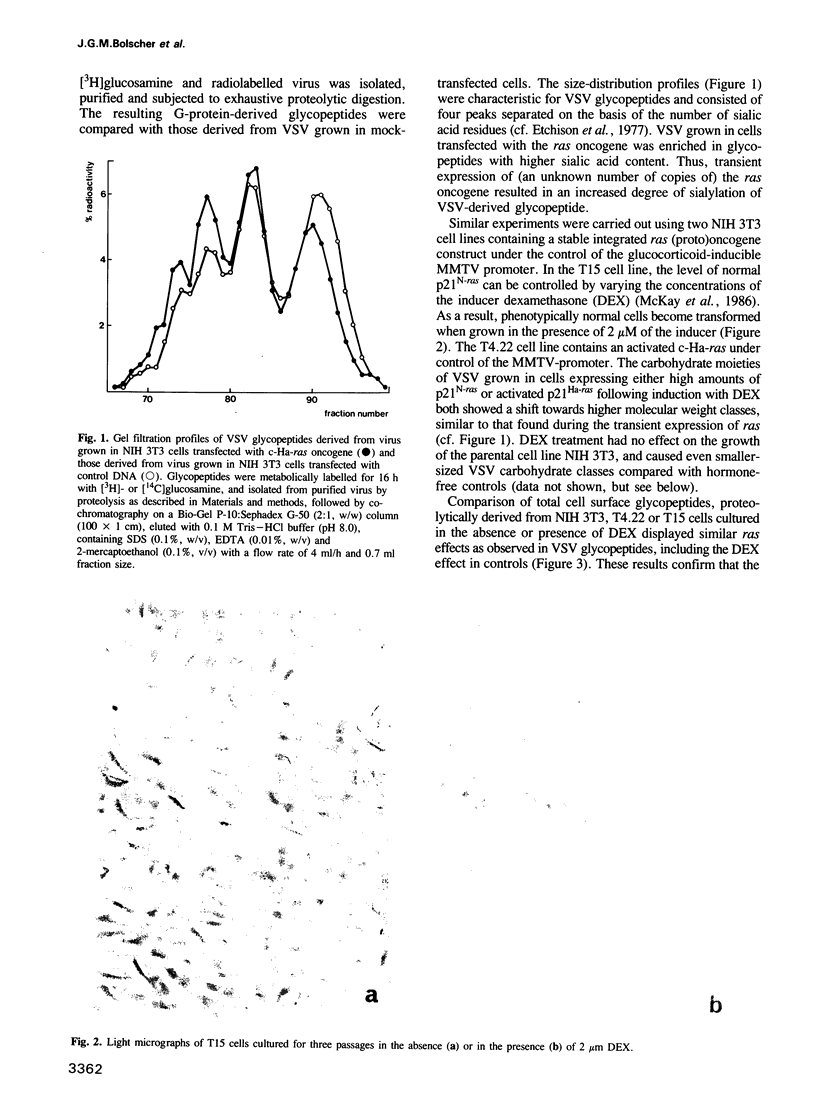
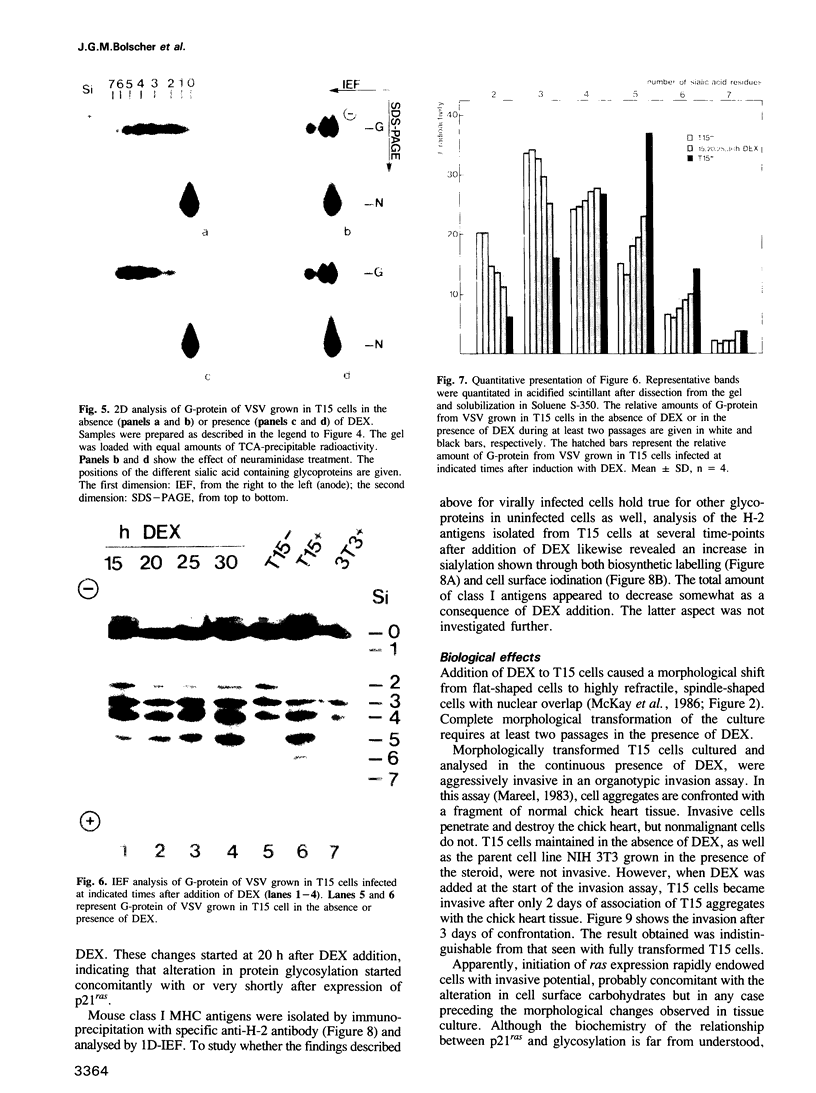
Abstract
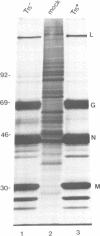
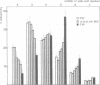
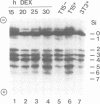
The effect of expression of the ras oncogene on protein glycosylation was studied. VSV G-protein and class I histocompatibility antigens were analysed to monitor ras-mediated changes in glycosylation. Transient expression of the c-Ha-ras oncogene, introduced into NIH 3T3 cells by the DEAE-dextran method, altered protein glycosylation within 25 h of transfection. The same result was obtained after dexamethasone-induced expression of p21-ras in stable NIH 3T3 transfectants containing either an activated Ha-ras oncogene or a normal N-ras proto-oncogene under control of the glucocorticoid-inducible MMTV promoter. The alteration of cell surface carbohydrates, induced by the ras (proto)oncogene and the subsequent acquisition of invasive potential, occurred prior to morphological transformation.
Full text
PDF
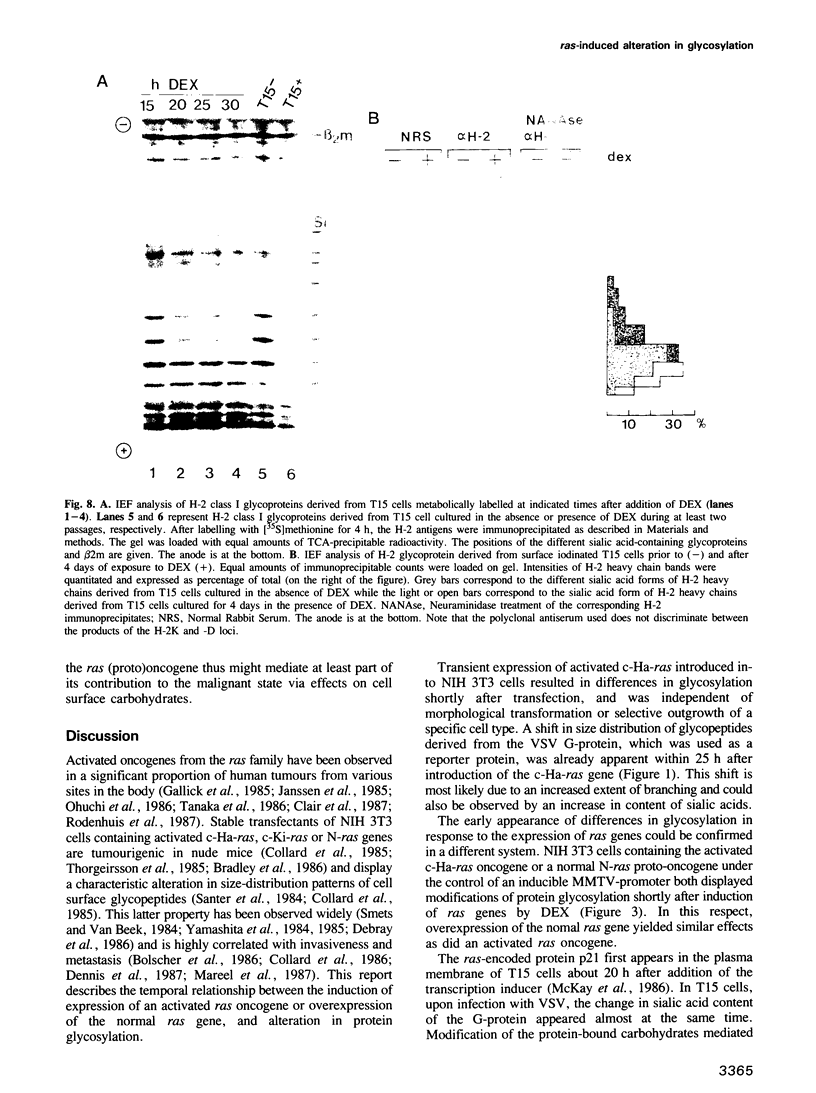
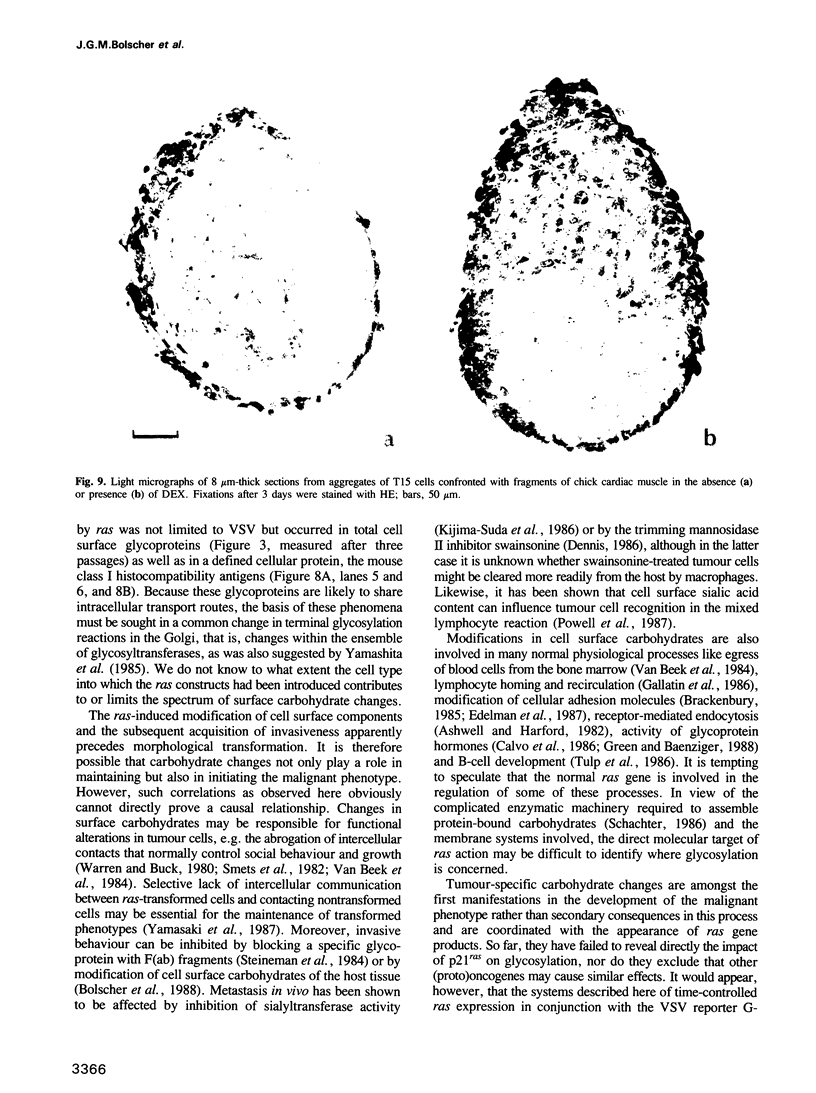


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashwell G., Harford J. Carbohydrate-specific receptors of the liver. Annu Rev Biochem. 1982;51:531–554. doi: 10.1146/annurev.bi.51.070182.002531. [DOI] [PubMed] [Google Scholar]
- Bolscher J. G., Schallier D. C., Smets L. A., van Rooy H., Collard J. G., Bruyneel E. A., Mareel M. M. Effect of cancer-related and drug-induced alterations in surface carbohydrates on the invasive capacity of mouse and rat cells. Cancer Res. 1986 Aug;46(8):4080–4086. [PubMed] [Google Scholar]
- Bolscher J. G., Schallier D. C., van Rooy H., Storme G. A., Smets L. A. Modification of cell surface carbohydrates and invasive behavior by an alkyl lysophospholipid. Cancer Res. 1988 Feb 15;48(4):977–982. [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Brackenbury R. Molecular mechanisms of cell adhesion in normal and transformed cells. Cancer Metastasis Rev. 1985;4(1):41–58. doi: 10.1007/BF00047736. [DOI] [PubMed] [Google Scholar]
- Bradley M. O., Kraynak A. R., Storer R. D., Gibbs J. B. Experimental metastasis in nude mice of NIH 3T3 cells containing various ras genes. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5277–5281. doi: 10.1073/pnas.83.14.5277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo F. O., Keutmann H. T., Bergert E. R., Ryan R. J. Deglycosylated human follitropin: characterization and effects on adenosine cyclic 3',5'-phosphate production in porcine granulosa cells. Biochemistry. 1986 Jul 1;25(13):3938–3943. doi: 10.1021/bi00361a030. [DOI] [PubMed] [Google Scholar]
- Chang E. H., Furth M. E., Scolnick E. M., Lowy D. R. Tumorigenic transformation of mammalian cells induced by a normal human gene homologous to the oncogene of Harvey murine sarcoma virus. Nature. 1982 Jun 10;297(5866):479–483. doi: 10.1038/297479a0. [DOI] [PubMed] [Google Scholar]
- Clair T., Miller W. R., Cho-Chung Y. S. Prognostic significance of the expression of a ras protein with a molecular weight of 21,000 by human breast cancer. Cancer Res. 1987 Oct 15;47(20):5290–5293. [PubMed] [Google Scholar]
- Collard J. G., Schijven J. F., Bikker A., La Riviere G., Bolscher J. G., Roos E. Cell surface sialic acid and the invasive and metastatic potential of T-cell hybridomas. Cancer Res. 1986 Jul;46(7):3521–3527. [PubMed] [Google Scholar]
- Collard J. G., van Beek W. P., Janssen J. W., Schijven J. F. Transfection by human oncogenes: concomitant induction of tumorigenicity and tumor-associated membrane alterations. Int J Cancer. 1985 Feb 15;35(2):207–213. doi: 10.1002/ijc.2910350211. [DOI] [PubMed] [Google Scholar]
- Cooper G. M., Neiman P. E. Transforming genes of neoplasms induced by avian lymphoid leukosis viruses. Nature. 1980 Oct 16;287(5783):656–659. doi: 10.1038/287656a0. [DOI] [PubMed] [Google Scholar]
- Debray H., Qin Z., Delannoy P., Montreuil J., Duś D., Radzikowski C., Christensen B., Kieler J. Altered glycosylation of membrane glycoproteins in human uroepithelial cell lines. Int J Cancer. 1986 Apr 15;37(4):607–611. doi: 10.1002/ijc.2910370421. [DOI] [PubMed] [Google Scholar]
- Dennis J. W. Effects of swainsonine and polyinosinic:polycytidylic acid on murine tumor cell growth and metastasis. Cancer Res. 1986 Oct;46(10):5131–5136. [PubMed] [Google Scholar]
- Dennis J. W., Laferte S. Tumor cell surface carbohydrate and the metastatic phenotype. Cancer Metastasis Rev. 1987;5(3):185–204. doi: 10.1007/BF00046998. [DOI] [PubMed] [Google Scholar]
- Dennis J. W., Laferté S., Waghorne C., Breitman M. L., Kerbel R. S. Beta 1-6 branching of Asn-linked oligosaccharides is directly associated with metastasis. Science. 1987 May 1;236(4801):582–585. doi: 10.1126/science.2953071. [DOI] [PubMed] [Google Scholar]
- Edelman G. M., Murray B. A., Mege R. M., Cunningham B. A., Gallin W. J. Cellular expression of liver and neural cell adhesion molecules after transfection with their cDNAs results in specific cell-cell binding. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8502–8506. doi: 10.1073/pnas.84.23.8502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchison J. R., Robertson J. S., Summers D. F. Partial structural analysis of the oligosaccharide moieties of the vesicular stomatitis virus glycoprotein by sequential chemical and enzymatic degradation. Virology. 1977 May 15;78(2):375–392. doi: 10.1016/0042-6822(77)90115-5. [DOI] [PubMed] [Google Scholar]
- Finkel T., Der C. J., Cooper G. M. Activation of ras genes in human tumors does not affect localization, modification, or nucleotide binding properties of p21. Cell. 1984 May;37(1):151–158. doi: 10.1016/0092-8674(84)90310-6. [DOI] [PubMed] [Google Scholar]
- Gallatin M., St John T. P., Siegelman M., Reichert R., Butcher E. C., Weissman I. L. Lymphocyte homing receptors. Cell. 1986 Mar 14;44(5):673–680. doi: 10.1016/0092-8674(86)90832-9. [DOI] [PubMed] [Google Scholar]
- Gallick G. E., Kurzrock R., Kloetzer W. S., Arlinghaus R. B., Gutterman J. U. Expression of p21ras in fresh primary and metastatic human colorectal tumors. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1795–1799. doi: 10.1073/pnas.82.6.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb M., Shimizu K., Perucho M., Wigler M. Isolation and preliminary characterization of a human transforming gene from T24 bladder carcinoma cells. Nature. 1982 Apr 1;296(5856):404–409. doi: 10.1038/296404a0. [DOI] [PubMed] [Google Scholar]
- Green E. D., Baenziger J. U. Asparagine-linked oligosaccharides on lutropin, follitropin, and thyrotropin. II. Distributions of sulfated and sialylated oligosaccharides on bovine, ovine, and human pituitary glycoprotein hormones. J Biol Chem. 1988 Jan 5;263(1):36–44. [PubMed] [Google Scholar]
- Hakomori S. Tumor-associated carbohydrate antigens. Annu Rev Immunol. 1984;2:103–126. doi: 10.1146/annurev.iy.02.040184.000535. [DOI] [PubMed] [Google Scholar]
- Hanley M. R., Jackson T. The ras gene. Transformer and transducer. Nature. 1987 Aug 20;328(6132):668–669. doi: 10.1038/328668a0. [DOI] [PubMed] [Google Scholar]
- Huang A. L., Ostrowski M. C., Berard D., Hager G. L. Glucocorticoid regulation of the Ha-MuSV p21 gene conferred by sequences from mouse mammary tumor virus. Cell. 1981 Dec;27(2 Pt 1):245–255. doi: 10.1016/0092-8674(81)90408-6. [DOI] [PubMed] [Google Scholar]
- Hurley J. B., Simon M. I., Teplow D. B., Robishaw J. D., Gilman A. G. Homologies between signal transducing G proteins and ras gene products. Science. 1984 Nov 16;226(4676):860–862. doi: 10.1126/science.6436980. [DOI] [PubMed] [Google Scholar]
- Janssen J. W., Steenvoorden A. C., Collard J. G., Nusse R. Oncogene activation in human myeloid leukemia. Cancer Res. 1985 Jul;45(7):3262–3267. [PubMed] [Google Scholar]
- Kijima-Suda I., Miyamoto Y., Toyoshima S., Itoh M., Osawa T. Inhibition of experimental pulmonary metastasis of mouse colon adenocarcinoma 26 sublines by a sialic acid:nucleoside conjugate having sialyltransferase inhibiting activity. Cancer Res. 1986 Feb;46(2):858–862. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lopata M. A., Cleveland D. W., Sollner-Webb B. High level transient expression of a chloramphenicol acetyl transferase gene by DEAE-dextran mediated DNA transfection coupled with a dimethyl sulfoxide or glycerol shock treatment. Nucleic Acids Res. 1984 Jul 25;12(14):5707–5717. doi: 10.1093/nar/12.14.5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mareel M. M. Invasion in vitro. Methods of analysis. Cancer Metastasis Rev. 1983;2(2):201–218. doi: 10.1007/BF00048970. [DOI] [PubMed] [Google Scholar]
- Mareel M., Kint J., Meyvisch C. Methods of study of the invasion of malignant C3H-mouse fibroblasts into embryonic chick heart in vitro. Virchows Arch B Cell Pathol Incl Mol Pathol. 1979 May 4;30(1):95–111. doi: 10.1007/BF02889094. [DOI] [PubMed] [Google Scholar]
- McCutchan J. H., Pagano J. S. Enchancement of the infectivity of simian virus 40 deoxyribonucleic acid with diethylaminoethyl-dextran. J Natl Cancer Inst. 1968 Aug;41(2):351–357. [PubMed] [Google Scholar]
- McGrath J. P., Capon D. J., Goeddel D. V., Levinson A. D. Comparative biochemical properties of normal and activated human ras p21 protein. Nature. 1984 Aug 23;310(5979):644–649. doi: 10.1038/310644a0. [DOI] [PubMed] [Google Scholar]
- McKay I. A., Marshall C. J., Calés C., Hall A. Transformation and stimulation of DNA synthesis in NIH-3T3 cells are a titratable function of normal p21N-ras expression. EMBO J. 1986 Oct;5(10):2617–2621. doi: 10.1002/j.1460-2075.1986.tb04542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neefjes J. J., Breur-Vriesendorp B. S., van Seventer G. A., Iványi P., Ploegh H. L. An improved biochemical method for the analysis of HLA-class I antigens. Definition of new HLA-class I subtypes. Hum Immunol. 1986 Jun;16(2):169–181. doi: 10.1016/0198-8859(86)90046-7. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L. Cell surface molecules and tumor metastasis. Regulation of metastatic phenotypic diversity. Exp Cell Res. 1984 Jan;150(1):3–22. doi: 10.1016/0014-4827(84)90696-7. [DOI] [PubMed] [Google Scholar]
- Ohuchi N., Thor A., Page D. L., Hand P. H., Halter S. A., Schlom J. Expression of the 21,000 molecular weight ras protein in a spectrum of benign and malignant human mammary tissues. Cancer Res. 1986 May;46(5):2511–2519. [PubMed] [Google Scholar]
- Olden K., Bernard B. A., White S. L., Parent J. B. Function of the carbohydrate moieties of glycoproteins. J Cell Biochem. 1982;18(3):313–335. doi: 10.1002/jcb.1982.240180306. [DOI] [PubMed] [Google Scholar]
- Phillips D. R., Morrison M. The arrangement of proteins in the human erythrocyte membrane. Biochem Biophys Res Commun. 1970 Jul 27;40(2):284–289. doi: 10.1016/0006-291x(70)91007-7. [DOI] [PubMed] [Google Scholar]
- Pierce M., Arango J. Rous sarcoma virus-transformed baby hamster kidney cells express higher levels of asparagine-linked tri- and tetraantennary glycopeptides containing [GlcNAc-beta (1,6)Man-alpha (1,6)Man] and poly-N-acetyllactosamine sequences than baby hamster kidney cells. J Biol Chem. 1986 Aug 15;261(23):10772–10777. [PubMed] [Google Scholar]
- Powell L. D., Whiteheart S. W., Hart G. W. Cell surface sialic acid influences tumor cell recognition in the mixed lymphocyte reaction. J Immunol. 1987 Jul 1;139(1):262–270. [PubMed] [Google Scholar]
- Pulciani S., Santos E., Long L. K., Sorrentino V., Barbacid M. ras gene Amplification and malignant transformation. Mol Cell Biol. 1985 Oct;5(10):2836–2841. doi: 10.1128/mcb.5.10.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodenhuis S., van de Wetering M. L., Mooi W. J., Evers S. G., van Zandwijk N., Bos J. L. Mutational activation of the K-ras oncogene. A possible pathogenetic factor in adenocarcinoma of the lung. N Engl J Med. 1987 Oct 8;317(15):929–935. doi: 10.1056/NEJM198710083171504. [DOI] [PubMed] [Google Scholar]
- Santer U. V., Gilbert F., Glick M. C. Change in glycosylation of membrane glycoproteins after transfection of NIH 3T3 with human tumor DNA. Cancer Res. 1984 Sep;44(9):3730–3735. [PubMed] [Google Scholar]
- Santos E., Tronick S. R., Aaronson S. A., Pulciani S., Barbacid M. T24 human bladder carcinoma oncogene is an activated form of the normal human homologue of BALB- and Harvey-MSV transforming genes. Nature. 1982 Jul 22;298(5872):343–347. doi: 10.1038/298343a0. [DOI] [PubMed] [Google Scholar]
- Schachter H. Biosynthetic controls that determine the branching and microheterogeneity of protein-bound oligosaccharides. Adv Exp Med Biol. 1986;205:53–85. doi: 10.1007/978-1-4684-5209-9_2. [DOI] [PubMed] [Google Scholar]
- Shih C., Shilo B. Z., Goldfarb M. P., Dannenberg A., Weinberg R. A. Passage of phenotypes of chemically transformed cells via transfection of DNA and chromatin. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5714–5718. doi: 10.1073/pnas.76.11.5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smets L. A., Enninga I. C., Van Rooy H. Cell communication reduced by changes in cell surface carbohydrates. Exp Cell Res. 1982 May;139(1):181–189. doi: 10.1016/0014-4827(82)90331-7. [DOI] [PubMed] [Google Scholar]
- Smets L. A., Van Beek W. P. Carbohydrates of the tumor cell surface. Biochim Biophys Acta. 1984;738(4):237–249. doi: 10.1016/0304-419x(83)90006-9. [DOI] [PubMed] [Google Scholar]
- Soto A. M., Murai J. T., Siiteri P. K., Sonnenschein C. Control of cell proliferation: evidence for negative control on estrogen-sensitive T47D human breast cancer cells. Cancer Res. 1986 May;46(5):2271–2275. [PubMed] [Google Scholar]
- Steinemann C., Fenner M., Binz H., Parish R. W. Invasive behavior of mouse sarcoma cells is inhibited by blocking a 37,000-dalton plasma membrane glycoprotein with Fab fragments. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3747–3750. doi: 10.1073/pnas.81.12.3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Slamon D. J., Battifora H., Cline M. J. Expression of p21 ras oncoproteins in human cancers. Cancer Res. 1986 Mar;46(3):1465–1470. [PubMed] [Google Scholar]
- Thorgeirsson U. P., Turpeenniemi-Hujanen T., Williams J. E., Westin E. H., Heilman C. A., Talmadge J. E., Liotta L. A. NIH/3T3 cells transfected with human tumor DNA containing activated ras oncogenes express the metastatic phenotype in nude mice. Mol Cell Biol. 1985 Jan;5(1):259–262. doi: 10.1128/mcb.5.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulp A., Barnhoorn M., Bause E., Ploegh H. Inhibition of N-linked oligosaccharide trimming mannosidases blocks human B cell development. EMBO J. 1986 Aug;5(8):1783–1790. doi: 10.1002/j.1460-2075.1986.tb04427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren L., Buck C. A. The membrane glycoproteins of the malignant cell. Clin Biochem. 1980 Oct;13(5):191–197. doi: 10.1016/s0009-9120(80)80022-1. [DOI] [PubMed] [Google Scholar]
- Warren L., Buck C. A., Tuszynski G. P. Glycopeptide changes and malignant transformation. A possible role for carbohydrate in malignant behavior. Biochim Biophys Acta. 1978 Sep 18;516(1):97–127. doi: 10.1016/0304-419x(78)90005-7. [DOI] [PubMed] [Google Scholar]
- Warren L., Fuhrer J. P., Buck C. A. Surface glycoproteins of normal and transformed cells: a difference determined by sialic acid and a growth-dependent sialyl transferase. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1838–1842. doi: 10.1073/pnas.69.7.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki H., Hollstein M., Mesnil M., Martel N., Aguelon A. M. Selective lack of intercellular communication between transformed and nontransformed cells as a common property of chemical and oncogene transformation of BALB/c 3T3 cells. Cancer Res. 1987 Nov 1;47(21):5658–5664. [PubMed] [Google Scholar]
- Yamashita K., Ohkura T., Tachibana Y., Takasaki S., Kobata A. Comparative study of the oligosaccharides released from baby hamster kidney cells and their polyoma transformant by hydrazinolysis. J Biol Chem. 1984 Sep 10;259(17):10834–10840. [PubMed] [Google Scholar]
- Yamashita K., Tachibana Y., Ohkura T., Kobata A. Enzymatic basis for the structural changes of asparagine-linked sugar chains of membrane glycoproteins of baby hamster kidney cells induced by polyoma transformation. J Biol Chem. 1985 Apr 10;260(7):3963–3969. [PubMed] [Google Scholar]
- Yogeeswaran G. Cell surface glycolipids and glycoproteins in malignant transformation. Adv Cancer Res. 1983;38:289–350. doi: 10.1016/s0065-230x(08)60191-8. [DOI] [PubMed] [Google Scholar]
- van Beek W. P., Smets L. A., Emmelot P. Increased sialic acid density in surface glycoprotein of transformed and malignant cells--a general phenomenon? Cancer Res. 1973 Nov;33(11):2913–2922. [PubMed] [Google Scholar]
- van Beek W., Tulp A., Bolscher J., Blanken G., Roozendaal K., Egbers M. Transient versus permanent expression of cancer-related glycopeptides on normal versus leukemic myeloid cells coinciding with marrow egress. Blood. 1984 Jan;63(1):170–176. [PubMed] [Google Scholar]