which was able to be continued during the trial.
Patients were excluded for severe central nervous system or organ-threatening lupus or any other active conditions requiring significant use of steroids or recent treatment with a cyclophosphamide or a calcineurin inhibitor (within 12 weeks of screening); a history of cancer or serious recurrent or chronic infection; uncontrolled medical disease; pregnancy or planning pregnancy; previous treatment with B cell–targeted therapy; aspartate aminotransferase or alanine aminotransferase level >2.5-fold the upper limit of normal (ULN); amylase or lipase level >2-fold the ULN; neutrophil count <1.0 × 103/μl; positive results of hepatitis B or hepatitis C serology; hemoglobin concentration <7 gm/dl (unless caused by hemolytic anemia due to SLE); platelet count <10,000/μl; and serum creatinine level >2.5 mg/dl.
Study design
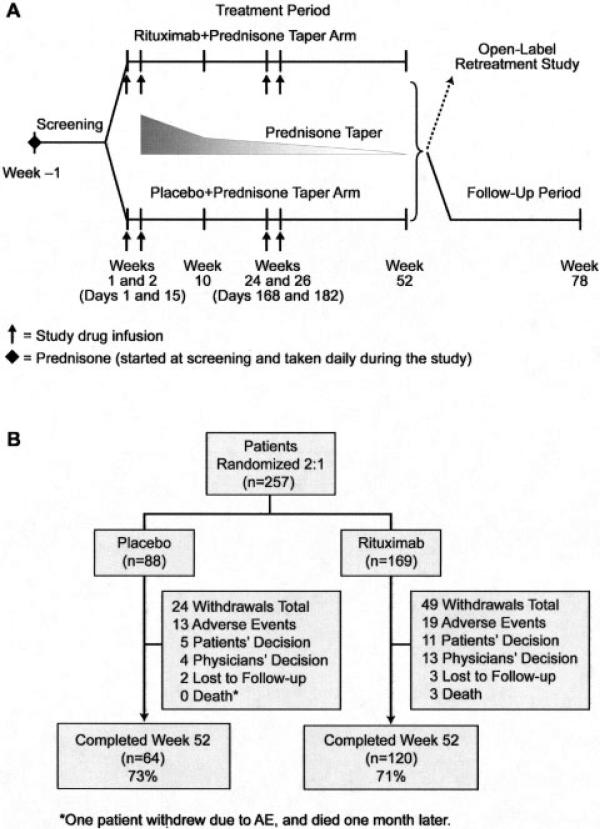
The EXPLORER trial was a randomized, double-blind, placebo-controlled, multicenter (55 centers) North American study evaluating the efficacy and safety of rituximab compared with placebo in patients with SLE who were receiving background immunosuppressants and prednisone (Figure 1A).
Figure 1.
A, Study design. B, Disposition of patients. AE = adverse event.
Patients were randomized at a 2:1 ratio to receive intravenous rituximab (2 1,000-mg doses given 14 days apart) or placebo on days 1, 15, 168, and 182, which was added to prednisone (given according to the protocol) and to the baseline immunosuppressive regimen. Each infusion was accompanied by intravenous administration of acetaminophen, diphenhydramine, and methylprednisolone (100 mg). The BILAG index was used to assess SLE activity. To ensure inclusion of only patients with significantly active disease, minimum disease severity at entry was stringently defined as ≥1 domain with a BILAG A score or ≥2 domains with a BILAG B score, despite background treatment with either azathioprine (AZA; 100–250 mg/day), mycophenolate mofetil (MMF; 1–4 gm/day), or methotrexate (MTX; 7.5–27.5 mg/week). After screening, eligible patients continued their immunosuppressant therapy and received additional daily oral prednisone (0.5 mg/kg, 0.75 mg/kg, or 1.0 mg/kg), based on the BILAG score at entry and the amount of steroids already being taken at the time of entry. Steroids were tapered beginning on day 16, with the goal of reaching a dosage of ≤10 mg/day over 10 weeks and ≤5 mg/day by week 52.
Clinical assessments
Patients were evaluated monthly with the BILAG index and the Lupus Quality of Life (LupusQol) index (based on the Short Form 36 [SF-36] with additional components including pain and fatigue) (36,37). The BILAG index was used to assess response, and scores were converted to numeric values (A = 9, B = 3, C = 1, D = 0, E = 0) (38) to enable evaluation of fluctuating global summary scores.
Primary end points
The effect of placebo versus rituximab in achieving and maintaining a major clinical response, a partial clinical response, or no clinical response at week 52 was assessed using each of the 8 BILAG index organ system scores.
A major clinical response was defined as achieving BILAG C scores or better in all organs at week 24 without experiencing a severe flare (1 new domain with a BILAG A score or 2 new domains with a BILAG B score) from day 1 to week 24, and maintaining this response without a moderate or severe flare (≥1 new domains with a BILAG A or B score) to week 52. A partial clinical response was defined as 1) achieving BILAG C scores or better at week 24 and maintaining this response without a new BILAG A or B score for 16 consecutive weeks, 2) achieving no more than 1 organ with a BILAG B score at week 24 without achieving ≥1 new BILAG A or B score to week 52, or 3) achieving a maximum of 2 BILAG B scores at week 24 without developing BILAG A or B scores in new domains until week 52 if the baseline BILAG score for the patient was 1 A score plus ≥2 B scores, ≥2 A scores, or ≥4 B scores. No clinical response was defined as failure to meet the definition of a major clinical response or a partial clinical response. Patients who terminated the study early were scored as having no clinical response.
Secondary end points
Secondary end points included: 1) the time-adjusted area under the curve minus baseline (AUCMB) of the BILAG score over 52 weeks, 2) the proportion of patients who achieved a major clinical response (excluding a partial clinical response) and the proportion of patients with a partial clinical response (including a major clinical response) at week 52, 3) the proportion of patients with a BILAG C score or better in all organs at week 24, 4) the time to the first moderate or severe disease flare, 5) improvement in quality of life as measured by the LupusQoL, and 6) the proportion of patients who achieved a major clinical response with a prednisone dosage of <10 mg/day from week 24 to week 52.
Subgroup analyses of the primary and secondary end points were preplanned for the following factors: race (African American/Hispanic versus others), age (≤40 years or >40 years), sex, assigned prednisone dose, background immunosuppressant, duration of lupus, long-term prednisone therapy, baseline BILAG A score, baseline BILAG-defined mucocutaneous or musculoskeletal system involvement, and baseline biomarkers.
Safety assessments
The incidence and severity of adverse events (AEs) were classified using the National Cancer Institute Common Toxicity Criteria for Adverse Events (version 3.0). Serious adverse events (SAEs), infusion-related AEs (an AE occurring during or within 24 hours following completion of the infusion of a study drug), and infection-related AEs were summarized independently. Serum chemistries, hematologic parameters, urinalysis results, quantitative IgG levels, T cell and B cell counts, human antichimeric antibody (HACA) levels, complement levels, and autoantibody levels were monitored.
Statistical analysis
The proportion of patients achieving a major clinical response, a partial clinical response, or no clinical response was compared between the placebo and rituximab groups using the stratified Wilcoxon's rank sum test, with the initial prednisone dose and race/ethnicity as stratification factors. Results were expressed as the proportion of patients in each of 6 cells (2 treatment groups × 3 response categories), and the P value was computed to compare the graded response across treatment arms. Two-sided P values less than 0.05 were considered significant. The analyses were performed using SAS software, version 9.1 (SAS Institute, Cary, NC).
RESULTS
Study population
Of the 257 patients, 88 were randomized to receive placebo, and 169 were assigned to the rituximab group. The demographic and disease characteristics of the patients are presented in Table 1. The mean age of the patients was 40.4 years, and the female:male ratio was 9:1. An African American, Hispanic, or Asian background was present in 42.1% of placebo-treated patients and in 41.5% of rituximab-treated patients.
Table 1.
Baseline demographic and disease characteristics of the patients*
| Characteristic | Placebo (n = 88) | Rituximab (n = 169) |
|---|---|---|
| Female sex | 93.2 | 89.9 |
| Age, mean ± SD years | 40.5 ± 12.8 | 40.2 ± 11.4 |
| Race, % | ||
| White | 55.7 | 56.2 |
| African American | 27.3 | 23.7 |
| Hispanic | 9.1 | 14.2 |
| Asian/Pacific Islander | 5.7 | 3.6 |
| Other | 2.2 | 1.1 |
| Disease duration, mean ± SD years | 8.7 ± 7.6 | 8.5 ± 7.2 |
| Long-term prednisone therapy† | 53.4 | 58.6 |
| Assigned prednisone dosage at screening, mg/kg/day | ||
| 0.5 | 61.4 | 62.7 |
| 0.75 | 29.5 | 32.0 |
| 1.0 | 9.1 | 5.3 |
| Background immunosuppressive drug | ||
| Azathioprine | 36.4 | 32.0 |
| Methotrexate | 27.3 | 27.8 |
| Mycophenolate mofetil | 36.4 | 39.6 |
| Disease activity | ||
| BILAG index score at entry | ||
| ≥1 A score | 56.0 | 51.0 |
| No A score, ≥3 B scores | 22.0 | 31.0 |
| 2 B scores only | 22.0 | 18.0 |
| BILAG index global score | ||
| Mean ± SD | 14.5 ± 5.6 | 14.0 ± 5.1 |
| Median (range) | 13 (7–33) | 13 (6–30) |
| SLE domains at baseline, no. (%) | ||
| General | ||
| BILAG A score | 11.0 (12.5) | 14.0 (8.3) |
| BILAG B score | 28.0 (31.8) | 53.0 (31.4) |
| Mucocutaneous | ||
| BILAG A score | 12.0 (13.6) | 27.0 (16.0) |
| BILAG B score | 51.0 (58.0) | 95.0 (56.2) |
| Neurologic | ||
| BILAG A score | 3.0 (3.4) | 3.0 (1.8) |
| BILAG B score | 6.0 (6.8) | 20.0 (11.8) |
| Musculoskeletal | ||
| BILAG A score | 27.0 (30.7) | 43.0 (25.4) |
| BILAG B score | 48.0 (54.5) | 93.0 (55.0) |
| Cardiovascular and respiratory | ||
| BILAG A score | 6.0 (6.8) | 8.0 (4.7) |
| BILAG B score | 13.0 (14.8) | 32.0 (18.9) |
| Vasculitis | ||
| BILAG A score | 3.0 (3.4) | 5.0 (3.0) |
| BILAG B score | 7.0 (8.0) | 23.0 (13.6) |
| Renal | ||
| BILAG A score | 0 (0) | 0 (0) |
| BILAG B score | 0 (0) | 3 (1.8) |
| Hematology | ||
| BILAG A score | 2.0 (2.3) | 3.0 (1.8) |
| BILAG B score | 17.0 (19.3) | 39.0 (23.1) |
Except where specified otherwise, values are the percent of patients. BILAG = British Isles Lupus Assessment Group; SLE = systemic lupus erythematosus.
Continuous use of corticosteroids for ≥6 months was required, as well as the inability to taper to a dosage of <10 mg/day without the recurrence of lupus activity.
After screening, 61.4% of placebo-treated patients and 62.7% of rituximab-treated patients received the lowest dosage of prednisone allowed (0.5 mg/kg/day). The mean ± SD prednisone dose at baseline was 45.9 ± 16.4 mg, and the median dose was 40.0 mg. Background immunosuppressive treatment included AZA, MMF, and MTX, and these treatments were distributed roughly evenly in the placebo group (36.4%, 36.4%, and 27.3%, respectively) and the rituximab group (32.0%, 39.6%, and 27.8%, respectively).
At baseline, most patients had disease activity in the mucocutaneous and musculoskeletal systems. In the placebo group, 58.0% and 13.6% of patients had BILAG B and BILAG A scores, respectively, for the mucocutaneous domain; in the rituximab group, these proportions were 56.2% and 16.0%, respectively. For the musculoskeletal domain, 54.5% and 30.7% of patients in the placebo group had BILAG B and BILAG A scores, respectively; in the rituximab group, these proportions were 55.0% and 25.4%, respectively. The most common concurrently active domains in the intent-to-treat population at entry were musculoskeletal and mucocutaneous (54.0%), musculoskeletal and general (constitutional features; 36.0%), and mucocutaneous and general (29.0%).
Seventy-three percent of patients in the placebo group and 71.0% in the rituximab group completed 52 weeks of treatment. Most withdrawals were due to adverse events or the patient's decision (Figure 1B).
Efficacy
Primary end point
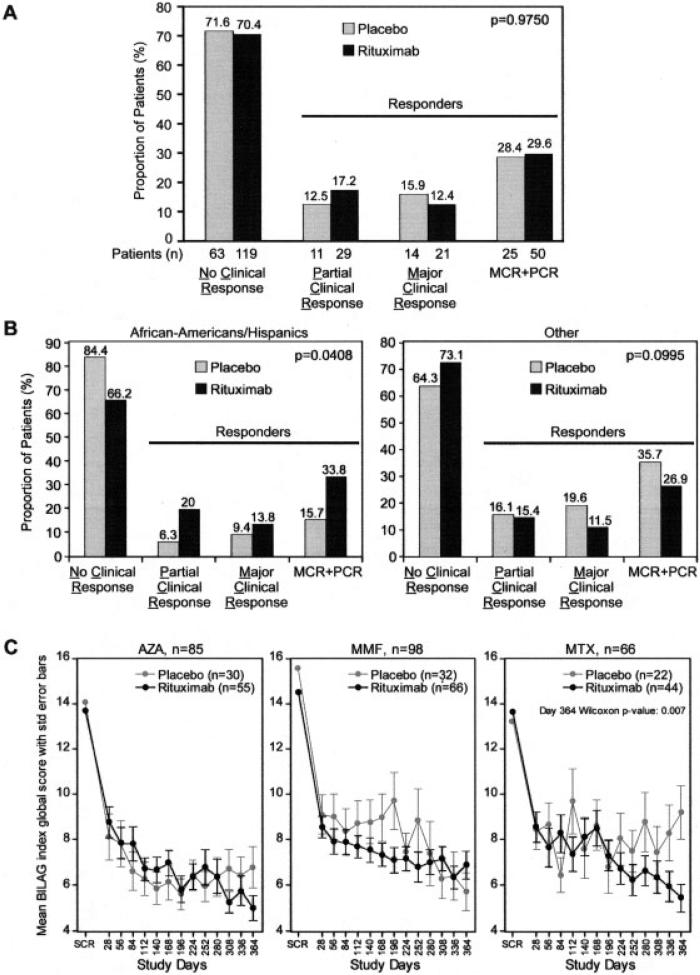
At week 52, no difference was noted in major clinical responses or partial clinical responses between the placebo group (15.9% had a major clinical response, and 12.5% had a partial clinical response) and the rituximab group (12.4% had a major clinical response, and 17.2% had a partial clinical response) (Figure 2A) relative to the overall response rate (28.4% versus 29.6%).
Figure 2.
A, Proportion of patients experiencing a major clinical response (MCR), a partial clinical response (PCR), and no clinical response (NCR) at 52 weeks. B, Responders with African American/Hispanic backgrounds. C, Responders, according to the background immunosuppressive drug. BILAG = British Isles Lupus Assessment Group; AZA = azathioprine; MMF = mycophenolate mofetil; MTX = methotrexate.
Subgroup analysis
There were no differences in the primary end point in prespecified subgroup analyses except in the African American/Hispanic group, which comprised approximately one-third of the patients in the study. Among the patients in this subgroup who received placebo, a major clinical response was observed in only 9.4%, and a partial clinical response was observed in only 6.3%. In contrast, among patients in this subgroup who received rituximab, a major clinical response was observed in 13.8%, and a partial clinical response was observed in 20.0% (P = 0.0408). This outcome was associated with a higher proportion of nonresponders in the placebo group compared with placebo-treated patients of other ethnic subgroups (Figure 2B). No difference in major secondary end points was observed in this subgroup.
None of the primary or secondary end points were met in the subgroup of patients on background MTX who were receiving rituximab. However, an ad hoc analysis showed that the rituximab-treated patients in this subgroup had improved mean BILAG global scores at week 52 compared with the placebo group (P = 0.007) (Figure 2C).
Secondary end point
In the intent-to-treat population, the mean ± SD time-adjusted AUCMB of the BILAG global score was −5.9 ± 4.5 in the placebo group compared with −5.8 ± 4.0 in the rituximab group, over 52 weeks (P = 0.8092). The proportion of patients with a BILAG C score or better in all domains in the first 24 weeks was the same in the placebo group (27.3%; 95% CI 18.0–36.6) and the rituximab group (24.9%; 95% CI 18.3–31.4 [P = 0.5602]). Patients with a major clinical response in whom prednisone was tapered to a dosage of <10 mg/day from week 24 to week 52 included 9 patients (10.2%) in the placebo group and 14 patients (8.3%) in the rituximab group.
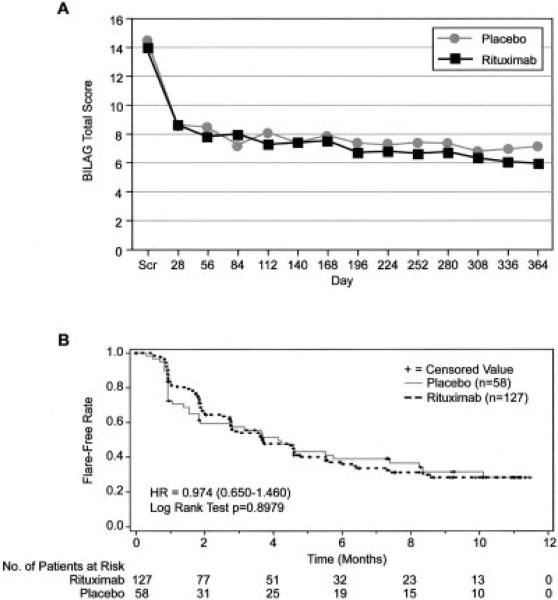
The mean ± SD change in the SF-36 quality of life physical component summary score from baseline to week 52 was the same in the placebo group (4.1 ± 17.0; 95% CI 0.3–7.9) and the rituximab group (8.2 ± 22.8; 95% CI 4.7–11.7 [P = 0.1277]). The mean BILAG global score over 52 weeks (Figure 3A) was also similar between these groups. The time to the first moderate or severe flare was calculated using Kaplan-Meier estimates of the flare-free time after the patient's first disease remission; the median was ~4 months in both groups (P = 0.8979) (Figure 3B).
Figure 3.
A, Mean British Isles Lupus Assessment Group (BILAG) index global scores over time. B, Kaplan-Meier curve showing the time to moderate/severe flare over 52 weeks. Scr = screening; HR = hazard ratio.
Immunologic parameters
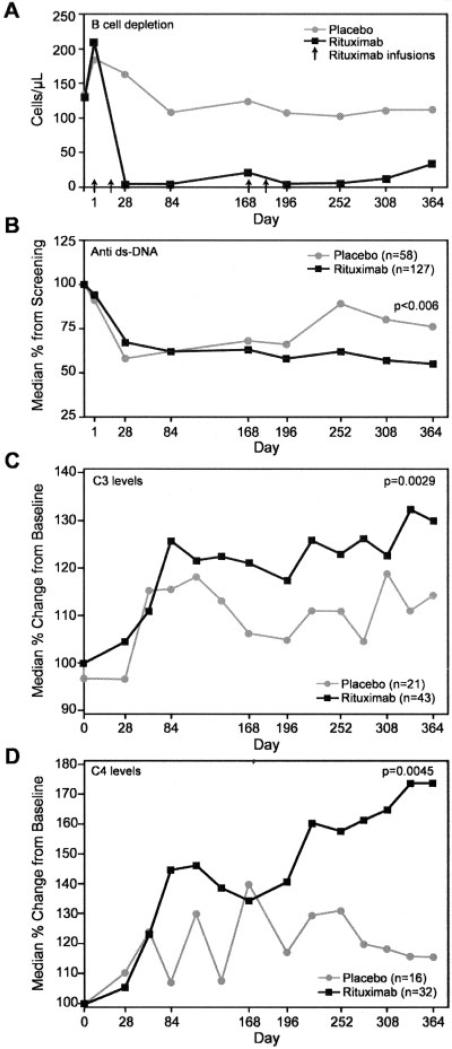
In the placebo group, levels of CD19-positive peripheral B lymphocytes remained stable for 52 weeks (>100 cells/μl). In contrast, rituximab treatment was associated with a rapid depletion of CD19-positive cells (<5 cells/μl) by 2 weeks after the infusions (Figure 4A). Approximately 9.5% of the treated patients did not achieve B cell depletion, having at least 10 cells/μl after the second infusion on day 15, with maintenance of this level until day 84. An ad hoc analysis removing patients with incomplete B cell depletion did not change the primary outcome.
Figure 4.
A, B cell depletion over time. Values are the means. B, Changes in the level of anti–double-stranded DNA (anti-dsDNA) over time. C and D, Changes in complement C3 and C4 levels over time in patients with low baseline levels of C3 and C4.
At screening, no patients in the placebo group or the rituximab group tested positive for HACAs to rituximab. At week 52, 3.4% patients in the placebo group had HACAs, and 26.0% of patients in the rituximab group tested positive for HACAs at any time. The results of HACA analysis did not affect the primary and secondary efficacy outcomes.
The levels of autoantibodies, including anti–double-stranded DNA (anti-dsDNA), anti-La, anti-Ro, anti-Sm, and anti-RNP, were examined. Among patients who began the study with detectable anti-dsDNA antibody levels, the decrease in rituximab-treated patients (n = 101) was significantly greater (median 76%) than that in the placebo group (n = 50; median 55% [P = 0.006]) (Figure 4B), despite the fact that both groups were receiving steroids. Among patients who began the trial with a low complement level, there was a greater increase in those treated with rituximab than in those treated with placebo for C3 (median 129% versus 114%; P = 0.0029) (Figure 4C) and for C4 (173% versus 115%; P = 0.0045) (Figure 4D). The percentage of patients with a low complement level at entry in whom the level had normalized at week 52 was also higher in the rituximab group compared with the placebo group (for C3, 40.6% versus 31.3% [P = 0.3711; for C4, 51.0% versus 21.7% [P = 0.0188]).
Safety
AEs in the intent-to-treat population are shown in Table 2. The proportion of patients with any treatment-emergent SAEs was similar in the placebo and rituximab groups (36.4% of placebo-treated subjects and 37.9% rituximab-treated patients experienced serious study-drug related AEs).
Table 2.
Adverse events in the safety population*
| Adverse event | Placebo (n = 88) | Rituximab (n = 169) |
|---|---|---|
| Any treatment-emergent SAE | 32 (36.4) | 64 (37.9) |
| Any treatment-emergent SAE reported in ≥5% of patients | ||
| Cardiac disorder | 5 (5.7) | 5 (3.0) |
| Infections and infestations | 15 (17.0) | 16 (9.5) |
| Gastrointestinal disorders | 7 (8.0) | 8 (4.7) |
| General disorder | 5 (5.7) | 7 (4.1) |
| Musculoskeletal and connective tissue disorders | 5 (5.7) | 9 (5.3) |
| Neutropenia | 0 (0) | 6 (3.6) |
| Any study drug–related treatment-emergent SAE | 8 (9.1) | 13 (7.7) |
| Any infusion-related AE | 34 (38.6) | 74 (43.8) |
| First infusion | 26 (29.5) | 46 (27.2) |
| Second infusion | 14 (16.5) | 29 (17.6) |
| Third infusion | 7 (10.0) | 23 (16.3) |
| Fourth infusion | 4 (5.9) | 25 (18.5) |
| Any infusion-related SAE | 15 (17.0) | 16 (9.5) |
| Any treatment-emergent infection-related SAE | 15 (17.0) | 16 (9.5) |
| Any treatment-emergent infection-related SAE reported in ≥2% of patients | ||
| Lower respiratory tract and lungs | 4 (4.5) | 5 (3.0) |
| Bacterial | 4 (4.5) | 4 (2.4) |
| Abdominal and gastrointestinal | 4 (4.5) | 2 (1.2) |
| Sepsis, bacteremia, viremia, and fungemia NEC | 3 (3.4) | 2 (1.2) |
| Death | 1 (1.1) | 4 (2.4) |
Values are the number (%). SAE = serious adverse event; NEC = not elsewhere classified.
Infusion-related AEs occurred in similar percentages of each group during the first course of infusions and decreased more in the placebo group than in the rituximab group during the second course (8.0% versus 13.6% for the first infusion, and 4.5% versus 14.8% for the second infusion). These were primarily mild and transient responses, although one SAE of angioedema was reported.
Four serum sickness adverse events (1 of which was an SAE) occurred in the rituximab group (3 of the 4 patients were HACA-positive, including the patient who experienced the SAE) in comparison with none in the placebo group. Two nonserious events were infusion related. Three of the 4 cases of serum sickness were resolved within 1 week, while 1 case (nonserious) was resolved within 3 weeks.
The most common AEs in the placebo group were nervous system (13.6%), general (10.2%), and gastrointestinal (10.2%) disorders, compared with nervous system (14.2%), gastrointestinal (14.2%), skin and subcutaneous tissue (11.8%), and general disorders (10.7%) in the rituximab group.
There were more grade 3 and grade 4 neutropenia events in the rituximab group (7.7%) compared with the placebo group (3.4%), including more cases of grade 4 neutropenia (6 cases versus 0), but this did not correlate with significant infectious events. Of the 6 patients in the rituximab group who had grade 4 neutropenia, 3 patients were receiving MMF as background therapy, 2 were receiving AZA, and 1 was receiving MTX. Most cases of neutropenia (5 of 6 patients with grade 4 neutropenia) were resolved at the subsequent monthly visit.
The proportion of patients experiencing infectious AEs was equivalent between the 2 groups (for rituximab, 82.2%; for placebo, 83.0%), with upper respiratory tract infections being the most common (for placebo, 46.6%; for rituximab, 49.1%). The proportion of patients with herpesvirus infections was lower in the placebo group (7 patients [8.0%]) than in the rituximab group (26 patients [15.4%]), including rare oral and genital outbreaks, as well as herpes zoster infection, which occurred in 3 placebo-treated patients (3.4%) and in 16 rituximab-treated patients (9.5%). In most patients (22 of 33), herpesvirus infections resolved within 1 month. Sepsis occurred in 2.3% of placebo-treated patients and in <1% of rituximab-treated patients.
The proportion of patients in whom serious infection developed (Table 2) was greater in the placebo group (17.0%) than in the rituximab group (9.5%). One patient in the placebo group died (cardiopulmonary arrest), and this death occurred 1 month after the patient had withdrawn from the study. Three patients in the rituximab group died, including 1 patient with a perforated bowel, 1 patient with multiple drug intoxication, and 1 patient in whom the cause of death was unknown. In addition, 1 neonatal death of a premature infant delivered at 33 weeks to a rituximab-treated patient was reported.
DISCUSSION
B cells are believed to play a central role in the pathogenesis of SLE. Preclinical studies (39) evaluating anti-CD20 monoclonal antibody therapy and single-arm open-label studies of patients with SLE refractory to treatment (40) provided the rationale for a more rigorous study of rituximab in SLE. We report that an adequately powered, double-blind, placebo-controlled trial of rituximab did not meet its prespecified primary and secondary end points after 52 weeks of treatment.
Significant B cell depletion was obtained within 2 weeks of rituximab administration; however, a subgroup of patients (9.5%) did not experience depletion. Incomplete B cell depletion has been observed in murine models and humans and may involve cellular reconstitution of naive B cells or incomplete depletion in other tissues (39,41,42). The significance of this is unknown, but removing this group of patients did not impact efficacy as measured by the primary and secondary end points.
The EXPLORER trial was designed to enroll a heterogeneous population of patients with significant SLE disease activity that was inadequately controlled by background immunosuppressants. The purpose of the design was to avoid the pitfalls observed in previous lupus trials in which insufficient lupus disease activity at entry might have impeded identification of differences between a test treatment and background therapy.
The EXPLORER population was maintained on the background treatment throughout the trial, and both arms were given a steroid initiation and taper to control immediate disease activity. Therefore, sicker patients could be enrolled, treated with an appropriate standard of care, and a novel definition of response could be investigated. The stringency of the standards for response in this study is illuminated by the fact that if any patient had 1 new BILAG B score during the second half of the study, that patient would be considered a treatment failure. Seventeen patients in the rituximab group and 4 patients in the placebo group were categorized as having no clinical response solely on the basis of achieving 1 new BILAG B score after 6 months. A BILAG B score signifies a range of moderate disease activity at any time in a 4-week period, and the minimal cutoff points can be reached through relatively mild and transient disease flares. This was confirmed by a study in which only 41% of patients with BILAG B disease flares were treated (43). Thus, in all cases, 1 relatively minor disease flare would count as a treatment failure. It was not known prior to this study whether its design would work for or against the differentiation of treatment effects in SLE. Because negative results occurred, there are no data to support or refute the robustness of this study design, although some hypotheses can be considered based on exploratory outcome data.
This study accomplished the important task of enrolling demonstrably ill patients. Eighty-one percent of patients entered with ≥1 BILAG A score or ≥3 BILAG B scores, and only 19.0% entered with the minimum of 2 BILAG B scores. Despite treatment with prednisone and immunosuppressants, most patients continued to experience some clinical activity: only 27.3% of placebo-treated patients and 24.9% of rituximab-treated patients achieved a BILAG C score or less activity in all organs at week 24, and the majority of withdrawals due to clinical disease activity occurred prior to this time point. Furthermore, the primary end point definition of response at week 52 was achieved in <30% of patients in either group.
Despite some continued disease activity in both groups, the results suggested that placebo-treated and rituximab-treated patients improved during the first month, when the largest steroid doses were given (Figures 2C and 3A). An ad hoc ACR committee has previously defined a 7-point decrease in the BILAG score as a clinically significant response (33). According to this definition, both the placebo and rituximab groups achieved a clinically significant response by day 28. The mean score did not subsequently increase again during the study in either group. This suggests that the benefit of initial steroid therapy was maintained by continued background immunosuppression in the placebo group, preventing an increase in disease activity (back to baseline levels) after tapering of steroids, and that rituximab did not add any incremental efficacy benefit compared with placebo.
One alternative interpretation of this observation is that both the placebo and rituximab groups received adequate treatment and achieved all that could be expected using current measures. In this case, although the efficacy of rituximab cannot be inferred, the possibility remains that potential benefits could have become apparent if patients in the placebo group had not maintained their initial responses during the course of the trial. This is supported by the suggested efficacy of rituximab observed in the African American/Hispanic subgroup, although this finding may be spurious in the setting of a trial with negative results and multiple analyses. The decision of how aggressive background treatment should be in a population of sick patients is a study design problem previously observed in trials of SLE (44). Because African American and Hispanic patients with SLE may have disease that is more refractory to treatment (45), another hypothesis is that a unique biology underlies these different results. It is possible that disease in these patients is more B cell driven, or that B cell depletion is less likely to stimulate other mechanisms that could confound the effects of rituximab. In either case, biologic differences before and after treatment could be tested in a future trial of B cell depletion therapy. A shared biology might be identified as more common in one ethnic group but is also likely to cross over ethnic differences and could be used to more rationally identify patients appropriate to treat.
At the end of the study, a difference in BILAG scores was observed between the placebo-treated and rituximab-treated patients whose background treatment was MTX. Caution should be used in interpreting this post hoc analysis, given that rituximab did not show benefit in this subgroup at the predefined primary and secondary analyses. In addition, the sample size was small at the week 52 visit, when the withdrawal rate in the 2 treatment groups was imbalanced (6 of 24 patients in the placebo group [25.0%] and 19 of 46 patients in the rituximab group [41.3%]). However, this does raise the possibility that those who remained in the MTX-treated placebo subgroup were beginning to experience a disease flare at the end of the study as compared with patients in the placebo group who were receiving background AZA or MMF. A separate ad hoc analysis of the population of MTX-treated patients also revealed a separation favoring rituximab when evaluating the physician's global score and the patient's global score over 52 weeks (data not shown).
Decreases in the level of anti-dsDNA autoantibodies and increases in complement C3 and C4 levels were also greater in the rituximab group than in the placebo group, confirming that rituximab had a biologic effect that was previously observed in preliminary studies. No significant change in the levels of anti–extractable nuclear antigen autoantibodies was observed, in contrast to what was observed in some previous studies (40,46).
The proportion of HACA-positive rituximab-treated patients (26.0%) was fairly high for this population of patients who were being treated with intravenous and oral steroids while receiving a background immunosuppressive drug. The incidences of AEs and SAEs were well-balanced between groups, although the frequency of treatment-emergent infectious SAEs was higher in the placebo group than in the group of patients receiving rituximab. Immunosuppressive drugs and prednisone could contribute to the AEs, but the balance of these background treatments between the groups would make it difficult to evaluate such a contribution. The incidence of infusion-related events, including hypotension and/or fever, was similar during the first treatment course, and the majority of events were mild-to-moderate in severity. During the second course of treatment, the number of infusion reactions was higher in the rituximab group. The incidence of neutropenia was also higher in the rituximab group, and the occurrences were distributed across all 3 background immunosuppressant therapies. Outside of the oncology setting, neutropenia has not been widely observed in patients receiving rituximab in combination with chemotherapeutic agents (47); however, a case of neutropenia in a patient with severe RA was recently reported (48). One case of neutropenia was associated with an infectious SAE. No cases of serum sickness were reported in the placebo group, although 1 SAE and 3 AEs of serum sickness were reported in the rituximab group. Three of the 4 patients who had serum sickness were HACA positive.
In summary, the EXPLORER trial demonstrated no difference in primary or secondary end points between the placebo group and the rituximab group over 52 weeks of treatment, in patients with moderate-to-severe SLE.
Acknowledgments
Supported by Genentech.
APPENDIX A: EXPLORER TRIAL CLINICAL CENTERS AND INVESTIGATORS
Investigators for the EXPLORER trial, including the authors, are as follows: for the University of Alabama at Birmingham, Graciela S. Alarcon; for Arizona Arthritis and Rheumatology Associates, Paradise Valley, Arizona, Ralph Bennett; for the East Bay Rheumatology Research Institute, San Leandro, California, C. Michael Neuwelt; for Cedars-Sinai Medical Center, Los Angeles, California, Daniel Wallace; for Stanford Health Services/Blake Wilbur Clinic, Palo Alto, California, Eliza F. Chakravarty, Mark Genovese; for the University of California, Los Angeles, Jennifer Grossman; for the University of California, San Francisco, John Davis, Maria Dall'Era; for the University of California, San Diego Thornton Hospital, La Jolla, Kenneth Kalunian; for the Family Arthritis Center, Jupiter, Florida, Howard Busch; for Arthritis and Rheumatic Disease Specialties, Aventura, Florida, Norman Gaylis; for Emory University School of Medicine, Atlanta, Georgia, S. Sam Lim; for the Coeur d'Alene Arthritis Clinic, Coeur d'Alene, Idaho, Craig Wiesenhutter; for the Intermountain Research Center, Boise, Idaho, James Loveless; for Northwestern University/Feinberg School of Medicine, Chicago, Illinois, Rosalind Ramsey-Goldman; for the University of Chicago, Chicago, Illinois, Tammy O. Utset; for the Tri-State Arthritis and Rheumatology Center, Evansville, Indiana, Moges Sisay; for the University of Kansas Medical Center, Kansas City, Kevin M. Latinis; for the Louisiana State University Health Science Center, Shreveport, Seth M. Berney; for Johns Hopkins University, Baltimore, Maryland, Michelle Petri; for The Center for Rheumatology and Bone Research, Wheaton, Maryland, Evan L. Siegel; for Tufts–New England Medical Center, Boston, Massachusetts, Elena Massarotti, Timothy E. McAlindon; for Beth Israel Deaconess Medical Center, Boston, Massachusetts, John Donohue, Lisa Fitzgerald; for the Michigan Arthritis Research Center, Brighton, James E. Dowd; for the University of Michigan Health System, Ann Arbor, Joseph McCune; for Buffalo Rheumatology, Orchard Park, New York, Joseph Grisanti; for the Feinstein Institute for Medical Research, Manhasset, New York, Meggan Mackay; for the Hospital for Special Surgery, New York, New York, Michael Lockshin; for the Seligman Center for Advanced Therapeutics, New York, New York, Jill Buyon; for the North Shore Long Island Jewish Health System, Lake Success, New York, Richard Furie; for Physician's East, Greenville, North Carolina, Jeff Alloway; for Duke University Medical Center, Durham, North Carolina, Stacy Ardoin, Meggan Clowse, Joseph C. Shanahan; for the University of North Carolina Hospital, Chapel Hill, Mary Anne Dooley; for the Ohio State University Medical Center, Columbus, Brad H. Rovin; for the Oklahoma Medical Research Foundation, Oklahoma City, Joan T. Merrill; for the Bone and Joint Hospital Research Department, Oklahoma City, Oklahoma, Larry Willis; for the Oklahoma Center for Arthritis Therapy and Research, Tulsa, Ellen Zanetakis; for East Penn Rheumatology Associates, Bethlehem, Pennsylvania, Allen Samuels; for the Albert Einstein Medical Center, Philadelphia, Pennsylvania, Lawrence Brent, Ramesh Pappu; for the University of Pennsylvania Medical Center, Philadelphia, Robert A. Eisenberg; for the University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, Susan Manzi; for the Medical University of South Carolina, Charleston, Gary Gilkeson, James C. Oates; for Arthritis Associates, Hixson, Tennessee, Joseph Huffstutter; for the Arthritis Centers of Texas, Dallas, Andrew Chubick, Linda Teague; for the Metroplex Clinical Research Center, Dallas, Texas, Stanley Cohen, Thomas D. Geppert; for the Texas Research Center, Sugar Land, Texas, Angela McCain; for the Houston Institute for Clinical Research, Houston, Texas, Dale Halter; for the Virginia Commonwealth University Clinical Research Center, Richmond, W. Neal Roberts; for the University of Washington, Seattle, Carin Dugowson; for Seattle Rheumatology Associates, Seattle, Washington, Philip Mease; for St. Joseph's Health Care, London, Ontario, Canada, Janet E. Pope; for the University of Manitoba, Manitoba, Canada, Christine Peschken.
Footnotes
ClinicalTrials.gov identifier: NCT00137969.
Dr. Merrill has received speaking fees, consulting fees, and/or honoraria from Genentech, Bristol-Myers Squibb, MedImmune, UCB, Wyeth, and Cephalon (less than $10,000 each), and has consulted with investment analysts. Dr. Wallace has received speaking fees, consulting fees, and/or honoraria from Genentech (less than $10,000). Dr. Shanahan has received speaking fees, consulting fees, and/or honoraria from Genentech, Actelion, and Cypress (less than $10,000 each), and owns stock or stock options in Schering-Plough. Dr. Latinis has received speaking fees, consulting fees, and/or honoraria from Genentech (less than $10,000). Dr. Oates has received speaking fees, consulting fees, and/or honoraria from Genentech (less than $10,000). Dr. Utset has received advisory board fees from Genentech (more than $10,000). Dr. Gordon has received speaking fees, consulting fees, and/or honoraria from Roche Pharmaceuticals and Genentech (less than $10,000 each). Dr. Hsieh owns stock or stock options in Genentech.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Merrill had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Merrill, Neuwelt, Wallace, Gordon, Isenberg, Hsieh, Zhang, Brunetta.
Acquisition of data. Merrill, Neuwelt, Shanahan, Oates, Utset.
Analysis and interpretation of data. Merrill, Neuwelt, Wallace, Latinis, Utset, Gordon, Isenberg, Hsieh, Zhang, Brunetta.
REFERENCES
- 1.Gill JM, Quisel AM, Rocca PV, Walters DT. Diagnosis of systemic lupus erythematosus. Am Fam Physician. 2003;68:2179–86. [PubMed] [Google Scholar]
- 2.Rahman A, Isenberg DA. Systemic lupus erythematosus. N Engl J Med. 2008;358:929–39. doi: 10.1056/NEJMra071297. [DOI] [PubMed] [Google Scholar]
- 3.Stoll T, Seifert B, Isenberg DA. SLICC/ACR damage index is valid, and renal and pulmonary organ scores are predictors of severe outcome in patients with systemic lupus erythematosus. Br J Rheumatol. 1996;35:248–54. doi: 10.1093/rheumatology/35.3.248. [DOI] [PubMed] [Google Scholar]
- 4.Dooley MA, Hogan S, Jennette C, Falk R, for the Glomerular Disease Collaborative Network Cyclophosphamide therapy for lupus nephritis: poor renal survival in black Americans. Kidney Int. 1997;51:1188–95. doi: 10.1038/ki.1997.162. [DOI] [PubMed] [Google Scholar]
- 5.Alarcon GS, Roseman JM, McGwin G, Jr, Uribe A, Bastian HM, Fessler BJ, et al. for the LUMINA study group Systemic lupus erythematosus in three ethnic groups. XX. Damage as a predictor of further damage. Rheumatology (Oxford) 2004;43:202–5. doi: 10.1093/rheumatology/keg481. [DOI] [PubMed] [Google Scholar]
- 6.Lam GK, Petri M. Assessment of systemic lupus erythematosus. Clin Exp Rheumatol. 2005;23(Suppl 39):S120–32. [PubMed] [Google Scholar]
- 7.Gladman DD, Urowitz MB, Rahman P, Ibanez D, Tam LS. Accrual of organ damage over time in patients with systemic lupus erythematosus. J Rheumatol. 2003;30:1955–9. [PubMed] [Google Scholar]
- 8.Borchers AT, Keen CL, Shoenfeld Y, Gershwin ME. Surviving the butterfly and the wolf: mortality trends in systemic lupus erythematosus. Autoimmun Rev. 2004;3:423–53. doi: 10.1016/j.autrev.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Lipsky PE. Systemic lupus erythematosus: an autoimmune disease of B cell hyperactivity. Nature Immunol. 2001;2:764–6. doi: 10.1038/ni0901-764. [DOI] [PubMed] [Google Scholar]
- 10.Mok CC, Lau CS. Pathogenesis of systemic lupus erythematosus. J Clin Pathol. 2003;56:481–90. doi: 10.1136/jcp.56.7.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Renaudineau Y, Pers JO, Bendaoud B, Jamin C, Youinou P. Dysfunctional B cells in systemic lupus erythematosus. Autoimmun Rev. 2004;3:516–23. doi: 10.1016/j.autrev.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 12.Swaak T, Smeenk R. Clinical significance of antibodies to double stranded DNA (dsDNA) for systemic lupus erythematosus (SLE). Clin Rheumatol. 1987;6:56–73. doi: 10.1007/BF02200721. [DOI] [PubMed] [Google Scholar]
- 13.Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349:1526–33. doi: 10.1056/NEJMoa021933. [DOI] [PubMed] [Google Scholar]
- 14.Anderson KC, Bates MP, Slaughenhoupt BL, Pinkus GS, Schloss-man SF, Nadler LM. Expression of human B cell-associated antigens on leukemias and lymphomas: a model of human B cell differentiation. Blood. 1984;63:1424–33. [PubMed] [Google Scholar]
- 15.Maloney DG, Liles TM, Czerwinski DK, Waldichuk C, Rosenberg J, Grillo-Lopez A, et al. Phase I clinical trial using escalating single-dose infusion of chimeric anti-CD20 monoclonal antibody (IDEC-C2B8) in patients with recurrent B-cell lymphoma. Blood. 1994;84:2457–66. [PubMed] [Google Scholar]
- 16.Reff ME, Carner K, Chambers KS, Chinn PC, Leonard JE, Raab R, et al. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood. 1994;83:435–45. [PubMed] [Google Scholar]
- 17.Edwards JC, Szczepanski L, Szechinski J, Filipowicz-Sosnowska A, Emery P, Close DR, et al. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med. 2004;350:2572–81. doi: 10.1056/NEJMoa032534. [DOI] [PubMed] [Google Scholar]
- 18.Cohen SB, Emery P, Greenwald MW, Dougados M, Furie RA, Genovese MC, et al. Rituximab for rheumatoid arthritis refractory to anti–tumor necrosis factor therapy: results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum. 2006;54:2793–806. doi: 10.1002/art.22025. [DOI] [PubMed] [Google Scholar]
- 19.Emery P, Fleischmann R, Filipowicz-Sosnowska A, Schechtman J, Szczepanski L, Kavanaugh A, et al. The efficacy and safety of rituximab in patients with active rheumatoid arthritis despite methotrexate treatment: results of a phase IIb randomized, double-blind, placebo-controlled, dose-ranging trial. Arthritis Rheum. 2006;54:1390–400. doi: 10.1002/art.21778. [DOI] [PubMed] [Google Scholar]
- 20.Albert D, Khan S, Stansberry J, Kolasinski S, Tsai D, Kamoun M, et al. A phase I trial of rituximab (anti-CD20) for treatment of systemic lupus erythematosus [abstract]. Arthritis Rheum. 2004;50(Suppl):S446–7. [Google Scholar]
- 21.Ryan JP, Singer NG, Scalzi LV. Treatment of resistant SLE with rituximab administered without cyclophosphamide [abstract]. Arthritis Rheum. 2004;50(Suppl):S413–4. [Google Scholar]
- 22.Leandro MJ, Edwards JC, Cambridge G, Ehrenstein MR, Isenberg DA. An open study of B lymphocyte depletion in systemic lupus erythematosus. Arthritis Rheum. 2002;46:2673–7. doi: 10.1002/art.10541. [DOI] [PubMed] [Google Scholar]
- 23.Leandro MJ, Cambridge G, Edwards JC, Ehrenstein MR, Isenberg DA. B-cell depletion in the treatment of patients with systemic lupus erythematosus: a longitudinal analysis of 24 patients. Rheumatology. 2005;44:1542–5. doi: 10.1093/rheumatology/kei080. [DOI] [PubMed] [Google Scholar]
- 24.Van Vollenhoven RF, Gunnarsson I, Welin-Henriksson E, Sun delin B, Osterborg A, Jacobson SH, et al. Biopsy-verified response of severe lupus nephritis to treatment with rituximab (anti-CD20 monoclonal antibody) plus cyclophosphamide after biopsy-documented failure to respond to cyclophosphamide alone. Scand J Rheumatol. 2004;33:423–7. doi: 10.1080/03009740410010227. [DOI] [PubMed] [Google Scholar]
- 25.Van Vollenhoven RF, Gunnarsson I, Welin-Henriksson E, Jonsdottir T, Sundelin B, Jacobsson S, et al. Rituximab plus cyclophosphamide in severe SLE: results in 15 patients who failed conventional immunosuppressive therapy [abstract]. Arthritis Rheum. 2005;52(Suppl):S741. [Google Scholar]
- 26.Cambridge G, Leandro MJ, Teodorescu M, Manson J, Rahman A, Isenberg DA, et al. B cell depletion therapy in systemic lupus erythematosus: effect on autoantibody and antimicrobial antibody profiles. Arthritis Rheum. 2006;54:3612–22. doi: 10.1002/art.22211. [DOI] [PubMed] [Google Scholar]
- 27.Smith KG, Jones RB, Burns SM, Jayne DR. Long-term comparison of rituximab treatment for refractory systemic lupus erythematosus and vasculitis: remission, relapse, and re-treatment. Arthritis Rheum. 2006;54:2970–82. doi: 10.1002/art.22046. [DOI] [PubMed] [Google Scholar]
- 28.Tokunaga M, Saito K, Kawabata D, Imura Y, Fujii T, Nakayamada S, et al. Efficacy of rituximab (anti-CD20) for refractory systemic lupus erythematosus involving the central nervous system. Ann Rheum Dis. 2007;66:470–5. doi: 10.1136/ard.2006.057885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sutter JA, Kwan-Morley J, Dunham J, Du YZ, Kamoun M, Albert D, et al. A longitudinal analysis of SLE patients treated with rituximab (anti-CD20): factors associated with B lymphocyte recovery. Clin Immunol. 2008;126:282–90. doi: 10.1016/j.clim.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 30.Lu TY, Jonsdottir T, van Vollenhoven RF, Isenberg DA. Prolonged B-cell depletion following rituximab therapy in systemic lupus erythematosus: a report of two cases. Ann Rheum Dis. 2008;67:1493–4. doi: 10.1136/ard.2008.091124. [DOI] [PubMed] [Google Scholar]
- 31.Kumar S, Benseler SM, Kirby-Allen M, Silverman ED. B-cell depletion for autoimmune thrombocytopenia and autoimmune hemolytic anemia in pediatric systemic lupus erythematosus. Pediatrics. 2009;123:159–63. doi: 10.1542/peds.2008-2361. [DOI] [PubMed] [Google Scholar]
- 32.Reynolds J, Toescu V, Yee C, Prabu A, Situnayake D, Gordon C. Effects of rituximab on resistant SLE disease including lung involvement. Lupus. 2009;18:67–73. doi: 10.1177/0961203308094653. [DOI] [PubMed] [Google Scholar]
- 33.American College of Rheumatology Ad Hoc Committee on Systemic Lupus Erythematosus Response Criteria The American College of Rheumatology response criteria for systemic lupus erythematosus clinical trials: measures of overall disease activity. Arthritis Rheum. 2004;50:3418–26. doi: 10.1002/art.20628. [DOI] [PubMed] [Google Scholar]
- 34.Hay EM, Bacon PA, Gordon C, Isenberg DA, Maddison P, Snaith ML, et al. The BILAG index: a reliable and valid instrument for measuring clinical disease activity in systemic lupus erythematosus. Q J Med. 1993;86:447–58. [PubMed] [Google Scholar]
- 35.Isenberg DA, Gordon C, the British Isles Lupus Assessment Group From BILAG to BLIPS: disease activity assessment in lupus past, present and future. Lupus. 2000;9:651–4. doi: 10.1191/096120300672904669. [DOI] [PubMed] [Google Scholar]
- 36.Yu EB, Shikiar R, Howard K, Kalunian KC, Petrillo J, Thompson C, et al. Validation of LUP-QOL: a lupus-specific measure of health-related quality of life (HRQL) [abstract]. Ann Rheum Dis. 2006;65(Suppl II):601. [Google Scholar]
- 37.Ware JE, Jr, Sherbourne CD. The MOS 36-item Short-Form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- 38.Ehrenstein MR, Conroy SE, Heath J, Latchman DS, Isenberg DA. The occurrence, nature and distribution of flares in a cohort of patients with systemic lupus erythematosus: a rheumatological view. Br J Rheumatol. 1995;34:257–60. doi: 10.1093/rheumatology/34.3.257. [DOI] [PubMed] [Google Scholar]
- 39.Ahuja A, Shupe J, Dunn R, Kashgarian M, Kehry MR, Shlomchik MJ. Depletion of B cells in murine lupus: efficacy and resistance. J Immunol. 2007;179:3351–61. doi: 10.4049/jimmunol.179.5.3351. [DOI] [PubMed] [Google Scholar]
- 40.Tanaka Y, Yamamoto K, Takeuchi T, Nishimoto N, Miyasaka N, Sumida T, et al. A multicenter phase I/II trial of rituximab for refractory systemic lupus erythematosus. Mod Rheumatol. 2007;17:191–7. doi: 10.1007/s10165-007-0565-z. [DOI] [PubMed] [Google Scholar]
- 41.Shlomchik MJ, Madaio MP, Ni D, Trounstein M, Huszar D. The role of B cells in lpr/lpr-induced autoimmunity. J Exp Med. 1994;180:1295–306. doi: 10.1084/jem.180.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gong Q, Ou Q, Ye S, Lee WP, Cornelius J, Diehl L, et al. Importance of cellular microenvironment and circulatory dynamics in B cell immunotherapy. J Immunol. 2005;174:817–26. doi: 10.4049/jimmunol.174.2.817. [DOI] [PubMed] [Google Scholar]
- 43.Gordon C, Sutcliffe N, Skan J, Stoll T, Isenberg DA. Definition and treatment of lupus flares measured by the BILAG index. Rheumatology. 2003;42:1372–9. doi: 10.1093/rheumatology/keg382. [DOI] [PubMed] [Google Scholar]
- 44.Lewis EJ, Hunsicker LG, Lan SP, Rohde RD, Lachin JM, for the Lupus Nephritis Collaborative Study Group A controlled trial of plasmapheresis therapy in severe lupus nephritis. N Engl J Med. 1992;326:1373–9. doi: 10.1056/NEJM199205213262101. [DOI] [PubMed] [Google Scholar]
- 45.Uribe AG, McGwin G, Jr, Reveille JD, Alarcon GS. What have we learned from a 10-year experience with the LUMINA (Lupus in Minorities; Nature vs. nurture) cohort? Where are we heading? Autoimmun Rev. 2004;3:321–9. doi: 10.1016/j.autrev.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 46.Anolik JH, Barnard J, Cappione A, Pugh-Bernard AE, Felgar RE, Looney RJ, et al. Rituximab improves peripheral B cell abnormalities in human systemic lupus erythematosus. Arthritis Rheum. 2004;11:3580–90. doi: 10.1002/art.20592. [DOI] [PubMed] [Google Scholar]
- 47.Voog E, Morschhauser F, Solal-Celigny P. Neutropenia in patients treated with rituximab. N Engl J Med. 2003;348:2691–4. doi: 10.1056/NEJM200306263482620. [DOI] [PubMed] [Google Scholar]
- 48.Marotte H, Paintaud G, Watier H, Miossec P. Rituximab-related late-onset neutropenia in a patient with severe rheumatoid arthritis. Ann Rheum Dis. 2008;67:893–4. doi: 10.1136/ard.2007.081166. [DOI] [PubMed] [Google Scholar]