Abstract
Parkinson’s disease (PD) is one of the most common degenerative disorders of the central nervous system among the elderly. The disease is caused by the slow deterioration of the dopaminergic neurons in the substantia nigra. Treatment strategies to protect dopaminergic neurons from progressive damage have received much attention. However there is no effective treatment for PD. Traditional Chinese medicines have shown potential clinical efficacy in attenuating the progression of PD. Increasing evidence indicates that constituents of some Chinese herbs include resveratrol, curcumin, and ginsenoside can be neuroprotective. Since pathologic processes in PD including inflammation, oxidative stress, apoptosis, mitochondrial dysfunction, and genetic factors lead to neuronal degeneration, and these Chinese herbs can protect dopaminergic neurons from neuronal degeneration, in this article, we review the neuroprotective roles of these herbs and summarize their anti-inflammatory, antioxidant, and anti-apoptotic effects in PD. In addition, we discuss their possible mechanisms of action in in vivo and in vitro models of PD. Traditional Chinese medicinal herbs, with their low toxicity and side-effects, have become the potential therapeutic interventions for prevention and treatment of PD and other neurodegenerative diseases.
Keywords: Chinese herbs, resveratrol, curcumin, ginsenoside, Parkinson’s disease, neuroprotection
Introduction
Parkinson’s disease (PD) is a common neurodegenerative disease characterized by motor symptoms of tremor, rigidity, bradykinesia, and postural instability. The main pathological change in PD is progressive loss of dopaminergic neurons in the substantia nigra of the midbrain, though the cause of cell death is unknown [1]. Treatments for PD include oral preparations of levodopa (L-DOPA) and dopamine (DA) receptor agonists and monoamine oxidase-B (MAO-B) inhibitors, and deep brain stimulation of the subthalamic nucleus and globus pallidus by surgically implanted electrodes, and stem cell transplantation into the striatum. Especially in recent years, experimental and clinical research on stem cells for treatment of PD has attracted increasing attention [2,3]. Such treatments have proven to have some positive results, but at present there is no effective treatment for PD. Neuroprotective treatment does not directly address the etiology of PD, but intervention in some intermediate links in pathogenesis can delay the development of disease. Traditional Chinese medicines have shown potential clinical efficacy in attenuating the progression of PD. Growing evidence indicates that some Chinese herbs contain neuroprotective compounds, such as resveratrol, curcumin, or ginsenoside, green tea polyphenols or catechins, triptolide, etc [4-8]. These herbs can protect dopaminergic neurons against the neurotoxins 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)- or 6-hydroxydopamine (6-OHDA)- induced cell degeneration. They may also increase antioxidant activity, impede dopamine loss, inhibit microglial activation and the subsequent reduction of proinflammatory factor release, modulate mRNA levels and protein expression of apoptosis-related factors, and prevent α-synuclein aggregation and fibrillation. In this article, we review the neuroprotective roles of resveratrol, curcumin, and panax ginseng/ginsenoside in PD and discuss their possible mechanisms of action (Table 1, Figure 1).
Table 1.
Neuroprotective effects of resveratrol, curcumin, and ginsenoside on PD
| Agent | Mechanism | In vivo/In vitro model | Protection | References |
|---|---|---|---|---|
| Resveratrol | Anti-Inflammation | N9 microglial cell | Decrease the mRNA levels of IL-1α and TNF-α; Decrease the levels of COX-2 expression; Decrease the levels of NO, TNF-α, IL-1β, IL-6, MCP-1; Suppress production of IL-12p40, IL-23 and C-reactive protein, and respective receptors; Down-regulate MPO; Modulate the activity of PGC-1α, Akt and NF-κB | [10,18-21] |
| 6-OHDA-induced rat | ||||
| MPTP-induced mice | ||||
| Primary microglia and astrocytes | ||||
| Primary mouse astrocytes | ||||
| BV2 microglial cells | ||||
| Anti-Apoptosis | PC12 cells | Reduce the activity of caspase-3 and the level of Bax; regulate DNA fragmentation and the mRNA levels and protein expression of Bax, Bcl-2, cleaved caspase-3, and cleaved PARP-1; Activate sirtuin deacetylases and PPAR-γ | [12,24,25,27] | |
| SH-SY5Y cells | ||||
| HtrA2 knockout mice | ||||
| Saccharomyces cerevisiae | ||||
| Antioxidation | PC12 cells | Diminish superoxide anion; Inhibit ROS generation; Up-regulate the antioxidant status and the expression of MsrA; Activate PPAR-γ, AMPK, SIRT1; Raise the mRNA expression of PGC-1α’s target genes, | [12,37,38,41] | |
| SKN-MC cells | ||||
| 6-OHDA-induced rat | ||||
| SH-SY5Y cells | ||||
| Primary fibroblast from PD patients with Park2 mutation Transgenic mice overexpressing PGC-1α DA SN4741 cells | ||||
| neurotrophic effect | Primary rat midbrain neuron-glia cultures | Increase neurotrophic factors release in the concentration- and time-dependent manners | [11] | |
| Curcumin | Anti-Inflammation | MES23.5 cells | Inhibit NF-κB translocation and AP-1 activation; Inhibit the protein expression of GFAP and iNOS, decrease activation of astrocytes and microglia, reduce pro-inflammatory cytokine, alleviate loss of TH-IR fibers, protect axon | [48-52] |
| Primary rat mesencephalic neuron-glia cultures | ||||
| MPTP-induced mice | ||||
| Anti-Apoptosis | PC12 cells | Reduce MMP loss, attenuate MPP(+)-induced an increase in intracellular ROS level, induce overexpression of BCl-2 and antagonize MPP+-induced overexpression of iNOS; Ease alphaS-induced toxicity; Protect DA neuron axon; Decrease the Bax/Bcl-2 ratio; Reduce the accumulation of A53Tα-synuclein; inhibit the JUN/c-Jun pathway; Block MPP(+) | [53-55,65] | |
| MPTP-induced mice | ||||
| SH-SY5Y cells | ||||
| Ts-1-infected mice | ||||
| A53T α-synuclein cell model | ||||
| DA neurons in Mpp(+) model | ||||
| Antioxidation | MES23.5 cells | Restore MMP, increase level of Cu-Zn superoxide dismutase, suppress ROS; Sustain SOD1 level; reduce the levels of p-p38, cleaved caspase-3 and quinoprotein formation; restore depletion of GSH levels, free radical scaveng; Inhibit oxidative stress and the mitochondrial cell death pathway; activate the Nrf2/ARE pathway; Reduce p53 phosphorylation | [48,51,57,62,64,66-68] | |
| 6-OHDA-induced mice | ||||
| SH-SY5Y cells | ||||
| 6-OHDA-induced rats | ||||
| A53T α-synuclein cell model | ||||
| Prevent α-synuclein aggregation and fibrillation | SH-SY5Y cells | Prevent α-synuclein aggregation and fibrillation; Destabilize preformed falphaS; Specifically binds to oligomeric intermediates | [60,71-74] | |
| Inhibit MAO-B | MPTP-induced mice | Inhibit MAO-B activity | [71,76] | |
| Ginsenoside | Anti-Inflammation | BV2 microglial cells | Suppress NO production and TNF-α secretion, inhibit the mRNA expressions of iNOS, TNF-α, IL-1β, COX-2 and MMP-9, inhibited the phophorylations of PI3K/Akt and MAPKs and the DNA binding activities of NF-kB and AP-1; Suppress phosphorylation and nuclear translocation of NF-κB/p65, phosphorylation and degradation of IκB and the phosphorylation of IKK; inhibit the activation of Akt and ERK1/2; Reduce NO-formation and PGE2 synthesis; attenuate up-regulation TNF-α, IL-1β and IL-6 mRNA, and iNOS and COX-2 expression | [5,84-86] |
| Rat primary microglia | ||||
| Mesencephalic primary cultures | ||||
| PC12 cells | ||||
| LPS-treated mice | ||||
| Anti-Apoptosis | PC12 cells | Inhibit the activation of caspase-3, reduce iNOS and NO production; Increased the phosphorylation inhibition of Bad through activation of the PI3K/Akt pathway; Enhance the expression of Bcl-2 protein and mRNA, reduce the expression of Bax, Bax mRNA, and iNOS, and attenuate the cleavage of caspase-3 | [87-91] | |
| Primary cultured nigral neurons | ||||
| MPTP-induced mice | ||||
| Antioxidation | PC12 cells | Reduce the generation of ROS and cytochrome c release, restore MMP, increased the phosphorylation inhibition of Bad through activation of the PI3K/Akt pathway; Decrease iron influx, inhibit IRPs; decrease DMT1-mediated ferrous iron uptake and iron-induced cell damage | [87,88,90,91] | |
| MES23.5 cells | ||||
| Primary cultured nigral neurons | ||||
| Neurotrophin-like effects | PC12 cells | Increase neurite outgrowth; Reversed MPTP-induced cell death | [92] | |
| SN-K-SH cells |
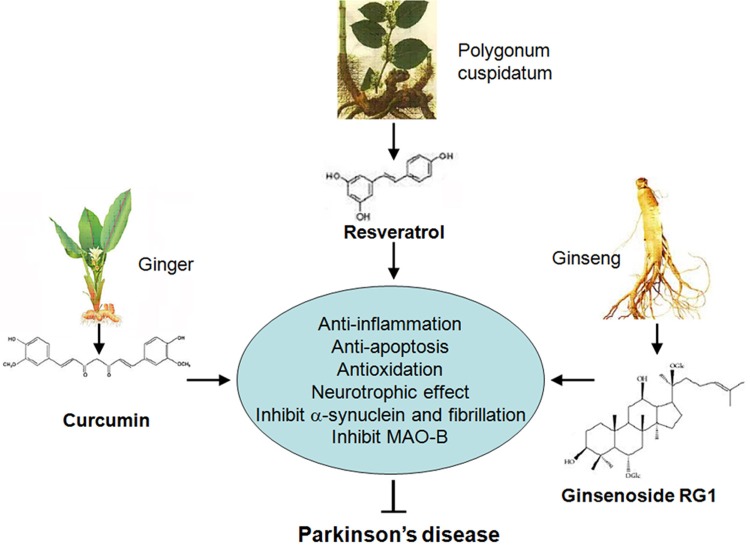
Figure 1.
The structures and protective mechanisms of resveratrol, curcumin, and ginsenoside on PD.
Resveratrol
Resveratrol, a polyphenolic compound naturally present in red wine and grapes, has a number of pharmacological effects including anti-inflammation, anti-apoptosis, antioxidation, antifungal, anticancer, and others [9-12]. It is also able to cross the blood-brain barrier and is water soluble [13]. The pathogenesis of PD is not clear, but in general is considered to be related to neuroinflammation, apoptosis, and oxidative stress [14-16]. Especially since the beginning of the 21st century, many research groups have explored the use of resveratrol in PD [17] and investigated its therapeutic effects from many angles. We summarize the neuroprotective roles of resveratrol in PD and discuss its possible mechanisms of action (Table 1, Figure 1).
Anti-inflammation
Glial activation and neuroinflammation have been found to be closely related to the pathogenesis of PD. Resveratrol strongly decreased the mRNA levels of two proinflammatory genes, interleukin 1-α (IL-1α) and tumor necrosis factor-α (TNF-α), in N9 microglial cells induced by lipopolysaccharide (LPS) [18]. Resveratrol treatment also significantly decreased the levels of cyclooxygenase-2 (COX-2) expression in the substantia nigra in the 6-hydroxydopamine (6-OHDA)-induced PD rat model [19]. Wight RD and colleagues tested the ability of resveratrol to inhibit LPS-induced production of inflammatory molecules by primary mouse astrocytes. They found that resveratrol inhibited LPS-induced production of nitric oxide (NO), cytokines such as TNF-α, interleukin 1-β (IL-1β), and Interleukin-6 (IL-6); and chemokine monocyte chemotactic protein-1 (MCP-1), which play critical roles in innate immunity. Resveratrol also suppressed astrocyte production of Interleukin-12p40 (IL-12p40) and Interleukin-23 (IL-23), which are known to alter the phenotype of T cells involved in adaptive immunity. Finally, resveratrol inhibited astrocyte production of C-reactive protein (CRP), which plays a role in a variety of chronic inflammatory disorders [10]. In 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated mice, resveratrol significantly reduced glial activation and decreased the levels of IL-1β, IL-6, and TNF-α as well as their respective receptors in the substantia nigra pars compacta (SNpc), according to Western blot, reverse transcription-polymerase chain reaction (RT-PCR), and quantitative PCR analysis [20]. Resveratrol protected DA neurons against LPS-induced neurotoxicity in a concentration- and time-dependent manner through the inhibition of microglial activation and the subsequent reduction of proinflammatory factor release [9]. Resveratrol significantly down-regulated myeloperoxidase (MPO) level (a key molecule in the host defense system against different pathogens), although it did not spark abnormal NO production in microglia and astrocytes. Moreover, resveratrol treatment restored the impaired responses of primary mixed glia from Mpo (-/-) mice to rotenone and attenuated rotenone-induced DA cell death. Furthermore, resveratrol plays the same regulatory roles on MPO levels in microglia treated with 1-methyl-4-phenylpyridinium (MPP)(+) [21]. Resveratrol suppressed the decrease of SIRT1 and increase of TNF-α and IL-6 induced by LPS, indicating that SIRT1 may participate in the regulation of proinflammatory cytokines derived from activated microglial activation [22]. Resveratrol treatment up-regulated the expression of the suppressor of cytokine signaling-1 (SOCS-1), supporting the hypothesis that it protects DA neurons of the SNpc against MPTP-induced cell loss by regulating inflammatory reactions, possibly through SOCS-1 induction [20]. Resveratrol also modulates the activity of numerous proteins, including peroxisome proliferator-activated receptor coactivator-1α (PGC-1α), members of the FOXO family, Akt (protein kinase B), and nuclear factor-κB (NF-κB) [23]. In conclusion, resveratrol protects DA neurons by reducing the inflammatory response.
Anti-apoptosis
Induced in a variety of ways, apoptosis of DA neurons leads to PD. Under microglial-neuronal coculture, treatment with resveratrol successfully reduced the inflammation-mediated apoptotic death of N9 microglial cells [18]. Resveratrol significantly reduced the activity of caspase-3 in neuroblastoma SH-SY5Y cells triggered by 6-OHDA [24], and decreased the level of Bax to the point of attenuating apoptosis [25]. Moreover, under high-glucose (HG) conditions, resveratrol significantly reduces HG-induced apoptosis in DA cells by regulating DNA fragmentation and the expression of several genes, such as Bax, Bcl-2, cleaved caspase-3, and cleaved poly(ADP-ribose)polymerase 1 (PARP-1) and also prevents the pro-apoptotic increase of p53 in the nucleus induced by HG [12]. Resveratrol showed anti-apoptotic activity in both rat and zebrafish brain synaptosomal fractions exposed to the neurotoxic agent rotenone by MTT assay [26]. Similarly, using PC12 cells, resveratrol greatly reduced PC12 death induced by MPP(+), which was related to modulation of the mRNA levels and protein expression of Bax and Bcl-2 [27]. Numerous studies have demonstrated the neuroprotective capability of resveratrol through activation of silent information regulator 1 (SIRT1) [28]. Resveratrol was shown to increase the lifespan of Saccharomyces cerevisiae [29], which was attributed to its ability to activate sirtuins, members of the histone deacetylase family [30]. There is evidence that resveratrol’s ability to attenuate tissue injury in the brain and restore mitochondrial function is partly attributable to its effect on SIRT1-dependent deacetylation of PGC-1α, a protein factor involved in mitochondrial biogenesis [23,31], and activation of peroxisome proliferator-activated receptor-γ (PPAR-γ), as a therapeutic target for neurodegenerative disease, due to PPAR-γ ability to protect against mitochondrial damage through upregulation of Bcl-2 [32,33]. Thus, resveratrol increases the levels of SIRT1 and related enzymes, which could change neuronal transcription profiles and enhance anti-apoptotic activity [34]. Moreover, resveratrol activates AMP-activated protein kinase (AMPK) to affect neuronal energy homeostasis, further contributing to neuroprotection [31]. AMPK and/or SIRT1 are required to induce resveratrol-mediated autophagy, and the AMPK-SIRT1-autophagy pathway plays an important role in neuroprotection by resveratrol in PD cellular models [35]. By activating autophagy, resveratrol prevented PrP (106-126)-induced neurotoxicityand reduction in mitochondrial potential, translocation of Bax to the mitochondria, and cytochrome c release [36]. To sum up, resveratrol plays its neuroprotective function by diminishing DA apoptosis.
Antioxidation
Many evidences showed that resveratrol exerted a neuroprotective effect on DA neurons by antioxidant. Excess reactive oxygen species (ROS) in the brain have been implicated as a likely potential risk factor for the pathogenesis of PD. Resveratrol scavenged ROS in a dose-dependent manner, and its antioxidant effects were further shown by protecting the enzymatic activity of the mitochondrial respiratory electron transport chain (complexes I and II) and pyruvate dehydrogenase in isolated liver mitochondria [37]. Resveratrol up-regulates antioxidant status and lowers DA loss in PD rat models [38], as well as prevents the formation of the DA-DNA adducts that could lead to gene mutations that cause PD [39]. Wang Y et al. showed that pretreatment of PD rats with resveratrol or resveratrol liposome (20 mg/kg per day) for 14 days greatly reduced abnormal rotational behavior and the loss and apoptosis of nigral cells, restored levels of total ROS, and significantly improved the total antioxidant capability of nigral tissues. Furthermore, resveratrol liposome showed even more profound effects than free resveratrol [40]. Methionine sulfoxide reductases A (MsrA) act as a catalytic antioxidant system and refers to the protection of oxidative stress-induced cell injury. Pretreatment with resveratrol up-regulated the expression of MsrA in human neuroblastoma SH-SY5Y cells [30]. It was also found that the expression and nuclear translocation of forkhead box group O 3a (FOXO3a), a transcription factor that activates the human MsrA promoter, were increased after resveratrol pretreatment [41]. In resveratrol (50 μM) coculture, PC12 cell death induced by DA (1 μM)-H2O2 (1 μM) was abolished, indicating resveratrol’s anti-oxidant capability [17]. Resveratrol protected DA neurons against HG-induced oxidative stress by diminishing cellular levels of superoxide anion [12]. Activation of PPAR-γ may also target the transcription of SOD and catalase genes through increasing the activity of the NF-E2-related factor 2 (Nrf2)/keap 1 pathway [42]. Many studies have confirmed that resveratrol suppresses neuroinflammation by inhibiting NADPH oxidase and attenuating NF-κB-induced expression of inducible nitric oxide synthase (iNOS), COX-2, and secretory phospholipase A2 (sPLA2) [9,43,44] and by activating the hormetic pathway, which involves the induction of SOD and catalase genes through stimulating the PI3K/Nrf2/keap 1 pathway [42]. Both in vivo (transgenic mice) and in vitro (SN4741 cells) studies showed that PGC-1α in DA neurons has the important function of resisting oxidative stress and improving neuronal viability, and resveratrol is neuroprotective via SIRT1/PGC-1α [45]. Nicotinamide is an inhibitor of SIRT1 and prevents resveratrol-induced elevation of FOXO3a and MsrA expression, demonstrating that the effect of resveratrol is mediated by a SIRT1-dependent pathway from another direction [41]. Recent research has shown that resveratrol regulates energy homeostasis through activation of AMPK and SIRT1 and raises the mRNA expression of a number of PGC-1α’s target genes, resulting in enhanced mitochondrial oxidative function; resveratrol treatment also causes an increase in complex I and citrate synthase activity, basal oxygen consumption, and mitochondrial ATP production and causes enhanced macro-autophagic flux through activation of an LC3-independent pathway [46].
Other effects
Resveratrol exerted neurotrophic effects on primary rat midbrain neuron-glial cultures; furthermore it increased the release of neurotrophic factors in a concentration- and time-dependent manner [11]. Polymorphisms of the cytochrome P450 (CYP/Cyp) 2D6 gene are related to PD. Resveratrol ameliorated the neurodegenerative changes by altering the expression of Cyp2d22, a mouse ortholog of human CYP2D6 as well as paraquat accumulation [47].
Curcumin
Curcumin, a natural polyphenol compound derived from the curry spice turmeric, is known for several biological and medicinal effects, such as anti-cancer, anti-microbial, anti-inflammatory, antioxidant, and antiproliferative activities. Curcumin has shown therapeutic potential for neurodegenerative diseases including PD, which has garnered great interest in recent years. We review the neuroprotective roles of curcumin in PD and discuss its possible mechanisms of action (Table 1, Figure 1).
Anti-inflammation
Both in vitro and in vivo studies showed that curcumin can protect DA neurons through anti-inflammatory effect. Curcumin pretreatment significantly inhibited both 6-OHDA-induced NF-κB translocation [48] and LPS-induced morphological changes in microglia, and dramatically suppressed the expression of many LPS-induced proinflammatory factors and their genes. Furthermore, curcumin treatment decreased LPS-induced activation of NF-κB and activator protein-1 (AP-1) [49]. Pretreatment with curcuminoids (150 mg/kg/day, oral administration) for 1 week prevented MPTP-mediated depletion of DA and tyrosine hydroxylase (TH) immunoreactivity and inhibited the protein expression of glial fibrillary acidic protein (GFAP) and iNOS. Likewise, curcumin pretreatment reduced pro-inflammatory cytokine (IL-6, IL-1β, TNF-α) and total nitrite generation in the striatum of MPTP-induced mice [50]. Curcumin alleviated loss of TH-IR fibers and decreased activation of astrocytes and microglia [51]. Recently, using a quantitative microfluidic-based methodology, Tegenge et al. showed that LPS-stimulated microglia release soluble factors, which when applied locally to axons, result in axon degeneration. Curcumin specifically protects axons, but not neuronal cell bodies, from NO-mediated degeneration [52].
Anti-apoptosis
Curcumin also protected DA neurons from apoptosis. It has been reported that curcumin protects PC12 cells against MPP(+)-induced cytotoxicity and apoptosis by reducing the loss of mitochondrial membrane potential (MMP), and its neuroprotective effects might be mediated by the Bcl-2-mitochondria-ROS-iNOS pathway because curcumin attenuates MPP(+)-induced an increase in intracellular ROS level, induces overexpression of BCl-2 and antagonizes MPP+-induced overexpression of iNOS [53]. Research using a PD cell model found that both intra- and extra-cellular alphaS may induce apoptosis of DA neurons; curcumin can ease alphaS-induced toxicity, decrease ROS levels, and protect cells against apoptosis [54]. In addition the death of DA neurons and the loss of DA axons in the striatum were significantly suppressed by curcumin in the MPTP mouse model [55]. Chiu et al. showed that liposomal-formulated curcumin [Lipocurc™] significantly blocked neuronal apoptosis and stimulated DA neurons in the substantia nigra [56]. Using 6-OHDA-induced neurotoxicity in the SH-sY5Y cells, curcumin decreased the Bax/Bcl-2 ratio at mRNA expression and protein level [57]. Curcumin specifically inhibits the JNK/c-Jun pathway [58] and can block MPP(+), which causes the upregulation of c-Jun N-terminal kinase (JNK) and DA neuronal death [59]. JNK phosphorylation induced by MPTP can cause translocation of Bax to mitochondria and the release of cytochrome c, which can be diminished by curcumin [55]. The axon degeneration induced by LPS is mediated by microglial MyD88/p38 MAPK signaling and concomitant production of NO. Through inhibiting JNK, curcumin protects axons from degeneration involving JNK phosphorylation [52]. Curcumin could reduce the accumulation of A53T α-synuclein through downregulation of the mTOR (mammalian target of rapamycin)/p70S6K signaling [60].
Antioxidation
The neuroprotective effects of curcumin to PD also related to its antioxidant properties. In 2005, Zbarsky V et al. pretreated rats with curcumin and showed clear protection of the number of TH-positive cells in the SN and DA levels in the striata [61]. Treatment of DA neurons and mice with curcumin restores depletion of glutathione (GSH) levels, protects against protein oxidation, preserves mitochondrial complex I activity that is normally is impaired due to GSH loss [62], and maintains SOD1 levels in the lesioned striatum of 6-OHDA mice [51]. Curcumin protected MES23.5 cells against the neurotoxin 6-OHDA by restoring mitochondrial membrane potential, increasing the levels of Cu-Zn superoxide dismutase, and suppressing an increase in intracellular ROS [48]. The effect of three bioconjugates of curcumin (involving diesters of demethylenated piperic acid, valine, and glutamic acid) against GSH depletion mediated oxidative stress in DA neurons [63]. Curcumin pretreatment of the human DA cell line SH-SY5Y exposed to 6-OHDA improved cell viability and significantly reduced ROS [57]. Curcuminoids were administered to rats (60 mg/kg, body weight, per oral) for three weeks followed by unilateral injection of 6-OHDA (10 μg/2 μL) into the right striatum on the 22nd day. The results showed that curcuminoids appear significant protection against progressive neuronal degeneration due to increased oxidative attack in 6-OHDA-lesioned rats through a free radical-scavenging mechanism [64]. Pretreatment of SH-SY5Y with curcumin I (diferuloylmethane) significantly decreased the formation of quinoprotein and reduced the levels of p-p38 and cleaved caspase-3 in a dose-dependent manner induced by 6-OHDA. Moreover, the levels of phospho-tyrosine hydroxylase (p-TH) were also dose-dependently increased by treatment with curcumin I. These results clearly demonstrate that curcumin I protects neurons against oxidative damage by attenuation of p-p38 expression, caspase-3-activation, and toxic quinoprotein formation and by restoration of p-TH levels [65].
Similarly, pretreatment with a pyrazole derivative of curcumin (CNB-001, 2 μM) 2 h before rotenone exposure (100 nM) to SK-N-SH cells increased cell viability, decreased ROS formation, maintained normal physiological mitochondrial membrane potential, and reduced apoptosis. Furthermore, CNB-001 inhibited downstream apoptotic cascade by increasing the expression of Bcl-2 and decreased the expression of Bax, caspase-3, and cytochrome C [66]. Curcumin has also been shown to detoxify peroxynitrite and protect against mitochondrial complex I (CI) inhibition and protein nitration [67]. Curcumin protects against A53T mutant α-synuclein-induced cell death via inhibition of oxidative stress and the mitochondrial cell death pathway [68]. Pretreatment with curcumin I (diferuloylmethane) protects SH-SY5Y cell from 6-OHDA-induced neurotoxicity. Curcumin I significantly improved cell viability, reduced ROS and p53 phosphorylation [61]. Curcumin has been shown to activate the Nrf2/ARE (antioxidant-response element) pathway that activates transcription of anti-inflammatory and antioxidant genes to produce neuroprotective effects [69]. The protective effects of curcumin against 6-OHDA may be attributable to its iron-chelating capability, suppressing the iron-induced degeneration of nigral DA neurons [70].
Other effects
In addition to preventing α-synuclein aggregation and fibrillation [71], curcumin also inhibits the formation of alpha-synuclein fibrils (falphaS) and destabilizes preformed falphaS at pH 7.5 at 37 degrees C in vitro [72]. Curcumin-glucoside (Curc-gluc), a modified form of curcumin, prevents oligomer formation and inhibits fibril formation. Curc-gluc inhibits aggregation in a dose-dependent manner and enhances the solubility of α-synuclein [73]. Curcumin efficiently reduces the accumulation of A53T α-synuclein [60]; however, fluorescence and two-dimensional nuclear magnetic resonance (2D-NMR) show that it binds not to monomeric α-Syn but instead to oligomeric intermediates [74]. A mixture of curcumin and β-cyclodextrin not only inhibited aggregation but also broke up the preformed aggregates [75].
In addition, curcumin inhibits monoamine oxidase B [71]. Systemic administration of curcumin (80 mg/kg i.p.) significantly reversed the MPTP-induced depletion of DA and dihydroxyphenylacetic acid (DOPAC). MAO-B activity was also significantly inhibited by curcumin and its metabolite tetrahydrocurcumin [76]. However, Ojha’s found the MAO-B activity only partially and not significantly altered [50]. In yeast, curcumin prevents formation of polyglutamine aggregates by inhibiting Vps36, a component of the ESCRT-II (endosomal sorting complex required for transport) complex [77].
The leucine-rich repeat kinase 2 (LRRK2) gene is most commonly associated with both familial and sporadic PD [78]. Employing both cell and Drosophila models to investigate the interaction between LRRK2 genetic mutations and oxidative stress, it was found that curcumin significantly redssuced LRRK2 kinase activity and the levels of oxidized proteins, and thus acted as not only an antioxidant but also a LRRK2 kinase inhibitor [79].
Ginsenoside
Ginseng, a traditional Chinese medicine, is widely used. Most ginseng species contain active constituents with beneficial effects, including ginsenosides, polysaccharides, peptides, polyacetylenic alcohols, and fatty acids. There are two major categories of ginsenosides: protopanaxadiols (PPD, e.g., Ra, Rb, Rc, Rd, Rg3, Rh2) and protopanaxatriols (PPT, e.g., Re, Rf, Rg1, Rg2, Rh1) [80]. Ginseng has many therapeutic effects, especially on nervous system diseases. Ginseng plays a neuroprotective roles in the regulation of synaptic plasticity, neuroinflammatory processes, and neurotransmitter release [6]. In vitro and in vivo studies have determined that ginsenosides, as the active compounds responsible for ginseng’s action, exert pharmacological effects against neuroinflammation, cerebral oxidative stress and radical formation, and apoptosis (Table 1 and Figure 1).
Anti-inflammation
Ginsenosides have clear anti-inflammatory effects. Ginsenoside Rg5 is one of the main constituents of steamed ginseng and belongs to the family of protopanaxadiol ginsenosides [81]. It has been shown to suppress NO production and proinflammatory TNF-α secretion in BV2 microglial cells and rat primary microglia, as well as inhibit the mRNA expression of iNOS, TNF-α, IL-1β, COX-2, and matrix metallopeptidase 9 (MMP-9) induced by LPS [82]. Drug screening determined that ginsenoside Re enhances the function of the defective PINK1-Hsp90/LRPPRC-Hsp60-complex IV signaling axis in PINK1-null neurons by restoring NO levels [83]. In addition, Re (2 μg/ml) protects against LPS (1 μg/ml)-treated microglial cells. The neuroprotective/anti-inflammatory effects induced by Re treatment appeared via the phospho-p38, iNOS, and COX2 signaling pathways in BV2 cells [80]. Rg1 obviously decreased the cytotoxicity induced by H2O2 in PC12 cells and suppressed phosphorylation and nuclear translocation of NF-κB/p65, phosphorylation and degradation of inhibitor protein of κB (IκB), and the phosphorylation of IB-kinase complex (IKK). Rg1 also inhibited the activation of Akt and extracellular signal-regulated kinase 1/2 (ERK1/2) [84]. Rd partially reduced the neurotoxic action of LPS on DA neurons, which could take place through a reduction of NO-formation and prostaglandin E2 (PGE2) synthesis [85]. In an in vivo animal model, pretreatment with Rg3 (orally with 10, 20, and 30 mg/kg 1 h prior to the LPS) significantly attenuated upregulation of TNF-α, IL-1β, and IL-6 mRNA in brain tissue at 4 h after LPS injection. Furthermore, iNOS and COX-2 expression in brain tissue were also attenuated [86]. Also, Rg5 inhibited the phosphorylation of PI3K/Akt and MAPKs and the DNA binding activity of NF-κB and AP-1, which are upstream molecules controlling inflammatory reactions [82]. In addition, ginsenoside’s neuroprotective effects via Rd may involve interference with the expression of iNOS and COX-2 [85].
Anti-apoptosis
Ginsenosides have anti-apoptotic effects. Pretreatment with ginsenoside Rg1 obviously inhibited the activation of caspase-3. In addition, Rg1 also reduced iNOS protein levels and NO production [87]. Rg1 increased the inhibition of phosphorylation of the pro-apoptotic protein Bad through activation of the PI3K/Akt pathway [88]. Pretreatment with Re markedly increased TH-positive neurons and decreased the TUNEL-positive ratio in a PD mouse model induced by MPTP. Furthermore, Re enhanced the expression of Bcl-2 protein and mRNA but reduced the expression of Bax, Bax mRNA, and iNOS and attenuated the cleavage of caspase-3 [89].
Antioxidation
Ginsenosides also have anti-oxidation function. Pretreatment with ginsenoside Rg1 evidently reduced the generation of DA-induced ROS and the release of mitochondrial cytochrome c into the cytosol [87]. Rg1 was shown to reduce rotenone-induced cell death in primary cultured nigral neurons and restored mitochondrial membrane potential. In addition, Rg1 prevented cytochrome c release from the mitochrondrial membrane [88]. Because iron accumulation is involved in the neurotoxicity of 6-OHDA, Rg1 pretreatment decreases iron influx by inhibiting 6-OHDA-induced up-regulation of an iron importer protein divalent metal transporter 1 with iron-responsive element (divalent metal transporter 1 (DMT1) + iron responsive element (IRE)), and the effect of Rg1 on DMT1 + IRE expression was due to its inhibition of iron-regulatory proteins (IRPs) by its antioxidant effect [90]. Pretreatment with Rg1 inhibited MPP(+)-induced up-regulation of DMT1-IRE in MES23.5 cells and significantly inhibited ROS production and translocation of NF-κB to nuclei. Rg1 decreased DMT1-mediated ferrous iron uptake and iron-induced cell damage by inhibiting the up-regulation of DMT1-IRE, likely by inhibiting the ROS-NF-κB pathway [91].
Other effects
Ginseng extract has been found to have neurotrophin-like effects. Both Rb1 and Rg1 increased neurite outgrowth of PC12 cells in the absence of NGF after 18 days in culture; in addition, both Rb1 and Rg1 reversed MPTP-induced SN-K-SH cell death [92].
Conclusion and perspective
PD is a chronic, progressive, and multifactorial neurologic disorder in which many pathologic processes including inflammation, oxidative stress, mitochondrial dysfunction, neurotransmitter imbalance, apoptosis, and genetic factors lead to neuronal degeneration. Thus, any therapeutic approach that limits itself to drugs against a single pathological process is invalid, and drug combinations with various pharmacological properties are likely to be more effective. Recently, traditional Chinese medicinal herbs, with their low toxicity and side-effects have become a popular topic when discussing new drugs for prevention and treatment of PD and other neurodegenerative diseases. It is known that Chinese herbs play various neuroprotective roles, including antioxidant, anti-inflammatory, free radicals-scavenging, anti-apoptosis, and chelating harmful metals through a variety of mechanisms [4,6,93,94]. Therefore, as therapeutic neuroprotective agents, Chinese herbs are attracting increasing attention for the treatment of PD patients. At present, for the prevention and treatment of PD, traditional Chinese herbs monomer and active ingredients with clear molecular structures contribute to its mechanism research, but there are some problems in the application of Chinese herbs. First, efficacy studies of traditional Chinese medicine monomer or effective ingredients for PD still remain in the stage of cell or animal model, and need further clinical observation and verification. Second, the efficacies of single use of traditional Chinese herbs monomer or effective components are very different from Chinese herbal compound used in clinic, and can not exploit advantages of traditional Chinese medicine on synergy effects and overall regulation of body. Third, some Chinese herbs ingredients have low bioavailability [93]. Future studies should be pay attention to the bioavailability of the traditional Chinese herb composition and the best combination of a variety of traditional Chinese medicine monomer and the effective composition to improve its therapeutic effect.
Acknowledgements
This work is supported by grants from the Muscular Dystrophy Association, the ALS Therapy Alliance, the Bill & Melinda Gates Foundation, and the Shandong Province Taishan Scholar Project the Natural Science Foundation of Shandong Province, the Key Project of Science and Technology Innovation Research Fund of Weifang Medical University.
Disclosure of conflict of interest
None.
Abbreviations
- AP-1
activator protein-1
- AMPK
AMP-activated protein kinase
- 6-OHDA
6-hydroxydopamine
- JNK
c-Jun N-terminal kinase
- CRP
C-reactive protein
- COX-2
cyclooxygenase-2
- DOPAC
dihydroxyphenylacetic acid
- DMT1
divalent metal transporter 1
- DA
dopamine
- ESCRT
endosomal sorting complex required for transport
- ERK1/2
extracellular signal-regulated kinase 1/2
- FOXO3a
forkhead box group O 3a
- GFAP
glial fibrillary acidic protein
- GSH
glutathione
- HG
high glucose
- iNOS
inducible nitric oxide synthase
- IκB
inhibitor protein of κB
- IKK
IκB-kinase complex
- IL-6
Interleukin-6
- IL-1α
interleukin 1-α
- IL-1β
interleukin 1-β
- IL-12p40
interleukin 12p40
- IL-23
interleukin 23
- IRPs
iron regulatory proteins
- IRE
iron responsive element
- L-DOPA
levodopa
- LRRK2
Leucinerich repeat kinase 2
- LPS
lipopolysaccharide
- MMP-9
matrix metallopeptidase 9
- MsrA
methionine sulfoxide reductases A
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- MPP+
1-methyl-4-phenylpyridinium
- MMP
mitochondria membrane potential
- MAO-B
monoamine oxidase-B
- MCP-1
monocyte chemotactic protein-1
- MPO
myeloperoxidase
- Nrf2
NF-E2-related factor 2
- NO
nitric oxide
- NF-κB
nuclear factor-κB
- PD
Parkinson’s disease
- PPAR-γ
peroxisome proliferator-activated receptor-γ
- PGC-1α
peroxisome proliferator-activated receptor-γcoactivator-1α
- PARP-1
poly(ADP-ribose)polymerase 1
- PGE2
prostaglandin E2
- ROS
reactive oxygen species
- RT-PCR
reverse transcription-polymerase chain reaction
- sPLA2
secretory phospholipase A2
- SIRT1
silent information regulator 1
- SNpc
substantia nigra pars compacta
- SOCS-1
suppressor of cytokine signaling 1
- TNF-α
tumor necrosis factor-α
- TH
tyrosine hydroxylase
References
- 1.Dauer W, Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 2.Fu W, Zheng Z, Zhuang W, Chen D, Wang X, Sun X, Wang X. Neural metabolite changes in corpus striatum after rat multipotent mesenchymal stem cells transplanted in hemiparkinsonian rats by magnetic resonance spectroscopy. Int J Neurosci. 2013;123:883–891. doi: 10.3109/00207454.2013.814132. [DOI] [PubMed] [Google Scholar]
- 3.Buzhor E, Leshansky L, Blumenthal J, Barash H, Warshawsky D, Mazor Y, Shtrichman R. Cell-based therapy approaches: the hope for incurable diseases. Regen Med. 2014;9:649–672. doi: 10.2217/rme.14.35. [DOI] [PubMed] [Google Scholar]
- 4.Virmani V, Pinto L, Binienda Z, Ali S. Food, nutrigenomics, and neurodegeneration--neuroprotection by what you eat! Mol Neurobiol. 2013;48:353–362. doi: 10.1007/s12035-013-8498-3. [DOI] [PubMed] [Google Scholar]
- 5.Lee WH, Loo CY, Bebawy M, Luk F, Mason RS, Rohanizadeh R. Curcumin and its derivatives: their application in neuropharmacology and neuroscience in the 21st century. Curr Neuropharmacol. 2013;11:338–378. doi: 10.2174/1570159X11311040002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim HJ, Kim P, Shin CY. A comprehensive review of the therapeutic and pharmacological effects of ginseng and ginsenosides in central nervous system. J Ginseng Res. 2013;37:8–29. doi: 10.5142/jgr.2013.37.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun AY, Wang Q, Simonyi A, Sun GY. Resveratrol as a therapeutic agent for neurodegenerative diseases. Mol Neurobiol. 2010;41:375–383. doi: 10.1007/s12035-010-8111-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen LW, Wang YQ, Wei LC, Shi M, Chan YS. Chinese herbs and herbal extracts for neuroprotection of dopaminergic neurons and potential therapeutic treatment of Parkinson’s disease. CNS Neurol Disord Drug Targets. 2007;6:273–281. doi: 10.2174/187152707781387288. [DOI] [PubMed] [Google Scholar]
- 9.Zhang F, Shi JS, Zhou H, Wilson B, Hong JS, Gao HM. Resveratrol protects dopamine neurons against lipopolysaccharide-induced neurotoxicity through its anti-inflammatory actions. Mol Pharmacol. 2010;78:466–477. doi: 10.1124/mol.110.064535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wight RD, Tull CA, Deel MW, Stroope BL, Eubanks AG, Chavis JA, Drew PD, Hensley LL. Resveratrol effects on astrocyte function: relevance to neurodegenerative diseases. Biochem Biophys Res Commun. 2012;426:112–115. doi: 10.1016/j.bbrc.2012.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang F, Wang YY, Liu H, Lu YF, Wu Q, Liu J, Shi JS. Resveratrol Produces Neurotrophic Effects on Cultured Dopaminergic Neurons through Prompting Astroglial BDNF and GDNF Release. Evid Based Complement Alternat Med. 2012;2012:937605. doi: 10.1155/2012/937605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Renaud J, Bournival J, Zottig X, Martinoli MG. Resveratrol protects DAergic PC12 cells from high glucose-induced oxidative stress and apoptosis: effect on p53 and GRP75 localization. Neurotox Res. 2014;25:110–123. doi: 10.1007/s12640-013-9439-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chao J, Yu MS, Ho YS, Wang M, Chang RC. Dietary oxyresveratrol prevents parkinsonian mimetic 6-hydroxydopamine neurotoxicity. Free Radic Biol Med. 2008;45:1019–1026. doi: 10.1016/j.freeradbiomed.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Su X, Federoff HJ. Immune responses in Parkinson’s disease: interplay between central and peripheral immune systems. Biomed Res Int. 2014;2014:275178. doi: 10.1155/2014/275178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu L, Arun A, Ellis L, Peritore C, Donmez G. SIRT2 enhances 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced nigrostriatal damage via apoptotic pathway. Front Aging Neurosci. 2014;6:184. doi: 10.3389/fnagi.2014.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Radi E, Formichi P, Battisti C, Federico A. Apoptosis and oxidative stress in neurodegenerative diseases. J Alzheimers Dis. 2014;42(Suppl 3):S125–152. doi: 10.3233/JAD-132738. [DOI] [PubMed] [Google Scholar]
- 17.Frankel D, Schipper HM. Cysteamine pretreatment of the astroglial substratum (mitochondrial iron sequestration) enhances PC12 cell vulnerability to oxidative injury. Exp Neurol. 1999;160:376–385. doi: 10.1006/exnr.1999.7214. [DOI] [PubMed] [Google Scholar]
- 18.Bureau G, Longpre F, Martinoli MG. Resveratrol and quercetin, two natural polyphenols, reduce apoptotic neuronal cell death induced by neuroinflammation. J Neurosci Res. 2008;86:403–410. doi: 10.1002/jnr.21503. [DOI] [PubMed] [Google Scholar]
- 19.Jin F, Wu Q, Lu YF, Gong QH, Shi JS. Neuroprotective effect of resveratrol on 6-OHDA-induced Parkinson’s disease in rats. Eur J Pharmacol. 2008;600:78–82. doi: 10.1016/j.ejphar.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Lofrumento DD, Nicolardi G, Cianciulli A, De Nuccio F, La Pesa V, Carofiglio V, Dragone T, Calvello R, Panaro MA. Neuroprotective effects of resveratrol in an MPTP mouse model of Parkinson’s-like disease: possible role of SOCS-1 in reducing pro-inflammatory responses. Innate Immun. 2014;20:249–260. doi: 10.1177/1753425913488429. [DOI] [PubMed] [Google Scholar]
- 21.Chang CY, Choi DK, Lee DK, Hong YJ, Park EJ. Resveratrol confers protection against rotenone-induced neurotoxicity by modulating myeloperoxidase levels in glial cells. PLoS One. 2013;8:e60654. doi: 10.1371/journal.pone.0060654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ye J, Liu Z, Wei J, Lu L, Huang Y, Luo L, Xie H. Protective effect of SIRT1 on toxicity of microglial-derived factors induced by LPS to PC12 cells via the p53-caspase-3-dependent apoptotic pathway. Neurosci Lett. 2013;553:72–77. doi: 10.1016/j.neulet.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 23.Pallas M, Casadesus G, Smith MA, Coto-Montes A, Pelegri C, Vilaplana J, Camins A. Resveratrol and neurodegenerative diseases: activation of SIRT1 as the potential pathway towards neuroprotection. Curr Neurovasc Res. 2009;6:70–81. doi: 10.2174/156720209787466019. [DOI] [PubMed] [Google Scholar]
- 24.Chao J, Li H, Cheng KW, Yu MS, Chang RC, Wang M. Protective effects of pinostilbene, a resveratrol methylated derivative, against 6-hydroxydopamine-induced neurotoxicity in SH-SY5Y cells. J Nutr Biochem. 2010;21:482–489. doi: 10.1016/j.jnutbio.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 25.Gerhardt E, Graber S, Szego EM, Moisoi N, Martins LM, Outeiro TF, Kermer P. Idebenone and resveratrol extend lifespan and improve motor function of HtrA2 knockout mice. PLoS One. 2011;6:e28855. doi: 10.1371/journal.pone.0028855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Makhija DT, Jagtap AG. Studies on sensitivity of zebrafish as a model organism for Parkinson’s disease: Comparison with rat model. J Pharmacol Pharmacother. 2014;5:39–46. doi: 10.4103/0976-500X.124422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bournival J, Quessy P, Martinoli MG. Protective effects of resveratrol and quercetin against MPP+ -induced oxidative stress act by modulating markers of apoptotic death in dopaminergic neurons. Cell Mol Neurobiol. 2009;29:1169–1180. doi: 10.1007/s10571-009-9411-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rasouri S, Lagouge M, Auwerx J. [SIRT1/ PGC-1: a neuroprotective axis?] . Med Sci (Paris) 2007;23:840–844. doi: 10.1051/medsci/20072310840. [DOI] [PubMed] [Google Scholar]
- 29.Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 30.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 31.Dasgupta B, Milbrandt J. Resveratrol stimulates AMP kinase activity in neurons. Proc Natl Acad Sci U S A. 2007;104:7217–7222. doi: 10.1073/pnas.0610068104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fuenzalida K, Quintanilla R, Ramos P, Piderit D, Fuentealba RA, Martinez G, Inestrosa NC, Bronfman M. Peroxisome proliferator-activated receptor gamma up-regulates the Bcl-2 anti-apoptotic protein in neurons and induces mitochondrial stabilization and protection against oxidative stress and apoptosis. J Biol Chem. 2007;282:37006–37015. doi: 10.1074/jbc.M700447200. [DOI] [PubMed] [Google Scholar]
- 33.Wu JS, Cheung WM, Tsai YS, Chen YT, Fong WH, Tsai HD, Chen YC, Liou JY, Shyue SK, Chen JJ, Chen YE, Maeda N, Wu KK, Lin TN. Ligand-activated peroxisome proliferator-activated receptor-gamma protects against ischemic cerebral infarction and neuronal apoptosis by 14-3-3 epsilon upregulation. Circulation. 2009;119:1124–1134. doi: 10.1161/CIRCULATIONAHA.108.812537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang BL, Chua CE. SIRT1 and neuronal diseases. Mol Aspects Med. 2008;29:187–200. doi: 10.1016/j.mam.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 35.Wu Y, Li X, Zhu JX, Xie W, Le W, Fan Z, Jankovic J, Pan T. Resveratrol-activated AMPK/ SIRT1/autophagy in cellular models of Parkinson’s disease. Neurosignals. 2011;19:163–174. doi: 10.1159/000328516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jeong JK, Moon MH, Bae BC, Lee YJ, Seol JW, Kang HS, Kim JS, Kang SJ, Park SY. Autophagy induced by resveratrol prevents human prion protein-mediated neurotoxicity. Neurosci Res. 2012;73:99–105. doi: 10.1016/j.neures.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 37.Long J, Gao H, Sun L, Liu J, Zhao-Wilson X. Grape extract protects mitochondria from oxidative damage and improves locomotor dysfunction and extends lifespan in a Drosophila Parkinson’s disease model. Rejuvenation Res. 2009;12:321–331. doi: 10.1089/rej.2009.0877. [DOI] [PubMed] [Google Scholar]
- 38.Khan MM, Ahmad A, Ishrat T, Khan MB, Hoda MN, Khuwaja G, Raza SS, Khan A, Javed H, Vaibhav K, Islam F. Resveratrol attenuates 6-hydroxydopamine-induced oxidative damage and dopamine depletion in rat model of Parkinson’s disease. Brain Res. 2010;1328:139–151. doi: 10.1016/j.brainres.2010.02.031. [DOI] [PubMed] [Google Scholar]
- 39.Zahid M, Saeed M, Yang L, Beseler C, Rogan E, Cavalieri EL. Formation of dopamine quinone-DNA adducts and their potential role in the etiology of Parkinson’s disease. IUBMB Life. 2011;63:1087–1093. doi: 10.1002/iub.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Y, Xu H, Fu Q, Ma R, Xiang J. Protective effect of resveratrol derived from Polygonum cuspidatum and its liposomal form on nigral cells in parkinsonian rats. J Neurol Sci. 2011;304:29–34. doi: 10.1016/j.jns.2011.02.025. [DOI] [PubMed] [Google Scholar]
- 41.Wu PF, Xie N, Zhang JJ, Guan XL, Zhou J, Long LH, Li YL, Xiong QJ, Zeng JH, Wang F, Chen JG. Resveratrol preconditioning increases methionine sulfoxide reductases A expression and enhances resistance of human neuroblastoma cells to neurotoxins. J Nutr Biochem. 2013;24:1070–1077. doi: 10.1016/j.jnutbio.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 42.Rubiolo JA, Mithieux G, Vega FV. Resveratrol protects primary rat hepatocytes against oxidative stress damage: activation of the Nrf2 transcription factor and augmented activities of antioxidant enzymes. Eur J Pharmacol. 2008;591:66–72. doi: 10.1016/j.ejphar.2008.06.067. [DOI] [PubMed] [Google Scholar]
- 43.Bi XL, Yang JY, Dong YX, Wang JM, Cui YH, Ikeshima T, Zhao YQ, Wu CF. Resveratrol inhibits nitric oxide and TNF-alpha production by lipopolysaccharide-activated microglia. Int Immunopharmacol. 2005;5:185–193. doi: 10.1016/j.intimp.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 44.Sun AY, Wang Q, Simonyi A, Sun GY. Botanical phenolics and brain health. Neuromolecular Med. 2008;10:259–274. doi: 10.1007/s12017-008-8052-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mudo G, Makela J, Di Liberto V, Tselykh TV, Olivieri M, Piepponen P, Eriksson O, Malkia A, Bonomo A, Kairisalo M, Aguirre JA, Korhonen L, Belluardo N, Lindholm D. Transgenic expression and activation of PGC-1alpha protect dopaminergic neurons in the MPTP mouse model of Parkinson’s disease. Cell Mol Life Sci. 2012;69:1153–1165. doi: 10.1007/s00018-011-0850-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ferretta A, Gaballo A, Tanzarella P, Piccoli C, Capitanio N, Nico B, Annese T, Di Paola M, Dell’aquila C, De Mari M, Aguirre JA, Korhonen L, Belluardo N, Lindholm D. Effect of resveratrol on mitochondrial function: implications in parkin-associated familiar Parkinson’s disease. Biochim Biophys Acta. 2014;1842:902–915. doi: 10.1016/j.bbadis.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 47.Srivastava G, Dixit A, Yadav S, Patel DK, Prakash O, Singh MP. Resveratrol potentiates cytochrome P450 2 d22-mediated neuroprotection in maneb- and paraquat-induced parkinsonism in the mouse. Free Radic Biol Med. 2012;52:1294–1306. doi: 10.1016/j.freeradbiomed.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 48.Wang J, Du XX, Jiang H, Xie JX. Curcumin attenuates 6-hydroxydopamine-induced cytotoxicity by anti-oxidation and nuclear factor-kappa B modulation in MES23.5 cells. Biochem Pharmacol. 2009;78:178–183. doi: 10.1016/j.bcp.2009.03.031. [DOI] [PubMed] [Google Scholar]
- 49.Yang S, Zhang D, Yang Z, Hu X, Qian S, Liu J, Wilson B, Block M, Hong JS. Curcumin protects dopaminergic neuron against LPS induced neurotoxicity in primary rat neuron/glia culture. Neurochem Res. 2008;33:2044–2053. doi: 10.1007/s11064-008-9675-z. [DOI] [PubMed] [Google Scholar]
- 50.Ojha RP, Rastogi M, Devi BP, Agrawal A, Dubey GP. Neuroprotective effect of curcuminoids against inflammation-mediated dopaminergic neurodegeneration in the MPTP model of Parkinson’s disease. J Neuroimmune Pharmacol. 2012;7:609–618. doi: 10.1007/s11481-012-9363-2. [DOI] [PubMed] [Google Scholar]
- 51.Tripanichkul W, Jaroensuppaperch EO. Ameliorating effects of curcumin on 6-OHDA-induced dopaminergic denervation, glial response, and SOD1 reduction in the striatum of hemiparkinsonian mice. Eur Rev Med Pharmacol Sci. 2013;17:1360–1368. [PubMed] [Google Scholar]
- 52.Tegenge MA, Rajbhandari L, Shrestha S, Mithal A, Hosmane S, Venkatesan A. Curcumin protects axons from degeneration in the setting of local neuroinflammation. Exp Neurol. 2014;253:102–110. doi: 10.1016/j.expneurol.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 53.Chen J, Tang XQ, Zhi JL, Cui Y, Yu HM, Tang EH, Sun SN, Feng JQ, Chen PX. Curcumin protects PC12 cells against 1-methyl-4-phenylpyridinium ion-induced apoptosis by bcl-2-mitochondria-ROS-iNOS pathway. Apoptosis. 2006;11:943–953. doi: 10.1007/s10495-006-6715-5. [DOI] [PubMed] [Google Scholar]
- 54.Wang MS, Boddapati S, Emadi S, Sierks MR. Curcumin reduces alpha-synuclein induced cytotoxicity in Parkinson’s disease cell model. BMC Neurosci. 2010;11:57. doi: 10.1186/1471-2202-11-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pan J, Li H, Ma JF, Tan YY, Xiao Q, Ding JQ, Chen SD. Curcumin inhibition of JNKs prevents dopaminergic neuronal loss in a mouse model of Parkinson’s disease through suppressing mitochondria dysfunction. Transl Neurodegener. 2012;1:16. doi: 10.1186/2047-9158-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chiu S, Terpstra KJ, Bureau Y, Hou J, Raheb H, Cernvosky Z, Badmeav V, Copen J, Husni M, Woodbury-Farina M. Liposomal-formulated curcumin [Lipocurc] targeting HDAC (histone deacetylase) prevents apoptosis and improves motor deficits in Park 7 (DJ-1)-knockout rat model of Parkinson’s disease: implications for epigenetics-based nanotechnology-driven drug platform. J Complement Integr Med. 2013:10. doi: 10.1515/jcim-2013-0020. [DOI] [PubMed] [Google Scholar]
- 57.Jaisin Y, Thampithak A, Meesarapee B, Ratanachamnong P, Suksamrarn A, Phivthong-Ngam L, Phumala-Morales N, Chongthammakun S, Govitrapong P, Sanvarinda Y. Curcumin I protects the dopaminergic cell line SH-SY5Y from 6-hydroxydopamine-induced neurotoxicity through attenuation of p53-mediated apoptosis. Neurosci Lett. 2011;489:192–196. doi: 10.1016/j.neulet.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 58.Kim HT, Qiang W, Liu N, Scofield VL, Wong PK, Stoica G. Up-regulation of astrocyte cyclooxygenase-2, CCAAT/enhancer-binding protein-homology protein, glucose-related protein 78, eukaryotic initiation factor 2 alpha, and c-Jun N-terminal kinase by a neurovirulent murine retrovirus. J Neurovirol. 2005;11:166–179. doi: 10.1080/13550280590922810. [DOI] [PubMed] [Google Scholar]
- 59.Sawada H, Ibi M, Kihara T, Honda K, Nakamizo T, Kanki R, Nakanishi M, Sakka N, Akaike A, Shimohama S. Estradiol protects dopaminergic neurons in a MPP+Parkinson’s disease model. Neuropharmacology. 2002;42:1056–1064. doi: 10.1016/s0028-3908(02)00049-7. [DOI] [PubMed] [Google Scholar]
- 60.Jiang TF, Zhang YJ, Zhou HY, Wang HM, Tian LP, Liu J, Ding JQ, Chen SD. Curcumin ameliorates the neurodegenerative pathology in A53T alpha-synuclein cell model of Parkinson’s disease through the downregulation of mTOR/p70S6K signaling and the recovery of macroautophagy. J Neuroimmune Pharmacol. 2013;8:356–369. doi: 10.1007/s11481-012-9431-7. [DOI] [PubMed] [Google Scholar]
- 61.Zbarsky V, Datla KP, Parkar S, Rai DK, Aruoma OI, Dexter DT. Neuroprotective properties of the natural phenolic antioxidants curcumin and naringenin but not quercetin and fisetin in a 6-OHDA model of Parkinson’s disease. Free Radic Res. 2005;39:1119–1125. doi: 10.1080/10715760500233113. [DOI] [PubMed] [Google Scholar]
- 62.Jagatha B, Mythri RB, Vali S, Bharath MM. Curcumin treatment alleviates the effects of glutathione depletion in vitro and in vivo: therapeutic implications for Parkinson’s disease explained via in silico studies. Free Radic Biol Med. 2008;44:907–917. doi: 10.1016/j.freeradbiomed.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 63.Harish G, Venkateshappa C, Mythri RB, Dubey SK, Mishra K, Singh N, Vali S, Bharath MM. Bioconjugates of curcumin display improved protection against glutathione depletion mediated oxidative stress in a dopaminergic neuronal cell line: Implications for Parkinson’s disease. Bioorg Med Chem. 2010;18:2631–2638. doi: 10.1016/j.bmc.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 64.Agrawal SS, Gullaiya S, Dubey V, Singh V, Kumar A, Nagar A, Tiwari P. Neurodegenerative Shielding by Curcumin and Its Derivatives on Brain Lesions Induced by 6-OHDA Model of Parkinson’s Disease in Albino Wistar Rats. Cardiovasc Psychiatry Neurol. 2012;2012:942981. doi: 10.1155/2012/942981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Meesarapee B, Thampithak A, Jaisin Y, Sanvarinda P, Suksamrarn A, Tuchinda P, Morales NP, Sanvarinda Y. Curcumin I mediates neuroprotective effect through attenuation of quinoprotein formation, p-p38 MAPK expression, and caspase-3 activation in 6-hydroxydopamine treated SH-SY5Y cells. Phytother Res. 2014;28:611–616. doi: 10.1002/ptr.5036. [DOI] [PubMed] [Google Scholar]
- 66.Jayaraj RL, Tamilselvam K, Manivasagam T, Elangovan N. Neuroprotective effect of CNB-001, a novel pyrazole derivative of curcumin on biochemical and apoptotic markers against rotenone-induced SK-N-SH cellular model of Parkinson’s disease. J Mol Neurosci. 2013;51:863–870. doi: 10.1007/s12031-013-0075-8. [DOI] [PubMed] [Google Scholar]
- 67.Mythri RB, Harish G, Dubey SK, Misra K, Bharath MM. Glutamoyl diester of the dietary polyphenol curcumin offers improved protection against peroxynitrite-mediated nitrosative stress and damage of brain mitochondria in vitro: implications for Parkinson’s disease. Mol Cell Biochem. 2011;347:135–143. doi: 10.1007/s11010-010-0621-4. [DOI] [PubMed] [Google Scholar]
- 68.Liu Z, Yu Y, Li X, Ross CA, Smith WW. Curcumin protects against A53T alpha-synuclein-induced toxicity in a PC12 inducible cell model for Parkinsonism. Pharmacol Res. 2011;63:439–444. doi: 10.1016/j.phrs.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 69.Beal MF. Therapeutic approaches to mitochondrial dysfunction in Parkinson’s disease. Parkinsonism Relat Disord. 2009;15(Suppl 3):S189–194. doi: 10.1016/S1353-8020(09)70812-0. [DOI] [PubMed] [Google Scholar]
- 70.Du XX, Xu HM, Jiang H, Song N, Wang J, Xie JX. Curcumin protects nigral dopaminergic neurons by iron-chelation in the 6-hydroxydopamine rat model of Parkinson’s disease. Neurosci Bull. 2012;28:253–258. doi: 10.1007/s12264-012-1238-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ji HF, Shen L. The multiple pharmaceutical potential of curcumin in Parkinson’s disease. CNS Neurol Disord Drug Targets. 2014;13:369–373. doi: 10.2174/18715273113129990077. [DOI] [PubMed] [Google Scholar]
- 72.Ono K, Hirohata M, Yamada M. Alpha-synuclein assembly as a therapeutic target of Parkinson’s disease and related disorders. Curr Pharm Des. 2008;14:3247–3266. doi: 10.2174/138161208786404191. [DOI] [PubMed] [Google Scholar]
- 73.Gadad BS, Subramanya PK, Pullabhatla S, Shantharam IS, Rao KS. Curcumin-glucoside, a novel synthetic derivative of curcumin, inhibits alpha-synuclein oligomer formation: relevance to Parkinson’s disease. Curr Pharm Des. 2012;18:76–84. doi: 10.2174/138161212798919093. [DOI] [PubMed] [Google Scholar]
- 74.Singh PK, Kotia V, Ghosh D, Mohite GM, Kumar A, Maji SK. Curcumin modulates alpha-synuclein aggregation and toxicity. ACS Chem Neurosci. 2013;4:393–407. doi: 10.1021/cn3001203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gautam S, Karmakar S, Bose A, Chowdhury PK. β-cyclodextrin and curcumin, a potent cocktail for disaggregating and/or inhibiting amyloids: a case study with alpha-synuclein. Biochemistry. 2014;53:4081–4083. doi: 10.1021/bi500642f. [DOI] [PubMed] [Google Scholar]
- 76.Rajeswari A, Sabesan M. Inhibition of monoamine oxidase-B by the polyphenolic compound, curcumin and its metabolite tetrahydrocurcumin, in a model of Parkinson’s disease induced by MPTP neurodegeneration in mice. Inflammopharmacology. 2008;16:96–99. doi: 10.1007/s10787-007-1614-0. [DOI] [PubMed] [Google Scholar]
- 77.Verma M, Sharma A, Naidu S, Bhadra AK, Kukreti R, Taneja V. Curcumin prevents formation of polyglutamine aggregates by inhibiting Vps36, a component of the ESCRT-II complex. PLoS One. 2012;7:e42923. doi: 10.1371/journal.pone.0042923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ortiz-Ortiz MA, Moran JM, Ruiz-Mesa LM, Niso-Santano M, Bravo-SanPedro JM, Gomez-Sanchez R, Gonzalez-Polo RA, Fuentes JM. Curcumin exposure induces expression of the Parkinson’s disease-associated leucine-rich repeat kinase 2 (LRRK2) in rat mesencephalic cells. Neurosci Lett. 2010;468:120–124. doi: 10.1016/j.neulet.2009.10.081. [DOI] [PubMed] [Google Scholar]
- 79.Yang D, Li T, Liu Z, Arbez N, Yan J, Moran TH, Ross CA, Smith WW. LRRK2 kinase activity mediates toxic interactions between genetic mutation and oxidative stress in a Drosophila model: suppression by curcumin. Neurobiol Dis. 2012;47:385–392. doi: 10.1016/j.nbd.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 80.Lee KW, Jung SY, Choi SM, Yang EJ. Effects of ginsenoside Re on LPS-induced inflammatory mediators in BV2 microglial cells. BMC Complement Altern Med. 2012;12:196. doi: 10.1186/1472-6882-12-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim SN, Ha YW, Shin H, Son SH, Wu SJ, Kim YS. Simultaneous quantification of 14 ginsenosides in Panax ginseng C. A. Meyer (Korean red ginseng) by HPLC-ELSD and its application to quality control. J Pharm Biomed Anal. 2007;45:164–170. doi: 10.1016/j.jpba.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 82.Lee YY, Park JS, Jung JS, Kim DH, Kim HS. Anti-Inflammatory Effect of Ginsenoside Rg5 in Lipopolysaccharide-Stimulated BV2 Microglial Cells. Int J Mol Sci. 2013;14:9820–9833. doi: 10.3390/ijms14059820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim KH, Song K, Yoon SH, Shehzad O, Kim YS, Son JH. Rescue of PINK1 protein null-specific mitochondrial complex IV deficits by ginsenoside Re activation of nitric oxide signaling. J Biol Chem. 2012;287:44109–44120. doi: 10.1074/jbc.M112.408146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu Q, Kou JP, Yu BY. Ginsenoside Rg1 protects against hydrogen peroxide-induced cell death in PC12 cells via inhibiting NF-kappaB activation. Neurochem Int. 2011;58:119–125. doi: 10.1016/j.neuint.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 85.Lin WM, Zhang YM, Moldzio R, Rausch WD. Ginsenoside Rd attenuates neuroinflammation of dopaminergic cells in culture. J Neural Transm Suppl. 2007;72:105–112. doi: 10.1007/978-3-211-73574-9_13. [DOI] [PubMed] [Google Scholar]
- 86.Park SM, Choi MS, Sohn NW, Shin JW. Ginsenoside Rg3 attenuates microglia activation following systemic lipopolysaccharide treatment in mice. Biol Pharm Bull. 2012;35:1546–1552. doi: 10.1248/bpb.b12-00393. [DOI] [PubMed] [Google Scholar]
- 87.Chen XC, Zhu YG, Zhu LA, Huang C, Chen Y, Chen LM, Fang F, Zhou YC, Zhao CH. Ginsenoside Rg1 attenuates dopamine-induced apoptosis in PC12 cells by suppressing oxidative stress. Eur J Pharmacol. 2003;473:1–7. doi: 10.1016/s0014-2999(03)01945-9. [DOI] [PubMed] [Google Scholar]
- 88.Leung KW, Yung KK, Mak NK, Chan YS, Fan TP, Wong RN. Neuroprotective effects of ginsenoside-Rg1 in primary nigral neurons against rotenone toxicity. Neuropharmacology. 2007;52:827–835. doi: 10.1016/j.neuropharm.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 89.Xu BB, Liu CQ, Gao X, Zhang WQ, Wang SW, Cao YL. Possible mechanisms of the protection of ginsenoside Re against MPTP-induced apoptosis in substantia nigra neurons of Parkinson’s disease mouse model. J Asian Nat Prod Res. 2005;7:215–224. doi: 10.1080/10286020410001690172. [DOI] [PubMed] [Google Scholar]
- 90.Xu H, Jiang H, Wang J, Xie J. Rg1 protects iron-induced neurotoxicity through antioxidant and iron regulatory proteins in 6-OHDA-treated MES23.5 cells. J Cell Biochem. 2010;111:1537–1545. doi: 10.1002/jcb.22885. [DOI] [PubMed] [Google Scholar]
- 91.Xu H, Jiang H, Wang J, Xie J. Rg1 protects the MPP+-treated MES23.5 cells via attenuating DMT1 up-regulation and cellular iron uptake. Neuropharmacology. 2010;58:488–494. doi: 10.1016/j.neuropharm.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 92.Rudakewich M, Ba F, Benishin CG. Neurotrophic and neuroprotective actions of ginsenosides Rb(1) and Rg(1) Planta Med. 2001;67:533–537. doi: 10.1055/s-2001-16488. [DOI] [PubMed] [Google Scholar]
- 93.Witkin JM, Li X. Curcumin, an active constiuent of the ancient medicinal herb Curcuma longa L. : some uses and the establishment and biological basis of medical efficacy. CNS Neurol Disord Drug Targets. 2013;12:487–497. doi: 10.2174/1871527311312040007. [DOI] [PubMed] [Google Scholar]
- 94.Song JX, Sze SC, Ng TB, Lee CK, Leung GP, Shaw PC, Tong Y, Zhang YB. Anti-Parkinsonian drug discovery from herbal medicines: what have we got from neurotoxic models? J Ethnopharmacol. 2012;139:698–711. doi: 10.1016/j.jep.2011.12.030. [DOI] [PubMed] [Google Scholar]