Abstract
The expression patterns of immunosuppressive molecules on regulatory T (Treg) cells have not been elucidated in non-small-cell lung cancer (NSCLC) patients. In this study, a total of 88 patients including 53 patients with NSCLC, 17 patients with lung non-malignant diseases, and 18 healthy volunteers were enrolled. Increased number of total CD4+CD25+FoxP3+ Treg cells and elevated expressions on the surface of several inhibitory molecules including CTLA-4, LAG-3 and PD-1 have been observed in the peripheral blood of NSCLC patients. We found that intratumoral Treg cells from NSCLC patients express the highest levels of co-inhibitory molecules compared to Treg cells isolated from tumor adjacent tissues or from peripheral blood of cancer patients, which is in consistent with the enhanced immunosuppressive function of these co-inhibitory molecules. Moreover, the number of Treg cells and their functional surface molecules increased during the progression of lung cancer. Elevated plasma levels of TGF-β and IL-10 in NSCLC patients were also observed in NSCLC patients compared to that in healthy volunteers. Our findings further support the role of Treg cells in the tumor microenvironments in NSCLC patients.
Keywords: Regulatory T cells, immunosuppressive molecules, tumor-infiltrating lymphocytes, non-small-cell lung cancer
Introduction
Lung cancer accounts for the largest number of cancer-related deaths worldwide, and more than 85% of cases are non-small-cell lung cancer (NSCLC). The predicted five-year survival rate of NSCLC is 16% due to limited improvements in the treatment of patients over the past few decades [1]. However, great progress has been made in understanding tumor development and lung cancer cell interactions with the tumor microenvironment; and this progress may help improve the survival rate of NSCLC patients through the development of novel therapeutic strategies.
T lymphocytes play a vital role in the immune response that controls tumor development. Increased number of conventional regulatory T (Treg) cells has been shown to inhibit anti-tumor immune response and reduce the effect of cancer immunotherapy [2]. Potential mechanisms underlying the immunosuppressive effects of Treg cells may include the production of inhibitory cytokines such as TGF-β and IL-10, as well as the suppression of T cell function by competitive binding of interleukin-2 (IL-2) via cell surface receptor CD25 (IL-2 receptor). In addition, expressions of several co-inhibitory molecules, such as cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) and glucocorticoid-induced tumor necrosis factor receptor (GITR), bind to ligands on effector T cells and directly contributes to the inhibitory function of Treg cells [3]. Although enrichment of Treg cells in both peripheral blood and the tumor microenvironment has been proposed to be responsible for suppressing anti-tumor immunity, key molecules that take part in the expansion and function of Treg remains largely undiscovered.
Recently, CTLA-4, programmed cell death-1 (PD-1), lymphocyte activation gene-3 (LAG-3), and ectonucleotidase CD39 have been proposed as critical immunosuppression molecules mediating Treg cell functions [4-7]. In this present study, we focused on the expression patterns of these four molecules on tumor-infiltrating and circulating Treg cells in NSCLC patients. CTLA-4 exerts immunosuppressive functions by inhibiting T cell proliferation and activation, and by inducing indoleamine 2,3-dioxygenase (IDO) activity in antigen presenting cells (APCs) [8]. It has been shown that CTLA-4 promotes the polarization of Treg cells from naive CD4+ T cells [4]. By combining to major histocompatibility complex-II (MHC-II) on APCs, LAG-3 on Treg cells is indispensable in the negative regulation of immune homeostasis and inhibition of effector T cell expansion [5]. PD-1 is a member of the CD28 family; which also plays an important role in peripheral tolerance, as well as in host anti-tumor immune response. PD-1 ligands, PD-L1 (B7-H1) and PD-L2 (B7-DC) [6] are widely expressed in immune cells, non-hematopoietic cells and tumor cells [3]. The interaction of PD-L1 on APCs with PD-1 on naive CD4+ T cells induces differentiation of Foxp3+ Treg cells [6]. Treg cells also express ectonucleotidase CD39; which has been proposed to be an important contributor to Treg-mediated immunosuppressive functions, and thereby promote tumor progression [9]. CD39 converts ATP and ADP into cAMP, and is further converted into adenosine, which is a potent immunosuppressive agent [9]. CTLA-4, PD-1, LAG-3 and CD39 are thereby very important not only for the inhibitory function of Treg cells, but also for regulating their differentiation and amplification. However, despite their importance in regulating the function of Treg cells, the comprehensive analysis of these molecules on Treg in NSCLC patients has not been reported.
We isolated lymphocytes from tumor tissues, tumor adjacent tissues and peripheral blood of NSCLC patients; and compared the expression of CTLA-4, PD-1, LAG-3 and CD39 on different sources of Treg cells. Levels of immunosuppressive molecules were elevated in circulating Treg cells from NSCLC patients compared to healthy volunteers. We demonstrated that intratumoral Treg cells have higher levels of immunosuppressive molecules than Tregs from tumor adjacent tissues or peripheral blood Treg cells. Our data suggest that elevated expressions of immunosuppressive molecules may contribute to the expansion and/or suppressive activity of Treg cells in both tumor sites and peripheral blood.
Materials and methods
Patients and specimens
Seventy patients, who were referred to the Shanghai Pulmonary Hospital affiliated to the Tongji University School of Medicine and the Changhai Hospital affiliated to the Second Military Medical University from August 2012 to March 2013, were included in this study. All patients were initially diagnosed with “lung cancer or lung placeholder”. Among the total patients enrolled in this study, 53 patients were diagnosed with NSCLC. Histological type was further classified according to the “The 2004 World Health Organization classification of lung tumors” [10], and TNM clinical staging was determined according to the 2009 “TNM Classification of Malignant Tumors” [11]. The remaining 17 patients were diagnosed with non-malignant diseases including lung tuberculosis (n=2), lung angioma (n=3), cryptogenic organizing pneumonia (COP, n=2), lung abscess (n=2), lung bullae (n=2), pulmonary infection (n=4) and lung sarcomatoid carcinoma (n=2). Peripheral blood samples and laboratory data were collected before chemotherapy, radiotherapy or surgery. Patients had no history of diabetes or other metabolic diseases. Further, 18 gender and age-matched healthy volunteers were also included in this study as controls. Thus, three groups were included in our study: NSCLC group, patients with NSCLC; non-malignant disease group, patients with non-malignant diseases; control group, healthy volunteers. According to TNM stage, the NSCLC group was further divided into two subgroups: early stage group, stage I-II patients; late stage group, stage III-IV patients. Thirteen paired tumor and normal (3-5 cm away from the tumor edge) tissues were obtained from NSCLC patients and analyzed. This present study was approved by the Shanghai Pulmonary Hospital and Changhai Hospital Institutional Review Board. Signed informed consent was obtained from each subject in accordance with the Declaration of Helsinki.
Antibodies and reagents
The following anti-human monoclonal antibodies (mAb) were used for staining: FITC-CD4, PE-PD-1, PE-CD39 (all obtained from eBioscience), PE-CTLA-4 (BD Biosciences), PE-LAG-3 (R&D Systems), Fc receptor blocking buffer (Miltenyi Biotec). TGF-β and IL-10 ELISA kits were obtained from eBioscience.
Collection of cancer tissues, adjacent tissues and peripheral blood samples
All blood samples were collected after overnight fasting. Part of the blood specimens was centrifuged, and plasma samples were stored at -20°C until use. The rest of the samples were directly labeled with antibodies for flow cytometry (FCM). Lung cancer tissues and adjacent tissues were obtained through conventional surgery or minimally invasive thoracoscopic surgery. Tissues were rinsed with PBS to remove blood and necrotic tissues, cut into pieces, and washed repeatedly with PBS for 3-4 times. Tissue fragments were digested with 0.1% type IV collagenase at 37°C for 1.5 hours, and were filtered through a 200 μm mesh stainless steel filter to obtain a single cell suspension. Cells were washed twice with PBS and re-suspended in 0.5 mL of PBS for FCM analysis.
Flow cytometry
Peripheral blood cells or cells isolated from tumor tissues or tumor adjacent tissues were blocked with 5 μL of Fc receptor blocking reagent for 10 minutes in 100 μL of staining buffer. Samples were stained with 5 μl of monoclonal antibodies to target different cell surface makers including CD4, CD25, CD39, CTLA4, LAG3 and PD-1 for 30 minutes at room temperature in the dark. Cells were washed with PBS and treated with 1 ml of erythrocyte lysis buffer for 10 minutes to remove red blood cells. Intracellular staining for FOXP3 was performed according to manufacturer’s protocol. Briefly, cells were washed with PBS, and subsequently fixed and permeabilized by Fix/Perm buffer. After being washed, cells were stained for Foxp3 in 50 μL of permeabilization solution for 4-6 hours in the dark, followed by another wash; and were re-suspended in 500 μl of PBS. FCM was performed using MACSQuant® Analyzer (Miltenyi Biotec), and data were analyzed using FlowJo software (Tree Star Inc.).
Treg cell suppression assays
Peripheral blood cells, as well as cells isolated from tumor tissues and tumor adjacent tissues as described above, were used for the isolation of CD4+CD25+ Treg cells. Isolation was performed using a human CD4+CD25+ Regulatory T Cell Isolation Kit (Miltenyi Biotec). Briefly, non-CD4+ cells were first magnetically labeled with a cocktail of biotin-conjugated antibodies and anti-biotin monoclonal antibodies conjugated to MicroBeads. Labeled cells were subsequently depleted by separation over a MACS® Column. In the second step, CD4+CD25+ Treg cells were directly labeled with CD25 MicroBeads, and isolated by positive selection from the pre-enriched CD4+ T cell fraction. The remaining CD4+CD25- pre-enriched CD4+ T cells obtained from peripheral blood were collected and used as effective T cells for Treg cell suppression assays. When CFSE dye was used, purified CD4+CD25- T cells were first co-incubated with 5 mM of CFSE for 15 minutes, and thoroughly washed before being co-cultured with Treg cells. Enriched CD4+CD25+ Treg cells and CFSE labeled CD4+CD25- effective T cells (1 × 105 cells/well) were mixed at 1:1, 0.5:1, 0.25:1 and 0:1 ratios; and were co-cultured in anti-CD3/anti-CD28 coated culture plates for four days before FCM analysis.
Enzyme-linked immunosorbent assay (ELISA)
Plasma samples were collected as described above, and TGF-β and IL-10 levels were detected according to manufacturer’s instructions.
Statistical analysis
All statistical analyses were performed with SPSS software (SPSS 17.0; Chicago, IL, USA). One-way ANOVA was used to compare the three groups (NSCLC, non-malignant disease and control groups), while Fisher’s LSD test was further used for pairwise comparisons. Data obtained from tumors and its adjacent tissues were analyzed with the paired-sample t-test. P values <0.05 were considered statistically significant.
Results
Clinical characteristics of patients
A total of 88 subjects were included in this study. There were no statistically significant differences in gender or age of patients in the NSCLC, non-malignant disease and control groups; or between NSCLC patients in the early and late stage groups (P>0.05). Clinical characteristics of these patients are listed in Table 1.
Table 1.
Clinical characteristics of all enrolled subjects
| NSCLC group | Non-malignant disease group | Control group | NSCLC subgroups | ||
|---|---|---|---|---|---|
|
| |||||
| Stage I-II | Stage III-IV | ||||
| Cases | 53 | 17 | 18 | 33 | 20 |
| Gender (male/female) | 39/14 | 11/6 | 14/4 | 22/11 | 17/3 |
| Age (years) | 60.50 ± 9.20 | 55.50 ± 10.00 | 57.60 ± 9.10 | 59.60 ± 9.80 | 61.90 ± 8.30 |
| Histology (Adenocarcinoma/squamous cell carcinoma | 39/14 | - | - | 27/6 | 12/8 |
| Differentiation (high/medium/low) | 7/29/17 | - | - | 6/20/7 | 1/9/10 |
Increased CTLA-4+, LAG-3+ and PD-1+ Treg cells in peripheral blood of NSCLC patients
We demonstrated that the number of CD4+CD25+FoxP3+ Treg cells was significantly elevated, and that CTLA-4, LAG-3 and PD-1 expressions were elevated in peripheral blood Treg cells of NSCLC patients, compared to healthy volunteers. In contrast, there was no significant difference in Treg cells of patients between the NSCLC and non-malignant disease groups. There was no statistically significant difference in the expression of CD39+ in peripheral blood Treg cells among the three groups (P>0.05, Table 2; Figure 1A).
Table 2.
Phenotypic characterization of Treg cells among different groups
| Patients Groups | n | Treg/CD4+ T (%) | CTLA-4+ Treg/Treg (%) | LAG-3+ Treg/Treg (%) | PD-1+ Treg/Treg (%) | CD39+ Treg/Treg (%) |
|---|---|---|---|---|---|---|
| NSCLC | 53 | 9.12 ± 3.57* | 6.01 ± 4.49* | 4.89 ± 4.80* | 20.14 ± 11.57** | 57.58 ± 20.84 |
| Non-malignant disease | 17 | 7.01 ± 2.89 | 6.02 ± 5.93 | 3.83 ± 4.39 | 20.17 ± 9.98 | 56.47 ± 30.26 |
| Control | 18 | 6.43 ± 2.48 | 2.53 ± 2.04 | 1.79 ± 2.18 | 11.68 ± 5.86 | 62.13 ± 30.60 |
Compared with the control group, P<0.05;
compared with the control group, P<0.01.
Figure 1.
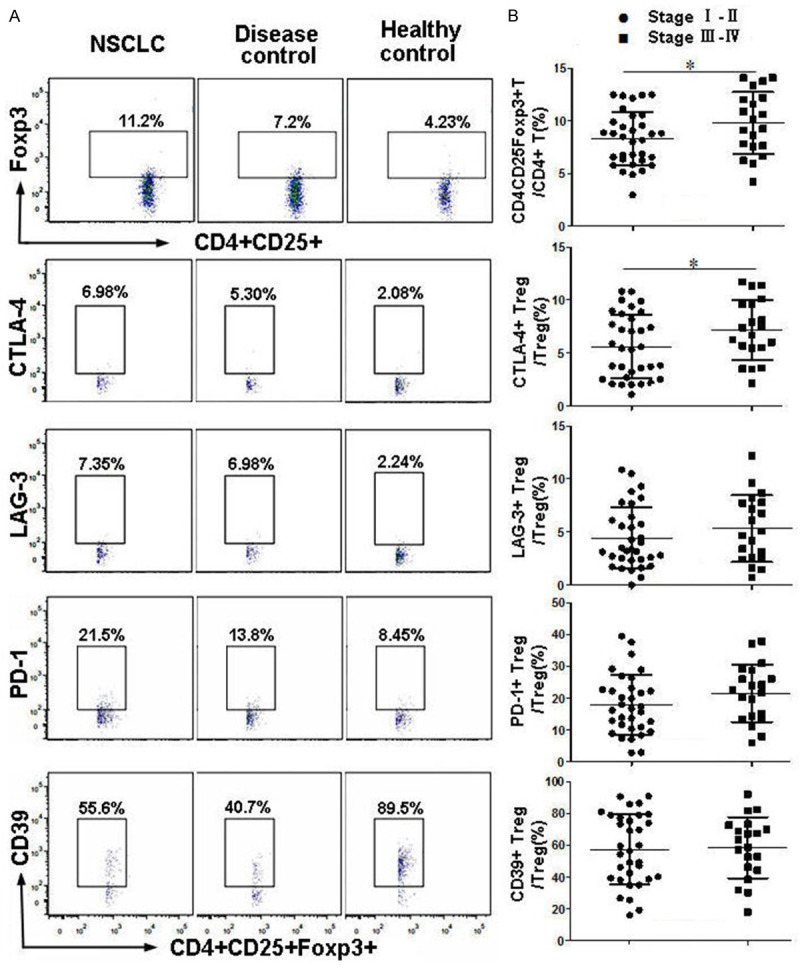
CTLA-4, LAG-3, PD-1 and CD39 expressions in peripheral blood Treg cells of NSCLC patients. Cells in different groups were labeled as described in the Materials and Methods, and detected by FCM. A. Representative flow cytometric analysis for detecting CD4+CD25+Fxop3+ Treg cells, or CTLA-4, LAG-3, PD-1 and CD39 on Treg cells. B. Treg cells and CTLA-4 expressions on Treg cells are elevated in peripheral blood of late stage NSCLC patients (*, P<0.05).
Next, we characterized circulating Treg cells in NSCLC patients with different TNM stages. The percentage of Treg cells in CD4+ T cells and CTLA-4+ Treg cells in the total CD4+CD25+FoxP3+ Treg cells in NSCLC patients with late stage disease (TNM stage III-IV) were statistically higher than in NSCLC patients with early stage disease (TNM stage I-II). There were no significant differences in LAG-3, PD-1 or CD39 expressions on Treg cells among the different NSCLC stages (Figure 1B).
Levels of CTLA-4, LAG-3 and PD-1 increased in tumor-infiltrating Treg cells compared to Treg cells in normal adjacent tissues
Paired tumor tissues and corresponding normal tissues were obtained from 13 NSCLC patients, and were analyzed by FCM to determine the ratio of Treg cells and expression levels of inhibitory molecules on their surface. As shown in Figure 2A and 2B, both the total Treg cells, as well as subsets of CTLA-4+, LAG-3+ and PD-1+ Treg cells, were significantly higher in tumors than in normal tissues (P<0.05). In contrast, no differences in CD39 expressions on Treg cells were found between tumor and non-malignant tissues.
Figure 2.
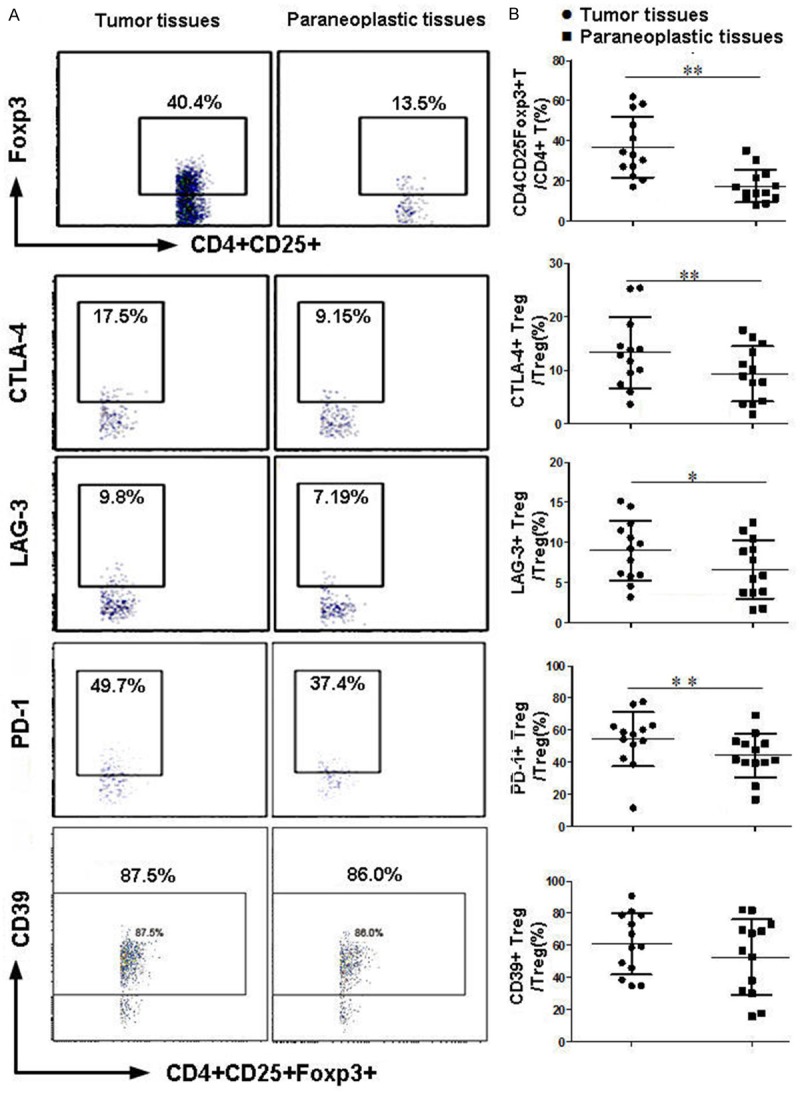
Comparison of Treg cells and expressions of inhibitory molecules in tumor tissues and corresponding adjacent tissues by flow cytometry. A. Representative flow cytometric analysis of a NSCLC patient; B. analysis of paired tumor and tumor-adjacent tissues obtained from 13 NSCLC patients (*P<0.05, **P<0.01).
Tumor infiltrating Treg cells express higher levels of inhibitory molecules compared to Treg cells in peripheral blood
Next, we compared the cell surface expression of CTLA-4, LAG-3, PD-1 and CD39 on tumor-infiltrating and circulating Treg cells in 13 NSCLC patients. Results revealed that the expression levels of LAG-3, PD-1 and CTLA-4 were higher in tumor-infiltrating Treg cells than in circulating Treg cells (Figure 3). In contrast, the expression of CD39 was comparable in tumor-infiltrating and peripheral blood Treg cells (data not shown).
Figure 3.
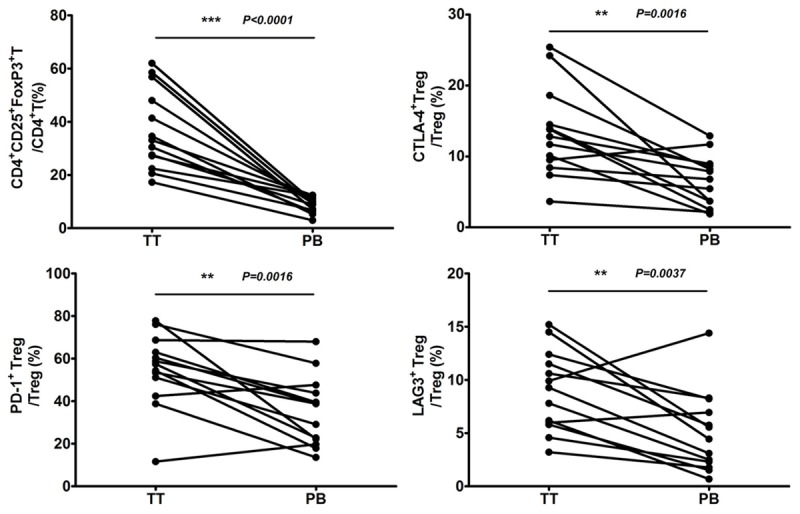
Treg cells and expressions of inhibitory molecules on Treg cells isolated from tumor tissues and peripheral blood. Local tumor-infiltrating or circulating Treg cells, as well as surface expressions of LAG-3, PD-1, and CTLA-4, were determined by flow cytometry. TT: tumor tissues; PB: peripheral blood.
Tumor-infiltrating Treg cells have increased suppressive properties
Since tumor-infiltrating Treg cells express higher levels of immunosuppressive molecules than Treg cells in normal tissues or in peripheral blood, we hypothesized that tumor-infiltrated Treg cells have increased suppressive properties. CD4+CD25+ T cells were isolated from tumor tissues, normal tissues and peripheral blood of two NSCLC patients; and Treg cell suppressive assays were performed as described in the Materials and Methods. Tumor-infiltrating Treg cells demonstrated increased suppressive ability on proliferation of effective T cells, compared to Treg cells obtained from peripheral blood or from normal tissues (Figure 4). These data confirms that tumor-infiltrating Treg cells have increased suppressive activity, which is likely to be at least in part attributed to elevated expressions of immunosuppressive molecules such as CTLA-4, PD-1 and LAG-3.
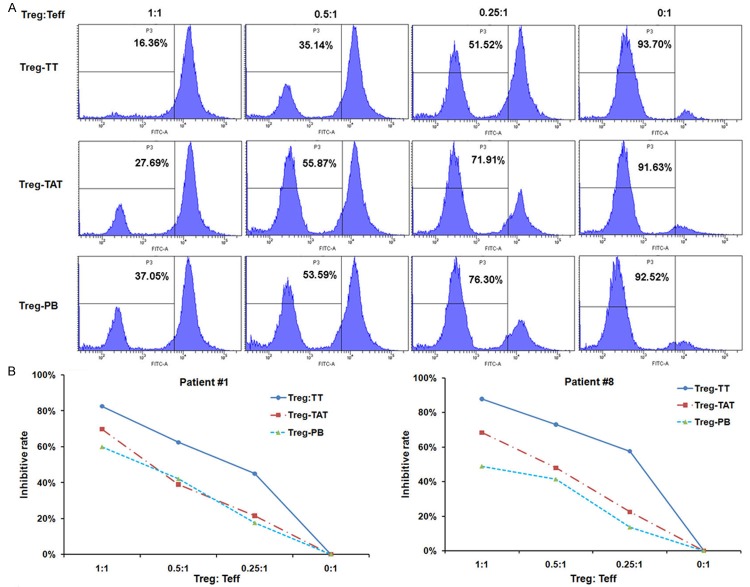
Figure 4.
Tumor-infiltrating Treg cells have increased suppressive properties. A. CFSE-labeled CD4+CD25- T cells were co-cultured with unlabelled CD4+CD25+ Treg cells from different sources at indicated ratios. After four days of treatment with anti-CD3 and anti-CD28 antibodies for stimulation of T cell proliferation, CD4+CFSE+ T cells were analyzed by flow cytometry. B. Inhibitory activity of Treg from different sources on T cells in Patient #1 and Patient #8. Treg: CD4+CD25+ regulatory T cells; Teff: CD4+CD25- effective T cells; Treg-TT: Treg cells in tumor tissues; Treg-TAT: Treg cells in tumor adjacent tissues; Treg-PB: Treg cells in peripheral blood.
Plasma levels of TGF-β and IL-10 are elevated in NSCLC patients
TGF-β and IL-10 have been shown to play important roles in the differentiation and function of Treg cells. We compared the plasma levels of TGF-β and IL-10 in the three experimental groups by ELISA, and found that TGF-β and IL-10 levels are elevated in NSCLC patients compared to healthy volunteers. Although the difference in serum levels of TGF-β between patients in the NSCLC and non-malignant disease groups were statistically significant, no differences in IL-10 levels were observed between these two groups (Figure 5, P>0.05). We found no correlation between the elevated levels of serum TGF-β or IL-10 and increased proportion of Treg cells in NSCLC patients (data not shown, P>0.05).
Figure 5.
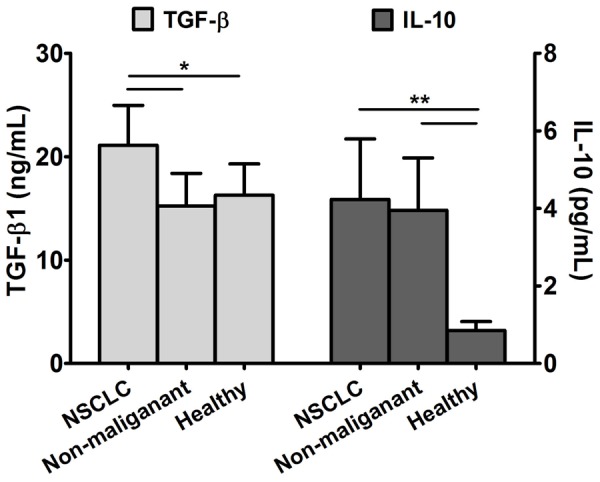
Plasma levels of TGF-β and IL-10 are elevated in NSCLC patients. All plasma samples of the 88 cases were collected and detected by ELISA (*P<0.05, **P<0.01).
Discussion
Treg cells suppress anti-tumor immune response and contribute to the immune escape mechanism of cancer [12]. Recently, the frequency and/or function of Treg cells have been reported to be elevated in peripheral blood and local tumor sites of patients with several types of cancers, including head and neck squamous cell carcinoma, breast cancer, pancreatic cancer, stomach cancer and liver cancer [9,12-16]. The increase in Treg cells correlates with tumor progression and poor prognosis of cancer patients [14,15]. Although it has been shown that the frequency of Treg cells increases during tumor progression in NSCLC [17], the mechanism in which tumor-associated Treg cells exert enhanced suppressive activity in NSCLC has not been elucidated.
Our data revealed that the percentage of Treg in CD4+ T cells in peripheral blood was significantly elevated in NSCLC patients, compared to healthy volunteers. Moreover, the number of Treg cells was significantly increased in tumor tissues, compared to normal tissues located 3-5 cm distal from the tumor. We found that in some patients, Treg cells account for as much as 60% of CD4+ T cells that infiltrate tumors. The proportion of intratumoral Treg cells was also significantly higher compared to circulating Treg cells. These results are consistent with previous studies in NSCLC and other cancer patients [12,18,19].
The analysis of cell surface molecules revealed that CTLA-4+, LAG-3+ and PD-1+ Treg cell frequencies in peripheral blood were elevated in NSCLC patients, compared to healthy volunteers. During the preparation of this study, Jie HB reported that CTLA-4, TIM-3, PD-1 and CD39 expressions were elevated in intratumoral Treg cells compared to circulating Treg cells in patients with head and neck squamous cell carcinoma [9]. We confirmed that tumor infiltrating Treg cells have increased suppressive activity compared to Treg cells in peripheral blood or in normal tissues. These data demonstrate that elevated Treg cells and increased expressions of co-inhibitory molecules might play critical roles in anti-tumor immune responses in NSCLC patients, which may contribute to tumor immune escape and tumor progression. In contrast, we found no differences in CD39 expressions between CD4+ T cells or Treg cells among the three experimental groups, and in CD39 expressions between tumor-infiltrating Treg cells or Treg cells from adjacent normal tissues.
The number of CD4+CD25+FOXP3+ Treg cells has been found to be elevated in the peripheral blood of NSCLC patients compared to patients with non-malignant lung diseases [12]. In contrast, we found no significant difference in the number of Treg cells or expression of co-inhibitory molecules between NSCLC patients and patients with non-malignant lung disease. These differences might be partly due to the different compositions of non-malignant diseases in the experimental group. Our cohort included patients with pulmonary tuberculosis, pulmonary hemangioma, cryptogenic organizing pneumonia, lung abscess and pulmonary bullae.
We found that plasma levels of both TGF-β and IL-10 were elevated in NSCLC patients. TGF-β regulates the proliferation and differentiation of a variety of immune cells, including dendritic cells, macrophages, natural killer cells, and functional subsets of CD4+ T cells. IL-10 is synthesized and secreted by several types of immune cells, epidermal cells, and some tumor cells. TGF-β and IL-10 secreted by Treg cells in the tumor microenvironment can inhibit the host’s anti-tumor immune response [20,21]. Due to limited blood samples obtained from patients, it is difficult to directly evaluate the potential relationship between the amount of TGF-β and IL-10 secreted by Treg cells and the immunosupression function of Treg cells in vitro. This issue should be further addressed in mice models of NSCLC.
Depletion of tumor-specific Treg cells and/or blockade of co-inhibitory molecules such as CTLA-4 and PD-1 are being tested for treating malignancies. It has been found that anti-CD25 monoclonal antibody could eradicate tumor cells in mice tumor models by eliminating Treg cells and inducing tumor-specific effector T cells [22]. Currently, anti-CD25 monoclonal antibody or IL-2 fusion toxin (Denileukin diftitox, Ontak) [23], anti-CCR4 monoclonal antibody [24], anti-PD-1 or anti-PD-L1 monoclonal antibody [25,26], and immunotherapy targeting GITR or OX40 on Treg cells have all been undergoing different stages of clinical trials [27]; and some of them have shown promising clinical outcomes [25,26]. Anti-CTLA-4 monoclonal antibody (Ipilimumab) has been approved by the FDA for treating malignant melanoma in 2010 [28,29], and anti-PD-1 antibody (Pembrolizumab) was approved for patients with advanced or unresectable melanoma in 2014 [30]. Therefore, targeting Treg cells and/or inhibitory molecules is also likely to benefit NSCLC patients.
In summary, we have demonstrated that the number of CD4+CD25+FoxP3+ Treg cells was elevated in the peripheral blood of NSCLC patients; and showed that the expressions of their cell surface inhibitory molecules were elevated, including CTLA-4, LAG-3 and PD-1. We have shown that tumor infiltrating Treg cells express higher levels of co-inhibitory molecules and have increased suppressive properties compared to circulating Treg cells or Treg cells isolated from tumor adjacent tissues. Increased plasma levels of TGF-β and IL-10 in NSCLC patients did not correlate with the number of Treg cells or expression of co-inhibitory molecules. This current research may provide new targets for tumor immunotherapy in NSCLC patients.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (81172883, 81102249). We thank Medjaden Bioscience Limited for their assistance on language in the preparation of this manuscript.
Disclosure of conflict of interest
None.
References
- 1.Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83:584–94. doi: 10.4065/83.5.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nishikawa H, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Curr Opin Immunol. 2014;27:1–7. doi: 10.1016/j.coi.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 3.Duraiswamy J, Kaluza KM, Freeman GJ, Coukos G. Dual blockade of PD-1 and CTLA-4 combined with tumor vaccine effectively restores T-cell rejection function in tumors. Cancer Res. 2013;73:3591–603. doi: 10.1158/0008-5472.CAN-12-4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He S, Li M, Ma X, Lin J, Li D. CD4+CD25+Foxp3+ regulatory T cells protect the proinflammatory activation of human umbilical vein endothelial cells. Arterioscler Thromb Vasc Biol. 2010;30:2621–30. doi: 10.1161/ATVBAHA.110.210492. [DOI] [PubMed] [Google Scholar]
- 5.Liang B, Workman C, Lee J, Chew C, Dale BM, Colonna L, Flores M, Li N, Schweighoffer E, Greenberg S, Tybulewicz V, Vignali D, Clynes R. Regulatory T cells inhibit dendritic cells by lymphocyte activation gene-3 engagement of MHC class II. J Immunol. 2008;180:5916–26. doi: 10.4049/jimmunol.180.9.5916. [DOI] [PubMed] [Google Scholar]
- 6.Rabe H, Nordström I, Andersson K, Lundell AC, Rudin A. Staphylococcus aureus convert neonatal conventional CD4+ T cells into FOXP3+CD25+CD127low T cells via the PD1/PD-L1 axis. Immunology. 2014;141:467–81. doi: 10.1111/imm.12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jenabian MA, Seddiki N, Yatim A, Carriere M, Hulin A, Younas M, Ghadimi E, Kök A, Routy JP, Tremblay A, Sévigny J, Lelievre JD, Levy Y. Regulatory T cells negatively affect IL-2 production of effector T cells through CD39/adenosine pathway in HIV Infection. PLoS Pathog. 2013;9:e1003319. doi: 10.1371/journal.ppat.1003319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y, Yang BH, Li H, Cao S, Ren XB, Yu JP. IDO+ DCs and signalling pathways. Curr Cancer Drug Targets. 2013;13:278–88. doi: 10.2174/15680096113139990073. [DOI] [PubMed] [Google Scholar]
- 9.Jie HB, Gildener-Leapman N, Li J, Srivastava RM, Gibson SP, Whiteside TL, Ferris RL. Intratumoral regulatory T cells upregulate immunosuppressive molecules in head and neck cancer patients. Br J Cancer. 2013;109:2629–35. doi: 10.1038/bjc.2013.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beasley MB, Brambilla E, Travis WD. The 2004 World Health Organization classification of lung tumors. Semin Roentgenol. 2005;40:90–7. doi: 10.1053/j.ro.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 11.Sobin LH, Gospodarowicz MK, Wittekind C. In: TNM Classification of Malignant Tumours. 7th Edition. Sobin LH, Gospodarowicz MK, Wittekind C, editors. Wiley-Blackwell; 2009. pp. 136–46. [Google Scholar]
- 12.Chen C, Chen D, Zhang Y, Chen Z, Zhu W, Zhang B, Wang Z, Le H. Changes of CD4+CD25+FOXP3+ and CD8+CD28- regulatory T cells in non-small cell lung cancer patients undergoing surgery. Int Immunopharmacol. 2014;18:255–61. doi: 10.1016/j.intimp.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Kim S, Lee A, Lim W, Park S, Cho MS, Koo H, Moon BI, Sung SH. Zonal difference and prognostic significance of foxp3 regulatory T cell infiltration in breast cance. J Breast Cancer. 2014;17:8–17. doi: 10.4048/jbc.2014.17.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vizio B, Novarino A, Giacobino A, Cristiano C, Prati A, Ciuffreda L, Montrucchio G, Bellone G. Potential plasticity of T regulatory cells in pancreatic carcinoma in relation to disease progression and outcome. Exp Ther Med. 2012;4:70–8. doi: 10.3892/etm.2012.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen LS, Wang J, Shen DF, Yuan XL, Dong P, Li MX, Xue J, Zhang FM, Ge HL, Xu D. CD4(+) CD25(+)CD127(low/-) regulatory T cells express Foxp3 and suppress effector T cell proliferation and contribute to gastric cancers progression. Clin Immunol. 2009;131:109–18. doi: 10.1016/j.clim.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 16.Mathai AM, Kapadia MJ, Alexander J, Kernochan LE, Swanson PE, Yeh MM. Role of Foxp3-positive tumor-infiltrating lymphocytes in the histologic features and clinical outcomes of hepatocellular carcinoma. Am J Surg Pathol. 2012;36:980–6. doi: 10.1097/PAS.0b013e31824e9b7c. [DOI] [PubMed] [Google Scholar]
- 17.Woo EY, Chu CS, Goletz TJ, Schlienger K, Yeh H, Coukos G, Rubin SC, Kaiser LR, June CH. Regulatory CD4+CD25+T cells in tumors from patients with early stage non small cell lung cancer and late stage ovarian cancer. Cancer Res. 2001;61:4766–72. [PubMed] [Google Scholar]
- 18.Black CC, Turk MJ, Dragnev K, Rigas JR. Adenocarcinoma contains more immune tolerance regulatory t-cell lymphocytes (versus squamous carcinoma) in non-small-cell lung cancer. Lung. 2013;191:265–70. doi: 10.1007/s00408-013-9455-7. [DOI] [PubMed] [Google Scholar]
- 19.Kinoshita T, Ishii G, Hiraoka N, Hirayama S, Yamauchi C, Aokage K, Hishida T, Yoshida J, Nagai K, Ochiai A. Forkhead box P3 regulatory T cells coexisting with cancer associated fibroblasts are correlated with a poor outcome in lung adenocarcinoma. Cancer Sci. 2013;104:409–15. doi: 10.1111/cas.12099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frimpong-Boateng K, van Rooijen N, Geiben-Lynn R. Regulation T cells suppress natural killer cells during plasmid DNA vaccination in mice, blunting the CD8 T cell immune response by the cytokines TGF beta. PLos One. 2010;5:e12281. doi: 10.1371/journal.pone.0012281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strauss L, Bergmann C, Szczepanski M, Gooding W, Johnson JT, Whiteside TL. A unique subset of CD4+CD25highFoxp3+T cells secreting interleukin-10 and transforming growth factorbeta mediates suppression in the tumor microvironment. Clin Cancer Res. 2007;13:4345–54. doi: 10.1158/1078-0432.CCR-07-0472. [DOI] [PubMed] [Google Scholar]
- 22.Rech AJ, Vonderheide RH. Clinical use of anti-CD25 antibody daclizumab to enhance immune responses to tumor antigen vaccination by targeting regulatory T cells. Ann N Y Acad Sci. 2009;1174:99–106. doi: 10.1111/j.1749-6632.2009.04939.x. [DOI] [PubMed] [Google Scholar]
- 23.Attia P, Maker AV, Haworth LR, Rogers-Freezer L, Rosenberg SA. Inability of a fusion protein of IL-2 and diphtheria toxin (Denileukin Diftitox, DAB389IL-2, ONTAK) to eliminate regulatory T lymphocytes in patients with melanoma. J Immunother. 2005;28:582–92. doi: 10.1097/01.cji.0000175468.19742.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sugiyama D, Nishikawa H, Maeda Y, Nishioka M, Tanemura A, Katayama I, Ezoe S, Kanakura Y, Sato E, Fukumori Y, Karbach J, Jäger E, Sakaguchi S. Anti-CCR4 mAb selectively depletes effector-type FoxP3+ CD4+ regulatory T cells, evoking antitumor immune responses in humans. Proc Natl Acad Sci U S A. 2013;110:17945–50. doi: 10.1073/pnas.1316796110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Voo KS, Bover L, Harline ML, Vien LT, Facchinetti V, Arima K, Kwak LW, Liu YJ. Antibodies targeting human OX40 expand effector T cells and block inducible and natural regulatory T cell function. J Immunol. 2013;191:3641–50. doi: 10.4049/jimmunol.1202752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simpson TR, Li F, Montalvo-Ortiz W, Sepulveda MA, Bergerhoff K, Arce F, Roddie C, Henry JY, Yagita H, Wolchok JD, Peggs KS, Ravetch JV, Allison JP, Quezada SA. Fc-dependent depletion of tumor-infiltrating regulatory T cells codefines the efficacy of anti–CTLA-4 therapy against melanoma. J Exp Med. 2013;210:1695–710. doi: 10.1084/jem.20130579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reichert JM. Antibodies to watch in 2015. MAbs. 2015;7:1–8. doi: 10.4161/19420862.2015.988944. [DOI] [PMC free article] [PubMed] [Google Scholar]