Abstract
Epidermal growth factor-like domain 7 gene (EGFL7) encodes an angiogenesis related factor and plays a crucial role in many human cancers. Previous studies have suggestedthat EGFL7 acts as a facilitator for tumor angiogenesis. However, little is known as to its role in osteosarcoma. Our aim was to investigate the expression of EGFL7 and to explore its correlation with the clinicopathological features of osteosarcoma. Tumor tissues from 32 Chinese young patients (below age of 24) with osteosarcoma were collected and subjected to EGFL7 detection by immunohistochemistry. The tissues from 10 patients with osteochondroma were collected and analyzed as controls. The intratumoral microvessel density (MVD) was examined by immunohistochemical staining using CD34 antibody. The results showed that patients with osteosarcoma had higher levels of EGFL7 in the tumor tissues compared to patients with osteochondroma (p<0.001). The expression of EGFL7 was significantly higher in advanced osteosarcoma (Enneking IIB-III) than that in early tumor stage (Enneking IA-IIA) (p<0.01). There is also a significant correlation between increased expression of EGFL7 and the Enneking stage (R = 0.714, p<0.001). Moreover, we detected a higher level of EGFL7 expression in tumor tissues of patients with recurrent or metastatic (bone or lung) osteosarcoma than those without recurrence or metastasis after 3 years’ follow-up (p<0.01). There is no detectable difference of EGFL7 expression between tumor tissues from different tumor location and sex. Finally, we showed that high level of EGFL7expression was significantly correlated with high MVD (R = 0.829, p<0.001). In conclusion, our study demonstrates for the first time that there was a tumor grade-dependent up-regulation of EGFL7 in osteosarcoma. Elevated EGFL7 expression correlated with poor clinical outcome: i.e. advanced tumor stage, recurrent and metastatic osteosarcoma. Our findings support EGFL7 as a potential prognostic marker, and may provide novel insights for the diagnostics and therapeutics of osteosarcoma.
Keywords: Osteosarcoma, epidermal growth factor-like domain 7 (EGFL7), tumor angiogenesis, microvessel density (MVD), clinicopathology, prognosis
Introduction
Osteosarcoma (OS) is the most commonly diagnosed primary malignant bone tumor. In the United States, osteosarcoma tends to occur more frequentlyin the age groups of adolescence and the elderly [1]. However, the incidence rate of osteosarcoma in Chinese people peaksonly in adolescence, which is around 6.8-9.2 per million. This means that there are at least 9,000 Chinese children and adolescents (age 24 or under)who suffer from thisdevastating bone cancer each year [2-4]. The major treatment for these osteosarcoma adolescents is combined limb-salvage surgery and neo-adjuvant chemotherapy, which has been demonstrated to lead to better survival time and function [5]. However, due to high prevalence and metastasis, the 5-year survival rate of Chinese adolescent patients is still lower than that in the United States [4,6]. Therefore, to find novel molecules that are relevant to osteosarcoma tumorigenesis and metastasis will help with the diagnosis and potentially better treatment and prognosis for Chinese young osteosarcoma patients.
Epidermal growth factor-like domain 7 (EGFL7) also known as vascular endothelial-statin (VE-statin) is a protein encoded by the EGFL7 gene. EGFL7 expression is mostly restricted to the endothelium, with high levels observed in proliferating endothelial cells during embryogenesis and patho-physiological angiogenesis [7]. EGFL7 expression in tumors has previously been shown to promote tumor progression possibly through its association with immune evasion. EGFL7 may reduce the expression of endothelial adhesion molecules in tumors and thus, fewer immune cell infiltrations [8]. EGFL7 may also play a key role in tumorigenesis through its function in angiogenesis. In fact, EGFL7 is aberrantly expressed in many human cancers, including colorectal cancer, hepatocellular carcinoma, breast cancer, laryngeal carcinoma, and malignant glioma. Notably, EGFL7 overexpression has been correlated with advanced stage, metastasis, vascular invasion, and poor prognosis of many solid tumors [9-13], whereas new blood vessel formation or angiogenesis is a prerequisite for tumor growth, local extension and distant metastasis of solid tumors, including osteosarcoma [14,15].
Despite its extensive tumor angiogenesis, until now, little is known about the correlation of EGFL7 expression with the clinical features and prognosis of osteosarcoma. In this study, we examined EGFL7 expression and microvessel density (MVD) of tumor tissues from Chinese young osteosarcoma patients, and evaluated their potential correlation with clinical features. The relationship between EGFL7 expression and MVD in osteosarcoma tumor tissues was also explored.
Materials and methods
Patients and tissue samples
The study was approved by the Ethics Committee of Central South University, and informed consent was obtained from each patient. Total 32 patients with osteosarcoma between January 2008 and January 2011 at Xiangya hospital in Central South University were enrolled. Tissues were obtained from patients undergone biopsy surgery but before neo-adjuvant chemotherapy. Patients under the age of 24 and tumors restricted to femoral and tibial locations were selected for current study. There were 17 male and 15 female patients with average age of 14.8 years (rang 7-24 years). 20 of the 32 osteosarcoma located at femora while the remaining 12 were tibial osteosarcoma. The detailed clinical information, including sex, age, tumor site/stage, and status of metastasis was summarized in Table 1. The 10 control osteochondroma (OC) patients included 6 males and 4 females with average age of 15.5 years (rang 8-23 years). Tumors were located at femora (6) and tibiae (4) respectively, and the tissues were obtained from resection surgery. The detailed information for OC patients was listed and compared to OS patients (Table 2). All tumors were confirmed by pathologicalexamination and all patients were regularly followed-up for 3 years, unless they died within 3 years which automatically ended the follow-up.
Table 1.
Information of patients with osteosarcoma
| Sex | AGE (year) | Tumor site | Enneking STAGE | Metastasis | EGFL7 expression | MVD |
|---|---|---|---|---|---|---|
| F | 18 | Femoral | IIB | Yes | +++ | 42.0 |
| F | 9 | Femoral | III | Yes | +++ | 41.7 |
| F | 20 | Femoral | I | No | - | 27.0 |
| F | 14 | Tibial | IIB | No | ++ | 38.3 |
| M | 18 | Femoral | III | Yes | +++ | 48.7 |
| M | 9 | Femoral | IIB | Yes | ++ | 37.7 |
| M | 15 | Tibial | IIB | Yes | +++ | 46.7 |
| M | 21 | Femoral | IIA | No | + | 29.3 |
| F | 15 | Femoral | IIA | No | ++ | 34.7 |
| M | 14 | Tibial | I | No | - | 23.3 |
| M | 9 | Femoral | III | Yes | +++ | 51.3 |
| M | 14 | Ffemoral | IIB | No | + | 37.0 |
| F | 10 | Femoral | I | No | + | 19.7 |
| M | 15 | Tibial | IIA | No | ++ | 33.0 |
| M | 19 | Femoral | IIA | No | +++ | 31.3 |
| F | 10 | Tibial | III | Yes | +++ | 47.0 |
| M | 13 | Tibial | III | Yes | +++ | 45.0 |
| M | 19 | Ttibial | III | Yes | ++ | 36.0 |
| F | 18 | Femoral | IIA | No | + | 32.7 |
| M | 17 | Femoral | IIA | No | ++ | 34.3 |
| F | 17 | Femoral | IIB | Yes | ++ | 38.7 |
| M | 15 | Femoral | IIB | No | ++ | 37.3 |
| M | 17 | Femoral | III | Yes | +++ | 43.0 |
| M | 9 | Femoral | III | Yes | +++ | 42.7 |
| M | 12 | Femoral | IIA | No | ++ | 33.3 |
| F | 10 | Femoral | IIB | No | ++ | 38.0 |
| M | 18 | Tibial | III | Yes | ++ | 36.7 |
| F | 19 | Tibial | III | Yes | +++ | 42.3 |
| F | 17 | Tibial | III | Yes | ++ | 39.0 |
| F | 7 | Tibial | III | Yes | +++ | 44.0 |
| F | 24 | Femoral | IIB | No | ++ | 35.7 |
| F | 11 | Tibial | IIA | No | + | 28.7 |
Table 2.
Comparison of information from OS and OC patients
| Sex (n) | Tumor site (n) | Average age (years) | |||
|---|---|---|---|---|---|
|
| |||||
| Male | Female | Tibial | Femoral | ||
| Osteosarcoma | 17 | 15 | 12 | 20 | 14.8 |
| Osteochondroma | 6 | 4 | 4 | 6 | 15.5 |
Immunohistochemistry
The tumor tissues from OS patients and OC control patients were fixed in 4% paraformaldehyde and paraffin-embedded and sectioned for immunohistochemistry (IHC) analysis. Specifically, each section was deparaffinized and dehydrated and subjected to antigen retrieval. The slides were placed in 0.01 mmol/L citrate buffer (pH 6.0) for 15 min in a microwave oven. Endogenous peroxidase activity was blocked with a 3% hydrogen peroxide solution for 10 min at room temperature. After rinsing with PBS, slides were incubated for 1 h at room temperature with primary antibodies. Primary antibodies rabbit anti-human-EFGL7 and rabbit anti-human-CD34 (both are polyclonal from Biosynthesis Biotech, Beijing, China) were 1:100 diluted. Sections were then washed in PBS three times, and incubated with anti-rabbit secondary antibody for 15 min at room temperature. Immunostaining was performed using the Envision system with diaminobenzidine (DakoCytomation, Glostrup, Denmark). Finally, the signal was developed with 3,3’-diaminobenzidine tetrahydrochloride (DAB), and all of the slides were counterstained with hematoxylin. Negative controls were treated with PBS instead of primary antibodies.
Scoring system for immunohistochemistry
Expression of EFGL7 was localized in the plasm of vascular endothelial cells [16]. Light-microscopic analysis was performed by manually counting positively stained cells in 5 separate areas of intratumoral regions under 400× high-power magnifications. The score was calculated by the sum of two parts: (1) percentage of positive cells (0 = 0-10% positive cells, 1 = 10-40% positive cells, and 2 = 40-70% positive cells, 3≥70% positive cells), and (2) staining intensity (0 = negative, 1 = weak, 2 = moderate, 3 = high). Scores <2 was regarded as negative (-), 2-3 as weak (+), 4-5 as moderate (++), >5 as strongly positive (+++), respectively.
Microvessel density (MVD) analysis
MVD was assessed by immunohistochemistry using CD34 markers [17]. The stained sections were screened at 100× magnification for hot spots. Five high-power fields in each specimen were selected randomly. Then, vessels were counted in 5 different fields at 400× magnification and an average number of microvessels were recorded. Two observers did the counting, and the mean value was used for analysis.
Statistical analysis
Statistical analysis was performed using the SPSS 13.0 for windows (SPSS, Chicago, IL, USA). Descriptive data were presented as mean ± SD. The chi-square tests were used to assess the different expression of EGFL7 between osteosarcoma and osteochondroma. The chi-square tests (Fisher’s exact) were used to assess the relationship between EGFL7 and clinicopathological features. Spearman correlation coefficient was calculated to further evaluate the correlation between EGFL7 and MVD, EGFL7 and Enneking stage. A p value less than 0.05 were considered significant.
Results
EGFL7 gene structure and functional domains
Based on latest NCBI (National Center for Biotechnology Information) database, the human EGFL7 gene consists of 11 exons with the coding region locates in exons 4-11 (Figure 1). There are four transcript variants (V1, V2, V3 and V4) of EGFL7 that encode three protein isoforms a, b, and c. Variant 1 is consisted of 11 exons, representing the longest transcript and encodes isoform a. Variant 2 lacks exon 2 compared to variant 1 and encodes a shorter isoform b. Variant 3 also lacks exon 2 and encodes the same isoform b. Variant 4 contains an alternate 5‘-exon and encodes isoform c that has a distinct and shorter N-terminus. Other variants were not listed or represented as non-coding. A miRNA, miR126, locates at the intronic region downstream of exon 8. The three functional domains (an EMI domain, a calcium-binding EGF-like domain, and a coagulation Factor Xa inhibitory site domain) of EGFL7 protein were as shown (Figure 1).
Figure 1.
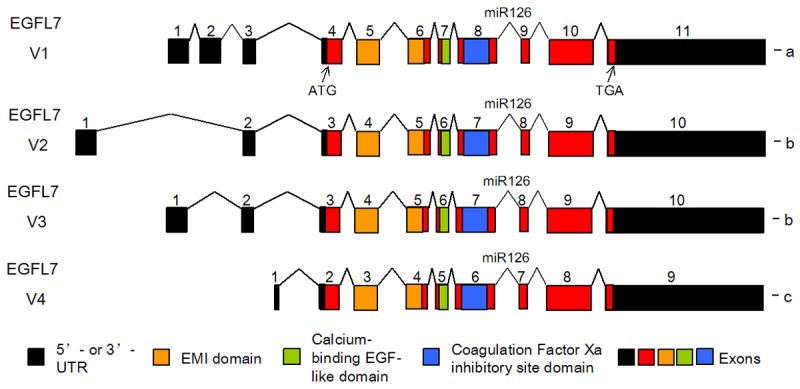
EGFL7 gene structure and its protein domains.The human EGFL7 gene is consisted four variants (V1, V2, V3 and V4) that encode three EGFL7 protein isoforms a-c. Compared to Variant 3, Variant 2 encodes a shorter isoform b. These variants encode three functional domains: an EMI domain (yellow box), a calcium-binding EGF-like domain (green box) and acoagulation Factor Xa inhibitory site domain (blue box). ATG: start codon; TGA: stop codon.
Increased expression of EGFL7 in patients with osteosarcoma
Expression of EGFL7 was predominantly localized in the cytoplasm of vascular endothelial cells and the surrounding osteosarcoma cells in tumor tissues. It was calculated by light-microscopic analysis. 93.8% osteosarcoma tissues had a positive EGFL7 expression. Only 1 sample had a weak expression in the control osteochondroma and almost no positive cells were appreciated in its tumor tissues (Figure 2). Increased expression of EGFL7 was shown in osteosarcoma tissues compared to that from controls (p<0.001) (Table 3, Figure 3).
Figure 2.
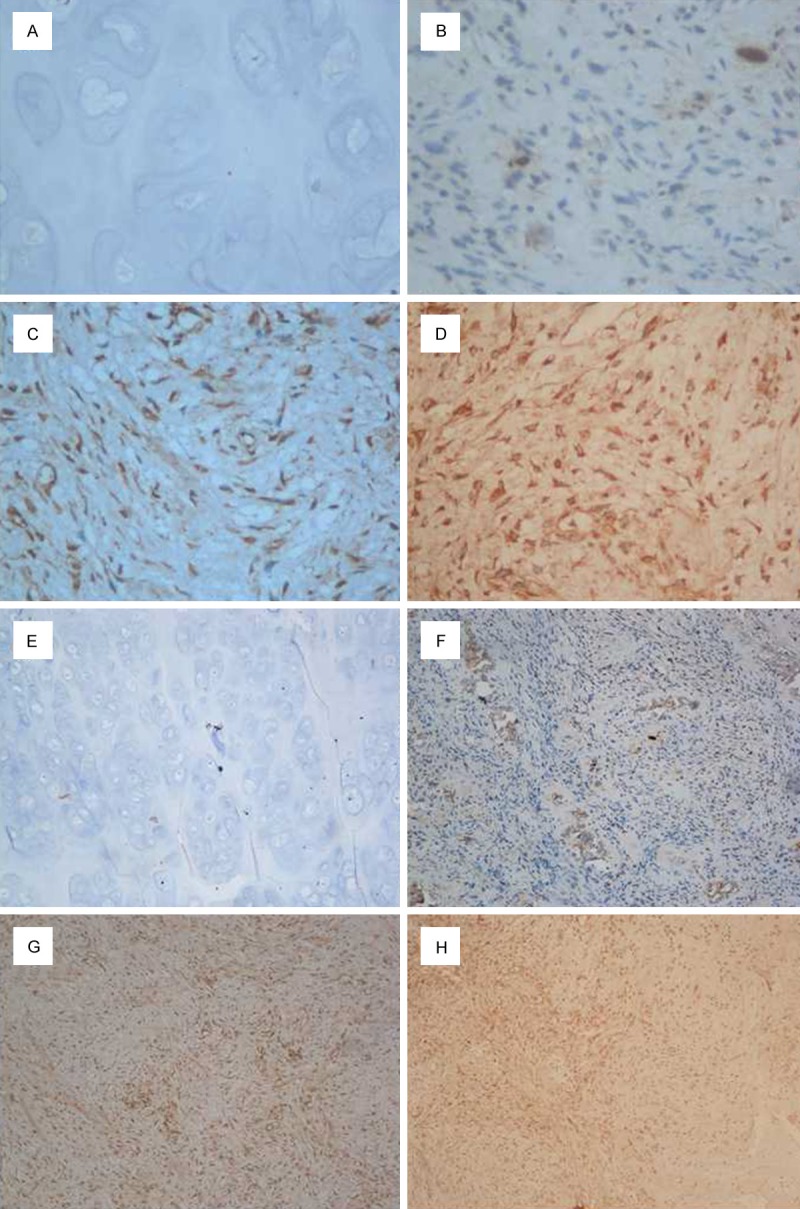
Varied EGFL7 expression in osteosarcoma patients and controls. (A, E) No expression of EGFL7 in controls, EGFL7 (-). (B-D, F-H) Expression of EGFL7 in different osteosarcoma tumor tissues: (B, F), EGFL7 (+); (C, G), EGFL7 (++); (D, H), EGFL7 (+++). (A-D, magnification ×400, E-H, magnification ×100).
Table 3.
Increased expression of EGFL7 in osteosarcoma tissues
| Group | n | EGFL7 expression | χ2 value | P value | |
|---|---|---|---|---|---|
|
| |||||
| - to + | ++ to +++ | ||||
| OS | 32 | 7 | 25 | 16.195 | 0.000* |
| OC | 10 | 10 | 0 | ||
OS, osteosarcoma, OC, osteochondroma,
p<0.001.
Figure 3.
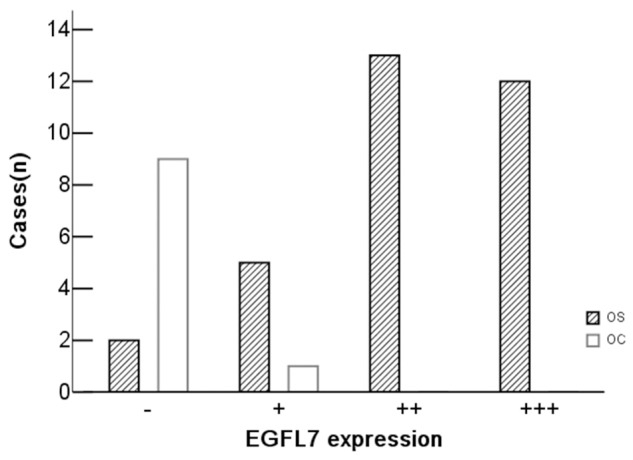
EGFL7 expression in osteosarcoma and osteochondroma patients. Increased expression of EGFL7 in osteosarcoma tissues compare to osteochondroma, 93.8% osteosarcoma tissues had a positive EGFL7 expression.
EGFL7 expression and clinicopathological features of osteosarcoma
We analyzed the relationship between EGFL7 expression and the clinical information of the osteosarcoma patients, including sex, tumor site/stage, and statu of metastasis.In tumor tissues, expression of EGFL7 was significantly higher in advanced osteosarcoma (Enneking IIB-III) than that in early tumor (Enneking IA-IIA) (p<0.01) (Table 4). There was a positive correlation between EGFL7 expression in tissues and Enneking stage in patient with osteosarcoma (R = 0.714, p<0.001) (Table 1, and Figure 4). There was also a higher EGFL7 expression of tumor tissues in osteosarcoma patients with recurrence or metastasis (bone or lung) than those without recurrence or metastasis in a 3 years follow-up period (p<0.01) (Table 4). No significant correlation was observed between EGFL7 expression and sex and tissues from different tumor location (Table 4).
Table 4.
EGFL7 and clinicopathological features of osteosarcoma
| Parameters | Cases | EGFL7 expression (n) | P value | |
|---|---|---|---|---|
|
| ||||
| - to ++ | ++ to +++ | |||
| Gender | 0.678 | |||
| Male | 17 | 3 | 14 | |
| Female | 15 | 4 | 11 | |
| Tumor site | 0.683 | |||
| Femoral | 20 | 5 | 15 | |
| Tibial | 12 | 2 | 10 | |
| Enneking stage | 0.003* | |||
| IA-IIA | 11 | 6 | 5 | |
| IIB-III | 21 | 1 | 20 | |
| Recurrence or metastasis | 0.007* | |||
| No | 16 | 7 | 9 | |
| Yes | 16 | 0 | 16 | |
The chi-square tests (Fisher’s exact),
p<0.01.
Figure 4.
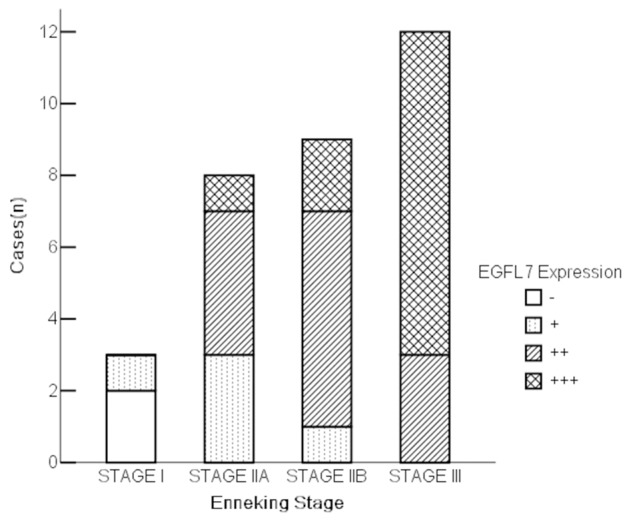
EGFL7 expression in different stages of osteosarcoma. Increased expression of EGFL7 in advanced osteosarcoma (Enneking Stage), there was a positive correlation between EGFL7 expression in tissues and Enneking stage in patient with osteosarcoma (R = 0.714, p<0.001).
EGFL7 expression and MVD in tumor tissues
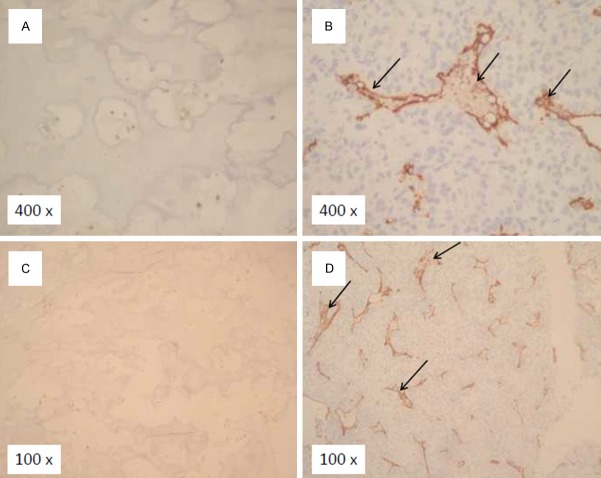
MVD staining was determined by CD34 immunohistochemistry analysis. After scoring and densitometric analysis, there was a very low MVD in tissues from osteochondroma control patients, but MVD staining was significantly higher in tissues from osteosarcoma patients (Figure 5A-D). The MVD for 32 osteosarcoma specimens ranged from 19.7 to 51.3 with a mean MVD of (37.4 ± 7.2) (Table 1). MVD was significantly higher in the tumor tissue specimen with higher level of EGFL7 expression than that with lower level of EGFL7 expression. Higher MVD staining was found significantly correlated with higher level of EGFL7 expression in OS tumor tissues (R = 0.829, p<0.001) (Figure 6).
Figure 5.
MVD staining in osteosarcoma patients and controls. CD34 staining was performed for MVD in tissues from osteosarcoma patients and osteochondroma control patients. The results showed that there were very low MVD staining in tissues from osteochondroma control patients (A, C), but significant higher MVD staining in tissues from osteosarcoma patients were observed (B, D, black arrows). (A, B, magnification 400×, C, D, magnification 100×).
Figure 6.
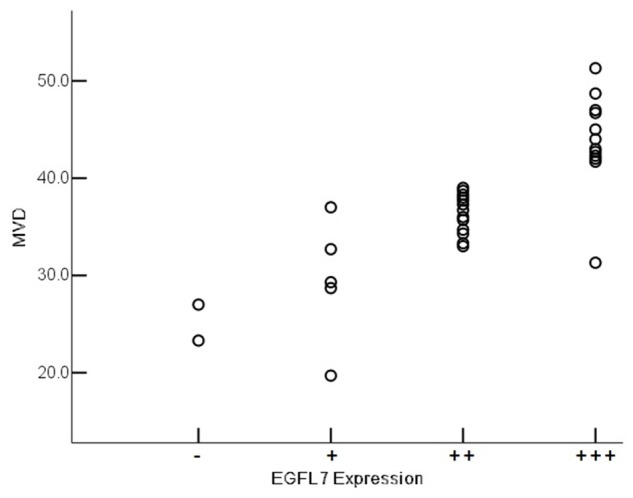
EGFL7 expression and MVD staining in osteosarcoma. Relationship between EGFL7 and MVD in CD34 staining for MVD in tumor tissues was shown. Scattered plots showed the distribution of MVD in different levels of EGFL7 expression. Increased MVD were found in osteosarcoma tissues with higher EGFL7 expression (staining intensity ++ and +++). High MVD was found significantly correlated with the expression of EGFL7 in tumor tissues (R = 0.829, p<0.001).
Discussion
Unlike in the United States and Europe, the incidence of osteosarcoma in children and adolescence in China is 6 times higher than that in the elderly [1]. Despite improved treatment, the prognosis for Chinese young osteosarcoma patients remains poor. There are many factors that affect the prognosis of osteosarcoma, including demographics (age, sex), tumor size, site, stage, and the response to chemotherapy. These prognostic factors are assessed or determined by varied criteria, which makes it difficult to compare between studies. Metastases and response to chemotherapy are probably the most important and accurate two prognostic determinants. However, these factors are usually determined at a late stage of tumor development [5,18]. There is an eager need to find general diagnostic and prognostic biomarkers of osteosarcoma progression so as to allow for early treatment of OS [5,19].
In this study, we reported elevated EGFL7 expression in tumor tissues of OS, especially in advanced, recurrent, and metastatic tumors, suggested a prognostic value of EGFL7 for OS. EGFL7 was produced by both osteosarcoma cells and vascular endothelial cells in tumor tissues. We found that high level of EGFL7 expression was associated with high MVD, while intratumoral expression of EGFL7 was associated with the clinical stages, as well as recurrence or (bone or lung) metastasis of OS, suggesting EGFL7 as a potential oncogenic/prognostic marker to promote OS progression via angiogenesis in tumor microenvironment. The correlation of high level of EGFL7 expression with poor outcome of OS providesnovel insights into the diagnosis and therapeutic intervention for osteosarcoma.
EGFL7 is a new secreted angiogenic factor controlling blood vessel development by creating a permissive environment for angiogenesis [7]. The EGFL7 protein contains a signal sequence, an EMI domain at the amino terminus, followedby two EGF-like domains and acoagulation factor Xa inhibitory site domain (Figure 1). EGFL7 is unique because it is highly conserved in vertebrates and may play specific function in endothelial cells. Unlike any other secreted angiogenic signaling molecules, EGFL7 expression is mostly restricted to the endothelium, with high levels observed in proliferating endothelial cells during embryogenesis and path-physiological angiogenesis [20-24]. EGFL7 has been shown to play important roles during repair of the microvasculature in response to vascular damage or injury and ischemia [20,25]. Notably, this pathological process is frequently observed in OS and the vascular endothelial cells may be the source of elevated EGFL7 expression in its tumor tissue, although the mechanisms underlying the regulated EGFL7 expression and its role in OS development remain largely unknown.
Recently, EGFL7 has been shown to be highly elevated in many human tumors, including kidney tumors, malignant gliomas, hepatocellular carcinomas, and colon cancers etc. High level of EGFL7 expression was also significantly correlated with pathological progression, poor prognosis, and tumor grade of these tumors [10,13,26]. EGFL7 may play a similar pro-tumor function as VEGF (vascular endothelial growth factor) by stimulating tumor angiogenesis and leading to irregular shape, tortuous and leaky vessels that are characteristic of the tumor vasculature [27]. EGFL7 has also been shown to promote cell motility by EGF receptor-dependent phosphorylation of focal adhesion kinase and thus enhance metastasis of cancer [13]. A recent study has shown that EGFL7 may promote tumor growth by protecting tumors from a host immune response, as EGFL7 enhanced tumor progression in immunocompromised mice and prevented host immune cell infiltration [8]. Whether EGFL7 contributes to OS tumorigenesis through above mechanisms is currently not clear. EGFL7 expression was localized to both vascular endothelial cells and the surrounding tumor cells in OS tissues, similar to that found in brain gliomas [10]. Although further investigation is needed, EGFL7 may exert its effect on the vasculature either in an autocrine manner, a paracrine fashion, or by cross talk between the 2 cell types.
Previous studies have shown that EGFL7 expression correlated well with MVD in human malignant gliomas, hepatocellular carcinomas, and colon cancers [10,23,26]. As to OS, little is known about the relationship between expression of EGFL7 and MVD before. However, EGFL7 expression in the local bony environment suggested its role in angiogenesis [28]. It was previously suggested that EGFL7 plays significant roles during pathological vessel formation [10,23,26]. Osteosarcomas are highly vascularized tumors, as demonstrated by our finding that the MVD in osteosarcoma tissues was significantly higher than that in osteochondroma. We also found that EGFL7 expression significantly correlated with recurrence and metastasisof OS, a process known to largely involve pathogenesis of angiogenesis. These findings supported that EGFL7 may participate in the progression of OS through association with angiogenesis.
We did not observe statistically significant correlation between EGFL7 expression and gender, although some data showed there was a different incidence of OS between males and females [1]. There was no difference of EGFL7 expression between femoral and tibial OS either. Due to limited number of the elderly who suffered from OS, we only analyzed young OS patients at age 24 or under. Further investigation using samples from the elderly is needed given their established differences in many clinical features.
In summary, to the best of our knowledge, we have for the first time systematically examined the expression of EGFL7 in OS. Our findings showed that increased expression of EGFL7 was seen in OS and there was a tumor grade-dependent up-regulation of EGFL7 in tumor tissues. In addition, there was a positive correlation between the expression of EGFL7 and MVD, suggesting the importance of EGFL7 in the angiogenesis, proliferation, and progression of OS. The high-level expression of EGFL7 within advanced, recurrent and metastatic OS, and its significant correlation with poor clinical outcome suggest that EGFL7 may be suitable as a predictive factor for OS progression and prognosis, and may prove useful as a therapeutic target to prevent OS metastasis by intervention of its pathological angiogenesis.
Acknowledgements
This work was supported by grants from National Natural Sciences Foundation of China (Project number: 81371955 and 81401838) and the Young Teacher’s Boosting Project of the Fundamental Research Funds for the Central Universities in Central South University, China (Project number: 2012QNZT095).
Disclosure of conflict of interest
None.
References
- 1.Mirabello L, Troisi RJ, Savage SA. International osteosarcoma incidence patterns in children and adolescents, middle ages and elderly persons. Int J Cancer. 2009;125:229–234. doi: 10.1002/ijc.24320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 3.Hung GY, Horng JL, Yen HJ, Yen CC, Chen WM, Chen PC, Wu HT, Chiou HJ. Incidence patterns of primary bone cancer in taiwan (2003-2010): a population-based study. Ann Surg Oncol. 2014;21:2490–2498. doi: 10.1245/s10434-014-3697-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang XD, Guo W, Yang R, Yang Y, Ji T. Limb salvage surgery for osteosarcoma around the knee in children and adolescent patients. Zhonghua Wai Ke Za Zhi. 2007;45:669–672. [PubMed] [Google Scholar]
- 5.Gazitt Y, Kolaparthi V, Moncada K, Thomas C, Freeman J. Targeted therapy of human osteosarcoma with 17AAG or rapamycin: characterization of induced apoptosis and inhibition of mTOR and Akt/MAPK/Wnt pathways. Int J Oncol. 2009;34:551–561. [PubMed] [Google Scholar]
- 6.Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004. Cancer. 2009;115:1531–1543. doi: 10.1002/cncr.24121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nichol D, Stuhlmann H. EGFL7: a unique angiogenic signaling factor in vascular development and disease. Blood. 2012;119:1345–1352. doi: 10.1182/blood-2011-10-322446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delfortrie S, Pinte S, Mattot V, Samson C, Villain G, Caetano B, Lauridant-Philippin G, Baranzelli MC, Bonneterre J, Trottein F. Egfl7 promotes tumor escape from immunity by repressing endothelial cell activation. Cancer Res. 2011;71:7176–7186. doi: 10.1158/0008-5472.CAN-11-1301. [DOI] [PubMed] [Google Scholar]
- 9.Fan C, Yang L, Wu F, Tao Y, Liu L, Zhang J, He Y, Tang L, Chen G, Guo L. The expression of Egfl7 in human normal tissues and epithelial tumors. Int J Biol Markers. 2013;28:71–83. doi: 10.5301/JBM.2013.10568. [DOI] [PubMed] [Google Scholar]
- 10.Huang C, Li X, Zhou Y, Luo Y, Li C, Yuan X. Expression and clinical significance of EGFL7 in malignant glioma. J Cancer Res Clin Oncol. 2010;136:1737–1743. doi: 10.1007/s00432-010-0832-9. [DOI] [PubMed] [Google Scholar]
- 11.Li J, Yang X, Wang S, Tang Q. Prognostic role of epidermal growth factor-like domain 7 protein expression in laryngeal squamous cell carcinoma. J Laryngol Otol. 2011;125:1152–1157. doi: 10.1017/S0022215111002441. [DOI] [PubMed] [Google Scholar]
- 12.Philippin LG, Baranzelli MC, Samson C, Fournier C, Pinte S, Mattot V, Bonneterre J, Soncin F. Expression of Egfl7 correlates with low-grade invasive lesions in human breast cancer. Int J Oncol. 2013;42:1367–1375. doi: 10.3892/ijo.2013.1820. [DOI] [PubMed] [Google Scholar]
- 13.Wu F, Yang LY, Li YF, Ou DP, Chen DP, Fan C. Novel role for epidermal growth factor-like domain 7 in metastasis of human hepatocellular carcinoma. Hepatology. 2009;50:1839–1850. doi: 10.1002/hep.23197. [DOI] [PubMed] [Google Scholar]
- 14.Vasudev NS, Reynolds AR. Anti-angiogenic therapy for cancer: current progress, unresolved questions and future directions. Angiogenesis. 2014;17:471–494. doi: 10.1007/s10456-014-9420-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaffe N. Pediatric and Adolescent Osteosarcoma. Springer; 2010. Osteosarcoma: review of the past, impact on the future. The American experience; pp. 239–262. [DOI] [PubMed] [Google Scholar]
- 16.Shimizu M, Saitoh Y, Itoh H. Immunohistochemical staining of Ha- ras oncogene product in normal, benign, and malignant human pancreatic tissues. Human Pathol. 1990;21:607–612. doi: 10.1016/s0046-8177(96)90006-4. [DOI] [PubMed] [Google Scholar]
- 17.Weidner N, Folkman J, Pozza F, Bevilacqua P, Allred EN, Moore DH, Meli S, Gasparini G. Tumor angiogenesis: a new significant and independent prognostic indicator in early-stage breast carcinoma. J Natl Cancer Inst. 1992;84:1875–1887. doi: 10.1093/jnci/84.24.1875. [DOI] [PubMed] [Google Scholar]
- 18.Clark JC, Dass CR, Choong PF. A review of clinical and molecular prognostic factors in osteosarcoma. J Cancer Res Clin Oncol. 2008;134:281–297. doi: 10.1007/s00432-007-0330-x. [DOI] [PubMed] [Google Scholar]
- 19.Trieb K, Kotz R. Proteins expressed in osteosarcoma and serum levels as prognostic factors. Int J Biochem Cell Biol. 2001;33:11–17. doi: 10.1016/s1357-2725(00)00066-2. [DOI] [PubMed] [Google Scholar]
- 20.Campagnolo L, Leahy A, Chitnis S, Koschnick S, Fitch MJ, Fallon JT, Loskutoff D, Taubman MB, Stuhlmann H. EGFL7 is a chemoattractant for endothelial cells and is up-regulated in angiogenesis and arterial injury. Am J Pathol. 2005;167:275–284. doi: 10.1016/S0002-9440(10)62972-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fitch MJ, Campagnolo L, Kuhnert F, Stuhlmann H. Egfl7, a novel epidermal growth factor-domain gene expressed in endothelial cells. Dev Dyn. 2004;230:316–324. doi: 10.1002/dvdy.20063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nichol D, Shawber C, Fitch MJ, Bambino K, Sharma A, Kitajewski J, Stuhlmann H. Impaired angiogenesis and altered Notch signaling in mice overexpressing endothelial Egfl7. Blood. 2010;116:6133–6143. doi: 10.1182/blood-2010-03-274860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parker LH, Schmidt M, Jin SW, Gray AM, Beis D, Pham T, Frantz G, Palmieri S, Hillan K, Stainier DY. The endothelial-cell-derived secreted factor Egfl7 regulates vascular tube formation. Nature. 2004;428:754–758. doi: 10.1038/nature02416. [DOI] [PubMed] [Google Scholar]
- 24.Soncin F, Mattot V, Lionneton F, Spruyt N, Lepretre F, Begue A, Stehelin D. VE-statin, an endothelial repressor of smooth muscle cell migration. EMBO J. 2003;22:5700–5711. doi: 10.1093/emboj/cdg549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gustavsson M, Mallard C, Vannucci SJ, Wilson MA, Johnston MV, Hagberg H. Vascular response to hypoxic preconditioning in the immature brain. J Cereb Blood Flow Metab. 2007;27:928–938. doi: 10.1038/sj.jcbfm.9600408. [DOI] [PubMed] [Google Scholar]
- 26.Díaz R, Silva J, García JM, Lorenzo Y, García V, Peña C, Rodriguez R, Muñoz C, Garcia F, Bonilla F. Deregulated expression of miR-106a predicts survival in human colon cancer patients. Genes Chromosomes Cancer. 2008;47:794–802. doi: 10.1002/gcc.20580. [DOI] [PubMed] [Google Scholar]
- 27.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chim SM, Tickner J, Chow ST, Kuek V, Guo B, Zhang G, Rosen V, Erber W, Xu J. Angiogenic factors in bone local environment. Cytokine Growth Factor Rev. 2013;24:297–310. doi: 10.1016/j.cytogfr.2013.03.008. [DOI] [PubMed] [Google Scholar]