Abstract
Accumulating evidence suggests that long non-coding RNA (lncRNA) HOTAIR participates in many types of cancer such as gastric cancer and may confer malignant phenotype to tumor cells. Fluorouracil and platinum combination chemotherapy is the first line therapy for gastric cancer. However, it is still unknown whether HOTAIR influences the outcome of cancer patients treated with chemotherapy. This study aimed to evaluate the association of HOTAIR expression with the prognosis of patients with advanced gastric adenocarcinoma (GA) receiving fluorouracil and platinum based chemotherapy. We examined the levels of HOTAIR in 168 GA samples using quantitative real-time PCR and analyzed its relationship with clinical features and prognosis of patients with advanced GA treated with fluorouracil and platinum based chemotherapy. Compared with paracancerous tissues, HOTAIR was significantly upregulated in GA tissues, especially in more advanced cases. High HOTAIR expression was an independent poor prognostic factor for patients with advanced GA. Further stratification analyses revealed that the association between HOTAIR expression and survival in patients with advanced GA remained significant in the subgroup of patients with TNM stages IIIA and IIIB, poorly differentiated, and smaller tumors. In conclusion, our results provide first evidence that HOTAIR may be served as a biomarker that predicts which patient with advanced GA will benefit from fluorouracil and platinum combination chemotherapy.
Keywords: Gastric cancer, long noncoding RNA, HOTAIR, chemotherapy, prognosis
Introduction
Gastric cancer is the fifth most common cancer in the world, accounting for 7% of the total new cancer cases and 9% of the total cancer deaths [1]. The incidence rate of gastric cancer is highest in Eastern Asia [2]. Gastric adenocarcinoma (GA) is the majority of gastric cancer, which can be further divided into intestinal and diffuse types according to Lauren classification.Although multimodality therapy is available to gastric cancer, approximately half of patients with advanced gastric cancer who undergo surgical resection still develop local or distant metastases and die from the cancer [3,4]. Therefore, it is urgent to identify biomarkers and explore the effective and personalized treatment.
Long noncoding RNAs (lncRNAs) are defined as transcripts > 200 nucleotides in length and are transcribed but non-translated noncoding RNAs in human genome [5,6]. There are convincing evidence that lncRNA are differentially expressed in various diseases including cancer and play a vital role in cancer progression and metastasis [7,8]. Functional studies of HOTAIR lifts lncRNAs to a new level with so many documents elucidated that HOTAIR is involved in the development and progression of various types of cancer, including ovarian [9], breast [10], pancreatic [11], and non-small cell lung cancers [12]. HOTAIR is overexpressed in gastric cancer, which promotes its carcinogenesis, invasion and metastasis [13-18]. Recent study revealed that when laryngeal squamous cell carcinoma was treated with increasing concentrations of drugs and extending the duration of treatment, the levels of HOTAIR were dramatically reduced. Furthermore, HOTAIR overexpression confers cisplatin resistance to lung adenocarcinoma cells and ovarian cancer cells [9,19]. However, it remains largely unknown whether HOTAIR overexpression influences the clinical outcome of cancer patients receiving chemotherapy. In this study, we examined the levels of HOTAIR in 168 patients with advanced GA receiving fluorouracil and platinum based chemotherapy, and evaluated the association of its expression with patient prognosis.
Materials and methods
Patients
A total of 168 patients with advanced GA were collected from Subei People’s Hospital and the Biobank of National engineering center for biochip at Shanghai. All patients were histologically confirmed and had TNM stage III-IV tumor. No patient had other cancer history. Samples were obtained before patients were treated with any anti-cancer therapy. This study was approved by the Ethics Committees of Subei People’s Hospital and National engineering center for biochip at Shanghai, and all subjects gave written informed consent before enrolling in this study.
Patients were treated with fluorouraciland platinum combination chemotherapy. There were 111 men and 57 women, ranging in age from 28 to 75 years. Clinical information such as date of birth, sex, histologic grade, tumor size, TNM stage, therapeutic regimen, and other necessary information were extracted from computerized clinical database or follow-up records.
Quantitative RT-PCR (qRT-PCR)
Total RNA was extracted from tissue samples using Trizol reagent (Invitrogen, CA, USA) according to the manufacturer’s protocols. The concentration of total RNA was quantified by Nanodrop ND-1000 spectrophotometer (Nanodrop, DE, USA). The first-strand cDNA was synthesized using a PrimeScript 1st Strand cDNA synthesis kit (TaKaRa, Dalian, China). Subsequently the cDNA template was amplified by qRT-PCR using SYBR Premix Ex Taq II (TliRNaseH Plus) (TaKaRa, Dalian, China) on an Applied Biosystems® 7900 Real-Time PCR Systems (Applied Biosystems, CA, USA). The relative expression level of HOTAIR was normalized to GAPDH and fold change was calculated using the equation 2-ΔΔCT. All experiments were performed in triplicate.
Statistical analyses
The level of HOTAIR was compared using Wilcoxon nonparametric test within patient groups. Patients were divided into high and low HOTAIR expression groups according to the median expression level of HOTAIR. Chi-square test or Fisher’s exact test were performed to determine the relationship between HOTAIR expression and clinical pathological parameters, as appropriate. Kaplan-Meier and log-rank analyses were carried out to evaluate the effect of HOTAIR expression on survival. Independent prognostic indicators were assessed in the multivariate analysis using Cox’s proportionalhazard model. All statistical analyses were performed using SPSS v20.0 (SPSS, IL, USA) and GraphPad Prism 5.0 (GraphPad Software, CA, USA). A P value less than 0.05 was considered statistically significant.
Results
The expression levels of HOTAIR in advanced GA
To investigate the levels of HOTAIR in advanced GA tissues, qRT-PCR was used to determine the levels of HOTAIR in 168 advanced GA. The levels of HOTAIR in GA tissues were significantly higher than those in paracancerous tissues (P < 0.001) (Figure 1A), and was positively related to tumor size (P = 0.034). Furthermore, when patients were subdivided into TNM stages IIIA+IIIB and IIIC+IV groups, the levels of HOTAIR in TNM stage IIIC+IV group were higher than those in TNM stage IIIA+IIIB group (P = 0.017, Figure 1B).
Figure 1.
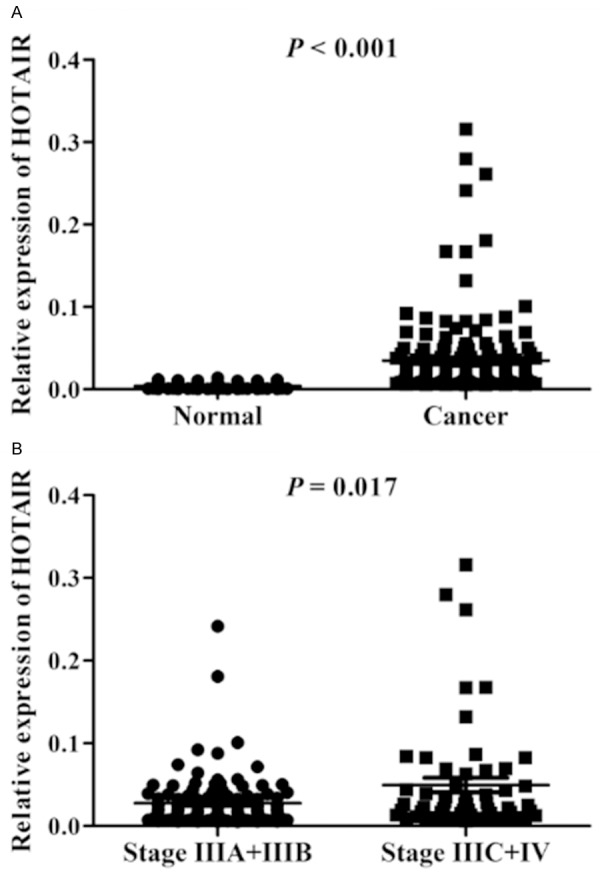
The expression levels of HOTAIR in advanced GA. A. The levels of HOTAIR in GA tissues were significantly upregulated compared paracancerous tissues. B. The levels of HOTAIR in cases with TNM stages IIIA and IIIB were higher than those with TNM stages IIIC and IV.
Association of HOTAIR expression with clinicopathologic features of patients with advanced GA
The association of HOTAIR expression with clinicopathologic parameters in patients with advanced GA was shown in Table 1. No difference was observed between HOTAIR expression and clinicopathologic parameters. However, there was a weak but not statistically significant association between HOTAIR expression and TNM stage (P = 0.092).
Table 1.
Association of HOTAIR expression with clinicopathologic parameters
| Clinicopathologic parameters | HOTAIR expression | P value | |
|---|---|---|---|
|
| |||
| High | Low | ||
| Age (years) | |||
| > 65 | 29 | 29 | 1.000 |
| ≤ 65 | 55 | 55 | |
| Sex | |||
| Male | 54 | 57 | 0.625 |
| Female | 30 | 27 | |
| Histologic grade | |||
| 1+2 | 16 | 13 | 0.540 |
| 3 | 68 | 71 | |
| Size (cm) | |||
| > 5 | 47 | 52 | 0.433 |
| ≤ 5 | 37 | 32 | |
| TNM stage | |||
| III | 66 | 75 | 0.092 |
| IV | 18 | 9 | |
Association of HOTAIR expression level with prognosis of patients with advanced GA
To investigate the effect of HOTAIR expression on survival, Kaplan-Meier curves were adopted in patients with advanced GA receiving fluorouracil and platinum based chemotherapy. As shown in Figure 2, median survival time (MST) of patients with high HOTAIR expression was 16.5 months, while MST in those with low HOTAIR expression was 21.5 months (P = 0.009). Multivariate Cox proportional hazard regression analysis that larger tumor size [adjusted hazard ratio [HR] = 1.470, 95% CI: 1.029-2.100, P = 0.034], TNM stages IIIC and IV (adjusted HR = 1.755, 95% CI: 1.227-2.511, P = 0.002), and high HOTAIR expression (adjusted HR = 1.466, 95% CI: 1.044-2.059, P = 0.027) were significant risk factors related to death status in patients with advanced GA (Table 2).
Figure 2.
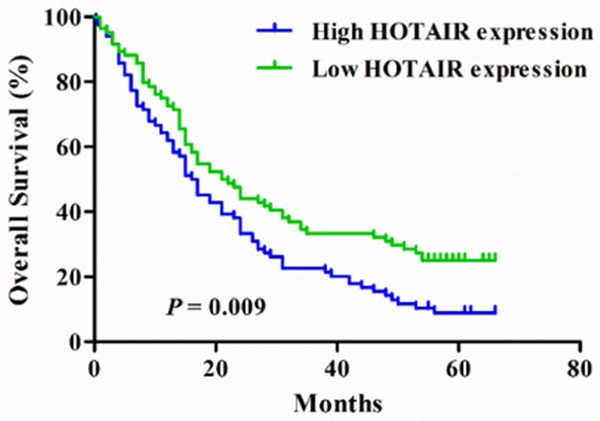
Kaplan-Meier curves of overall survival in 168 patients with advanced gastric adenocarcinoma according to HOTAIR expression.
Table 2.
Univariate and multivariate Cox regression analysis of overall survival in 168 patients with advanced GA
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
|
|
|
|||
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age (≤ 65 years vs > 65 years) | 0.818 (0.578-1.157) | 0.255 | ||
| Sex (male vs female) | 0.938 (0.654-1.344) | 0.726 | ||
| Histologic grade (3 vs 1, 2) | 1.169 (0.752-1.814) | 0.488 | ||
| Size (> 5 cm vs ≤ 5 cm) | 1.627 (1.150-2.303) | 0.006 | 1.470 (1.029-2.100) | 0.034 |
| TNM (IIIC, IV vs IIIA, IIIB) | 2.014 (1.424-2.847) | < 0.001 | 1.755 (1.227-2.511) | 0.002 |
| HOTAIR (high vs low) | 1.547 (1.105-2.167) | 0.011 | 1.466 (1.044-2.059) | 0.027 |
Given that HOTAIR expression was correlated with clinical features, further stratification analysis was undertaken to elucidate the exact role of HOTAIR expression in patients with different clinicopathologic parameter. As shown in Table 3, among patients with poorly differentiated tumors, high HOTAIR expression conferred an increased risk of mortality (adjusted HR = 1.679, 95% CI: 1.148-2.456, P = 0.008) (Figure 3). Among patients with smaller tumors, those with high HOTAIR expression had a 1.856-fold (95% CI: 1.024-3.363) higher risk of mortality compared with those with low HOTAIR expression. In addition, among patients with TNM stages IIIA and IIIB, high HOTAIR expression was associated with an elevated risk of mortality (adjusted HR = 1.718, 95% CI: 1.113-2.651, P = 0.015).
Table 3.
Stratification analysis of HOTAIR associated with survival of patients with advanced GA
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
|
|
|
|||
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age, years | ||||
| ≤ 65 | 1.489 (0.979-2.265) | 0.063 | 1.249 (0.818-1.908) | 0.304 |
| > 65 | 1.669 (0.944-2.950) | 0.078 | 1.588 (0.894-2.821) | 0.115 |
| Sex | ||||
| Male | 1.487 (0.984-2.248) | 0.060 | 1.350 (0.890-2.048) | 0.159 |
| Female | 1.696 (0.938-3.065) | 0.080 | 1.542 (0.849-2.799) | 0.155 |
| Histologic grade | ||||
| 1+2 | 0.769 (0.343-1.725) | 0.524 | 0.780 (0.347-1.751) | 0.546 |
| 3 | 1.802 (1.241-2.617) | 0.002 | 1.679 (1.148-2.456) | 0.008 |
| Tumor size | ||||
| ≤ 5 cm | 1.881 (1.066-3.319) | 0.029 | 1.856 (1.024-3.363) | 0.042 |
| > 5 cm | 1.405 (0.922-2.141) | 0.113 | 1.302 (0.848-1.999) | 0.227 |
| TNM | ||||
| IIIA+IIIB | 1.594 (1.038-2.448) | 0.033 | 1.718 (1.113-2.651) | 0.015 |
| IIIC+IV | 1.255 (0.728-2.162) | 0.414 | 1.263 (0.732-2.178) | 0.401 |
Figure 3.
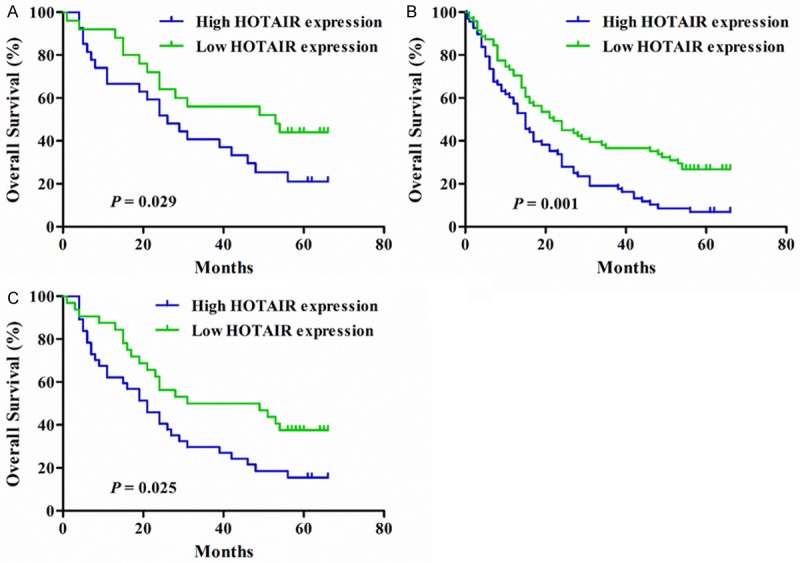
Kaplan-Meier curves stratified by clinical features. A. Kaplan-Meier curves for overall survival of patients with TNM stages IIIA and IIIB according to HOTAIR expression. B. Kaplan-Meier curves for overall survival of patients with poor differentiated tumors according to HOTAIR expression. C. Kaplan-Meier curves for overall survival of patients with smaller tumors according to HOTAIR expression.
Discussion
Gastric cancer is a leading cause responsible for cancer deaths [1], of which the vast majority are GA and associated with infection of Helicobacter pylori and Epstein-Barr virus [20,21]. The management for gastric cancer is complicated and constantly evolving. Although surgery resection is regarded as the only option for cure, most patients exhibit an advanced disease at diagnosis and usually have a poor prognosis [22]. Furthermore, fluorouracil and platinum combination chemotherapy is the first line therapy for gastric cancer. Therefore, a potential biomarker is urgent for identifying the outcome of patients with advanced gastric cancer who received fluorouracil and platinum based chemotherapy.
Dysregulation of HOTAIR is correlated with multiple types of cancer [9-12,23-25]. Recent studies have revealed that HOTAIR can act as a scaffold and interact with at least two distinct histone modification complexes to regulate the expression of multiple genes involved in various cellular functions [10,26-28]. HOTAIR binds polycomb repressive complex 2 (PRC2) and LSD1/CoREST/REST complexes via its 5’ and 3’ domains, respectively, leading to H3K27 methylation and H3K4 demethylation and ultimately gene silencing [29-31]. Previous study have shown that the level of HOTAIR positively correlates with TNM stage and metastasis of gastric cancer [13,17]. In this study, although high HOTAIR expression was predominant in cases with distant metastasis, the difference did not reach statistical significance. This may be explained that all cases are advanced gastric adenocarcinoma in our study. HOTAIR promotes gastric cancer metastasis by inhibiting Poly r(C)-Binding Protein 1 [32]. Inhibition of HOTAIR impairs gastric cancer cell viability, induces apoptosis, and suppresses the growth of gastric cancer xenograft [15,16,33]. Furthermore, HOTAIR acts as a competing endogenous RNA to regulate HER2 expression by sponging miR-331-3p [34]. Given that trastuzumab is used as first line therapy for HER2-positive gastric cancer, HOTAIR is potential therapeutic target for gastric cancer.
It is well known that drug resistance is a main obstacle and challenge in cancer treatment. Previous studies have revealed that HOTAIR overexpression is related to cisplatin resistance [9,19]. Upregulation of HOTAIR contributes to cisplatin resistance via downregulation ofp21 (WAF1/CIP1) expression in lung adenocarcinoma and exhibits downregulation in cisplatin-responding lung adenocarcinoma tissues [19]. Downregulation of HOTAIR restores cisplatin sensitivity in cisplatin-resistant ovarian cancer cells [9]. In addition, decreased expression of HOTAIR is associated with good response to treatment in sarcoma patient [35]. It has been demonstrated that HOTAIR expression affect the prognosis of patients with many types of cancer [12,18,23,27,33]. In the present study, we found that high HOTAIR expression was associated with worse survival in patients with advanced GA receiving fluorouracil and platinum combination chemotherapy, especially those with TNM stages IIIA and IIIB, poorly differentiated, and smaller tumors. Therefore, drug resistance caused by HOTAIR overexpression is one plausible reason for the association of HOTAIR overexpression with poor prognosis of cancer patients.
In summary, our findings provide the first evidence that HOTAIR influences the prognosis of patients with advanced GA treated with fluorouracil and platinum combination chemotherapy. HOTAIR may be served as a biomarker classifier for identifying the subset of patients who will benefit from fluorouracil and platinum combination chemotherapy.
Acknowledgements
This work was supported by the Fund for International Scientific Cooperation of Shanghai Committee of Science and Technology, China (grant No. 13440701500).
Disclosure of conflict of interest
None.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 3.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Ruschoff J, Kang YK. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 4.Lee J, Lim do H, Kim S, Park SH, Park JO, Park YS, Lim HY, Choi MG, Sohn TS, Noh JH, Bae JM, Ahn YC, Sohn I, Jung SH, Park CK, Kim KM, Kang WK. Phase III trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: the ARTIST trial. J. Clin. Oncol. 2012;30:268–273. doi: 10.1200/JCO.2011.39.1953. [DOI] [PubMed] [Google Scholar]
- 5.Carninci P, Kasukawa T, Katayama S, Gough J, Frith MC, Maeda N, Oyama R, Ravasi T, Lenhard B, Wells C, Kodzius R, Shimokawa K, Bajic VB, Brenner SE, Batalov S, Forrest AR, Zavolan M, Davis MJ, Wilming LG, Aidinis V, Allen JE, Ambesi-Impiombato A, Apweiler R, Aturaliya RN, Bailey TL, Bansal M, Baxter L, Beisel KW, Bersano T, Bono H, Chalk AM, Chiu KP, Choudhary V, Christoffels A, Clutterbuck DR, Crowe ML, Dalla E, Dalrymple BP, de Bono B, Della Gatta G, di Bernardo D, Down T, Engstrom P, Fagiolini M, Faulkner G, Fletcher CF, Fukushima T, Furuno M, Futaki S, Gariboldi M, Georgii-Hemming P, Gingeras TR, Gojobori T, Green RE, Gustincich S, Harbers M, Hayashi Y, Hensch TK, Hirokawa N, Hill D, Huminiecki L, Iacono M, Ikeo K, Iwama A, Ishikawa T, Jakt M, Kanapin A, Katoh M, Kawasawa Y, Kelso J, Kitamura H, Kitano H, Kollias G, Krishnan SP, Kruger A, Kummerfeld SK, Kurochkin IV, Lareau LF, Lazarevic D, Lipovich L, Liu J, Liuni S, McWilliam S, Madan Babu M, Madera M, Marchionni L, Matsuda H, Matsuzawa S, Miki H, Mignone F, Miyake S, Morris K, Mottagui-Tabar S, Mulder N, Nakano N, Nakauchi H, Ng P, Nilsson R, Nishiguchi S, Nishikawa S, Nori F, Ohara O, Okazaki Y, Orlando V, Pang KC, Pavan WJ, Pavesi G, Pesole G, Petrovsky N, Piazza S, Reed J, Reid JF, Ring BZ, Ringwald M, Rost B, Ruan Y, Salzberg SL, Sandelin A, Schneider C, Schonbach C, Sekiguchi K, Semple CA, Seno S, Sessa L, Sheng Y, Shibata Y, Shimada H, Shimada K, Silva D, Sinclair B, Sperling S, Stupka E, Sugiura K, Sultana R, Takenaka Y, Taki K, Tammoja K, Tan SL, Tang S, Taylor MS, Tegner J, Teichmann SA, Ueda HR, van Nimwegen E, Verardo R, Wei CL, Yagi K, Yamanishi H, Zabarovsky E, Zhu S, Zimmer A, Hide W, Bult C, Grimmond SM, Teasdale RD, Liu ET, Brusic V, Quackenbush J, Wahlestedt C, Mattick JS, Hume DA, Kai C, Sasaki D, Tomaru Y, Fukuda S, Kanamori-Katayama M, Suzuki M, Aoki J, Arakawa T, Iida J, Imamura K, Itoh M, Kato T, Kawaji H, Kawagashira N, Kawashima T, Kojima M, Kondo S, Konno H, Nakano K, Ninomiya N, Nishio T, Okada M, Plessy C, Shibata K, Shiraki T, Suzuki S, Tagami M, Waki K, Watahiki A, Okamura-Oho Y, Suzuki H, Kawai J, Hayashizaki Y. The transcriptional landscape of the mammalian genome. Science. 2005;309:1559–1563. doi: 10.1126/science.1112014. [DOI] [PubMed] [Google Scholar]
- 6.Kapranov P, Cheng J, Dike S, Nix DA, Duttagupta R, Willingham AT, Stadler PF, Hertel J, Hackermuller J, Hofacker IL, Bell I, Cheung E, Drenkow J, Dumais E, Patel S, Helt G, Ganesh M, Ghosh S, Piccolboni A, Sementchenko V, Tammana H, Gingeras TR. RNA maps reveal new RNA classes and a possible function for pervasive transcription. Science. 2007;316:1484–1488. doi: 10.1126/science.1138341. [DOI] [PubMed] [Google Scholar]
- 7.Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136:629–641. doi: 10.1016/j.cell.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Wang X, Song X, Glass CK, Rosenfeld MG. The long arm of long noncoding RNAs: roles as sensors regulating gene transcriptional programs. Cold Spring Harb Perspect Biol. 2011;3:a003756. doi: 10.1101/cshperspect.a003756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, Wang H, Song T, Zou Y, Jiang J, Fang L, Li P. HOTAIR is a potential target for the treatment of cisplatin resistant ovarian cancer. Mol Med Rep. 2015;12:2211–6. doi: 10.3892/mmr.2015.3562. [DOI] [PubMed] [Google Scholar]
- 10.Gupta RA, Shah N, Wang KC, Kim J, Horlings HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, Wang Y, Brzoska P, Kong B, Li R, West RB, van de Vijver MJ, Sukumar S, Chang HY. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464:1071–1076. doi: 10.1038/nature08975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim K, Jutooru I, Chadalapaka G, Johnson G, Frank J, Burghardt R, Kim S, Safe S. HOTAIR is a negative prognostic factor and exhibits pro-oncogenic activity in pancreatic cancer. Oncogene. 2013;32:1616–1625. doi: 10.1038/onc.2012.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu XH, Liu ZL, Sun M, Liu J, Wang ZX, De W. The long non-coding RNA HOTAIR indicates a poor prognosis and promotes metastasis in non-small cell lung cancer. BMC Cancer. 2013;13:464. doi: 10.1186/1471-2407-13-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hajjari M, Behmanesh M, Sadeghizadeh M, Zeinoddini M. Up-regulation of HOTAIR long non-coding RNA in human gastric adenocarcinoma tissues. Med Oncol. 2013;30:670. doi: 10.1007/s12032-013-0670-0. [DOI] [PubMed] [Google Scholar]
- 14.Zhang ZZ, Shen ZY, Shen YY, Zhao EH, Wang M, Wang CJ, Cao H, Xu J. HOTAIR Long Noncoding RNA Promotes Gastric Cancer Metastasis through Suppression of Poly r(C)-Binding Protein (PCBP) 1. Mol Cancer Ther. 2015;14:1162–70. doi: 10.1158/1535-7163.MCT-14-0695. [DOI] [PubMed] [Google Scholar]
- 15.Okugawa Y, Toiyama Y, Hur K, Toden S, Saigusa S, Tanaka K, Inoue Y, Mohri Y, Kusunoki M, Boland CR, Goel A. Metastasis-associated long non-coding RNA drives gastric cancer development and promotes peritoneal metastasis. Carcinogenesis. 2014;35:2731–2739. doi: 10.1093/carcin/bgu200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee NK, Lee JH, Park CH, Yu D, Lee YC, Cheong JH, Noh SH, Lee SK. Long non-coding RNA HOTAIR promotes carcinogenesis and invasion of gastric adenocarcinoma. Biochem Biophys Res Commun. 2014;451:171–178. doi: 10.1016/j.bbrc.2014.07.067. [DOI] [PubMed] [Google Scholar]
- 17.Emadi-Andani E, Nikpour P, Emadi-Baygi M, Bidmeshkipour A. Association of HOTAIR expression in gastric carcinoma with invasion and distant metastasis. Adv Biomed Res. 2014;3:135. doi: 10.4103/2277-9175.133278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Endo H, Shiroki T, Nakagawa T, Yokoyama M, Tamai K, Yamanami H, Fujiya T, Sato I, Yamaguchi K, Tanaka N, Iijima K, Shimosegawa T, Sugamura K, Satoh K. Enhanced expression of long non-coding RNA HOTAIR is associated with the development of gastric cancer. PLoS One. 2013;8:e77070. doi: 10.1371/journal.pone.0077070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Z, Sun M, Lu K, Liu J, Zhang M, Wu W, De W, Wang Z, Wang R. The long noncoding RNA HOTAIR contributes to cisplatin resistance of human lung adenocarcinoma cells via down-regualtion of p21(WAF1/CIP1) expression. PLoS One. 2013;8:e77293. doi: 10.1371/journal.pone.0077293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784–789. doi: 10.1056/NEJMoa001999. [DOI] [PubMed] [Google Scholar]
- 21.Bertuccio P, Chatenoud L, Levi F, Praud D, Ferlay J, Negri E, Malvezzi M, La Vecchia C. Recent patterns in gastric cancer: a global overview. Int J Cancer. 2009;125:666–673. doi: 10.1002/ijc.24290. [DOI] [PubMed] [Google Scholar]
- 22.Wagner AD, Unverzagt S, Grothe W, Kleber G, Grothey A, Haerting J, Fleig WE. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev. 2010:CD004064. doi: 10.1002/14651858.CD004064.pub3. [DOI] [PubMed] [Google Scholar]
- 23.Yang Z, Zhou L, Wu LM, Lai MC, Xie HY, Zhang F, Zheng SS. Overexpression of long noncoding RNA HOTAIR predicts tumor recurrence in hepatocellular carcinoma patients following liver transplantation. Ann Surg Oncol. 2011;18:1243–1250. doi: 10.1245/s10434-011-1581-y. [DOI] [PubMed] [Google Scholar]
- 24.Niinuma T, Suzuki H, Nojima M, Nosho K, Yamamoto H, Takamaru H, Yamamoto E, Maruyama R, Nobuoka T, Miyazaki Y, Nishida T, Bamba T, Kanda T, Ajioka Y, Taguchi T, Okahara S, Takahashi H, Nishida Y, Hosokawa M, Hasegawa T, Tokino T, Hirata K, Imai K, Toyota M, Shinomura Y. Upregulation of miR-196a and HOTAIR drive malignant character in gastrointestinal stromal tumors. Cancer Res. 2012;72:1126–1136. doi: 10.1158/0008-5472.CAN-11-1803. [DOI] [PubMed] [Google Scholar]
- 25.Tang L, Zhang W, Su B, Yu B. Long noncoding RNA HOTAIR is associated with motility, invasion, and metastatic potential of metastatic melanoma. Biomed Res Int. 2013;2013:251098. doi: 10.1155/2013/251098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tao S, He H, Chen Q. Estradiol induces HOTAIR levels via GPER-mediated miR-148a inhibition in breast cancer. J Transl Med. 2015;13:131. doi: 10.1186/s12967-015-0489-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu Y, Zhang L, Zhang L, Wang Y, Li H, Ren X, Wei F, Yu W, Liu T, Wang X, Zhou X, Yu J, Hao X. Long non-coding RNA HOTAIR promotes tumor cell invasion and metastasis by recruiting EZH2 and repressing E-cadherin in oral squamous cell carcinoma. Int J Oncol. 2015;46:2586–2594. doi: 10.3892/ijo.2015.2976. [DOI] [PubMed] [Google Scholar]
- 28.Zhang H, Diab A, Fan H, Mani SK, Hullinger R, Merle P, Andrisani O. PLK1 and HOTAIR accelerate proteasomal degradation of SUZ12 and ZNF198 during hepatitis B virus-induced liver carcinogenesis. Cancer Res. 2015;75:2363–74. doi: 10.1158/0008-5472.CAN-14-2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rinn JL, Kertesz M, Wang JK, Squazzo SL, Xu X, Brugmann SA, Goodnough LH, Helms JA, Farnham PJ, Segal E, Chang HY. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell. 2007;129:1311–1323. doi: 10.1016/j.cell.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woo CJ, Kingston RE. HOTAIR lifts noncoding RNAs to new levels. Cell. 2007;129:1257–1259. doi: 10.1016/j.cell.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 31.Tsai MC, Manor O, Wan Y, Mosammaparast N, Wang JK, Lan F, Shi Y, Segal E, Chang HY. Long noncoding RNA as modular scaffold of histone modification complexes. Science. 2010;329:689–693. doi: 10.1126/science.1192002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang ZZ, Shen ZY, Shen YY, Zhao EH, Wang M, Wang CJ, Cao H, Xu J. HOTAIR Long Noncoding RNA Promotes Gastric Cancer Metastasis through Suppression of Poly r(C)-Binding Protein (PCBP) 1. Mol Cancer Ther. 2015;14:1162–1170. doi: 10.1158/1535-7163.MCT-14-0695. [DOI] [PubMed] [Google Scholar]
- 33.Xu ZY, Yu QM, Du YA, Yang LT, Dong RZ, Huang L, Yu PF, Cheng XD. Knockdown of long non-coding RNA HOTAIR suppresses tumor invasion and reverses epithelial-mesenchymal transition in gastric cancer. Int J Biol Sci. 2013;9:587–597. doi: 10.7150/ijbs.6339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu XH, Sun M, Nie FQ, Ge YB, Zhang EB, Yin DD, Kong R, Xia R, Lu KH, Li JH, De W, Wang KM, Wang ZX. Lnc RNA HOTAIR functions as a competing endogenous RNA to regulate HER2 expression by sponging miR-331-3p in gastric cancer. Mol Cancer. 2014;13:92. doi: 10.1186/1476-4598-13-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Milhem MM, Knutson T, Yang S, Zhu D, Wang X, Leslie KK, Meng X. Correlation of MTDH/ AEG-1 and HOTAIR Expression with Metastasis and Response to Treatment in Sarcoma Patients. J Cancer Sci Ther. 2011;S5:004. [PMC free article] [PubMed] [Google Scholar]


