Abstract
The house dust mite (HDM), Dermatophagoidesfarinae (D. farina), is one of the most important indoor allergen sources and a major elicitor of allergic asthma; itscharacterization is important in the diagnosis and immunotherapy of mite allergen-relevant diseases. This study aims to characterize a novel allergen, the D. farinae-derived serpin (Der f 27). In this study, the total RNA of D. farinae was extracted, and the Der f 27 gene was cloned and expressed. The allergenicity of recombinant Der f 27 protein was determined by enzyme-linked immunosorbent assay, and Western-blotting with the sera of asthma patients, and skin prick test (SPT) in allergic human subjects. A r-Der f 27 allergic asthma mouse model was established. The cloned Der f 27 gene has been presented at the Gene Bank with an accession number of KM009995. The IgE levels of r-Der f 27 in the serum from r-Der f 27 SPT positive allergic patients were 3 folds more than healthy subjects. The Der f 27 SPT positive ratewas 42.1% in 19 DM-SPT positive patients. Airway hyperresponsiveness, serum specific IgE, and levels of interleukin-4 in the spleen cell culture supernatant were significantly increased in allergic asthma mice sensitized to r-Der f 27. In conclusion, Der f 27 is a new subtype of house mite allergen.
Keywords: Dermatophagoides farinae, serpin, Der f 27, allergic asthma, mouse model
Introduction
Allergic diseases, such as asthma, atopic dermatitis, or rhinitis, affecting 30% to 40% population in the world, and the prevalence of allergy is increasing in the recent decades. Allergic diseases have a greater negative impact on patients’ work and social lives and have become a costly global health problem [1,2]. Allergens from house dust mites (HDM) are major environmental trigger factors for allergic diseases [3]. It is responsible for the sensitization of more than 50% of airway allergic patients [4,5]. At present, SIT (specific immunotherapy) with house dust mite extracts is the most efficient treatment for dust mite allergy diseases. However, crude extracts of house dust mites not only contain allergens, but also include inflammatory molecules, such as ceramides, kallikreins and the ever popular endotoxin, which have been proved to have side effects in the clinical treatment [6]. Therefore, recombinant allergens have been regarded as a substitute for crude mite extracts used in clinical immunotherapy, which are expected to improve the efficacy and safety of SIT with house dust-mite allergens.
Extensive studies have been conducted to understand the structure, chemical and biological properties of dust mite allergens. Two groups of mite allergens (group 1 and 2) have been proven as the major allergens in Dermtophagoidesfarina (D. farina). They are a cysteine protease and anepididymal protein, respectively. More than 80% of humans with house dust mite allergy have IgE antibody to the group 1 and more than 90% of the group 2 [7-10]. However, about 20% of patients do not have IgE antibody to the two major allergen groups [7]. It is estimated that there are at least 30 allergens in the extracts of D. Farina and many other HDM allergens which containing high IgE binding activity, with low and variable concentrations in mite extracts, which are usually less than 1% of the group 1 and 2 mite allergens [7,11]. Although allergic diseases caused by serpin have been reported [12,13], there are no reports about the characterization of serpin from D. farina. Therefore, it is necessary to characterize the D. farina-derived Der f 27 in the initiation of allergic responses. In this study, high purity Der f 27 was purified from D. Farina; the immunological characterization was analyzed. We cloned, expressed and purified the serpin (r-Der f 27) from HDM, established a mouse model of r-Der f 27 induced-allergic asthma, and evaluated the allergenicity of r-Der f 27.
Materials and methods
Mice
Six-to-eight-week-old female BALB/c mice, weighting 18-20 g, were obtained from Southern Medical University Laboratory Animal Center (Guangzhou, China). The mice were exposed to a 12-h light/dark cycle, maintained under conditions of constant humidity (60%) and temperature (24°C), and provided tap water to drink, as well as SPF level standard mouse food. The experimental procedures were approved by the Institutional Ethics Committee at Shenzhen University.
Sera
The sera of patients with allergic disorders were obtained from the First Affiliated Hospital of Guangzhou Medical University. The Sera from non-allergic individuals were used as normal controls. The using human sera in the present study was approved by the Human Ethic Committee at Shenzhen University.
D. farinae RNA sample preparation, cDNA synthesis, and polymerase chain reaction (PCR)
The gene sequence of Der f 27 (Serpin, KM009995) was obtained by blast with the whole genome of Dermatophagoides farina. Total RNA was isolated from the homogenates of mite with Trizol following the manufacturer’s instructions.The cDNA was synthesized with a SMARTerTM PCR cDNA Synthesis Kit (Clontech Laboratories, Inc.) following the manufacturer’s protocol. Briefly, 1 μg total RNA in 3.5 μl double-distilled H2O was mixed with 1 μl 3’ SMART CDS Primer IIA and then incubated serially at 72°C for 3 min and at 42°C for 2 min. The reaction was transferred to a 5.5-μl aliquot of master mix containing 2 μl 5× First-strand buffer, 0.25 μl DTT (100 mM), 1 μl dNTP (10 mM), 1 μl SMARTer IIA oligonucleotide (12 μM), 0.25 RNase inhibitor, and 1 μl SMARTScribeTM reverse transcriptase (RT, 100 U). The RT reaction was allowed to proceed at 42°C for 1 h, followed by heating at 70°C for 10 min, and then a transfer to a 90-μl aliquot of PCR master mix containing 10 μl 10× Advantage 2 PCR buffer, 2 μl 50× dNTP (10 mM), 2 μl 5’ PCR Primer2A (12 μM), and 2 μl 50× Advantage 2 polymerase. The PCR conditions were as follows: 95°C for 1 min followed by 15 cycles at 95°C for 15 s, 65°C for 30 s, and 68°C for 3 min. The quality of the cDNA was confirmed by a Bioanalyzer.
Expression and purification of the recombinant Der f 27
The PCR products were ligated into pMD18-T vector (Takara), the constructed plasmids were transformed into E. coli Top10. The positive clones were sequenced by BGI (Shenzhen, China). The pMD18-T vector with Der f 27 product were digested. Der f 27 fragments were recycled from the gel and ligated into the pET44-a expression vector. Initial cloning was done in E. coli strain Top10. DNA extracted from positive clones were digested with the BamH I and XhoI restriction enzymes and followed by sequencing. The target plasmids were transformed into E. coli BL21 cells for expression. The cells were grown in LB-medium supplemented with 50 µg/ml ampicillin to an absorbance of 0.6 at 600 nm. Expression of Der f 27 was induced by adding isopropyl-D-thiogalactopyranoside (IPTG) to LB medium at a final concentration of 0.1 mM and the incubation was continued for 4 h at 37°C. The cells were harvested by centrifugation and resuspended in 50 mM Tris-HCl, 100 mM NaCl, pH 7.5. The cells were sonicated at an amplitude of 38% for 5 min (1 s pulse on and 0.1 s pulse off) followed by centrifugation at 10,000 rev/min at 4°C for 20 min. Finally the recombinant protein was purified by affinity chromatography using the Ni+ as solid phase. After vigorous washing with a washing buffer (50 mM Tris, 40 mM imidazole and 0.5 M NaCl, pH 8), the protein was eluted slowly using an elution buffer (50 mM Tris, 0.3 M imidazole and 0.2 M NaCl, pH 8). The elution peak was collected and purified again by molecular sieve chromatography.
SDS-PAGE and protein quantification
Theprotein concentration was determined by a protein assay kit (Bio-Rad, Hercules, CA, USA) with Bovine serum albumin (BSA) as a standard. SDS-PAGE of the protein was carried out using a Biorad Powerpac Instrument apparatus in a discontinuous buffer system. Protein samples were boiled for 10 min in the presence of 5 x loading buffer before the application to polyacrylamide gels (12% or 8%). Reducing conditions were achieved with 5% β-mercaptoethanol. Each Gel electrophoresis hole was added 10 µL samples, and they were stained using coomassie brilliant blue R250 stain after electrophoresis, Then decolored in acetic acid buffer.
Skin prick test of restructuring dust mites Der f 27
SPTs with purified and endotoxin removed r-Der f 27 was dissolved in pH 7.4 phosphate buffer (50 mM PB, 100 mM NaCl). Glycerin was added to 50% final concentration. The Sample concentration was 0.01 mg/ml controlled with both positive histamine phosphate (0.1%) and negative (saline), and checking the test results 20 minutes after SPT. The judgments of the result: if the prick spot became a wheal and fleck surrounding the wheal, it was positive (+). 4+: the response was stronger than histamine control; 3+: the response was almost the same as histamine control; 2+: the response was weaker than histamine, but stronger than negative control; 1+: the response was significantly weaker than histamine, but slightly stronger than the negative control; negative: No response. Approval to conduct these studies was obtained from the ethics committee of the hospital. All participants were provided written informed consent for the use of these clinical materials for research purposes and skin test before study entry.
Immunoblotting analysis with sera from allergic patients to dust mites
The r-Der f 27 protein was prepared with 0.05 M KH2PO4 (pH 7.4) buffer. The proteins were subjected to SDS-PAGE (sodium dodecyl sulfate polyacryl-amide gel electrophoresis) and electro-transferred onto a nitrocellulose membrane (Bio-Rad, Hercules, CA, USA) for immunoblotting analysis. The membrane was blocked for 2 hours with 3% BSA (Bovine Serum Albumin) in room temperature, incubated with the sera from patients sensitized to dust mite. Dilutedwith 5% BSA-PBST (PBST: PBS containing 0.05% Tween 20) for an hour under constant agitation on a rotary shaker at room temperature. After washing three times with TBS-T (Tris-buffered saline containing triton X-100), the membrane was incubated with the secondary antibodies (mouse anti human IgE) for 60 minutes. In the end, after washing with TBS-T for three times, the membrane was developed with the DAB kit (Invitrogen, USA). Stop developing reaction with ddH2O.
Enzyme-linked immunosorbent assay (ELISA)
Sera IgE antibodies specific for purifying allergens were measured by indirect ELISA. Briefly, the ELISA microtiter plates were coated over-night at 4°C with at 100 ng/well in Carbonate buffered solution (CBS. pH 9.5). Then blocked at 37°C with 200 μl 3% bovine serum albumin (BSA) in PBS for 120 min. The serum (diluted to 5 times) or BSA (using as a negative control) was then added to each well (100 μL/well) and incubated for 60 min at room temperature. After IgE binding, the plates were incubated with peroxidase-labeled goat anti-human IgE (1:2000) for 60 min at 37°C. Each incubating step was followed by 3 washes with PBST. The color was developed by adding tetramethylbenzidine (TMB; 100 uL/well) and stopped by the addition of 2 M H2SO4 (50 μL/well). The plates were read by an ELx808 absorbance microplate reader (BioTek, Shanghai, China) at 450 nm.
Development of a mouse model of allergic asthma
Eighteen mice were divided into three groups: ① HDM group, ② Der f 27 group, ③ PBS group. In the HDM group and the Der f 27 group, mice were inoculated with 100 μg extracts from Dermtophagoides farina or r-Der f 27 group with 4 mg aluminum hydroxide as adjuvants, respectively. PBS was used as an alternative in the control group. Sensitization was completed after boosting on day 0, 3, 7. From day 14 on, the challenge was performed once a day for a week. HDM and Der f 27 group was challenged with 50 μg crude extract of Dermtophagoidesfarina or Der f 27 proteins, respectively. The vehicle group was treated with the same procedure, but received PBS instead of the allergen. Following the challenge, Airway hyperresponsiveness to methacholine was measured using unrestrained whole-body plethysmography (WBP). The mice were subjected to progressively increasing doses of methacholine (0, 6.25, 12.5, 25, 50, 100 mg/mL), and the Penh value was recorded.
Analysis of specific IgE and IgG1 in serum
The blood was collected from retrobulbarvenousplexus by removing eyeball after the last challenge. Serum was collected by centrifugation of whole blood at 5000 rpm for 20 min. IgE and IgG1 were detected by ELISA according to manufacturers’ instructions. Briefly, the ElISA microtiter plates were coated over-night at 4°C with at 100 ng/well in Carbonate buffered solution (CBS. pH 9.5). Then blocked at 37°C with 200 μl 3% bovine serum albumin (BSA) in PBS for 120 min. The serum (diluted to 5 times) or BSA (using as a negative control) was then added to each well (100 μL/well) and incubated for 60 min at room temperature. After IgE binding, the plates were incubated with peroxidase-labeled goat anti-human IgE (1:2000) for 60 min at 37°C. Each incubating step was followed by 3 washes with PBST. The color was developed by adding tetramethylbenzidine (TMB; 100 uL/well) and stopped by the addition of 2 M H2SO4 (50 μL/well). The plates were read by an ELx808 absorbance microplate reader (BioTek, Shanghai, China) at 450 nm.
Bronchoalveolar lavage cytokine analysis
Bronchoalveolar lavage (BAL) and cell counts were performed as described previously [16]. IL-4 and IFN-γ levels in bronchoalveolar lavage fluid were detected by Multiplex cytokine assays.
Proliferative and cytokine responses of splenocytes
Spleen cells were prepared according to Gunzer M et al. Splenocytes (1×106/well) were incubated with 0.5 mg/ml HDM, Der f 27 or culture medium in 96-well plate for 72 h at 37°C. Subsequently, MTS was added to the medium and incubated for 4 h and then the results were recorded by a microplate reader at 490 nm. To analyze cytokine responses, splenocytes (1×106/well) were cultured in 24-well plate and stimulated with 0.2 mg/ml HDM, Der f 27 or normal saline for 72 h. IL-4 and IFN-γ levels in the supernatants were measured by multiplex cytokine assays.
Histology
The lung tissue was fixed with 4% paraformaldehyde for 24 h. After dehydration, lung tissue were embedded in paraffin. The tissues were cut into 5-µm thick sections, and then mounted onto glass slides. Lung sections were stained with Hematoxylin and Eosin (HE), and observed under a light microscope.
Data statistics
All data were expressed as mean ± standard deviation and processed with SPSS statistical software. One-way ANOVA was used for comparison of multiple groups and t-test was performed to determine the mean differences between two groups. p value < 0.05 was considered as significant.
Results
Cloning, expression and purification of Der f 27
The amplification products showed a bright band at around 1300 bp by agarose gel electrophoresis (Figure 1A) and double enzymatic digestion (Figure 1B). The full length of D. farinaeSerpincDNA, named Der f 27 (KM009995), was 1284 bp, encoding a 427 amino acid polypeptide, respectively (Figure 1C). The molecular evolutionary tree was constructed by software MEGA5. 1. The results showed that D. Farinaeserpen has a close relationship with Sarcoptesscabiei type hominis, Rhipicephalusmicroplus, Pedosphaeraparvula and Columba Livia (Figure 1D). In order to obtain Der f 27 protein for the subsequent experiments, we cloned Der f 27 cDNA into the plasmid, pET-44a(+), expressed and purified r-Der f 27. The result showed that the His-tag Der f 27 was successfully expressed as shown by chromatography and Western blotting (Figure 2A-C).
Figure 1.
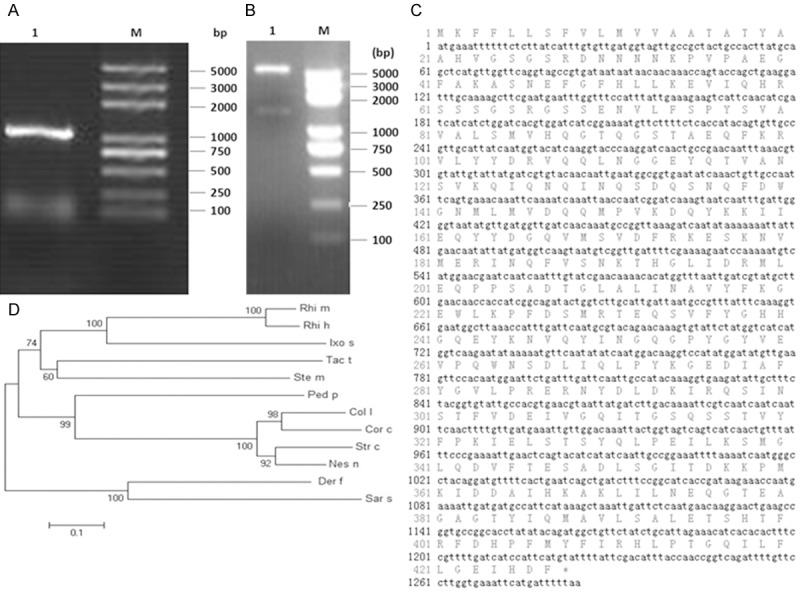
Cloning and sequence alignment of the Der f 27 gene. A. Cloning and PCR of Der f 27 cDNA (M; DNA Marker 1; Der f 27 gene). B. Restriction enzymatic digestion results of recombinant Hyastatin-pET-32a (+) plasmid (M: DNA Marker; 1: Der f 27 DNA). C. The amino acid sequences of Der f 27. D. The phylogenetic tree analysis; Der f (Dermatophagoidesfarinae); Sars (Sarcoptesscabiei type hominis); Rhim (Rhipicephalusmicroplus); Ped p (Pedosphaeraparvula); Coll (Columba livia); Rhi h (Rhipicephalushaemaphysaloides); Ixos (Ixodesscapularis); Tact (Tachypleustridentatus); Stem (Stegodyphusmimosarum); Strc (Struthiocamelusaustralis); Nesn (Nestor notabilis); Corc (Corvuscornixcornix).
Figure 2.
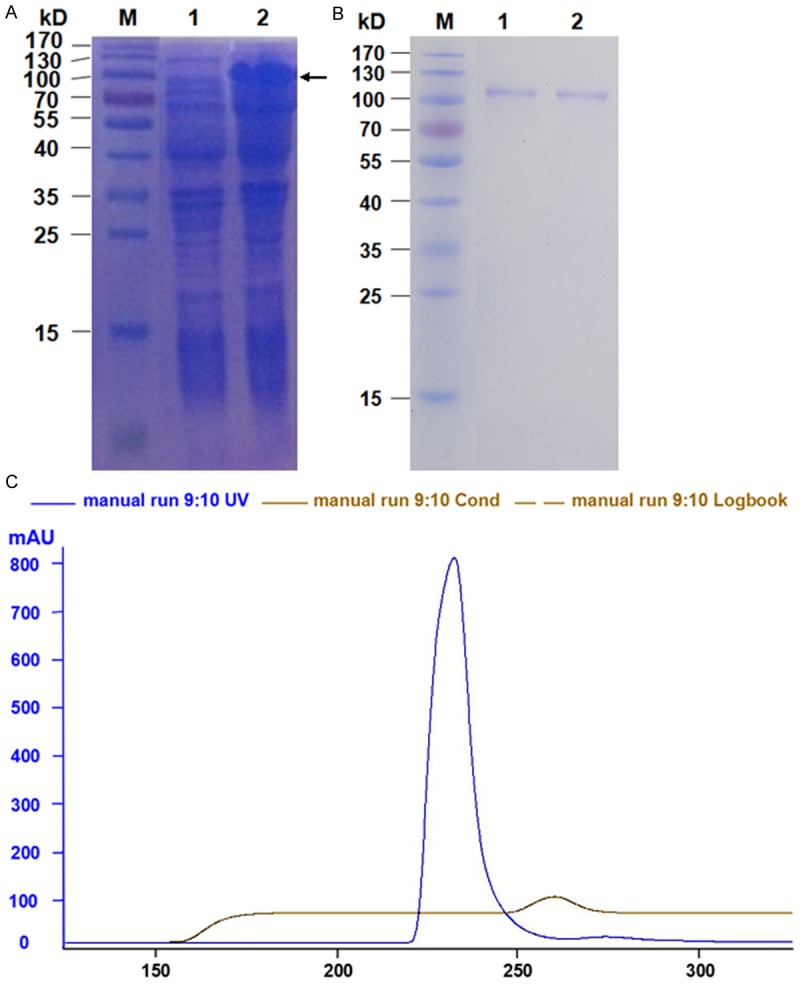
Expression and purification of Der f (A) SDS-PAGE analysis of the protein expressed from the pET-44a (+)-Der f 27 recombinant plasmids in E. coli BL21 cells. Lane M: Protein Marker; Lane 1, E. coli BL21 transformed with pET-Der f 27 without IPTG induction; Lane 2, E. coli BL21 transformed with pet-Der f 27 with IPTG induction. (B) The peak figure of recombinant Der f 27 purified by molecular sieve chromatography. The longitudinal axis (mAu) is a protein absorption peak; Horizontal axis (ml) for volume; Blue curve for protein absorption peak; Gray curve for conductance value. (C) SDS-PAGE analysis of the final purified r-Der f 27 from E. coli BL21 cells. Lane M: Protein Marker. Lane 1-2: r-Der f 27 purified by molecular sieve chromatography.
The allergenicityof Der f 27
We next determined the allergenicity of Der f 27. The results (Table 1) showed that 8 out of 19 (42.1%) allergic patients had positive SPTresponses to Der f 27. Then, to determine the allergenicity of the Der f 27 protein, immunoblotting was performed using the sera from three DME-allergic patients. The result demonstrated that the serum IgE from the 3 allergic patients reacted to the Der f 27 protein as shown by the IgE-binding bands with molecular weight around 110 kD (PET-44a-Der f 27). The sera from healthy controls showed negative results (Figure 3A). The specific immunoreactivity of IgE against purified Der f 27 was further confirmed by ELISA. Compared with the sera from healthy controls, the IgE reactivity of r-Der f 27 from allergic patients increased by more than 3 folds (Figure 3B). The results suggest that r-Der f 27 may be an allergen from dust mite allergen family that is involved in type I hypersensitivity.
Table 1.
Demographic data of allergic patients and the skin test results of r-Der f 27
| Subjects | Gender/Age | Diagnosis | Net wheal size (mm), Level | |||
|---|---|---|---|---|---|---|
|
| ||||||
| DME | Histamine | PS | Der f 27 | |||
| 1 | Male/15 | BA | 3, + | 6.25 | 0 | 0 |
| 2 | Female/24 | BA, AR, AD, FA, DA | 8.5, +++ | 7 | 0 | 3, + |
| 3 | Female/10 | BA, AR | 8.5, +++ | 5.5 | 0 | 3, ++ |
| 4 | Female/18 | BA, AR, DA | 10, +++ | 6 | 0 | 1.75, + |
| 5 | Male/54 | BA, AR, AD | 1.5, + | 4.5 | 0 | 0 |
| 6 | Female/41 | BA, AR, DA | 10.5, +++ | 4 | 0 | 3.5, ++ |
| 7 | Female/13 | BA, AR | 7, +++ | 5 | 0 | 0 |
| 8 | Male/17 | BA, AR, AD, FA, DA | 0.25, + | 1 | 0 | 0 |
| 9 | Female/31 | AD | 9.5, +++ | 2.5 | 0 | 0 |
| 10 | Male/47 | BA, AR | 3.5, ++ | 5.5 | 0 | 0 |
| 11 | Male/45 | AR | 2, + | 7.5 | 0 | 0 |
| 12 | Female/52 | AR, FA | 1.5, + | 5.5 | 0 | 0 |
| 13 | Male/20 | BA, FA | 1.5, + | 5 | 0 | 0 |
| 14 | Female/20 | BA | 2, ++ | 4 | 0 | 0 |
| 15 | Female/13 | AR | 1.25, ++ | 6 | 0 | 3.5, ++ |
| 16 | Female/66 | BA, AR, DA | 9, +++ | 5.5 | 0 | 6, +++ |
| 17 | Female/64 | BA | 2.25, + | 6 | 0 | 2.5, + |
| 18 | Female/41 | BA, AR | 3.5, ++ | 5.5 | 0 | 0 |
| 19 | Female/44 | BA, AR | 4.5, +++ | 4.5 | 0 | 1.5, + |
BA (Bronchial asthma); AR (Allergic rhinitis); AD (Atopic dermatitis); FA (Food allergy); DA (Drug allergy); PS (physiological saline). Positive: ≥ 1; Negative: 0.
Figure 3.
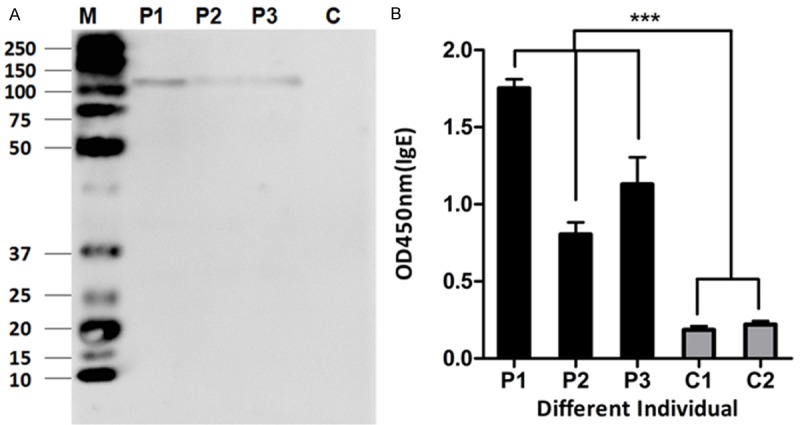
Immunological characterization of Der f 27. A. Immunobloting analysis of specific IgE reactivity to allergen r-Der f 27 in the serum from r-Der f 27 SPT positive individuals. Lanes 1-3 indicatethe IgE positive serum from allergic subjects. Lane 4 indicatescontrol serum from healthy subjects using as negative controls. B. The specific IgE reactivity to allergen r-Der f 27 by ELISA. P1-P3, the serum from r-Der f 27 positive patients; C1 and C2, the serum from healthy control subjects.
Establishment of a mouse model of allergic asthma with r-Der f 27
An r-Der f 27-induced mouse model of allergic asthma was developed (Figure 4A). The airway hyperresponsiveness was assessed by the methacholine-induced airflow obstruction. As shown in Figure 4B, the airway hyperresponsiveness of the mice in the r-Der f 27 group was weaker than that in HDM group, while the PBS control group showed the lowest reaction. The levels of IL-4 and γ-IFN in bronchoalveolar lavage (BAL) of HDM group and r-Der f 27 group were increased significantly (Figure 4C and 4D). The frequency of eosinophils in the HDM group and the r-Der f 27 group was higher than that in the control group (Figure 4E). In normal lung tissue, the contour of bronchial tube was clear and there wasa low frequency of inflammatory cell infiltration around the bronchial submucosa. In the HDM group and the r-Der f 27 group, a large amount of exudate was observed in the airway lumen, the structure of bronchial mucosa was partially damaged and a high frequency of inflammatory cell infiltration was observed (Figure 4F-H). The results indicate that the house dust mite extract and the r-Der f 27 protein can induce pulmonary inflammation.
Figure 4.
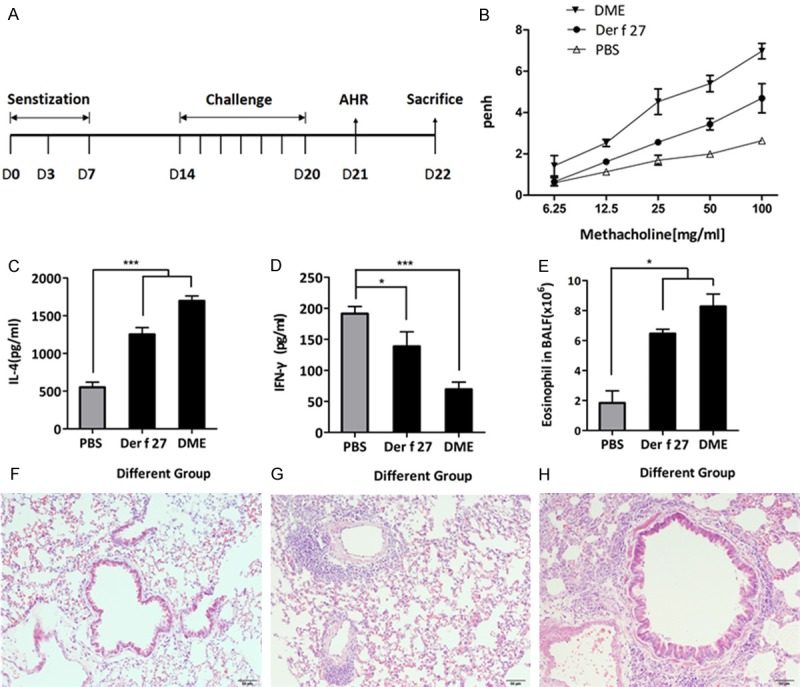
Establishment of a mouse model of allergic asthma by r-Der f 27. A. The protocol for a mouse model of r-Der f 27-induce allergic asthma. B. Airway hyper-responsiveness were measured using the unrestrained whole-body plethysmography. The Penh values were recorded when the mice were stimulated with the increasing concentration of methacholine after the challenge. C and D. The level of IL-4 and INF-γ in BLAF were detected by ELISA. Samples of BLAF were collected on day 22, and levels of IL-4 and INF-γ were measured by ELISA. E. The number of eosinophil cells was counted in the BLAF. F-H. The pathologic changes of pulmonary tissue. F: The PBS group. G: The r-Der f 27 group. H: The DME group.
Specific immune response induced by r-Der f 27
The levels of specific IgE and IgG1 in the mouse serum were increased. As shown in the Figure 5A and 5B, the levels of r-Der f 27 specific IgE and IgG1 in the HDM group and the r-Der f 27 group were significantly higher than that in the normal control group. The results suggest that the crude extracts of dust mites and r-Der f 27 can induce the antigen specific IgE and IgG1 in mice.
Figure 5.
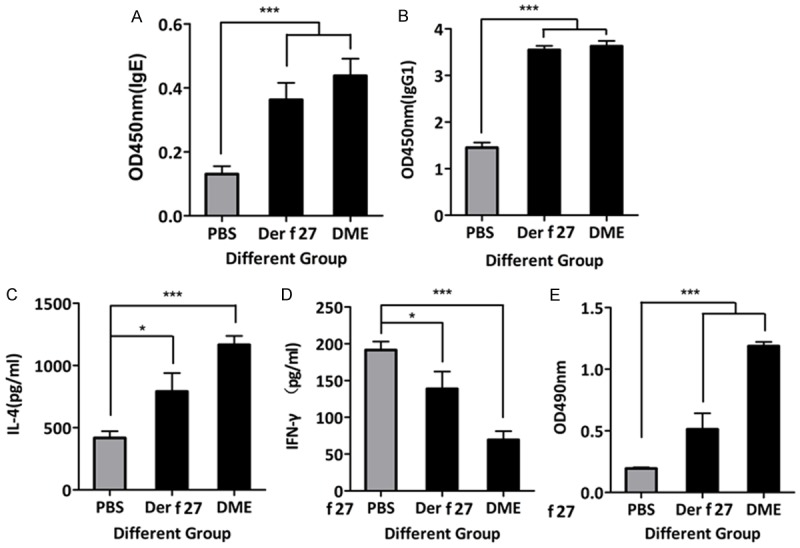
Specific immune response induced by r-Der f 27. A and B. The level of IgE and IgG1 were detected using ELISA. Serum was collected on day 22, and the levels of specific serum IgG1 and IgE were measured. C and D. The levels of specific splenocyte IL-4 and IFN-γ were measured by ELISA.
To assess whether r-Der f 27 affected the systemic adaptive immune response, splenocytes were isolated and stimulated with HDM and r-Der f 27 respectively in the culture; the cytokines were assayed in the culture supernatants. The results showed that the HDM and r-Der f 27 stimulation of splenocytes induced a significant increase in IL-4 and IFN-γ in the culture compared with the normal control group (Figure 5C and 5D). To characterize the specific cellular immune response, splenocytes were counted. The results showed an increase insplenocytes in r-Der f 27-treated mice compared to the normal control group (Figure 5E).
Discussion
Cumulative reports indicate thatthe allergens of house dust mites are diverse; many of them have not been identified and characterized [17]. The serpin has been reported as an allergen from wheat flour and Schistosomahaematobiumbut has not beenreported as an allergen in HDM [12,13]. In this study, through cloning, expression, and purification of full length recombinant serpin (r-Der f 27), we identified r-Der f 27 is an allergen in D. farina.
Using the SPT technique, we found that there were 8 out of 19 mite allergic patients (42%) showeda positive reaction to r-Der f 27, indicating that r-Der f 27 has the ability to bind IgEin allergic patients. Moreover, a well characterized mouse modelof allergic airways disease was induced by the r-Der f 27 was created successfully. Both DME and r-Der f 27 can induce mouse pulmonary inflammatory changes. The spleen cells produced and release large amounts of Th2 cytokines, indicating that the r-Der f 27 mediated Th2 type allergic reactions. This investigation also found that mice exposed to the r-Der f 27 showed higher levels of specific IgE in the serum compared with the mouse that were exposed to PBS. The results suggest thatDer f 27 is a new subtype allergen of HDM.
Here we provide the first evidence that serpin is a new subtype of house dust mite allergen, which can be usefulin the HDM allergy diagnosis and specific immunotherapy. Besides, it may provide further insights to understand the diversity, characteristics of D. farinae allergens.
Acknowledgements
This study was supported by grants from the Natural Science Foundation of China (No. 31328014 and 31400786), Guangdong Provincial University International-Hong Kong, Marco, Taiwan Scientific Innovention Projects (No. 2012gjhz0009), Guangdong Engineering Technology Research Center Project (No. 2013158925), Guangdong Scientific Technology Social Development Project (No. 20133180002), Guangdong Foreign Scientific Technology Cooperative Project (No. 2013B051000088), Guangdong Natural Science Foundation PhD Study Initiative Project (No. 2014A030310192), Shenzhen Scientific Techology Project International Cooperative Project (GJHZ20130408174112021), Shenzhen Scientific Techology Basic Research Projects (No. JCYJ20120613100657482, JCYJ20130329110735981, JCYJ20120613173233810, JCYJ20140828163633992, JCYJ20140418095735538) and Nanshan District Promoting Project for Innovation Institution, Nanshan District Innovation Project (KC2014JSCX0055A).
Disclosure of conflict of interest
None.
References
- 1.Arlian LG. House-dust-mite allergens: a review. Exp Appl Acarol. 1991;10:167–186. doi: 10.1007/BF01198649. [DOI] [PubMed] [Google Scholar]
- 2.Platts-Mills TAE, Thomas WR, Aalberse R, Vervloet D, Champman MD. Dust mite allergens and asthma: report of a second international workshop. J Allergy Clin Immunol. 1992;89:1046–1060. doi: 10.1016/0091-6749(92)90228-t. [DOI] [PubMed] [Google Scholar]
- 3.Tovey ER, Chapman MD, Platts-Mills TA. Mite faeces are a major source of house dust mite allergens. Nature. 1981;289:592–593. doi: 10.1038/289592a0. [DOI] [PubMed] [Google Scholar]
- 4.Vrtala S, Huber H, Thomas WR. Recombinant house dust mite allergens. Methods. 2014;66:67–74. doi: 10.1016/j.ymeth.2013.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.An S, Shen C, Liu X, Chen L, Xu X, Rong M, Liu Z, Lai R. Alpha-actinin is a new type of house dust mite allergen. PLoS One. 2013;8:e81377. doi: 10.1371/journal.pone.0081377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valenta R, Linhart B, Swoboda I, Niederberger V. Recombinant allergens for allergen-specific immunotherapy: 10 years anniversary of immunotherapy with recombinant allergens. Allergy. 2011;66:775–783. doi: 10.1111/j.1398-9995.2011.02565.x. [DOI] [PubMed] [Google Scholar]
- 7.Thomas WR, Smith WA, Hales BJ. The allergenic specificities of the house dust mite. Chang Gung Med J. 2004;27:563–569. [PubMed] [Google Scholar]
- 8.Van Der Veen MJ, Jansen HM, Aalberse RC, Van der Zee JS. Der p 1 and Der p 2 induce less severe late asthmatic responses than native Dermatophagoides pteronyssinus extract after a similar early asthmatic response. Clin Exp Allergy. 2001;31:705–714. doi: 10.1046/j.1365-2222.2001.01120.x. [DOI] [PubMed] [Google Scholar]
- 9.Trombone AP, Tobias KR, Ferriani VP, Schuurman J, Aalberse RC, Smith AM, Chapman MD, Arruda LK. Use of a chimeric ELISA to investigate immunoglobulin E antibody responses to Der p 1 and Der p 2 in mite-allergic patients with asthma, wheezing and/or rhinitis. Clin Exp Allergy. 2002;32:1323–1328. doi: 10.1046/j.1365-2745.2002.01455.x. [DOI] [PubMed] [Google Scholar]
- 10.Meyer CH, Bond JF, Chen MS, Kasaian MT. Comparison of the levels of the major allergens Der p I and Der p II in standardized extract of the house dust mite, Dermatophagoides pteronyssinus. Clin Exp Allergy. 1994;24:1041–1048. doi: 10.1111/j.1365-2222.1994.tb02741.x. [DOI] [PubMed] [Google Scholar]
- 11.Le Mao J, Mayer CE, Peltre G, Desvaux FX, David B, Weyer A, Sénéchal H. Mapping of Dermatophagoides farinae mite allergens by two-dimensional immunoblotting. J Allergy Clin Immunol. 1998;102:631–636. doi: 10.1016/s0091-6749(98)70280-5. [DOI] [PubMed] [Google Scholar]
- 12.Sander I, Rozynek P, Rihs HP, van Kampen V, Chew FT, Lee WS, Kotschy-Lang N, Merget R, Brüning T, Raulf-Heimsoth M. Multiple wheat flour allergens and cross-reactive carbohydrate determinants bind IgE in baker’s asthma. Allergy. 2011;66:1208–15. doi: 10.1111/j.1398-9995.2011.02636.x. [DOI] [PubMed] [Google Scholar]
- 13.King CL, Ogundipe JO, Licate LS, Blanton RE. Preferential recognition by human IgE and IgG4 of a species-specific Schistosoma haematobium serine protease inhibitor. J Infect Dis. 1995;171:416–22. doi: 10.1093/infdis/171.2.416. [DOI] [PubMed] [Google Scholar]
- 14.Dreborg S, Foucard T. Allergy to apple, carrot and potato in children with birch pollen allergy. Allergy. 1983;38:167–172. doi: 10.1111/j.1398-9995.1983.tb01602.x. [DOI] [PubMed] [Google Scholar]
- 15.Chan TF, Ji KM, Yim AK, Liu XY, Zhou JW, Li RQ, Yang KY, Li J, Li M, Law PT, Wu YL, Cai ZL, Qin H, Bao Y, Leung RK, Ng PK, Zou J, Zhong XJ, Ran PX, Zhong NS, Liu ZG, Tsui SK. The draft genome, transcriptome, and microbiome of Dermatophagoides farinae reveal a broad spectrum of dust mite allergens. J Allergy Clin Immunol. 2015;135:539–48. doi: 10.1016/j.jaci.2014.09.031. [DOI] [PubMed] [Google Scholar]
- 16.Chang YS, Kim YK, Jeon SG, Kim SH, Kim SS, Park HW, Min KU, Kim YY, Cho SH. Influence of the Adjuvants and Genetic Background on the Asthma Model Using Recombinant Der f 2 in Mice. Immune Netw. 2013;13:295–300. doi: 10.4110/in.2013.13.6.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le Mao J, Mayer CE, Peltre G, Desvaux FX, David B, Weyer A, Sénéchal H. Mapping of Dermatophagoides farinae mite allergens by two-dimensional immunoblotting. J Allergy Clin Immunol. 1998;102:631–636. doi: 10.1016/s0091-6749(98)70280-5. [DOI] [PubMed] [Google Scholar]
- 18.Shen DH, Chua KY, Lin WL, Hsieh KH, Thomas WR. Characterization of the house dust mite allergen Der p 7 by monoclonal antibodies. Clin Exp Allergy. 1995;25:416–422. doi: 10.1111/j.1365-2222.1995.tb01072.x. [DOI] [PubMed] [Google Scholar]
- 19.Aki T, Kodama T, Fujikawa A, Miura K, Shigeta S, Wada T, Jyo T, Murooka Y, Oka S, Ono K. Immunochemical characterization of recombinant and native tropomyosins as a new allergen from the house dust mite, Dermatophagoides farinae. J Allergy Clin Immunol. 1995;96:74–83. doi: 10.1016/s0091-6749(95)70035-8. [DOI] [PubMed] [Google Scholar]
- 20.Hales BJ, Martin AC, Pearce LJ, Laing IA, Hayden CM, Goldblatt J, Le Souëf PN, Thomas WR. IgE and IgG anti-house dust mite specificities in allergic disease. J Allergy Clin Immunol. 2006;118:361–367. doi: 10.1016/j.jaci.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 21.Lin KL, Hsieh KH, Thomas WR, Chiang BL, Chua KY. Characterization of Der p V allergen, cDNA analysis, and IgE mediated reactivity to the recombinant protein. J Allergy Clin Immunol. 1994;94:989–996. doi: 10.1016/0091-6749(94)90117-1. [DOI] [PubMed] [Google Scholar]


